Abstract
Objective
To examine the efficacy of methotrexate monotherapy relative to etanercept monotherapy and the value of combining methotrexate and etanercept for the treatment of patients with psoriatic arthritis (PsA).
Methods
In this double‐blind study, 851 patients with PsA were randomized to 1 of 3 treatment arms, as follows: oral methotrexate (20 mg) plus subcutaneous placebo given weekly (n = 284), subcutaneous etanercept (50 mg) plus oral placebo given weekly (n = 284), or subcutaneous etanercept (50 mg) plus oral methotrexate (20 mg) given weekly (combination therapy; n = 283). The American College of Rheumatology 20% improvement (ACR20) response and Minimal Disease Activity (MDA) response at week 24 were the primary end point and key secondary end point, respectively. Other measures of inflammatory arthritis, radiographic progression, and nonarticular disease manifestations were also assessed.
Results
Patients with PsA had a mean ± SD age of 48.4 ± 13.1 years, and the mean ± SD duration of PsA was 3.2 ± 6.3 years (median 0.6 years). ACR20 and MDA response rates at week 24 were significantly greater in patients who received etanercept monotherapy compared with those who received methotrexate monotherapy (ACR20, 60.9% versus 50.7% of patients [P = 0.029]; MDA, 35.9% versus 22.9% of patients [P = 0.005]), and both were significantly greater in the combination therapy group compared with the methotrexate monotherapy group at week 24 (ACR20, 65.0% versus 50.7% of patients [P = 0.005]; MDA, 35.7% versus 22.9% of patients [P = 0.005]). Other secondary outcomes (ACR50 and ACR70 response rates, proportions of patients achieving a Very Low Disease Activity score, and PsA disease activity scores) showed between‐group differences that were consistent with the primary and key secondary end point results. Furthermore, patients in both etanercept treatment arms showed less radiographic progression at week 48 compared with patients who received methotrexate monotherapy. Outcomes were similar in the combination therapy and etanercept monotherapy groups, except for some skin end points. No new safety signals were seen.
Conclusion
Etanercept monotherapy and combination therapy with etanercept and methotrexate showed greater efficacy than methotrexate monotherapy in patients with PsA, according to the ACR and MDA response rates and extent of radiographic progression at follow‐up. Overall, combining methotrexate and etanercept did not improve the efficacy of etanercept.
Introduction
Psoriatic arthritis (PsA) is a chronic, systemic inflammatory arthritis of the peripheral joints and axial skeleton that is commonly associated with psoriasis 1. Clinical manifestations include dactylitis, enthesitis, and nail changes, as well as joint erosions frequently seen on radiographs 1. PsA occurs in up to 30% of patients with psoriasis 2. The annual incidence of PsA in patients with psoriasis has been reported to be 1–3% 3, 4, 5.
Early treatment of PsA may help prevent the impaired function and deformities caused by joint destruction 6, 7, 8. Agents used to treat PsA include disease‐modifying antirheumatic drugs (DMARDs) such as methotrexate and tumor necrosis factor (TNF) inhibitors 9, 10. Additional agents that have recently been approved for use in PsA include biologic inhibitors of the interleukin‐12 (IL‐12)/IL‐23 and IL‐17 pathways 11, 12, 13 and small molecule inhibitors of janus kinase 14 and phosphodiesterase 4 15. Although methotrexate is widely used to treat PsA and is approved by the US Food and Drug Administration (FDA) for use in psoriasis, it is not approved by the FDA for the treatment of PsA. Therefore, there is a need to better understand its efficacy in PsA 16, 17, 18.
Prior trials comparing methotrexate with a biologic agent included patients who were inadequate responders to methotrexate 19, thus limiting the ability to clearly understand the efficacy of methotrexate in comparison with an established biologic therapy in methotrexate‐naive patients. In the Remicade Study in Psoriatic Arthritis Patients of Methotrexate‐Naive Disease (RESPOND) trial 20, investigators studied the efficacy of methotrexate in methotrexate‐naive patients, but it was an open‐label study that compared methotrexate with infliximab in combination with methotrexate, obscuring the ability to directly compare the efficacy of methotrexate and infliximab as monotherapies. The Methotrexate in Psoriatic Arthritis (MIPA) study, a randomized clinical trial comparing methotrexate with placebo in methotrexate‐naive patients, failed to demonstrate statistically significant differences between the 2 study arms at 24 weeks 21. However, the overall findings were inconclusive, possibly because of a high dropout rate and use of a submaximal methotrexate target dosage of 15 mg/week 21.
The efficacy of TNF inhibitors has been demonstrated in PsA 22, 23, 24, 25, 26, 27, but the benefit of combining methotrexate and TNF inhibitors remains unclear. In rheumatoid arthritis, the Trial of Etanercept and Methotrexate with Radiographic Patient Outcomes (TEMPO) study 28 (and analogous trials with other TNF inhibitors) have established that methotrexate used in combination with a TNF inhibitor increases the efficacy of the TNF inhibitor. No comparable study has been conducted in PsA, and results of observational studies have suggested that, unlike in rheumatoid arthritis, no additional efficacy is added by combining methotrexate with a TNF inhibitor in PsA 29, 30.
We therefore undertook the current randomized, controlled trial to examine the comparative efficacy of methotrexate monotherapy relative to TNF inhibitor monotherapy in methotrexate‐naive patients with PsA early in the course of their disease. This trial also assessed the effect of combining methotrexate with a TNF inhibitor for the treatment of PsA in this patient population.
Patients and methods
Study design and population
The Study of Etanercept and Methotrexate in Subjects with Psoriatic Arthritis (SEAM‐PsA) trial was a 48‐week phase III, multicenter, randomized, double‐blind international study 31. Following the 48‐week double‐blind period, there was a 30‐day safety follow‐up period. Study patients were adults age ≥18 years with active PsA (based on the Classification Criteria for Psoriatic Arthritis 32) who were naive to treatment with etanercept and other biologic agents, and had no prior use of methotrexate for PsA. Prior treatment with methotrexate was allowed for psoriasis if discontinuation had not been due to toxicity or intolerance, and if the methotrexate had been discontinued ≥6 months prior to initiation of the investigational product. Patients had to have 3 tender joints and 3 swollen joints (based on 68‐ and 66‐joint counts, respectively) at screening and at baseline, and an active psoriatic skin lesion that was ≥2 cm in diameter. For patients who were receiving nonsteroidal antiinflammatory drugs (NSAIDs), the dose had to be stable ≥2 weeks prior to initiation of the investigational product. For patients receiving oral corticosteroids, the dose had to be stable (not to exceed the equivalent of 10 mg prednisone per day) ≥4 weeks prior to initiation of the investigational product (for a complete description of the patient inclusion and exclusion criteria, see Supplementary Methods, available on the Arthritis & Rheumatology web site at http://onlinelibrary.wiley.com/doi/10.1002/art.40851/abstract).
Patients were enrolled in the study at 124 hospital centers or clinics in 17 countries (for a list of the investigators and countries in which the study was conducted, see Supplementary Methods at http://onlinelibrary.wiley.com/doi/10.1002/art.40851/abstract). Patients were randomized 1:1:1 to 1 of 3 treatment arms, as follows: oral methotrexate (target dose 20 mg) plus subcutaneous placebo given weekly, subcutaneous etanercept (target dose 50 mg) plus oral placebo given weekly, or subcutaneous etanercept (target dose 50 mg) plus oral methotrexate (target dose 20 mg) given weekly. At or after week 24, patients with an inadequate response to treatment (i.e., showing <20% improvement from baseline in tender joint counts and <20% improvement from baseline in swollen joint counts) received rescue therapy with etanercept plus methotrexate until week 48. Patients who were withdrawn or removed were not replaced. Patients were randomized via an interactive voice‐response system, based on a computer‐generated randomization schedule that was prepared by the study sponsor before the start of the trial. Each patient was assigned a single, unique randomization number. Treatment assignments were maintained within the interactive voice‐response system.
Both patients and investigators were blinded with regard to the randomized treatment assignments. Original treatment assignments remained blinded until patients reached week 48 or had an early termination visit (whichever occurred first). Treatment assignment was only unblinded when knowledge of treatment was essential to further patient care. Blinding was achieved by using matching prefilled syringes for the etanercept and injectable placebo administrations, and by using matching capsules for the methotrexate and oral placebo administrations.
