Abstract
Background:
Role of serum amyloid A (SAA) protein as a biomarker for the disease activity in juvenile idiopathic arthritis (JIA) has not been explored. This study was done to find its role as marker of disease activity in JIA.
Methods:
A case–control study with 50 newly diagnosed cases of JIA of all subtypes and 40 healthy controls was done. Serum amyloid A (SAA), erythrocyte sedimentation rate (ESR), and C-reactive protein (CRP) were measured in both patients and healthy controls. Quantitative measurement of SAA level was measured by using standard human SAA enzyme-linked immunosorbent assay (ELISA). Disease activity was assessed clinically and by ultrasonography (USG) score determined by examining eight large joints (bilateral elbow, wrist, knee, ankle). Serum SAA levels were found significantly higher in JIA patients as compared with healthy controls (P < 0.001). Significant positive correlations were found between SAA with presence of active joints (r = 0.64, P < 0.001), ESR (r = 0.39, P < 0.05), and CRP (r = 0.36, P < 0.05). However, significant correlations was not found between ESR and the presence of active joints (r = 0.21, P = 0.225) and between CRP and the presence of active joints (r = 0.034, P = 0.855). The mean USG score of patients with increased SAA level was significantly higher than that of patients with normal SAA level (P < 0.05).
Conclusion:
A significant increase in SAA levels was found in JIA patients with strong positive correlation between SAA level and JIA disease activity. The study discerned SAA to be a more sensitive laboratory marker than ESR and CRP for evaluating the presence of active joints.
Keywords: C-reactive protein, juvenile idiopathic arthritis, serum amyloid A
Introduction
Juvenile idiopathic arthritis (JIA) reflects all forms of arthritis that begin before the age of 16 years and usually persist for >6 weeks.[1] The disease is characterized by inflammation of the synovium and the periarticular tissues. JIA just like other inflammatory arthritis is characterized by chronic inflammation with variable disease presentation. However, the mechanism of this variability of inflammation which changes the course of disease needs to be deciphered. The International League of Associations for Rheumatology (ILAR) has provided the most recent classification for JIA.[2] Since very long, acute phase reactant, erythrocyte sedimentation rate (ESR), and C-reactive protein (CRP) have been used as markers of inflammation.[3,4] Advantage of these markers for being cost-effective and easy applicable are also associated with limitation of sensitivity and specificity especially in cases of rheumatological disorders.[5] The need of new markers of inflammation to predict disease activity and to monitoring remission and relapse is warranted in era of biological therapy for various arthritis including JIA.[6]
SAA belonging to the apolipoproteins family synthesized by hepatocytes, adipocytes, macrophages, and fibroblast-like synoviocytes is a newer acute phase reactants.[7] It acts through toll-like receptor (TLR) 2, nuclear factor-κβ activation, and binding to the G-protein-coupled formyl peptide receptor like-1 (FPRL1).[8] This results in release of various inflammatory cytokines such as TNF-α, IL-1,6 and granulocyte-colony stimulating factor (G-CSF) and stimulates tissue factor, angiogenesis, and matrix metalloproteinases.[9,10] SAA can be used for marker for local and systemic inflammation as it is produced by cells involved in inflammation locally and has systematic spill.[9]
Although several studies have validated the use of serum amyloid A (SAA) protein as a biomarker for the disease activity in some rheumatoid conditions such as rheumatoid arthritis (RA), polymyalgia rheumatica (PMR), ankylosing spondylitis (AS), and sarcoidosis.[11,12,13,14] SAA is also part of “multi-biomarkers disease activity test (MBDA)” developed to monitor disease activity in RA.[15] However, studies evaluating the role of SAA in JIA as inflammatory marker are sparse.
This study was done to see the level of SAA in JIA patients and to correlation its level with disease activity and compare with known markers of inflammation such as CRP and ESR.
Materials and Methods
Patient selection
A case–control study was done at eastern Uttar Pradesh, India, in a tertiary care center from September 2015 to June 2016. Fifty newly diagnosed cases of JIA (ILAR criteria 2001), covering all subtypes and 40 healthy controls of same age and sex, were included in this study.
Ethical approval: Ethical approval was taken by institute ethical committee (Letter No. Dean/2014-15/EC/1364).
Methodology
Every patient was evaluated systematically and findings were recorded in standard rheumatology case record forms. Evaluations included general demographics, anthropometric measurements, patient narrative (complaints, co-morbidity, and drug use/toxicity), disease activity (global assessments by patient and doctor), and physical and rheumatology examination (including joint counts for pain/tenderness and swelling).
