Abstract
Background:
Vitamin D is an important vitamin required to maintain normal skeletal as well as nonskeletal functions. The daily supplementation of vitamin D not only have poor adherence to the regimen but also of doubtful efficacy in deficient patients.
Objectives:
The aim of this study was to compare the effect of oral high-dose vitamin D regimens (60,000 IU weekly) and daily low-dose vitamin D regimen of 1000 IU in mitigating symptoms and increase in serum levels of vitamin D in patients with hypovitaminosis D.
Materials and Methods:
A total of 90 patients aged 18–60 years with vitamin D deficiency (serum levels < 30 ng/mL) were enrolled. A total of 38 subjects received 60,000 IU of vitamin D weekly with 500 mg/day calcium and 40 subjects received a dose of 1000 IU of vitamin D daily with 500 mg/day calcium for 10 weeks. Baseline and follow-up total serum vitamin D levels and improvement in symptoms were measured within and between groups.
Results:
For high-dose vitamin D (60,000 IU weekly), the increase in mean serum vitamin D levels from baseline was 28.33 ng/mL over 10 weeks’ treatment period; whereas for the low-dose group (1000 IU daily) the mean increment in serum vitamin D was 6.79 ng/mL for the same period. The mean difference in increase in serum vitamin D between two groups was highly significant (P < 0.001). In both the groups, decrease in myalgia as evaluated on visual analog scale was observed after 10 weeks.
Conclusions:
High-dose vitamin D (60,000 IU weekly) regimen rapidly normalized 25(OH) D levels and ensure symptomatic relief earlier than daily dosing of 1000 IU vitamin D for same duration.
Keywords: Dosage forms, hypovitaminosis, muscle pain, regimens, visual analog scale, Vitamin D
Introduction
Vitamin D deficiency has been a global pandemic.[1] Its deficiency leads to secondary hyperparathyroidism, increased bone turnover, and bone loss, predisposing individuals to osteoporosis and osteoporotic fractures.[2,3] The optimal serum level of vitamin D required for maintenance of bone health.[4] However, the appropriate dosage for replacement therapy is still debatable. Though, many experts agree that vitamin D deficiency is defined as circulating levels of 25-hydroxyvitamin D (25[OH] D) less than 20 ng/mL.[5]
Many studies from various parts of our country have revealed widespread vitamin D deficiency in India in all age groups. Such a large population is largely catered by the primary care physicians and family physicians. It was found that 98% of the general population may be benefitted by vitamin D supplements.[6] We were of the opinion that the daily dosages of vitamin D supplementation has not only poor adherence to the regimen but also of doubtful efficacy in deficient patients. We hypothesized that instead of small daily dosages, high vitamin D doses at less frequent intervals would address the issue of compliance with improved efficacy. Scientific literature search did not provide any clue on consensus over dose and duration to be used in different populations. Therefore, we determined the efficacy of two common regimens (1000 or 60,000 IU) along with compliance in an open label randomized study.
Methods
Study design
A prospective, randomized, observational study was conducted in Department of General Medicine at a tertiary care teaching hospital. The study took overall 6 months for recruitment and follow-up. Adults (18–65 years of age) with musculoskeletal symptoms attending Medicine Outpatient Department (OPD) were included in the study based on the following criteria:
Inclusion criteria
Patients of either sex having musculoskeletal features of hypovitaminosis D like muscle pain, fatigue, body ache, lethargy, and numbness.
Patients between 18 and 65 years, of either gender.
Exclusion criteria
Pregnant and lactating women.
Patients above 65 years.
A patient who has taken vitamin D in the last 3 months.
History thyroid, parathyroid, renal, or any metabolic disease.
Patients on steroid, antiepileptics, statins, proton pump inhibitors, and diuretics.