Etanercept is a dimeric fusion protein (comprising the extracellular ligand‐binding domain of the human TNF p75 receptor linked to the Fc portion of human IgG1) that inhibits the activity of TNFα and TNFβ 33. For the SEAM‐PsA trial, etanercept was manufactured and provided by Immunex (a subsidiary of Amgen Inc.) in single‐use, prefilled 1‐ml syringes. Etanercept was administered at a dosage of 50 mg/week by subcutaneous injection, as recommended in the US prescribing information for use of etanercept in PsA 33.
The first dose of etanercept was administered at the study site, and subsequent injections were administered by the patient or a caregiver. Methotrexate, supplied as 2.5‐mg tablets in capsules (for blinding purposes), was initiated at a dosage of 10 mg/week and titrated up to 20 mg/week over a 4‐week period. If patients developed a toxic reaction to methotrexate, the methotrexate dosage could be reduced to as low as 10 mg/week. Folic acid was administered at 5–7 mg/week. Patients entering the study taking oral corticosteroids had to remain on a stable dose up to week 24. Those taking acetaminophen, narcotic analgesics, or NSAIDs had to remain on a stable dose up to week 24 and could not take these agents within 12 hours (24 hours for oxycontin) before a scheduled study visit.
Registration and ethics review
The SEAM‐PsA study has been completed, and is registered at https://ClinicalTrials.gov . The study was conducted in accordance with the Declaration of Helsinki. All patients provided written informed consent, and each participating site obtained protocol approval by an institutional review board. A data monitoring committee did not oversee this study.
Study outcome measures
Outcomes were measured at baseline and at weeks 4, 8, 12, 16, 24, 36, and 48. The primary end point was achievement of ≥20% improvement in the American College of Rheumatology response (ACR20) criteria 34 at week 24. (For detailed descriptions and scales for all of the outcome measures used, see Supplementary Table 1, available on the Arthritis & Rheumatology web site at http://onlinelibrary.wiley.com/doi/10.1002/art.40851/abstract.) The key secondary end point was the Minimal Disease Activity (MDA) response 35 at week 24. Secondary end points included the ACR20 and MDA response rates analyzed at other time points, as well as ACR50 and ACR70 response rates analyzed at all time points.
Additional end points, assessed at weeks 24 and 48, included the following: Very Low Disease Activity (VLDA) response 35, Psoriatic Arthritis Disease Activity Score (PASDAS) 36, Disease Activity Index for Psoriatic Arthritis (DAPSA) score 37, change from baseline in both the Leeds Dactylitis Index (LDI) 38 and the Spondyloarthritis Research Consortium of Canada (SPARCC) Enthesitis Index 39, dermatologic end points of the percentage of psoriasis‐affected body surface area (BSA) 40, the static Physician's Global Assessment (sPGA) of psoriasis 41, and modified Nail Psoriasis Severity Index (mNAPSI) 42, and patient‐reported outcomes from the Health Assessment Questionnaire disability index (HAQ DI) 43 and the 36‐item Short Form (SF‐36) Health Survey 44.
Radiographic end points included change from baseline in radiographic progression scores using the van der Heijde modification of the total Sharp score (SHS) 45, and rates of radiographic nonprogression at weeks 24 and 48. Radiographs of the hands and feet were obtained from patients locally and read centrally by 2 independent readers to assess erosions and joint space narrowing.
Safety analyses examined the incidence of adverse events, serious adverse events, and adverse events leading to discontinuation of the investigational product at weeks 24 and 48.
Statistical analysis
For the SEAM‐PsA study, we estimated that a sample size of 840 patients would provide a marginal power of >90% to detect treatment differences in the ACR20 response at week 24 between the etanercept‐containing arms and the methotrexate monotherapy arm, at a 2‐sided significance level of 0.025. This estimate assumed a randomization ratio of 1:1:1 to the 3 study arms (adjusting for an anticipated 10% dropout rate) and assumed an ACR20 response rate of 65% for the etanercept plus methotrexate combination arm, 60% for the etanercept monotherapy arm, and 44% for the methotrexate monotherapy arm. A sample size of 840 patients was also estimated to provide a marginal power of >90% to detect treatment differences in the MDA response at week 24 between the etanercept‐containing arms and the methotrexate monotherapy arm, at a 2‐sided significance level of 0.025, assuming response rates of 35% for the etanercept plus methotrexate combination therapy arm, 30% for the etanercept monotherapy arm, and 15% for the methotrexate monotherapy arm. These assumed response rates for the ACR20 and MDA responses were estimates based on data from previous studies 21, 22, 29, 35, 46 and on the assumption that generally higher responses than those reported in the literature would occur in the SEAM‐PsA trial since it enrolled patients whose disease course was anticipated to be in a relatively early phase and who were naive to treatment with biologics and methotrexate for PsA.
Analyses were conducted using SAS version 9.4 (SAS Institute). The main analyses of the primary and key secondary end points were based on the full analysis set, which included all randomized patients analyzed according to randomization assignment (intent‐to‐treat analysis). Missing data were imputed as nonresponder data. The treatment differences between etanercept plus methotrexate combination therapy and methotrexate monotherapy, and between etanercept monotherapy and methotrexate monotherapy were tested in a Bonferroni‐based gatekeeping chain procedure 47 to control the family‐wise, 2‐sided Type I error rate at 0.05 (see Supplementary Figure 1, available on the Arthritis & Rheumatology web site at http://onlinelibrary.wiley.com/doi/10.1002/art.40851/abstract). Between‐treatment comparisons were adjusted using the Cochran‐Mantel‐Haenszel test, with baseline body mass index status (≤30 kg/m2 or >30 kg/m2) and prior treatment with a non–biologic DMARD being used as stratification factors. The risk difference between groups was estimated using the Mantel‐Haenszel estimate of common risk difference (using the same stratification factors mentioned above).
Efficacy end points other than the primary and key secondary end points were analyzed as observed and not adjusted for multiplicity; therefore, P values are unadjusted and considered descriptive. Safety end points were summarized descriptively based on the safety analysis set, which included patients who received ≥1 dose of active investigational product. Patients were analyzed according to actual treatment received.
Results
Between March 3, 2015 and July 7, 2017, a total of 851 patients (of 1,080 screened) were enrolled in the SEAM‐PsA trial (229 patients did not meet the eligibility criteria) and randomized to receive a treatment (Figure 1). The last patient completed the study on July 6, 2018. Of the 851 enrolled patients, 284 were randomized to receive methotrexate monotherapy, 284 to receive etanercept monotherapy, and 283 to receive methotrexate plus etanercept (combination therapy) (Figure 1). Of the 851 patients, 44% were from North America, 27% from Europe, 16% from Latin America, 9% from Russia, and 4% from South Africa. The number of patients who received their intended treatment was 282 (99.3%) in the methotrexate monotherapy arm, 284 (100%) in the etanercept monotherapy arm, and 282 (99.6%) in the combination therapy arm. A total of 691 patients (81.2%) completed the trial. The most common reason for study discontinuation was withdrawal of consent. The primary end point was analyzed using the full analysis set of 851 randomized patients.
Figure 1.
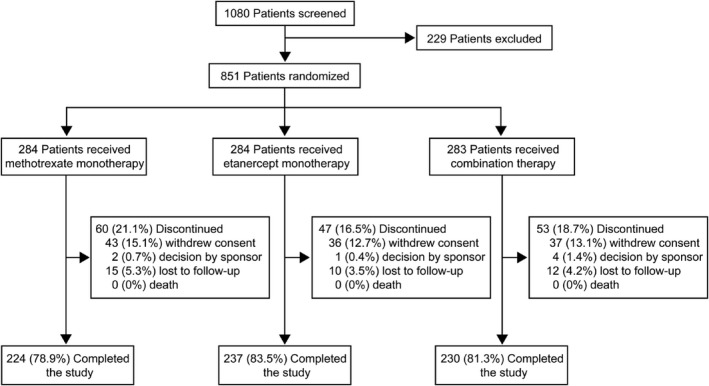
Diagram of patient allocation.
The 3 treatment arms were generally balanced with respect to baseline demographic and disease characteristics (Table 1). Most patients were white (90.7%), and the mean ± SD age was 48.4 ± 13.1 years. The duration of PsA was a mean ± SD 3.2 ± 6.3 years (median 0.6 years); overall, 56% of patients had a disease duration of ≤2 years. In total, 12.6% of the patients had received prior treatment with a nonbiologic DMARD (42 patients [5%] had received methotrexate prior to the study). Prior treatment with a nonbiologic DMARD occurred in 13.4%, 9.2%, and 15.2% of patients in the methotrexate monotherapy, etanercept monotherapy, and combination arms, respectively (Table 1). From weeks 4 to 24, patients in both of the methotrexate‐containing treatment arms achieved and maintained a mean methotrexate dose of >18.8 mg (median 20 mg) (average weekly doses are listed in Supplementary Table 2, available on the Arthritis & Rheumatology web site at http://onlinelibrary.wiley.com/doi/10.1002/art.40851/abstract). The percentage of patients who received rescue therapy at or after week 24 was 24.3% in the methotrexate monotherapy arm, 16.2% in the etanercept monotherapy arm, and 12.7% in the combination therapy arm.
