Abstract
Objective
We investigated whether cognitive reserve modifies the risk of dementia attributable to apolipoprotein ε4 (APOE‐ε4), a well‐known genetic risk factor for dementia.
Methods
We followed 2,556 cognitively intact participants aged ≥60 years from the ongoing prospective community‐based Swedish National Study on Aging and Care in Kungsholmen (SNAC‐K). Dementia was ascertained through clinical and neuropsychological assessments and diagnosed according to Diagnostic and Statistical Manual of Mental Disorders, 4th edition criteria. Structural equation modeling was used to generate a cognitive reserve indicator from 4 previously validated contributors: early life education, midlife substantive work complexity, late life leisure activities, and late life social networks. Cox proportional hazard models estimated dementia risk in relation to cognitive reserve indicator. The interaction between the cognitive reserve indicator and APOE‐ε4 was assessed on multiplicative and additive scales.
Results
After an average of 6.3 years (range = 2.1–10.7) of follow‐up, 232 dementia cases were ascertained. Relative to individuals in the lowest tertile of cognitive reserve indicator, those with moderate and high reserve were at a reduced risk of dementia. There was no multiplicative interaction between APOE‐ε4 status and cognitive reserve indicator (p = 0.113). Additive interaction was statistically significant. Relative to APOE‐ε4 carriers with low cognitive reserve, ε4 carriers with high reserve had a reduced risk of dementia (hazard ratio [HR] = 0.28, 95% confidence interval [CI] = 0.13–0.59). The magnitude of risk reduction was similar in ε4 noncarriers with a high cognitive reserve indicator (HR = 0.24, 95% CI = 0.15–0.40).
Interpretation
Lifelong engagement in reserve‐enhancing activities attenuates the risk of dementia attributable to APOE‐ε4. Promoting cognitive reserve might be especially effective in subpopulations with high genetic risk of dementia. ANN NEUROL 2019
Cognitive reserve has been proposed to account for the mismatch between the extent of brain damage and its clinical manifestation in the form of dementia diagnosis.1 People with more reserve might withstand dementia pathology as a result of active compensatory mechanisms that enhance brain network efficiency and flexibility.2 Education, but also stimulating occupational and social environments have been suggested as factors contributing to reserve.3, 4
Measuring cognitive reserve is often a challenging task.5 For instance, it is becoming increasingly clear that cognitive reserve develops from continued engagement in multiple activities throughout the life course.6 As a result, focusing on contributors occurring at a single period makes it difficult to capture either the full effect of prolonged exposure, or the important modifying effects of several reserve‐enhancing factors.7, 8 In a previous study, we attempted to uncover the relative importance of life stages (early, mid‐, and late life) at which exposure to reserve‐enhancing factors was most associated with the risk of dementia.9 Due to the strong correlation between the early and the midlife reserve indicators, we were unable to assess the relative importance of each life period. However, we found that having a high reserve score in all 3 life stages was associated with the largest reduction in dementia risk.9 This suggested that continued engagement in activities with reserve‐enhancing properties might be especially important.
The interaction between lifelong cognitive reserve and genetic predisposition to dementia remains poorly understood. Some have suggested that carriers of the apolipoprotein ε4 (APOE‐ε4) allele might be more susceptible to the beneficial effects of physical activities or healthy lifestyles,10 although this conclusion has not been universally supported.11 Others, focusing on single contributors to reserve, such as education, have reported both increased protection in those with genetic risk12 and equivalent levels of dementia risk reduction in both ε4 carriers and noncarriers with high education.13 A comprehensive examination of cognitive reserve's ability to mitigate ε4‐related risk in a large population‐based cohort comprising reserve contributors from several stages over the life course, as well as a clinically ascertained diagnosis of dementia, is yet to be attempted.
Building on our previous work, the aim of this study is to generate a lifelong cognitive reserve indicator that is not specific to life stage, but that emphasizes the accumulation of mental and social inputs across the entire life course. Importantly, and in contrast with our previous study, in deriving this measure we also extract information on late life social network, which has been repeatedly suggested as a viable input into cognitive reserve.14, 15 Our reserve indicator incorporates education (early adulthood), substantive work complexity (accumulated across 5 occupations in midlife), social network (late life), and leisure activities (late life). Using a different study population that is not just larger, but also much more diverse with respect to gender and age/birth cohort mix, we examine the association between the cumulative measure of cognitive reserve and dementia risk over an average of 6 years of follow‐up. Finally, as an expansion of our previous work, we explore in much greater detail the relationship between cumulative cognitive reserve and genetic predisposition to dementia (APOE‐ε4 status).
Subjects and Methods
Study Population
The study population was from the Swedish National Study on Aging and Care in Kungsholmen (SNAC‐K), an ongoing community‐based longitudinal study of adults aged ≥60 years, living at home or in an institution in a district of Stockholm between 2001 and 2004.16 This study population is different from the Kungsholmen Project17 data used in our previous study, which was both smaller and less heterogeneous with respect to age/gender mix. SNAC‐K participants were randomly drawn from 11 age cohorts. At baseline, 3,363 individuals (participation rate = 73.3%) underwent nurse interviews, clinical examinations, cognitive assessments, and laboratory testing. Participants aged 60 to 72 years were re‐examined every 6 years, whereas those aged ≥78 years were re‐examined every 3 years. For cognitively impaired subjects, a proxy or a caregiver was interviewed. SNAC‐K was approved by the Regional Ethical Review Board in Stockholm, and written informed consent was obtained from participants or their next of kin.
Of the 3,363 participants, 322 were diagnosed with dementia at baseline and were excluded from the study. Individuals who did not attend any of the follow‐up examinations (n = 272) and those with schizophrenia or developmental disorders (n = 16) were excluded, resulting in 2,753 subjects eligible for inclusion (see Fig 1 for flowchart). After removing 197 participants with missing data on covariates, the study population amounted to 2,556 individuals. The average follow‐up time was 6.3 years (range = 2.1–10.7).
Figure 1.
