Abstract
Background
Glaucoma is the world's second biggest cause of blindness, and patients progressively lose their eyesight. The current clinical treatment for glaucoma involves controlling intraocular pressure with drugs or surgery; however, some patients still progressively lose their eyesight. This treatment is also similar to the treatment of traumatic optic neuropathy. Thus, saving retinal ganglion cells (RGCs) from apoptosis is essential.
Methods
The role of Acteoside on autophagy modulation in the 661 W cell line.
Results
In this study, we first find that Acteoside inhibits autophagy, Rapamycin alleviates this inhibition and the PI3K inhibitor, 3‐MA or LY294002, synergistically promotes it. In a mechanistic study, we find that Optineurin (OPTN) mediates Acteoside regulation of autophagy. OPTN overexpression or knockdown activates or inhibits autophagy, respectively. OPTN is inhibited by autophagy inhibitors, such as Acteoside and 3‐MA and is promoted by the autophagy activator, Rapamycin. Meanwhile, PI3K and AKT are elevated by Acteoside and 3‐MA and inhibited by Rapamycin. Finally, we find that Acteoside inhibits apoptosis in parallel to autophagy and that this inhibition is also mediated by OPTN.
Conclusion
In summary, we conclude that Acteoside inhibits autophagy‐induced apoptosis in RGCs through the OPTN and PI3K/AKT/mTOR pathway, and glaucoma patients may benefit from Acteoside treatment alone or in combination with other autophagy inhibitors.
Keywords: Acteoside, apoptosis, autophagy, glaucoma
1. INTRODUCTION
Glaucoma is the second leading cause of blindness in the world, and it is characterized by optic atrophy together with visual field defects. Pathological high intraocular pressure is the main reason for glaucoma, and most patients do not have subjective symptoms because of occult onset. A survey on ocular hypertension shows that untreated subjects have a 5‐year glaucomatous optic neuropathy incidence of 9.5% and a 13‐year incidence of 22%.1, 2 Traumatic optic neuropathy is usually caused by various kinds of accidents, whereas most patients lose their sight immediately; optic atrophy actually occurs in the following 4 to 8 weeks.3 Glaucoma and traumatic optic neuropathy share a similar disease progression process, including impairments to retinal ganglion cells (RGCs), RGC apoptosis, and eventually optic atrophy. All clinical treatments including corticosteroids or intraocular pressure, controlled through drugs or surgery, cannot effectively prevent RGC apoptosis; thus, rescuing RGCs from apoptosis is an essential approach to keeping patients' eyesight.4
Acteoside is the main constituent of Yunnan Ilex latifolia (Ligustrum purpurascens Y. C. Yang), and it has been proven to have numerous biological functions, including anti‐inflammatory, antioxidant, and neuron protection.5 Acteoside downregulates the TGF‐β1/smad signaling pathway, inhibiting hepatic stellate cell activation and hepatic fibrosis.6 Acteoside remarkably increases the learning and memory ability of mice with Alzheimer's disease, and it inhibits NO, nitric oxide synthase, and caspase 3 expression.7 Acteoside shows a protective effect on X‐ray‐induced injury through enforced ROS elimination, a decreased Bax/Bcl‐2 ratio and downregulated procaspase‐3 expression.8 Thus, Acteoside may act as an antiapoptosis drug to rescue certain kinds of degenerative diseases.
RGC apoptosis occurs in the early stage of glaucoma and traumatic optic neuropathy, and autophagy has been reported as a cause of optic nerve apoptosis. In an optic nerve crush model in rats, autophagy was found to rapidly and constantly increase after optic nerve injury, and the autophagy inhibitor 3‐MA attenuated the axonal degeneration significantly.9 In a chronic hypertensive glaucoma rat model, a sustained increase of autophagosomes and LC3‐II was observed over 8 weeks of chronic intraocular pressure elevation, and 3‐MA significantly decreased RGC apoptosis in this model.10 Optineurin (OPTN) is a classical autophagy receptor, and the OPTN gene mutation leads to familial glaucoma and amyotrophic lateral sclerosis. A mutation of the OPTN Ubiquitin binding domain shows the dominant negative effect on autophagosome maturation and thus inhibits OPTN mediated autophagy.11 An OPTN‐M98K mutant was shown to induce retinal cell apoptosis, and OPTN knockdown reduces autophagosome formation.12 Autophagy is regulated through multiple signaling, including AMPK, mTOR, and PI3K. AMPK phosphorylation activates autophagy,13, 14, 15 mTOR inhibition by Rapamycin activates autophagy,16 and specific PI3K inhibitors, such as 3‐MA and LY‐294002 inhibit autophagosome fusion with lysosomes, thus inhibiting autophagy.17
On the basis of the effect of causing apoptosis on glaucoma and traumatic optic neuropathy‐induced blindness, as well as the protective effect of Acteoside on autophagic apoptosis, we hypothesize that Acteoside may protect glaucoma or traumatic optic neuropathy‐induced RGC apoptosis through the inhibition of autophagy.
