Abstract
CSL112 (Apolipoprotein A‐I [human]) is an intravenous preparation of apolipoprotein A‐I (apoA‐I), formulated with phosphatidylcholine (PC) and stabilized with sucrose, in development to prevent early recurrent cardiovascular events following acute myocardial infarction (AMI). This phase 1 study was designed to determine if moderate renal impairment (RI) influenced the pharmacokinetics (PK) and safety of CSL112. Thirty‐two subjects, 16 with moderate RI (estimated glomerular filtration rate [eGFR] ≥ 30 and < 60 mL/min/1.73 m2) and 16 age‐, sex‐, and weight‐matched subjects with normal renal function (eGFR ≥ 90 mL/min/1.73 m2) were randomized 3:1 to receive a single infusion of CSL112 2 g (n = 6) or placebo (n = 2), or CSL112 6 g (n = 6) or placebo (n = 2). PK sampling was at prespecified times from 48 hours prior to 144 hours following infusions, with final safety assessments at 90 days. Renal and hepatic safety, and adverse events (AEs) were monitored throughout the study. Plasma apoA‐I and PC PK profiles were similar between renal function cohorts at both doses. For CSL112 6 g mean ± SD apoA‐I AUC0 ‐ last was 7670 ± 1900 and 9170 ± 2910 mg·h/dL in normal renal function and moderate RI subjects, respectively. Renal apoA‐I clearance was <1% of CSL112 dose. In moderate RI, sucrose clearance was slower; however, approximately 70% was excreted within 48 hours in both renal function cohorts. No CSL112‐related serious AEs or clinically significant renal or hepatic safety changes were observed. Dose adjustment of CSL112 is not required in subjects with moderate RI, supporting its further investigation in AMI patients with moderate RI.
Keywords: pharmacokinetics, atherosclerosis, chronic kidney disease, lipids, randomized controlled trial
CSL112 (Apolipoprotein A‐I [human]) is a novel formulation of intravenous plasma‐derived apolipoprotein A‐I (apoA‐I) formulated with phosphatidylcholine (PC) and stabilized with sucrose. CSL112 is being developed to enhance cholesterol efflux from coronary plaque to reduce the risk of recurrent cardiovascular events in the first weeks and months following an acute myocardial infarction (AMI),1 when the risk of experiencing such events is high.2, 3, 4 Importantly, reduced renal function is known to increase the risk of having a recurrent AMI, and patients with moderate/severe renal impairment (RI) (estimated glomerular filtration rate [eGFR] <40 mL/min/1.73 m2) have been shown to have a significantly higher rate of reinfarction (approximately 3‐fold) than those with an eGFR ≥ 75.0 mL/min/1.73 m.5
The pharmacokinetics (PK), safety, and tolerability of CSL112 in healthy subjects were previously reported in single‐ and multiple‐ascending dose studies.6 In the multiple‐ascending dose study, 36 healthy subjects with normal renal function were randomized to receive CSL112 once weekly (3.4 or 6.8 g) or twice weekly (3.4 g).6 Peak plasma concentrations of apoA‐I were observed by the end of the 2‐hour infusion, followed by a biphasic decline and a dose‐dependent increase in Cmax. No accumulation of apoA‐I or PC was observed with weekly dosing. The PK profile of PC, a formulation component of CSL112, which is used to reconstitute apoA‐I into disc‐shaped particles suitable for intravenous infusion, showed a pattern similar to that of apoA‐I, with Cmax reached by the end of the 2‐hour infusion. Again, similar to apoA‐I, this was followed by a biphasic decline, albeit with a shorter half‐life than apoA‐I. The volume of distribution of CSL112 was found to be slightly higher than total plasma volume, indicating that CSL112 undergoes a degree of distribution into extravascular fluids and tissues. To date, the PK of sucrose following CSL112 administration has not been studied; however, it is known that sucrose undergoes significant renal excretion.7
In a phase 2a single‐ascending dose study, the PK and safety of CSL112 were investigated in subjects with mild RI (eGFR ≥ 60 to < 90 mL/min/1.73 m2) and stable atherosclerotic disease.8 Similar to results observed in the phase 1 study, rapid dose‐dependent increases in serum apoA‐I (up to 244% of baseline levels with the 6.8 g dose) were noted, with no signs of drug‐related renal toxicity detected. Furthermore, a recent phase 2b study of 4 weekly doses of CSL112 in subjects with AMI included 419 patients with mild RI. For both the low (2 g) and high (6 g) doses, PK profiles were similar in subjects with normal renal function and mild RI.9 In addition, both doses were well tolerated, with no significant renal safety changes compared with placebo.10 These findings established that there is no need for dose adjustment in AMI patients with mild RI.
RI is known to be a common comorbidity in cardiovascular disease; therefore, AMI patients with RI will likely constitute a significant proportion of the patient population that may benefit from CSL112. Consequently, preliminary PK and safety profile data for CSL112 in patients with moderate RI are required prior to their inclusion in further clinical studies of CSL112 to determine whether dose adjustment is required in this population. Here we report the findings of a phase 1 PK and safety study in subjects with moderate RI (eGFR ≥ 30 and <60 mL/min/1.73 m2) compared with age‐, weight‐, and sex‐matched subjects with normal renal function (eGFR ≥ 90 mL/min/1.73 m2).