Each joint was counted individually. Carpal joints of each hand and tarsal joints of each foot were counted as one joint each. Each metacarpo-phalangeal, metatarso-phalangeal, and proximal interphalangeal joint was counted as a separate joint. Enthesitis was defined by a history of moderately severe persistent pain and demonstration of tenderness at one or more bony insertion sites of ligaments/tendons/fascia, especially around the heel and knee region. The children were especially questioned about early-morning painful gait or a limp due to pain in the heel-foot region.
Patients were investigated for various hematological and biochemical parameters with specific serological assessment plus radiological evaluation (depending upon the case). Investigations included complete blood count, ESR, CRP, renal function test, liver function test, urine – routine, and microscopic examination.
In patients with polyarticular/oligoarticular onset, additionally rheumatoid factor, anti-cyclic citrullinated antibodies, anti-nuclear antibodies (ANA), digital X-ray bilateral hand, or the affected joint were done.
In patients with enthesitis/low back pain/psoriasis, digital X-ray bilateral sacroiliac joint (AP view), and bilateral foot (lateral view) for tendoachilles involvement was done along with ANA and HLA B27. Only children with obvious eye complaints and ANA positive were referred to ophthalmologists to look for uveitis. Patients with psoriasis and those having distal interphalangeal joint involvement were also referred to dermatologist for a good dermatological examination and expert opinion.
Serum amyloid A (SAA), ESR, and CRP were measured in both patients and healthy controls. Quantitative measurement of SAA level was measured by using standard human SAA enzyme-linked immunosorbent assay (ELISA) [RayBio®, GA, USA, with coefficient of variation per cent (CV %), <10%].
Disease activity was assessed by number of large joints affected which was detected by the ultrasonography (USG) score as well as clinical examination in all patients. Four large joints of the body (viz., wrist, elbow, knee, and ankle) were assessed bilaterally, plus the joints in which complaints were made, were also assessed using both gray-scale B mode Doppler and power Doppler studies. Radiological features as synovial hypertrophy, increased vascularity, erosions, effusions, tenosynovitis, reduced joint space, and enthesitis were studied.
Statistical analysis
The statistical analysis was done using statistical package for social sciences software (SPSS, Inc., Chicago, IL, USA) for windows (Version 16.0). Fisher's exact test was used for the categorical variable because the sample size was small. The normality of the data was checked by the Kolmogorov–Smirnov test, and it was normal. Since there were no any outliers, so we preferred Pearson correlation. P < 0.05 is considered as statistically significant.
Results
Out of 50 cases 31 patients were boys. Mean age of patients and of controls were 12.7 years and 13.4, respectively.
Table 1 shows baseline demographic profile of cases and control. Table 2 shows the different forms of JIA in the study. Among 50 patients, maximum number of patients were of enthesitis related arthritis group (n = 25; 50%). Table 3 shows the various characteristics of patients with regard to symptoms, involvement of joints and various laboratory investigations. Serum SAA was raised in 46 patients (92%), whereas CRP and ESR was raised in 32 (64%) and 37 (74%) patients respectively. In 9 patients (18%), SAA was raised with normal ESR, and in 14 (28%) patients, SAA was raised with normal CRP.
Table 1.
Baseline characteristics of two groups
| Characteristics | JIA | Control |
|---|---|---|
| No | 50 | 40 |
| Avg. age | 12.7 | 13.4 |
| M/F | 31/19 (1.63:1) | 26/14 |
Table 2.
Distribution of patients of JIA among different subtypes
| Subtypes | No. of patients (n) | Percentage |
|---|---|---|
| Enthesitis related arthritis | 25 | 50 |
| Polyarticular (RF +) | 8 | 16 |
| Polyarticular (RF -) | 6 | 12 |
| Oligoarticular | 8 | 16 |
| Systemic onset | 1 | 2 |
| Psoriatic | 1 | 2 |
| Undifferentiated | 1 | 2 |
RF: Rheumatoid factor
Table 3.