Sample Size: In view of availability of patients, time constraint and resources, no formal sample size calculation was done. It was decided to include a reasonable number of patients to have meaningful, valid, and credible results. A total of 90 patients (age between 18 and 65 years) attending medicine OPD were randomized after obtaining approval from Institutional Ethics Committee (IEC). Patients with musculoskeletal features like muscle pain, fatigue, body ache, lethargy, and numbness suggestive of vitamin D deficiency and fulfilling inclusion criteria were offered participation in the study. Informed consent was taken from all patients. Patient's demographic data were collected at the time of visit to the medicine OPD.
Randomization: The randomization list was computer generated prior to the start of the study and kept with third party who was not involved with the study at all. Block randomization with block size of 6 and 4 was taken to ensure equal patient number in both the groups. Further, to ensure concealment, the block sizes were also randomized. It was a single-blinded study and patients involved in the study were unaware of assignment to treatment groups. Subjects eligible for the study were divided in two groups. Each group consisted of 45 subjects. Vitamin D supplementation was given irrespective of their baseline vitamin D levels (<30 ng/dL). Group 1 received 60,000 IU/week of vitamin D3 for 10 weeks along with calcium carbonate (500 mg elemental calcium/day) and group 2 received 1,000 IU/day of vitamin D3 for 10 weeks along with calcium carbonate (500 mg elemental calcium/day). The treatment was given free of cost to the patients. In the study, compliance to the study medication was assessed by verbal questioning from the patient and was relied upon whatever patient told to us. After 10 weeks, serum vitamin D levels were measured.
A clinical profile sheet was specifically designed to capture information like onset of symptoms and musculoskeletal symptoms like myalgia. Patients were asked questions regarding muscle pain and scoring on pain grade is given by visual analog scale.[7]
Procedure of estimation of vitamin D
Venous blood samples were collected to measure the level of Vitamin D levels. 25-hydroxy vitamin D estimation was done by chemiluminescence immunoassay, a quantitative immunoassay method processed by a fully automated analyzer, (DiaSorin LIAISON, Germany). The venous samples collected were immediately transported to the laboratory for estimation.
Cut-off to define an inefficient/insufficient serum vitamin D levels (circulating concentration of 25(OH) D is above 20 ng/dL but less than equal to 29 ng/dL), while concentrations lower than 20 ng/dL are categorized as deficient. Subjects who were having serum vitamin D level ≥30 ng/dL were classified as not deficient (ND) or normal.
Ethical consideration
Ethical clearance was taken from the Institutional Ethical Committee with Ethical Clearance Certificate No. AIIMS/IEC/2017/759. The study was also registered with Clinical Trial Registry, India with Reference No. REF/2018/02/017336.
Statistical analysis
Data were compiled using Microsoft Excel and analyzed using SPSS software (IBM-SPSS statistics 20.0; SPSS Inc., Chicago, IL, USA). Quantitative variables were analyzed using mean and standard deviation. Baseline as well as follow-up characteristics between groups (vitamin D 60,000 IU/week and vitamin D 1000 IU/day) were compared using an independent t-test for numerical variables and Chi-square test for categorical variables. The data were used to analyze the difference in quantitative variables. Two tailed P value less than 0.05 was considered significant.
Results
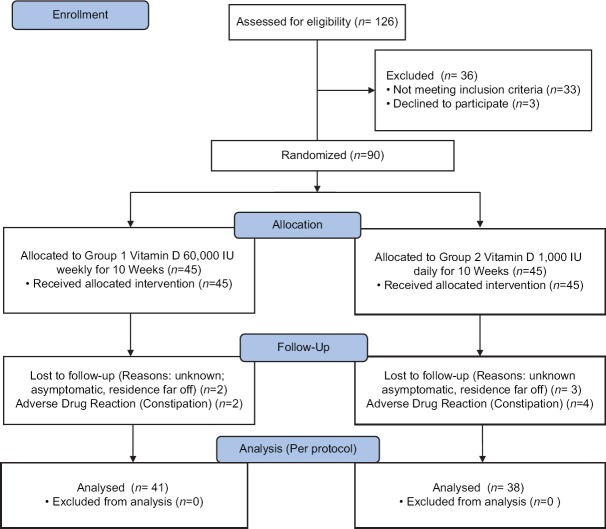
A total of 126 patients were enrolled in the study. After screening, 90 patients were found to be eligible. The subjects were equally divided in 1:1 ratio as per computer-generated randomization tables into two groups:First group received 60,000 IU vitamin D per week and the second group received 1,000 IU vitamin D per day [Figure 1]. The study population comprised of 51.22% female and 48.88% male. The mean age between the group was statistically significant (33.17 ± 10.53 years versus 39.67 ± 11.37 years for groups one and two, respectively). Sociodemographic data, diet, sun-exposure, weight, body mass index, pulse, and vitamin D level at baseline were all nonsignificant except systolic blood pressure and diastolic blood pressure of the study participants at baseline [Table 1].