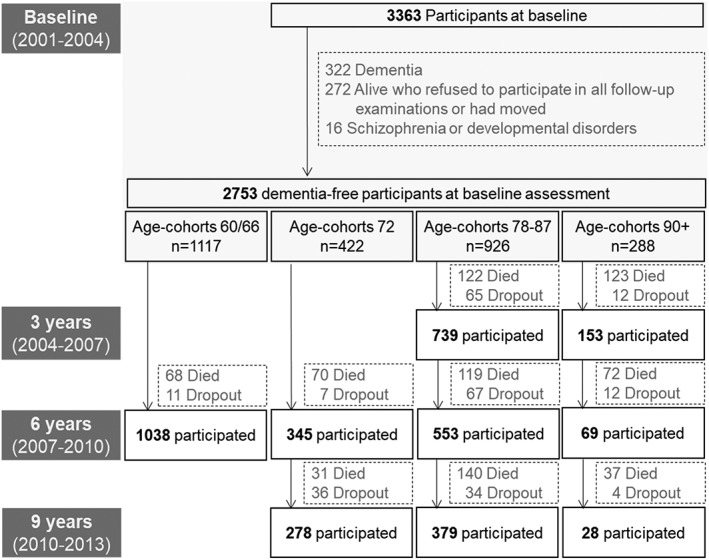
Flowchart of study population in SNAC‐K (Swedish National Study on Aging and Care in Kungsholmen). Baseline dementia cases (n = 322) include both definite (n = 252) and questionable cases (n = 70). Dementia status was available for deceased study participants as a result of comprehensive assessment of inpatient and cause of death records. Age‐cohort 60/66 is composed of individuals aged 60 and 66 years at baseline who were followed up after six years. Age‐cohort 72 is made up of individuals aged 72 years at baseline who were followed up for the first time after six years and then three years after that. Age‐cohort 78‐87 includes individuals aged 78, 81, 84, 87 years who were followed‐up every three years since baseline. Age cohort 90+ is made up of individuals aged 90 years or older at baseline who were followed up every three years.
Dementia Diagnosis
Dementia was ascertained through clinical and cognitive examinations administered according to Diagnostic and Statistical Manual of Mental Disorders, 4th edition criteria. A 3‐step procedure was employed,18 where 2 physicians working independently made a preliminary diagnosis and a third opinion was sought from the senior neurologist in the event of discordant assessments. For deceased participants, dementia diagnosis was based on death certificates, hospital discharge registers, and hospital records.
Assessment of Cognitive Reserve Indicator over the Life Course
An indicator of cognitive reserve combined 4 measures of stimulating mental, social, and physical environments over the life course: early life education, midlife substantive work complexity, and indices of late life social network and leisure activities.
Years of Education (Early Life)
Years of education were assessed during the nurse interview at baseline. The levels of education and their corresponding mean durations were unfinished primary (7 years), elementary (9.5 years), vocational training (11 years), high school (11.6 years), unfinished higher education (13.8 years), completed university (16.1 years), and doctoral studies (20.6 years).
Substantive Work Complexity (Midlife)
During the baseline interview, participants were inquired about their 5 longest‐held occupations, which we ranked according to substantive complexity. Substantively complex occupations require thought and independent judgment, especially when making decisions involving poorly defined outcomes.19 Substantive complexity ratings were originally derived from the US Dictionary of Occupational Titles and were matched with analogous Swedish occupations by 2 independent raters, one based in the United States and the other in Sweden.20 Mean complexity score across all reported occupations throughout life indicated accumulated complexity. The resulting variable was a continuous score ranging from 0.7 to 10 (higher values indicated greater substantive complexity).
Social Network Index (Late Life)
Aspects of social network were considered along the dimensions of size and support in accordance with a previously validated procedure.21 To capture network size, we used information on marital status, living arrangement, number of children, frequency of contact with relatives or friends, and the number of contacts available to turn to. Network support was assessed by inquiring about satisfaction with social connections, the extent of perceived material and psychosocial support received, and the sense of affinity and belonging to the various groups. All indicators were converted into z scores and averaged separately across the components of network size and support. Because the correlation between the two measures was considerable (0.76), we computed the social network index by averaging the 2 subindices. The resulting variable was a continuous score ranging from −2 to 1.22 (high scores indicate richer social network).
Leisure Activities Index (Late Life)
During the baseline interview, participants were read a list of 26 activities and were instructed to indicate which ones they practiced and specify the frequency of engagement over the preceding year. Leisure activities were categorized as mental, social, or physical. For the mental and social components, the richness of engagement was categorized into 3 levels (low, moderate, high) according to the number of activities performed (mental component: low engagement [≤1 activity], moderate engagement [2–3 activities], and high [≥4 activities]; social component: low engagement [no activities], moderate engagement [1 activity], high engagement [≥2 activities]). For the physical component, richness was defined based on the frequency of engagement and categorized as: low (less than once per week), moderate (at least once per week), and high (more than once per week). As a previous study has reported similar dementia risk reduction across all 3 leisure components, with the largest protection being associated with rich engagement in all domains,15 we generated a global measure of leisure richness by summing the mental, social, and physical scores, resulting in a continuous variable (range = 0–6; higher scores denote greater leisure engagement).
Genetic Predisposition
Genomic DNA was extracted from peripheral blood samples collected at baseline. APOE allelic status was determined using a microsequencing method (AffiGen APOE; Sangtec Medical, Stockholm, Sweden) based on a polymerase chain reaction with biotinylated primers.22 APOE‐ε4 status was dichotomized (any ε4 carriers vs noncarriers) in the analysis.
Covariates
In addition to age and sex, we considered smoking, body mass index (BMI), hypertension, cerebro‐ and cardiovascular diseases, and diabetes. Disease status came from physician examinations, medication use, and the Swedish National Patient Register. Vital status came from the Swedish Cause of Death Register and hospital discharge records.