Methods
This study (NCT02427035) was conducted at 4 centers in the European Union: Quintiles Drug Research Unit at Guy's Hospital, London, United Kingdom; CMFT, Manchester, United Kingdom; APEX, Munich, Germany and Charite Research, Berlin, Germany. The protocol was approved by the respective institutional review boards at each study site. Written informed consent was obtained from all subjects, in line with the International Conference on Harmonization Good Clinical Practice guidelines and the Declaration of Helsinki.
Study Design
This was a phase 1, double‐blind, placebo‐controlled single‐ascending dose study, with a 1:1 ratio between subjects with moderate RI and subjects with normal renal function matched for sex, age, and weight. Subjects were randomized 3:1 within a renal function cohort to receive a single 2‐hour intravenous infusion of CSL112 (2 or 6 g) or placebo. The cohort receiving 2 g CSL112 and matched placebo was dosed first; dosing in the 6 g cohort was commenced after review of 2 g data by the Data and Safety Monitoring Board (Figure 1A).
Figure 1.
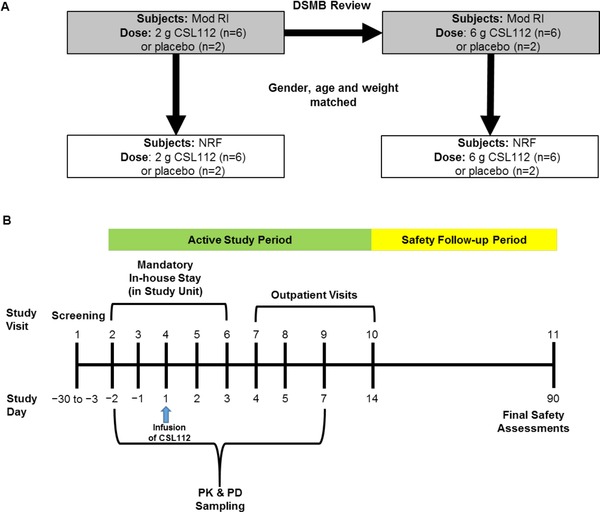
Study design (A) and schedule of visits and assessments (B). Dose escalation to 6 g CSL112 was recommended following Data Safety and Monitoring Board review of data for the 2 g CSL112 dose. DSMB, Data and Safety Monitoring Board; Mod RI, moderate renal impairment; NRF, normal renal function; PD, pharmacodynamics; PK, pharmacokinetics.
The study consisted of a 28‐day screening period, a 16‐day active treatment period that included a mandatory in‐house study unit stay, several outpatient visits, and a 76‐day safety follow‐up period (Figure 1B).
The primary end points were the plasma PK parameters for apoA‐I and PC, and the urinary excretion profile of apoA‐I. Secondary end points included the plasma PK and urinary excretion profile of sucrose and the safety and tolerability of CSL112.
CSL112
CSL112 is an investigational product containing apoA‐I purified from human plasma and formulated with PC, with sucrose as a stabilizing agent.1 CSL112 was administered as a dose of 2 or 6 g. Subjects randomized to receive placebo were given a volume of 0.9% saline matched for each dose of CSL112.
Study Population
Adults with moderate RI, as defined by an eGFR ≥ 30 and <60 mL/min/1.73 m2, and sex‐, age‐, and weight‐matched subjects with normal renal function (eGFR ≥ 90 mL/min/1.73 m2) were enrolled; eGFR was determined using the Chronic Kidney Disease Epidemiology Collaboration (CKD‐EPI) equation.11 The study included male and female subjects aged 18 to 85 years with a body weight of at least 50 kg at screening. Details of the subject‐matching process between renal function cohorts can be found in Supplementary Table 1. Key exclusion criteria were evidence of an unstable or uncontrolled medical condition, disorder, or disease; evidence of hepatobiliary disease; evidence of hemodynamic instability by heart rate or blood pressure criteria; known history of hypersensitivity to soy or peanuts or an IgA deficiency; other severe comorbid condition; concurrent medication or other issue that renders the subject unsuitable for participation in the study (eg, acute renal failure, history of organ transplant, cancer, thrombocytopenia or coagulopathy, HIV infection, pregnancy, and alcohol or substance abuse), and current participation in another clinical trial.
