Abstract
Amyloid-β (Aβ), major constituent of senile plaques in Alzheimer's disease (AD), is generated by proteolytic processing of the amyloid precursor protein (APP) by β- and γ-secretase. Several lipids, especially cholesterol, are associated with AD. Phytosterols are naturally occurring cholesterol plant equivalents, recently been shown to cross the blood–brain-barrier accumulating in brain. Here, we investigated the effect of the most nutritional prevalent phytosterols and cholesterol on APP processing. In general, phytosterols are less amyloidogenic than cholesterol. However, only one phytosterol, stigmasterol, reduced Aβ generation by (1) directly decreasing β-secretase activity, (2) reducing expression of all γ-secretase components, (3) reducing cholesterol and presenilin distribution in lipid rafts implicated in amyloidogenic APP cleavage, and by (4) decreasing BACE1 internalization to endosomal compartments, involved in APP β-secretase cleavage. Mice fed with stigmasterol-enriched diets confirmed protective effects in vivo, suggesting that dietary intake of phytosterol blends mainly containing stigmasterol might be beneficial in preventing AD.
Introduction
Alzheimer's disease (AD) is the most prevalent progressive neurodegenerative disorder in the elderly, pathologically characterized by extracellular deposition of amyloid-β (Aβ) in the brain (Glenner and Wong, 1984). Aβ peptides are released by sequential proteolytic cleavage of the amyloid precursor protein (APP), a large type I transmembrane protein (Sisodia and St George-Hyslop, 2002). For the generation of Aβ peptides APP is first cleaved within its ectodomain by β-secretase BACE1, generating β-secreted APP (sAPPβ) and a C-terminal membrane-tethered fragment (β-CTF) consisting of the Aβ domain and a short cytoplasmic tail (Sinha et al., 1999; Vassar et al., 1999). β-CTF is subsequently cleaved within its transmembrane domain by γ-secretase, releasing Aβ peptides. γ-secretase has been identified as a heterotetrameric protein complex and consists of at least four proteins: Presenilin 1 (PS1) or presenilin 2 (PS2), which constitute the active site of the protease, nicastrin (NCSTN), anterior pharynx-defective 1 (APH1A and APH1B), and presenilin enhancer 2 (PEN2) (De Strooper, 2003; Kimberly et al., 2003).
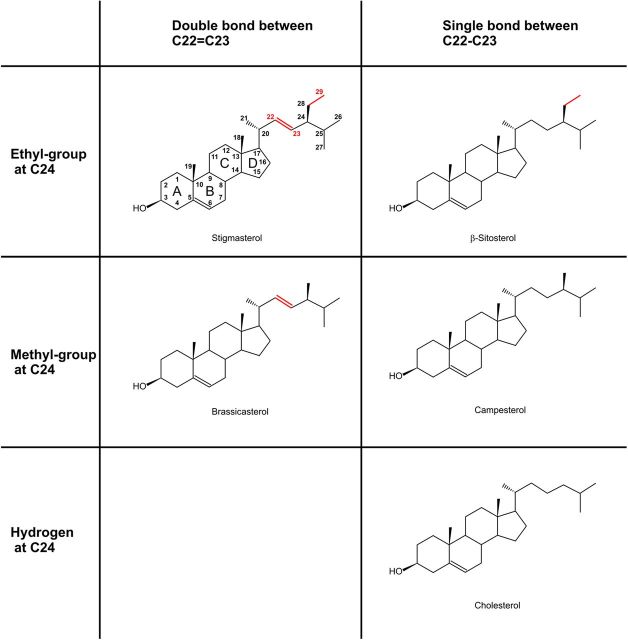
As γ-secretase has peculiar intramembrane proteolysis activity and all APP cleaving proteases and APP itself are integral membrane proteins, alterations in the lipid composition of cellular membranes are discussed to influence proteolytic processing of APP (Grimm et al., 2005, 2011b, 2012; Osenkowski et al., 2008; Lemkul and Bevan, 2011). However, previous studies mainly focused on cholesterol, which has been associated with AD pathogenesis (Corder et al., 1993; Kivipelto et al., 2001; Wolozin, 2001; Puglielli et al., 2003). High cholesterol level have been shown to increase Aβ generation whereas suppression of cholesterol de novo synthesis strongly reduces Aβ release in vivo and in vitro (Fassbender et al., 2001; Refolo et al., 2001; Shie et al., 2002; Ghribi et al., 2006). Cholesterol is an essential component of cellular membranes and is highly enriched in detergent-resistant membrane microdomains, called lipid rafts (Simons and Ikonen, 1997), which have been implicated in amyloidogenic APP processing (Ehehalt et al., 2003; Vetrivel and Thinakaran, 2010). Phytosterols are the naturally occurring cholesterol-equivalents in plants and are contained in dietary sources like nuts, seeds, legumes, and unrefined plant oils (Weihrauch and Gardner, 1978; Piironen et al., 2000). Plant sterols reduce intestinal resorption of cholesterol and are able to cross the blood–brain barrier and accumulate in the brain (Vanmierlo et al., 2012). They have a structure very similar to cholesterol with some modifications in the acyl chain, containing an additional double bond at C-atom C22–C23 (Δ22) or an alkyl-group (methyl- or ethyl-group) at C24 (Fig. 1). Due to their structural similarity to cholesterol, plant sterols may interfere with cholesterol-dependent cellular processes in the brain. Furthermore, plant sterols have also been shown to be organized in lipid rafts within plant cell membranes (Mongrand et al., 2004, 2010) and might therefore alter amyloidogenic processing of APP. In the present study we investigated the influence of the most prevalent plant sterols—stigmasterol, brassicasterol, β-sitosterol, and campesterol (Gunstone et al., 1994; Dufourc, 2008)—and cholesterol on the amyloidogenic proteolytic cleavage of APP.
Figure 1.
Chemical structure of stigmasterol, brassicasterol, β-sitosterol, campesterol, and cholesterol.
Materials and Methods
Chemical reagents and sterols
Unless otherwise indicated, all chemical reagents and sterols were obtained from Sigma Aldrich and were from the highest commercially available purity.
Animals
Eighteen male, nine-week-old C57BL/6J mice were obtained from Charles River. The animals were kept in groups of six animals per cage. The environmental parameters were adjusted to a temperature at 20−22°C, humidity at 50–60%, and lights on for 12 h every day. Water and food were available ad libitum throughout this study.
All experiments were performed in accordance with Dutch laws governing the use of laboratory animals.
The diets were AIN-93M-based (Reeves et al., 1993) and obtained from Research Diet Services. Total fat-content was adjusted to 5% in all diets. Animals were fed 4–6 weeks with one of three isocaloric diets. Next to the control diet, stigmasterol-enriched diets containing 0.19, 0.25, or 0.39% of stigmasterol (CarboMer) were administered.
After administration of the diet, animals were killed by CO2 gas inhalation and decapitation by guillotine. Brains were quickly removed from the scull, snapfrozen in liquid nitrogen, and stored at −80°C until analysis.
Brains were homogenized either in 2 ml of aqua destillata or 4× volume of lysis buffer (50 mm Tris/HCl pH7.4, 1% NP-40, 0.25% sodium-desoxycholat, 150 mm NaCl, 1 mm EDTA, 1× protease inhibitor cocktail containing AEBSF, Calbiochem) using a Teflon homogenizer.
Cell culture and sterol treatment
Human neuroblastoma SH-SY5Y wt cells and SH-SY5Y cells, stably transfected with human APP695 or SPC99 (Grimm et al., 2003), were cultivated as described previously (Grimm et al., 2011b). Cells were cultured until they attained confluence. Sixteen hours before sterol treatment, serum in the culture medium was reduced (0.1% FBS). Plant sterols and cholesterol were used at a final concentration of 10 μm and were incubated for 8 h + 16 h in serum-reduced culture medium containing 0.1% (w/v) fatty acid free BSA. In controls, solvent ethanol was adjusted to 1‰, according to solvent content in sterol-treated cells.
Antibodies
For Western blot (WB) analysis, the following antibodies were used: anti-presenilin1 sc7860, 1:500 (Santa Cruz Biotechnology), human anti-BACE1 B0806, 1: 1000 (Sigma Aldrich), mouse anti-BACE PC529, 1:1000 (Calbiochem), anti-flotillin 610821, 1:250 (BD Biosciences), anti-cadherin ab6528, 1:1000 (Abcam), anti-actin ab1801, 1:2000 (Abcam), anti-EEA1 ab2900, 1:1000 (Abcam), anti-APP/anti-βCTF/anti-Aβ W02, 1 μg/ml (Ida et al., 1996) As secondary antibodies, anti-rabbit-HRP W401B, 1:5000 (Promega) and anti-mouse-HRP 1:5000 (DAKO) were used. Proteins were detected using ECL-method. Data are presented as percentage of density compared with solvent control. Densiometric quantification was performed with Image Gauge software. As control for the determination of BACE1 and PS1 protein level in presence of plant sterols or cholesterol, we analyzed protein level of actin, which was unchanged in presence of sterols (Fig. 2E).
Figure 2.
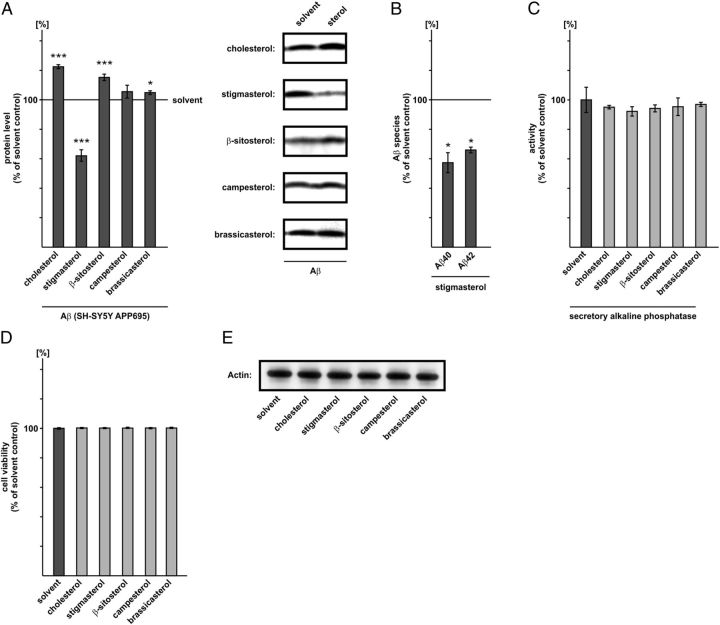
Effect of plant sterols and cholesterol on Aβ secretion. A, Human neuroblastoma SH-SY5Y cells stably transfected with APP695 were incubated with stigmasterol, β-sitosterol, campesterol, brassicasterol, or cholesterol (final concentration, 10 μm) and solvent control. Immunoprecipitation of secreted Aβ and WB analysis was performed with antibody W02. Representative WBs are shown. Error bars represent SD of the mean. B, Determination of Aβ40 and Aβ42 peptides in stigmasterol-treated APP695 transfected SH-SY5Y cells using ELISA technique. C, Determination of SEAP activity in presence of sterols (10 μm) in SH-SY5Y cells transiently transfected with SEAP. D, Lactate dehydrogenase assay in cells incubated with cholesterol, stigmasterol, β-sitosterol, campesterol, and brassicasterol in a final concentration of 10 μm compared with solvent control. No signs for increased cytotoxicity were observed in cells treated with sterols. Asterisks show the statistical significance compared with control (*p ≤ 0.05; **p ≤ 0.01; ***p ≤ 0.001). Quantified data represent an average of at least three independent experiments (n ≥ 3). E, Representative WB analysis of actin protein level in SH-SY5Y cells incubated with plant sterols and cholesterol (final concentration of 10 μm).
Constructs and transient transfection of secreted alkaline phosphatase
For transient transfection of SH-SY5Y wt cells, expression vector pVectOZ-secreted alkaline phosphatase (SEAP; OZ Biosciences) was used at 1 μg/transfection. Lipofectamine 2000 (Invitrogen) was used as transfection reagent at 1.5 μl/transfection. Briefly, cells were washed once with OptiMEM + GlutaMAX serum-reduced medium (Invitrogen), and DNA/Lipofectamine complexes were incubated for 5 h in OptiMEM + GlutaMAX. After transfection, medium was removed and cells were cultured in growth medium (DMEM, 10% FBS, 1× MEM) for 48 h. Subsequently, cells were treated with plant sterols like described before.
SEAP-assay
Culture media were collected after plant sterol treatment of SEAP-transfected SH-SY5Y cells. Samples were centrifuged at 3000 rpm at 4°C and boiled at 65°C for 10 min, and 100 μl sample was analyzed in a 96-well plate after adding 100 μl of 1-Step PNPP solution (Thermo Scientific) by monitoring OD405 nm. SEAP expression was shown to be detectable at least for 96 h after transfection in SH-SY5Y cells. To exclude variations in SEAP secretion due to heterogeneous transfection, SEAP secretion was also monitored before plant sterol treatment of the cells.