Table 1.
Baseline demographic and disease characteristics of the patients in each treatment groupa
| Methotrexate monotherapy (n = 284) | Etanercept monotherapy (n = 284) | Combination therapy (n = 283) | |
|---|---|---|---|
| Age, mean ± SD years | 48.7 ± 13.1 | 48.5 ± 13.5 | 48.1 ± 12.7 |
| Sex, no. (%) female | 160 (56.3) | 133 (46.8) | 139 (49.1) |
| Race, no. (%) white | 255 (89.8) | 252 (88.7) | 265 (93.6) |
| Duration of PsA, mean ± SD years (no. assessed) | 3.6 ± 6.8 (231) | 3.1 ± 6.0 (222) | 3.0 ± 6.0 (231) |
| Prior use of nonbiologic DMARD, no. (%) | 38 (13.4) | 26 (9.2) | 43 (15.2) |
| Body mass index, mean ± SD kg/m2 (no. assessed) | 30.6 ± 7.1 (284) | 30.4 ± 6.6 (283) | 30.0 ± 6.7 (283) |
| ≤30 kg/m2, no. (%) | 146 (51.4) | 153 (53.9) | 160 (56.5) |
| >30 kg/m2, no. (%) | 138 (48.6) | 130 (45.8) | 123 (43.5) |
| Swollen joint count (of 66 joints), mean ± SD (no. assessed) | 12.9 ± 9.9 (284) | 11.5 ± 9.6 (283) | 11.2 ± 9.1 (282) |
| Tender joint count (of 68 joints), mean ± SD (no. assessed) | 20.9 ± 15.0 (284) | 18.8 ± 14.5 (283) | 20.0 ± 15.3 (282) |
| PASDAS, mean ± SEM (no. assessed) | 6.09 ± 0.07 (282) | 6.05 ± 0.07 (279) | 6.04 ± 0.07 (280) |
| DAPSA, mean ± SEM (no. assessed) | 46.5 ± 1.4 (283) | 43.4 ± 1.4 (281) | 43.8 ± 1.4 (281) |
| LDI | |||
| Patients with score >0 at baseline, no. (%) | 98 (34.5) | 96 (33.8) | 90 (31.8) |
| Mean ± SEM score (no. assessed) | 164.9 ± 26.9 (98) | 147.6 ± 20.8 (96) | 138.2 ± 23.9 (90) |
| SPARCC Enthesitis Index | |||
| Patients with score >0 at baseline, no. (%) | 191 (67.3) | 189 (66.5) | 196 (69.3) |
| Mean ± SEM score (no. assessed) | 5.7 ± 0.3 (191) | 5.5 ± 0.3 (189) | 5.9 ± 0.3 (196) |
| Psoriasis‐affected BSA, mean ± SD % | 12.7 ± 18.8 | 10.8 ± 14.7 | 10.7 ± 15.6 |
| Patients with ≥3% affected BSA at baseline, no. (%) | 192 (67.6) | 179 (63.0) | 177 (62.5) |
| Mean ± SEM % affected BSA | 18.1 ± 1.5 | 16.4 ± 1.2 | 16.4 ± 1.3 |
| Patients with ≥10% affected BSA at baseline, no. (%) | 99 (34.9) | 97 (34.2) | 90 (31.8) |
| Mean ± SEM % affected BSA | 30.3 ± 2.3 | 25.9 ± 1.7 | 27.3 ± 2.0 |
| sPGA | |||
| All patients, mean ± SD score | 2.6 ± 1.1 | 2.6 ± 1.0 | 2.5 ± 1.0 |
| Patients with ≥3% affected BSA at baseline, mean ± SEM score (no. assessed) | 2.9 ± 0.1 (191) | 2.9 ± 0.1 (179) | 2.9 ± 0.1 (177) |
| Patients with ≥10% affected BSA at baseline, mean ± SEM score (no. assessed) | 3.2 ± 0.1 (99) | 3.1 ± 0.1 (97) | 3.3 ± 0.1 (90) |
| mNAPSI | |||
| Patients with score >0 at baseline, no. (%) | 185 (65.1) | 206 (72.5) | 197 (69.6) |
| Mean ± SEM score (no. assessed) | 3.4 ± 0.2 (183) | 3.5 ± 0.2 (205) | 3.6 ± 0.2 (195) |
| HAQ DI, mean ± SEM score (no. assessed) | 1.27 ± 0.04 (283) | 1.15 ± 0.04 (284) | 1.15 ± 0.04 (282) |
| SF‐36, mean ± SEM score (no. assessed) | |||
| Overall | 80.8 ± 0.9 (282) | 82.9 ± 0.9 (284) | 83.6 ± 0.9 (282) |
| MCS | 45.2 ± 0.7 (282) | 45.1 ± 0.7 (284) | 46.3 ± 0.7 (282) |
| PCS | 35.6 ± 0.5 (282) | 37.8 ± 0.5 (284) | 37.4 ± 0.6 (282) |
| SHS | |||
| Total, mean ± SEM score (no. assessed) | 2.76 ± 0.12 (269) | 2.97 ± 0.13 (273) | 2.70 ± 0.12 (274) |
| Erosion score >0, no./total no. (%) | 223/269 (82.9) | 220/273 (80.6) | 224/274 (81.8) |
PsA = psoriatic arthritis; DMARD = disease‐modifying antirheumatic drug; PASDAS = Psoriatic Arthritis Disease Activity Score; DAPSA = Disease Activity Index for Psoriatic Arthritis; LDI = Leeds Dactylitis Index; SPARCC = Spondyloarthritis Research Consortium of Canada; BSA = body surface area; sPGA = static Physician's Global Assessment (of psoriasis); mNAPSI = modified Nail Psoriasis Severity Index; HAQ DI = Health Assessment Questionnaire disability index; SF‐36 = Short‐Form 36 Health Survey; MCS = mental component summary; PCS = physical component summary; SHS = van der Heijde modification of the total Sharp score (of radiographic progression).
The proportion of patients achieving an ACR20 response at week 24 (primary end point) was significantly greater among those receiving etanercept monotherapy compared with those receiving methotrexate monotherapy (173 [60.9%] of 284 patients versus 144 [50.7%] of 284 patients; adjusted P = 0.029) and significantly greater among those receiving combination therapy compared with those receiving methotrexate monotherapy (184 [65.0%] of 283 patients versus 144 [50.7%] of 284 patients; adjusted P = 0.005) (Figure 2 and Table 2). The proportion of patients achieving an MDA response at week 24 (key secondary end point) was significantly greater among patients receiving etanercept monotherapy compared with those receiving methotrexate monotherapy (102 [35.9%] of 284 patients versus 65 [22.9%] of 284 patients; adjusted P = 0.005) and significantly greater among those receiving combination therapy compared with those receiving methotrexate monotherapy (101 [35.7%] of 283 patients versus 65 [22.9%] of 284 patients; adjusted P = 0.005) (Table 2 and Figure 2).
Figure 2.