Pharmacokinetics of Apoa‐I, PC, and Sucrose
Blood samples were collected 2 days prior and immediately before dosing and 2, 4, 6, 8, 12, 24, 48, 72, 96, and 144 hours after infusion. Urine sample collection took place over the following time intervals: 24‐20, 20‐16, 16‐12, and 12‐0 hours prior to infusion and 0‐4, 4‐8, 8‐12, 12‐24, 24‐36, and 36‐48 hours postinfusion. ApoA‐I was measured in plasma and urine using immunoturbidometric (Roche/Hitachi Modular P automated clinical chemistry analyzer and K‐Assay Apo AI assay; Kamiya Biomedical Company, Tukwila, Washington) and enzyme‐linked immunosorbent assays (Molecular Devices VersaMaz plate reader and AssayMax Apolipoprotein A‐I ELISA kit; AssayPro, St. Charles, Missouri), respectively. PC was quantified enzymatically in plasma using phospholipase and choline oxidase forming hydrogen peroxide (Wako Phospholipids C assay; Wako Pure Chemical Industries, Osaka, Japan); the resultant blue pigment was measured spectrophotometrically at a primary wavelength of 600 nm and a secondary wavelength of 700 nm (Roche/Hitachi Modular P automated clinical chemistry analyzer; Kamiya Biomedical Company, Tukwila, Washington). Sucrose was measured in plasma and urine using a 2‐step reaction whereby invertase (Invertase from baker's yeast; Fluka, distributed by Sigma Aldrich, St. Louis, Missouri) converted sucrose into fructose and glucose, which was then measured using a hexokinase automated chemistry assay (Roche/Hitachi Modular P automated clinical chemistry analyzer; Kamiya Biomedical Company, Tukwila, Washington).
The following plasma PK parameters for apoA‐I, PC, and sucrose were calculated by noncompartmental PK analysis on unadjusted and baseline‐corrected data using Phoenix WinNonlin, version 6.3: AUC0‐inf, area under the plasma concentration‐time curve from baseline extrapolated to infinity; AUC0‐last, area under the plasma concentration‐time curve from baseline to the last quantifiable point before analyte first returns to baseline; Cmax, observed maximum plasma concentration; Tmax, time to reach Cmax; Vz, volume of distribution during terminal phase after intravenous administration; CL, systemic plasma clearance; and t1/2, terminal half‐life. For urinary excretion of apoA‐I and sucrose, the following parameters were calculated: Ae0‐t, amount excreted over a collection interval 0‐t; %Fe0‐t, percent fraction excreted over time interval 0‐t, calculated as Ae0‐t/dose × 100; CLR, renal clearance calculated as Ae0‐48/AUC0‐48.
Safety
Safety end points were: the occurrence of any adverse event (AE) after the start of the infusion, renal and hepatic safety and tolerability, clinically significant changes in laboratory tests, physical examinations, body weight, vital signs, and immunogenicity.
Statistical Analysis
The study was exploratory and not designed to evaluate formal hypotheses. Sample sizes were deemed sufficient to assess PK and safety. Descriptive analysis of concentration data was performed for plasma apoA‐I, PC, and sucrose.
Results
Study Population
A total of 76 subjects provided written consent and were screened for inclusion in the study. Of these, 32 subjects were enrolled, 16 subjects with moderate RI and 16 subjects with normal renal function. Subjects were randomly assigned within renal function groups to receive CSL112 2 g (n = 12), CSL112 6 g (n = 12), or placebo (n = 8). Safety and tolerability were assessed in all 32 subjects and PK parameters in the 24 subjects randomized to receive CSL112. Study population demographics and baseline characteristics are provided in Table 1. Baseline group mean eGFR values were 100.5 and 49.1 mL/min/1.73 m2 for the normal renal function and moderate RI groups, respectively. Moderate RI subjects were older than those with normal renal function (mean age, 69 vs 55 years) but were well matched for weight (mean weight, 80.5 vs 78 kg).
Table 1.
Study Population Demographics and Baseline Characteristics
| Renal Function/Treatment Group | ||||||
|---|---|---|---|---|---|---|
| Normal Renal Function | Moderate RI | |||||
| CSL112 | CSL112 | CSL112 | CSL112 | |||
| 2 g | 6 g | Placebo | 2 g | 6 g | Placebo | |
| (n = 6) | (n = 6) | (n = 4) | (n = 6) | (n = 6) | (n = 4) | |
| Age, years | 57 ± 5 | 54 ± 10 | 56 ± 5 | 71 ± 8 | 66 ± 12 | 71 ± 5 |
| Sex, n (%) | ||||||
| Male | 5 (83.3) | 3 (50.0) | 3 (75.0) | 5 (83.3) | 4 (66.7) | 2 (50.0) |
| Female | 1 (16.7) | 3 (50.0) | 1 (25.0) | 1 (16.7) | 2 (33.3) | 2 (50.0) |
| Race, n (%) | ||||||
| White | 6 (100) | 6 (100) | 4 (100) | 6 (100) | 6 (100) | 4 (100) |
| Weight, kg | 82.0 ± 7.2 | 74.6 ± 13.4 | 77.2 ± 11.9 | 80.1 ± 11.9 | 72.8 ± 18.6 | 92.7 ± 15.5 |
| eGFR, mL/min/1.73 m2 | 99.1 ± 5.5 | 100.5 ± 6.5 | 102.7 ± 6.9 | 47.6 ± 9.1 | 51.2 ± 6.5 | 48.3 ± 8.6 |
| Plasma apoA‐I, mg/dL | 133 ± 26.3 | 150 ± 12.6 | ND | 147 ± 24.5 | 135 ± 17.8 | ND |
| Plasma PC, mg/dL | 196 ± 31.9 | 206 ± 38.0 | ND | 226 ± 26.1 | 197 ± 27.3 | ND |
| Plasma sucrose, mg/dL | BLQ | 0.7 | ND | BLQ | BLQ | ND |
Values are mean ± standard deviation unless otherwise stated.