Determination of protein concentration
Protein determination was performed according to Smith et al. (1985).
Detection of secreted Aβ
Generation of conditioned media and detection of secreted Aβ of sterol-treated cells was performed as described previously (Grimm et al., 2003).
Determination of Aβ40 and Aβ42 by ELISA
Levels of soluble Aβ in brains of stigmasterol-fed mice were determined using a commercially available ELISA Kit (Mouse Aβ ELISA Kit, Invitrogen) according to manufacturer's protocol. Mouse brains were weighted and homogenized in 4× volume of lysis buffer (50 mm Tris/HCl pH7.4, 1% NP-40, 0.25% sodium-desoxycholat, 150 mm NaCl, 1 mm EDTA, 1× protease inhibitor cocktail containing AEBSF, Calbiochem). Homogenates were incubated on ice for 15 min and centrifuged for 10 min at 3000 rpm at 4°C. Supernatant was removed and pellet was solubilized in lysis buffer containing protease inhibitors using a 24 gauge × 1” needle. Samples were incubated on ice for 90 min, followed by a centrifugation at 13,000 rpm at 4°C for 20 min. Supernatants, containing soluble Aβ peptides, were transferred to a new tube and protein concentration was determined as described. Samples were diluted 1:2 in standard dilution buffer (kit content) and 225 μg/100 μl was used for each assay. Aβ in mouse brains was calculated at means of a serial dilution of mouse synthetic peptide and were presented as pg/mg wet weight tissue.
Level of Aβ40 and Aβ42 in cell culture supernatant of stigmasterol-incubated SH-SY5Y cells stably expressing human APP695 were determined using commercially available ELISA Kits (Human Aβ ELISA Kits KHB3481/KHB3544, Invitrogen) according to manufacturer's protocol. Samples were diluted in standard dilution buffer and the linear range of the ELISA was verified by a serial dilution of synthetic peptides.
Preparation of cell lysates
After washing cells three times with ice-cold PBS, cells were scraped off in PBS, containing complete protease inhibitor cocktail (Roche). Cells were centrifuged at 13,000 rpm for 10 min at 4°C. Supernatant was removed and cells were lysed with 75 μl of lysis buffer (50 mm Tris/HCl pH7.4, 1% NP-40, 0.25% sodium-desoxycholat, 150 mm NaCl, 1 mm EDTA, complete protease inhibitor cocktail) and solubilized using a 23× gauge needle. Samples were centrifuged again at 13,000 rpm for 10 min at 4°C and supernatants were used for further analysis. Protein concentrations were determined as described. Samples were adjusted to equal protein amounts (60 μg) and were used for the analysis of BACE1 and PS1 protein levels.
In vitro and ex vivo determination of secretase activities
Activities of β- and γ-secretase were measured either in vitro in postnuclear fractions (PNFs) of SH-SY5Y wt cells or ex vivo in brain PNFs of C57BL/6 mice in presence of plant sterols, cholesterol, or solvent control in at least four independent experiments (implying independent sterol-treatments/PNF, each analyzed in triplicate, n ≥ 4). Additionally, β- and γ-secretase activities were determined in brain homogenates of six stigmasterol-fed mice, compared with six mice, fed with control diet (n = 6).
Preparation of postnuclear fractions
Confluent SH-SY5Y wt cells were cultured for 48 h in incubation medium (DMEM, 0.1% FBS, 1% MEM). Medium was changed after 24 h. Briefly, cells were harvested and cells or mouse brains were homogenized in EDTA-sucrose buffer (10 mm Tris-HCl pH7.4, 1 mm EDTA, 200 mm sucrose). Samples were adjusted to a protein concentration of 2 mg/ml and centrifuged at 900 rpm for 10 min at 4°C. Postnuclear supernatants were transferred in a new tube and stored at −80°C until analysis.
Treatment of postnuclear fractions with plant sterols/cholesterol
Postnuclear fractions (PNFs) of SH-SY5Y cells or C57BL/6 mouse brains were transferred in a glass tube and plant sterols or cholesterol were added to a final concentration of 50 nMol/mg protein. All samples contained equal solvent concentrations (1% ethanol). Samples were incubated for 15 min at 37°C in a Heidolph Multi Reax Shaker (Heidolph Instruments GmbH and Co. KG) at maximum speed.
Preparations of total membranes
After incubation of PNFs, total membranes were prepared by centrifugation at 55,000 rpm for 75 min at 4°C in an Optima MAX Ultracentrifuge using a TLA-55 rotor (Beckmann Coulter). The resulting pellet was resuspended in the corresponding EDTA-sucrose buffer. In all cases, the pellet was resolubilized by passing the sample through needles with decreasing diameters (0.6 mm, 0.4 mm, 0.33 mm) (BD).
Measurement of secretase activities
Activities of either β- or γ-secretase were determined in a black 96-well plate (BD) by continuous measurement of the cleavage of a specific substrate in a Safire2 Fluorometer (Tecan), resulting in an increasing fluorescent signal. For the assay of γ-secretase, 100 μl of sample was used, corresponding to 250 μg of total protein for SH-SY5Y wt cells and 500 μg for mouse brain homogenates. γ-secretase substrate (Calbiochem) was added to a final concentration of 10 μm. Measurement parameters were adjusted to an excitation wavelength of 355 nm (bandwidth 10 nm) and an emission wavelength of 440 nm (bandwidth 10 nm). Kinetic measurement was performed for 50 cycles with intervals of 180 s. For the determination of γ-secretase activity in lipid rafts and nonraft gradient fractions, 100 μl of each fraction was used.
For determination of β-secretase activity, samples were diluted 1:1 in PBS, pH4.5, and 100 μl of this dilution was used for the assay, corresponding to 125 μg of total protein for SH-SY5Y wt cells and 250 μg for mouse brain homogenates. The β-secretase substrate IV (Calbiochem) was used at a final concentration of 20 μm. Measurement parameters were adjusted to an excitation wavelength of 345 nm (bandwidth 5 nm) and an emission wavelength of 500 nm (bandwidth 10 nm). Kinetic was performed for 180 cycles with intervals of 60 s.
Quantitative real-time PCR
Total RNA extraction and real-time PCR (RT-PCR) was performed as described previously (Grimm et al., 2011a). The following primer sequences were used:
Aph1a: 5′-CAG CCA TTA TCC TGC TCC AT-3′ and 5′-GGA ATG TCA GTC CCG ATG TC-3′; Aph1b: 5′-GTG TCA GCC CAG ACC TTC AT-3′ and 5′-CAG GCA GAG TTT CAG GCT TC-3′; Bace1: 5′-AAT ACC TGC GGT GGA AGA TG-3′ and 5′-GCC CTC CAT GAT AAC AGC TC-3′; Ncstn: 5′-CTG TAC GGA ACC AGG TGG AG-3′ and 5′-GAG AGG CTG GGA CTG ATT TG-3′; Psen1: 5′-CTC AAT TCT GAA TGC TGC CA-3′ and 5′-GGC ATG GAT GAC CTT ATA GCA-3′; Psen2: 5′-GAT CAG CGT CAT CGT GGT TA-3′ and 5′-GGA ACA GCA GCA TCA GTG AA-3′; Psenen2: 5′-CAT CTT CTG GTT CTT CCG AGA G-3′ and 5′-AGA AGA GGA AGC CCA CAG C-3′; β-actin: 5′-CTT CCT GGG CAT GGA GTC-3′ and 5′-AGC ACT GTG TTG GCG TAC AG-3′.
Lipid raft preparation
Lipid rafts were prepared as described previously using Triton X-100 and ultracentrifugation method (Grimm et al., 2011b).
Cholesterol distribution in lipid rafts
Confluent SH-SY5Y wt cells were washed two times with incubation medium (DMEM, 0.1% FBS, 1% MEM) and preincubated for 4 h under serum-reduced conditions. After this, cells were pulsed for 16 h with 0.4 μCi/ml [14C]-cholesterol (45–60 mCi/mmol, PerkinElmer) in incubation medium. Subsequent to cholesterol treatment, cells were incubated with plant sterols like described before. Lipid rafts were prepared as described previously (Grimm et al., 2011b) and distribution of incorporated [14C]-cholesterol in collected fractions was determined via liquid scintillation counting in a Tri-Carb2800TR (PerkinElmer).
Preparation of endosomal fractions
Endosomal fractions were prepared with OptiPrep (Iodixanol; Axis-Shield) density gradient centrifugation according to Woods et al. (2004) with minor modifications. After incubation with stigmasterol, cells were washed with ice-cold PBS and homogenized in sucrose buffer containing 140 mm NaCl, 1 mm EDTA, and 20 mm Tris/HCl, pH 8,0, using a PotterS (Braun). For WB analysis of APP, Bace1 and β-CTF in endosomal fractions sucrose buffer additionally contains complete protease inhibitor cocktail. Cell debris and nuclei were removed by centrifugation at 800 × g for 5 min. Gradient solutions with 10, 20, 30, and 40% iodixanol were made by diluting 60% Iodixanol stock solution with dilution buffer containing 250 mm sucrose, 140 mm NaCl, 3 mm EDTA, and 60 mm Tris/HCl, pH 8,0. A discontinuous 10–40% density gradient was prepared with 2.5 ml layers of each solution in 13.2 ml ultracentrifugation tubes (Beckman Coulter). Tubes were turned in a horizontal position for 1 h at room temperature (RT), resulting in a continuous gradient. One milliliter of postnuclear fraction containing 5 mg of protein was layered on top of each gradient and gradients were centrifuged for 18 h at 48,000 × g at 4°C in a swinging-bucket rotor (SW41, Beckman Coulter). Gradients were unloaded from the top collecting 20 fractions à 550 μl. Endosomal fractions were identified by WB with anti-EEA1 antibody (ab2900, Abcam). Early endosomal antigen 1 (EEA1)-positive fractions were pooled for further analyzing β-secretase activity, BACE1, and β-CTF protein levels.
Cell surface biotinylation
Cells were cultivated until confluence on 10 cm cell culture dishes. After incubation with stigmasterol, cells were washed three times with ice-cold PBS containing 1 mm MgCl2 and 0.1 mm CaCl2 (PBS/CM), scraped off, and incubated in PBS/CM supplemented with 0.5 mg/ml EZ-Link Sulfo-NHS-LC-Biotin (Pierce) for 30 min at 4°C with rotating. Cells were washed once with PBS/CM and biotinylation was quenched with a 100 mm glycine/PBS wash, followed by three washes with PBS/CM. After lysis, samples were centrifuged with 14,500 rpm for 15 min at 4°C and biotinylated proteins were immunoprecipitated with 100 μl of 50% streptavidin agarose (Pierce) overnight at 4°C. After washing three times with lysis buffer, proteins were eluted by boiling for 5 min in 3× SDS sample buffer (187.5 mm Tris/HCl pH6.8, 6% SDS, 30% glycerol, 15% β-mercaptoethanol, 0.03% bromphenol blue) and analyzed by WB as described above.
Determination of cholesterol and plant sterol uptake in SH-SY5Y cells and membrane microdomains after treatment
Sample preparation.
Cells were harvested and homogenized by ultrasound treatment (30 s, 50% intensity) and adjusted to 175 μg/20 μl aqua destillata. For sterol-determination in membrane microdomains, fractions 6–9 (raft) and fractions 12–16 (nonraft) were pooled. Lipids were extracted using a modified method according to Bligh and Dyer (1959).
Sterol extraction.
As internal standard, cholesterol (D7, 5 μg/sample; Avanti Polar Lipids) was added. One-hundred microliters of dry 1,4-dioxane was added to each sample. The samples were mixed, sonicated for 10 s, and dried in a vacuum concentrator. For sterol extraction, samples were resolubilized in 50 μl of 1,4-dioxane, sonicated, and centrifuged at 20,800 × g for 20 min at 15°C. Resulting supernatants were transferred in new microcentrifuge tubes and dried in a vacuum concentrator.
Derivatization.
Sterols were derivatized by a method from the study by Duff (1949). Briefly, 20 μl of a freshly prepared sulfur trioxide pyridine complex solution (25 mg of sulfur trioxide pyridine complex in 5 ml of pyridine) was added to the samples and sonicated for 10 s. The derivatization reaction was performed for 30 min at RT, followed by addition of 2.1 μl of barium acetate solution (314.1 mm). The samples were sonicated for 10 s and incubated for 10 min at RT followed by 60 min at 4°C. Finally, 120 μl of methanol was added and samples were centrifuged at 20,800 × g for 10 min at 15°C. Supernatants were transferred into a 96-deep well plate (Nunc), 600 μl of methanol was added to each sample, and sterols were analyzed by mass spectrometry.
Mass spectrometry.