Figure 1.
Consort Diagram
Table 1.
Comparison of 1000 IU and 60000 IU of vitamin D on patient’s parameters at baseline.
| Variables | 60000 IU | 1000 IU | P (two-sided) |
|---|---|---|---|
| Age (years) | 33.17±10.53 | 39.67±11.37 | 0.025 |
| Male, n (%) | 19 (42.22) | 25 (55.55) | 0.187 |
| Female, n (%) | 26 (57.77) | 20 (44.44) | 0.187 |
| Sun exposure (Hrs) | 0.75±0.53 | 1.25±1.76 | 0.147 |
| Diet (V/NV), n (%) | 31 (68.88)/14 (31.11) | 35 (77.77)/10 (22.22) | 0.084 |
| Vitamin D (ng/dL) | 11.58±7.30 | 11.70±5.39 | 0.942 |
| Weight (kg) | 66.70±15.70 | 69.10±14.92 | 0.546 |
| BMI (kg/m2) | 24.29±4.39 | 25.80±6.01 | 0.273 |
| SBP (mmHg) | 116.93±15.13 | 125.07±14.48 | 0.038 |
| DBP (mmHg) | 77.93±11.04 | 83.40±9.11 | 0.041 |
| Pulse (beats/min) | 74.37±6.73 | 77.77±7.61 | 0.072 |
Abbreviations: Mean±SD=Mean plus minus standard deviation, n=Number, % = Percentage, Mean±SD=Mean plus minus standard deviation, Hrs=Hours, V=Vegetarian, NV=Nonvegetarian, BMI=Body mass index, SBP=Systolic blood pressure, DBP=Diastolic blood pressure and IU=International units. P value<0.05 is considered significant
Nearly 41 participants completed the course of 60,000 IU vitamin D per week for 10 weeks. Two participants complained of constipation and discontinued. Other two subjects lost to follow-up and did not turn up at the end of 10 weeks [Table 2]. At the end of 10 weeks, the participants taking 60,000 IU/week showed significant improvement in vitamin D level from baseline (11.58 ± 7.30) to follow-up (39.91 ± 12.79) at 10 weeks with mean difference of 28.33 ng/dL. There was significant improvement in myalgia in higher dose group compared to low dose group [Graph 1].
Table 2.
Change in the parameters in study groups over a period of 10 weeks (intention to treat analysis)
| Variable | Group 1 - Vitamin D 60,000 IU (n=45) | Group 2 - Vitamin D 1,000 IU (n=45) | Difference between Groups-60,000 IU vs 1000 IU | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Baseline | Follow-up | Mean Difference | P | Baseline | Follow-up | Mean Difference | P | Mean Difference | P | |
| Vitamin D (ng/dl) | 11.58±7.30 | 39.91±12.79 | 28.33 | 0.000 | 11.79±5.39 | 18.49±10.34 | 6.79 | 0.003 | 21.42 | 0.000 |
| Weight (Kg) | 66.70±15.70 | 65.95±16.36 | 0.75 | 0.116 | 69.10±14.92 | 68.65±14.85 | 0.45 | 0.405 | -2.7 | 0.506 |
| BMI (kg/m2) | 24.29±4.39 | 24.01±4.54 | 0.28 | 0.108 | 25.80±6.01 | 25.61±5.91 | 0.19 | 0.351 | -1.6 | 0.246 |
| SBP (mmHg) | 116.93±15.13 | 117±16.34 | 0.067 | 0.977 | 125.07±14.48 | 116±10.9 | 8.2 | 0.000 | 0.13 | 0.970 |
| DBP (mmHg) | 77.93±11.04 | 77.67±9.20 | 0.267 | 0.870 | 83.40±9.11 | 76.93±8.41 | 6.46 | 0.000 | 0.74 | 0.749 |
| Pulse (beats/min) | 74.37±6.73 | 72.20±6.13 | 2.16 | 0.007 | 77.77±7.61 | 74.20±6.89 | 3.5 | 0.405 | -2 | 0.240 |
All data in mean±SD. BMI=Body mass index, SBP=Systolic blood pressure, DBP=Diastolic blood pressure and IU=International units. P value<0.05 is considered significant
Graph 1.