ApoA‐I, apolipoprotein A‐I; BLQ, below the limit of quantification; eGFR, estimated glomerular filtration rate; ND, not determined; PC, phosphatidylcholine; RI, renal impairment.
Primary End Points: PK Profiles for Apoa‐I and PC
Mean apoA‐I and PC plasma concentrations were similar between renal function and dose groups at baseline (Table 1). Following infusion of CSL112, mean (unadjusted and baseline corrected [data not shown]) apoA‐I and PC plasma concentration‐time profiles were between in renal function groups (Figure 2). The increase in apoA‐I was dose dependent and peaked at the end of the infusion, with a Tmax of approximately 2 hours for all dose levels and renal function groups. In addition, there were dose‐dependent increases in Cmax, AUC0‐72, and AUC0‐last for both apoA‐I and PC, irrespective of renal function (Table 2). Based on comparison of geometric least‐square means of Cmax and AUC at each dose level, there were no significant differences in plasma exposure to CSL112 (ie, apoA‐I and PC) between renal function groups.
Figure 2.
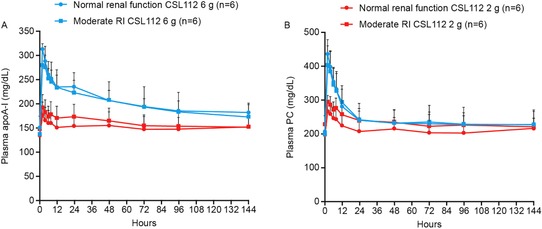
Plasma pharmacokinetic profiles of apolipoprotein A‐I and phosphatidylcholine following CSL112 infusion. Shown are mean + standard deviation unadjusted (A) apolipoprotein A‐I (apoA‐I) and (B) phosphatidylcholine (PC) plasma concentration‐time profiles by renal function group, moderate renal impairment (RI), and normal renal function following infusion of CSL112 2 or 6 g. Please note that the small differences in the 2 g curves are attributable to differences in values at baseline rather than differences in response to CSL112.
Table 2.
Plasma Pharmacokinetic Parameters (Baseline Corrected) for Apolipoprotein A‐I (apoA‐I), Phosphatidylcholine (PC), and Sucrose (Unadjusted) Following Infusion of CSL112
| CSL112 2 g | CSL112 6 g | |||
|---|---|---|---|---|
| Normal Renal Function | Moderate RI | Normal Renal Function | Moderate RI | |
| ApoA‐I | ||||
| Cmax, mg/dL | 42.7 ± 3.50 | 44.0 ± 12.0 | 162 ± 13.0 | 152 ± 24.3 |
| Tmax, h | 2.05 (2.03‐2.10) | 2.00 (2.00‐8.00) | 2.01 (2.00‐2.22) | 2.00 (2.00‐4.00) |
| AUC0‐last, mg·h/dL | 2570 ± 1620 | 2030 ± 862 | 7670 ± 1900 | 9170 ± 2910 |
| AUC0‐72, mg·h/dL | 1550 ± 585 | 1510 ± 219 | 5140 ± 847 | 5890 ± 1560 |
| t1/2, h | ND | 36.8 ± 23.8 | 97.8 ± 59.3 | 91.8 ± 28.0 |
| PC | ||||
| Cmax, mg/dL | 68.5 ± 6.60 | 67.2 ± 15.7 | 228 ± 14.3 | 208 ± 28.9 |
| Tmax, h | 2.05 (2.03‐2.10) | 2.00 (2.00‐8.00) | 2.01 (2.00‐2.22) | 2.00 (2.00‐4.00) |
| AUC0‐last, mg·h/dL | 2230 ± 1610 | 1620 ± 1530 | 5250 ± 1870 | 6400 ± 2950 |
| AUC0‐72, mg·h/dL | 745 ± 107 | 749 ± 164 | 2320 ± 233 | 2420 ± 539 |
| t1/2, h | ND | 28.6 ± 28.9 | ND | ND |
| Sucrose | ||||
| Cmax, mg/dL | 10.0 ± 1.0 | 12.9 ± 1.7 | 35.6 ± 2.6 | 35.7 ± 14.3 |
| Tmax, h | 2.05 (2.03‐2.10) | 2.05 (2.00‐4.00) | 2.01 (2.00‐2.22) | 2.00 (2.00‐8.00) |
| AUC0‐last, mg·h/dL | 29.3 ± 13.3 | 76.5 ± 41.1 | 116 ± 21.2 | 199 ± 157 |
Values are the arithmetic mean ± standard deviation or median (range).