2. MATERIAL AND METHODS
2.1. Cell culture and small interfering RNA/plasmid transfection
661 W cells were maintained in DMEM high glucose (HyClone, Cat.No.SH30022.01B) supplemented with 10% fetal bovine serum (HyClone, Cat.No. SH30087.01) and penicillin/streptomycin (HyClone, Cat.No. SH30010). OPTN siRNA was purchased from GUANGZHOU RIBIO CO., LTD. Transfection was performed using Lipofectamine RNAiMAX (Invitrogen, Cat.No. 13778075) following the manufacturer's instruction. In brief, 1.25 μL small interfering RNA (siRNA) stock solution (50 nM) was added into 100 μL Opti‐MEM (Invitrogen, Cat.No.31985070) to make solution A, and 1 μL Lipofectamine RNAiMAX was added into 100 μL Opti‐MEM to make solution B. We mixed each solution separately and waited for 5 minutes, and then we added solution A into B and mixed; after 20 minutes, we transferred the mixture into a p24 well with 300 µL complete medium without penicillin/streptomycin and culture in a 5% CO2, 37℃ incubator. The OPTN CDS was ordered from GENEWIZ, Inc. and inserted into the KpnI and BamHI site of the pCDNA3.1 vector to make the OPTN overexpression plasmid. Plasmid transfection was performed using a Lipofectamine 3000 (Invitrogen, Cat.No.L3000015) following the manufacturer's instructions.
2.2. Western blotting and immunofluorescence
For Western blotting, the cells were washed twice with DPBS, put on ice, lysed in radioimmunoprecipitation assay (50 mM Tris–HCl, pH 7.4, 150 mM NaCl, 0.25% Sodium deoxycholate, 0.1% Nonidet P‐40, 0.1% Triton X‐100) and supplemented with 10 mM Na3VO4, 50 mM NaF, protease inhibitor cocktails (Roche) and 1 mM PMSF, following by being scraped off. The cell lysates were sonicated, mixed with the loading buffer, boiled, vortexed, centrifuged, subjected to sodium dodecyl sulfate‐polyacrylamide gel electrophoresis and transferred to a PVDF membrane (Millipore) according to the standard protocol. After blocking with 5% nonfat milk in TBST (50 mM Tris–HCl at pH 8.0, 150 mM NaCl, 0.1% Tween 20), the membrane was incubated with 5% nonfat milk‐diluted primary and secondary antibodies. Signals were detected with Immobilon Western Chemiluminescent HRP Substrate (Millipore, Cat.No.WBKLS0500). The anti‐LC3 (#10837‐1‐AP) and anti‐OPTN (#12135‐1‐AP) were from Proteintech. The anti‐PI3K (#ab86712), anti‐AKT (#ab8805), and anti‐pAKT (#ab38449) were from Abcam. The rabbit Anti‐Mouse IgG (H + L) (#6170‐05) and Goat Anti‐Rabbit IgG (H + L chain specific; #4050‐05) were from Southern Biotech. The Peroxidase‐ Rabbit Anti‐Goat IgG (#BA1060) and the Peroxidase‐Rabbit Anti‐Rat IgG (#BA1058) were from Wuhan Boster Biological Technology Co., Ltd. For immunofluorescence, the cells were fixed in 4% paraformaldehyde for 30 minutes, washed, blocked, and permeabilized in blocking solution (phosphate‐buffered saline containing 3% fetal bovine serum and 0.2% Triton X‐100) for 30 minutes. The primary antibodies were incubated overnight at 4°C in blocking solution, washed twice, and incubated with the corresponding secondary antibodies for 2 hours at room temperature. The cells were washed twice and stained with 4′,6‐diamidino‐2‐phenylindole (Sigma) for 5 minutes. The antibodies including Goat Anti‐Rabbit IgG (H + L), DylightTM488 conjugated (#35552) and Goat Anti‐Mouse IgG (H + L), DylightTM594 conjugated (#35510) were from Thermo.
