Abstract
SGLT-2 inhibitors are a novel class of anti-diabetic agents which act by inhibiting glucose reabsorption in proximal convoluted tubules of kidney. Apart from maintaining glucose homeostasis they exert a number of positive effects on the cardiovascular system like weight loss, decreasing blood pressure, preserving renal function, reducing triglycerides, natriuresis and improving endothelial dysfunction. In large clinical trials, all the three prototype agents – Empaglifozin, Canaglifozin and dapaglifozin have shown reductions in major adverse cardiovascular events including cardiovascular deaths, non fatal MI, stroke and heart failure (HF) hospitalizations. The reduction in heart failure hospitalization is a surprising finding and trials of these drug are now underway for HF also. More surprising is the fact that the benefits are comparable or even better that achieved by recently approved novel drugs for HF. In this review, we briefly discuss the pathophysiology of HF in diabetes, describe the prototype SGLT-2 molecules available, their data from large cardiovascular outcome trials till date and their role in current practice of diabetes management.
Keywords: Diabetes mellitus, heart failure, sodium glucose cotransporter 2 inhibitor
Introduction
Diabetes mellitus (DM) has been strongly associated with cardiovascular diseases (CVDs). Various randomized studies showed that a significant proportion of diabetic patients suffer from heart failure (HF, Figure 1). This is due to the abnormal cardiac handling of glucose and free fatty acids (FFAs) and the effect of the metabolic derangements of diabetes on the cardiovascular (CV) system. Also, studies have reported that incidence of HF in diabetic patients is significantly correlated with HbA1c levels.[1,2,3] Hyperglycemia is an independent risk factor for ischemic heart disease (IHD) as several mechanisms lead to vascular damage due to long-term hyperglycemia.[4] It is known that approximately, 50% cases of DM suffer from heart failure with preserved ejection fraction (HFpEF).[5] Therefore, DM has been perceived as a responsible factor for HF.
Figure 1.
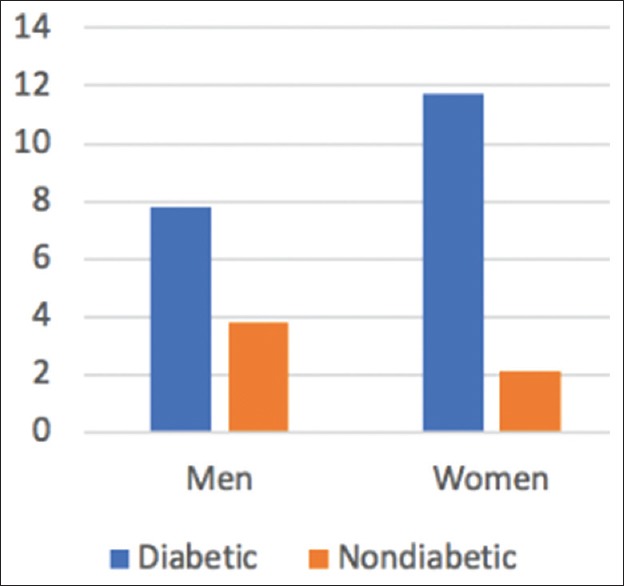
Incidence of HF as a function of DM per 1000 person years
Epidemiology
In India, the prevalence of diabetes is projected to increase from 32 million (2000) to 70 million (2025). The incidence of HF has been demonstrated to increase from 2.3 per 1000 person-years for an HbA1c <6% to 11.9 per 1000 person-years for an HbA1c >11.9%. Therefore, the annual incidence of HF due to diabetes may increase from 73 600 (2000) to 161 000 (2025).[6] In patients with DM, the prevalence of HF is greater than that of the general population. The predominance of HF is more prominent in DM patients than that of the all-inclusive community [Figure 1]. In DM patients, 1% increase in HbA1c is related with an 8% increased risk for HF. The connection among DM and HF is bidirectional, with each disease independently increasing the risk for the other.[7] Also, the prevalence of hospitalizations due to HFpEF comparative to heart failure with reduced ejection fraction (HFrEF) is increasing at a rate of 1% per year.[5]
Pathophysiology of HF in diabetes
To clarify the mechanisms responsible for diminished myocardial contractility in the diabetic population, a few speculations have been proposed as elucidated in Figure 2. Long-standing metabolic and functional alterations ultimately prompt irreversible basic changes.[7,8] In the patients with diabetes, altered metabolism was associated with increased myocardial oxygen consumption and increased concentrations of free fatty acids (FFA) in serum. The overstated FFA uptake and use in HF with DM eventually leads to dysfunction of myocardial mitochondria.[7] Abnormalities in contractile and regulatory protein expression and cardiomyocyte Ca2+ sensitivity are also found in DM. In diabetes, reduced activity of the sarcoplasmic reticular (SR) calcium pump and rate of Ca2+ removal from the cytoplasm in diastole may be responsible for diastolic dysfunction.[7,8] The main pathogenic mechanism in HF and DM that leads to structural alteration is hyperglycemia. Hyperglycemia leads to glycation of numerous macromolecules that result into decreased elasticity of the vessel walls and myocardial dysfunction.[7,8]
Figure 2.