AUC, area under the curve; 0‐last, time zero to the last quantifiable point (following baseline correction); Cmax, maximum concentration in plasma; ND, not determined; RI, renal impairment; Tmax, time to maximal plasma concentration; t1/2, terminal half‐life
Linear regression analysis showed that plasma clearance of apoA‐I was largely unaffected by lower eGFR in subjects with moderate RI (Figure 3). At the CSL112 6 g dose level, it was possible to calculate mean ± standard deviation t1/2 for baseline‐corrected apoA‐I for subjects with moderate RI and for those matched subjects with normal renal function (91.8 ± 28.0 and 97.8 ± 59.3 hours, respectively).
Figure 3.
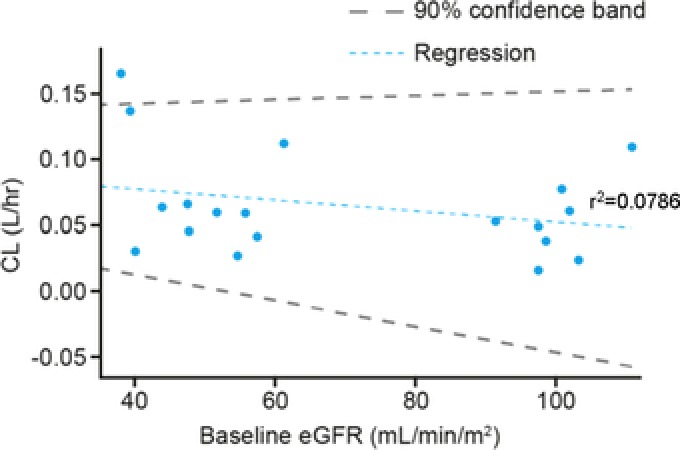
Relationship between plasma clearance and baseline eGFR for apolipoprotein A‐I. Slope (90% confidence band), −0.000417 (−0.0015 to 0.000314). CL, systematic plasma clearance; eGFR, estimated glomerular filtration rate.
The fraction of apoA‐I excreted in the urine 48 hours after infusion of CSL112 was <1% across all dose and renal function cohorts.
PK Profile of Sucrose
At baseline, plasma sucrose levels were below the limit of quantification or negligible in all groups (Table 1). Mean unadjusted Cmax values for sucrose were similar for both renal function groups at each dose level (Table 2). However, mean unadjusted plasma sucrose concentration‐time profiles in subjects with moderate RI showed slower elimination when compared with subjects with normal renal function at both doses (Figure 4). Despite this, levels returned to baseline after 24 hours regardless of renal function (Figure 4).
Figure 4.
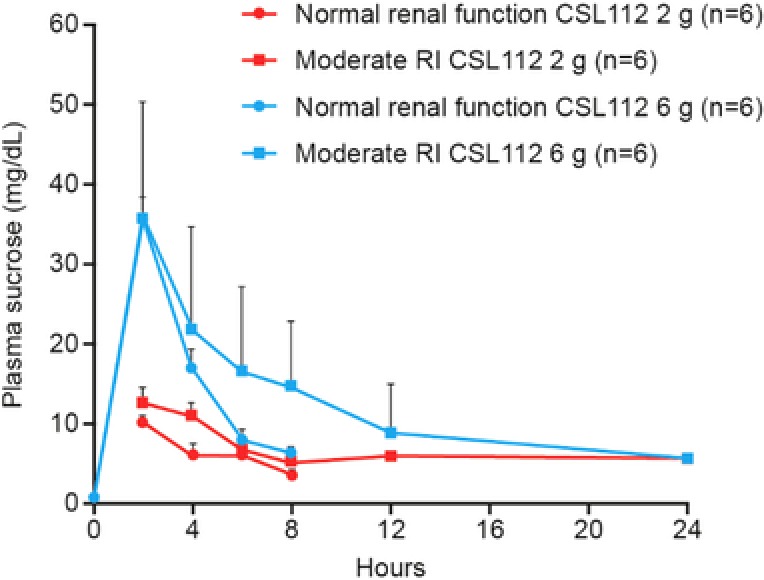
Pharmacokinetic profile of sucrose in plasma following CSL112 infusion. Shown are mean + standard deviation unadjusted sucrose plasma concentration‐time profiles for each renal function group, moderate renal impairment (RI), and normal renal function following infusion of CSL112 2 or 6 g.
There was a dose‐dependent increase in the amount of sucrose in urine in both renal function cohorts. In the first 12 hours after administration of CSL112, 55%‐70% of the infused sucrose was excreted, with 30%‐40% excreted within the first 4 hours (Table 3). At the 6 g CSL112 dose, the time course for excretion of sucrose was slower in subjects with moderate RI; however, after 48 hours of urine collection, the total amount excreted by each group was similar.
Table 3.