Mass spectrometry analyses were performed with a 4000 quadrupole linear-ion trap (QTrap) with a Turbo Spray ion source (AB SCIEX) connected to a 1200 Agilent HPLC (Agilent). Twenty microliters of each sample was injected and precursor ion scan (negative mode) with m/z 97 set as precursor ion (loss of HSO4−) was performed using the following HPLC gradient: Step 0: 0.00 min; 30 μl/min; water/methanol (3/97; v/v). Step 1: 1.60 min; 30 μl/min; water/methanol (3/97; v/v), Step 2: 2.40 min; 200 μl/min; water/methanol (3/97; v/v). Step 3: 2.80 min; 200 μl/min; water/methanol (3/97; v/v). Step 4: 3.00 min; 30 μl/min; water/methanol (3/97; v/v). The method properties were set as following: Curtain Gas (CUR): 10.0; Collision Gas (CAD): Medium; Ionspray voltage (IS): −4500.00 and Ion source gas (GS1): 19.0. For each spectrum (m/z 400–500) 50 repetitive scans with duration of 3 s each were averaged. For quantification of sterol-sulfates from treated cells, the following m/z-values were used: cholesterol-sulfate 465.3 m/z; stigmasterol-sulfate 491.3 m/z; brassicasterol-sulfate 477.3 m/z; campesterol-sulfate 479.3 m/z; β-sitopsterol-sulfate 493.3 m/z; cholesterol(D7)-sulfate (standard) 472.3 m/z.
Sterol extraction and determination in brain and serum samples of stigmasterol-fed mice
Samples were frozen and stored at −80°C until analysis. Cholesterol and stigmasterol were extracted from brain and serum using chloroform-methanol and analyzed after derivatization.
Cholesterol content in brains and serum of stigmasterol-fed mice and control mice was determined by gas chromatography-flame ionization detection (GC-FID) as previously described (Kölsch et al., 2010). Stigmasterol content of the same samples was analyzed by a method of combined gas chromatography and mass spectrometry (GC-MS-SIM). Both techniques were performed as previously described (Teunissen et al., 2003; Kölsch et al., 2010). Cholesterol content was determined by means of 5α-cholestan as internal standard and stigmasterol levels by means of epicoprostanol.
Gas chromatography–flame ionization detection
Plasma and brain cholesterol was analyzed on a HP 6890 seriesII plus GC (Agilent Technologies). As an internal standard, 5α-cholestane was used. Briefly, samples were injected in a splitless mode at 280°C, using an injector (HP 7683) and hydrogen as carrier gas (inlet pressure 9.9 psi, total gas-flow 1.1 ml/min). For separation of sterols, a cross-linked methyl-silicone DB-XLB 122–1232 fused silica capillary column (J&W; 30 m × 0.25 mm and 0.25 μm film thickness) was used. The initial temperature of 150°C was kept for 3 min and then increased stepwise at 30°C to reach a final temperature of 290°C. For determination of absolute cholesterol concentrations, ratios of cholesterol areas to areas of the internal standard 5α-cholestane were defined and multiplied by the amount of 5 α-cholestane (50 μg), added to a defined sample volume.
GC-MS-SIM
The technique was performed on a HP GC-MSD system (HP 5890, series II GC) in combination with a 5971 mass selective detector (Agilent Technologies). Briefly, samples were injected in the splitless mode with helium as carrier gas (flow rate 1 ml/min). The sterols were separated on a DB-XLB 122–1232 fused silica capillary column (J&W; 30 m × 0.25 mm and 0.25 μm film thickness). A temperature of 150°C was initially kept at 150°C for 1 min, followed by a stepwise increase at 20°C/min to 260°C, followed by 10°C/min to 280°C.
Stigmasterol and epicoprostanol (internal standard) were monitored as their TMSi derivates in the selected ion monitoring mode, based on following masses: stigmasterol m/z 484 [M+] and epicoprostanol (internal standard) m/z 370 [M+-OTMSi]. Stigmasterol was analyzed from selected ion monitoring analysis against internal standard epicoprostanol using standard curves. The identity of sterols was validated by the use of additional qualifier ions, as well as comparison with full-scan mass spectra of authentic compound (m/z 50–500).
Statistical analysis
All quantified data represent an average of at least three independent experiments. Statistical significance was determined by two-tailed Student's t test.
Results
Effect of plant sterols and cholesterol on Aβ secretion
To analyze whether the nutritional most relevant plant sterols affect amyloidogenic processing of APP, we incubated human neuroblastoma SH-SY5Y cells with stigmasterol (Δ22, ethyl group at C24), β-sitosterol (ethyl group at C24), brassicasterol (Δ22, methyl group at C24) and campesterol (methyl group at C24) (Fig. 1). To validate potential effects found by plant sterols, we used cholesterol throughout our study as the impact of cholesterol on APP processing has been at least in some respect already intensively investigated.
For the detection of secreted Aβ levels we used SH-SY5Y cells stably transfected with APP695, the predominant APP isoform in neurons (Rohan de Silva et al., 1997). As expected cholesterol significantly increased Aβ levels (122.4% ± 1.3, p ≤ 0.001) compared with the solvent control (Fig. 2A) (Refolo et al., 2000; Fassbender et al., 2001; Refolo et al., 2001; Ghribi et al., 2006; Xiong et al., 2008). Stigmasterol strongly reduced Aβ levels (62.00% ± 3.9, p = 0.0005) (Fig. 2A), whereas β-sitosterol significantly increased Aβ secretion (115.2% ± 2.2, p ≤ 0.001). For campesterol and brassicasterol Aβ levels were only slightly elevated compared with solvent control. The determination of Aβ40 and Aβ42 peptides using ELISA technique revealed that both Aβ species were significantly reduced in presence of stigmasterol: Aβ40 peptides were decreased to 58% (58.4% ± 6.8, p = 0.0102), Aβ42 peptides to 66% (65.9% ± 1.9, p = 0.049) compared with control cells (Fig. 2B).
To exclude that the observed changes in Aβ secretion in presence of plant sterols are caused by general alterations in protein secretion, we transfected SH-SY5Y wild-type (wt) cells with secretory alkaline phosphatase (SEAP). SEAP secretion was not significantly changed in presence of plant sterols (Fig. 2C). In addition the sterols were significantly taken up by the cells and the uptake was not significantly altered between single plant sterols (uptake = 4.1 μg ± 0.4 μg plant sterol/cholesterol per milligrams protein). Lactate-dehydrogenase-assay (LDH) analysis revealed no signs for elevated cytotoxicity or reduced membrane integrity in presence of sterols compared with solvent control (Fig. 2D).
Cholesterol elevates Aβ generation by increasing gene expression of β- and γ-secretase and by directly affecting β- and γ-secretase enzymatic activity
To evaluate whether the observed changes in Aβ secretion are caused by alterations in β- and/or γ-secretase processing of APP, we systematically investigated the effect of cholesterol and plant sterols on β- and γ-secretase activity, BACE1 and PS1 protein level and gene expression of BACE1 and the components of the γ-secretase complex. The β- and γ-secretase activities were determined in purified membranes of SH-SY5Y wt cells or mouse brains in presence of sterols and a specific fluorogenic peptide. The use of a cell-free assay using purified membranes, allows identifying the direct influence of sterols on secretase-activities independent of alterations in gene expression and protein level. The specificity of the assay was determined by using specific inhibitors (Fig. 3C,D).
Figure 3.
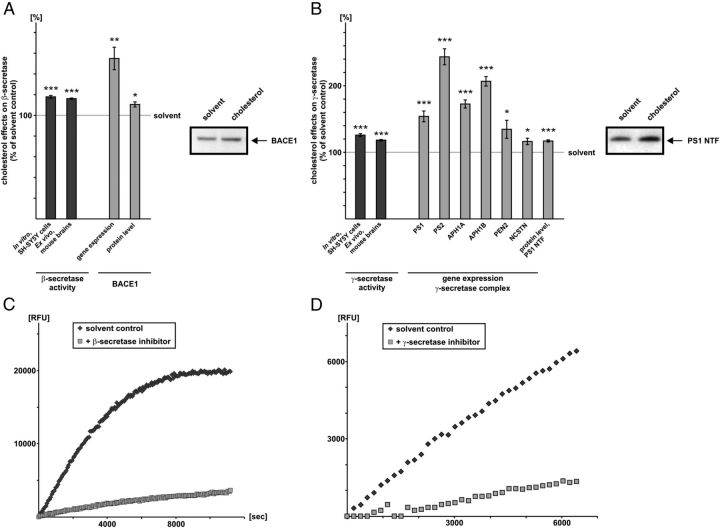
Effect of cholesterol on amyloidogenic β- and γ-secretase cleavage. A, Effect of cholesterol on β-secretase BACE1. Dark-gray bars, Influence of cholesterol on β-secretase activity in purified membranes of human SH-SY5Y wt cells and ex vivo in purified membranes of mouse brains. Purified membranes were incubated with cholesterol (50nMol/mg protein) or solvent control. The β-secretase activity was determined by a fluorometric assay. Light-gray bars, Influence of cholesterol on gene expression and protein level of BACE1. SH-SY5Y wt cells were incubated with cholesterol (10 μm) or solvent control; total RNA was prepared and mRNA level of BACE1 was determined via RT-PCR. For the determination of BACE1 protein level, cell lysates were prepared, protein level adjusted, and equal volumes of cell lysates loaded and separated on 10–20% Tris-Tricine gels. WB analysis was performed with antibody B0806. A representative WB is shown. B, Influence of cholesterol on γ-secretase. Dark-gray bars, Influence of cholesterol on γ-secretase activity in purified membranes of human SH-SY5Y wt cells and ex vivo in purified membranes of mouse brains. Purified membranes were incubated with cholesterol (50nMol/mg protein) or solvent control and γ-secretase activity was determined by a fluorometric assay. Light-gray bars, Influence of cholesterol on gene expression of the γ-secretase components and on the protein level of the PS1 N-terminal fragment (PS1 NTF). SH-SY5Y wt cells were incubated with cholesterol (10 μm) or solvent control; total RNA was prepared and mRNA levels were determined via RT-PCR for PS1, PS2, APH1A, APH1B, PEN2, and NCSTN. For the analysis of the PS1 protein level, cell lysates were prepared, protein level adjusted, and equal volumes of cell lysates loaded and separated on 10–20% Tris-Tricine gels. WB analysis was performed with antibody sc7860. A representative WB is shown. A, B, Illustration and statistical significance as described for Figure 2; n ≥ 4. C, Determination of specificity of the β-secretase assay. The assay was performed as described using β-secretase inhibitor II (Calbiochem). D, Determination of specificity of the γ-secretase assay. The assay was performed as described using γ-secretase-inhibitor X (Calbiochem).
In agreement with increased Aβ levels in APP695-expressing SH-SY5Y cells, cholesterol significantly increased β-secretase activity in purified membranes of SH-SY5Y wt cells (119.9% ± 1.4, p ≤ 0.001) and mouse brains (116.2% ± 0.6, p ≤ 0.001) compared with the solvent control (Fig. 3A), indicating a direct effect of cholesterol on β-secretase enzymatic activity. Elevated β-secretase activity in presence of cholesterol has been already recently described (Ghribi et al., 2006; Xiong et al., 2008; Liu et al., 2009), validating the results obtained with the β-secretase assay used in this study. Beside the direct effect of cholesterol on β-secretase activity, RT-PCR analysis of SH-SY5Y wt cells incubated with cholesterol showed increased gene expression of BACE1 (154.9% ± 10.9, p = 0.002) (Fig. 3A). To analyze whether the BACE1 protein level is also changed in presence of cholesterol, we performed WB analysis of BACE1. In accordance with the changed BACE1 expression, BACE1 protein level was also significantly increased in SH-SY5Y wt cells exposed to cholesterol (110.5% ± 2.5, p = 0.022) (Fig. 3A). These findings are in line with the results obtained by Ghribi et al. describing increased BACE 1 protein level in rabbits fed with 1% cholesterol for 7 months (Ghribi et al., 2006).
As the generation of Aβ depends on the successive action of β- and γ-secretase activity, we next determined the direct effect of cholesterol on γ-secretase activity in purified membranes of SH-SY5Y wt cells and mouse brains. Similarly to the direct effect of cholesterol on β-secretase activity, cholesterol directly increased γ-secretase activity in purified membranes of SH-SY5Y wt cells (125.9% ± 2.2, p ≤ 0.001) and mouse brains (118.4% ± 1.0, p ≤ 0.001) (Fig. 3B), according to previous studies (Xiong et al., 2008). In addition, cholesterol significantly increased gene expression of all γ-secretase components, PS1, PS2, APH1A, APH1B, PEN2 and NCSTN, attended by a significant increase in the PS1 protein level (117.1% ± 1.7, p ≤ 0.001) (Fig. 3B).
These results indicate that cholesterol increases Aβ generation by directly affecting β- and γ-secretase activity and by increasing the expression of BACE1 and the γ-secretase complex.