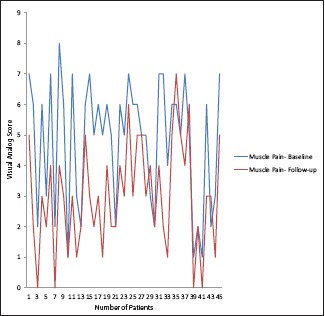
Comparison of muscle pain at baseline and follow-up by visual analog scale after giving 60000 IU of vitamin D
A total of 38 participants completed the course of 1000 IU vitamin D per day for 10 weeks. Four participants complained of constipation so dropped out of the study and the rest three did not turn up at week 10 [Table 2]. At the end of 10 weeks, the participants showed statistically significant improvement in vitamin D level from baseline (11.79 ± 5.39) to follow-up (18.49 ± 10.34) at 10 weeks with mean difference of 6.79 ng/dL. There was gradual improvement in self-reported myalgia after taking 1000 IU vitamin D daily for 10 weeks [Graph 2]. Comparison of two groups at the end of the treatment showed that subjects getting 60,000 IU of vitamin D per weeks has a mean difference of 21.42 ng/dL over subjects getting 1000 IU of vitamin D daily which is highly significant statistically.
Graph 2.
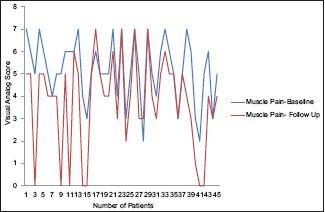
Comparison of muscle pain at baseline and follow-up by visual analog scale after giving 1000 IU of vitamin D
Discussion
Many randomized clinical trials have linked vitamin D with bone mineralization and fracture risk.[8,9] Many observational studies have revealed an inverse relationship between vitamin D status and noncommunicable disease like cancer, cardiac disease, diabetes, infertility, cognitive decline, and autoimmune diseases.[10] Despite extensive research, there is no guidelines when to treat and how to supplement vitamin D in deficient patients.[11] There is inadequacy of well-defined recommendations for vitamin D supplementation and its reference concentration in the serum that leads to diversity in treatment regimens (daily or weekly or monthly).[8]
Studies have shown that in vitamin D deficient person, high-dose vitamin D supplementation (≥40,000 IU/week) for a short duration (4-16 weeks) often achieve an optimal 25-OH vitamin D level.[12] On the contrary, Holick et al. and National Academy of Medicine, USA recommends 800–1000 IU of vitamin D as the daily dose as it is demonstrated to raise 25-OHD concentrations.[13,14] Further, the Food and Nutrition Board and Endocrine Society Clinical Practice guidelines recommended 1,500–2,000 IU a day of vitamin D as the upper intake level which was found to be well tolerated among the participant subjects in the previous studies.[15] It has been observed that different regimens have different outcomes in terms of symptomatic relief and effect on serum level of vitamin D. In our study, supplementing vitamin D as 60,000 IU/week and 1000 IU/day for 10 weeks was chosen based on the evidences provided by observational studies.[16]
By measuring vitamin D before and after supplementation using two different regimens, we were able to demonstrate that baseline serum levels and supplementation dose directly influence the dose-response curve of vitamin D in the deficient subjects as compared to previous studies. Our study is showing similar results to studies by Whiting et al., Von Groningen et al. and Rolland et al.[17,18,19]
In the present study, 1000 IU of vitamin D supplementation did not achieve sufficiency level of 25-OHD i.e. ≥30 ng/mL as defined in many studies[20,21,22] in contrast to several randomized placebo-controlled trials taking 1000 IU or less of vitamin D daily.[23]
