Abstract
Background
Routine colonoscopy was traditionally recommended after acute diverticulitis to exclude coexistent malignancy. Improved CT imaging may make routine colonoscopy less required over time but most guidelines still recommend it. The aim of this review was to assess the role of colonoscopy in patients with CT‐proven acute diverticulitis.
Methods
PubMed and Embase were searched for studies reporting the prevalence of advanced colorectal neoplasia (ACN) or colorectal carcinoma in patients who underwent colonoscopy within 1 year after CT‐proven left‐sided acute diverticulitis. The prevalence was pooled using a random‐effects model and, if possible, compared with that among asymptomatic controls.
Results
Seventeen studies with 3296 patients were included. The pooled prevalence of ACN was 6·9 (95 per cent c.i. 5·0 to 9·4) per cent and that of colorectal carcinoma was 2·1 (1·5 to 3·1) per cent. Only two studies reported a comparison with asymptomatic controls, showing comparable risks (risk ratio 1·80, 95 per cent c.i. 0·66 to 4·96). In subgroup analysis of patients with uncomplicated acute diverticulitis, the prevalence of colorectal carcinoma was only 0·5 (0·2 to 1·2) per cent.
Conclusion
Routine colonoscopy may be omitted in patients with uncomplicated diverticulitis if CT imaging is otherwise clear. Patients with complicated disease or ongoing symptoms should undergo colonoscopy.
Introduction
The incidence rates of acute diverticulitis have been increasing rapidly over recent decades1, 2, 3. Acute diverticulitis has traditionally been associated with an increased risk of colorectal malignancy, which has led to routine colonic evaluation by colonoscopy after the episode of acute diverticulitis has resolved. However, a causal association between colonic diverticulitis or diverticulosis and malignancy has never been found. The association is most likely explained by misdiagnosis of colorectal malignancy as acute diverticulitis. The fact that acute diverticulitis used to be diagnosed based on the clinical picture or barium enemas, and later on by ultrasonography, probably increased the number of misdiagnoses and thereby played a role in establishing the association between acute diverticulitis and colorectal malignancy4, 5. CT has a higher accuracy for the detection of an alternative diagnosis such as colorectal carcinoma6, 7. If this were accurate enough, colonoscopy would not be needed in every patient, thereby reducing the healthcare burden and colonoscopy‐related morbidity8, 9, 10.
Even though the risk of colorectal carcinoma in patients with acute diverticulitis has been the topic of debate in multiple studies11, 12, 13, 14, 15, 16, 17, 18, there remains a lack of clarity. The majority of guidelines11, 13, 14, 15, 17, 18 still recommends routine colonoscopy after an episode of acute diverticulitis. The objective of this review was to assess the role of colonoscopy in patients with CT‐proven acute diverticulitis in detecting a colorectal carcinoma that is presenting as acute diverticulitis or is masked by acute diverticulitis.
Methods
Study identification
Two authors searched PubMed and Embase databases independently for studies published up to April 2018, using the following search terms: diverticulitis, diverticular, colonoscopy, colonic evaluation, colon cancer, colon carcinoma, colorectal cancer, colorectal carcinoma, sigmoid cancer and sigmoid carcinoma. The search strategy is shown in Appendix S1 (supporting information). Additionally, a manual cross‐reference search of the reference lists of relevant articles was performed to identify other studies not found in the initial search. No language limits were applied. MOOSE guidelines19 for reporting were followed. A review protocol for this systematic review was not published or registered before the study was undertaken.
Study selection
Studies eligible for inclusion were: RCTs or observational cohort studies including patients with CT‐proven acute colonic diverticulitis and reporting rates of advanced colorectal neoplasia (ACN) or colorectal carcinoma found at colonoscopy. Only patients who underwent colonoscopy within 1 year after the acute diverticulitis diagnosis were included, to enable assessment of the risk of having a colorectal carcinoma at the time of the acute diverticulitis diagnosis rather than the risk of developing a colorectal carcinoma several years later that is unrelated to the acute diverticulitis. Studies of Western origin that did not quantify the number of patients with right‐sided diverticulitis were included on the assumption that the vast majority of cases in the Western world (usually above 90 per cent20, 21, 22) comprise left‐sided diverticulitis. Right‐sided diverticulitis was defined as diverticulitis located proximal to the splenic flexure. Reviews, conference abstracts, letters to the editor, animal studies and studies with fewer than ten patients were excluded. For studies with overlapping patient cohorts, the largest study was included. The two reviewers independently considered all studies retrieved from the search for eligibility against these criteria. Any disagreements in any phase of the study selection, quality assessment or data extraction were resolved by discussion.
Quality assessment
The two reviewers appraised each study critically using the Newcastle–Ottawa Scale for cohort studies23.
Data extraction
Data from each included study were extracted by two reviewers independently using a predefined extraction table. These data included: study setting, study design (prospective or retrospective data collection), type of patient (uncomplicated versus complicated diverticulitis), number of patients, patient age, proportion of complete colonoscopies (caecal intubation), time interval between acute diverticulitis episode and colonoscopy, number of colonoscopy‐related complications (complications as reported by the studies), number of patients with ACN, number of patients with colorectal carcinoma and tumour location, and outcome results from logistic regression analyses. For some studies, only a subgroup of patients who fulfilled the inclusion and exclusion criteria for the present systematic review was included in the analysis: only patients who underwent colonoscopy, or only those who had colonoscopy within 1 year after the acute diverticulitis episode.