β-Sitosterol increases Aβ generation by increasing gene expression of β-secretase, but not of the γ-secretase complex, and by directly elevating β- and γ-secretase enzymatic activity
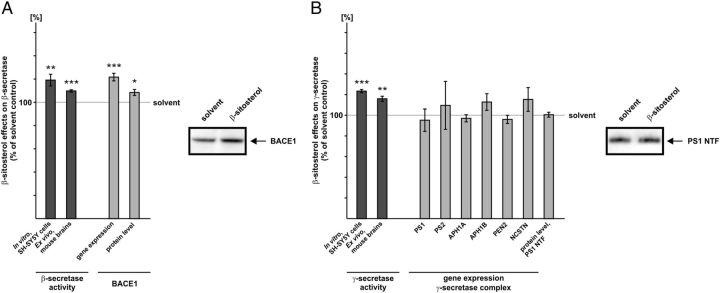
Among the plant sterols analyzed, β-sitosterol showed the strongest increase in Aβ secretion. However, the increase in Aβ generation found for β-sitosterol was lower compared with cholesterol. Interestingly we found for β-sitosterol a nearly identical increase in β-secretase activity in purified membranes of SH-SY5Y wt cells (β-sitosterol: 119.1% ± 5.0, p = 0.002) (Fig. 4A) as shown for cholesterol (cholesterol: 119.9% ± 1.4, p ≤ 0.001). Also in purified membranes of mouse brains, β-sitosterol significantly elevated β-secretase activity (109.8% ± 1.1, p ≤ 0.001) (Fig. 4A), however the observed increase was not as strong as found with SH-SY5Y membranes. RT-PCR analysis of SH-SY5Y wt cells exposed to β-sitosterol also revealed increased gene transcription of BACE1 (121.7% ± 3.3, p ≤ 0.001), attended by an increase in BACE1 protein level (108.5% ± 2.6, p = 0.034) (Fig. 4A).
Figure 4.
Influence of β-sitosterol on β- and γ-secretase. A, Influence of β-sitosterol on β-secretase activity, gene expression, and protein level of BACE1. B, Influence of β-sitosterol on γ-secretase activity, gene expression of the γ-secretase complex, and PS1 protein level. A, B, Sterol concentration used and analysis of secretase activities, gene expression, and protein level as described for Figure 3. Illustration and statistical significance as described for Figure 2; n ≥ 6.
Similarly to the effect of β-sitosterol on β-secretase enzymatic activity, β-sitosterol also showed a nearly identical potency to increase γ-secretase activity directly as found for cholesterol using either purified membranes of SH-SY5Y wt cells (123.3% ± 2.4, p ≤ 0.001) or of mouse brains (115.9% ± 2.5, p ≤ 0.001) (Fig. 4B). However, in contrast to cholesterol, β-sitosterol showed no significant effect on the gene expression of the γ-secretase complex and as a consequence no effect on the PS1 protein level (100.5% ± 2.4, p = 0.881) (Fig. 4B). Since β-sitosterol shows no additional effect on the gene expression and protein level of the γ-secretase complex compared with cholesterol, this should result in lower secreted Aβ levels compared with cholesterol which was indeed found for β-sitosterol.
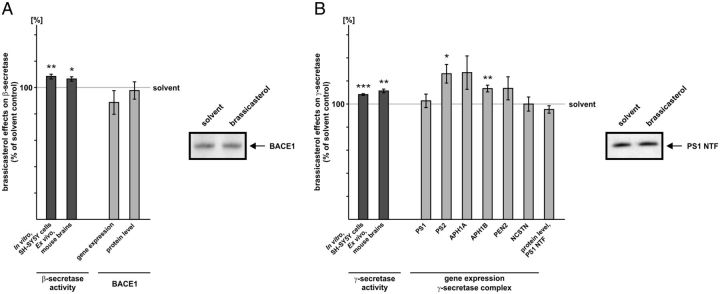
Brassicasterol slightly increases Aβ generation by directly affecting β- and γ-secretase activity
For brassicasterol we found only slightly elevated secreted Aβ levels using APP695-expressing SH-SY5Y cells. The analysis of the β-secretase cleavage in presence of brassicasterol revealed slightly, however significantly increased β-secretase enzymatic activity. Brassicasterol directly increased β-secretase activity in purified membranes of SH-SY5Y wt cells (108.5% ± 1.9, p = 0.0013) and mouse brains (106.6% ± 1.7, p = 0.018) (Fig. 5A). Gene expression and protein level of BACE1 remained unaffected (Fig. 5A). Similar to the direct effect of brassicasterol on β-secretase activity, γ-secretase activity was also slightly, however again significantly elevated in purified membranes of SH-SY5Y wt cells (108.2% ± 1.0, p ≤ 0.001) and mouse brains (111.4% ± 1.6%, p = 0.005) (Fig. 5B). Significantly altered gene expression was only found for the γ-secretase components PS2 and APH1B, but not for the homologues PS1 and APH1A, PEN2 and NCSTN (Fig. 5B). Protein level of PS1 was also unchanged in presence of brassicasterol (Fig. 5B).
Figure 5.
Influence of brassicasterol on β- and γ-secretase. A, Effect of brassicasterol on β-secretase activity, gene expression, and protein level of BACE1. B, Influence of brassicasterol on γ-secretase activity, gene expression of the γ-secretase complex, and PS1 protein level. A, B, Sterol concentration used and analysis of secretase activities, gene expression, and protein level as described for Figure 3. Illustration and statistical significance as described for Figure 2; n ≥ 6.
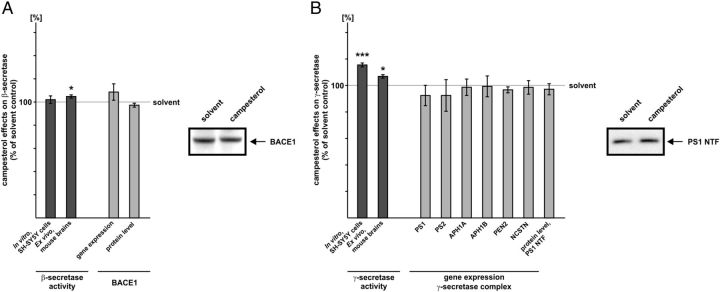
Campesterol slightly increases Aβ secretion by directly affecting γ-secretase activity
Beside brassicasterol, campesterol only marginally affected Aβ generation. Analyzing β- and γ-secretase activity, gene expression and protein level of BACE1 and the γ-secretase complex, we found that the slight increase in Aβ secretion obtained with APP695-expressing SH-SY5Y cells in presence of campesterol is mainly attributed to increased γ-secretase activity. The β-secretase activity was only marginally, but significantly elevated in purified membranes of mouse brains (104.8% ± 1.4, p = 0.049), whereas we found no direct effect of campesterol in SH-SY5Y wt membranes (Fig. 6A). In line with the unaffected gene expression of BACE1, the protein level of BACE1 was unchanged (Fig. 6A). However, campesterol significantly increased γ-secretase activity in purified membranes of SH-SY5Y wt cells (115.5% ± 3.0, p ≤ 0.001) and mouse brains (106.9% ± 1.3, p = 0.046) (Fig. 6B). Gene expression of all components of the γ-secretase complex remained unaffected in presence of campesterol, reflected by unchanged PS1 protein level (Fig. 6B).
Figure 6.
Influence of campesterol on β- and γ-secretase. A, Effect of campesterol on β-secretase activity, gene expression, and protein level of BACE1. B, Influence of campesterol on γ-secretase activity, gene expression of the γ-secretase complex, and PS1 protein level. A, B, Sterol concentration used and analysis of secretase activities, gene expression, and protein level as described for Figure 3. Illustration and statistical significance as described for Figure 2; n ≥ 5.
Pleiotropic mechanisms are involved in reducing Aβ secretion in presence of stigmasterol
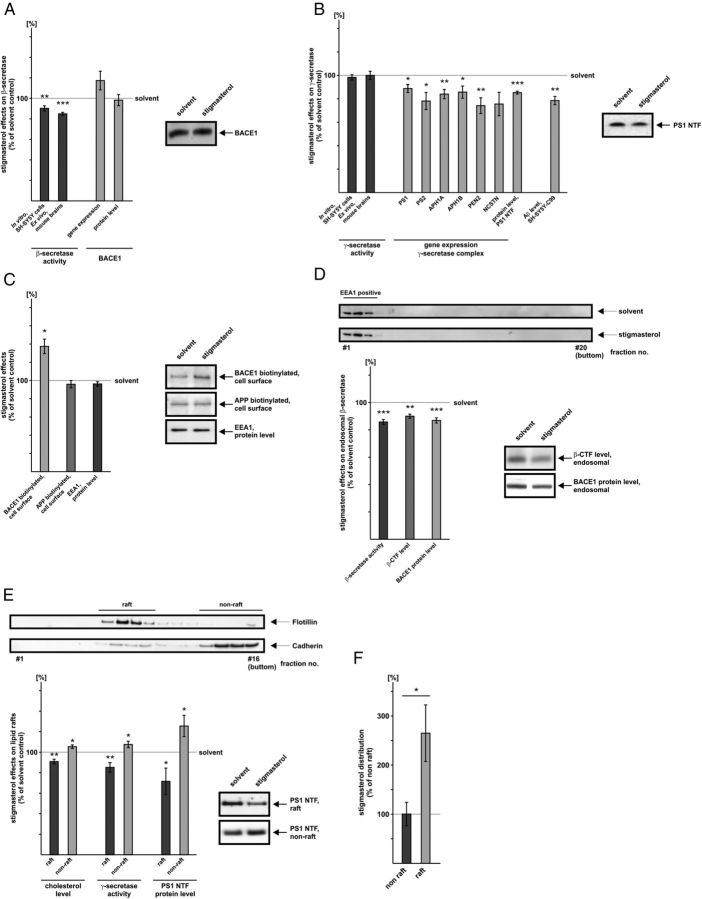
Stigmasterol directly reduces β-secretase activity and decreases gene expression of the γ-secretase complex
Stigmasterol, the plant sterol having the strongest structurally differences compared with cholesterol, was found to be the only sterol to reduce Aβ generation. The analysis of the β-secretase activity in purified membranes in presence of stigmasterol revealed slightly however significantly reduced β-secretase enzymatic activity. For SH-SY5Y wt membranes we found β-secretase activity to be decreased to 90% (90.3% ± 2.5, p = 0.002), for mouse brain membranes to 85% (84.9% ± 1.5, p ≤ 0.001) (Fig. 7A). In contrast to the direct effect of stigmasterol on β-secretase activity, no significant changes on gene expression and protein level of BACE1 were obtained (Fig. 7A). Similarly, stigmasterol does not directly affect γ-secretase activity as we observed no alterations in γ-secretase enzymatic activity in purified membranes of SH-SY5Y wt cells and mouse brains (Fig. 7B). Instead, in presence of stigmasterol mRNA levels of all γ-secretase components were significantly reduced to 74%–89%, attended by a significant decrease in the PS1 protein level (85.3% ± 1.2, p ≤ 0.001) (Fig. 7B). To evaluate whether the observed reduced gene expression of the γ-secretase complex results in decreased Aβ levels, we analyzed Aβ secretion in SH-SY5Y cells stably transfected with the truncated APP construct SPC99. SPC99 represents the C-terminal fragment of β-secretase cleaved APP, which allows studying the influence of sterols on γ-secretase cleavage, independent of β-secretase activity. Indeed, Aβ generation was also significantly reduced to 78% in stigmasterol-treated SH-SY5Y cells stably transfected with the truncated APP construct SPC99 (78.5% ± 3.6, p = 0.009) (Fig. 7B).
Figure 7.