Summary of Urinary Excretion Parameters for Sucrose After Infusion of CSL112
| CSL112 2 g | CSL112 6 g | |||
|---|---|---|---|---|
| Normal Renal Function | Moderate RI | Normal Renal Function | Moderate RI | |
| Ae0‐4, g | 1.21 ± 0.371 | 1.01 ± 0.281 | 4.66 ± 0.569 | 2.68 ± 0.630 |
| Ae0‐12, g | 2.13 ± 0.369 | 2.05 ± 0.128 | 6.82 ± 0.419 | 5.60 ± 0.750 |
| Ae0‐48, g | 2.23 ± 0.378 | 2.39 ± 0.178 | 7.08 ± 0.486 | 7.03 ± 0.718 |
| %Fe0‐4 | 36.6 ± 11.3 | 30.5 ± 8.56 | 47.1 ± 5.76 | 27.1 ± 6.34 |
| %Fe0‐12 | 64.6 ± 11.1 | 62.1 ± 3.91 | 68.9 ± 4.23 | 56.6 ± 7.58 |
| %Fe0‐48 | 67.4 ± 11.4 | 72.3 ± 5.40 | 71.5 ± 4.88 | 71.0 ± 7.23 |
Values are the arithmetic mean ± standard deviation.
Ae0‐4, 0‐12, or 0‐48, amount of sucrose excreted over the collection interval 0‐12 or 0‐48 hours; %Fe0‐4, 0‐12, or 0‐48, percent fraction of sucrose excreted in urine over collection interval 0‐12 or 0‐48 hours; RI, renal impairment.
Safety
Adverse Events
Fourteen of 32 subjects (2 of 8 placebo [25%] and 12 of 24 CSL112 [50%]) experienced AEs (Table 4). Overall, fewer subjects with moderate RI than with normal renal function had at least 1 AE: n = 4 versus n = 10, respectively. Rates of AEs were highest at the CSL112 6 g dose for both subjects with moderate RI (3 of 6 subjects [50.0%]) and subjects with normal renal function (5 of 6 subjects [83.3%]). No subjects had serious treatment‐related AEs. No deaths occurred during the study, and no subjects discontinued from the study because of an AE.
Table 4.
Summary of Adverse Events by Renal Function and Treatment Groups
| Number of Subjects (%) | ||||||
|---|---|---|---|---|---|---|
| Normal Renal Function | Moderate RI | |||||
| CSL112 2 g | CSL112 6 g | Placebo | CSL112 2 g | CSL112 6 g | Placebo | |
| (n = 6) | (n = 6) | (n = 4) | (n = 6) | (n = 6) | (n = 4) | |
| Any TEAE | 3 (50.0) | 5 (83.3) | 2 (50.0) | 1 (16.7) | 3 (50.0) | 0 |
| Blood bilirubin increased | 0 | 0 | 0 | 0 | 2 (33.3) | 0 |
| Headache | 0 | 2 (33.3) | 1 (25.0) | 0 | 0 | 0 |
| Serious AE | 0 | 2 (33.3) | 0 | 0 | 0 | 0 |
| Procedure‐related AEs | 1 (16.7) | 2 (33.3) | 1 (25.0) | 1 (16.7) | 0 | 0 |
| Treatment‐related AEs | 0 | 2 (33.3) | 1 (25.0) | 1 (16.7) | 3 (50.0) | 0 |
AE, adverse event; RI, renal impairment; TEAE, treatment‐emergent adverse event.
A total of 7 subjects (4 with moderate RI and 3 with normal renal function) had treatment‐related AEs, which were considered mild or moderate in severity. The most frequently reported AEs, which were more common in subjects dosed with CSL112, were headache, in 2 of 6 patients with normal renal function receiving CSL112 6 g (33.3%), and increased blood bilirubin (<1.5 × the upper limit of normal [ULN] and resolved within 24 hours) in 2 of 6 patients with moderate RI receiving CSL112 6 g (33.3%). There were no subjects with clinically significant elevations in liver transaminases and no subjects with concomitant elevations in total bilirubin and liver transaminases.
Renal and Hepatic Safety
Overall, no clinically meaningful renal safety changes were observed in any of the dose/renal function groups. In all subjects, there were no clinically significant increases in serum creatinine levels, that is, a ≥1.5‐fold increase from baseline values during the treatment period. In addition, there were no clinically significant changes in eGFR.
At baseline, cystatin C values were higher in subjects with moderate RI compared with normal renal function subjects. Following infusion of CSL112, shifts in cystatin C above the ULN occurred in 3 subjects with moderate RI (2 subjects in the 2 g CSL112 dose group and 1 subject in 6 g CSL112 dose group). Furthermore, over 24 hours, absolute mean changes in cystatin C were similar between renal function groups. There were also transient dose‐dependent increases in urinary cystatin C/creatinine ratios following infusion of CSL112 in all renal function groups, with maximal ratios observed at the 0‐ to 4‐hour interval (Supplementary Table 2); however, this was not related to changes in eGFR (Supplementary Figure 1).
Urine KIM‐1/creatinine ratios were higher in the group of patients with moderate RI receiving CSL112 6 g; however, this was because of 2 individuals with higher baseline values. Ratios were higher across each interval in these individuals but remained stable. Overall, KIM‐1/creatinine ratios were similar across renal function and dose groups (Supplementary Table 2).