Statistical Analysis
Structural equation modeling (SEM) was performed to derive a best‐fitting measurement model incorporating the 4 observed factors occurring over the life course: education, substantive complexity, social network, and leisure activities. Model fit was assessed using χ2 goodness of fit, the comparative fit index, and the root mean square error of approximation. Omitted paths were explored using modification indices. Predicted values of the latent variable, called the reserve indicator, were obtained by summing the products of standardized indicators and the corresponding SEM‐factor score weights.
Minimally (age, sex) and fully adjusted (smoking, BMI, heart disease, hypertension, cerebrovascular diseases, diabetes, and APOE‐ε4 status) Cox proportional hazard models estimated the risk of dementia conditional on the cognitive reserve indicator predicted from the SEM. The reserve indicator was operationalized continuously and categorically (tertile distribution; reference: bottom tertile). Follow‐up time was computed as time since entry until dementia diagnosis, death, or the last examination.
Both multiplicative and additive interactions between reserve indicator and APOE‐ε4 status were examined. Because estimation of the additive interaction may be biased when preventive factors are considered,23 the reserve indicator was recoded as a binary risk factor (0: middle or top tertile; 1: bottom tertile) and excess risk of dementia in various strata of reserve and ε4‐status was estimated relative to the most optimal scenario: “high reserve and APOE‐non‐ε4.” For the additive interaction, relative excess risk due to interaction, the attributable proportion, and the synergy index were computed. If statistically significant, these measures would suggest that the relative risk of dementia when the 2 factors were present was significantly greater than the sum of the relative risks associated with the presence of just the ε4 allele or just the low score on the reserve indicator. This is in contrast with the multiplicative interaction, which would quantify the extent to which the effect of both exposures together exceeds the product of the effects of the 2 exposures considered separately. To further explore the interrelationship between reserve indicator and genetic predisposition, we generated a categorical variable designating 6 combinations of the life course reserve indicator (in tertiles: bottom, middle, top) and APOE‐ε4 status (no vs yes). We examined the effect of each level of this variable on dementia, relative to the group: “low reserve and APOE‐ε4”.
In sensitivity analyses, multiple imputation by chained equation was used to impute data for 197 participants with missing data on covariates. Cox models were re‐estimated without 86 incident dementia cases from the first follow‐up to reduce potential bias of preclinical dementia at baseline. We also excluded 308 individuals with possible cognitive impairment (Mini‐Mental State Examination [MMSE] ≤ 27).
Results
After an average of 6.3 years (range = 2.1–10.7) of follow‐up of 2,556 participants (15,606 person‐years), 232 dementia cases were ascertained (incidence rate = 14.9 cases per 1,000 person‐years; 95% confidence interval [CI] = 13.1–16.9). Baseline characteristics of the study population according to incident dementia status are in Table 1. Additionally, tabulating baseline characteristics according to APOE‐ε4 status (not presented) revealed that ε4 carriers were slightly younger at baseline (72.1 vs. 73.8 years) and more likely to smoke (proportion current/ever smoking: 56.9% [APOE‐ε4 carrier] vs 52.1% [noncarrier]). Baseline MMSE levels did not differ across APOE‐ε4 subsamples, suggesting limited selection of cognitively impaired participants with ε4 allele into the study.
Table 1.
Baseline Characteristics of Study Population by Incident Dementia during Follow‐up
| Characteristic | Dementia‐Free, n = 2324 | Incident Dementia, n = 232 | p |
|---|---|---|---|
| Demographics | |||
| Age, yr | 72.3 ± 10.1 | 82.7 ± 6.8 | <0.001 |
| 60–66 | 1,066 (45.9) | 3 (1.3) | |
| 72–78 | 704 (30.3) | 85 (36.7) | |
| 81–87 | 378 (16.2) | 87 (37.5) | |
| 90+ | 176 (7.6) | 57 (24.5) | |
| Female | 1,435 (61.8) | 162 (69.8) | 0.015 |
| Education | <0.001 | ||
| Elementary | 328 (14.1) | 46 (19.8) | |
| Professional schools | 968 (41.7) | 126 (54.3) | |
| High school | 233 (10.0) | 19 (8.2) | |
| University | 794 (34.2) | 41 (17.7) | |
| VRFs and medical conditions | |||
| Current/ever smoking | 1,255 (54.0) | 111 (47.8) | 0.073 |
| Alcohol consumption | 1,633 (70.5) | 109 (47.2) | <0.001 |
| BMI, kg/m2 | <0.001 | ||
| Underweight, <20 | 112 (4.8) | 28 (12.1) | |
| Normal, 20–24.9 | 917 (41.8) | 115 (49.6) | |
| Overweight, 25–29.9 | 941 (40.4) | 72 (31.0) | |
| Obese, ≥30 | 300 (12.9) | 17 (7.3) | |
| Hypertension | 1,627 (70.0) | 166 (71.5) | 0.624 |
| Heart diseases | 516 (22.2) | 103 (37.1) | 0.001 |
| Cerebrovascular diseases | 127 (5.5) | 38 (13.8) | 0.001 |
| Type 2 diabetes | 190 (8.2) | 37 (14.7) | 0.007 |
| Any APOE ε4 | 640 (27.5) | 89 (37.9) | 0.001 |
| MMSE | 28.9 ± 1.34 | 27.4 ± 2.01 | <0.001 |
| Cognitive reserve–enhancing factors | |||
| Years of education | 12.3 ± 4.0 | 10.7 ± 3.6 | <0.001 |
| Work complexity score | 5.0 ± 1.8 | 4.4 ± 1.8 | <0.001 |
| Leisure activities score | 2.51 ± 1.47 | 1.69 ± 1.34 | <0.001 |
| Social network score | 0.09 ± 0.51 | −0.19 ± 0.6 | <0.001 |
Data are presented as mean ± standard deviation or number (%).
APOE ε4 = apolipoprotein ε4 allele; BMI = body mass index; MMSE = Mini‐Mental State Examination; VRF = vascular risk factor.
The 197 individuals who were excluded due to missing data on covariates did not differ from the analyzed population with respect to sex (odds ratio [OR] = 1.00, 95% CI = 0.72–1.39) or the probability of university education (OR = 0.64, 95% CI = 0.40–1.01). They were more likely to be older (OR = 1.05, 95% CI = 1.03–1.06) and had more cerebrovascular disorders (OR = 1.81, 95% CI = 1.16–2.84).