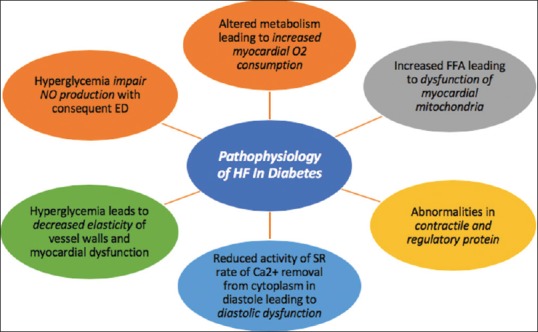
Mechanisms responsible for pathophysiology of HF in DM
Hyperglycemia induces diabetes-specific changes in microvascular architecture such as impaired nitric oxide production with consequent endothelial dysfunction, decreased vessel density, and increased permeability.[7] The rennin–angiotensin–aldosterone system (RAAS) is activated early in DM. As HF progresses, activation of the sympathetic nervous system (SNS) and RAAS increases that lead to worsening of CV and renal function.[7,8] Above data suggest that various mechanisms contribute to the impairment in systolic and diastolic function in patients with diabetes and these patients develop HF independent of the presence of coronary artery disease or its associated risk factors. Therefore, screening for HF may help to prevent its progression and reduce mortality in diabetic patients.[9]
Current therapy and challenges
Increasing pandemic of diabetes and healthcare cost for HF hospitalizations demands for appropriate selection of antidiabetic drug. Routinely used drugs like insulin therapy in Diabetes mellitus insulin-glucose infusion in acute myocardial infarction (DIGAMI) study showed a significant reduction in mortality in DM patients with CVD; however, some studies reported that insulin failed to provide CV benefit when compared to standard of care with oral therapies. Further, United Kingdom Prospective Diabetes Study (UKPDS) data reported that metformin-treated patients with DM and a history of HF had significantly decreased all-cause mortality and lower risk of readmission for HF. FIGHT, LEADER, and SUSTAIN-6 trials demonstrated that glucagon-like peptide 1 (GLP-1) agonists provide more benefit for vascular disease than for HF.
Further, it is important to reduce doses of thiazolidinediones in those with class II HF and they are best avoided in patients with class III and IV symptoms. Also, some retrospective trials reported an increased risk of HF with first and second generation sulfonylureas (SUs). Moreover, dipeptidyl peptidase-4 (DPP4) inhibitors are associated with an increase in hospitalizations for HF and in 2016; the food and drug administration (FDA) released an alert for the increased risk of HF associated with both alogliptin and saxagliptin but not with sitagliptin.[10] According to American association of clinical endocrinologists (AACE) 2017 guidelines metformin, alpha-glucosidase inhibitors possess neutral effects on HF; whereas, insulin, SUs are associated with increased risk of HF.[11] Hence, a positive strategy to address heart failure risk in diabetes is needed.
The first-line drug to manage hyperglycemia in type 2 DM is metformin. The add on drug classes recommended by the American Diabetes Association (ADA) and European Association for the Study of Diabetes for combination therapy on top of metformin are SGLT2-inhibitors (SGLT-2is), GLP-1 RAs, dipeptidyl peptidase 4 inhibitors, sulfonylureas, thiazolidinediones, and basal insulin [Figure 3].
Figure 3.
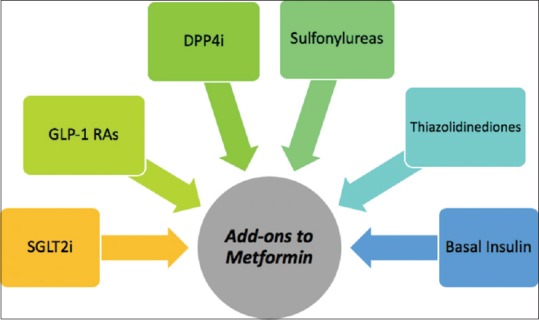
Various add-on drug options to metformin
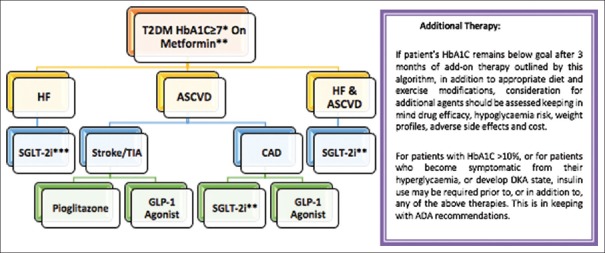
The diabetic patient with HF represents particular challenges with the pharmacologic treatment of diabetes. Current management strategies focus on known modifiable risk factors like glucose, lipids and blood pressure (BP) that produce modest effects. Even though these are important risk markers, none of these interventions substantially prevents HF or improves its outcomes.[12] Figure 4 depicts an algorithm that approaches patients with HF and atherosclerotic cardiovascular disease risk.[10] The potential importance of SGLT2 inhibition to HF and its outcomes is the important development in this field.
Figure 4.
A new algorithm for choosing therapy for a patient with T2DM. *Cardiovascular outcome data for these drug classes are available for patients with A1C of 7% or higher, but achieving an A1C 6.5% or less should be the goal for most patients, as suggested by the ADA. **If GFR > 30 mL/min/1.7 m2 and there is no other contraindication to metformin use. For GFR of 30–45 mL/min/1.7m2 assess the benefits versus risks before starting. ***For GFR < 45 mL/min/1.7m2 or less, patients should not be offered SGLT-2 inhibitors
SGLT-2 inhibitors: An overview
SGLT-2is are oral antidiabetic agents that inhibit glucose reabsorption in the renal proximal tubules and excrete glucose into the urine thus lowering blood glucose. SGLT2-is are unique antidiabetic agents as it lowers blood glucose independently of insulin and suppresses insulin secretion. In addition to glycemic control, SGLT2i also carries several pleiotropic effects. These are the only class of agents which reported to decrease the risk of CV events primarily through reduction of HF development or progression. In addition, SGLT-2 inhibitors might improve the efficiency of myocardial energetics by oxidation of β-hydroxybutyrate and increase hematocrit thus improving oxygen transport. Finally, decreased vascular stiffness and improved endothelial function are observed with the use of SGLT-2Is in diabetes.[13,14,15] Clinically available SGLT2 inhibitors are canagliflozin, dapagliflozin, empagliflozin, and ertugliflozin as approved by FDA which can be used as monotherapy as well as combination therapy. Table 1 enumerates the silent features of this new class of oral hypoglycemic agents (OHAs).[16,17] Table 2 mentions their pleiotropic effects.[17,18,19,20,21,22,23,24] Table 3 mentions the important side effects of SGLT2i.[25,26,27,28,29,30,31] Table 4 compares the pharmacokinetic, pharmacodynamic properties, clinical efficacy, and adverse events of SGLT2 inhibitors.[25,26,27,28,29,30,31]
Table 1.
