Abstract
The sense of touch allows an organism to detect and respond to physical environmental stimuli. Mechanosensitive proteins play a crucial role in this process by converting the mechanical cue into a biological response. Recently, the Piezo family of stretch-activated ion channels has been identified as genuine mechanosensitive proteins. We set out to determine whether any of these genes are involved in touch response during zebrafish development. In situ hybridization indicates that piezo2b is specifically expressed in a subset of neurons (Rohon–Beard cells) responsible for detecting light touch. Using morpholino-mediated knockdown, we specifically targeted piezo2b and determined that it is involved in mediating touch-evoked response.
Introduction
Touch is a fundamental ability that allows organisms to sense external environmental forces and elicit an appropriate response. Touch can generate a diverse range of behavioral responses depending on the initial stimuli; for example, touch can induce avoidance from a potential hazard or elicit social interactions (Lumpkin et al., 2010). To detect and ultimately produce a specific response, a multitude of specialized cell types have arisen, possessing specific mechanosensory molecules dedicated toward determining particular environmental forces (Lumpkin et al., 2010). Mechanosensitive ion channels, present in the cell membrane, represent a class of such molecules that can detect mechanical stimuli and convert them into a biological response (Xiao and Xu, 2010). Although mechanosensitive ion channels have been detected in virtually all organisms via electrophysiological recordings, the difficulties associated with expressing putative channels in systems that would allow for a detailed analysis have meant that their identities remain largely elusive (Hamill and Martinac, 2001; Xiao and Xu, 2010). Recently, the family of PIEZO proteins have been identified as genuine mechanosensitive ion channels (Coste et al., 2010). These stretch-activated channels are able detect changes in cell membrane tension resulting in the opening of the channel and an influx of cations (Coste et al., 2010; Gottlieb and Sachs, 2012). Homologs of the piezo genes have been identified in a variety of species ranging from protozoa to vertebrates (Coste et al., 2010). Mammals have two piezo genes, piezo1 (fam38A) and piezo2 (fam38B). These genes are expressed in a number of mechanosensitive tissues such as kidney, lung, and colon. In particular, piezo1 is highly expressed in the skin, while piezo2 shows an elevated expression pattern in dorsal root ganglion cells (Coste et al., 2010).
Determining the biological processes regulated by the piezo genes is currently the subject of intense investigation (Nilius and Honoré, 2012). Recently, the piezo genes have been linked to mechanical nociception. The fruit fly Drosophila melanogaster has a single homolog Dmpiezo. Consequently, Dmpiezo knock-out larvae fail to respond to noxious mechanical stimuli yet retain normal responsiveness to touch and other noxious stimuli (Kim et al., 2012).
We sought to determine whether any of the piezo genes were involved in regulating touch response in vertebrates. Zebrafish acquire the ability to respond to light touch between 24 and 27 hours post-fertilization (hpf), as evidenced by the writhing behavioral response exhibited by embryos following tactile stimulation (Pietri et al., 2009). The mechanosensitive Rohon–Beard cells innervating the skin mediate touch responsiveness in zebrafish embryos via their free nerve endings (Metcalfe et al., 1990; Nakano et al., 2010). Here we report that piezo2b is expressed in Rohon–Beard cells of developing zebrafish embryos and is essential for mediating touch response.
Materials and Methods
Zebrafish strains and husbandry.
Zebrafish were maintained under standardized conditions, and experiments were conducted in accordance with the European Communities council directive of November 1986. Embryos were staged according to Kimmel et al. (1995); however, it is not possible to determine the sex at these stages of development.
Morpholinos and injections.
Morpholino oligonucleotides (MOs) were obtained from Gene Tools and were injected into one-cell stage embryos. The Pz2b MO1 morpholino targets the start codon of piezo2b. The Pz2b MO2 morpholino targets the 5′ untranslated region (UTR) of piezo2b and does not overlap with the Pz2b 1 morpholino. The Pz2b MO3 morpholino targets the exon1/intron1 splice site of piezo2b. The trpa1b MO targets the start codon of trpa1b. The sequences of the injected MOs are as follows: Pz2b MO1(ATG), 5′-GCGCAGATGTAGCTCTGCTTCATGA-3′; Pz2b MO2(5′UTR), 5′-ACTTGAACACTTTGACGAGAGCTGC-3′; Pz2b MO3(splice), 5′-GTCTATATTATACCATCATAGCGAT-3′; and Trpa1b MO(ATG), 5′-TCACTAACTCCTTTCCAAACTGCAT-3′.