The result that higher dose of 60,000 IU of vitamin D weekly for 10 weeks improved vitamin D status was comparable with the results shown by Malabanan et al. who used ergocalciferol in the dosage of >40,000 IU once a week for 8 weeks.[24] Our results are consistent with the finding of Ish-Shalom et al. who showed that the total dose of vitamin D was more predictive than the frequency of dosing in the treatment of vitamin D deficiency.[25]
After 10 weeks, subjects receiving high-dose vitamin D supplementation had an average increase in 25-OH vitamin D of 28.33 ng/mL, which was significant (P < 0.001) and greater than the increase (6.79 ng/mL) in the low-dose vitamin D group. This shows that daily low-dose vitamin D supplementation may not be enough to normalize vitamin D levels for those who are already deficient at the baseline.[26,27] These results are in agreement with other studies that also show that daily low-dose vitamin D (≤1,000 IU/day) is not sufficient to correct vitamin D deficiency.[28,29] Therefore, compared to daily low dose supplementation, high dosage weekly vitamin D supplementation may thus be the preferred strategy for vitamin D deficiency.[29,30]
There were few limitations of our study. First, the sample size was relatively small and around 10% of subjects were lost to follow-up or due to adverse reactions. Second, 100% compliance or adherence to vitamin D supplementation could not be confirmed, although the subjects were given comprehensive counseling and education on the compliance and importance of vitamin D supplementation. Third, due to the limited resources, the effect of vitamin D on parathyroid hormone, phosphate, and calcium could not be investigated.
Although the study investigated smaller number of subjects but its results may provide basis for further studies with a larger sample size and of a longer duration of follow-up to validate the effect of vitamin D supplementation on its serum levels. Lack of similar studies from Indian subcontinent forced us to conduct a preliminary investigation without formally calculating sample size.
Conclusion
We found that a high proportion of subjects with musculoskeletal symptoms suffered from vitamin D deficiency/insufficiency. The findings of this randomized, prospective study revealed that in vitamin D deficient subject, the weekly dose of 60,000 IU of vitamin D was more efficacious than daily dose of 1000 IU in increasing the vitamin D level to normal range as well as in mitigating the deficiency symptoms. Thus, high dose regimen may be a more convenient, effective, and economical mode of treatment for patients with vitamin D deficiency. It will help the primary care physicians, family physicians, and specialist doctors to follow the latest, evidence-based practice and maintain the standards of care for the population.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Acknowledgements
We would like to thank Lupin Limited and Sun Pharmaceutical Industries Limited for providing tablets. All the authors contributed to the paper as work assigned to them and there was no conflict of interest among the authors.