Outcome measures
Primary outcome measures were the prevalence of colorectal carcinoma and ACN at follow‐up colonoscopy. These rates were compared with those in asymptomatic control cohorts, when this information was reported by the studies. A sensitivity analysis was undertaken to assess rates of colorectal carcinoma and ACN in patients with uncomplicated and complicated acute diverticulitis separately, but only for studies that reported these numbers specifically for one or both of these patient subgroups. ACN was defined by colorectal carcinoma or advanced adenoma, according to the most advanced lesion per patient. ACN prevalence was analysed in addition to colorectal carcinoma prevalence only because it is a clinically relevant additional finding, given the potential of advanced adenomas to progress into malignancy24. Advanced adenoma was defined as an adenoma either larger than 10 mm, or with more than 25 per cent villous features (also classified as tubulovillous or villous histology), or with high‐grade dysplasia25. Uncomplicated diverticulitis was defined by peridiverticular inflammation, and complicated diverticulitis by diverticular abscess, perforation or fistula. The secondary outcome was colonoscopy‐related adverse events.
Statistical analysis
Prevalence rates of colorectal carcinoma and ACN were pooled using a DerSimonian and Laird random‐effects model and displayed using forest plots. The result of comparison between groups of patients with diverticulitis and asymptomatic controls was expressed as a pooled risk ratio with 95 per cent confidence intervals. Statistical heterogeneity was assessed using χ2 analysis and I 2 values. Funnel plots were used to assess publication bias. Statistical analyses were conducted using RStudio® (RStudio, Boston, Massachusetts, USA).
Results
Study selection
The search retrieved 4164 records (Fig. 1). After removal of 752 duplicates, 3412 records were screened based on title and abstract, and 136 full‐text articles were assessed for eligibility. Cross‐referencing did not identify additional relevant studies. Seventeen studies fulfilled the inclusion and exclusion criteria, and were included in this systematic review. The reasons for exclusion of full‐text articles are available in Table S1 (supporting information).
Figure 1.
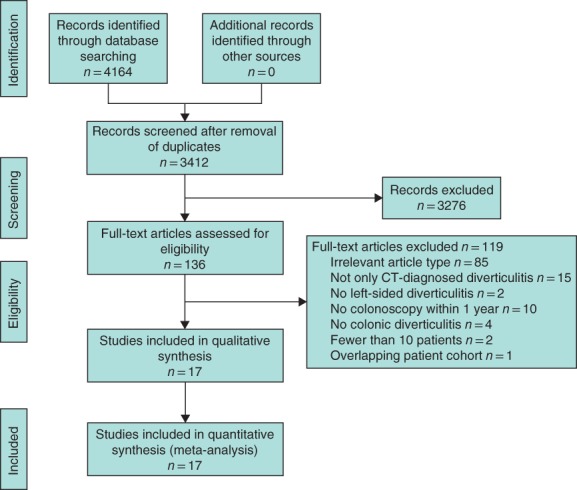
PRISMA flow diagram showing selection of articles for this review
Study characteristics
All studies were published after 2003. There were four prospective cohort studies26, 27, 28, 29 and 13 retrospective cohort studies30, 31, 32, 33, 34, 35, 36, 37, 38, 39, 40, 41, 42 (Table 1). Four studies26, 32, 34, 36 included only patients with left‐sided acute diverticulitis, and only the subgroup of patients with left‐sided diverticulitis from one Korean study33 was included in the present review. Twelve studies27, 28, 29, 30, 31, 35, 37, 38, 39, 40, 41, 42 did not report the proportion of left‐sided diverticulitis, but were conducted in the West. Most studies included all patients with acute diverticulitis; two28, 30 included only patients with uncomplicated diverticulitis. Eight studies26, 28, 29, 32, 33, 34, 36, 37 excluded patients who underwent colonoscopy before (varying from 6 months to 2 years) the diagnosis of acute diverticulitis. Colorectal carcinoma was reported in all 17 studies26, 27, 28, 29, 30, 31, 32, 33, 34, 35, 36, 37, 38, 39, 40, 41, 42. Eight studies28, 31, 32, 33, 34, 37, 38, 40 used the correct definition of advanced adenoma and could therefore be used for analysis of ACN (colorectal carcinoma and advanced adenoma combined). Only two studies34, 37 included a group of asymptomatic controls from a screening colonoscopy cohort; one study37 matched each patient with diverticulitis to a control patient based on sex and age, and the other34 selected patients aged between 50 and 75 years, of similar age to patients with acute diverticulitis. Ten of the 17 studies reported whether patients had undergone a complete colonoscopy. Six studies29, 30, 31, 34, 36, 37 reported caecal intubation rates above 90 per cent; the caecal intubation rate in the other four studies28, 32, 38, 39 ranged from 76 to 88 per cent.
Table 1.
Summary of study characteristics
| Reference | Setting | Design | Left‐sided (%) | Caecal intubation (%) | Age (years)* |
|---|---|---|---|---|---|
| Alexandersson et al.30 | Iceland | Retrospective | n.r. | 91 | 58 (50–67)† |
| Andrade et al.31 | Portugal | Retrospective | n.r. | 100 | 55 (11·1)† |
| Brar et al.32 | Canada | Retrospective | 100 | 86 | 55 (27–90)‡ |
| Chabok et al.26 | Sweden | Prospective | 100 | n.r. | 56 (27–84)§ |
| Choi et al.33 | Korea | Retrospective | 100 | n.r. | n.r. |
| Daniels et al.34 | Netherlands | Retrospective | 100 | 91 | 57 (49–65)† |
| Elmi et al.35 | USA | Retrospective | n.r. | n.r. | n.r. |
| Hjern et al.27 | Sweden | Prospective | n.r. | n.r. | 56 (29–79)‡ |
| Lahat et al.28 | Israel | Prospective | n.r. | 88 | 60(12·7) |
| Lau et al.36 | Australia | Retrospective | 100 | 93 | n.r. |
| Lecleire et al.37 | France | Retrospective | n.r. | 97 | 60·9(12·6) |
| Ou et al.38 | Canada | Retrospective | n.r. | 80 | 59·4(15·1) |
| Sakhnini et al.29 | Israel | Prospective | n.r. | 98 | 63 (30–89)§ |
| Sallinen et al.39 | Finland | Retrospective | n.r. | 76 | 58·3(13·9) |
| Schmilovitz‐Weiss et al.40 | Israel | Retrospective | n.r. | n.r. | 61·8(14·3) |
| Suhardja et al.41 | Australia | Retrospective | n.r. | n.r. | 58·8 (47–71)† |
| Zaman et al.42 | UK | Retrospective | n.r. | n.r. | n.r. |
Values are mean(s.d.) unless indicated otherwise; values are
median (i.q.r.),
mean (range) and
median (range). n.r., Not reported.