Characteristics of patients
| Characteristics | No of patients (n=50) |
|---|---|
| Peripheral symptoms | 40 (80%) |
| Axial involvement (X-ray/MRI) | 14 (28%) |
| PIP/DIP Joint* (examination) | 13 (26%) |
| Wrist joint ( USG) | 20 (40%) |
| Elbow joint (USG) | 11 (22%) |
| Knee joint (USG) | 35 (70%) |
| Ankle joint (USG) | 30 (60%) |
| Symmetry | 20 (40%) |
| RF positivity | 10 (20%) |
| Anti CCP positivity | 9 (18%) |
| HLA-B27 positivity | 22 (44%) |
| ANA positivity | 4 (8%) |
| Enthesitis | 6 (12%) |
| Uveitis | 3 (6%) |
*Involvement of Proximal Interphalangeal (PIP) and Distal Interphalangeal (DIP) joint was assessed only by clinical examination; USG: Assessment by Ultrasonography (USG); Cyclic citrullinated antibodies
The mean SAA level of JIA patient was significantly high 18.64 ± 9.09 mg/L as compared with the mean SAA level of control (2.36 ± 4.11 mg/L) (P < 0.05).
As compared with SAA and ESR, CRP levels were slightly less sensitive as acute phase reactant [Graph 1].
Graph 1.
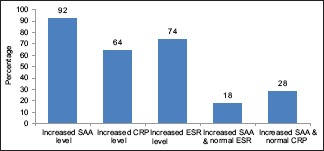
Acute phase reactants in JIA patients
Forty-six (n = 46) patients were having increased level of SAA with mean value of 20.5 ± 14.05 mg/L and mean USG score of 4.82 ± 1.3. Four patients were having normal SAA level with mean value of 2.82 ± 1.59 mg/L and mean USG score of 3.06 ± 1.1. The mean USG score of patients with increased SAA level was significantly higher than that of patients with normal SAA level (P < 0.05) [Table 4].
Table 4.
Correlation between SAA, USG score, ESR and CRP
| Parameters | r | P |
|---|---|---|
| USG v/s SAA | 0.64 | <0.001 |
| USG v/s ESR | 0.39 | <0.05 |
| USG v/s CRP | 0.36 | <0.05 |
| CRP v/s ESR | 0.57 | <0.001 |
| ESR v/s SAA | 0.36 | <0.05 |
| CRP v/s SAA | 0.29 | <0.05 |
USG: Ultrasonography score; CRP: C-Reactive Protein; SAA: Serum Amyloid A; ESR: Erythrocyte sedimentation rate
SAA levels showed highly significant positive correlation with USG score (r = 0.64, P < 0.001). Similarly, significant correlation was found with ESR (r = 0.39, P < 0.05) and CRP (r = 0.36, P < 0.05). The correlation between CRP and ESR was also significant (r = 0.57, P ≤ 0.001).
Out of 46 patients with increased SAA level, 9 patients were having normal ESR level and 14 patients were having normal CRP level. The mean SAA level for patients with normal ESR and normal CRP level was raised with values of 17.33 ± 8.65 and 15.61 ± 10.61 mg/L, respectively. The mean USG scores of these patients were also high with values of 4.06 ± 1.83 and 3.69 ± 1.44, respectively. Hence, a positive correlation between SAA and USG score was found in these patients, though statistical not significant [Table 5].
Table 5.
Correlation between SAA and USG score in patients with normal CRP and normal ESR and increased SAA level
| Parameter | Mean USG | Mean SAA (mg/L) | r | P |
|---|---|---|---|---|
| Normal ESR (n=9) | 4.06±1.83 | 17.33±8.65 | 0.65 | 0.06 |
| Normal CRP (n=14) | 3.69±1.44 | 15.61±10.6 | 0.35 | 0.08 |
Discussion
Several studies have shown that SAA has higher sensitivity and specificity than CRP and ESR as marker for disease activity in rheumatoid conditions.[16,17] In a study, it was found that SAA was better biomarker than CRP in RA to measure disease activity in group treated with tumor necrosis factor antagonists.[18] High SAA and CRP levels were found to serve as biomarkers for pulmonary involvement in diffuse systemic sclerosis.[19] Also, it has been found that patients of JIA with high SAA levels are associated with the polyarticular and systemic forms of the disease.[20]
The study was done to find out a new marker SAA as a new marker for disease activity in JIA. Overall in the study population, male-to-female ratio was 1.63:1 and the median age of onset was 12.7 years, which is exactly same as in study of Kunjir et al.; however, it was slightly higher in Spanish study (16 years).[7,8,9]
Out of 50 newly diagnosed JIA patients included, 46 patients were having increased level of SAA and mean USG score of these patients was also significantly higher as compared with controls, showing strongly positive correlation between SAA levels and disease activity (USG score).