In situ hybridization.
In situ hybridization were performed as described previously (Jopling and den Hertog, 2005). The piezo probes were generated by cloning an ∼500 bp fragment of each gene from embryonic cDNA. The trpa1b probe was a gift from Alex Schier (Harvard University, Cambridge, MA; Prober et al., 2008). Fragments were ligated into the pGEM-T EASY vector and subsequently linearized. Antisense probes were synthesized as described previously (Jopling and den Hertog, 2005). Two-color and high-resolution fluorescent, double-whole-mount in situ hybridizations were performed as described previously (Jowett, 2001; Brend and Holley, 2009).
Immunohistochemistry.
For immunohistochemistry, embryos were fixed overnight at 4°C in a solution of 4% paraformaldehyde in PBS. After fixation, samples were washed in PBS with 0.1% Tween-20 (PBST) and blocked at room temperature with 10% bovine serum albumin in PBST. Primary antibody incubation was conducted overnight at 4°C in blocking buffer with the zn-12 antibody (ZIRC, University of Oregon, Eugene, OR) at a 1:100 dilution. Secondary-antibody incubation was conducted overnight at 4°C in PBST with an Alexa Fluor-594 anti-mouse antibody (Jackson ImmunoResearch) at a 1:1000 dilution.
Light touch response.
Touch-evoked behaviors were elicited by touching an embryo/larvae up to three times with a pair of No. 5 forceps between 24 and 27 hpf, as previously described (Low et al., 2010). A response was deemed positive when writhing behavior was observed.
Tail pinch assay.
Tail pinch-evoked behaviors were elicited by pinching the tail of an embryo/larvae with a pair of No. 5 forceps between 24 and 27 hpf, as previously described (Low et al., 2010). A response was deemed positive when writhing behavior was observed.
Mustard oil assay.
Chemosensory-evoked behavior was elicited by placing embryos in a 500 μm mustard oil solution diluted in embryo media (06019–10M, Fluka), as previously described (Low et al., 2010). A response was deemed positive when writhing behavior was observed. For more detailed analysis, time-lapse images were recorded. Single writhes were counted when the tail was coiled either to the left or right of the embryo.
Mosaic Rohon–Beard cell labeling and counting.
A HuC:mem-TdTomato construct was used to mosaically label Rohon–Beard cells, as described previously (Zhao et al., 2006; Faucherre et al., 2009). For counting Rohon–Beard cells, embryos were first labeled with the zn-12 antibody. Subsequently, a bright-field image was made to identify the somite boundaries, then the Rohon–Beard soma in the closest focal plane were counted.
Imaging.
Behaviors and still images of fixed embryos were recorded using a CoolSNAP Color cf camera (Photometrics) mounted on a stereomicroscope (Zeiss). Images were acquired with MetaMorph software. Fluorescent in situ hybridization and immunohistochemistry images were acquired with a Leica TCS SP8 inverted confocal laser scanning microscope with a 20×, 40×, or 63× oil-immersion objective. z-stacks were acquired at 0.5, 2, or 3 μm intervals, using Alexa Fluor-594 for imaging (561 nm excitation, 585–610 nm emission). Maximal projections and analysis were performed with ImageJ software.
Results
The zebrafish homologs of piezo1 and piezo2a have been previously identified (GenBank accession No. XP_696355.4 and XP_002666625, respectively; Coste et al., 2010; Faucherre et al., 2013). However, in silico analysis using the Ensembl database indicates a third homolog, piezo2b (GenBank accession No. XP_003198010), is also present in zebrafish. Gene duplication is not uncommon in zebrafish and is most likely caused by a whole-genome duplication event during zebrafish evolution (Hultman et al., 2007). Protein sequence analysis indicates that the zebrafish PIEZOS show a similar homology to their mammalian counterparts: human PIEZO1 versus zebrafish PIEZO1, 59.2%; human PIEZO2 versus zebrafish PIEZO2A, 66.9%; and human PIEZO2 versus zebrafish PIEZO2B, 62.6%. To determine the expression of the piezo genes during early development, we designed antisense RNA probes covering the initial 500 bp of each gene. In situ hybridization analysis reveals that only piezo2b is expressed in the Rohon–Beard cells throughout the tail and in the trigeminal ganglion in the head, similar to the chemosensory trpa1b ion channel (Prober et al., 2008; Fig. 1A–G). Consequently, we focused our attention on piezo2b to determine whether it played a role in the touch response elicited by this set of neurons.