Population characteristics
A total of 3296 patients with acute diverticulitis was included in this review. The subgroup with uncomplicated acute diverticulitis across studies consisted of 959 patients.
Critical appraisal
Results of the risk‐of‐bias analysis are shown in Table S2 (supporting information). The quality of studies varied from moderate to good, ranging from four to seven stars on the Newcastle–Ottawa Scale. Most studies were mainly biased by the lack of a control group or the limited comparability between patients with acute diverticulitis and groups of asymptomatic controls. Most studies did not state which patients, among all those diagnosed with acute diverticulitis, eventually underwent colonoscopy. Patients with a deviant clinical course, for example persistent or progressive disease, may have undergone surgery before colonoscopy could be performed, risking selection bias for the patients who did undergo colonoscopy. The risk of publication bias was assessed for two outcomes: prevalence of colorectal carcinoma in all patients with acute diverticulitis and prevalence of colorectal carcinoma in the subgroup with uncomplicated acute diverticulitis (Fig. S1, supporting information). The funnel plot of studies assessing all patients with acute diverticulitis was slightly asymmetrical regarding small studies. Some small studies with a higher proportion of colorectal carcinoma may be considered missing. The funnel plot of the subgroup analysis in uncomplicated acute diverticulitis was symmetrical.
Prevalence of colorectal cancer and advanced colorectal neoplasia
The risk of colorectal carcinoma in patients with acute diverticulitis was comparable to that in asymptomatic controls (risk ratio 1·80, 95 per cent c.i. 0·66 to 4·96) in the meta‐analysis of data from only two studies34, 37 with a control group. All 17 studies reported rates of colorectal carcinoma in patients with acute diverticulitis, yielding a pooled colorectal carcinoma prevalence of 2·1 (95 per cent c.i. 1·5 to 3·1) per cent (I 2 = 40 per cent) (Fig. 2). The pooled prevalence of ACN was 6·9 (5·0 to 9·4) per cent (I 2 = 61 per cent) based on eight studies28, 31, 32, 33, 34, 37, 38, 40 (Fig. 3). The subgroup analysis of 959 patients with uncomplicated acute diverticulitis from six studies28, 30, 31, 32, 40, 41 showed a pooled colorectal carcinoma prevalence of 0·5 (0·2 to 1·2) per cent (I 2 = 0 per cent) (Fig. 4 a). Subgroup analysis of 197 patients with complicated acute diverticulitis from four studies31, 32, 40, 41 showed a pooled colorectal carcinoma prevalence of 8·3 (4·2 to 15·8) per cent (I 2 = 40 per cent) (Fig. 4 b).
Figure 2.
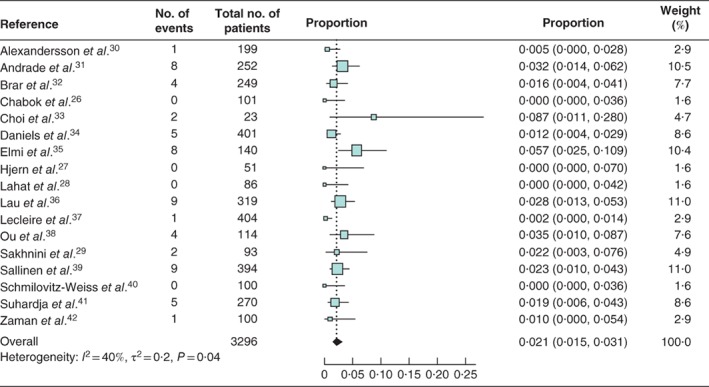
Forest plot of colorectal cancer prevalence in patients with acute diverticulitis A random‐effects model was used for meta‐analysis. Proportions are shown with 95 per cent confidence intervals.
Figure 3.
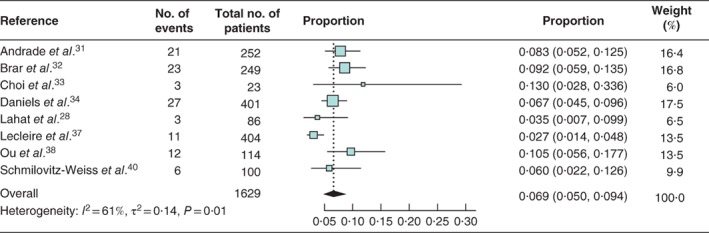
Forest plot of advanced colorectal neoplasia prevalence in patients with acute diverticulitis A random‐effects model was used for meta‐analysis. Proportions are shown with 95 per cent confidence intervals.
Figure 4.