The best‐fitting SEM with 4 cognitive reserve–enhancing factors is presented in Figure 2. A unique value of the latent variable was predicted for each individual using the SEM factor score weights, and the resulting continuous variable, the reserve indicator, was normally distributed with a mean of zero (range = −4.28 to 3.62).
Figure 2.
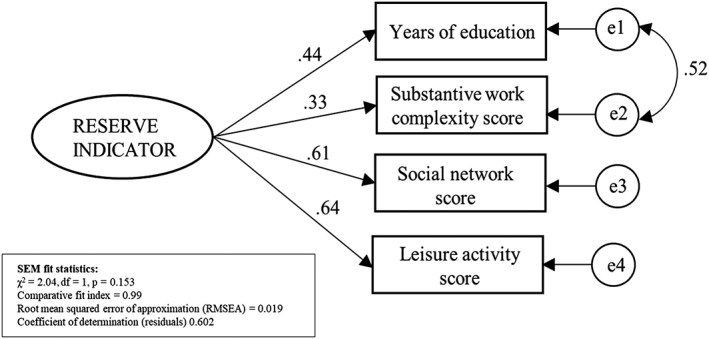
Standardized estimates from the structural equation model (SEM) with 4 observed cognitive reserve–enhancing factors. For years of education, range = 3 to 23 years. Substantive work complexity score is the average complexity score for all reported occupations during professional life (range = 0.7–10). Social network score was obtained by averaging z‐standardized components of network size and network satisfaction. Leisure activity score was obtained by examining 26 leisure activities across mental/physical/social domains of leisure. For details about the operationalization of the cognitive reserve–enhancing factors, see the Subjects and Methods section.
In the basic adjusted model (age cohort and sex), the continuous operationalization of the cognitive reserve indicator, predicted from SEM, was associated with a reduced risk of dementia over the follow‐up (Table 2, first row). This association was only marginally attenuated in the multiadjusted model.
Table 2.
The Association between the Cognitive Reserve Indicator and Dementia
| Reserve Indicator | Subjects, n | Cases, n | Basic Adjusted, HR (95% CI)a | p | Multiadjusted, HR (95% CI)b | p |
|---|---|---|---|---|---|---|
| Continuous | 2,556 | 232 | 0.76 (0.68–0.85) | <0.001 | 0.77 (0.69–0.86) | <0.001 |
| By tertile | ||||||
| Low | 816 | 133 | Reference | Reference | ||
| Moderate | 849 | 68 | 0.69 (0.51–0.93) | <0.001 | 0.68 (0.50–0.92) | 0.011 |
| High | 891 | 41 | 0.40 (0.27–0.60) | <0.001 | 0.42 (0.28–0.63) | <0.001 |
| p for trend | 0.65 (0.54–0.78) | <0.001 | 0.65 (0.54–0.79) | <0.001 | ||
Cox proportional hazard models. Separate Cox models estimated for continuous and categorical versions of reserve indicator.
Adjusted for age cohorts and sex.
Adjusted for age cohorts, sex, smoking, body mass index, heart disease, hypertension, cerebrovascular diseases, diabetes, and APOE‐ε4.
CI = confidence interval; HR = hazard ratio.
In a categorical specification, relative to those in the bottom tertile of the reserve indicator, the middle and the top tertiles were at a reduced risk of dementia (see Table 2, rows 2–4). The dose‐response trend was statistically significant (p < 0.01). Adjustment for health behaviors, comorbidities, and genetic predisposition did not alter the results.
The multiplicative interaction between the continuous reserve indicator and APOE‐ε4 did not reach statistical significance (p = 0.113). For the additive interaction, we recoded cognitive reserve indicator as a binary risk factor “low reserve” (0: middle or top tertile vs 1: bottom tertile of reserve) to avoid biased computation in the presence of preventive factors with relative risks below 1. The relative excess risk due to interaction (1.28, 95% CI = 0.19–2.36), the attributable proportion due to interaction (0.38, 95% CI = 0.12–0.64), and the synergy index (2.18, 95% CI = 1.01–4.72) all indicated the presence of an additive interaction (Table 3). This suggests that the relative risk of dementia when both low reserve and APOE‐ε4 were present was significantly greater than the sum of the relative risks associated with the presence of just the ε4 allele or just the low score on the reserve indicator.
Table 3.
Combined Effect of Dichotomized Reserve Indicator (High vs Low) and Genetic Risk of Dementia (Any APOE‐ε4 Carrier vs Noncarrier) Used for the Calculation of Additive Interaction
| Joint Effect | Basic Adjusteda | Multiadjustedb | ||||
|---|---|---|---|---|---|---|
| Cognitive Reserve | APOE‐ε4 | Subjects (cases) | HR (95% CI) | p | HR (95% CI) | p |
| High | No | 1,236 (65) | Reference | Reference | ||
| Low | No | 592 (79) | 1.53 (1.09–2.14) | 0.014 | 1.56 (1.11–2.19) | 0.010 |
| High | Yes | 504 (34) | 1.56 (1.03–2.37) | 0.036 | 1.54 (1.01–2.34) | 0.043 |
| Low | Yes | 224 (54) | 3.30 (2.29–4.74) | <0.001 | 3.25 (2.25–4.69) | <0.001 |
Measures of additive interaction (from the model with multiple confounder adjustment): relative excess risk due to interaction, 1.276, 95% CI = 0.189–2.363; attributable proportion due to interaction, 0.380, 95% CI = 0.123–0.638; synergy index, 2.183, 95% CI = 1.010–4.720.
Adjusted for baseline age and sex.
Adjusted for baseline age cohorts, sex, smoking, body mass index, heart disease, hypertension, cerebrovascular diseases, and diabetes.
CI = confidence interval; HR = hazard ratio.