Figure 1.
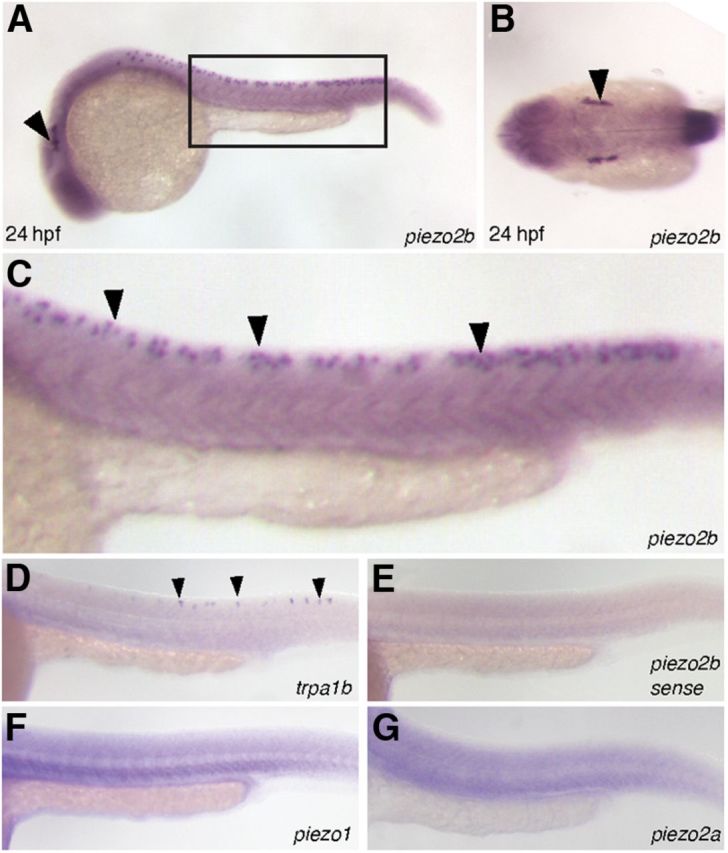
The expression pattern of piezo2b during zebrafish development. A–C, In situ hybridization analysis using an antisense piezo2b probe at 24 hpf. A, Piezo2b expression can be observed in the trigeminal ganglion (arrowhead) and Rohon–Beard cells (box). B, Dorsal view showing piezo2b expression in the trigeminal ganglion (arrowhead). C, Higher magnification of the boxed region in A. Piezo2b expression can be detected in the Rohon–Beard cells (arrowheads). E, To ensure that these observations were specific, we performed an identical assay using a sense piezo2b probe, which did not label anything. D, In situ hybridization analysis using an antisense trpa1b probe indicates that this gene is also expressed in Rohon–Beard neurons (arrowheads). F, G, The other two zebrafish piezo homologs, piezo1 and piezo2a, do not appear to be expressed in Rohon–Beard cells.