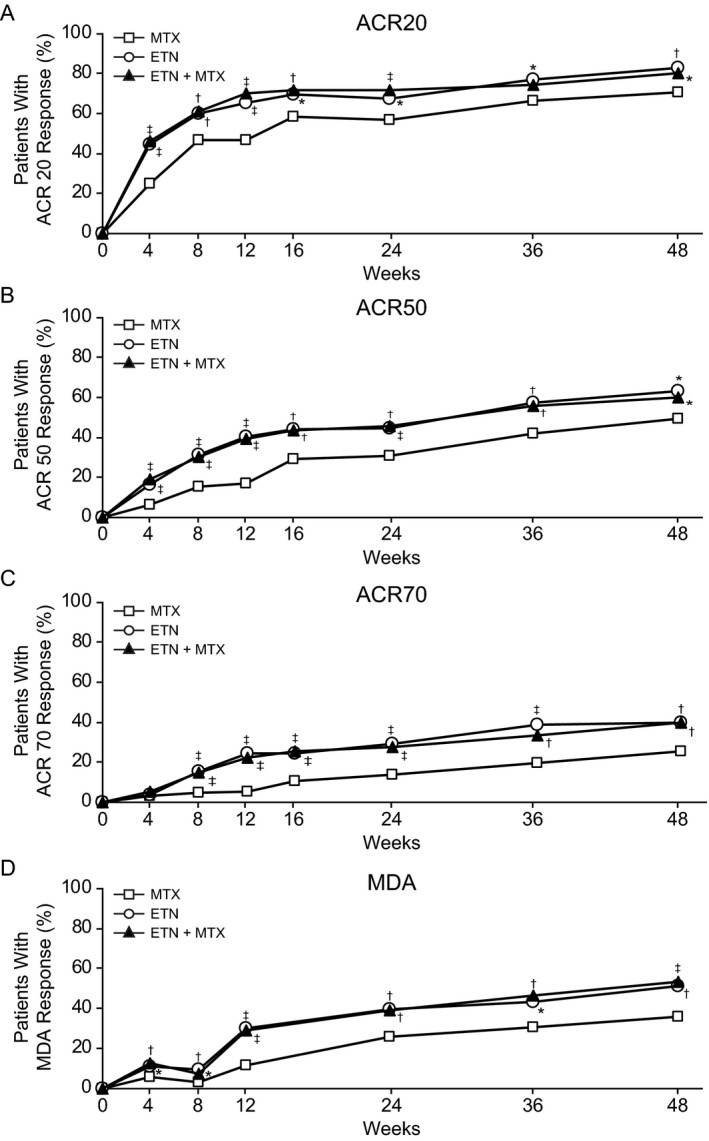
Percentage of patients achieving treatment responses based on the American College of Rheumatology 20% improvement criteria (ACR20) (A), ACR50 (B), and ACR70 (C) and the Minimal Disease Activity (MDA) response (D) over time (from baseline to week 48). Data are reported as observed (patients who received rescue therapy remained in their randomized treatment arm). * = unadjusted P < 0.05; † = unadjusted P < 0.01; ‡ = unadjusted P ≤ 0.001, versus methotrexate (MTX) monotherapy. ETN = etanercept.
Table 2.
Primary, key secondary, and select other end points at 24 weeksa
| Methotrexate monotherapy (n = 284) | Etanercept monotherapy (n = 284) | P, etanercept monotherapy vs. methotrexate monotherapy | Combination therapy (n = 283) | P, combination therapy vs. methotrexate monotherapy | |
|---|---|---|---|---|---|
| ACR improvement response, no./total (%) | |||||
| ACR20 | 144/284 (50.7) | 173/284 (60.9) | 0.029b | 184/283 (65.0) | 0.005b |
| ACR50 | 77/252 (30.6) | 114/257 (44.4) | 0.006 | 117/256 (45.7) | <0.001 |
| ACR70 | 35/253 (13.8) | 75/257 (29.2) | <0.001 | 71/256 (27.7) | <0.001 |
| MDA response, no./total (%) | 65/284 (22.9) | 102/284 (35.9) | 0.005b | 101/283 (35.7) | 0.005b |
| VLDA response, no./total (%) | 12/252 (4.8) | 39/257 (15.2) | <0.001 | 37/258 (14.3) | <0.001 |
| PASDAS, mean ± SEM change from baseline (no. assessed) | −1.98 ± 0.10 (246) | −2.64 ± 0.10 (250) | <0.001 | −2.63 ± 0.11 (255) | <0.001 |
| DAPSA, mean ± SEM change from baseline (no. assessed) | −22.6 ± 1.4 (251) | −25.0 ± 1.3 (253) | 0.24 | −24.9 ± 1.4 (256) | 0.23 |
| LDI | |||||
| Mean ± SEM change from baseline (no. assessed) | −128.8 ± 26.8 (89) | −119.1 ± 20.7 (89) | 0.85 | −110.2 ± 22.7 (87) | 0.68 |
| Resolution, no./total (%) | 58/89 (65.2) | 68/89 (76.4) | 0.12 | 69/87 (79.3) | 0.057 |
| SPARCC Enthesitis Index | |||||
| Mean ± SEM change from baseline (no. assessed) | −3.1 ± 0.3 (167) | −3.0 ± 0.3 (173) | 0.93 | −2.9 ± 0.3 (179) | 0.70 |
| Resolution, no./total (%) | 72/167 (43.1) | 91/173 (52.6) | 0.11 | 85/179 (47.5) | 0.55 |
| Psoriasis‐affected BSA,&error;mean ± SEM % improvement&error;from baseline (no. assessed) | |||||
| Patients with ≥3% BSA affected at baseline | 66.1 ± 2.8 (179) | 69.8 ± 2.7 (166) | 0.49 | 75.5 ± 3.7 (163) | 0.031 |
| Patients with ≥10% BSA affected at baseline | 65.7 ± 3.7 (92) | 74.2 ± 3.3 (91) | 0.12 | 81.6 ± 2.6 (86) | <0.001 |
| sPGA, status clear or almost&error;clear, no./total (%)c | |||||
| Patients with ≥3% BSA affected at baseline | 118/178 (66.3) | 120/166 (72.3) | 0.40 | 125/161 (77.6) | 0.019 |
| Patients with ≥10% BSA affected at baseline | 54/91 (59.3) | 72/91 (79.1) | 0.012 | 67/85 (78.8) | 0.004 |
| mNAPSI | |||||
| Mean ± SEM change from baseline (no. assessed) | −1.1 ± 0.2 (121) | −1.5 ± 0.2 (115) | 0.10 | −1.7 ± 0.2 (123) | 0.020 |
| Patients achieving a score of 1, no./total (%) | 47/121 (38.8) | 50/115 (43.5) | 0.44 | 60/123 (48.8) | 0.14 |
| HAQ DI, mean ± SEM change from baseline (no. assessed) | −0.41 ± 0.04 (252) | −0.44 ± 0.04 (258) | 0.67 | −0.47 ± 0.04 (257) | 0.34 |
| SF‐36, mean ± SEM changefrom baseline (no. assessed) | |||||
| Total score | 9.2 ± 0.8 (253) | 10.6 ± 0.8 (256) | 0.31 | 11.3 ± 0.9 (257) | 0.11 |
| PCS score | 6.0 ± 0.6 (253) | 7.8 (0.6) (256) | 0.033 | 8.0 ± 0.6 (257) | 0.015 |
| MCS score | 3.3 ± 0.6 (253) | 2.8 (0.6) (256) | 0.56 | 3.3 ± 0.6 (257) | 0.97 |
| SHS score | |||||
| Mean ± SEM change from baseline at week 48 (no. assessed) | 0.08 ± 0.03 (216) | −0.04 ± 0.04 (225) | 0.014 | −0.01 ± 0.03 (226) | 0.041 |
| Nonprogression at week 48, no./total (%)d | 193/216 (89.4) | 213/225 (94.7) | 0.088 | 214/226 (94.7) | 0.033 |
All end points were measured at 24 weeks, except for the van der Heijde modification of the total Sharp score (SHS) of radiographic progression, which was measured at 48 weeks. VLDA = Very Low Disease Activity; PASDAS = Psoriatic Arthritis Disease Activity Score; DAPSA = Disease Activity Index for Psoriatic Arthritis; LDI = Leeds Dactylitis Index; SPARCC = Spondyloarthritis Research Consortium of Canada; BSA = (psoriasis‐affected) body surface area; mNAPSI = modified Nail Psoriasis Severity Index; HAQ DI = Health Assessment Questionnaire disability index; SF‐36 = Short‐Form 36 Health Survey; PCS = physical component summary; MCS = mental component summary.
P values for the American College of Rheumatology 20% improvement (ACR20) response rates at week 24 (primary end point) and Minimal Disease Activity (MDA) response rates at week 24 (key secondary end point) were tested in a Bonferroni‐based gatekeeping procedure and stratified by body mass index category and prior nonbiologic disease‐modifying antirheumatic drug use. All other P values were unadjusted.
A status of clear on the static Physician's Global Assessment (sPGA) of psoriasis was defined as a score of 0, and a status of almost clear was defined as a score of 1. The score scale ranges from 0 (clear) to 5 (severe).
Radiographic nonprogression was defined as a change in the SHS of ≤0 from baseline to week 48.