The joint effect of reserve indicator and APOE‐ε4 status was further examined using categorical indicators (Fig 3). Relative to ε4 carriers with a low score on the cognitive reserve indicator (bottom tertile), ε4 carriers with moderate reserve had a reduced risk of dementia (hazard ratio [HR] = 0.60, 95% CI = 0.37–0.96). APOE‐ε4 carriers with a high reserve indicator score were at a further reduced risk (HR = 0.28, 95% CI = 0.13‐0.59). APOE‐ε4 noncarriers had their dementia risk reduced proportionally to an increase in the cognitive reserve indicator, from low (HR = 0.48, 95% CI = 0.34–0.69), through moderate (HR = 0.35, 95% CI = 0.23–0.53), to high (HR = 0.24, 95% CI = 0.15–0.40).
Figure 3.
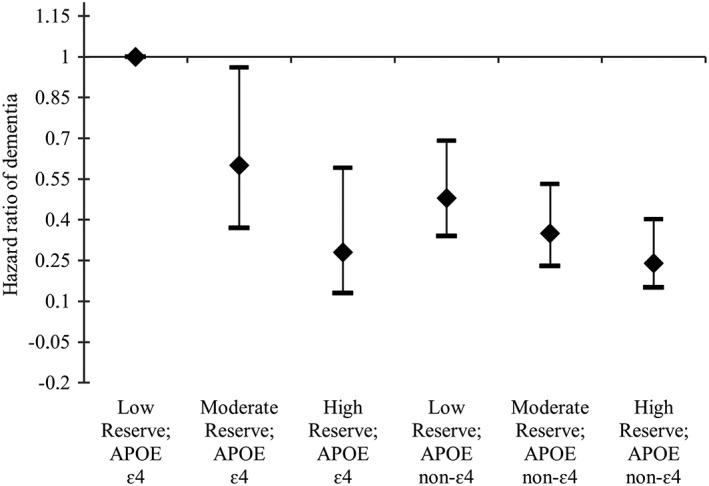
Combined effect of the cognitive reserve indicator and APOE‐ε4 status on the risk of dementia estimated from a fully adjusted Cox proportional hazard model. Hazard ratios and 95% confidence intervals are shown. Multiple confounder adjustment included age cohort, sex, smoking, body mass index, heart disease, hypertension, cerebrovascular diseases, and diabetes.
The substantive conclusions remained unchanged after excluding 86 individuals with incident dementia at the first follow‐up, or omitting 308 participants with baseline MMSE ≤ 27 (Tables 4 and 5).
Table 4.
Sensitivity Analyses
| Reserve Indicator | Subjects, na, b | Cases, na, b | Excluding Incident Dementia During First Follow‐up | Excluding MMSE ≤ 27 | ||
|---|---|---|---|---|---|---|
| HR (95% CI) | p | HR (95% CI) | p | |||
| Continuous | 2,470 (2,248) | 146 (143) | 0.75 (0.65–0.86) | <0.001 | 0.75 (0.65–0.86) | <0.001 |
| Low | 761 (635) | 78 (78) | Reference | Reference | ||
| Moderate | 824 (761) | 43 (42) | 0.69 (0.47–1.01) | 0.058 | 0.63 (0.43–0.93) | 0.021 |
| High | 885 (852) | 25 (23) | 0.45 (0.28–0.72) | 0.001 | 0.42 (0.26–0.68) | <0.001 |
| p for trend | 0.67 (0.54–0.84) | <0.001 | 0.64 (0.51–0.82) | <0.001 | ||
Cox regression HRs and 95% CIs for the association between levels of cognitive reserve and dementia, multiple adjustment.
After excluding n = 86 participants with dementia at first follow‐up.
After excluding n = 308 participants with MMSE ≤ 27.
Multiple confounder adjustment: age cohorts, sex, smoking, body mass index, heart disease, hypertension, cerebrovascular diseases, diabetes, and APOE‐ε4.
Table 5.
Sensitivity Analyses
| Joint Effect | Excluding Incident Dementia during First Follow‐up | Excluding MMSE ≤ 27 | |||||
|---|---|---|---|---|---|---|---|
| Reserve Indicator | APOE‐ε4 | Subjects (cases), na | Subjects (cases), nb | HR (95% CI)a | p | HR (95% CI)b | p |
| Low | Yes | 206 (36) | 174 (38) | Reference | Reference | ||
| Moderate | Yes | 247 (15) | 236 (18) | 0.49 (0.27–0.91) | 0.025 | 0.53 (0.30–0.94) | 0.031 |
| High | Yes | 245 (7) | 235 (6) | 0.29 (0.13–0.67) | 0.004 | 0.24 (0.10–0.57) | 0.001 |
| Low | No | 555 (42) | 461 (40) | 0.42 (0.27–0.66) | <0.001 | 0.36 (0.23–0.56) | <0.001 |
| Moderate | No | 577 (28) | 525 (24) | 0.36 (0.22–0.59) | <0.001 | 0.26 (0.15–0.44) | <0.001 |
| High | No | 640 (18) | 617 (17) | 0.24 (0.14–0.43) | <0.001 | 0.21 (0.11–0.37) | <0.001 |
HRs and 95% CIs of dementia for the combined effect of APOE‐ε4 in Cox models with different inclusion criteria. Multiple confounder adjustment: age cohorts, sex, smoking, body mass index, heart disease, hypertension, cerebrovascular diseases, and diabetes.
After excluding n = 86 participants with dementia at first follow‐up.
After excluding n = 308 participants with MMSE ≤ 27.
CI = confidence interval; HR = hazard ratio; MMSE = Mini‐Mental State Examination.
Discussion
In this population‐based prospective study, we found that a cognitive reserve indicator comprised of 4 observed factors over the life course—education, substantive work complexity, social network, and leisure activities—was associated with a reduced risk of dementia in a dose‐response manner. Additive interaction between the reserve indicator and APOE‐ε4 status was observed. When combinations of the reserve indicator and genetic risk were examined, cognitive reserve appeared to override the detrimental effect of genetic predisposition, as the risk of dementia among APOE‐ε4 carriers was similar to that of the noncarriers.