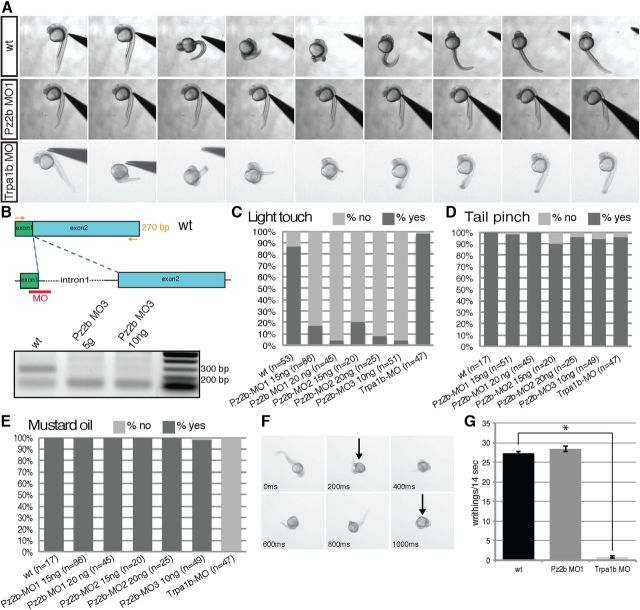
To target piezo2b during zebrafish development, we used nonoverlapping antisense morpholino oligonucleotides targeting either the start codon or the 5′ UTR (Pz2b-MO1 and Pz2b-MO2). Morpholinos were titrated to concentrations that did not cause any gross developmental defects/necrosis. To determine whether piezo2b genes have a role in touch response, we performed a previously described light touch assay (Low et al., 2010). Control embryos responded normally to light touch stimulation exhibiting a writhing behavioral response (Fig. 2A,C). Conversely, piezo2b morphants showed a complete lack of touch response to tactile stimulation (Fig. 2A,C).
Figure 2.
Piezo2b morphants are unresponsive to light touch but respond normally to mechanical and chemical stimuli. Touch response stimulation was performed on 24–27 hpf wild-type and piezo2b morphant embryos. A, wt panel, Time-lapse images of a wild-type embryo's response to touch-evoked stimuli. Piezo2b morphant embryos fail to respond to touch response stimulation (Pz2b MO panel). Trpa1b morphants respond normally to light touch (Trpa1b MO panel). B, RT-PCR analysis of Pz2b-MO3 morphant embryos. The Pz2b-MO3 targets the exon1/intron1 splice site. Primers designed to the start of exon1 and the end of exon 2 (arrows) are able to amplify the correct 270 bp fragment (top band) from wild-type embryo cDNA (lane 1). With morphant embryo cDNA, this band disappears, indicating that the correct splicing of piezo2b has been disrupted (excess primers form the lower band in all lanes). C, Quantitative analysis of wild-type, piezo2b, and trpa1b morphants subjected to touch response stimulation. Piezo2b morphants generated with morpholinos targeting the start codon (Pz2b-MO1), a nonoverlapping morpholino directed toward the 5′ UTR (Pz2b-MO2), or a splice-blocking morpholino targeting the exon1/intron1 boundary (Pz2b-MO3) fail to respond to tactile stimulation. The effect is enhanced slightly by increasing the concentration of morpholinos. Trpa1b morphants (Trpa1b-MO) respond normally in this assay (n = cumulative number of embryos from three independent assays). D, A tail pinch assay was performed on the same wild-type, piezo2b, and trpa1b morphants used in the previous light touch assay. The response of piezo2b and trpa1b morphants was indistinguishable from that of wild-type control embryos (n = cumulative number of embryos from three independent assays). E, Piezo2b morphants respond the same as control embryos when challenged with mustard oil. The assay was performed on the same wild-type and piezo2b morphants used in the previous light touch assay. However, mustard oil fails to elicit a response from trpa1b morphants (Trpa1b-MO; n = cumulative number of embryos from three independent assays). To analyze this phenotype in more detail, time-lapse images were recorded allowing us to count the number of writhings in response to mustard oil stimulation, an example of a wild-type embryo response is shown in F (black arrow indicates when a single writhe has been initiated). G, Piezo2b morphants respond to mustard oil with a similar number of writhings to that observed in wild-type embryos, while trpa1b morphants barely elicit a response to mustard oil (n = 10 embryos analyzed for each condition). Error bars indicate ±SEM. *p < 0.001, two-tailed Student's t test.