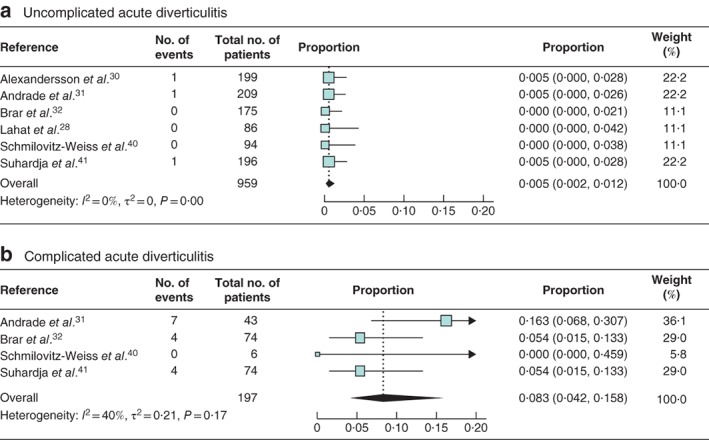
Forest plot of colorectal cancer prevalence according to severity of acute diverticulitis a Uncomplicated and b complicated acute diverticulitis. A random‐effects model was used for meta‐analysis. Proportions are shown with 95 per cent confidence intervals.
Ten studies29, 30, 32, 34, 35, 36, 37, 38, 39, 42, including 43 patients with colorectal carcinoma, reported the location of the carcinoma. Almost all tumours (41 of 43) were found at the site of the presumed acute diverticulitis.
Two studies31, 32 used logistic regression analyses to assess risk factors for ACN in the groups of patients with acute diverticulitis included in the present systematic review. Both found that patients with an abscess were at significantly higher risk of having ACN than those with uncomplicated acute diverticulitis, with multivariable odds ratios of 3·15 (95 per cent c.i. 1·59 to 11·59) and 4·15 (1·68 to 10·30) respectively. Older age was an independent predictor of the presence of ACN in both studies. Two other studies36, 39 used logistic regression, but performed these analyses on a combined group of patients who underwent colonoscopy and, if no colonoscopy had been performed, patients whose follow‐up data were collected using a cancer registry. These two studies assessed the risk of colorectal carcinoma instead of ACN. Nevertheless, their results were comparable to those of studies that included only patients who underwent colonoscopy; diverticular abscess was an independent risk factor for (the presence of) colorectal carcinoma in both studies.
Colonoscopy‐related adverse events
Only four studies28, 29, 37, 40 reported colonoscopy‐related adverse events. In two studies28, 40 (86 and 100 patients respectively), no complications occurred. In the other two studies29, 37 (93 and 404 patients respectively), three patients in total developed a perforation after colonoscopy; one was secondary to polypectomy and two were diverticular perforations.
Discussion
The risk of having colorectal carcinoma seemed to be comparable in patients with acute diverticulitis and asymptomatic controls, but only two studies could be included in this comparison, limiting firm conclusions. Three systematic reviews24, 43, 44 on this topic were published in 2014. The selection of studies included varied between these reviews and differed substantially from that in the present systematic review. Few studies in the previous reviews were included in the present systematic review, mainly because of differences in inclusion and exclusion criteria (Table S3, supporting information). Only one systematic review44 assessed the prevalence of colorectal carcinoma in all patients with acute diverticulitis, yielding a prevalence of 1·6 (95 per cent c.i. 0·9 to 2·8) per cent. The slightly lower prevalence compared with the present result may be explained by the fact that the 2014 meta‐analysis44 included two studies45, 46 in which some patients were diagnosed by ultrasonography, one study47 in which colonic evaluation was performed by barium enema or CT colonography in some patients, one study48 that undertook colonoscopies up to 2 years before the episode of acute diverticulitis, two studies28, 49 with overlapping patients cohorts, and one conference abstract50 that was never published as a full paper.
The three previous systematic reviews found a slightly higher prevalence of colorectal carcinoma in patients with uncomplicated acute diverticulitis than the present systematic review: 1·5 (95 per cent c.i. 1·0 to 2·3) per cent24, 1·2 (0·7 to 1·9) per cent43 and 0·7 (0·3 to 1·4 per cent)44. Two reviews24, 44 included studies26, 29, 35, 36 of patients with complicated diverticulitis rather than the intended uncomplicated disease only. Furthermore, these previous systematic reviews included several studies or subgroups of patients specifically excluded from the present review because the diverticulitis diagnoses were made partly using ultrasonography45, 46, 51 and not CT, colonoscopies were performed up to 2 years before48 or up to 11 years after35 the acute diverticulitis episode, or studies were only published as a conference abstract50, 52, 53. Moreover, one of the systematic reviews43 reported a crude mean proportion instead of using a fixed‐ or random‐effects model with a pooled, weighted mean proportion. The prevalence of ACN was reported in only one previous systematic review24, with a prevalence of 5·0 (3·8 to 6·7) per cent, comparable to that in the present review.
To assess the role of colonoscopy after an episode of acute diverticulitis, the prevalence of colorectal carcinoma and ACN needs to be compared with that in healthy individuals without acute diverticulitis, comprising asymptomatic controls. Because studies including such a control group were scarce, the only other way is to compare prevalence in the present systematic review with that in published data from cohorts of asymptomatic individuals54, 55, 56, 57, 58, 59, 60, 61. Eight studies assessed the prevalence of colorectal carcinoma and ACN in asymptomatic individuals who underwent screening colonoscopy (not related to diverticulitis). One study56 included asymptomatic individuals aged over 40 years, and the other seven54, 55, 57, 58, 59, 60, 61 included only those over 50 years of age, comparable to the age of patients with acute diverticulitis included in the present systematic review.