The proportion of patients achieving an ACR50 response at week 24 was greater for the etanercept monotherapy group compared with the methotrexate monotherapy group (114 [44.4%] of 257 patients versus 77 [30.6%] of 252 patients; unadjusted P = 0.006) and for the combination therapy group compared with the methotrexate monotherapy group (117 [45.7%] of 256 patients versus 77 [30.6%] of 252 patients; unadjusted P < 0.001) (Table 2 and Figure 2). Similarly, the proportion of patients achieving an ACR70 response at week 24 was greater with etanercept monotherapy compared with methotrexate monotherapy (75 [29.2%] of 257 patients versus 35 [13.8%] of 253 patients; unadjusted P < 0.001) and greater with combination therapy compared with methotrexate monotherapy (71 [27.7%] of 256 patients versus 35 [13.8%] of 253; unadjusted P < 0.001) (Table 2 and Figure 2). Although not formally tested for comparison, the ACR improvement responses and MDA response were similar between the combination therapy and etanercept monotherapy arms (Table 2). The proportions of patients achieving ACR20, ACR50, ACR70, and MDA responses at week 48 were consistent with those seen at week 24 (results for all 48‐week outcomes are shown in Supplementary Table 3, available on the Arthritis & Rheumatology web site at http://onlinelibrary.wiley.com/doi/10.1002/art.40851/abstract).
The mean change in SHS score from baseline at week 48 indicated that there was less radiographic progression in patients who received etanercept monotherapy compared with those who received methotrexate monotherapy (mean ± SEM change −0.04 ± 0.04 versus 0.08 ± 0.03; unadjusted P = 0.014), and less radiographic progression in those who received combination therapy compared with those who received methotrexate monotherapy (mean ± SEM change −0.01 ± 0.03 versus 0.08 ± 0.03; unadjusted P = 0.041) (Table 2). The cumulative probability plot of the change in SHS from baseline at week 48 showed a lower likelihood of radiographic progression in the etanercept‐containing treatment arms compared with the methotrexate monotherapy arm (Figure 3).
Figure 3.
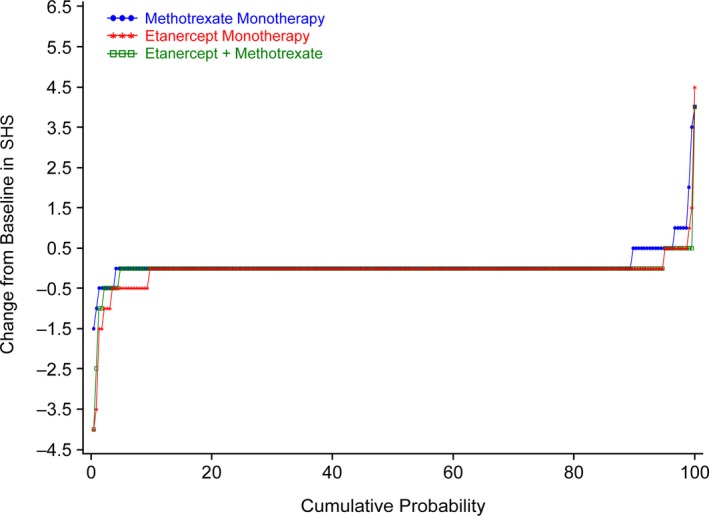
Cumulative probability plot of the change from baseline in the van der Heijde modification of the total Sharp score (SHS) of radiographic progression at week 48 in the methotrexate monotherapy, etanercept monotherapy, and combination therapy study arms. At week 48, progression (change from baseline in SHS >0) was seen in 23 patients (10.6%), 12 patients (5.3%), and 12 patients (5.3%) in the methotrexate monotherapy, etanercept monotherapy, and combination therapy arms, respectively.
Consistent results were seen with rates of radiographic progression at week 48 (see Supplementary Figure 2, available on the Arthritis & Rheumatology web site at http://onlinelibrary.wiley.com/doi/10.1002/art.40851/abstract). Moreover, the rates of radiographic nonprogression were similar between each etanercept‐containing treatment arm.
The VLDA response at week 24 was greater in patients receiving etanercept monotherapy compared with those receiving methotrexate monotherapy (39 [15.2%] of 257 patients versus 12 [4.8%] of 252 patients; unadjusted P < 0.001), and in those receiving combination therapy compared with those receiving methotrexate monotherapy (37 [14.3%] of 258 patients versus 12 [4.8%] of 252 patients; unadjusted P < 0.001) (Table 2).
Analysis of changes in disease activity levels showed that the mean improvement in the PASDAS score from baseline at week 24 was greater in the etanercept‐containing treatment arms compared with the methotrexate monotherapy arm (Table 2). Furthermore, the mean improvement in the DAPSA score from baseline at week 24 was numerically greater in the etanercept‐containing treatment arms compared with the methotrexate monotherapy arm, but the differences were modest (Table 2). The results for these end points were similar for both the etanercept monotherapy and combination therapy arms (Table 2).
Although numerical trends were observed, no meaningful differences were seen in the percentage of patients who achieved clear scores on the LDI or SPARCC Enthesitis Index by week 24 between the etanercept‐containing and methotrexate monotherapy treatment arms (Table 2). Furthermore, although not formally tested, results for the LDI and SPARCC Enthesitis Index were similar in both etanercept‐containing treatment arms (Table 2).
In patients with ≥3% or ≥10% BSA affected by psoriasis at baseline, analyses at week 24 showed that there was greater mean percentage improvement in psoriasis‐affected BSA in patients who received combination therapy compared with those who received methotrexate monotherapy. Moreover, a greater percentage of patients in the combination therapy group had clear or almost clear sPGA scores compared with those in the methotrexate monotherapy arm (Table 2).
The number of patients who experienced improvements in psoriasis‐affected BSA was numerically higher in the etanercept monotherapy arm compared with the methotrexate monotherapy arm. Moreover, a greater percentage of patients receiving etanercept monotherapy had clear or almost clear sPGA scores compared with patients receiving methotrexate monotherapy.
In contrast to the distributions of ACR and MDA response rates, more patients in the combination therapy group experienced numerically higher improvements in skin end points compared with patients in the etanercept monotherapy group (Table 2). At week 24, none of the patients achieved a clear mNAPSI score, but when the percentage of patients achieving an mNAPSI score of 1 was examined, small differences between the treatment arms were seen (Table 2).
In each of the 3 study arms, there were improvements from baseline at week 24 in the HAQ DI and SF‐36 total health function scores. Differences in the SF‐36 total score and SF‐36 mental component summary score were minimal between the study arms, but the SF‐36 physical component summary score showed a greater mean change from baseline at week 24 in the etanercept‐containing arms compared with the methotrexate monotherapy arm (Table 2).
Over this 48‐week study, rates of adverse events, serious adverse events, and adverse events that led to discontinuation of the investigational product were similar across the 3 study arms (Table 3). The rates of any adverse events were 212 (75.2%) of 282 patients receiving methotrexate monotherapy, 191 (67.7%) of 282 patients receiving etanercept monotherapy, and 216 (76.1%) of 284 patients receiving combination therapy. Of note, nausea occurred more commonly in the methotrexate‐containing arms (37 [13.1%] of 282 patients in the methotrexate monotherapy arm and 41 [14.4%] of 284 patients in the combination therapy arm compared with 18 [6.4%] of 282 patients in the etanercept monotherapy arm). Injection site reactions occurred more commonly in the etanercept‐containing arms (5 [1.8%] of 282 patients in the etanercept monotherapy arm and 8 [2.8%] of 284 patients in the combination therapy arm compared with 1 [0.4%] of 282 patients in the methotrexate monotherapy arm). No fatal adverse events were reported.
Table 3.
Summary of safety results at 48 weeksa
| Methotrexate monotherapy (n = 282) | Etanercept monotherapy (n = 282) | Combination therapy (n = 284) | |
|---|---|---|---|
| Any adverse event | 212 (75.2) | 191 (67.7) | 216 (76.1) |
| Serious adverse eventb | 16 (5.7) | 19 (6.7) | 17 (6.0) |
| Adverse events leading to discontinuation of investigational product | 19 (6.7) | 16 (5.7) | 20 (7.0) |
| Treatment‐related adverse events | 64 (22.7) | 71 (25.2) | 79 (27.8) |
| Fatal adverse events | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Adverse events occurring in ≥5%of patients | |||
| Nausea | 37 (13.1) | 18 (6.4) | 41 (14.4) |
| Nasopharyngitis | 22 (7.8) | 21 (7.4) | 27 (9.5) |
| Upper respiratory tract infection | 21 (7.4) | 18 (6.4) | 23 (8.1) |
| Diarrhea | 17 (6.0) | 13 (4.6) | 14 (4.9) |
| Headache | 15 (5.3) | 12 (4.3) | 17 (6.0) |
| Bronchitis | 9 (3.2) | 14 (5.0) | 19 (6.7) |
| Vomiting | 15 (5.3) | 7 (2.5) | 10 (3.5) |
Adverse events were categorized using the Medical Dictionary for Regulatory Activities, version 21. Values are the number (%) of patients.