To ensure that the defect we observed in piezo2b morphants was specific for the light touch response, and was not due to a global neural defect, we performed a number of other behavioral assays. Tail pinching also elicits a similar writhing behavior; however, in mutant zebrafish lacking a light touch response, this assay still produces an effect, indicating that the sensory systems used to detect either stimulus are distinct (Low et al., 2010). We found that piezo2b morphants respond normally to the tail pinch assay compared with control embryos, indicating that the mechanical nociceptive system used to detect these stimuli is unaffected by piezo2b knockdown (Fig. 2D). As an additional control, we designed a third piezo2b morpholino (Pz2b-MO3) targeting the exon1/intron1 splice site (Fig. 2B). RT-PCR analysis of Pz2b-MO3 morphant embryos confirmed that normal splicing of the target mRNA had been disrupted (Fig. 2B). We subsequently found that knockdown of piezo2b using the Pz2b-MO3 morpholino also resulted in a loss of light touch response without affecting the embryos' ability to react to tail pinch stimuli (Fig. 2C,D). Zebrafish embryos have previously been shown to respond to noxious chemical stimuli such as mustard oil with increased locomotive activity (Prober et al., 2008). The ion channel trpa1b, which is also expressed in Rohon–Beard neurons (Fig. 1D), is essential for this chemosensation. Consequently, trpa1b mutant embryos do not respond to mustard oil; however, they do retain touch responsiveness (Prober et al., 2008). These observations suggest that Rohon–Beard neurons are able to detect diverse sensory stimuli. To further investigate these intriguing observations we also designed a morpholino to trpa1b (trpa1b-MO). We found that knockdown of trpa1b did not affect either the light touch or mechanical nociceptive response of zebrafish embryos (Fig. 2A,C,D). However, trpa1b morphants did display a severely reduced chemosensory response to mustard oil (Fig. 2E–G). Conversely, we were unable to detect any observable difference in the response of piezo2b morphants to mustard oil when compared with control embryos (Fig. 2E–G). Together, these results indicate that the loss of light touch response observed in piezo2b morphants is not due to a global neural defect.
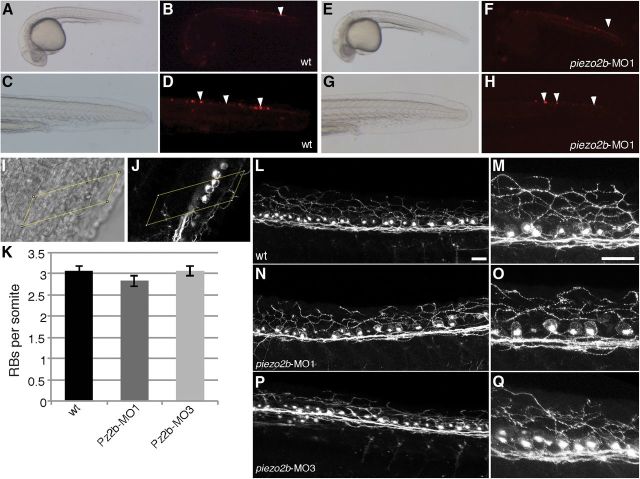
Next we assessed whether piezo2b knockdown affected Rohon–Beard cell development/homeostasis. One possible explanation for the light touch response defect we observed in piezo2b morphants is that knockdown of this gene results in the loss of or disrupts the function of Rohon–Beard cells. To analyze this, we used a previously reported HuC:mem-TdTomato construct, which, when injected into zebrafish embryos, mosaically labels a subset of neurons including Rohon–Beard cells (Zhao et al., 2006; Faucherre et al., 2009; Fig. 3A–H). piezo2b morphants were first selected by performing the light touch stimulation to ensure that they did not respond to this assay. In this manner, we were able to clearly detect Rohon–Beard cells in piezo2b morphants, indicating that these neurons are still present (Fig. 3A–H). To further ensure that Rohon–Beard cell numbers did not differ between wild-type and morphant embryos, we performed immunohistochemistry using the zn-12 antibody, which effectively labels Rohon–Beard cells (Metcalfe et al., 1990). In this manner, we were able to determine that the average number of Rohon–Beard cells/somites did not differ significantly between wild-type and morphant embryos (Fig. 3I–K). More detailed analysis revealed that the gross morphology of neurites extending from Rohon–Beard soma appears to be unaffected in piezo2b morphants (Fig. 3L–Q). Together, these results indicate that knockdown of piezo2b does not affect Rohon–Beard cell development or homeostasis as these neurons are present and appear normal in piezo2b morphant embryos.
Figure 3.