The eight screening colonoscopy studies reported a prevalence of colorectal carcinoma of between 0·4 and 1·0 per cent, compared with 2·1 per cent among all patients with acute diverticulitis in the present systematic review. The prevalence of colorectal carcinoma in patients with acute diverticulitis therefore seems to be higher than that in asymptomatic screening subjects. However, the 0·5 per cent prevalence of colorectal carcinoma in patients with uncomplicated acute diverticulitis is comparable to the prevalence in controls. The prevalence of ACN ranged from 3·8 to 10·3 per cent in the eight studies with asymptomatic controls, and seems comparable to that in patients with acute diverticulitis here (6·9 per cent). However, it is possible that the true ACN prevalence may be slightly higher in patients with acute diverticulitis owing to incomplete colonoscopies. Caecal intubation at colonoscopy may be more difficult after acute diverticulitis as luminal narrowing, spasm, muscular hypertrophy and fixation can cause technical difficulties8, 9, 62. Of the ten studies in the present review that reported the proportion of complete colonoscopies (caecal intubation), four did not have adequate caecal intubation rates (defined as at least 90 per cent63), which could have led to underestimation of the ACN prevalence. As almost all colorectal carcinomas are found at the site where acute diverticulitis is diagnosed, the effect of these lower caecal intubation rates is considered to be limited for the prevalence of colorectal carcinoma, but may be important for the prevalence of ACN.
It has been proposed that the association between acute diverticulitis and colorectal malignancy is not causal64. It is likely that colorectal carcinomas are sometimes misdiagnosed as acute diverticulitis because they have similar clinical and radiological signs. As the risk of malignancy is increased predominantly in complicated acute diverticulitis, misdiagnosis seems to be an issue particularly in this group. The comparable prevalence of colorectal carcinoma in asymptomatic screening controls and patients with uncomplicated diverticulitis also supports this misdiagnosis hypothesis. Apparently, a colorectal malignancy is not easily missed in a radiological image of uncomplicated diverticulitis, but it can be missed in an image of complicated diverticulitis.
A limitation of this review is the lack of studies including asymptomatic controls. Only two studies included such a control group, so conclusions are based mainly on comparison with the colorectal carcinoma prevalence in asymptomatic controls reported in the literature. Although a direct comparison would have been preferable, the consistency of the published prevalence seems to be a sign of the robustness of this comparison; the colorectal carcinoma prevalence in all screening colonoscopy studies from the literature (0·4–1·0 per cent) is below the 95 per cent confidence interval of the prevalence in patients with acute diverticulitis in the present review (95 per cent c.i. 1·5 to 3·1 per cent), but within the 95 per confidence interval of the prevalence in patients with uncomplicated acute diverticulitis (0·2 to 1·2 per cent). Another potential limitation is that the group of patients who underwent colonoscopy may have been subject to selection bias. Patients with a protracted clinical course may have undergone surgery before a colonoscopy could have been done, leading to possible underestimation of the prevalence of malignancy. On the other hand, patients with uncomplicated diverticulitis who did not develop persistent complaints are less likely to have undergone colonoscopy owing to doctor or patient preferences, which means that the prevalence of colorectal carcinoma may have been overestimated.
Several national and international guidelines11, 12, 13, 14, 15, 16, 17, 18 on acute diverticulitis have been published in recent years. The recommendations in these guidelines are conflicting, and the evidence on which they based differs. Most of the guidelines (6 of 8)11, 13, 14, 15, 17, 18 recommend routine colonoscopy after an episode of acute diverticulitis to rule out malignancy, although the American Gastroenterological Association (AGA)17 suggests that previous colonoscopies, co‐morbidities, persistent symptoms and patients' preferences may influence the decision. The scientific grounds for the recommendations in these six guidelines are noteworthy. Whereas the AGA17 and German15 guidelines are based on a previous systematic review, two other guidelines11, 18 used only two observational studies, and an Italian guideline13 published in 2015 stated that evidence‐based data were not available. The American Society of Colon and Rectal Surgeons14 made strong recommendations based on studies with a low quality of evidence, but based their recommendation for routine colonoscopy mainly on studies that assessed the risk of malignancy in a selected group of patients with signs suggestive of malignancy, such as colonic wall thickening or mass on CT. Two guidelines12, 16 recommend not performing routine colonoscopy after an episode of acute diverticulitis; of these, the World Society of Emergency Surgery (WSES)16 recommends omitting colonoscopy only in patients with uncomplicated acute diverticulitis. The fact that the AGA17 and WSES16 guidelines make opposite recommendations based on the same systematic reviews from 2014 highlights the interpretational uncertainty of previous evidence. The more robust evidence in the present systematic review may reduce the conflicting interpretation of evidence, and may result in higher levels of consensus on this topic.
Supporting information
Fig. S1 Funnel plot of CRC prevalence in a all acute diverticulitis patients and b subgroup of uncomplicated acute diverticulitis patients
Appendix S1. Supporting Information.
Acknowledgements
S.J.R. and S.T.v.D. contributed equally to this study. No preregistration exists for the studies reported in this article. Data, methods used in the analysis, and material used to conduct the research will be made available on request to any researcher for the purposes of reproducing the results or replicating the procedure.
Disclosure: The authors declare no conflict of interest.