Protective effects of single‐factor measures of cognitive reserve on dementia, such as higher education or complex occupations, have been reported previously.3 Focusing on single reserve contributors makes it difficult to disentangle reserve pathways of efficiency or compensation from nonreserve mechanisms, for example those involving socioeconomic disadvantage. Instead, a shift toward more integrated measures, akin to a lifelong reserve indicator operationalized here using SEM, has been discussed in literature, although this approach is not without methodological and theoretical shortcomings.5 Cognitive reserve is modeled as a reflective indicator here, meaning that the measurement model treats observable factors whose shared covariation identifies the latent variable as downstream outcomes of the latent reserve construct. This causal ordering, however, is not entirely consistent with the theory of cognitive reserve according to which it is the unique contribution (and not the shared covariation) of lifelong inputs that forms the reserve. This suggests that cognitive reserve is a formative, rather than a reflective latent indicator.5 Respecifying reflective measurement models as formative is methodologically unfeasible, as the resulting specification becomes statistically indistinguishable from the multiple regression and does not allow for assessing the validity of the hypothesized latent variable. Even though several neuroimaging and clinicopathological studies have largely supported the presence of a neural basis for efficiency and compensation,24, 25 there is still much need for methodological development of the cognitive reserve concept.26
A life course perspective to cognitive reserve in dementia has been suggested previously,6 but only a handful of studies were able to implement it.27 A recent study from our group has attempted to test the relevant importance of stages (early, mid‐, and late life) at which cognitive reserve exerted the largest protection against dementia risk.9 Building on the findings from that study, which suggested that cumulative engagement in reserve‐enhancing activities might be more relevant than the specific stages at which these contributors were acquired, we devised a cognitive reserve indicator that considered simultaneous contributions of several inputs. Among these, we also included social network and accumulated substantive complexity across 5 midlife occupations that were not considered in the previous study. Our findings confirmed that lifelong accumulation of stimulating experiences, supplying the reserve, is associated with the lowest risk of dementia and could be viewed as a viable prevention target.27
Based on the SEM factor loads, it appeared that the contribution of late life inputs, specifically social network and leisure activities, to the cumulative cognitive reserve indicator was the largest. This is in agreement with our previous study where mental, social, and physical components of late life leisure also had high factor loadings. Notably, aspects of social network were not taken into account in that earlier analysis. It appears from these results that inputs into cognitive reserve continue well into old age and may be especially relevant at a time when protection afforded by the earlier sources of resilience (eg, education) will have been exhausted. However, some of this association may also reflect reverse causality between preclinical dementia and social network or leisure activities, whereby individuals in the early stages of the disease disengage from social participation. However, sensitivity analysis in which we excluded incident dementia cases after 3 years of follow‐up revealed only negligible deviation from the original results, strengthening the conclusions of this study.
Our study is one of the few to report evidence of an additive interaction between the composite cognitive reserve indicator and genetic predisposition to dementia (APOE‐ε4 status). It suggests that if the reserve indicator were to be expressed as a risk factor (ie, lowest tertile of the index, as selected here), the APOE‐ε4 subgroup of the population who also had low reserve would experience a risk of dementia that was significantly in excess of the risk associated with the presence of only ε4 allele or only low reserve. This is in contrast to a multiplicative interaction, which would quantify the extent to which the effect of both low reserve and ε4‐positivity would exceed the product of their separate effects. It has been suggested in epidemiological literature that additive interactions are preferable to multiplicative interactions due to their greater public health relevance.28 Some have also argued that additive interactions may be more in line with the biological notion of synergism.29, 30
Previous literature on the interplay between cognitive reserve and genetic predisposition to dementia has reported conflicting findings. Some have found that protection against dementia as a result of physical leisure activities was more pronounced in ε4 carriers than in noncarriers,10 whereas others have reported the opposite pattern, with physical leisure favoring the noncarriers more.11 This is in accordance with another study of physical leisure that reported a tendency for an additive interaction between physical activity and APOE‐ε4 status, although only for Alzheimer disease.31 When it comes to factors other than physical engagement, a multicenter study has found that dementia risk was halved in ε4 carriers with >7 years of schooling.12 Another study, examining education, vascular risk factors, and leisure activities separately, has reported comparable magnitudes of dementia risk reduction among both carriers and noncarriers of APOE‐ε4 in the presence of optimal levels of modifiable risk factors,13 which was also in accordance with our results. An imaging study has shown that higher educated ε4 carriers exhibited similar levels of metabolism in medial temporal and prefrontal areas as the noncarriers,32 suggesting that stimulating environments could help postpone cognitive changes in carriers of genetic risk for dementia. Another study has examined the contribution of behavioral, reserve‐stimulating, and cardiometabolic factors to the rate of cognitive ageing in black and white APOE‐ε4 carriers and reported that a number of factors were able to provide resilience against cognitive ageing (measured by MMSE) even in ε4‐positive subjects.33
It may appear counterintuitive that we simultaneously found identical risks of dementia in both APOE‐ε4 carriers and noncarriers with higher cognitive reserve (suggesting independence between the factors), as well as an additive interaction between reserve and APOE‐ε4 status (suggesting a synergistic relationship between reserve and genetic predisposition). Reconciling the 2 findings is possible once the hazards of dementia in the 2 APOE‐ε4 subgroups with low reserve are compared. The HRs are nonoverlapping, and the ε4‐positive group with low reserve has a higher risk of dementia than the ε4‐negative subgroup with low reserve. It is precisely this difference that gives rise to the additive interaction reported here. At the same time, the HRs overlap across APOE‐ε4 status once the low scores on reserve are avoided, with the risk estimates becoming virtually identical in the high reserve subgroup. These 2 distinct, yet equally important findings—(1) excess dementia risk in the face of low reserve and ε4‐positivity; and (2) similarly lowered risk of dementia in the face of high reserve irrespective of APOE‐ε4 status—are the key pieces of new evidence generated by this study on the interplay between cognitive reserve and genetic risk for dementia. These findings are especially timely, given that a recent study examining the interaction between the APOE genotype and other genetic risk factors concluded that dementia risk of ε4 carriers was most receptive to modification.34 Accordingly, it has been argued that identifying resources that improve cognitive reserve should be a priority for patients with high genetic risk.35 Our findings suggest that a cognitive reserve indicator comprising several activities could be one such resource.