Rohon–Beard cells develop normally in piezo2b morphants. A–D, Rohon–Beard cells are clearly visible in wild-type embryos mosaically expressing mem-TdTomato under the control of the HuC promoter. A, Bright-field image of a wild-type embryo at 24 hpf mosaically expressing HuC:mem-TdTomato. B, The same embryo viewed with RFP-filtered UV light. The Rohon–Beard cells are indicated with arrowheads. C, D, The same embryo at higher magnification. The Rohon–Beard cells are indicated with arrowheads. E, Bright-field image of a piezo2b morphant embryo at 24 hpf mosaically expressing HuC:mem-TdTomato. F, The same embryo viewed with RFP-filtered UV light. The Rohon–Beard cells are indicated with arrowheads. G, H, The same embryo at higher magnification. The Rohon–Beard cells are indicated with arrowheads. Note: because the HuC promoter-driven mem-TdTomato expression is mosaic, this is not a quantitative assay and only illustrates that Rohon–Beard cells are present in piezo2b morphants. I–K, The average number of Rohon–Beard cells does not change following piezo2b knockdown. I, Bright-field image of a 24 hpf embryo immunolabeled with the zn-12 antibody; the yellow box delineates the somite boundaries. J, Fluorescent image of the same embryo. Three Rohon–Beard cell soma are clearly visible within the somite boundary (yellow box). K, Graph indicating the average number of Rohon–Beard cells (RB)/somite in wild-type (wt) embryos, and in two different piezo2b morphant groups (n = 6 embryos/condition, 5 somites/embryo). Error bar indicates ±SEM. Somite data were pooled, and no statistical difference was observed between these groups. L–Q, The gross morphology of Rohon–Beard soma and neurites is unaffected following piezo2b knockdown. L, Fluorescent image of a wild-type embryo labeled with the zn-12 antibody; peripheral neurites can be seen extending from individual Rohon–Beard soma to innervate the skin. Scale bar, 25 μm. M, The same image at a higher magnification. Scale bar, 25 μm. N–Q, Similar images taken from two different piezo2b morphant groups; the gross morphology of the Rohon–Beard soma and their neurites appears normal when compared with that of the wild-type controls (n = 6 embryos analyzed/condition).
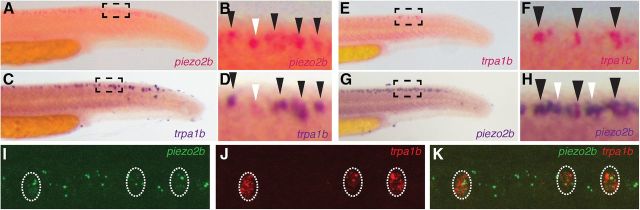
Because piezo2b and trpa1b are both expressed in Rohon–Beard neurons, this raises two possible scenarios regarding the differences in the behavioral responses we have observed between these respective morphant embryos. First, piezo2b and trpa1b could be expressed in distinct subsets of Rohon–Beard neurons; consequently, the detection of different sensory stimuli could be restricted to defined populations of these cells. The second possibility is that both of these genes are expressed in the same neurons that subsequently perform a multisensory role. To ascertain which of these hypotheses is correct, we performed a variety of double in situ hybridization assays on zebrafish embryos. Using a two-color approach, we found that 100% of trpa1b-positive Rohon–Beard neurons also express peizo2b (Fig. 4A–H); however, it was also possible to detect numerous piezo2b-positive/trpa1b-negative Rohon–Beard cells (n = 34 embryos analyzed; Fig. 4A–H). This indicates that trpa1b is expressed in a subset of piezo2b-positive Rohon–Beard neurons. To further substantiate these findings, we performed double-fluorescent in situ hybridization assays for both piezo2b and trpa1b. Consequently, we found that 100% of trpa1b-positive Rohon–Beard cells were also positive for piezo2b, and, as we had observed in the previous assays, we were also able to detect piezo2b-positive/trpa1b-negative Rohon–Beard neurons (n = 16 embryos analyzed; Fig. 4I–K). Because all trpa1b-positive Rohon–Beard neurons are also positive for piezo2b, we determined that knockdown of piezo2b does not disrupt the overall functionality of these cells. Despite being unable to sense light touch due to the loss of piezo2b, zebrafish are still perfectly capable of responding to chemosensory stimuli. Together, this leads us to conclude that piezo2b is essential for sensing light touch.