Stigmasterol reduces Aβ generation via pleiotropic mechanisms. A, Effect of stigmasterol on β-secretase BACE1. Dark-gray bars, Stigmasterol reduces β-secretase activity directly in purified membranes of SH-SY5Y wt cells and of mouse brains. Light-gray bars, Stigmasterol has no effect on BACE1 expression. Sterol concentration used and analysis of secretase activities and BACE1 expression as described for Figure 3A. B, Influence of stigmasterol on γ-secretase. Dark-gray bars, Stigmasterol does not affect γ-secretase activity directly in purified membranes of SH-SY5Y wt cells and of mouse brains. Light-gray bars, left, Stigmasterol significantly decreases gene expression of all γ-secretase components, PS1, PS2, APH1A, APH1B, PEN2, and NCSTN, resulting in a reduced PS1 protein level. Sterol concentration, analysis of gene transcription, and PS1 protein level as described for Figure 3B. Light gray bar, right, Influence of stigmasterol on secreted Aβ levels in SH-SY5Y cells stably expressing SPC99. SPC99-expressing SH-SY5Y cells were incubated with 10 μm stigmasterol or solvent control. Secreted Aβ levels were immunoprecipitated with antibody W02, and WB analysis was performed with antibody W02. C, Biotinylation of cell surface localized proteins in presence of stigmasterol. SH-SY5Y wt cells were incubated with 10 μm stigmasterol or solvent control, scraped off, and biotinylation was performed as described in Material and Methods. Biotinylated proteins were immunoprecipitated using streptavidin agarose. WB analysis of biotinylated APP was performed with antibody W02, WB analysis of biotinylated BACE1 with antibody B0806. Cell-lysates were analyzed for total protein level of EEA1 of stigmasterol-treated cells or control cells. WB analysis was performed with antibody ab2900. D, Stigmasterol reduces BACE1 internalization. Stigmasterol-treated cells (10 μm) and control cells were fractionated using OptiPrep density gradient centrifugation. Collected fractions were loaded and separated on 10–20% Tris-Tricine gels. WB analysis was performed with anti-EEA1 antibody. EEA1-positive fractions were pooled and analyzed for BACE1 protein level as described for Figure 3A. β-CTF was detected using antibody W02 for WB analysis. EEA1-positive fractions were also analyzed for β-secretase activity. E, Stigmasterol affects cholesterol level, γ-secretase activity, and PS1 protein level in lipid rafts. Stigmasterol-treated cells (10 μm) and control cells were subjected to buoyant sucrose density centrifugation and 16 fractions were collected. Fractions were loaded, separated on 10–20% Tris-Tricine gels and subjected to WB analysis. For the detection of raft-positive gradient fractions, the antibody anti-flotillin 610821 was used, for the detection of nonraft fractions the antibody anti-cadherin ab6528. Determination of cholesterol level, γ-secretase activity, and PS1 protein level in raft and nonraft membrane microdomains. For the determination of the cholesterol content, SH-SY5Y wt cells were pulsed with 14C-cholesterol and afterward incubated with 10 μm stigmasterol as described in Materials and Methods. Cholesterol levels were determined by liquid scintillation counting in a Tri-Carb2800TR, γ-secretase activity via the fluorescent γ-secretase assay as described in Material and Methods. PS1 protein level was detected using antibody sc7860. A–E, Representative WBs for protein analysis experiments are shown. Illustration and statistical significance as described for Figure 2; n ≥ 4. F, Stigmasterol distribution to raft and nonraft microdomains. Pooled raft/nonraft samples were subjected to derivatization and mass spectrometry as described in Materials and Methods to determine stigmasterol content in raft and nonraft membrane microdomains.
Stigmasterol decreases β-secretase cleavage by reducing BACE1 internalization to the endosomal compartments
As we observed only a minor effect of stigmasterol on directly decreasing β-secretase activity we asked whether β-secretase cleavage of APP is affected by additional mechanisms in presence of stigmasterol. Since the endosomal compartments have been found to be the major cellular site of BACE1 activity (Huse et al., 2000), we analyzed whether stigmasterol interferes with BACE1 internalization from the plasma membrane to the endosomes. Biotinylation of cell surface localized proteins revealed a strong increase in biotinylated BACE1 in presence of stigmasterol (137.4% ± 8.0, p = 0.016) compared with control cells, indicating that BACE1 accumulates at the plasma membrane (Fig. 7C). Levels of biotinylated APP were unchanged (Fig. 7C), suggesting that the observed effect is specific for BACE1. Similarly, protein level of the EEA1 was not affected in cell-lysates of stigmasterol-treated SH-SY5Y wt cells compared with control cells. BACE1 accumulation at the plasma membrane might be caused by reduced internalization of BACE1 to the endosomal compartments. To analyze whether stigmasterol might additionally decrease β-secretase cleavage of APP by diminishing BACE1 internalization, we isolated endosomes by density centrifugation. Cell fractionation followed by gel electrophoresis and WB analysis of the collected fractions resulted in three fractions positive for the EEA1 (Fig. 7D). EEA1-positive fractions were pooled and analyzed for β-secretase activity, the C-terminal fragment generated by β-secretase processing of APP (β-CTF) and for BACE1 protein level. Indeed, the protein level of BACE1 was significantly reduced in EEA1-positive fractions in presence of stigmasterol (86.9% ± 1.9, p ≤ 0.001), accompanied by a similar decrease in β-secretase activity (85.8 ± 1.8, p ≤ 0.001) and the product of β-secretase-mediated APP cleavage, β-CTF (90.0% ± 1.5, p = 0.003) (Fig. 7D).Together, these results indicate that stigmasterol also decreases β-secretase cleavage of APP and thus Aβ generation, by diminishing BACE1 internalization from the cell surface to the endosomal compartments.
Stigmasterol decreases γ-secretase activity by affecting cholesterol distribution in lipid rafts
Cholesterol within mammalian cells as well as plant sterols within plant cell membranes have been shown to be organized in specialized microdomains of the plasma membrane, also called lipid rafts (Simons and Ikonen, 1997; Mongrand et al., 2010). Lipid rafts are involved in β- and γ-secretase cleavage of APP (Ehehalt et al., 2003; Vetrivel and Thinakaran, 2010) and in line with our results, it has been shown that alterations in the cholesterol level influence Aβ generation (Refolo et al., 2000; Fassbender et al., 2001; Wahrle et al., 2002; Ghribi et al., 2006). As plant sterols, compared with cholesterol, have a similar sterol backbone, we analyzed whether stigmasterol affects cholesterol distribution and thus γ-secretase activity in lipid raft membrane microdomains. To address this question, SH-SY5Y wt cells were incubated with stigmasterol or the solvent control and raft and nonraft membrane microdomains were obtained by sucrose buoyant density centrifugation. To identify raft and nonraft membrane microdomains, collected gradient fractions were immunoblotted against raft marker protein flotillin or cadherin for the determination of gradient fractions representing nonraft membrane microdomains (Fig. 7E). The analysis of the cholesterol content in the collected fractions revealed that the cholesterol level was significantly reduced in raft-positive fractions when cells were incubated with stigmasterol (cholesterol/rafts: 90.9% ± 2.3, p = 0.007) (Fig. 7E). As a consequence of the reduced cholesterol level in lipid rafts, γ-secretase activity should be decreased since we have shown that cholesterol directly activates γ-secretase activity. Indeed, γ-secretase activity was also significantly reduced in raft membrane microdomains (γ-sec/rafts: 85.2% ± 4.6, p = 0.009), whereas activity of γ-secretase was slightly, however, significantly increased in nonraft gradient fractions (γ-sec/nonrafts: 107.5% ± 3.2, p = 0.039) (Fig. 7E). The increase in γ-secretase activity in nonraft microdomains might be caused by the elevated cholesterol level in nonraft gradient fractions in stigmasterol-incubated cells (cholesterol/nonrafts: 105.4% ± 1.7, p = 0.021) (Fig. 7E). Notably, stigmasterol distribution to lipid raft-positive fractions were 2.56fold increased compared with stigmasterol content in nonraft membrane microdomains (Fig. 7F). The stigmasterol-induced shift of cholesterol and γ-secretase activity from rafts to nonraft membrane microdomains was attended by a shift of PS1 protein from raft to nonraft gradient fractions (PS1/rafts: 71.6% ± 12.9, p = 0.033; PS1/nonrafts: 125.5% ± 10.4, p = 0.026 (Fig. 7E), also contributing to a reduced γ-secretase cleavage in lipid rafts. These data indicate that stigmasterol reduces Aβ generation by incorporating in lipid raft membrane microdomains, thus displacing cholesterol and PS1 out of lipid rafts and therefore reducing γ-secretase cleavage in lipid rafts.
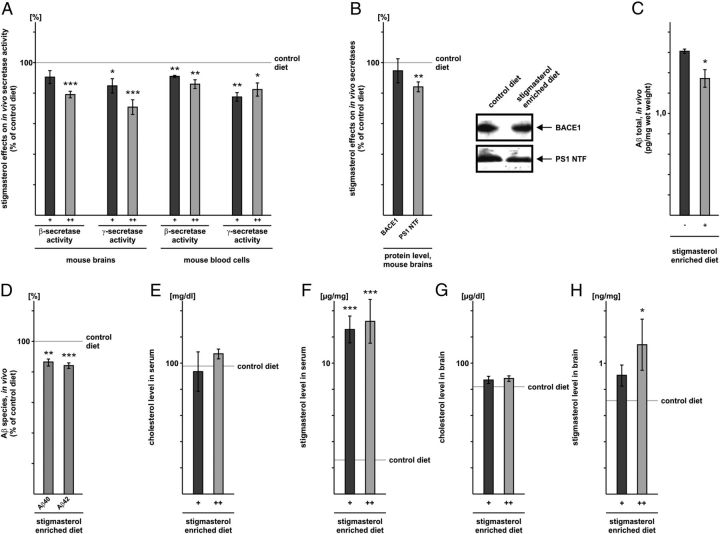
Stigmasterol-enriched diets decrease amyloidogenic APP processing in vivo
To examine whether stigmasterol, the only plant sterol showing anti-amyloidogenic properties, might also be beneficial in vivo, mice were fed with two stigmasterol-enriched diets, containing different amounts of stigmasterol. Whereas brain β-secretase activity from mice fed with a diet containing 0.19% stigmasterol (+) was not significantly reduced (β-sec/+: 90.5% ± 4.3, n.s.), β-secretase activity was significantly decreased when the diet contained 0.39% of stigmasterol (++) (β-sec/++: 79.1% ± 2.1, p ≤ 0.001) compared with mice fed with normal chow (Fig. 8A). For mouse blood cells we found significantly decreased β-secretase activity for both stigmasterol-enriched diets (β-sec/+: 91.0% ± 0.6, p = 0.0013; β-sec/++: 85.9% ± 2.9, p = 0.003) (Fig. 8A). The γ-secretase activity was significantly and dose-dependently decreased in mouse brains obtained from mice fed with the stigmasterol-enriched diets compared with control mice (γ-sec/+: 84.8% ± 3.2, p = 0.008; γ-sec/++: 70.8% ± 4.8, p ≤ 0.001) (Fig. 8A). Significantly reduced γ-secretase activity was also obtained for blood cells of these mice (γ-sec/+: 77.5% ± 2.8, p = 0.003; γ-sec/++: 82.3% ± 4.3, p = 0.021) (Fig. 8A).
Figure 8.
Effect of stigmasterol on proteolytic processing of APP in mice fed with stigmasterol-enriched diets. A, Determination of β- and γ-secretase activity in brains and blood cells of mice fed with a diet containing 0.19% (+) or 0.39% (++) of stigmasterol. B, Protein level BACE1 and PS1 in mice fed with the stigmasterol-enriched diet containing higher amounts (++) of stigmasterol. Brain homogenates were prepared and analyzed by WB using antibody PC529 (anti-BACE1) and sc7860 (anti-PS1). C, Determination of soluble Aβ level (Aβ40 and Aβ42) in brains of mice fed with a stigmasterol-enriched diet or the control diet. D, Determination of Aβ40 and Aβ42 peptides in brains of mice fed with a stigmasterol-enriched diet or the control diet. A–D, Illustration and statistical significance as described for Figure 2. E, Determination of cholesterol level in serum of stigmasterol-fed mice containing 0.19% (+) or 0.39% (++) of stigmasterol. F, Determination of stigmasterol level in serum of stigmasterol-fed mice containing 0.19% (+) or 0.39% (++) of stigmasterol. G, Determination of cholesterol level in brain of stigmasterol-fed mice containing 0.19% (+) or 0.39% (++) of stigmasterol. H, Determination of stigmasterol level in brain of stigmasterol-fed mice containing 0.19% (+) or 0.39% (++) of stigmasterol. E, G, Cholesterol content was determined by GC-FID. F, H, Stigmasterol content was analyzed by a method of GC-MS. All quantified data represent an average of six independent mouse brains/blood cells (n = 6).
In accordance to the reduced PS1 protein level and unchanged BACE1 protein level in cells incubated with stigmasterol (Fig. 7A,B), PS1 protein level was also significantly decreased in stigmasterol (++)-fed mice (PS1: 84.1% ± 3.2, p = 0.008) (Fig. 8B), and protein level of BACE1 was not significantly changed. As a consequence of reduced β- and γ-secretase activity (Fig. 8A), soluble Aβ levels were also decreased in mouse brains fed with a stigmasterol-enriched diet (Fig. 8C). Similar to the results obtained with cell culture experiments, both Aβ species were significantly reduced in stigmasterol-fed mice (Aβ40: 86.5% ± 2.3, p = 0.0018; Aβ42: 84.2% ± 1.8, p ≤ 0.001) (Fig. 8D). These data indicate that indeed nutritional intake of stigmasterol can reduce the cerebral activity of amyloidogenic enzymes in vivo and thus Aβ levels in brain.