References
- 1. Bharucha AE, Parthasarathy G, Ditah I, Fletcher JG, Ewelukwa O, Pendlimari R et al Temporal trends in the incidence and natural history of diverticulitis: a population‐based study. Am J Gastroenterol 2015; 110: 1589–1596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Jamal Talabani A, Lydersen S, Endreseth BH, Edna TH. Major increase in admission‐ and incidence rates of acute colonic diverticulitis. Int J Colorectal Dis 2014; 29: 937–945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Peery AF, Dellon ES, Lund J, Crockett SD, McGowan CE, Bulsiewicz WJ et al Burden of gastrointestinal disease in the United States: 2012 update. Gastroenterology 2012; 143: 1179–1187.e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Boulos PB, Cowin AP, Karamanolis DG, Clark CG. Diverticula, neoplasia, or both? Early detection of carcinoma in sigmoid diverticular disease. Ann Surg 1985; 202: 607–609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Boulos PB, Karamanolis DG, Salmon PR, Clark CG. Is colonoscopy necessary in diverticular disease? Lancet 1984; 1: 95–96. [DOI] [PubMed] [Google Scholar]
- 6. Laméris W, van Randen A, Bipat S, Bossuyt PM, Boermeester MA, Stoker J. Graded compression ultrasonography and computed tomography in acute colonic diverticulitis: meta‐analysis of test accuracy. Eur Radiol 2008; 18: 2498–2511. [DOI] [PubMed] [Google Scholar]
- 7. van Randen A, Laméris W, van Es HW, van Heesewijk HP, van Ramshorst B, Ten Hove W et al; OPTIMA Study Group. A comparison of the accuracy of ultrasound and computed tomography in common diagnoses causing acute abdominal pain. Eur Radiol 2011; 21: 1535–1545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Dafnis G, Granath F, Påhlman L, Ekbom A, Blomqvist P. Patient factors influencing the completion rate in colonoscopy. Dig Liver Dis 2005; 37: 113–118. [DOI] [PubMed] [Google Scholar]
- 9. Herman LL, Kurtz RC, McKee KJ, Sun M, Thaler HT, Winawer SJ. Risk factors associated with vasovagal reactions during colonoscopy. Gastrointest Endosc 1993; 39: 388–391. [DOI] [PubMed] [Google Scholar]
- 10. Williams C, Teague R. Colonoscopy. Gut 1973; 14: 990–1003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Andersen JC, Bundgaard L, Elbrønd H, Laurberg S, Walker LR, Støvring J; Danish Surgical Society . Danish national guidelines for treatment of diverticular disease. Dan Med J 2012; 59: C4453. [PubMed] [Google Scholar]
- 12. Andeweg CS, Mulder IM, Felt‐Bersma RJ, Verbon A, van der Wilt GJ, van Goor H et al; Netherlands Society of Surgery; Working group from Netherlands Societies of Internal Medicine, Gastroenterologists, Radiology, Health Technology Assessment and Dieticians. Guidelines of diagnostics and treatment of acute left‐sided colonic diverticulitis. Dig Surg 2013; 30: 278–292. [DOI] [PubMed] [Google Scholar]
- 13. Binda GA, Cuomo R, Laghi A, Nascimbeni R, Serventi A, Bellini D et al; Italian Society of Colon and Rectal Surgery. Practice parameters for the treatment of colonic diverticular disease: Italian Society of Colon and Rectal Surgery (SICCR) guidelines. Tech Coloproctol 2015; 19: 615–626. [DOI] [PubMed] [Google Scholar]
- 14. Feingold D, Steele SR, Lee S, Kaiser A, Boushey R, Buie WD et al Practice parameters for the treatment of sigmoid diverticulitis. Dis Colon Rectum 2014; 57: 284–294. [DOI] [PubMed] [Google Scholar]
- 15. Kruis W, Germer CT, Leifeld L; German Society for Gastroenterology, Digestive and Metabolic Diseases and German Society for General and Visceral Surgery . Diverticular disease: Guidelines of the German Society for Gastroenterology, Digestive and Metabolic Diseases and the German Society for General and Visceral Surgery. Digestion 2014; 90: 190–207. [DOI] [PubMed] [Google Scholar]
- 16. Sartelli M, Catena F, Ansaloni L, Coccolini F, Griffiths EA, Abu‐Zidan FM et al WSES Guidelines for the management of acute left sided colonic diverticulitis in the emergency setting. World J Emerg Surg 2016; 11: 37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Stollman N, Smalley W, Hirano I; AGA Institute Clinical Guidelines Committee . American Gastroenterological Association Institute guideline on the management of acute diverticulitis. Gastroenterology 2015; 149: 1944–1949. [DOI] [PubMed] [Google Scholar]
- 18. Wilkins T, Embry K, George R. Diagnosis and management of acute diverticulitis. Am Fam Physician 2013; 87: 612–620. [PubMed] [Google Scholar]
- 19. Stroup DF, Berlin JA, Morton SC, Olkin I, Williamson GD, Rennie D et al Meta‐analysis of observational studies in epidemiology: a proposal for reporting. Meta‐analysis Of Observational Studies in Epidemiology (MOOSE) group. JAMA 2000; 283: 2008–2012. [DOI] [PubMed] [Google Scholar]
- 20. Hall JF, Roberts PL, Ricciardi R, Marcello PW, Scheirey C, Wald C et al Colonic diverticulitis: does age predict severity of disease on CT imaging? Dis Colon Rectum 2010; 53: 121–125. [DOI] [PubMed] [Google Scholar]
- 21. Hjern F, Josephson T, Altman D, Holmström B, Johansson C. Outcome of younger patients with acute diverticulitis. Br J Surg 2008; 95: 758–764. [DOI] [PubMed] [Google Scholar]
- 22. Horesh N, Saeed Y, Horesh H, Berger Y, Speter C, Pery R et al Colonoscopy after the first episode of acute diverticulitis: challenging management paradigms. Tech Coloproctol 2016; 20: 383–387. [DOI] [PubMed] [Google Scholar]