Figure 4.
A subset of piezo2b-expressing Rohon–Beard cells also express trpa1b. A–D, Two-color in situ hybridization with piezo2b labeled in red (A, B) and trpa1b in blue (C, D). Individual embryos were initially imaged to detect the red stain. Subsequently, following the blue stain, the same individual embryos were imaged again, allowing us to determine coexpression. All of the trpa1b-positive cells are also positive for piezo2b (B, D; black arrowheads); however, it is also possible to detect piezo2b-positive/trpa1b-negative Rohon–Beard cells (D; white arrowhead). E–H, A similar in situ hybridization assay performed with trpa1b in red (E, F) and piezo2b in blue (G, H). All of the trpa1b-positive cells are also positive for piezo2b (F, H; black arrowheads); however, it is also possible to detect piezo2b positive/trpa1b-negative Rohon–Beard cells (H; white arrowheads). I, J, Double-fluorescent in situ hybridization for piezo2b (I; green) and trpa1b (J; red). K, All trpa1b-positive cells also express piezo2b.
Discussion
Of the three zebrafish piezo homologs, only piezo2b showed a specific neural expression pattern, appearing in the trigeminal and Rohon–Beard neurons that innervate the skin and are involved in sensing external stimuli (Prober et al., 2008). In particular, Rohon–Beard cells are specified very early during zebrafish embryogenesis and provide initial mechanosensation, typified by the light touch response (Reyes et al., 2004; Rossi et al., 2009). Similarly, piezo2 is expressed in mammals in dorsal root ganglion neurons, which relay sensory information, such as touch, to the CNS (Coste et al., 2010). Because piezo2b morphants lack a light touch response, it has been suggested that this gene is involved in the mechanosensory function of this subset of neurons. A recent study in D. melanogaster has indicated that the fly homolog Dmpiezo is involved in mechanical nociception. Mutant larvae that lack Dmpiezo do not respond to noxious mechanical stimulation but still retain a light touch response (Kim et al., 2012). However, we found that piezo2b morphants responded normally in a mechanical nociception assay (tail pinch), similar to a previously reported zebrafish mutant that also lacks a light touch response (Low et al., 2010). This suggests that there is an evolutionary divergence in the sensory role played by piezo between vertebrates and nonvertebrates. It is unlikely that the mechanosensory function of the PIEZO protein itself differs drastically between these two species and is most likely due to a difference in sensory neural expression of piezo between Drosophila larvae and zebrafish embryos.
Another possible explanation for the lack of touch response observed in piezo2b morphants is that neural function in general has been perturbed in some manner. However, the fact that we could not observe any noticeable differences between piezo2b morphants and wild-type control embryos when we performed chemosensory or mechanical nociceptive assays indicates that this is unlikely. Furthermore, analysis of Rohon–Beard cells and neurites indicates that these appear to be unaffected by piezo2b knockdown, indicating that the function, rather than the development of these neurons has been perturbed in piezo2b morphants. Interestingly, another ion channel, trpa1b, which plays a chemosensory role, is also expressed in Rohon–Beard neurons (Prober et al., 2008). We found that trpa1b morphants do not react to mustard oil stimulation but still retain a light touch response. In piezo2b morphants, the reverse is true: light touch sensation is lost, while chemosensitivity is retained. Because all of the trpa1b-positive Rohon–Beard neurons also express piezo2b, we concluded that knockdown of piezo2b does not affect the differentiation/homeostasis of the particular neurons it is expressed in or indeed the ability of these cells to sense noxious chemical stimuli. Rather, it points to a direct role for piezo2b in the mechanosensation of light touch. In agreement with our findings, a recent study used a combination of transgenic reporter lines to show that different subtypes of Rohon–Beard cells are present in developing zebrafish embryos (Palanca et al., 2013). Our results indicate that the chemosensory trpa1b-positive Rohon–Beard neurons are a subset of the light touch-sensing piezo2b cells.