About 92% patient with JIA were having increased SAA level in our study in comparison to Sang Youn Jung et al. (in AS patients), where only 52.6% patient were having increased SAA level.[17] Raised SAA with normal CRP was comparable in both the studies (28% and 23.6%) whereas raised SAA with normal ESR was in less patients (18%) in our study as compared with the other study (42.5%) [Table 6].
Table 6.
Relation between different Acute phase reactants
| Parameters | Present study (n=50) | Sang Youn Jung et al. 2007 (n=38)[17] |
|---|---|---|
| Increased SAA level | 92% | 52.6% |
| Increased SAA & normal ESR | 18% | 42.5% |
| Increased SAA & normal CRP | 28% | 23.6% |
Though ample efforts have been made to demonstrate the correlation between SAA levels and disease activity in different rheumatological disorders, its role in JIA has been studied by Cantarini et al.[20] Disease activity in form of active joints were determined in this study by both physical examination and USG (more sensitive), whereas in the study by Cantarini et al. (Italy), it was done by only physical examination [Table 7].
Table 7.
Correlation among disease activity and different acute phase reactants
| Characters | Present study (n=50) | Cantarini et al., 2011 (n=41)[20] | ||
|---|---|---|---|---|
| r | P | r | P | |
| Active joints vs SAA | 0.64 | <0.001 | 0.42 | <0.005 |
| Active joints vs CRP | 0.36 | <0.05 | 0.03 | 0.859 |
| ESR vs SAA | 0.36 | <0.05 | 0.70 | <0.005 |
| CRP vs SAA | 0.29 | <0.05 | 0.82 | <0.001 |
In our study, correlation between disease active joints (USG score) and SAA level was statistically significant and better (r-value = 0.64, P value < 0.001) than ESR and CRP (r-value = 0.36, P value < 0.05) and was in accordance to the observation made by Cantarini et al., in Italian population of 41 JIA patients.
Although correlation between SAA with CRP and ESR titer was higher in the study by Cantarini et al., it was also statistically significant in our study. Our study showed poorer correlation between CRP titer and disease activity as compared with SAA, whereas the study by Cantarini et al. showed no correlation.
The study had few limitations that only large joints were assessed and small joints of body as proximal interphalangeal and meta-carpophalangeal joints were not included in the study. Also, every patient with doubtful X-ray could not afford MRI due to financial constraints. Every patient did not repeat the serological tests and radiological tests due to financial constraints and only a few could do it. Further, not all the patients of ERA and oligoarticular category underwent ophthalmological examination and only patients with ocular complaints could do it. Also, no specific disease activity index was available to compare all the subtypes of JIA simultaneously, so disease activity was assessed by number of active joints documented by USG-Power Doppler assessment only.
Our study concluded that SAA levels were significantly increased in JIA patients and correlated well with disease activity. We also discerned SAA to be a more sensitive laboratory marker than ESR and CRP for evaluating the presence and number of active joints. However, more studies with larger sample size are needed to delineate SAA sensitivity as marker of disease activity in JIA.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- 1.Cassidy JT, Petty RE. Chronic Arthritis in Childhood. In: Cassidy JT, Petty RE, Laxer R, Lindlsy C, editors. Textbook of Pediatric Rheumatology. 5th ed. Philadelphia: Elsevier Saunders; 2005. pp. 206–60. [Google Scholar]
- 2.Petty RE, Southwood TR, Manners P, Baum J, Glass DN, Goldenberg J, et al. International League of Associations for Rheumatology classification of juvenile idiopathic arthritis: Second revision, Edmonton, 2001. J Rheumatol. 2004;31:390. [PubMed] [Google Scholar]
- 3.Ravelli A, Martini A. Juvenile idiopathic arthritis. Lancet. 2007;369:767–78. doi: 10.1016/S0140-6736(07)60363-8. [DOI] [PubMed] [Google Scholar]
- 4.Martini A, Lovell DJ. Juvenile idiopathic arthritis: State of the art and future perspectives. Ann Rheum Dis. 2010;69:1260–3. doi: 10.1136/ard.2010.133033. [DOI] [PubMed] [Google Scholar]