Strengths of this study include a prospective cohort design with a relatively long follow‐up period. Dementia diagnosis was reliably ascertained in a clinical setting following a comprehensive 3‐step assessment. Limitations include the difficulty of timing dementia given the interexamination time gaps of 3 (or 6) years. For individuals who died during this period, linkages to hospitalization and cause‐of‐death registers were available to determine dementia status at death. In ascertaining dementia status among the deceased participants, SNAC‐K physicians do not just accept the diagnoses listed on medical/cause of death records. Instead, they consult full medical charts, allowing them to independently determine participants’ dementia status. This allows for better precision of diagnosing dementia in deceased participants. Nonetheless, we accept that some underestimation of dementia cases among the deceased during the interexamination window may have occurred. Reverse causality between preclinical dementia and self‐reported exposure information at baseline could have occurred. To help reduce it, we restricted the study to cognitively intact dementia‐free participants at baseline and conducted sensitivity analyses where incident cases during the first follow‐up were excluded. Information on observed contributors to cognitive reserve was self‐reported, potentially increasing measurement error. Our usage of an SEM‐based latent variable approach allowed for the correction of unreliability in observed factors by attenuating measurement error. Nonresponse bias may have occurred, although the proportion of dropouts due to refusals was low, and those excluded were older and frailer than the analyzed population, suggesting effect underestimation. In sensitivity analyses, multiple imputation produced estimates similar to the complete‐case analysis. The rate of dementia occurrence in our study population was arguably lower than expected. One possibility is the restriction of the study sample to cognitively intact baseline participants who were not in the preclinical stage of dementia. Additionally, the positive selectivity of the study population (more fit and of higher socioeconomic status), a recent decline in dementia occurrence observed in Sweden and internationally,36 and the low sensitivity of diagnoses among deceased participants could have lowered the rate of dementia occurrence here.
In conclusion, we found that when 4 reserve‐enhancing factors from distinct periods during the life course—early life education, midlife substantive work complexity, and late life social network and leisure activities—were combined into a reserve indicator, a reduced risk of dementia in late life was demonstrated. The risk‐reducing effects of the reserve indicator were such that it largely negated the effects of a genetic predisposition to dementia. These findings underscore the clinical relevance of interventions aimed at comprehensively enhancing cognitive reserve throughout life, which might be an especially viable preventive strategy in at‐risk subpopulations genetically predisposed to dementia.
Author Contributions
S.D., A.M., W.X., A.D.‐M., and L.F. contributed to the conception and design of the study. All authors contributed to the acquisition and analysis of the data. S.D. and A.M. contributed to drafting the text and preparing the figures.
Potential Conflicts of Interest
Nothing to report.
Acknowledgment
SNAC‐K (http://www.snac.org) is financially supported by the Swedish Ministry of Health and Social Affairs, participating county councils and municipalities, and the Swedish Research Council. The authors acknowledge support from the Swedish Research Council, Konung Gustaf V:s och Drottning Victorias Frimurare Foundation, Alzeimerfonden, Gun och Bertil Stohnes Stiftelsen, Stiftelsen För Gamla Tjänarinnor, and Marianne and Marcus Wallenberg Foundation. This project is part of CoSTREAM (www.costream.eu) and received funding from the European Union's Horizon 2020 research and innovation program (No. 667375).
We thank the participants and staff involved in data collection and management in the SNAC‐K study; and E. Heiland for language editing the manuscript.
References
- 1. Stern Y. Cognitive reserve in ageing and Alzheimer's disease. Lancet Neurol 2012;11:1006–1012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Tucker AM, Stern Y. Cognitive reserve in aging. Curr Alzheimer Res 2011;8:354–360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Valenzuela MJ, Sachdev P. Brain reserve and dementia: a systematic review. Psychol Med 2006;36:441–454. [DOI] [PubMed] [Google Scholar]
- 4. Harrison SL, Sajjad A, Bramer WM, et al. Exploring strategies to operationalize cognitive reserve: a systematic review of reviews. J Clin Exp Neuropsychol 2015;37:253–264. [DOI] [PubMed] [Google Scholar]
- 5. Jones RN, Manly J, Glymour MM, et al. Conceptual and measurement challenges in research on cognitive reserve. J Int Neuropsychol Soc 2011;17:593–601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Richards M, Deary IJ. A life course approach to cognitive reserve: a model for cognitive aging and development? Ann Neurol 2005;58:617–622. [DOI] [PubMed] [Google Scholar]
- 7. Fratiglioni L, Paillard‐Borg S, Winblad B. An active and socially integrated lifestyle in late life might protect against dementia. Lancet Neurol 2004;3:343–353. [DOI] [PubMed] [Google Scholar]
- 8. Reed BR, Dowling M, Farias ST, et al. Cognitive activities during adulthood are more important than education in building reserve. J Int Neuropsychol Soc 2011;17:615–624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Wang H‐X, MacDonald SWS, Dekhtyar S, Fratiglioni L. Association of lifelong exposure to cognitive reserve‐enhancing factors with dementia risk: a community‐based cohort study. PLoS Med 2017;14:e1002251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Rovio S, Kareholt I, Helkala EL, et al. Leisure‐time physical activity at midlife and the risk of dementia and Alzheimer's disease. Lancet Neurol 2005;4:705–711. [DOI] [PubMed] [Google Scholar]