Total bilirubin, alanine aminotransferase (ALT), aspartate aminotransferase (AST), and alkaline phosphatase were measured to assess hepatic safety, and no clinically meaningful changes in hepatic function were observed. There were no potential Hy's Law cases (ie, concomitant elevation of ALT/AST and total bilirubin). One subject receiving CSL112 6 g in the normal renal function group had a postbaseline increase in total bilirubin of ˃1.5 to ≤2.0 × ULN at study visit 5 (day 2); however, bilirubin levels were within the normal range at study visit 8 (day 5) — this event was not reported as an AE.
Other Safety Measures
No clinically meaningful changes in other clinical laboratory tests, physical examinations, body weight, or vital signs were observed. No subjects developed anti‐apoA‐I or anti‐CSL112 antibodies.
Discussion
The aim of the present investigation was to determine the PK profile, safety, and tolerability of CSL112 in subjects with moderate RI. The primary outcomes of interest were the PK of apoA‐I and PC. We found that following a single infusion of CSL112, PK parameters for apoA‐I and PC increased in a dose‐dependent manner, and there were no significant differences in CSL112 plasma exposure (Cmax and AUC) between subjects with normal renal function and subjects with moderate RI. As a secondary outcome, the PK profile of sucrose was assessed. As expected, plasma sucrose had a slower elimination in subjects with moderate RI when compared with subjects with normal renal function at both doses. However, despite this, approximately 70% of sucrose was excreted in the 48 hours postinfusion in both renal function groups. In addition, results of the safety analysis showed that CSL112 was well tolerated in both treatment groups; no clinically significant renal function changes were observed, and there were no serious treatment‐related AEs.
In this study, infusion of CSL112 resulted in elevations of plasma apoA‐I and PC up to the end of the 2‐hour infusion, with subsequent biphasic decline, consistent with previous studies.6, 12 In a phase 2a study, subjects with mild RI and stable atherosclerosis had slightly increased apoA‐I and PC exposure compared with subjects with normal renal function.8 This is believed to be because of the difference in weight observed between renal function groups — body weight was included as a covariate in a previously conducted population PK model of CSL112 and was found to have a significant impact on clearance of apoA‐I.13 After accounting for the differences in weight, renal function was not found to be a significant covariate in the clearance of apoA‐I.13 In contrast, in the present study, groups were well matched by mean weight, and no differences in apoA‐I and PC exposure were observed between renal function cohorts.
We observed that <1% of administered apoA‐I was excreted in urine as intact apoA‐I. However, this finding does not rule out substantial renal filtration and metabolism of apoA‐I.14 The proximal tubules in the kidney express cubilin, a membrane protein that binds apoA‐I in the ultrafiltrate and salvages the filtered apoA‐I.15 Animals15 and humans16 with a cubilin deficiency show high levels of intact apoA‐I in the urine. Our finding of low apoA‐I in the urine thus indicates that the ability of the proximal tubule to reabsorb apoA‐I via the cubilin pathway is preserved in moderate RI.
A further consideration is the renal handling of sucrose. It is known that sucrose in plasma, in contrast to glucose, is subject to limited tubular reabsorption in the kidney and therefore undergoes significant renal excretion.7 In line with this, in the present study, it was observed that following CSL112 infusion, the majority of the administered sucrose was rapidly cleared from the blood via glomerular filtration. Therefore, it was not unexpected that sucrose had a longer plasma elimination time in moderate RI subjects compared with subjects with normal renal function. However, importantly, the overall amount of sucrose excreted over 48 hours was similar to that in those with normal renal function and there was no indication of any renal toxicity.
The safety results of this study show that moderate RI did not influence the incidence or severity of AEs, and no clinically significant renal or hepatic function changes were observed. These findings are consistent with those from a previous single‐dose study in stable atherosclerotic disease patients with mild RI.8 The present results also add to the favorable safety profile of CSL112; multiple doses in healthy subjects and in mild RI patients post‐AMI have been shown to be safe and well tolerated.6, 10
In conclusion, moderate RI does not impact the PK or safety profile of CSL112 (Apolipoprotein A‐I [human]), and a dose adjustment is not required. Therefore, this study supports the dose selection of CSL112 6 g for further clinical trials in patients who experience an AMI, including those with moderate RI.
Supporting information
Supplementary Table 1. Subject‐Matching Process Between Renal Function Cohorts
Supplementary Table 2. Urine Biomarkers of Renal Safety
Supplementary Figure 1. Serum cystatin C versus eGFR during the active treatment period.
Acknowledgments
The authors thank the participants of the study in addition to the contract research organization (QuintilesIMS, Reading, UK), who assisted with study coordination, monitoring, data management, and biostatistics. The authors also thank CSL Limited Central Laboratory Services (Parkville, Australia) for providing clinical/analytical support and ICON Laboratory Services, Inc. (Dublin, Ireland) and Pacific Biomarkers (Seattle, Washington) for conducting the PK bioanalyses. Timothy Mant is supported by the National Institute for Health Research Biomedical Research Centre at Guy's and St Thomas’ National Health Service Foundation Trust and King's College London.