- 23. Deeks JJ, Dinnes J, D'Amico R, Sowden AJ, Sakarovitch C, Song F et al; International Stroke Trial Collaborative Group; European Carotid Surgery Trial Collaborative Group. Evaluating non‐randomised intervention studies. Health Technol Assess 2003; 7 iii–x, 1–173. [DOI] [PubMed] [Google Scholar]
- 24. Daniels L, Unlü C, de Wijkerslooth TR, Dekker E, Boermeester MA. Routine colonoscopy after left‐sided acute uncomplicated diverticulitis: a systematic review. Gastrointest Endosc 2014; 79: 378–389. [DOI] [PubMed] [Google Scholar]
- 25. Schlemper RJ, Riddell RH, Kato Y, Borchard F, Cooper HS, Dawsey SM et al The Vienna classification of gastrointestinal epithelial neoplasia. Gut 2000; 47: 251–255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Chabok A, Smedh K, Nilsson S, Stenson M, Påhlman L. CT‐colonography in the follow‐up of acute diverticulitis: patient acceptance and diagnostic accuracy. Scand J Gastroenterol 2013; 48: 979–986. [DOI] [PubMed] [Google Scholar]
- 27. Hjern F, Jonas E, Holmström B, Josephson T, Mellgren A, Johansson C. CT colonography versus colonoscopy in the follow‐up of patients after diverticulitis – a prospective, comparative study. Clin Radiol 2007; 62: 645–650. [DOI] [PubMed] [Google Scholar]
- 28. Lahat A, Yanai H, Menachem Y, Avidan B, Bar‐Meir S. The feasibility and risk of early colonoscopy in acute diverticulitis: a prospective controlled study. Endoscopy 2007; 39: 521–524. [DOI] [PubMed] [Google Scholar]
- 29. Sakhnini E, Lahat A, Melzer E, Apter S, Simon C, Natour M et al Early colonoscopy in patients with acute diverticulitis: results of a prospective pilot study. Endoscopy 2004; 36: 504–507. [DOI] [PubMed] [Google Scholar]
- 30. Alexandersson BT, Hreinsson JP, Stefansson T, Jonasson JG, Bjornsson ES. The risk of colorectal cancer after an attack of uncomplicated diverticulitis. Scand J Gastroenterol 2014; 49: 576–580. [DOI] [PubMed] [Google Scholar]
- 31. Andrade P, Ribeiro A, Ramalho R, Lopes S, Macedo G. Routine colonoscopy after acute uncomplicated diverticulitis – challenging a putative indication. Dig Surg 2017; 34: 197–202. [DOI] [PubMed] [Google Scholar]
- 32. Brar MS, Roxin G, Yaffe PB, Stanger J, MacLean AR, Buie WD. Colonoscopy following nonoperative management of uncomplicated diverticulitis may not be warranted. Dis Colon Rectum 2013; 56: 1259–1264. [DOI] [PubMed] [Google Scholar]
- 33. Choi YH, Koh SJ, Kim JW, Kim BG, Lee KL, Im JP et al Do we need colonoscopy following acute diverticulitis detected on computed tomography to exclude colorectal malignancy? Dig Dis Sci 2014; 59: 2236–2242. [DOI] [PubMed] [Google Scholar]
- 34. Daniels L, Ünlü Ç, de Wijkerslooth TR, Stockmann HB, Kuipers EJ, Boermeester MA et al Yield of colonoscopy after recent CT‐proven uncomplicated acute diverticulitis: a comparative cohort study. Surg Endosc 2015; 29: 2605–2613. [DOI] [PubMed] [Google Scholar]
- 35. Elmi A, Hedgire SS, Pargaonkar V, Cao K, McDermott S, Harisinghani M. Is early colonoscopy beneficial in patients with CT‐diagnosed diverticulitis? AJR Am J Roentgenol 2013; 200: 1269–1274. [DOI] [PubMed] [Google Scholar]
- 36. Lau KC, Spilsbury K, Farooque Y, Kariyawasam SB, Owen RG, Wallace MH et al Is colonoscopy still mandatory after a CT diagnosis of left‐sided diverticulitis: can colorectal cancer be confidently excluded? Dis Colon Rectum 2011; 54: 1265–1270. [DOI] [PubMed] [Google Scholar]
- 37. Lecleire S, Nahon S, Alatawi A, Antonietti M, Chaput U, Di‐Fiore A et al Diagnostic impact of routine colonoscopy following acute diverticulitis: a multicenter study in 808 patients and controls. United European Gastroenterol J 2014; 2: 301–306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Ou G, Rosenfeld G, Brown J, Chan N, Hong T, Lim H et al Colonoscopy after CT‐diagnosed acute diverticulitis: is it really necessary? Can J Surg 2015; 58: 226–231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Sallinen V, Mentula P, Leppäniemi A. Risk of colon cancer after computed tomography‐diagnosed acute diverticulitis: is routine colonoscopy necessary? Surg Endosc 2014; 28: 961–966. [DOI] [PubMed] [Google Scholar]
- 40. Schmilovitz‐Weiss H, Yalunin E, Boaz M, Sehayek‐Shabbat V, Levin I, Chervinski A et al Does a colonoscopy after acute diverticulitis affect its management?: a single center experience. J Clin Gastroenterol 2012; 46: 317–320. [DOI] [PubMed] [Google Scholar]
- 41. Suhardja TS, Norhadi S, Seah EZ, Rodgers‐Wilson S. Is early colonoscopy after CT‐diagnosed diverticulitis still necessary? Int J Colorectal Dis 2017; 32: 485–489. [DOI] [PubMed] [Google Scholar]
- 42. Zaman S, Chapman W, Mohammed I, Gill K, Ward ST. Patients with computed tomography‐proven acute diverticulitis require follow‐up to exclude colorectal cancer. Intest Res 2017; 15: 195–202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. de Vries HS, Boerma D, Timmer R, van Ramshorst B, Dieleman LA, van Westreenen HL. Routine colonoscopy is not required in uncomplicated diverticulitis: a systematic review. Surg Endosc 2014; 28: 2039–2047. [DOI] [PubMed] [Google Scholar]