The most common serious adverse events were of the system organ class infections and infestations, which occurred in 1.1% of patients in the methotrexate monotherapy arm, 2.8% of patients in the etanercept monotherapy arm, and 2.5% of patients in the combination therapy arm.
Discussion
The SEAM‐PsA trial contributes to our understanding of how best to treat patients early in the course of PsA who are naive to treatment with methotrexate and to biologic DMARDs. The results of this study demonstrate that etanercept as monotherapy and in combination with methotrexate is superior to methotrexate monotherapy when assessed according to the proportion of patients experiencing ACR20 and MDA responses (the primary and key secondary end points of the trial, respectively). The ACR50 and ACR70 responses and the PsA‐specific composite measures (VLDA and PASDAS) also showed greater efficacy with etanercept monotherapy and combination therapy compared with methotrexate monotherapy. The etanercept‐containing treatment arms also showed less radiographic progression compared with the methotrexate monotherapy arm. In general, the addition of methotrexate to etanercept did not contribute meaningfully to the overall efficacy of etanercept monotherapy, except in the case of some of the skin end points, for which combination therapy appeared to have increased efficacy.
The results of this study represent a detailed characterization of the efficacy of methotrexate monotherapy relative to a TNF inhibitor across a broad spectrum of PsA clinical manifestations in patients with early PsA. To overcome a key criticism of the MIPA trial 21, in which the target methotrexate dosage was 15 mg/week, the SEAM‐PsA trial stipulated a target dosage of 20 mg/week and achieved mean dosages of methotrexate in the 2 methotrexate‐containing arms of >18.8 mg/week from weeks 4 to 24.
As a comparator study, the SEAM‐PsA trial had no separate placebo arm, so the interpretation of the efficacy of methotrexate across the various clinical end points needs to be considered relative to etanercept. Nevertheless, methotrexate showed generally good efficacy across multiple domains, which is consistent with the Tight Control of PsA (TICOPA) study, which examined methotrexate monotherapy in PsA 48. Although numerical trends were observed, no substantial differences were noted between the etanercept and methotrexate monotherapy arms in terms of enthesitis and dactylitis scores and change in psoriasis‐affected BSA from baseline (when examined among patients with ≥3% BSA involved with psoriasis at baseline). However, patients with ≥10% BSA involved with psoriasis at baseline were more likely to achieve clear or almost clear status by week 24 in the etanercept monotherapy or combination therapy groups compared with the methotrexate monotherapy group.
In addition, no clear differences in the HAQ DI or SF‐36 scores were noted between the 3 study arms, except for the SF‐36 physical component summary score, which showed a greater mean change from baseline at week 24 in the etanercept‐containing treatment arms compared with the methotrexate monotherapy arm. Overall, these results support the use of etanercept as monotherapy without the need for concomitant methotrexate, and also provide information about methotrexate when used as monotherapy.
Some limitations of this study should be noted. The study did not consider axial disease in the eligibility criteria. In addition, the study included patients with mostly polyarticular disease (with mean swollen joint counts and tender joint counts of ~12 and ~30, respectively), which limits the generalizability of the results to those patients with oligoarticular disease and/or axial involvement. While the study was designed to characterize the relative efficacy of etanercept and methotrexate, there is significant interest in the study data with regard to efficacy of methotrexate monotherapy. However, the absence of a placebo group in this study limits the ability to draw firm conclusions about the efficacy of methotrexate monotherapy.
Disease modification is an important clinical consideration in the treatment of PsA. Investigators have demonstrated the ability of etanercept to inhibit radiographic progression in PsA 49, but the effect of methotrexate on radiographic progression has not been studied in a placebo‐controlled trial. Since the SEAM‐PsA study did not enrich for patients with radiographic evidence of PsA, only a small subset of patients, as expected, showed radiographic changes over a 48‐week period. Moreover, any differences between the methotrexate monotherapy arm and the etanercept monotherapy arm at week 48 would have been blunted by the 24.3% of patients in the methotrexate monotherapy arm who received rescue combination therapy starting at or after week 24. These results demonstrate that etanercept is associated with a lower degree of radiographic progression compared with methotrexate, and that combining methotrexate and etanercept may not have any discernible effect on radiographic progression as compared with etanercept monotherapy. Although the magnitude of the effects was small, the differences were clear for this clinically important end point. The lack of a placebo arm limits the ability to interpret the effect of methotrexate alone on radiographic progression, and these results (as for the other end points) should be interpreted relative to those in the other treatment arms.
MDA response was included as the key secondary end point in this trial to highlight the importance of more comprehensively capturing the unique clinical manifestations of PsA when compared with the “joint‐centric” ACR response measures. The MDA criteria evaluate PsA disease activity using measures of joint and entheseal inflammation, skin disease, patient‐reported outcomes, and functional disability 24, 35, 50, 51, 52. The MDA response rates in this study were consistent with the ACR20, ACR50, and ACR70 response rates in terms of showing greater responses in the etanercept‐containing treatment arms compared with the methotrexate monotherapy arm; no additional efficacy was observed with combination therapy compared with etanercept monotherapy.
In addition to the MDA response, other PsA‐specific outcome measures were included in this study in support of the overall findings. The VLDA response has recently become a clinically relevant benchmark that utilizes the same components as the MDA but with response defined as meeting all 7 MDA criteria. PASDAS and DAPSA are additional measures (with PASDAS covering a broader spectrum of PsA manifestations) that assess a binary outcome in PsA and also provide a continuous measure of disease activity. The PASDAS scores were consistent with those seen for the ACR and MDA responses, but the DAPSA scores showed similar outcomes across all 3 study arms. Consistent with the other end points, methotrexate did not contribute additional efficacy to etanercept alone.
Many patients in this trial had a moderate‐to‐severe level of psoriasis, as assessed by the percentage of psoriasis‐affected BSA. Results with regard to the dermatologic end points showed that etanercept and methotrexate had good efficacy, with a suggestion that the combination treatment arm had slightly greater efficacy than either of the monotherapy treatment arms in terms of improvement in the percentage of psoriasis‐affected BSA, sPGA scores, and mNAPSI scores. The fact that etanercept was more effective in combination with methotrexate for the skin‐related end points is consistent with results reported in a prior study 53; it should also be noted that etanercept monotherapy was administered at 50 mg once per week in this study, but is indicated for use at 50 mg twice per week to treat psoriasis in adults 33.
The observed varied impact of methotrexate, both alone and as an addition to etanercept, across these individual manifestations provides insights about the potentially context‐dependent and heterogeneous drivers of PsA pathology. Taken as a whole, the SEAM‐PsA trial results are remarkably different from those of the TEMPO trial in rheumatoid arthritis 28, in which the combination of etanercept and methotrexate was more effective than etanercept monotherapy, which underscores the pathophysiologic differences between rheumatoid arthritis and PsA 54.
Differences among TNF inhibitors have been noted in terms of their effects on long‐term survival in patients in clinical registries, when the impact of using a TNF inhibitor as monotherapy has been compared with a TNF inhibitor in combination with methotrexate 29, 55. These differences should be taken into account when considering the use of TNF inhibitors as monotherapy or in combination with methotrexate. Because of patients’ and physicians’ concerns with regard to the tolerability and safety of methotrexate, a clear understanding of its clinical benefits, alone or in combination with TNF inhibitors, is an important aspect of clinical decision‐making. Thus, the characterization in this trial of the safety and tolerability of both methotrexate monotherapy and biologic monotherapy provides important information when considering the safety profile (including immunogenicity) associated with PsA treatment approaches.
Overall, these results provide information that is of practical value for clinical practice, in terms of both demonstrating the relative efficacy of methotrexate and etanercept and characterizing the impact of combining methotrexate and etanercept across a broad range of PsA clinical manifestations.
Author contributions
All authors were involved in drafting the article or revising it critically for important intellectual content, and all authors approved the final version to be published. Dr. Mease had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Study conception and design
Mease, Gladman, Collier, Ritchlin, Kricorian, Chung.
Acquisition of data
Mease, Gladman, Collier, Ritchlin, Helliwell, Chung.
Analysis and interpretation of data
Mease, Gladman, Collier, Ritchlin, Helliwell, Liu, Kricorian, Chung.