2.3. Transmission electron microscopy
Cell samples were processed according to a previous publication1 with minor modifications. In brief, the cells were washed three times with DPBS and fixed with 2.5% electron microscopy grade glutaraldehyde (Sigma) and 2% paraformaldehyde (Sigma) in 0.1 M sodium cacodylate‐HCl, pH 7.4 for 30 minutes at room temperature. The cells were washed with DPBS twice and put into a 4°C refrigerator. Before further processing, the cells were scraped, collected, and stained with osmium tetroxide. The cells were then embedded, sectioned, and examined using an Hitachi H‐7500 transmission electron microscope.
2.4. Statistical analysis
Statistical analyses were performed using a two‐tailed t test. The values are shown as the mean ± the standard error of the mean (SEM) for multiple independent experiments, not technical replicates.
3. RESULTS
3.1. Acteoside inhibits autophagy through OPTN
To investigate the function of Acteoside on the autophagy of retinal ganglion cells, LC3‐II expression in 661 W cells was detected. As shown in Figure 1A, Acteoside decreased LC3‐II moderately but statistically significantly. Moreover, OPTN is reported to regulate autophagy, therefore, we further explored the effects of Acteoside on LC3‐II level by OPTN overexpression and siRNA knockdown. Western blot showed that OPTN overexpression elevated the LC3‐II level dramatically, but LC3‐II expression was significantly decreased in Acteoside‐treated OPTN overexpression cells. Besides, si‐OPTN contributed to the downregulation of LC3‐II expression, and Acteoside treatment further decreased the LC3‐II level to some extent (Figure 1A). Meanwhile, we also performed immunofluorescence to confirm the anti‐autophagic role of Acteoside. 661 W cells were treated with OPTN overexpression or siRNA in combination with the Acteoside treatment. Antibodies targeting OPTN or LC3 were incubated with the treated cells. We used LC3 puncta per cell as the quantification criteria for the autophagy level. According to Figure 1B, Acteoside treatment alone showed a moderate but statistically significant decrease of autophagy, whereas OPTN overexpression or siRNA alone induced increased or decreased autophagy, and the addition of Acteoside inhibited autophagy in both conditions. Taken together, we conclude that OPTN potently regulates autophagy, and Acteoside inhibits autophagy at least partially through OPTN.
Figure 1.
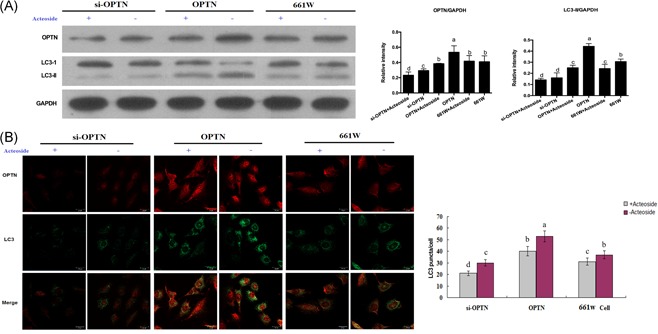
A, Western blotting showing the inhibition of Acteoside on the autophagy of OPTN overexpression and OPTN knockdown 661 W cells. Results are given as mean ± SEM. Differences between groups were determined by two‐tailed t test (n = 3). B, Immunofluorescence showing the inhibition of Acteoside on the autophagy of OPTN overexpression and OPTN knockdown 661 W cells. The red fluorescence labels the OPTN and the green fluorescence labels the LC3. The number of LC3 puncta/cells indicates autophagic activity. Results are given as mean ± SEM. Differences between groups were determined by two‐tailed t test. Three fields per cell and thirty cells per group were analyzed. Different letters indicate values significantly different among groups (P < .05). OPTN, optineurin; SEM, standard error of mean
3.2. Acteoside inhibits autophagy through the PI3K/AKT/mTOR pathway
Autophagy is tightly regulated by the PI3K/AKT/mTOR pathway. 3‐MA is a selective PI3K and autophagy inhibitor, and Rapamycin is a widely accepted inhibitor of mTOR and activator of autophagy.18 To elucidate the detailed mechanism of Acteoside autophagy regulation, we investigated the combined effect of Acteoside, 3‐MA, and Rapamycin on the PI3K/AKT/mTOR pathway and autophagy activity. The Western blot shows that Acteoside or 3‐MA alone activates PI3K and AKT at the same time that autophagy is inhibited. Rapamycin inhibits PI3K and AKT, and meanwhile, autophagy is activated. The addition of Acteoside to 3‐MA or Rapamycin dramatically activates PI3K and AKT, and autophagy is significantly inhibited (Figure 2A). For further confirmation, we performed transmission electron microscopy (TEM) to detect autophagosomes. Autophagosomes are double membrane particles that have engulfed substrates for degradation, they show an electron dense character in the TEM.19 Rapamycin treatment induces much more autophagosomes in 661 W cells (Figure 2B and 2D), and Acteoside addition alleviates this effect (Figure 2D); 3‐MA or Acteoside alone reduces autophagosomes (Figure 2C and 2D), and a combination shows even less (Figure 2D). From these results, we conclude that Acteoside regulates autophagy not only through OPTN signaling but also through the PI3K/AKT/mTOR pathway.