| Average HbA1c drop |
| 0.79% in monotherapy |
| 0.61% in combination therapy |
| More effective at higher baseline sugar; higher filtered sugar load |
| Glucose-lowering efficacy parallels renal function |
| eGFR 30-59 mL/min/1.73 m2: HbA1c reductions 0.3-0.4% |
| eGFR <30 mL/min/1.73 m2: HbA1c no effect |
| Increases plasma glucagon levels and stimulate hepatic glucose production, which limits efficacy |
| Insulin-independent action hence effective in all stages of DM |
| Carries various pleiotropic effects |
| Well tolerated |
| Low hypoglycemia risk in patients not using sulfonylureas or insulin |
Table 2.
| Induce weight loss of 2-3 kg (initially by osmotic diuresis and later by reductions in fat mass[18,19] |
| Reductions in systolic BP (SBP) and diastolic BP (DBP) of 5 and 2 mmHg, respectively[20,21] by |
| Plasma volume contraction by osmotic diuresis |
| Weight loss |
| Improvements in vascular stiffness by reductions in body weight, hyperglycemia-associated oxidative stress, and/or endothelial glycocalyx protection from sodium overload |
| Reduced SNS activity |
| Lower serum uric acid concentrations |
| Modestly alter lipid profiles by reductions in plasma triglycerides and increases in HDL and LDL cholesterol[17] |
| Attenuate several factors associated with NASH and NAFLD, such as weight gain, elevated alanine aminotransferase, high liver fat index, and visceral fat[22] |
| Induce natriuresis, which might improve whole-body sodium balance and volume status[23] |
| Improved endothelial function and reduced vascular stiffening, decreasing the demand placed on cardiac tissue that causes left ventricular hypertrophy[24] |
| Reduction in intraglomerular pressure, hence attenuating albuminuria by 30-40%[23] |
Table 3.
| Increase risk of genital mycotic infections (4-5 times) |
| Rare DKA episodes due to reduced availability of carbohydrates caused by glycosuria, a shift in substrate utilization from glucose to fat oxidation, and promotion of hyperglucagonemia, stimulating ketogenesis |
| Canagliflozin association with higher risk of bone fractures and lower limb amputations (CANVAS Program) |
| Osmotic diuresis leads to volume depletion and orthostatic hypotension |
Table 4.
Pharmacokinetics, pharmacodynamics, clinical efficacy, and adverse events of canagliflozin, dapagliflozin, empagliflozin
| Rapidly absorbed | Oral administration | ||
|---|---|---|---|
| Canagliflozin[28,29] | Dapagliflozin[30] | Empagliflozin[27] | |
| tmax | 1.5-2.0 h | 2 h | 1.33-3.0 h, then declining in a biphasic fashion |
| Terminal elimination half-life (t ½) | 14-16 h | 13 h | 5.6-13.1 h (single dose) 10.3-18.8 h (multiple dose) |
| Renal clearance (CLR) over 72 h | <1% of the dose (100 and 200 mg dose) | - | 32.1-51.3 mL/mi |
| UGE0-24h | 100 g (100 and 300 mg dose) | - | 71.7 g (50 mg dose) |
| Clinical Efficacy[27] | |||
| HbA1c reduction* | 0.74% (Inagaki) and 0.77% (CANTATA-M)- P<0.001, 100 mg/day | 0.45% (Kaku) and 1.11% (Ji) - P<0.0001, 10 mg/day | 0.66 (Roden, the monotherapy trial) and 0.83% (Lewin)- 10 mg/day |
| Weight reduction* | 2.5 and 2.6 kg, 100 mg/day | 2.2 and 3.2 kg, 10 mg/day | 2.2 and 2.5 kg, 10 or 25 mg/day |
| Lipid* | LDL-C: ↑ by 0 and 0.15 mmol/l (100 mg/day) | LDL-C: ↑ by 0.19 and -0.03 mmol/l | LDL-C: ↑ by 0.06 and 0.11 mmol/l |
| HDL-C: ↑ by 0.07 and 0.11 mmol/l (100 mg/day) | HDL-C: ↑ by 0.16 and 0.3 mmol/l | HDL-C: ↑ by 0.10 and 0.13 mmol/l | |
| TC: ↓ by 0.12 mmol/l (300 mg/day) | TC: ↓ by 0.01-0.06 mmol/l (10-mg/day) | TC: ↓ by 0.07-0.2 mmol/l | |
| Systolic blood pressure* | ↓ by 3.3 and 7.9 mmHg (P<0.001, 100 mg/day) | ↓ by 2.3 and 3.6 mmHg (10 mg/day) | ↓ by 2.9 and 3.7 mmHg (10 or 25 mg/day) |
| ↓ by 5.0 mmHg (300 mg/day) | |||
| Adverse events**[25,26] | |||
| Urogenital tract infections | Infrequent, mild, managed with standard treatments and did not recur in any of the patients | Mild to moderate in severity, tended to occur during the first 6 months of dapagliflozin therapy | Urogenital infections were more common in women, generally mild to moderate in severity and amenable to standard treatment |
| Diabetic ketoacidosis | Very low incidences-0.5 per 1000 patient-years in 100 mg daily dose, 0.8 in 300 mg daily dose | - | - |
| Bone health | Decreased bone density and an increased risk of fractures (Incidence per 1000 patient-years was 18.1 for canagliflozin regimens and 14.2 for other regimens) | Risk of fractures (Kohan et al., 9.4% suffered fractures in 10 mg group) | - |
#Type 2 diabetes mellitus and moderate renal impairment, h: hours, UGE: urinary glucose excretion, LDL-C: low-density lipoprotein cholesterol, HDL-C: high-density lipoprotein cholesterol, TC: total cholesterol, *compared with placebo, **adverse events reported with combination and monotherapy
Clinical evidence with SGLT2-i [Table 5]
Table 5.