However, as plant sterols interfere with the intestinal resorption of cholesterol (Ostlund, 2002; Richelle et al., 2004), the observed effect on amyloidogenic processing in stigmasterol-fed mice might be a consequence of altered cholesterol level in the brains of these animals. To evaluate whether the reduced amyloidogenic processing in vivo is caused by stigmasterol itself and not accompanied by reduced cholesterol, we examined stigmasterol and cholesterol level in serum and brain samples of these animals. The analysis of cholesterol and stigmasterol in serum samples of stigmasterol (+)- and (++)-fed mice revealed no change in the cholesterol level, whereas stigmasterol was significantly increased compared with serum samples of control animals (Fig. 8E,F), indicating efficient dietary stigmasterol uptake. In brain samples, cholesterol level was also unchanged, the level of stigmasterol was dose-dependently increased compared with the control diet (Fig. 8G,H), emphasizing that stigmasterol passed the blood–brain-barrier and is the cause for the observed reduced β- and γ-secretase activities.
Discussion
To evaluate whether dietary intake of plant sterols might be beneficial for the prevention of AD, we analyzed the effect of plant sterols and cholesterol on the molecular mechanisms of APP processing.
We found that stigmasterol was the only plant sterol that significantly decreased Aβ levels. Compared with stigmasterol, β-sitosterol and cholesterol significantly increased Aβ levels, whereas campesterol and brassicasterol showed minor or no effect on Aβ secretion. Interestingly, the Aβ-reducing plant sterol stigmasterol is structurally the most distinctive from cholesterol, having an additional double-bond at C22–C23 and an ethyl-group at C24. The analysis of the molecular mechanisms revealed that stigmasterol directly inhibited β-secretase activity and further reduced β-secretase cleavage of APP by decreasing BACE1 internalization from the plasma membrane to the endosomal compartments, known to have an optimal acidic pH for BACE1 cleavage of APP (Vassar et al., 1999; Huse et al., 2000). In addition, the stigmasterol-induced reduction of secreted Aβ levels, Aβ40 and Aβ42, is caused by reduced gene transcription of all γ-secretase components, further validated by a decrease in the protein level of PS1, the catalytic active part of the heterotetrameric γ-secretase (Herreman et al., 2000). Interestingly, we found that stigmasterol can incorporate in lipid raft membrane microdomains of mammalian cells, accompanied by a reduction in the cholesterol content in lipid rafts. The decrease in the cholesterol content in lipid raft membrane microdomains to 90% in presence of stigmasterol might be rather small, however, as cholesterol has been shown to alter Aβ production via multiple mechanisms, a 10% decreased cholesterol level in lipid rafts might have a more pronounced effect on Aβ generation.
First, we and others could show that cholesterol directly activates γ-secretase activity (Wahrle et al., 2002; Xiong et al., 2008). We found γ-secretase activity to be reduced in lipid raft membrane microdomains in presence of stigmasterol, which might be a consequence of the reduced cholesterol level. Taken into consideration that in the present study as well as in previous studies cholesterol also directly increased β-secretase activity (Ghribi et al., 2006; Liu et al., 2009), a reduced level of cholesterol in lipid rafts should further decrease Aβ generation, as β-secretase BACE1 has been shown to be raft-associated (Riddell et al., 2001; Sakurai et al., 2008).
Second, cholesterol has been shown to increase the accessibility of BACE1 to its substrate APP by relocalization of APP from nonraft membrane microdomains to lipid rafts, already containing BACE1, followed by rapid endocytosis (Marquer et al., 2011). Furthermore, it has been shown that cholesterol depletion decreases endocytosis, which is crucial for BACE1 cleavage of APP (Huse et al., 2000). As we found reduced cholesterol level in lipid rafts in presence of stigmasterol, one might speculate that the reduced internalization of BACE1 that we found in presence of stigmasterol might be caused by the reduced cholesterol level in lipid raft membrane microdomains. However, in this context one has to consider that lipid raft-associated proteins are known to be generally internalized through clathrin-independent pathways, whereas cholesterol depletion mainly affects clathrin-dependent endocytosis (Rodal et al., 1999; Subtil et al., 1999). However, recent evidence suggests a link between lipid rafts and clathrin-mediated endocytosis as it has been shown that lipid-raft associated proteins can be internalized by clathrin-coated pits (Stoddart et al., 2002; Rollason et al., 2007). Furthermore, cholera and anthrax toxins enter cells through lipid rafts by clathrin-dependent mechanisms (Abrami et al., 2003; Hansen et al., 2005). Interestingly, in presence of stigmasterol, cholesterol content, γ-secretase activity, and PS1 protein level was increased in nonraft membrane microdomains. In contrast to the lipid raft-associated amyloidogenic APP processing (Vetrivel and Thinakaran, 2010), nonamyloidogenic processing of APP by α-secretases preventing Aβ generation is discussed to take place outside of lipid rafts (Kojro et al., 2001; Parr-Sturgess et al., 2010), suggesting that stigmasterol might shift APP cleavage toward nonamyloidogenic processing. A similar shift of cholesterol and γ-secretase activity toward nonamyloidogenic processing of APP in nonraft membrane microdomains was recently reported to be induced by the ω-3 fatty acid docosahexaenoic acid (Grimm et al., 2011b), indicating that in general a slight shift of cholesterol from raft to nonraft membrane microdomains might be beneficial to reduce Aβ generation.
Stigmasterol-induced effects are all between 10% and 30% and less pronounced compared with e.g., synthetic secretase inhibitors (Shearman et al., 2000; Probst et al., 2013). However, these obviously minor effects on different cellular processes, including direct modulation of enzymes, gene expression, and subcellular transport processes, result in a 38% reduced Aβ generation. Furthermore, it has to be considered that BACE1 and γ-secretase not only cleave APP. BACE1 substrates include proteins with important neuronal functions, like the β2-subunit of the voltage-gated sodium channel (Navβ2) (Wong et al., 2005) and neuregulin-1 (Willem et al., 2006), suggesting that a strong therapeutic inhibition of BACE1 activity might affect neuronal membrane excitability. It has been reported that BACE activity is increased in sporadic AD (Yang et al., 2003). Some current approaches therefore target this elevated BACE activity to decrease it to a physiological level instead of completely inhibiting BACE activity. In line, the moderate inhibition of BACE activity by stigmasterol could facilitate physiological BACE activity. Similarly, total inhibition of γ-secretase activity might not be suitable to treat AD. The γ-secretase is beside APP essential for the proteolysis of many type-I transmembrane proteins, e.g., Notch, ErbB4, CD44, LRP1, and jagged (Parks and Curtis, 2007). Therefore, a slight and combined modulation of β- and γ-secretase activity affecting different cellular processes as observed for stigmasterol might be more suitable.
Beside directly increasing β- and γ-secretase activity, we found that cholesterol significantly elevates gene expression of all γ-secretase components and of β-secretase BACE1 attended by an increase of PS1 and BACE1 protein level. Increased BACE1 gene expression was already described in low density lipoprotein receptor (LDLR)-deficient mice fed with a high fat/cholesterol diet for an 8 week period (Thirumangalakudi et al., 2008). In addition, Parsons et al. (2007) reported reduced BACE1 expression in human embryonic kidney cells when cholesterol synthesis was inhibited. In line with our in vitro findings, BACE1 protein level has been shown to be increased in rabbits fed with 1% cholesterol for 7 months (Ghribi et al., 2006). Cholesterol increases Aβ generation by directly activating β- and γ-secretase enzyme activity and by elevating gene expression of β- and γ-secretase. For β-sitosterol we observed a nearly identical increase in β- and γ-secretase activity as obtained for cholesterol, resulting in significantly increased Aβ levels. However, in contrast to cholesterol, β-sitosterol only elevates gene expression of BACE1 but not of the γ-secretase components. As β-sitosterol only elevates gene transcription of BACE1 resulting in increased BACE1 protein level but unchanged protein level of PS1, it is not remarkable that for β-sitosterol the observed increase in the Aβ level is reduced compared with cholesterol.
Based on these findings, cholesterol is highly amyloidogenic whereas all analyzed plant sterols are not as amyloidogenic as cholesterol to the point of stigmasterol that significantly decreased amyloidogenic processing of APP, suggesting that a diet enriched in plant sterols mainly containing stigmasterol might be beneficial for AD. Indeed, in vivo, β- and γ-secretase activities were also significantly decreased in mice fed with a stigmasterol-enriched diet, accompanied by reduced brain Aβ levels, Aβ40 and Aβ42. Although phytosterols reduce the intestinal resorption of cholesterol (Richelle et al., 2004), brain cholesterol level of stigmasterol-fed mice were unaffected, whereas brain stigmasterol levels were increased. This is in line with the findings that brain cholesterol homeostasis is widely independent of ingested cholesterol (Vance et al., 2005). The above mentioned reduction of secretase activities in vivo might be attributed to a reduction of cholesterol in brain lipid rafts as we found reduced cholesterol and increased stigmasterol level in rafts after stigmasterol incubation, thus reducing secretase activities in rafts.
The estimated daily dietary intake of naturally occurring phytosterols ranges from 100 to 450 mg (Berger et al., 2004). Among edible oils, maize, wheat germ, and rapeseed oils have been shown to be the richest phytosterol sources (Phillips et al., 2002; Schwartz et al., 2008), however, higher stigmasterol level have been found for soybean oil (up to 82,4 mg/100 g) (Lechner et al., 1999). Normen et al. (2002) reported a mean phytosterol concentration for cereals of 49 mg/100 g; the median content of stigmasterol was 2 mg/100 g. A recent study analyzing ingredients used to enrich foods with phytosterols revealed high stigmasterol contents in two ingredients (6.6% stigmasterol and 17.8% stigmasterol of total phytosterols) (González-Larena et al., 2011). Compared with the stigmasterol content used for our in vivo studies, 190 mg/100 g and 390 mg/100 g, the stigmasterol contents in natural foods are lower. However, it has to be mentioned that mice were fed 6 weeks with the stigmasterol-enriched diets, and that this short time period already showed anti-amyloidogenic effects. In humans, the time period of dietary phytosterol intake would be expected to be longer and continuously. Therefore, functional foods might be suitable to achieve higher stigmasterol uptake in the human diet. However, whether the stigmasterol concentrations in functional foods are indeed sufficient to reduce Aβ generation in humans has still to be elucidated.
Conclusion
Food products containing plant sterols have been widely used as therapeutic diets or food supplements to lower plasma cholesterol, atherosclerosis risk, and the risk for coronary heart diseases (Plat and Mensink, 2001; Ostlund, 2002; Acuff et al., 2007; Calpe-Berdiel et al., 2009). However, up to now it has been controversially discussed whether these lipids are indeed protective. It is important to notice that mainly blends containing different phytosterols are used as food supplements and that studies systematically investigating potential differences between these phytosterols in respect to their biological properties and activities are still missing. It was recently shown that plant sterols can pass the blood–brain barrier and accumulate in the brain, underlying the importance of the question whether plant sterols are biologically active compounds in the brain. Their similarity to cholesterol also raises the question what kind of properties phytosterols might have in respect to AD. This issue was addressed by our study, where we investigated the effect of the major plant sterols—β-sitosterol, stigmasterol, brassicasterol, and campesterol—and cholesterol on their impact on the molecular mechanism leading to AD.
Our data show that plant sterols are biologically active food compounds interfering with important functional processes in the brain. In respect to Alzheimer's disease we could demonstrate that plant sterols are not as amyloidogenic as cholesterol, and that in particular stigmasterol decreases amyloidogenic processing and could therefore be an interesting food compound being beneficial in Alzheimer's disease.
However, we could also show that the underlying mechanisms, making phytosterols an interesting target for Alzheimer's disease, also affect membrane organization like cholesterol-enriched lipid raft membrane microdomains and endocytosis, which might also be relevant for other brain functions. Therefore, we cannot exclude that plant sterols might interfere with further critical lipid raft membrane functions, including cell signaling, neurotransmission, and membrane protein trafficking (Tsui-Pierchala et al., 2002), which should be addressed by further studies.
Footnotes
The research leading to these results has received funding from the EU FP7 project LipiDiDiet, Grant Agreement No 211696 (T.H.), the DFG (T.H.), the Bundesministerium für Bildung, Forschung, Wissenschaft und Technologie via NGFNplus and KNDD (T.H.), and the HOMFOR (M.O.W.G.) and HOMFOR excellence (M.O.W.G.). We thank Inge Tomic and Anja Kerksiek for technical assistance.
The authors declare no conflicts of interest.