Declaration of Conflicting Interests
M.A.T., D.D., R.E., J.F., S.D.W., and A.G. are employees of CSL Behring/CSL Limited. D.D. was an employee of CSL Behring at the time of the study. T.G.K.M. is an employee and shareholder of Quintiles Limited (now IQIVA). Medical writing assistance was provided by Steven Foster and Kate Holliday of Meridian HealthComms, Plumley, funded by CSL Behring, in accordance with Good Publication Practice (GPP3).
Funding
The clinical trial described here (NCT02427035) was funded by CSL Behring. Individual participant data will not be shared.
Author Contributions
All authors contributed substantially to the design of the study and/or assisted with the data analysis and with interpretation of the data, assisted in the preparation of the manuscript, reviewed the manuscript, provided their approval for submission, and agree to be accountable for all aspects of the work presented.
References
- 1. Diditchenko S, Gille A, Pragst I, et al. Novel formulation of a reconstituted high‐density lipoprotein (CSL112) dramatically enhances ABCA1‐dependent cholesterol efflux. Arterioscler Thromb Vasc Biol. 2013;33(9):2202–2211. [DOI] [PubMed] [Google Scholar]
- 2. Dharmarajan K, Hsieh AF, Kulkarni VT, et al. Trajectories of risk after hospitalization for heart failure, acute myocardial infarction, or pneumonia: retrospective cohort study. BMJ. 2015;350:h411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Jernberg T, Hasvold P, Henriksson M, Hjelm H, Thuresson M, Janzon M. Cardiovascular risk in post‐myocardial infarction patients: nationwide real world data demonstrate the importance of a long‐term perspective. Eur Heart J. 2015;36(19):1163–1170. [DOI] [PubMed] [Google Scholar]
- 4. Smolina K, Wright FL, Rayner M, Goldacre MJ. Long‐term survival and recurrence after acute myocardial infarction in England, 2004 to 2010. Circ Cardiovasc Qual Outcomes. 2012;5(4):532–540. [DOI] [PubMed] [Google Scholar]
- 5. Anavekar NS, McMurray JJ, Velazquez EJ, et al. Relation between renal dysfunction and cardiovascular outcomes after myocardial infarction. N Engl J Med. 2004;351(13):1285–1295. [DOI] [PubMed] [Google Scholar]
- 6. Easton R, Gille A, D'Andrea D, Davis R, Wright SD, Shear C. A multiple ascending dose study of CSL112, an infused formulation of ApoA‐I. J Clin Pharmacol. 2014;54(3):301–310. [DOI] [PubMed] [Google Scholar]
- 7. Steinitz K. The renal excretion of sucrose in normal man; comparison with inulin. Am J Physiol 1940;129(2):252–258. [Google Scholar]
- 8. Tricoci P, D'Andrea DM, Gurbel PA, et al. Infusion of reconstituted high‐density lipoprotein, CSL112, in patients with atherosclerosis: safety and pharmacokinetic results from a phase 2a randomized clinical trial. J Am Heart Assoc. 2015;4(8):e002171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Tortorici MA, Gille A, Liss C, et al. Direct augmentation of cholesterol efflux capacity in AMI patients: a PKPD substudy of AEGIS‐I. Eur Heart J. 2017;38(suppl 1):P1106. [Google Scholar]
- 10. Gibson CM, Korjian S, Tricoci P, et al. Safety and tolerability of CSL112, a reconstituted, infusible, plasma‐derived apolipoprotein A‐I, after acute myocardial infarction: the AEGIS‐I Trial (ApoA‐I Event Reducing in Ischemic Syndromes I). Circulation. 2016;134(24):1918–1930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Levey AS, Stevens LA, Schmid CH, et al. A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009;150(9):604–612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Gille A, Easton R, D'Andrea D, Wright SD, Shear CL. CSL112 enhances biomarkers of reverse cholesterol transport after single and multiple infusions in healthy subjects. Arterioscler Thromb Vasc Biol. 2014;34(9):2106–2114. [DOI] [PubMed] [Google Scholar]
- 13. Yao Z, Pfister M, Comisar C, et al. ApoA‐I enhancement, by CSL112 administration, leads to a linear increase in total cholesterol efflux capacity. Poster presented at the American Conference of Pharmacometrics (ACoP). 2014.
- 14. Glass C, Pittman RC, Weinstein DB, Steinberg D. Dissociation of tissue uptake of cholesterol ester from that of apoprotein A‐I of rat plasma high density lipoprotein: selective delivery of cholesterol ester to liver, adrenal, and gonad. Proc Natl Acad Sci U S A. 1983;80(17):5435–5439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Aseem O, Smith BT, Cooley MA, et al. Cubilin maintains blood levels of HDL and albumin. J Am Soc Nephrol. 2014;25(5):1028–1036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Storm T, Emma F, Verroust PJ, Hertz JM, Nielsen R, Christensen EI. A patient with cubilin deficiency. N Engl J Med. 2011;364(1):89–91. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Table 1. Subject‐Matching Process Between Renal Function Cohorts
Supplementary Table 2. Urine Biomarkers of Renal Safety
Supplementary Figure 1. Serum cystatin C versus eGFR during the active treatment period.