References
- 1.Prentice A. Vitamin D deficiency: A global perspective. Nutr Rev. 2008;66:S153–64. doi: 10.1111/j.1753-4887.2008.00100.x. [DOI] [PubMed] [Google Scholar]
- 2.Holick MF. Sunlight and vitamin D for bone health and prevention of autoimmune diseases, cancers, and cardiovascular disease. Am J ClinNutr. 2004;80(Suppl 6):1678S–8S. doi: 10.1093/ajcn/80.6.1678S. [DOI] [PubMed] [Google Scholar]
- 3.Kotwal N, Upreti V, Nachankar A, Kumar KVSH. A prospective, observational study of osteoporosis in men. Indian J Endocrinol Metab. 2018;22:62–6. doi: 10.4103/ijem.IJEM_414_16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Guntona JE, Girgisa CM. Vitamin D and muscle. Bone Reports. 2018;8:163–7. doi: 10.1016/j.bonr.2018.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Holick MF. Vitamin D deficiency. N Engl J Med. 2007;357:266–81. doi: 10.1056/NEJMra070553. [DOI] [PubMed] [Google Scholar]
- 6.Qari FA. Practical approach for the prevention and management of vitamin D deficiency in adults. J Family Med Prim Care. 2013;2:315–8. doi: 10.4103/2249-4863.123776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mattacola CG, Perrin DH, Gansneder BM, Allen JD, Mickey CA. A comparison of visual analog and graphic rating scales for assessing pain following delayed onset muscle soreness. J Sport Rehabil. 1997;6:36–8. [Google Scholar]
- 8.Brouwer-Brolsma EM, Bischoff-Ferrari HA, Bouillon R, Feskens EJ, Gallagher CJ, Hypponen E, et al. Vitamin D: Do we get enough? A discussion between vitamin D experts in order to make a step towards the harmonisation of dietary reference intakes for vitamin D across Europe. Osteoporos Int. 2013;24:1567–77. doi: 10.1007/s00198-012-2231-3. [DOI] [PubMed] [Google Scholar]
- 9.Nikiphorou E, Uksila J, Sokka T. A cross-sectional study of vitamin D levels in a large cohort of patients with rheumatic diseases. Clin Rheumatol. 2018;37:803–10. doi: 10.1007/s10067-017-3870-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cranney A, Horsley T, O’Donnell S, Weiler H, Puil L, Ooi D, et al. Effectiveness and safety of vitamin D in relation to bone health. Evid Rep Technol Assess (Full Rep) 2007;158:1–235. [PMC free article] [PubMed] [Google Scholar]
- 11.Hiremath GS, Cettomai D, Baynes M, Ratchford JN, Newsome S, Harrison D, et al. Vitamin D status and effect of low-dose cholecalciferol and high-dose ergocalciferol supplementation in multiple sclerosis. Mult Scler. 2009;15:735–40. doi: 10.1177/1352458509102844. [DOI] [PubMed] [Google Scholar]
- 12.Cannell JJ, Hollis BW, Zasloff M, Heaney RP. Diagnosis and treatment of vitamin D deficiency. Expert Opin Pharmacother. 2008;9:107–18. doi: 10.1517/14656566.9.1.107. [DOI] [PubMed] [Google Scholar]
- 13.Holick MF, Biancuzzo RM, Chen TC, Klein EK, Young A, Bibuld D, et al. Vitamin D2 is as effective as vitamin D3 in maintaining circulating concentrations of 25-hydroxyvitamin D. J Clin Endocrinol Metab. 2008;93:677–81. doi: 10.1210/jc.2007-2308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bacon CJ, Gamble GD, Horne AM, Scott MA, Reid IR. High-dose oral vitamin D3 supplementation in the elderly. Osteoporos Int. 2009;20:1407–15. doi: 10.1007/s00198-008-0814-9. [DOI] [PubMed] [Google Scholar]
- 15.Burton JM, Kimball S, Vieth R, Bar-Or A, Dosch HM, Cheung R, et al. A phase I/II dose-escalation trial of vitamin D3 and calcium in multiple sclerosis. Neurology. 2010;74:1852–9. doi: 10.1212/WNL.0b013e3181e1cec2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kennel KA, Drake MT, Hurley DL. Vitamin D deficiency in adults: When to test and how to treat. Mayo Clin Proc. 2010;85:752–7. doi: 10.4065/mcp.2010.0138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Whiting SJ, Calvo MS. Correcting poor vitamin D status: Do older adults need higher repletion doses of vitamin D3 than younger adults? Mol Nutr Food Res. 2010;54:1077–84. doi: 10.1002/mnfr.200900536. [DOI] [PubMed] [Google Scholar]