Role of the study sponsor
Amgen Inc., the sponsor of the SEAM‐PsA trial, designed the trial in collaboration with academic investigators, oversaw data collection, performed the data analyses, and supported the development of this manuscript. Data interpretation and writing of the manuscript were performed by both the Amgen and non‐Amgen authors. The corresponding author had full access to all of the data in the study. The sponsor and the corresponding author had the final responsibility for the decision to submit this report for publication. Linda Rice, PhD (Amgen Inc.) and Julia R. Gage (on behalf of Amgen Inc.) assisted in writing the manuscript and preparing the tables and figures. Publication of this article was contingent upon approval by Amgen Inc. and all of the authors.
Supporting information
ClinicalTrials.gov identifier: NCT02376790.
Supported by Amgen Inc.
Dr. Mease has received consulting fees, speaking fees, and/or honoraria from Bristol‐Myers Squibb, Celgene, Galapagos, Genentech, Sun, and UCB (less than $10,000 each) and from AbbVie, Amgen, Janssen, Eli Lilly, Novartis, and Pfizer (more than $10,000 each). Dr. Gladman has received consulting fees from AbbVie, Amgen, Bristol‐Myers Squibb, Celgene, Eli Lilly, Gilead, Janssen, Novartis, Pfizer, and UCB (less than $10,000 each) and research support from those companies. Drs. Collier, Liu, Kricorian, and Chung own stock or stock options in Amgen Inc. Dr. Ritchlin has received consulting fees from Amgen, UCB, and Eli Lilly (less than $10,000 each) and from AbbVie, Novartis, Janssen, and Pfizer (more than $10,000 each). Dr. Helliwell has received consulting fees and honoraria from AbbVie, Amgen, Pfizer, UCB, Celgene, and Galapagos (less than $10,000 each) and research support from Novartis, AbbVie, and Janssen.
There is a plan to share data. This may include deidentified individual patient data for variables necessary to address the specific research question in an approved data sharing request, and also may include related data dictionaries, study protocol, statistical analysis plan, informed consent form, and/or clinical study report. Data sharing requests relating to data from this report will be considered after the publication date. There is no end date for eligibility to submit a data sharing request for these data. Qualified researchers may submit a request containing the research objectives, the Amgen products and Amgen study/studies in scope, end points/outcomes of interest, statistical analysis plan, data requirements, publication plan, and qualifications of the researchers. In general, Amgen does not grant external requests for individual patient data for the purpose of reevaluating safety and efficacy issues already addressed in the product labeling. A committee of internal advisors will review requests. If not approved, requests may be further arbitrated by a Data Sharing Independent Review Panel. Requests that pose a potential conflict of interest or an actual or potential competitive risk may be declined at Amgen's sole discretion and without further arbitration. Upon approval, information necessary to address the research question will be provided under the terms of a data sharing agreement. This may include anonymized individual patient data and/or available supporting documents, containing fragments of analysis code where provided in analysis specifications. Further details are available at https://wwwext.amgen.com/science/clinical-trials/clinical-data-transparency-practices/.
References
- 1. Ritchlin CT, Colbert RA, Gladman DD. Psoriatic arthritis. N Engl J Med 2017;376:957–70. [DOI] [PubMed] [Google Scholar]
- 2. Ogdie A, Weiss P. The epidemiology of psoriatic arthritis. Rheum Dis Clin North Am 2015;41:545–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Eder L, Haddad A, Rosen CF, Lee KA, Chandran V, Cook R, et al. The incidence and risk factors for psoriatic arthritis in patients with psoriasis: a prospective cohort study. Arthritis Rheumatol 2016;68:915–23. [DOI] [PubMed] [Google Scholar]
- 4. Koo J. Population‐based epidemiologic study of psoriasis with emphasis on quality of life assessment. Dermatol Clin 1996;14:485–96. [DOI] [PubMed] [Google Scholar]
- 5. Biondi Oriente C, Scarpa R, Pucino A, Oriente P. Psoriasis and psoriatic arthritis: dermatological and rheumatological co‐operative clinical report. Acta Derm Venereol Suppl (Stockh) 1989;146:69–71. [PubMed] [Google Scholar]
- 6. Gladman DD, Stafford‐Brady F, Chang CH, Lewandowski K, Russell ML. Longitudinal study of clinical and radiological progression in psoriatic arthritis. J Rheumatol 1990;17:809–12. [PubMed] [Google Scholar]
- 7. McHugh NJ, Balachrishnan C, Jones SM. Progression of peripheral joint disease in psoriatic arthritis: a 5‐yr prospective study. Rheumatology (Oxford) 2003;42:778–83. [DOI] [PubMed] [Google Scholar]
- 8. Michelsen B, Fiane R, Diamantopoulos AP, Soldal DM, Hansen IJ, Sokka T, et al. A comparison of disease burden in rheumatoid arthritis, psoriatic arthritis and axial spondyloarthritis. PLoS One 2015;10:e0123582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Coates LC, Kavanaugh A, Mease PJ, Soriano ER, Acosta‐Felquer ML, Armstrong AW, et al. Group for Research and Assessment of Psoriasis and Psoriatic Arthritis 2015 treatment recommendations for psoriatic arthritis. Arthritis Rheumatol 2016;68:1060–71. [DOI] [PubMed] [Google Scholar]
- 10. Gossec L, Smolen JS, Ramiro S, de Wit M, Cutolo M, Dougados M, et al. European League Against Rheumatism (EULAR) recommendations for the management of psoriatic arthritis with pharmacological therapies: 2015 update. Ann Rheum Dis 2016;75:499–510. [DOI] [PubMed] [Google Scholar]
- 11. Taltz (ixekizumab) prescribing information. Indianapolis (IN): Eli Lilly and Company; 2018. [Google Scholar]
- 12. Stelara (ustekinumab) prescribing information. Horsham (PA): Janssen Biotech; 2017. [Google Scholar]
- 13. Cosentyx (secukinumab) prescribing information. East Hanover (NJ): Novartis Pharmaceuticals Corporation; 2018. [Google Scholar]
- 14. Xeljanz (tofacitinib) prescribing information. New York (NY): Pfizer; 2018. [Google Scholar]
- 15. Otezla (apremilast) prescribing information. Summit (NJ): Celegene Corporation; 2017. [Google Scholar]
- 16. Ianculescu I, Weisman MH. The role of methotrexate in psoriatic arthritis: what is the evidence? Clin Exp Rheumatol 2015;33:S94–7. [PubMed] [Google Scholar]
- 17. Ogdie A, Schwartzman S, Husni ME. Recognizing and managing comorbidities in psoriatic arthritis. Curr Opin Rheumatol 2015;27:118–26. [DOI] [PubMed] [Google Scholar]
- 18. Tilling L, Townsend S, David J. Methotrexate and hepatic toxicity in rheumatoid arthritis and psoriatic arthritis. Clin Drug Investig 2006;26:55–62. [DOI] [PubMed] [Google Scholar]
- 19. Kavanaugh A, Husni ME, Harrison DD, Kim L, Lo KH, Leu JH, et al. Safety and efficacy of intravenous golimumab in patients with active psoriatic arthritis: results through week twenty‐four of the GO‐VIBRANTstudy. Arthritis Rheumatol 2017;69:2151–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Baranauskaite A, Raffayova H, Kungurov NV, Kubanova A, Venalis A, Helmle L, et al. Infliximab plus methotrexate is superior to methotrexate alone in the treatment of psoriatic arthritis in methotrexate‐naive patients: the RESPOND study. Ann Rheum Dis 2012;71:541–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Kingsley GH, Kowalczyk A, Taylor H, Ibrahim F, Packham JC, McHugh NJ, et al. A randomized placebo‐controlled trial of methotrexate in psoriatic arthritis. Rheumatology (Oxford) 2012;51:1368–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Mease PJ, Goffe BS, Metz J, VanderStoep A, Finck B, Burge DJ. Etanercept in the treatment of psoriatic arthritis and psoriasis: a randomised trial. Lancet 2000;356:385–90. [DOI] [PubMed] [Google Scholar]
- 23. Mease PJ, Kivitz AJ, Burch FX, Siegel EL, Cohen SB, Ory P, et al. Etanercept treatment of psoriatic arthritis: safety, efficacy, and effect on disease progression. Arthritis Rheum 2004;50:2264–72. [DOI] [PubMed] [Google Scholar]