- 44. Sharma PV, Eglinton T, Hider P, Frizelle F. Systematic review and meta‐analysis of the role of routine colonic evaluation after radiologically confirmed acute diverticulitis. Ann Surg 2014; 259: 263–272. [DOI] [PubMed] [Google Scholar]
- 45. Pradel JA, Adell JF, Taourel P, Djafari M, Monnin‐Delhom E, Bruel JM. Acute colonic diverticulitis: prospective comparative evaluation with US and CT. Radiology 1997; 205: 503–512. [DOI] [PubMed] [Google Scholar]
- 46. van de Wall BJ, Reuling EM, Consten EC, van Grinsven JH, Schwartz MP, Broeders IA et al Endoscopic evaluation of the colon after an episode of diverticulitis: a call for a more selective approach. Int J Colorectal Dis 2012; 27: 1145–1150. [DOI] [PubMed] [Google Scholar]
- 47. Chabok A, Påhlman L, Hjern F, Haapaniemi S, Smedh K; AVOD Study Group . Randomized clinical trial of antibiotics in acute uncomplicated diverticulitis. Br J Surg 2012; 99: 532–539. [DOI] [PubMed] [Google Scholar]
- 48. Westwood DA, Eglinton TW, Frizelle FA. Routine colonoscopy following acute uncomplicated diverticulitis. Br J Surg 2011; 98: 1630–1634. [DOI] [PubMed] [Google Scholar]
- 49. Lahat A, Yanai H, Sakhnini E, Menachem Y, Bar‐Meir S. Role of colonoscopy in patients with persistent acute diverticulitis. World J Gastroenterol 2008; 14: 2763–2766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Elramah MM, Horwitz JP, Einstein MM, Qureshi J, Leo J, Omballi M et al Colonoscopic evaluation after acute diverticulitis – is there an association with colonic malignancy? Gastrointest Endosc 2010; 71: AB188. [Google Scholar]
- 51. Schout PJ, Spillenaar Bilgen EJ, Groenen MJ. Routine screening for colon cancer after conservative treatment of diverticulitis. Dig Surg 2012; 29: 408–411. [DOI] [PubMed] [Google Scholar]
- 52. Ahmeidat H, Kai‐Yip Fung A, McAteer D, Aly E. CT diagnosed acute diverticulitis: is the cost for subsequent colonoscopy to exclude associated carcinoma justified? Colorectal Dis 2012; 14: 19.23136820 [Google Scholar]
- 53. Alexandersson BT, Hreinsson JP, Stefánsson T, Bjorrnsson E. Tu1184 the risk of colorectal cancer is not increased in patients after an attack of diverticulitis. Gastroenterology 2013; 144: S‐783. [Google Scholar]
- 54. Blumenstein I, Tacke W, Bock H, Filmann N, Lieber E, Zeuzem S et al Prevalence of colorectal cancer and its precursor lesions in symptomatic and asymptomatic patients undergoing total colonoscopy: results of a large prospective, multicenter, controlled endoscopy study. Eur J Gastroenterol Hepatol 2013; 25: 556–561. [DOI] [PubMed] [Google Scholar]
- 55. Bokemeyer B, Bock H, Hüppe D, Düffelmeyer M, Rambow A, Tacke W et al Screening colonoscopy for colorectal cancer prevention: results from a German online registry on 269 000 cases. Eur J Gastroenterol Hepatol 2009; 21: 650–655. [DOI] [PubMed] [Google Scholar]
- 56. Hilsden RJ, Dube C, Heitman SJ, Bridges R, McGregor SE, Rostom A. The association of colonoscopy quality indicators with the detection of screen‐relevant lesions, adverse events, and postcolonoscopy cancers in an asymptomatic Canadian colorectal cancer screening population. Gastrointest Endosc 2015; 82: 887–894. [DOI] [PubMed] [Google Scholar]
- 57. Lisi D, Hassan C, Crespi M; AMOD Study Group . Participation in colorectal cancer screening with FOBT and colonoscopy: an Italian, multicentre, randomized population study. Dig Liver Dis 2010; 42: 371–376. [DOI] [PubMed] [Google Scholar]
- 58. Niv Y, Hazazi R, Levi Z, Fraser G. Screening colonoscopy for colorectal cancer in asymptomatic people: a meta‐analysis. Dig Dis Sci 2008; 53: 3049–3054. [DOI] [PubMed] [Google Scholar]
- 59. Quintero E, Castells A, Bujanda L, Cubiella J, Salas D, Lanas Á et al; COLONPREV Study Investigators. Colonoscopy versus fecal immunochemical testing in colorectal‐cancer screening. N Engl J Med 2012; 366: 697–706. [DOI] [PubMed] [Google Scholar]
- 60. Segnan N, Senore C, Andreoni B, Azzoni A, Bisanti L, Cardelli A et al; SCORE3 Working Group—Italy. Comparing attendance and detection rate of colonoscopy with sigmoidoscopy and FIT for colorectal cancer screening. Gastroenterology 2007; 132: 2304–2312. [DOI] [PubMed] [Google Scholar]
- 61. Stoop EM, de Haan MC, de Wijkerslooth TR, Bossuyt PM, van Ballegooijen M, Nio CY et al Participation and yield of colonoscopy versus non‐cathartic CT colonography in population‐based screening for colorectal cancer: a randomised controlled trial. Lancet Oncol 2012; 13: 55–64. [DOI] [PubMed] [Google Scholar]
- 62. Anderson JC, Messina CR, Cohn W, Gottfried E, Ingber S, Bernstein G et al Factors predictive of difficult colonoscopy. Gastrointest Endosc 2001; 54: 558–562. [DOI] [PubMed] [Google Scholar]
- 63. Rex DK, Schoenfeld PS, Cohen J, Pike IM, Adler DG, Fennerty MB et al Quality indicators for colonoscopy. Am J Gastroenterol 2015; 110: 72–90. [DOI] [PubMed] [Google Scholar]
- 64. Granlund J, Svensson T, Granath F, Hjern F, Ekbom A, Blomqvist P et al Diverticular disease and the risk of colon cancer – a population‐based case–control study. Aliment Pharmacol Ther 2011; 34: 675–681. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fig. S1 Funnel plot of CRC prevalence in a all acute diverticulitis patients and b subgroup of uncomplicated acute diverticulitis patients
Appendix S1. Supporting Information.