- 18.Van Groningen L, Opdenoordt S, Van Sorge A, Telting D, Giesen A, de Boer H. Cholecalciferol loading dose guideline for vitamin D-deficient adults. Eur J Endocrinol. 2010;162:805–11. doi: 10.1530/EJE-09-0932. [DOI] [PubMed] [Google Scholar]
- 19.Rolland Y, de Souto BP, Abellan Van KG, Annweiler C, Beauchet O, Bischoff-Ferrari H, et al. Vitamin D supplementation in older adults: Searching for specific guidelines in nursing homes. J Nutr Health Aging. 2013;17:402–12. doi: 10.1007/s12603-013-0007-x. [DOI] [PubMed] [Google Scholar]
- 20.Bruyere O, Malaise O, Neuprez A, Collette J, Reginster JY. Prevalence of vitamin D inadequacy in European post-menopausal women. Curr Med Res Opin. 2007;23:1939–44. doi: 10.1185/030079907X219562. [DOI] [PubMed] [Google Scholar]
- 21.Holick MF. Too little vitamin D in premenopausal women: Why should we care? Am J Clin Nutr. 2002;76:3–4. doi: 10.1093/ajcn/76.1.3. [DOI] [PubMed] [Google Scholar]
- 22.Vieth R. Vitamin D supplementation, 25-hydroxyvitamin D concentrations, and safety. Am J Clin Nutr. 1999;69:842–56. doi: 10.1093/ajcn/69.5.842. [DOI] [PubMed] [Google Scholar]
- 23.Heaney RP. The vitamin D requirement in health and disease. J Steroid Biochem Mol Biol. 2005;97:9–13. doi: 10.1016/j.jsbmb.2005.06.020. [DOI] [PubMed] [Google Scholar]
- 24.Malabanan A, Veronikis IE, Holick MF. Redefining vitamin D insufficiency. Lancet. 1998;351:805–6. doi: 10.1016/s0140-6736(05)78933-9. [DOI] [PubMed] [Google Scholar]
- 25.Ish-Shalom S, Segal E, Salganik T, Raz B, Bromberg IL, Vieth R. Comparison of daily, weekly, and monthly vitamin D3 in ethanol dosing protocols for two months in elderly hip fracture patients. J Clin Endocrinol Metab. 2008;93:3430–5. doi: 10.1210/jc.2008-0241. [DOI] [PubMed] [Google Scholar]
- 26.Bischoff-Ferrari HA, Dawson-Hughes B, Staehelin HB, Orav JE, Stuck AE, Theiler R, et al. Fall prevention with supplemental and active forms of vitamin D: A meta-analysis of randomised controlled trials. BMJ. 2009;339:b3692. doi: 10.1136/bmj.b3692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Crew KD, Shane E, Cremers S, McMahon DJ, Irani D, Hershman DL. High prevalence of vitamin D deficiency despite supplementation in premenopausal women with breast cancer undergoing adjuvant chemotherapy. J Clin Oncol. 2009;27:2151–6. doi: 10.1200/JCO.2008.19.6162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Khan QJ, Reddy PS, Kimler BF, Sharma P, Baxa SE, O’Dea AP, et al. Effect of vitamin D supplementation on serum 25-hydroxy vitamin D levels, joint pain, and fatigue in women starting adjuvant letrozole treatment for breast cancer. Breast Cancer Res Treat. 2010;119:111–8. doi: 10.1007/s10549-009-0495-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wu F, Staykova T, Horne A, Clearwater J, Ames R, Mason B, et al. Efficacy of an oral, 10-day course of high-dose calciferol in correcting vitamin D deficiency. NZ Med J. 2003;116:U536. [PubMed] [Google Scholar]
- 30.Lagowska K, Bajerska J, Jamka M. The role of vitamin D oral supplementation in insulin resistance in women with polycystic ovary syndrome: A systematic review and meta-analysis of randomized controlled trials? Nutrients. 2018;10:E1637. doi: 10.3390/nu10111637. doi: 10.3390/nu10111637. [DOI] [PMC free article] [PubMed] [Google Scholar]