References
- Abrami L, Liu S, Cosson P, Leppla SH, van der Goot FG. Anthrax toxin triggers endocytosis of its receptor via a lipid raft-mediated clathrin-dependent process. J Cell Biol. 2003;160:321–328. doi: 10.1083/jcb.200211018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Acuff RV, Cai DJ, Dong ZP, Bell D. The lipid lowering effect of plant sterol ester capsules in hypercholesterolemic subjects. Lipids Health Dis. 2007;6:11. doi: 10.1186/1476-511X-6-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berger A, Jones PJ, Abumweis SS. Plant sterols: factors affecting their efficacy and safety as functional food ingredients. Lipids Health Dis. 2004;3:5. doi: 10.1186/1476-511X-3-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bligh EG, Dyer WJ. A rapid method of total lipid extraction and purification. Can J Biochem Physiol. 1959;37:911–917. doi: 10.1139/o59-099. [DOI] [PubMed] [Google Scholar]
- Calpe-Berdiel L, Escolà-Gil JC, Blanco-Vaca F. New insights into the molecular actions of plant sterols and stanols in cholesterol metabolism. Atherosclerosis. 2009;203:18–31. doi: 10.1016/j.atherosclerosis.2008.06.026. [DOI] [PubMed] [Google Scholar]
- Corder EH, Saunders AM, Strittmatter WJ, Schmechel DE, Gaskell PC, Small GW, Roses AD, Haines JL, Pericak-Vance MA. Gene dose of apolipoprotein E type 4 allele and the risk of Alzheimer's disease in late onset families. Science. 1993;261:921–923. doi: 10.1126/science.8346443. [DOI] [PubMed] [Google Scholar]
- De Strooper B. Aph-1, Pen-2, and Nicastrin with Presenilin generate an active gamma-Secretase complex. Neuron. 2003;38:9–12. doi: 10.1016/S0896-6273(03)00205-8. [DOI] [PubMed] [Google Scholar]
- Duff RB. Carbohydrate sulphuric esters. Part V. The demonstration of Walden inversion on hydrolysis of barium 1:6-anhydro-beta-d-galactose 2-sulfate. J Am Chem Soc. 1949:1597–1600. [Google Scholar]
- Dufourc EJ. Sterols and membrane dynamics. J Chem Biol. 2008;1:63–77. doi: 10.1007/s12154-008-0010-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehehalt R, Keller P, Haass C, Thiele C, Simons K. Amyloidogenic processing of the Alzheimer beta-amyloid precursor protein depends on lipid rafts. J Cell Biol. 2003;160:113–123. doi: 10.1083/jcb.200207113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fassbender K, Simons M, Bergmann C, Stroick M, Lutjohann D, Keller P, Runz H, Kuhl S, Bertsch T, von Bergmann K, Hennerici M, Beyreuther K, Hartmann T. Simvastatin strongly reduces levels of Alzheimer's disease beta-amyloid peptides Abeta 42 and Abeta 40 in vitro and in vivo. Proc Natl Acad Sci U S A. 2001;98:5856–5861. doi: 10.1073/pnas.081620098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghribi O, Larsen B, Schrag M, Herman MM. High cholesterol content in neurons increases BACE, beta-amyloid, and phosphorylated tau levels in rabbit hippocampus. Exp Neurol. 2006;200:460–467. doi: 10.1016/j.expneurol.2006.03.019. [DOI] [PubMed] [Google Scholar]
- Glenner GG, Wong CW. Alzheimer's disease: initial report of the purification and characterization of a novel cerebrovascular amyloid protein. Biochem Biophys Res Commun. 1984;120:885–890. doi: 10.1016/S0006-291X(84)80190-4. [DOI] [PubMed] [Google Scholar]
- González-Larena M, García-Llatas G, Vidal MC, Sánchez-Siles LM, Barberá R, Lagarda MJ. Stability of plant sterols in ingredients used in functional foods. J Agric Food Chem. 2011;59:3624–3631. doi: 10.1021/jf1044102. [DOI] [PubMed] [Google Scholar]
- Grimm HS, Beher D, Lichtenthaler SF, Shearman MS, Beyreuther K, Hartmann T. gamma-Secretase cleavage site specificity differs for intracellular and secretory amyloid beta. J Biol Chem. 2003;278:13077–13085. doi: 10.1074/jbc.M210380200. [DOI] [PubMed] [Google Scholar]
- Grimm MO, Grimm HS, Pätzold AJ, Zinser EG, Halonen R, Duering M, Tschäpe JA, De Strooper B, Müller U, Shen J, Hartmann T. Regulation of cholesterol and sphingomyelin metabolism by amyloid-beta and presenilin. Nat Cell Biol. 2005;7:1118–1123. doi: 10.1038/ncb1313. [DOI] [PubMed] [Google Scholar]
- Grimm MO, Kuchenbecker J, Rothhaar TL, Grösgen S, Hundsdörfer B, Burg VK, Friess P, Müller U, Grimm HS, Riemenschneider M, Hartmann T. Plasmalogen synthesis is regulated via alkyl-dihydroxyacetonephosphate-synthase by amyloid precursor protein processing and is affected in Alzheimer's disease. J Neurochem. 2011a;116:916–925. doi: 10.1111/j.1471-4159.2010.07070.x. [DOI] [PubMed] [Google Scholar]
- Grimm MO, Kuchenbecker J, Grösgen S, Burg VK, Hundsdörfer B, Rothhaar TL, Friess P, de Wilde MC, Broersen LM, Penke B, Péter M, Vígh L, Grimm HS, Hartmann T. Docosahexaenoic acid reduces amyloid beta production via multiple pleiotropic mechanisms. J Biol Chem. 2011b;286:14028–14039. doi: 10.1074/jbc.M110.182329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grimm MO, Rothhaar TL, Grösgen S, Burg VK, Hundsdörfer B, Haupenthal VJ, Friess P, Kins S, Grimm HS, Hartmann T. Trans fatty acids enhance amyloidogenic processing of the Alzheimer amyloid precursor protein (APP) J Nutr Biochem. 2012;23:1214–1223. doi: 10.1016/j.jnutbio.2011.06.015. [DOI] [PubMed] [Google Scholar]
- Gunstone FD, Harwood JL, Padley FB. The lipid handbook, second edition. London: Chapman and Hall; 1994. [Google Scholar]
- Hansen GH, Dalskov SM, Rasmussen CR, Immerdal L, Niels-Christiansen LL, Danielsen EM. Cholera toxin entry into pig enterocytes occurs via a lipid raft- and clathrin-dependent mechanism. Biochemistry. 2005;44:873–882. doi: 10.1021/bi047959+. [DOI] [PubMed] [Google Scholar]
- Herreman A, Serneels L, Annaert W, Collen D, Schoonjans L, De Strooper B. Total inactivation of gamma-secretase activity in presenilin-deficient embryonic stem cells. Nat Cell Biol. 2000;2:461–462. doi: 10.1038/35017105. [DOI] [PubMed] [Google Scholar]
- Huse JT, Pijak DS, Leslie GJ, Lee VM, Doms RW. Maturation and endosomal targeting of beta-site amyloid precursor protein-cleaving enzyme. The Alzheimer's disease beta-secretase. J Biol Chem. 2000;275:33729–33737. doi: 10.1074/jbc.M004175200. [DOI] [PubMed] [Google Scholar]
- Ida N, Hartmann T, Pantel J, Schröder J, Zerfass R, Förstl H, Sandbrink R, Masters CL, Beyreuther K. Analysis of heterogeneous A4 peptides in human cerebrospinal fluid and blood by a newly developed sensitive Western blot assay. J Biol Chem. 1996;271:22908–22914. doi: 10.1074/jbc.271.37.22908. [DOI] [PubMed] [Google Scholar]
- Kimberly WT, LaVoie MJ, Ostaszewski BL, Ye W, Wolfe MS, Selkoe DJ. Gamma-secretase is a membrane protein complex comprised of presenilin, nicastrin, Aph-1, and Pen-2. Proc Natl Acad Sci U S A. 2003;100:6382–6387. doi: 10.1073/pnas.1037392100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kivipelto M, Helkala EL, Laakso MP, Hänninen T, Hallikainen M, Alhainen K, Soininen H, Tuomilehto J, Nissinen A. Midlife vascular risk factors and Alzheimer's disease in later life: longitudinal, population based study. BMJ. 2001;322:1447–1451. doi: 10.1136/bmj.322.7300.1447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kojro E, Gimpl G, Lammich S, Marz W, Fahrenholz F. Low cholesterol stimulates the nonamyloidogenic pathway by its effect on the alpha-secretase ADAM 10. Proc Natl Acad Sci U S A. 2001;98:5815–5820. doi: 10.1073/pnas.081612998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kölsch H, Heun R, Jessen F, Popp J, Hentschel F, Maier W, Lütjohann D. Alterations of cholesterol precursor levels in Alzheimer's disease. Biochim Biophys Acta. 2010;1801:945–950. doi: 10.1016/j.bbalip.2010.03.001. [DOI] [PubMed] [Google Scholar]
- Lechner M, Reiter B, Lorbeer E. Determination of tocopherols and sterols in vegetable oils by solid-phase extraction and subsequent capillary gas chromatographic analysis. J Chromatogr A. 1999;857:231–238. doi: 10.1016/S0021-9673(99)00751-7. [DOI] [PubMed] [Google Scholar]
- Lemkul JA, Bevan DR. Lipid composition influences the release of Alzheimer's amyloid beta-peptide from membranes. Protein Sci. 2011;20:1530–1545. doi: 10.1002/pro.678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu WW, Todd S, Coulson DT, Irvine GB, Passmore AP, McGuinness B, McConville M, Craig D, Johnston JA. A novel reciprocal and biphasic relationship between membrane cholesterol and beta-secretase activity in SH-SY5Y cells and in human platelets. J Neurochem. 2009;108:341–349. doi: 10.1111/j.1471-4159.2008.05753.x. [DOI] [PubMed] [Google Scholar]
- Marquer C, Devauges V, Cossec JC, Liot G, Lécart S, Saudou F, Duyckaerts C, Lévêque-Fort S, Potier MC. Local cholesterol increase triggers amyloid precursor protein-Bace1 clustering in lipid rafts and rapid endocytosis. FASEB J. 2011;25:1295–1305. doi: 10.1096/fj.10-168633. [DOI] [PubMed] [Google Scholar]
- Mongrand S, Morel J, Laroche J, Claverol S, Carde JP, Hartmann MA, Bonneu M, Simon-Plas F, Lessire R, Bessoule JJ. Lipid rafts in higher plant cells: purification and characterization of Triton X-100-insoluble microdomains from tobacco plasma membrane. J Biol Chem. 2004;279:36277–36286. doi: 10.1074/jbc.M403440200. [DOI] [PubMed] [Google Scholar]
- Mongrand S, Stanislas T, Bayer EM, Lherminier J, Simon-Plas F. Membrane rafts in plant cells. Trends Plant Sci. 2010;15:656–663. doi: 10.1016/j.tplants.2010.09.003. [DOI] [PubMed] [Google Scholar]
- Normen L, Bryngelsson S, Johnsson M, Evheden P, Ellegard L, Brants H, Andersson H, Dutta P. The phytosterol content of some cereal foods commonly consumed in Sweden and in The Netherlands. J Food Compost Anal. 2002;15:693–704. doi: 10.1006/jfca.2002.1098. [DOI] [Google Scholar]
- Osenkowski P, Ye W, Wang R, Wolfe MS, Selkoe DJ. Direct and potent regulation of gamma-secretase by its lipid microenvironment. J Biol Chem. 2008;283:22529–22540. doi: 10.1074/jbc.M801925200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ostlund RE., Jr Phytosterols in human nutrition. Annu Rev Nutr. 2002;22:533–549. doi: 10.1146/annurev.nutr.22.020702.075220. [DOI] [PubMed] [Google Scholar]
- Parks AL, Curtis D. Presenilin diversifies its portfolio. Trends Genet. 2007;23:140–150. doi: 10.1016/j.tig.2007.01.008. [DOI] [PubMed] [Google Scholar]
- Parr-Sturgess CA, Rushton DJ, Parkin ET. Ectodomain shedding of the Notch ligand Jagged1 is mediated by ADAM17, but is not a lipid-raft-associated event. Biochem J. 2010;432:283–294. doi: 10.1042/BJ20100321. [DOI] [PubMed] [Google Scholar]
- Parsons RB, Subramaniam D, Austen BM. A specific inhibitor of cholesterol biosynthesis, BM15.766, reduces the expression of beta-secretase and the production of amyloid-beta in vitro. J Neurochem. 2007;102:1276–1291. doi: 10.1111/j.1471-4159.2007.04619.x. [DOI] [PubMed] [Google Scholar]
- Phillips KM, Ruggio DM, Toivo JI, Swank MA, Simpkins AH. Free and esterified sterol composition of edible oils and fats. J Food Compost Anal. 2002;15:123–142. doi: 10.1006/jfca.2001.1044. [DOI] [Google Scholar]