- 5.Manners PJ, Diepeveen DA. Prevalence of juvenile chronic arthritis in a population of 12-year-old children in urban Australia. Pediatrics. 1996;98:84–90. [PubMed] [Google Scholar]
- 6.Demirkaya E, Ozen S, Bilginer Y, Ayaz N, Makay B, Unsal E, et al. The distribution of juvenile idiopathic arthritis in the eastern Mediterranean: Results from the registry of the Turkish Paediatric Rheumatology Association. Clin Exp Rheumatol. 2011;29:111. [PubMed] [Google Scholar]
- 7.Merino R, De Inocencio J, García-Consuegra J. Evaluation of ILAR classification criteria for juvenile idiopathic arthritis in Spanish children. J Rheumatol. 2001;28:2731–6. [PubMed] [Google Scholar]
- 8.Stabile A, Avallone L, Compagnone A, Ansuini V, Bertoni B, Rigante D. Focus on juvenile idiopathic arthritis according to the 2001 Edmonton revised classification from the International League of Associations for Rheumatology: An Italian experience. Eur Rev Med Pharmacol Sci. 2006;10:229. [PubMed] [Google Scholar]
- 9.Kunjir V, Venugopalan A, Chopra A. Profile of Indian patients with juvenile onset chronic inflammatory joint disease using the ILAR classification criteria for JIA: A community-based cohort study. J Rheumatol. 2010;37:1756–62. doi: 10.3899/jrheum.090937. [DOI] [PubMed] [Google Scholar]
- 10.John J, Chandran L. Arthritis in children and adolescents. Pediatr Rev. 2011;32:470. doi: 10.1542/pir.32-11-470. [DOI] [PubMed] [Google Scholar]
- 11.Shah C, Hari-Dass R, Raynes JG. Serum amyloid A is an innate immune opsonin for Gram-negative bacteria. Blood. 2006;108:1751–7. doi: 10.1182/blood-2005-11-011932. [DOI] [PubMed] [Google Scholar]
- 12.Seok A, Lee HJ, Lee S, Lee J, Mun S, Park A, et al. Identification and validation of SAA4 as a rheumatoid arthritis prescreening marker by liquid chromatography tandem-mass spectrometry. Molecules. 2017;22:805. doi: 10.3390/molecules22050805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.O’Hara R, Murphy EP, Whitehead AS, FitzGerald O, Bresnihan B. Acute-phase serum amyloid A production by rheumatoid arthritis synovial tissue. Arthritis Res Ther. 2000;2:142. doi: 10.1186/ar78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shimojima Y, Matsuda M, Gono T, Ishii W, Ikeda S. Serum amyloid A as a potent therapeutic marker in a refractory patient with polymyalgia rheumatica. Intern Med. 2005;44:1009–12. doi: 10.2169/internalmedicine.44.1009. [DOI] [PubMed] [Google Scholar]
- 15.Centola M, Cavet G, Shen Y, Ramanujan S, Knowlton N, Swan KA, et al. Development of a multi-biomarker disease activity test for rheumatoid arthritis. PLoS One. 2013;8:e60635. doi: 10.1371/journal.pone.0060635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Scheinberg MA, Hubscher O, Morteo OG, Benson MD. Serum amyloid protein levels in south american children with rheumatoid arthritis: A co-operative study. Ann Rheum Dis. 1980;39:228–30. doi: 10.1136/ard.39.3.228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jung SY, Park M-C, Park Y-B, Lee S-K. Serum amyloid a as a useful indicator of disease activity in patients with ankylosing spondylitis. Yonsei Med J. 2007;48:218–24. doi: 10.3349/ymj.2007.48.2.218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hwang YG, Balasubramani GK, Metes ID, Levesque MC, Bridges SL, Jr, Moreland LW. Differential response of serum amyloid A to different therapies in early rheumatoid arthritis and its potential value as a disease activity biomarker. Arthritis Res Ther. 2016;18:108. doi: 10.1186/s13075-016-1009-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lis-Święty A, Widuchowska M, Brzezińska-Wcisło L, Kucharz E. High acute phase protein levels correlate with pulmonary and skin involvement in patients with diffuse systemic sclerosis. J Int Med Res. 2018;46:1634–9. doi: 10.1177/0300060518760955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cantarini L, Giani T, Fioravanti A, Iacoponi F, Simonini G, Pagnini I, et al. Serum amyloid A circulating levels and disease activity in patients with juvenile idiopathic arthritis. Yonsei Med J. 2012;53:1045–8. doi: 10.3349/ymj.2012.53.5.1045. [DOI] [PMC free article] [PubMed] [Google Scholar]