Figure 2.
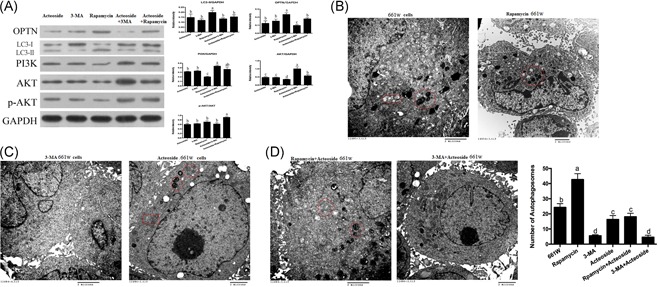
A, Western blotting showing the effects of Acteoside on the PI3K/AKT/mTOR signaling pathway. 611 W cells were treated as follows: Acteoside, 3‐MA, rapamycin, Acteoside + 3‐MA and rapamycin + 3‐MA. B, TEM shows that Rapamycin induces increased autophagy. The double membrane electro dense particles indicate autophagosomes. C, TEM shows that 3‐MA and Acteoside inhibit autophagy. The double membrane electro dense particles indicate autophagosomes. D, TEM shows the synergistic result of Acteoside with Rapamycin or 3‐MA on the autophagy level. The double membrane electro dense particles indicate autophagosomes (the scar bar represents 2 μm). Results are given as mean ± SEM. Differences between groups were determined by two‐tailed t test (n = 3). Different letters indicate values significantly different among groups (P < .05). SEM, standard error of mean; TEM, transmission electron microscopy
3.3. Acteoside operates synergistically with OPTN and the PI3K/AKT/mTOR pathway
On the basis of our observation that Acteoside separately cooperates with OPTN and the PI3K/AKT/mTOR pathway, we begin to wonder whether there is a synergistic effect of these three. We incorporated Acteoside with OPTN modulation and PI3K inhibitors together and detected the autophagy level. Western blot results show that even in the presence of Acteoside, the PI3K inhibitor LY294002 still dramatically inhibits OPTN and autophagy, regardless of OPTN overexpression or siRNA (Figure 3A). Immunofluorescence results show that 3‐MA further reduces OPTN and LC3 puncta in the context of Acteoside, and Rapamycin reverses the effect of Acteoside (Figure 3B). TEM shows that although si‐OPTN has fewer autophagosomes than OPTN overexpression, the addition of Acteoside reduces the autophagosome number in both conditions. The addition of LY294002 further reduces the autophagosome number to an even lower level (Figure 3C). From these results, we conclude that Acteoside operates synergistically with OPTN and the PI3K/AKT/mTOR pathway, and a combination of Acteoside with 3‐MA or LY294002 can inhibit autophagy to an even lower level.
Figure 3.