- 11. Podewils LJ, Guallar E, Kuller LH, et al. Physical activity, APOE genotype, and dementia risk: findings from the Cardiovascular Health Cognition Study. Am J Epidemiol 2005;161:639–651. [DOI] [PubMed] [Google Scholar]
- 12. Wang HX, Gustafson DR, Kivipelto M, et al. Education halves the risk of dementia due to apolipoprotein epsilon4 allele: a collaborative study from the Swedish Brain Power Initiative. Neurobiol Aging 2012;33:1007.e1–1007.e7. [DOI] [PubMed] [Google Scholar]
- 13. Ferrari C, Xu WL, Wang HX, et al. How can elderly apolipoprotein E epsilon4 carriers remain free from dementia? Neurobiol Aging 2013;34:13–21. [DOI] [PubMed] [Google Scholar]
- 14. Bennett DA, Schneider JA, Tang Y, et al. The effect of social networks on the relation between Alzheimer's disease pathology and level of cognitive function in old people: a longitudinal cohort study. Lancet Neurol 2006;5:406–412. [DOI] [PubMed] [Google Scholar]
- 15. Wang H‐X, Karp A, Winblad B, Fratiglioni L. Late‐life engagement in social and leisure activities is associated with a decreased risk of dementia: a longitudinal study from the Kungsholmen project. Am J Epidemiol 2002;155:1081–1087. [DOI] [PubMed] [Google Scholar]
- 16. Lagergren M, Fratiglioni L, Hallberg IR, et al. A longitudinal study integrating population, care and social services data. The Swedish National study on Aging and Care (SNAC). Aging Clin Exp Res 2004;16:158–168. [DOI] [PubMed] [Google Scholar]
- 17. Fratiglioni L, Viitanen M, Bäckman L, et al. Occurrence of dementia in advanced age: the study design of the Kungsholmen Project. Neuroepidemiology 1992;11(suppl 1):29–36. [DOI] [PubMed] [Google Scholar]
- 18. Fratiglioni L, Viitanen M, von Strauss E, et al. Very old women at highest risk of dementia and Alzheimer's disease: incidence data from the Kungsholmen Project, Stockholm. Neurology 1997;48:132–138. [DOI] [PubMed] [Google Scholar]
- 19. Schooler C, Mulatu MS, Oates G. The continuing effects of substantively complex work on the intellectual functioning of older workers. Psychol Aging 1999;14:483. [DOI] [PubMed] [Google Scholar]
- 20. Andel R, Crowe M, Pedersen NL, et al. Complexity of work and risk of Alzheimer's disease: a population‐based study of Swedish twins. J Gerontol B Psychol Sci Soc Sci 2005;60:P251–P258. [DOI] [PubMed] [Google Scholar]
- 21. Cornwell EY, Waite LJ. Measuring social isolation among older adults using multiple indicators from the NSHAP study. J Gerontol B Psychol Sci Soc Sci 2009;64(suppl 1):i38–i46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Basun H, Corder EH, Guo Z, et al. Apolipoprotein E polymorphism and stroke in a population sample aged 75 years or more. Stroke 1996;27:1310–1315. [DOI] [PubMed] [Google Scholar]
- 23. Knol MJ, VanderWeele TJ, Groenwold RH, et al. Estimating measures of interaction on an additive scale for preventive exposures. Eur J Epidemiol 2011;26:433–438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Arenaza‐Urquijo EM, Bejanin A, Gonneaud J, et al. Association between educational attainment and amyloid deposition across the spectrum from normal cognition to dementia: neuroimaging evidence for protection and compensation. Neurobiol Aging 2017;59:72–79. [DOI] [PubMed] [Google Scholar]
- 25. EClipSE Collaborative Members , Brayne C, Ince PG, Keage HAD, et al. Education, the brain and dementia: neuroprotection or compensation? Brain 2010;133:2210–2216. [DOI] [PubMed] [Google Scholar]
- 26. Chapko D, McCormack R, Black C, et al. Life‐course determinants of cognitive reserve (CR) in cognitive aging and dementia—a systematic literature review. Aging Ment Health 2018;22:915–926. [DOI] [PubMed] [Google Scholar]
- 27. Dekhtyar S, Wang H‐X, Scott K, et al. A life‐course study of cognitive reserve in dementia—from childhood to old age. Am J Geriatr Psychiatry 2015;23:885–896. [DOI] [PubMed] [Google Scholar]
- 28. Rothman KJ, Greenland S, Walker AM. Concepts of interaction. Am J Epidemiol 1980;112:467–470. [DOI] [PubMed] [Google Scholar]
- 29. Rothman KJ. Causes. Am J Epidemiol 1995;141:90–95. [DOI] [PubMed] [Google Scholar]
- 30. VanderWeele TJ, Robins JM. The identification of synergism in the sufficient‐component‐cause framework. Epidemiology 2007;18:329–339. [DOI] [PubMed] [Google Scholar]
- 31. Luck T, Riedel‐Heller SG, Luppa M, et al. Apolipoprotein E epsilon 4 genotype and a physically active lifestyle in late life: analysis of gene–environment interaction for the risk of dementia and Alzheimer's disease dementia. Psychol Med 2013;44:1319–1329. [DOI] [PubMed] [Google Scholar]
- 32. Arenaza‐Urquijo EM, Gonneaud J, Fouquet M, et al. Interaction between years of education and APOE ε4 status on frontal and temporal metabolism. Neurology 2015;85:1392–1399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Kaup AR, Nettiksimmons J, Harris TB, et al. Cognitive resilience to apolipoprotein e ε4: contributing factors in black and white older adults. JAMA Neurol 2015;72:340–348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. van der Lee SJ, Wolters FJ, Ikram MK, et al. The effect of APOE and other common genetic variants on the onset of Alzheimer's disease and dementia: a community‐based cohort study. Lancet Neurol 2018;17:434–444. [DOI] [PubMed] [Google Scholar]
- 35. Dufouil C, Glymour MM. Prediction to prevention in Alzheimer's disease and dementia. Lancet Neurol 2018;17:388–389. [DOI] [PubMed] [Google Scholar]
- 36. Wu Y‐T, Beiser AS, Breteler MMB, et al. The changing prevalence and incidence of dementia over time—current evidence. Nat Rev Neurol 2017;13:327–339. [DOI] [PubMed] [Google Scholar]