- 24. Antoni C, Krueger GG, de Vlam K, Birbara C, Beutler A, Guzzo C, et al. Infliximab improves signs and symptoms of psoriatic arthritis: results of the IMPACT 2 trial. Ann Rheum Dis 2005;64:1150–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Kavanaugh A, McInnes I, Mease P, Krueger GG, Gladman D, Gomez‐Reino J, et al. Golimumab, a new human tumor necrosis factor α antibody, administered every four weeks as a subcutaneous injection in psoriatic arthritis: twenty‐four–week efficacy and safety results of a randomized, placebo‐controlled study. Arthritis Rheum 2009;60:976–86. [DOI] [PubMed] [Google Scholar]
- 26. Mease PJ, Fleischmann R, Deodhar AA, Wollenhaupt J, Khraishi M, Kielar D, et al. Effect of certolizumab pegol on signs and symptoms in patients with psoriatic arthritis: 24‐week results of a phase 3 double‐blind randomised placebo‐controlled study (RAPID‐PsA). Ann Rheum Dis 2014;73:48–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Mease PJ, Gladman DD, Ritchlin CT, Ruderman EM, Steinfeld SD, Choy EH, et al. Adalimumab for the treatment of patients with moderately to severely active psoriatic arthritis: results of a double‐blind, randomized, placebo‐controlled trial. Arthritis Rheum 2005;52:3279–89. [DOI] [PubMed] [Google Scholar]
- 28. Klareskog L, van der Heijde D, de Jager JP, Gough A, Kalden J, Malaise M, et al. Therapeutic effect of the combination of etanercept and methotrexate compared with each treatment alone in patients with rheumatoid arthritis: double‐blind randomised controlled trial. Lancet 2004;363:675–81. [DOI] [PubMed] [Google Scholar]
- 29. Fagerli KM, Lie E, van der Heijde D, Heiberg MS, Lexberg AS, Rødevand E, et al. The role of methotrexate co‐medication in TNF‐inhibitor treatment in patients with psoriatic arthritis: results from 440 patients included in the NOR‐DMARD study. Ann Rheum Dis 2014;73:132–7. [DOI] [PubMed] [Google Scholar]
- 30. Mease PJ, Collier DH, Saunders KC, Li G, Kremer JM, Greenberg JD. Comparative effectiveness of biologic monotherapy versus combination therapy for patients with psoriatic arthritis: results from the Corrona registry. RMD Open 2015;1:e000181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Mease PJ, Gladman DD, Samad AS, Coates LC, Liu LX, Aras GA, et al. Design and rationale of the Study of Etanercept and Methotrexate in Combination or as Monotherapy in Subjects with Psoriatic Arthritis (SEAM‐PsA). RMD Open 2018;4:e000606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Taylor W, Gladman D, Helliwell P, Marchesoni A, Mease P, Mielants H, et al. Classification criteria for psoriatic arthritis: development of new criteria from a large international study. Arthritis Rheum 2006;54:2665–73. [DOI] [PubMed] [Google Scholar]
- 33. Enbrel (etanercept) prescribing information. Thousand Oaks (CA): Immunex Corporation; 2017. [Google Scholar]
- 34. Felson DT, Anderson JJ, Boers M, Bombardier C, Furst D, Goldsmith C, et al. American College of Rheumatology preliminary definition of improvement in rheumatoid arthritis. Arthritis Rheum 1995;38:727–35. [DOI] [PubMed] [Google Scholar]
- 35. Coates LC, Fransen J, Helliwell PS. Defining minimal disease activity in psoriatic arthritis: a proposed objective target for treatment. Ann Rheum Dis 2010;69:48–53. [DOI] [PubMed] [Google Scholar]
- 36. Helliwell PS, FitzGerald O, Fransen J, Gladman DD, Kreuger GG, Callis‐Duffin K, et al. The development of candidate composite disease activity and responder indices for psoriatic arthritis (GRACE project). Ann Rheum Dis 2013;72:986–91. [DOI] [PubMed] [Google Scholar]
- 37. Schoels M, Aletaha D, Funovits J, Kavanaugh A, Baker D, Smolen JS. Application of the DAREA/DAPSA score for assessment of disease activity in psoriatic arthritis. Ann Rheum Dis 2010;69:1441–7. [DOI] [PubMed] [Google Scholar]
- 38. Helliwell PS, Firth J, Ibrahim GH, Melsom RD, Shah I, Turner DE. Development of an assessment tool for dactylitis in patients with psoriatic arthritis. J Rheumatol 2005;32:1745–50. [PubMed] [Google Scholar]
- 39. Maksymowych WP, Mallon C, Morrow S, Shojania K, Olszynski WP, Wong RL, et al. Development and validation of the Spondyloarthritis Research Consortium of Canada (SPARCC) Enthesitis Index. Ann Rheum Dis 2009;68:948–53. [DOI] [PubMed] [Google Scholar]
- 40. Ashcroft DM, Wan Po AL, Williams HC, Griffiths CE. Clinical measures of disease severity and outcome in psoriasis: a critical appraisal of their quality. Br J Dermatol 1999;141:185–91. [DOI] [PubMed] [Google Scholar]
- 41. Langley RG, Ellis CN. Evaluating psoriasis with Psoriasis Area and Severity Index, Psoriasis Global Assessment, and Lattice System Physician's Global Assessment. J Am Acad Dermatol 2004;51:563–9. [DOI] [PubMed] [Google Scholar]
- 42. Cassell SE, Bieber JD, Rich P, Tutuncu ZN, Lee SJ, Kalunian KC, et al. The modified Nail Psoriasis Severity Index: validation of an instrument to assess psoriatic nail involvement in patients with psoriatic arthritis. J Rheumatol 2007;34:123–9. [PubMed] [Google Scholar]
- 43. Fries JF, Spitz P, Kraines RG, Holman HR. Measurement of patient outcome in arthritis. Arthritis Rheum 1980;23:137–45. [DOI] [PubMed] [Google Scholar]
- 44. Ware JE Jr, Snow KK, Kosinski M, Gandek B. SF‐36 health survey: manual and interpretation guide. Boston: The Health Institute, New England Medical Center; 1993. [Google Scholar]
- 45. Van der Heijde D, Sharp J, Wassenberg S, Gladman DD. Psoriatic arthritis imaging: a review of scoring methods. Ann Rheum Dis 2005;64 Suppl 2:ii61–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Kavanaugh A, McInnes IB, Mease PJ, Krueger GG, Gladman D, Xu S, et al. Impact of persistent minimal disease activity on long‐term outcomes in psoriatic arthritis: results from 5 years of the long term extension of a randomized, placebo‐controlled, study. Ann Rheum Dis 2014;73:92. [Google Scholar]
- 47. Burman CF, Sonesson C, Guilbaud O. A recycling framework for the construction of Bonferroni‐based multiple tests. Stat Med 2009;28:739–61. [DOI] [PubMed] [Google Scholar]
- 48. Coates LC, Helliwell PS. Methotrexate efficacy in the Tight Control in Psoriatic Arthritis study. J Rheumatol 2016;43:356–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Mease PJ, Kivitz AJ, Burch FX, Siegel EL, Cohen SB, Ory P, et al. Continued inhibition of radiographic progression in patients with psoriatic arthritis following 2 years of treatment with etanercept. J Rheumatol 2006;33:712–21. [PubMed] [Google Scholar]
- 50. Antoni CE, Kavanaugh A, Kirkham B, Tutuncu Z, Burmester GR, Schneider U, et al. Sustained benefits of infliximab therapy for dermatologic and articular manifestations of psoriatic arthritis: results from the Infliximab Multinational Psoriatic Arthritis Controlled Trial (IMPACT). Arthritis Rheum 2005;52:1227–36. [DOI] [PubMed] [Google Scholar]
- 51. Coates LC, Helliwell PS. Validation of minimal disease activity criteria for psoriatic arthritis using interventional trial data. Arthritis Care Res (Hoboken) 2010;62:965–9. [DOI] [PubMed] [Google Scholar]
- 52. Tugwell P, Boers M, Brooks P, Simon L, Strand V, Idzerda L. OMERACT: an international initiative to improve outcome measurement in rheumatology. Trials 2007;8:38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Gottlieb AB, Langley RG, Strober BE, Papp KA, Klekotka P, Creamer K, et al. A randomized, double‐blind, placebo‐controlled study to evaluate the addition of methotrexate to etanercept in patients with moderate to severe plaque psoriasis. Br J Dermatol 2012;167:649–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Coates LC, FitzGerald O, Helliwell PS, Paul C. Psoriasis, psoriatic arthritis, and rheumatoid arthritis: is all inflammation the same? Semin Arthritis Rheum 2016;46:291–304. [DOI] [PubMed] [Google Scholar]
- 55. Fagerli KM, Lie E, van der Heijde D, Heiberg MS, Kalstad S, Rodevand E, et al. Switching between TNF inhibitors in psoriatic arthritis: data from the NOR‐DMARD study. Ann Rheum Dis 2013;72:1840–4. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials