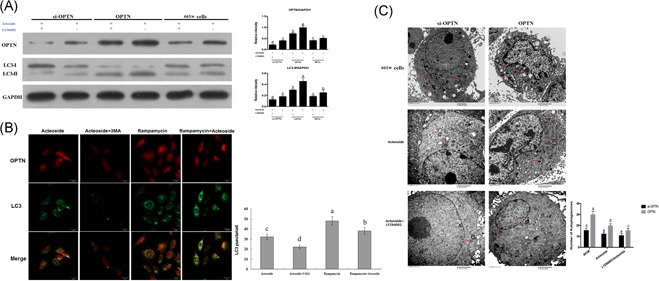
A, Western blotting showing the synergistic influence of Acteoside and LY294002 on the autophagy. B, Immunofluorescence showing the synergistic influence of Acteoside with 3‐MA or Rapamycin on autophagy. The red fluorescence labels the OPTN and the green fluorescence labels the LC3, the number of LC3 puncta/cells indicates the autophagic activity. C, TEM showing the synergistic influence of Acteoside and LY294002 on autophagy. The double membrane electro dense particles indicate autophagosomes (the scar bar represents 2 μm). Results are given as mean ± SEM. Differences between groups were determined by two‐tailed t test (n = 3). For immunofluorescence, three fields per cell and thirty cells per group were analyzed. Different letters indicate values significantly different among groups (P < .05). OPTN, Optineurin; SEM, standard error of mean; TEM, transmission electron microscopy
3.4. The effect of Acteoside on autophagy inhibition correlates with apoptosis inhibition
In the neuron system, autophagy usually correlates with apoptosis, so we detected the apoptosis enzymes caspase 3 and caspase7 to elucidate the relationship between Acteoside and apoptosis.20 Western blot results show that similar to autophagy, caspase 3 and caspase7 are negatively regulated by Acteoside. OPTN overexpression elevates caspase 3/7, si‐OPTN decreases caspase 3/7 and Acteoside inhibits caspase 3/7 in all conditions regardless of OPTN modulation (Figure 4A and 4B). From these observations, we conclude that Acteoside inhibits not only autophagy but also autophagic apoptosis in 661 W cells.
Figure 4.
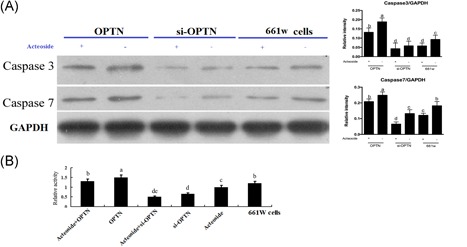
A, Western blotting showing the influence of Acteoside on the apoptosis. B, The effects of Acteoside on the relative activities of caspase 3/7. Results are given as mean ± SEM. Differences between groups were determined by two‐tailed t test (n = 3 for Western blot; n = 6 for the caspase 3/7 activity). Different letters indicate values significantly different among groups (P < .05). SEM, standard error of mean
4. DISCUSSION
Autophagy has recently gained much attention as a vital mechanism for neuronal homeostasis, and defects in the autophagic machinery have also been described in several neurodegenerative diseases.21 Autophagic dysfunctions have been found in chronic neurodegenerative diseases including Alzheimer's disease,22 Parkinson's disease (PD),23 and Huntington's disease,24 as well as in acute diseases, such as brain hypoxia/ischemia, trauma,25 and other pathologies of the nervous system, such as neuropathic pain.26
Autophagy is particularly crucial for postmitotic cells, such as neurons, because misfolded proteins and damaged or aged organelles can only be removed by autophagy; if not efficiently removed, they accumulate and lead to neuronal degeneration and death.27 Indeed, neuron‐specific loss of the core autophagic proteins (Atg7 and Atg5) in mice generates a neurodegenerative phenotype.28, 29 Several studies provide evidence for the modulation of autophagy as an attractive therapy for neurodegenerative diseases. Indeed, enhancing the autophagic efficacy might (1) lower the amount of toxic protein aggregates, (2) provide a more effective response to stress by degrading nonessential components to gain energy and to support adaptive protein synthesis, and (3) prevent or reduce apoptotic cell death.30, 31
Glaucoma is conventionally defined as a chronic optic neuropathy characterized by the progressive loss of RGCs whose cell bodies lie on the inner surface of the retina projecting their axon along the optic nerve to the lateral geniculate nucleus. The structures affected by glaucoma include the retina and optic nerve; taken together with the pathological features and the recent findings that glaucomatous injury is related to both RGCs and the central areas of the visual system,32 glaucoma is being classified among the neurodegenerative disorders. As a neurodegenerative disease, neuroprotective strategies aimed at protecting or maintaining RGC survival or enhancing RGC function have been explored as possible approaches for glaucoma treatment.4 Furthermore, being able to recognize the neurodegenerative nature of glaucoma indicates that this eye disease might share common mechanisms with central nervous system degenerative diseases.
Most evidence supports the concept of autophagy as a mechanism of stress adaptation that promotes cell survival; however, there is also evidence suggesting that autophagy can be associated with cell death, mainly by overactivation, leading to self‐digestion.33, 34, 35 Furthermore, although autophagy and apoptosis are distinct cellular processes, their signaling pathways are strictly interconnected through several crosstalk mechanisms; therefore, the perturbation of the balance between these two processes might be associated with the development of pathological conditions.36, 37 Only recently, several groups have focused their research on the role of autophagy in the neurodegeneration associated with glaucoma, producing a body of literature that sets the basis for future studies.
It has to be stressed here that the still limited comprehension of the cellular and molecular pathology of glaucoma does not allow designing an ideal experimental setting under which to study the autophagic process in the context of glaucoma neurodegeneration. A wide variety of glaucoma animal models are available based on the induction of axonal injury at the optic nerve head, and they all lead to the ultimate death of RGCs. For other neurodegenerative diseases, none of these models can give an exhaustive view of the pathological process, but each system mimics at least one component of glaucomatous optic neuropathy helping to gain information and test potential therapies. The limits of each model, being set on a single and specific type of insult, which does not resemble the multifactorial pathogenesis of glaucoma, can be overcome only by integrating the data from different experimental settings and drawing a more comprehensive view.
5. CONCLUSION
In our study, we tested the function of Acteoside on autophagy modulation in the 661 W cell line. We find that Acteoside inhibits autophagy and autophagic apoptosis through OPTN and the PI3K/AKT/mTOR pathway. Treatment with the mTOR inhibitor Rapamycin neutralizes this inhibition, and treatment with the PI3K inhibitors 3‐MA or LY294002 has a synergistic effect with Acteoside. However, there are still three points that need further investigation. The first one is that we are studying everything in a rat retinal ganglion 661 W cell line. This cell line may vary from human physiological conditions, and in the next step, we need to further confirm these phenotypes in human retinal ganglion cells and mouse glaucoma models. The second point is that we did not detect the autophagy flux because the elevated LC3‐II or autophagosome number may also be a result of blocked autophagic degradation, so incorporation of an autophagosome‐lysosome fusion inhibitor is necessary to distinguish between activated autophagy and blocked degradation. The third point is that to eventually restore visual function for patients with glaucoma or traumatic optic neuropathy, it is crucial that the axonal connection from the RGCs to the brain is rebuilt; thus, the amount of axonal degeneration and regeneration in the optic nerve should be evaluated in future studies.
CONFLICT OF INTEREST
The authors declare that there is no conflict of interest.
AUTHOR CONTRIBUTIONS
Li Yan designed the study and wrote the manuscript; Xiaoting Xi and Qianbo Chen performed the experiments; Yong Zeng and Zhendan He analyzed the data; Jianfeng Zhao revised the language editing.
ACKNOWLEDGMENT
This work was financially supported by National Science Fund of China (No. 81060077) and Medical and Health Research Projects of Yunnan Province (No. 2014NS183).
Chen Q, Xi X, Zeng Y, He Z, Zhao J, Li Y. Acteoside inhibits autophagic apoptosis of retinal ganglion cells to rescue glaucoma‐induced optic atrophy. J Cell Biochem. 2019;120:13133‐13140. 10.1002/jcb.28586
Qianbo Chen and Xiaoting Xi contributed equally to this work.