Comparison of EMPA-REG OUTCOME, CANVAS, DECLARE-TIMI 58, and CVD-REAL Study
| Parameters | EMPA-REG OUTCOME[32,33] (Empagliflozin) | CANVAS Program[25,34] (Canagliflozin) | DECLARE-TIMI 58[35] (Dapagliflozin) | CVD-REAL Study[36] (SGLT-2i versus other glucose-lowering drugs) |
|---|---|---|---|---|
| Patients | 7028 | 10142- Largest SGLT2i CVOT till date | 17,160 | 235,064 in SGLT-2i group. 235,064 in other glucose-lowering drugs group |
| Study duration | ~4 years (Medium term) | ~6 years (Longer term) | 4.2 years (Medium term) | ~14 months (Medium term) |
| Duration of diabetes | >1 to 5 years: 15.2% >5 to 10 years: 25.1% >10 years: 57.0% | 13.5 years (mean) | - | - |
| Primary prevention | No | 35% of patients | 59% of patients | 73% of patients |
| Secondary prevention | Yes | 65% of patients | 41% of patients | 27% of patients |
| Types of patients | All patients with established CVD | 65% patients with established CVD (Secondary Prevention) 35% patients with CV risk factors* (Primary Prevention) *Diabetes duration ≥10 years, SBP>140 mmHg on ≥1 medication, current smoker, micro- or macro-albuminuria, or HDL cholesterol <1 mmol/L | ≥40 year old with T2DM | All T2DM >18 years old |
| Results | ||||
| MACE | 0.86 (0.74-0.99), P=0.04 | 0.86 (0.75-0.97), P=0.02 | 0.93; 95% CI, 0.84-1.03; P=0.17 | |
| CV deaths | 0.62 (0.49-0.77), P<0.001 | 0.86 (0.75-0.97) | 0.83; 95% CI, 0.73 to 0.95; P=0.005) | 0.49; 95% CI, 0.41-0.57; P<0.001 |
| Nonfatal MI | 0.87 (0.70-1.09), P=0.23 | 0.85 (0.69-1.05) | 0.89; 95% CI, 0.77-1.01 | 0.81; 95% CI, 0.74-0.88: P<0.001 |
| Nonfatal stroke | 1.24 (0.92-1.67), P=0.16 | 0.90 (0.71-1.15) | ||
| Fatal or nonfatal stroke | 1.18 (0.89-1.56), P=0.26 | 0.87 (0.69-1.09) | 1.01; 95% CI, 0.84-1.21 | 0.68; 95% CI, 0.55-0.84; P<0.001 |
| CV death | 0.62 (0.49-0.77), P<0.001 Treatment Vs. Placebo: 12.4/1000 patient-years vs. 20.2/1000 patient-years | 0.87 (0.72-1.06) 11.6/1000 patient-years vs. 12.8/1000 patient-years | 0.83; 95% CI, 0.73 to 0.95; P=0.005) | 0.49; 95% CI, 0.41-0.57; P<0.001 |
| Heart failure benefits | 35% reduction in HHF risk | 33% reduction in HHF risk | 0.73; 95% CI: 0.61 to 0.88 | 0.64; 95% CI: 0.50 to 0.82; P<0.001 |
| Risk of stroke | Nonsignificant increase in risk of stroke with | Nonsignificant reduction in stroke risk | ||
| Composite renal endpoint | 46% reduction (post hoc analysis) | 40% reduction (prespecified outcome) | 0.76; 95% CI: 0.67 to 0.87 |
The first large-scale CV safety trial with SGLT2 inhibitors in type 2 diabetes mellitus patients (EMPA-REG OUTCOME) trial reported beneficial effects on the CV events and hospitalization for HF in patients without HF at baseline, which suggests SGLT2I is a unique glucose-lowering agent and possess multifaceted effects on hemodynamic and metabolic parameters.[32] Figure 5 elicits the various potential mechanisms of CV benefits of SGLT2i.[16] SGLT2i can be used in selected patients’ population with substantial CV risk, but it should be prescribed with high caution in high-risk patients, especially those taking diuretics.[29]
Figure 5.
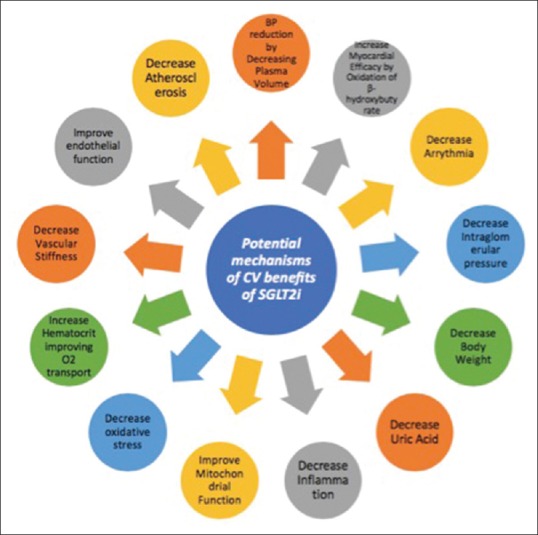
Various potential mechanisms of cardiovascular benefits of SGLT2i
EMPA-REG OUTCOME trial with empagliflozin reported that patients with type 2 diabetes and high CV risk who received empagliflozin as add-on therapy to standard-of-care drugs as compared to placebo showed a lower rate of occurrence of primary CV outcomes and overall mortality. Also, the trial reported 0.38%–0.85% reduction in HbA1c levels with empagliflozin than placebo.[32,33] Another study analyzed data from two cohorts of patients participating in randomized trials, one treated with double-blind EMPA versus placebo for 12 weeks (cohort 1) and one treated with double-blind EMPA versus placebo for 24 weeks (cohort 2) to assess the effects of empagliflozin on adiposity indices among patients with DM. The study reported that empagliflozin reduced weight, waist circumference, and adiposity indices. Mean change from baseline in empagliflozin versus placebo was −1.7 kg and −1.9 kg for body weight (P < 0.001); −1.3 cm and −1.3 cm for waist circumference (P < 0.001); −0.2% and −0.3% P = 0.08) for estimated total body fat; −0.007 and − 0.008 for index of central obesity (P < 0.001); and − 0.3 and − 0.4 (P = 0.003) for visceral adiposity in cohorts 1 and 2, respectively. The study concluded that empagliflozin significantly reduced weight and adiposity indices with the potential to improvement in cardiometabolic risk among patients with DM.[32] However, in the EMPA-REG OUTCOME trial, in the first 30 days more events of acute kidney injury were reported in the empagliflozin-treated group (0.9%) versus the placebo group (0.7%), which highlights the importance of pragmatic use of SGLT2i to optimize the possible benefits and minimize associated risk.[32]
The Canagliflozin Cardiovascular Assessment (CANVAS) Study assessed the efficacy, safety, and durability of canagliflozin in more than 10,000 patients with type 2 diabetes, who had either a prior history of CV disease or at least two CV risk factors. The results showed that canagliflozin reduced the CV and nonfatal myocardial infarction (26.9 vs. 31.5%). The drug also demonstrated potential renal protective effects. Further, canagliflozin was found to increase the risk of amputation—a result corroborated in the CANVAS and CANVAS-R studies.[25,31,34] Also, European Medicines Agency focused on potential increased risk of lower limb amputation in patients taking the SGLT2 inhibitors canagliflozin, dapagliflozin, and empagliflozin.[29] Another study with DM patients (with moderate renal impairment and elevated CV risk) showed that treatment with canagliflozin was associated with clinically significant, dose-dependent reductions in HbA1c, as monotherapy and as part of combination therapy. In addition to reducing HbA1c levels, phase 3 studies of canagliflozin reported dose-dependent reductions in body weight that are enhanced by reductions in visceral adiposity, which may reduce CV complications and mortality.[29] Additional study reported the effects of canagliflozin on CV biomarkers in older patients with DM. The study showed that serum N-terminal pro-B-type natriuretic peptide, high-sensitivity troponin I, and soluble ST2 remained unchanged in canagliflozin. Serum galectin-3 modestly increased from baseline with canagliflozin versus placebo. These cardiac biomarker data support for the beneficial CV effect of SGLT2Is in DM patients.[29]
The DECLARE TIMI 58 trial (Dapagliflozin Effect on Cardiovascular Events-Thrombolysis in Myocardial Infarction 58)[35] assessed the cardiovascular safety profile of dapagliflozin. It evaluated 17,160 patients, including 10,186 without atherosclerotic CV disease, who were followed for a median of 4.2 years. In the primary safety outcome analysis, dapagliflozin met the prespecified criterion for noninferiority to placebo with respect to major adverse cardiovascular events (MACE upper boundary of the 95% confidence interval [CI], <1.3; P < 0.001 for noninferiority). In the two primary efficacy analyses, dapagliflozin did not result in a lower rate of MACE (8.8% in the dapagliflozin group and 9.4% in the placebo group; hazard ratio, 0.93; 95% CI, 0.84 to 1.03; P = 0.17) but did result in a lower rate of CV death or hospitalization for HF (HHF) (4.9% vs. 5.8%; hazard ratio, 0.83; 95% CI, 0.73–0.95; P = 0.005), which reflected a lower rate of HHF (hazard ratio, 0.73; 95% CI, 0.61–0.88); there was no between-group difference in CV death (hazard ratio, 0.98; 95% CI, 0.82–1.17). A renal event occurred in 4.3% in the dapagliflozin group and in 5.6% in the placebo group (hazard ratio, 0.76; 95% CI, 0.67–0.87), and death from any cause occurred in 6.2% and 6.6%, respectively (hazard ratio, 0.93; 95% CI, 0.82–1.04). Diabetic ketoacidosis (DKA) was more common with dapagliflozin than with placebo (0.3% vs. 0.1%, P = 0.02), as was the rate of genital infections that led to discontinuation of the regimen or that were considered to be serious adverse events (0.9% vs. 0.1%, P < 0.001). The trial concluded that in patients with type 2 diabetes who had or were at risk for atherosclerotic CV disease, treatment with dapagliflozin did not result in a higher or lower rate of MACE than placebo but did result in a lower rate of CV death or HHF.
In CVD-REAL Study (Comparative Effectiveness of Cardiovascular Outcomes in New Users of Sodium-Glucose Cotransporter-2 Inhibitors),[36] there were 309,056 patients newly initiated on either SGLT-2i or other glucose-lowering drugs (154 528 patients in each treatment group). Canagliflozin, dapagliflozin, and empagliflozin accounted for 53%, 42%, and 5% of the total exposure time in the SGLT-2i class, respectively. Baseline characteristics were balanced between the two groups. There were 961 HHF cases during 190 164 person-years follow-up (incidence rate, 0.51/100 person-years). Of 215,622 patients in the United States, Norway, Denmark, Sweden, and the United Kingdom, death occurred in 1334 (incidence rate, 0.87/100 person-years), and HHF or death in 1983 (incidence rate, 1.38/100 person-years). Use of SGLT-2i, versus other glucose-lowering drugs, was associated with lower rates of HHF (hazard ratio, 0.61; 95% confidence interval, 0.51-0.73; P < 0.001); death (hazard ratio, 0.49; 95% CI, 0.41-0.57; P < 0.001); and HHF or death (hazard ratio, 0.54; 95% confidence interval, 0.48-0.60; P < 0.001) with no significant heterogeneity by country. It was concluded that treatment with SGLT-2i versus other glucose-lowering drugs was associated with a lower risk of HHF and death. These analyses suggest that in real-world practice SGLT-2i can be applicable to a broad population of patients with type 2 DM.
SGLT2i versus other HF Medications
When pitted against the two other new HF medication approved in the last 5 years (Ivarbradine and Angiotensin Receptor blocker and Neprilysin inhibitor –ARNI), SGLT-2i have shown comparable benefits although they were not primarily developed for HF patients.
In the SHIFT study, heart rate reduction with ivabradine improved clinical outcomes in HF.[37] In this study, 6558 patients were randomly assigned to treatment groups (3268 ivabradine, 3290 placebo). Median follow-up was 22.9 months. 793 (24%) patients in the ivabradine group and 937 (29%) of those taking placebo had a primary endpoint event (HR 0.82, 95% CI 0.75–0.90, P < 0.0001). The effects were driven mainly by hospital admissions for worsening HF (672 [21%] placebo vs 514 [16%] ivabradine; HR 0.74, 0.66–0.83; P < 0.0001) and deaths due to HF(151 [5%] vs 113 [3%]; HR 0.74, 0.58–0.94, P = 0.014). On the other hand, in the more recent PARADIGM-HF trial, it was shown that sacubitril/valsartan combination (better known as ARNI) was associated with a reduction in cardiovascular death or HF Hospitalizations (HFH).[38] In this trial 8442 patients with class II- IV HF and an ejection fraction of 40% or less were randomly assigned to receive either sacubitril/valsartan (ARNI) (at a dose of 200 mg twice daily) or enalapril (at a dose of 10 mg twice daily), in addition to recommended therapy. The primary outcome was a composite of death from cardiovascular causes or HFH. After a median follow-up of 27 months, there was 20% reduction in the primary outcome in the ARNI group when comopared to the enalapril group (21.8% vs. 26.5%; HR in ARNI group, 0.80; 95% CI 0.73 to 0.87; P < 0.001). Death form any cause was reduced by 16% in pateints receiving ARNI (0.84; 95% CI, 0.76 to 0.93; P < 0.001) while deaths attributed to cardiovascular causes were laso recuded by 20% (HR, 0.80; 95% CI, 0.71 to 0.89; P < 0.001). On the same note, sacubitril/valsartan also reduced the risk of HFH by 21% (P < 0.001) and decreased the symptoms and physical limitations of HF (P = 0.001).
Table 6 compares the newer HF medications ARNI and ivabradine to SGLT-2i with respect to their heart failure benefits. We can see that the SGLT-2i provide comparable MACE reducitons while providing better redcutions in HF hopsitalizations despite not being primarily approved for reatment of HF. The promising reduction in HF hospitalizations are almost 10% higher than that achieved by recently apporved HF medications. Although the trials of SGLT-2i did not strictly include HF patients per se but diabetes mellitus remians an imoprtant driver and risk factor for HF. It will be interesting to see how these drugs will behave in patients with Heart failure per se. Nevertherless, empaglifozin is being tried in HF patients with both preserved and reduced ejection fraction cohorts (EMPEROR HF-Reduced; NCT03057977 and EMPEROR HF-Preserved; NCT03057951).
Table 6.
Comparison of SGLT-2i with the newer HF medication approved in the last decade
| CVOT Parameters | SGLT-2i | Ivabradine (SHIFT Study) | Sacubitril/Valsartan (PARADIGM-HF Trial) |
|---|---|---|---|
| MACE Reduction | Empagliflozin: 14% | 18% | 16% |
| Canagliflozin: 14% | |||
| Dapagliflozin: 7% | |||
| CV deaths Reduction | Empagliflozin: 38% | 9% | 20% |
| Canagliflozin: 14% | |||
| Dapagliflozin: 17% | |||
| HF Hospitalization Reduction | Empagliflozin: 35% | 26% | 21% |
| Canagliflozin: 33% | |||
| Dapagliflozin: 27% |
CVOT- Cardiovascular outcome trials; MACE – major adverse cardiovascular events; SHIFT – Systolic heart failue treatment with the IF inhibitor ivabradine trial; PARADIGH-HF – propsrective comprison of ARNi with ACEI ot determine impact on global mortality & morbidity in heart failure trial
Implications for Clinical Practice
As we see in real world scenario, primary care physician is the first contact of a patient for the consultation of his/her illness. India being the world's diabetes capital, makes it the most commonly consulted noncommunicable disease. Hence, it is essential that primary care physician should be well versed with first-line therapies of this epidemiological illness. SGLT2-i's have been approved as first-line antihyperglycemic therapies along with metformin in recent guidelines. In addition to the primary benefit of antihyperglycemic agents, these drugs possess several pleiotropic effects, thus providing benefits beyond glycemic control. Various studies have shown beneficial effects on CV risk factors by reduction in BP, improvement in endothelial function and arterial stiffness, promoting weight loss, improving lipid profile, attenuating non-alcoholic steatohepatitis (NASH) and nonalcoholic fatty liver disease (NAFLD) etc.[17,18,19,20,21,22,23,24] Further, the incidences of adverse events in clinical trials of SGLT2i were similar to that observed with other antidiabetic drugs. The overall incidences of serious events have varied between 1.0% and 12.6%. The most frequently noticed adverse events in subjects on SGLT2i are urogenital tract infections. The osmotic diuresis leads to the volume depletion and orthostatic hypotension with SGLT2i use. The risk of hypoglycemia is minimal with SGLT2is.[25,26,27,39] These extraordinary features of this new class have attracted attention of endocrinologists as well as cardiologists, and has earned the status of first line OHAs in leading guidelines. Hence, more and more diabetic patients are being prescribed these drugs. Hence, it is imperative for the primary care physician to be well versed with doses, titration, and follow-up of patients on SGLT2 inhibitors.
Conclusion
The safety and efficacy data of SGLT2is (including canagliflozin, dapagliflozin, and empagliflozin) so far suggest that SGLT2is could add a new dimension to the management o DM. On the contrary, significant reductions of CV death and heart failure hospitalizations with these agents have opened up a new avenue for management of HF with DM. The benefits in HHF achieved by these drugs are comparable to that achieved by novel heart failure medications approved. Future research is on regarding the potential role these agents in heart failure per se. The success of these drugs advocate to move away from a “Glucocentric” approach in DM to a more wholesome “Cardiovascular”or “Cardiocentric” approach.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- 1.Bauters C, Lamblin N, Mc Fadden EP, Van Belle E, Millaire A, de Groote P, et al. Influence of diabetes mellitus on heart failure risk and outcome. Cardiovasc Diabetol. 2003;2:1. doi: 10.1186/1475-2840-2-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Johansson I, Dahlström U, Edner M, Näsman P, Rydén L, Norhammar A. Risk factors, treatment and prognosis in Men and Women with heart failure with and without diabetes. Heart. 2015;10:1139–48. doi: 10.1136/heartjnl-2014-307131. [DOI] [PubMed] [Google Scholar]
- 3.Rosano GMC, Vitale C, Seferovic P. Heart failure in patients with diabetes mellitus. Card Fail Rev. 2017;3:52–5. doi: 10.15420/cfr.2016:20:2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dresslerová I, Vojácek J. Diabetes Mellitus And Ischemic Heart Disease. Vnitr Lek. 2010;56:301–6. [PubMed] [Google Scholar]
- 5.Kumar M, Gautham RK, Singh PS, Kumar G, Kant P, Sharma H, et al. A study of diastolic dysfunction in patients of type-2 diabetes mellitus in rural population of western Uttar Pradesh. J Evid Based Med Healthc. 2017;4:1608–11. [Google Scholar]
- 6.Huffman MD, Prabhakaran D. Heart failure: Epidemiology and prevention in India. Natl Med J India. 2010;23:283–8. [PMC free article] [PubMed] [Google Scholar]
- 7.Cas AD, Khan SS, Butler J, Mentz RJ, Bonow RO, Avogaro A, et al. Impact of diabetes on epidemiology, treatment, and outcomes of patients with heart failure. JACC. 2015;3:136–45. doi: 10.1016/j.jchf.2014.08.004. [DOI] [PubMed] [Google Scholar]
- 8.Kasznicki J, Drzewoski J. Heart failure in the diabetic population-pathophysiology, diagnosis and management. Arch Med Sci. 2014;10:546–56. doi: 10.5114/aoms.2014.43748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lehrke M, Marx N. Diabetes mellitus and heart failure. Am J Med. 2017;130:S40–50. doi: 10.1016/j.amjmed.2017.04.010. [DOI] [PubMed] [Google Scholar]
- 10.Valentina R, Weiss MC, Weintraub H, Goldberg IJ, Schwartzbard A. Cardiovascular disease leads to a new algorithm for diabetes treatment. J Clin Lipidol. 2017;11:1126–33. doi: 10.1016/j.jacl.2017.07.004. [DOI] [PubMed] [Google Scholar]
- 11.Garber AJ, Abrahamson MJ, Barzilay JI, Blonde L, Bloomgarden ZT, Bush MA, et al. Consensus statement by the american association of clinical endocrinologists and american college of endocrinology on the comprehensive type 2 diabetes management algorithm-2017 executive summary. Endocr Pract. 2017;23:207–38. doi: 10.4158/EP161682.CS. [DOI] [PubMed] [Google Scholar]
- 12.Thomas MC. Type 2 diabetes and heart failure: Challenges and solutions. Curr Cardiol Rev. 2016;12:249–55. doi: 10.2174/1573403X12666160606120254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Packer M, Anker SD, Butler J, Filippatos G, Zannad F. Effects of Sodium-Glucose Cotransporter 2 Inhibitors for the Treatment of Patients With Heart Failure: Proposal of a Novel Mechanism of Action. JAMA Cardiol. 2017;2:1025–9. doi: 10.1001/jamacardio.2017.2275. [DOI] [PubMed] [Google Scholar]
- 14.Kimura G. Diuretic action of Sodium-glucose cotransporter 2 inhibitors and its importance in the management of heart failure. Circ J. 2016;80:2277–81. doi: 10.1253/circj.CJ-16-0780. [DOI] [PubMed] [Google Scholar]
- 15.Martens P, Mathieu C, Verbrugge FH. Promise of SGLT2 inhibitors in heart failure: Diabetes and beyond. Curr Treat Options Cardiovasc Med. 2017;19:23. doi: 10.1007/s11936-017-0522-x. [DOI] [PubMed] [Google Scholar]
- 16.Vasilakou D, Karagiannis T, Athanasiadou E, Mainou M, Liakos A, Bekiari E, et al. Sodium-glucose cotransporter 2 inhibitors for type 2 diabetes: A systematic review and metaanalysis. Ann Intern Med. 2013;159:262–74. doi: 10.7326/0003-4819-159-4-201308200-00007. [DOI] [PubMed] [Google Scholar]
- 17.van Bommel EJ, Muskiet MH, Tonneijck L, Kramer MH, Nieuwdorp M, van Raalte DH. SGLT2 inhibition in the diabetic kidney-from mechanisms to clinical outcome. Clin J Am Soc Nephrol. 2017;12:700–10. doi: 10.2215/CJN.06080616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sha S, Polidori D, Heise T, Natarajan J, Farrell K, Wang SS, et al. Effect of the sodium glucose co-transporter 2 inhibitor canagliflozin on plasma volume in patients with type 2 diabetes mellitus. Diabetes Obes Metab. 2014;16:1087–95. doi: 10.1111/dom.12322. [DOI] [PubMed] [Google Scholar]
- 19.Ferrannini G, Hach T, Crowe S, Sanghvi A, Hall KD, Ferrannini E. Energy balance after sodium- glucose cotransporter 2 inhibition. Diabetes Care. 2015;38:1730–5. doi: 10.2337/dc15-0355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Baker WL, Buckley LF, Kelly MS, Bucheit JD, Parod ED, Brown R, et al. Effects of sodium-glucose cotransporter 2 inhibitors on 24-hour ambulatory blood pressure: A systematic review and meta-analysis. J Am Heart Assoc. 2017;6:e005686. doi: 10.1161/JAHA.117.005686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Muskiet MHA, van Bommel EJM, van Raalte DH. Antihypertensive effects of SGLT2 inhibitors in type 2 diabetes. Lancet Diabetes Endocrinol. 2016;4:188–9. doi: 10.1016/S2213-8587(15)00457-X. [DOI] [PubMed] [Google Scholar]
- 22.Sumida Y, Yoneda M. Current and future pharmacological therapies for NAFLD/NASH. J Gastroenterol. 2018;53:362–76. doi: 10.1007/s00535-017-1415-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Heerspink HJ, Perkins BA, Fitchett DH, Husain M, Cherney DZ. Sodium glucose cotransporter 2 inhibitors in the treatment of diabetes mellitus: Cardiovascular and kidney effects, potential mechanisms, and clinical applications. Circulation. 2016;134:752–72. doi: 10.1161/CIRCULATIONAHA.116.021887. [DOI] [PubMed] [Google Scholar]
- 24.Lytvyn Y, Bjornstad P, Udell JA, Lovshin JA, Cherney DZI. Sodium glucose cotransporter-2 inhibition in heart failure: Potential mechanisms, clinical applications, and summary of clinical trials. Circulation. 2017;136:1643–58. doi: 10.1161/CIRCULATIONAHA.117.030012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Neal B, Perkovic V, Mahaffey KW, de Zeeuw D, Fulcher G, Erondu N, et al. Canagliflozin and cardiovascular and renal events in type 2 diabetes. N Engl J Med. 2017;377:644–57. doi: 10.1056/NEJMoa1611925. [DOI] [PubMed] [Google Scholar]
- 26.Rosenstock J, Ferrannini E. Euglycemic diabetic ketoacidosis: A predictable, detectable and preventable safety concern with SGLT2 inhibitors. Diabetes Care. 2015;38:1638–42. doi: 10.2337/dc15-1380. [DOI] [PubMed] [Google Scholar]
- 27.Scheen AJ. Pharmacokinetic and pharmacodynamic profile of empagliflozin, a sodium glucose co-transporter 2 inhibitor. Clin Pharmacokinet. 2014;53:213–25. doi: 10.1007/s40262-013-0126-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Devineni D, Curtin CR, Polidori D, Gutierrez MJ, Murphy JBS, Rusch S, et al. Pharmacokinetics and pharmacodynamics of canagliflozin, a Sodium glucose co-transporter 2 inhibitor, in subjects with type 2 diabetes mellitus. J Clin Pharmacol. 2013;53:601–10. doi: 10.1002/jcph.88. [DOI] [PubMed] [Google Scholar]
- 29.Inagaki N, Kondo K, Yoshinari T, Ishii M, Sakai M, Kuki H, et al. Pharmacokinetic and pharmacodynamic profiles of canagliflozin in Japanese patients with type 2 diabetes mellitus and moderate renal impairment. Clin Drug Investig. 2014;34:731–42. doi: 10.1007/s40261-014-0226-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Saeed MA, Narendran P. Dapagliflozin for the treatment of type 2 diabetes: A review of the literature. Drug Des Devel Ther. 2014;8:2493–505. doi: 10.2147/DDDT.S50963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tucker ME. CANVAS: Canagliflozin Reduces CV Events, But At Cost Of Amputations. Medscape-June 12. 2017 [Google Scholar]
- 32.Saiz LC. The EMPA-REG OUTCOME Trial (Empagliflozin).A Critical Appraisal-The Power of Truth, The Truth of Power. Drug and Therapeutics Bulletin of Navarre. 2016;24:1–14. [Google Scholar]
- 33.Alzaid A. Empa's new clothes: The untold story of the Empa-Reg outcome trial. Diabetes Technol Ther. 2017;19:324–7. doi: 10.1089/dia.2017.0033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Canagliflozin Cardiovascular Assessment Study-CANVAS. Available from: http://www.Acc.Org/Latest-In-Cardiology/Clinical-Trials/2017/06/12/16/25/Canvas .
- 35.Wiviott SD, Raz I, Bonaca MP, Mosenzon O, Kato ET, Cahn A, et al. Dapagliflozin and cardiovascular outcomes in type 2 diabetes. N Engl J Med. 2019;380:347–57. doi: 10.1056/NEJMoa1812389. [DOI] [PubMed] [Google Scholar]
- 36.Kosiborod M, Cavender MA, Az F, Wilding JP, Khunti K, Holl RW, et al. Lower risk of heart failure and death in patients initiated on Sodium-glucose cotransporter-2 inhibitors versus other glucose-lowering drugs: The CVD-REAL study (Comparative effectiveness of cardiovascular outcomes in new users of sodium-glucose cotransporter-2 inhibitors) Circulation. 2017;136:249–59. doi: 10.1161/CIRCULATIONAHA.117.029190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Swedberg K, Komjada M, Bohm M, Borer JS, Ford I, Dubost-brama A, et al. Ivabradine and outcomes in chronic heart failure(SHIFT): a randomised placebo controlled study. Lancet. 2010;376:875–885. doi: 10.1016/S0140-6736(10)61198-1. [DOI] [PubMed] [Google Scholar]
- 38.Mc Murray JJ, Packer M, Desai AS, Gong J, Lefkowitz MP, Rizkala AR, et al. Angiotensin–Neprilysin Inhibition versus Enalapril in Heart Failure (PARADIGM-HF) N Engl J Med. 2014;371:993–1004. doi: 10.1056/NEJMoa1409077. [DOI] [PubMed] [Google Scholar]
- 39.Prasanna Kumar K, Ghosh S, Canovatchel W, Garodia N, Sujith Rajashekar. A review of clinical efficacy and safety of canagliflozin 300 mg in the management of patients with type 2 diabetes mellitus. Indian J Endocrinol Metab. 2017;21:196–209. doi: 10.4103/2230-8210.196016. [DOI] [PMC free article] [PubMed] [Google Scholar]