- Piironen V, Lindsay DG, Miettinen TA, Toivo J, Lampi AM. Plant sterols: biosynthesis, biological function and their importance to human nutrition. J Sci Food Agric. 2000;80:939–966. doi: 10.1002/(SICI)1097-0010(20000515)80:7<939::AID-JSFA644>3.0.CO;2-C. [DOI] [Google Scholar]
- Plat J, Mensink RP. Effects of plant sterols and stanols on lipid metabolism and cardiovascular risk. Nutr Metab Cardiovasc Dis. 2001;11:31–40. [PubMed] [Google Scholar]
- Probst G, Aubele DL, Bowers S, Dressen D, Garofalo AW, Hom RK, Konradi AW, Marugg JL, Mattson MN, Neitzel ML, Semko CM, Sham HL, Smith J, Sun M, Truong AP, Ye XM, Xu YZ, Dappen MS, Jagodzinski JJ, Keim PS, et al. Discovery of (R)-4-Cyclopropyl-7,8-difluoro-5-(4-(trifluoromethyl)phenylsulfonyl)-4,5-dihydro-1H-pyrazolo[4,3-c]quinoline (ELND006) and (R)-4-Cyclopropyl-8-fluoro-5-(6-(trifluoromethyl)pyridin-3-ylsulfonyl)-4,5-dihydr o-2H-pyrazolo[4,3-c]quinoline (ELND007): metabolically stable gamma-secretase inhibitors that selectively inhibit the production of amyloid-beta over notch. J Med Chem. 2013 doi: 10.1021/jm301741t. doi: 10.1021/jm301741t. Advance online publication. Retrieved June 20, 2103. [DOI] [PubMed] [Google Scholar]
- Puglielli L, Tanzi RE, Kovacs DM. Alzheimer's disease: the cholesterol connection. Nat Neurosci. 2003;6:345–351. doi: 10.1038/nn0403-345. [DOI] [PubMed] [Google Scholar]
- Reeves PG, Nielsen FH, Fahey GC., Jr AIN-93 purified diets for laboratory rodents: final report of the American Institute of Nutrition ad hoc writing committee on the reformulation of the AIN-76A rodent diet. J Nutr. 1993;123:1939–1951. doi: 10.1093/jn/123.11.1939. [DOI] [PubMed] [Google Scholar]
- Refolo LM, Malester B, LaFrancois J, Bryant-Thomas T, Wang R, Tint GS, Sambamurti K, Duff K, Pappolla MA. Hypercholesterolemia accelerates the Alzheimer's amyloid pathology in a transgenic mouse model. Neurobiol Dis. 2000;7:321–331. doi: 10.1006/nbdi.2000.0304. [DOI] [PubMed] [Google Scholar]
- Refolo LM, Pappolla MA, LaFrancois J, Malester B, Schmidt SD, Thomas-Bryant T, Tint GS, Wang R, Mercken M, Petanceska SS, Duff KE. A cholesterol-lowering drug reduces beta-amyloid pathology in a transgenic mouse model of Alzheimer's disease. Neurobiol Dis. 2001;8:890–899. doi: 10.1006/nbdi.2001.0422. [DOI] [PubMed] [Google Scholar]
- Richelle M, Enslen M, Hager C, Groux M, Tavazzi I, Godin JP, Berger A, Métairon S, Quaile S, Piguet-Welsch C, Sagalowicz L, Green H, Fay LB. Both free and esterified plant sterols reduce cholesterol absorption and the bioavailability of beta-carotene and alpha-tocopherol in normocholesterolemic humans. Am J Clin Nutr. 2004;80:171–177. doi: 10.1093/ajcn/80.1.171. [DOI] [PubMed] [Google Scholar]
- Riddell DR, Christie G, Hussain I, Dingwall C. Compartmentalization of beta-secretase (Asp2) into low-buoyant density, noncaveolar lipid rafts. Curr Biol. 2001;11:1288–1293. doi: 10.1016/S0960-9822(01)00394-3. [DOI] [PubMed] [Google Scholar]
- Rodal SK, Skretting G, Garred O, Vilhardt F, van Deurs B, Sandvig K. Extraction of cholesterol with methyl-beta-cyclodextrin perturbs formation of clathrin-coated endocytic vesicles. Mol Biol Cell. 1999;10:961–974. doi: 10.1091/mbc.10.4.961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rohan de Silva HA, Jen A, Wickenden C, Jen LS, Wilkinson SL, Patel AJ. Cell-specific expression of beta-amyloid precursor protein isoform mRNAs and proteins in neurons and astrocytes. Brain Res Mol Brain Res. 1997;47:147–156. doi: 10.1016/S0169-328X(97)00045-4. [DOI] [PubMed] [Google Scholar]
- Rollason R, Korolchuk V, Hamilton C, Schu P, Banting G. Clathrin-mediated endocytosis of a lipid-raft-associated protein is mediated through a dual tyrosine motif. J Cell Sci. 2007;120:3850–3858. doi: 10.1242/jcs.003343. [DOI] [PubMed] [Google Scholar]
- Sakurai T, Kaneko K, Okuno M, Wada K, Kashiyama T, Shimizu H, Akagi T, Hashikawa T, Nukina N. Membrane microdomain switching: a regulatory mechanism of amyloid precursor protein processing. J Cell Biol. 2008;183:339–352. doi: 10.1083/jcb.200804075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz H, Ollilainen V, Piironen V, Lampi AM. Tocopherol, tocotrienol and plant sterol contents of vegetable oils and industrial fats. J Food Compost Anal. 2008;21:152–161. doi: 10.1016/j.jfca.2007.07.012. [DOI] [Google Scholar]
- Shearman MS, Beher D, Clarke EE, Lewis HD, Harrison T, Hunt P, Nadin A, Smith AL, Stevenson G, Castro JL. L-685,458, an aspartyl protease transition state mimic, is a potent inhibitor of amyloid beta-protein precursor gamma-secretase activity. Biochemistry. 2000;39:8698–8704. doi: 10.1021/bi0005456. [DOI] [PubMed] [Google Scholar]
- Shie FS, Jin LW, Cook DG, Leverenz JB, LeBoeuf RC. Diet-induced hypercholesterolemia enhances brain A beta accumulation in transgenic mice. Neuroreport. 2002;13:455–459. doi: 10.1097/00001756-200203250-00019. [DOI] [PubMed] [Google Scholar]
- Simons K, Ikonen E. Functional rafts in cell membranes. Nature. 1997;387:569–572. doi: 10.1038/42408. [DOI] [PubMed] [Google Scholar]
- Sinha S, Anderson JP, Barbour R, Basi GS, Caccavello R, Davis D, Doan M, Dovey HF, Frigon N, Hong J, Jacobson-Croak K, Jewett N, Keim P, Knops J, Lieberburg I, Power M, Tan H, Tatsuno G, Tung J, Schenk D, Seubert P, Suomensaari SM, Wang S, Walker D, Zhao J, McConlogue L, John V. Purification and cloning of amyloid precursor protein beta-secretase from human brain. Nature. 1999;402:537–540. doi: 10.1038/990114. [DOI] [PubMed] [Google Scholar]
- Sisodia SS, St George-Hyslop PH. gamma-Secretase, Notch, Abeta and Alzheimer's disease: where do the presenilins fit in? Nat Rev Neurosci. 2002;3:281–290. doi: 10.1038/nrn785. [DOI] [PubMed] [Google Scholar]
- Smith PK, Krohn RI, Hermanson GT, Mallia AK, Gartner FH, Provenzano MD, Fujimoto EK, Goeke NM, Olson BJ, Klenk DC. Measurement of protein using bicinchoninic acid. Anal Biochem. 1985;150:76–85. doi: 10.1016/0003-2697(85)90442-7. [DOI] [PubMed] [Google Scholar]
- Stoddart A, Dykstra ML, Brown BK, Song W, Pierce SK, Brodsky FM. Lipid rafts unite signaling cascades with clathrin to regulate BCR internalization. Immunity. 2002;17:451–462. doi: 10.1016/S1074-7613(02)00416-8. [DOI] [PubMed] [Google Scholar]
- Subtil A, Gaidarov I, Kobylarz K, Lampson MA, Keen JH, McGraw TE. Acute cholesterol depletion inhibits clathrin-coated pit budding. Proc Natl Acad Sci U S A. 1999;96:6775–6780. doi: 10.1073/pnas.96.12.6775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teunissen CE, De Vente J, von Bergmann K, Bosma H, van Boxtel MP, De Bruijn C, Jolles J, Steinbusch HW, Lütjohann D. Serum cholesterol, precursors and metabolites and cognitive performance in an aging population. Neurobiol Aging. 2003;24:147–155. doi: 10.1016/S0197-4580(02)00061-1. [DOI] [PubMed] [Google Scholar]
- Thirumangalakudi L, Prakasam A, Zhang R, Bimonte-Nelson H, Sambamurti K, Kindy MS, Bhat NR. High cholesterol-induced neuroinflammation and amyloid precursor protein processing correlate with loss of working memory in mice. J Neurochem. 2008;106:475–485. doi: 10.1111/j.1471-4159.2008.05415.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsui-Pierchala BA, Encinas M, Milbrandt J, Johnson EM., Jr Lipid rafts in neuronal signaling and function. Trends Neurosci. 2002;25:412–417. doi: 10.1016/S0166-2236(02)02215-4. [DOI] [PubMed] [Google Scholar]
- Vance JE, Hayashi H, Karten B. Cholesterol homeostasis in neurons and glial cells. Semin Cell Dev Biol. 2005;16:193–212. doi: 10.1016/j.semcdb.2005.01.005. [DOI] [PubMed] [Google Scholar]
- Vanmierlo T, Weingärtner O, van der Pol S, Husche C, Kerksiek A, Friedrichs S, Sijbrands E, Steinbusch H, Grimm M, Hartmann T, Laufs U, Böhm M, de Vries HE, Mulder M, Lütjohann D. Dietary intake of plant sterols stably increases plant sterol levels in the murine brain. J Lipid Res. 2012;53:726–735. doi: 10.1194/jlr.M017244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vassar R, Bennett BD, Babu-Khan S, Kahn S, Mendiaz EA, Denis P, Teplow DB, Ross S, Amarante P, Loeloff R, Luo Y, Fisher S, Fuller J, Edenson S, Lile J, Jarosinski MA, Biere AL, Curran E, Burgess T, Louis JC, Collins F, Treanor J, Rogers G, Citron M. Beta-secretase cleavage of Alzheimer's amyloid precursor protein by the transmembrane aspartic protease BACE. Science. 1999;286:735–741. doi: 10.1126/science.286.5440.735. [DOI] [PubMed] [Google Scholar]
- Vetrivel KS, Thinakaran G. Membrane rafts in Alzheimer's disease beta-amyloid production. Biochim Biophys Acta. 2010;1801:860–867. doi: 10.1016/j.bbalip.2010.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wahrle S, Das P, Nyborg AC, McLendon C, Shoji M, Kawarabayashi T, Younkin LH, Younkin SG, Golde TE. Cholesterol-dependent gamma-secretase activity in buoyant cholesterol-rich membrane microdomains. Neurobiol Dis. 2002;9:11–23. doi: 10.1006/nbdi.2001.0470. [DOI] [PubMed] [Google Scholar]
- Weihrauch JL, Gardner JM. Sterol content of foods of plant origin. J Am Diet Assoc. 1978;73:39–47. [PubMed] [Google Scholar]
- Willem M, Garratt AN, Novak B, Citron M, Kaufmann S, Rittger A, DeStrooper B, Saftig P, Birchmeier C, Haass C. Control of peripheral nerve myelination by the beta-secretase BACE1. Science. 2006;314:664–666. doi: 10.1126/science.1132341. [DOI] [PubMed] [Google Scholar]
- Wolozin B. A fluid connection: cholesterol and Abeta. Proc Natl Acad Sci U S A. 2001;98:5371–5373. doi: 10.1073/pnas.101123198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong HK, Sakurai T, Oyama F, Kaneko K, Wada K, Miyazaki H, Kurosawa M, De Strooper B, Saftig P, Nukina N. beta Subunits of voltage-gated sodium channels are novel substrates of beta-site amyloid precursor protein-cleaving enzyme (BACE1) and gamma-secretase. J Biol Chem. 2005;280:23009–23017. doi: 10.1074/jbc.M414648200. [DOI] [PubMed] [Google Scholar]
- Woods AJ, White DP, Caswell PT, Norman JC. PKD1/PKCmu promotes alphavbeta3 integrin recycling and delivery to nascent focal adhesions. EMBO J. 2004;23:2531–2543. doi: 10.1038/sj.emboj.7600267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiong H, Callaghan D, Jones A, Walker DG, Lue LF, Beach TG, Sue LI, Woulfe J, Xu H, Stanimirovic DB, Zhang W. Cholesterol retention in Alzheimer's brain is responsible for high beta- and gamma-secretase activities and Abeta production. Neurobiol Dis. 2008;29:422–437. doi: 10.1016/j.nbd.2007.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang LB, Lindholm K, Yan R, Citron M, Xia W, Yang XL, Beach T, Sue L, Wong P, Price D, Li R, Shen Y. Elevated beta-secretase expression and enzymatic activity detected in sporadic Alzheimer disease. Nat Med. 2003;9:3–4. doi: 10.1038/nm0103-3. [DOI] [PubMed] [Google Scholar]