References
REFERENCES
- 1. Gordon MO, Beiser JA, Brandt JD, et al. The ocular hypertension treatment study: baseline factors that predict the onset of primary open‐angle glaucoma. Arch Ophthalmol. 2002;120:714‐720. discussion 829‐830 [DOI] [PubMed] [Google Scholar]
- 2. Kass MA, Heuer DK, Higginbotham EJ, et al. The ocular hypertension treatment study: a randomized trial determines that topical ocular hypotensive medication delays or prevents the onset of primary open‐angle glaucoma. Arch Ophthalmol. 2002;120:701‐713. discussion 829‐830 [DOI] [PubMed] [Google Scholar]
- 3. Quigley HA. Glaucoma. Lancet. 2011;377:1367‐1377. 10.1016/S0140-6736(10)61423-7 [DOI] [PubMed] [Google Scholar]
- 4. Baltmr A, Duggan J, Nizari S, Salt TE, Cordeiro MF. Neuroprotection in glaucoma ‐ is there a future role? Exp Eye Res. 2010;91:554‐566. 10.1016/j.exer.2010.08.009 [DOI] [PubMed] [Google Scholar]
- 5. He J, Hu XP, Zeng Y, et al. Advanced research on acteoside for chemistry and bioactivities. J Asian Nat Prod Res. 2011;13:449‐464. 10.1080/10286020.2011.568940 [DOI] [PubMed] [Google Scholar]
- 6. You SP, Ma L, Zhao J, Zhang SL, Liu T. Phenylethanol glycosides from cistanche tubulosa suppress hepatic stellate cell activation and block the conduction of signaling pathways in TGF‐β1/smad as potential anti‐hepatic fibrosis agents. Molecules. 2016;21:102 10.3390/molecules21010102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Peng XM, Gao L, Huo SX, Liu XM, Yan M. The mechanism of memory enhancement of acteoside (Verbascoside) in the senescent mouse model induced by a combination of d‐gal and AlCl3. Phytother Res. 2015;29:1137‐1144. 10.1002/ptr.5358 [DOI] [PubMed] [Google Scholar]
- 8. Yang J, Yan Y, Liu H, Wang J, Hu J. Protective effects of acteoside against Xrayinduced damage in human skin fibroblasts. Mol Med Rep. 2015a;12:2301‐2306. 10.3892/mmr.2015.3630 [DOI] [PubMed] [Google Scholar]
- 9. Knoferle J, Koch JC, Ostendorf T, et al. Mechanisms of acute axonal degeneration in the optic nerve in vivo. Proc Natl Acad Sci USA. 2010;107:6064‐6069. 10.1073/pnas.0909794107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Park HY, Kim JH, Park CK. Activation of autophagy induces retinal ganglion cell death in a chronic hypertensive glaucoma model. Cell Death Dis. 2012;3:e290‐e290. 10.1038/cddis.2012.26 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Shen WC, Li HY, Chen GC, Chern Y, Tu PH. Mutations in the ubiquitin‐binding domain of OPTN/optineurin interfere with autophagy‐mediated degradation of misfolded proteins by a dominant‐negative mechanism. Autophagy. 2015;11:685‐700. 10.4161/auto.36098 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Sirohi K, Kumari A, Radha V, Swarup G. A glaucoma‐associated variant of optineurin, M98K, activates Tbk1 to enhance autophagosome formation and retinal cell death dependent on Ser177 phosphorylation of optineurin. PLoS One. 2015;10:e0138289 10.1371/journal.pone.0138289 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Park SK, Seong RK, Kim JA, et al. Oligonol promotes anti‐aging pathways via modulation of SIRT1‐AMPK‐autophagy pathway. Nutr Res Pract. 2016;10:3‐10. 10.4162/nrp.2016.10.1.3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Wu X, Fleming A, Ricketts T, et al. Autophagy regulates Notch degradation and modulates stem cell development and neurogenesis. Nat Commun. 2016;7:10533 10.1038/ncomms10533 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Yang JY, Park MY, Park SY, et al. Nitric oxide‐induced autophagy in MC3T3‐E1 cells is associated with cytoprotection via AMPK activation. Korean J Physiol Pharmacol. 2015b;19:507‐514. 10.4196/kjpp.2015.19.6.507 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Chen R, Wang B, Chen L, et al. DNA damage‐inducible transcript 4 (DDIT4) mediates methamphetamine‐induced autophagy and apoptosis through mTOR signaling pathway in cardiomyocytes. Toxicol Appl Pharmacol. 2016;295:1‐11. 10.1016/j.taap.2016.01.017 [DOI] [PubMed] [Google Scholar]
- 17. Button RW, Vincent JH, Strang CJ, Luo S. Dual PI‐3 kinase/mTOR inhibition impairs autophagy flux and induces cell death independent of apoptosis and necroptosis. Oncotarget. 2016;7:5157‐5175. 10.18632/oncotarget.6986 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Heras‐Sandoval D, Perez‐Rojas JM, Hernandez‐Damian J, Pedraza‐Chaverri J. The role of PI3K/AKT/mTOR pathway in the modulation of autophagy and the clearance of protein aggregates in neurodegeneration. Cell Signal. 2014;26:2694‐2701. 10.1016/j.cellsig.2014.08.019 [DOI] [PubMed] [Google Scholar]
- 19. Wong YC, Holzbaur EL. Autophagosome dynamics in neurodegeneration at a glance. J Cell Sci. 2015;128:1259‐1267. 10.1242/jcs.161216 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Shalini S, Dorstyn L, Dawar S, Kumar S. Old, new and emerging functions of caspases. Cell Death Differ. 2015;22:526‐539. 10.1038/cdd.2014.216 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Frake RA, Ricketts T, Menzies FM, Rubinsztein DC. Autophagy and neurodegeneration. J Clin Invest. 2015;125:65‐74. 10.1172/JCI73944 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Nixon RA, Wegiel J, Kumar A, et al. Extensive involvement of autophagy in Alzheimer disease: an immuno‐electron microscopy study. J Neuropathol Exp Neurol. 2005;64:113‐122. [DOI] [PubMed] [Google Scholar]
- 23. Pan T, Kondo S, Le W, Jankovic J. The role of autophagy‐lysosome pathway in neurodegeneration associated with Parkinson's disease. Brain. 2008;131:1969‐1978. 10.1093/brain/awm318 [DOI] [PubMed] [Google Scholar]
- 24. Ravikumar B, Vacher C, Berger Z, et al. Inhibition of mTOR induces autophagy and reduces toxicity of polyglutamine expansions in fly and mouse models of Huntington disease. Nat Genet. 2004;36:585‐595. 10.1038/ng1362 [DOI] [PubMed] [Google Scholar]
- 25. Wong E, Cuervo AM. Autophagy gone awry in neurodegenerative diseases. Nat Neurosci. 2010;13:805‐811. 10.1038/nn.2575 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Berliocchi L, Russo R, Maiaru M, Levato A, Bagetta G, Corasaniti MT. Autophagy impairment in a mouse model of neuropathic pain. Mol Pain. 2011;7:83 10.1186/1744-8069-7-83 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Marino G, Madeo F, Kroemer G. Autophagy for tissue homeostasis and neuroprotection. Curr Opin Cell Biol. 2011;23:198‐206. 10.1016/j.ceb.2010.10.001 [DOI] [PubMed] [Google Scholar]
- 28. Hara T, Nakamura K, Matsui M, et al. Suppression of basal autophagy in neural cells causes neurodegenerative disease in mice. Nature. 2006;441:885‐889. 10.1038/nature04724 [DOI] [PubMed] [Google Scholar]
- 29. Komatsu M, Wang QJ, Holstein GR, et al. Essential role for autophagy protein Atg7 in the maintenance of axonal homeostasis and the prevention of axonal degeneration. Proc Natl Acad Sci USA. 2007;104:14489‐14494. 10.1073/pnas.0701311104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Ravikumar B, Rubinsztein DC. Can autophagy protect against neurodegeneration caused by aggregate‐prone proteins? Neuroreport. 2004;15:2443‐2445. [DOI] [PubMed] [Google Scholar]
- 31. Boya P, Kroemer G. Lysosomal membrane permeabilization in cell death. Oncogene. 2008;27:6434‐6451. 10.1038/onc.2008.310 [DOI] [PubMed] [Google Scholar]
- 32. Nucci C, Martucci A, Cesareo M, et al. Brain involvement in glaucoma: advanced neuroimaging for understanding and monitoring a new target for therapy. Curr Opin Pharmacol. 2013;13:128‐133. 10.1016/j.coph.2012.08.004 [DOI] [PubMed] [Google Scholar]
- 33. Kroemer G, Levine B. Autophagic cell death: the story of a misnomer. Nat Rev Mol Cell Biol. 2008;9:1004‐1010. 10.1038/nrm2529 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Denton D, Nicolson S, Kumar S. Cell death by autophagy: facts and apparent artefacts. Cell Death Differ. 2012;19:87‐95. 10.1038/cdd.2011.146 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Fulda S, Kogel D. Cell death by autophagy: emerging molecular mechanisms and implications for cancer therapy. Oncogene. 2015;34:5105‐5113. 10.1038/onc.2014.458 [DOI] [PubMed] [Google Scholar]
- 36. Rubinstein AD, Kimchi A. Life in the balance ‐ a mechanistic view of the crosstalk between autophagy and apoptosis. J Cell Sci. 2012;125:5259‐5268. 10.1242/jcs.115865 [DOI] [PubMed] [Google Scholar]
- 37. Booth LA, Tavallai S, Hamed HA, Cruickshanks N, Dent P. The role of cell signalling in the crosstalk between autophagy and apoptosis. Cell Signal. 2014;26:549‐555. 10.1016/j.cellsig.2013.11.028 [DOI] [PMC free article] [PubMed] [Google Scholar]