At present, little is known about the mechanisms involved in touch mechanosensation. However, it has been suggested that stretch can evoke calcium influx in neurons (Bhattacharya et al., 2008), a feature that could potentially be regulated by stretch-induced ion channels such as piezo2b. Furthermore, a recent study into the neuropathy mechanical allodynia found that knockdown of piezo2 could attenuate the light touch-evoked pain associated with this condition (Eijkelkamp et al., 2013). Consequently, identification of a specific piezo2 blocker will have an enormous impact on the lives of patients suffering from this condition.
Footnotes
A.F. is supported by a Fondation pour la Recherche Médicale postdoctoral fellowship. This work was supported by an INSERM ATIP-AVENIR grant (to C.J.) and the Agence Nationale pour la Recherche (ANR) Grant ANR-2010-BLAN-1128-01 (to M.E.M.). A.F., J.N., M.E.M., and C.J. are members of the Laboratory of Excellence “Ion Channel Science and Therapeutics,” which is supported by a grant from ANR.
The authors declare no competing financial interests.
References
- Bhattacharya MR, Bautista DM, Wu K, Haeberle H, Lumpkin EA, Julius D. Radial stretch reveals distinct populations of mechanosensitive mammalian somatosensory neurons. Proc Natl Acad Sci U S A. 2008;105:20015–20020. doi: 10.1073/pnas.0810801105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brend T, Holley SA. Zebrafish whole mount high-resolution double fluorescent in situ hybridization. J Vis Exp. 2009;pii:1229. doi: 10.3791/1229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coste B, Mathur J, Schmidt M, Earley TJ, Ranade S, Petrus MJ, Dubin AE, Patapoutian A. Piezo1 and Piezo2 are essential components of distinct mechanically activated cation channels. Science. 2010;330:55–60. doi: 10.1126/science.1193270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eijkelkamp N, Linley JE, Torres JM, Bee L, Dickenson AH, Gringhuis M, Minett MS, Hong GS, Lee E, Oh U, Ishikawa Y, Zwartkuis FJ, Cox JJ, Wood JN. A role for Piezo2 in EPAC1-dependent mechanical allodynia. Nat Commun. 2013;4:1682. doi: 10.1038/ncomms2673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faucherre A, Pujol-Martí J, Kawakami K, López-Schier H. Afferent neurons of the zebrafish lateral line are strict selectors of hair-cell orientation. PLoS One. 2009;4:e4477. doi: 10.1371/journal.pone.0004477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faucherre A, Kissa K, Nargeot J, Mangoni M, Jopling C. Piezo1 plays a role in erythrocyte volume homeostasis. Haematologica. 2013 doi: 10.3324/haematol.2013.086090. doi: 10.3324/haematol.2013.086090. Advance online publication. Retrieved September 25, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gottlieb PA, Sachs F. Piezo1: properties of a cation selective mechanical channel. Channels (Austin) 2012;6:214–219. doi: 10.4161/chan.21050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamill OP, Martinac B. Molecular basis of mechanotransduction in living cells. Physiol Rev. 2001;81:685–740. doi: 10.1152/physrev.2001.81.2.685. [DOI] [PubMed] [Google Scholar]
- Hultman KA, Bahary N, Zon LI, Johnson SL. Gene Duplication of the zebrafish kit ligand and partitioning of melanocyte development functions to kit ligand a. PLoS Genet. 2007;3:e17. doi: 10.1371/journal.pgen.0030017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jopling C, den Hertog J. Fyn/Yes and non-canonical Wnt signalling converge on RhoA in vertebrate gastrulation cell movements. EMBO Rep. 2005;6:426–431. doi: 10.1038/sj.embor.7400386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jowett T. Double in situ hybridization techniques in zebrafish. Methods. 2001;23:345–358. doi: 10.1006/meth.2000.1147. [DOI] [PubMed] [Google Scholar]
- Kim SE, Coste B, Chadha A, Cook B, Patapoutian A. The role of Drosophila Piezo in mechanical nociception. Nature. 2012;483:209–212. doi: 10.1038/nature10801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimmel CB, Ballard WW, Kimmel SR, Ullmann B, Schilling TF. Stages of embryonic development of the zebrafish. Dev Dyn. 1995;203:253–310. doi: 10.1002/aja.1002030302. [DOI] [PubMed] [Google Scholar]
- Low SE, Ryan J, Sprague SM, Hirata H, Cui WW, Zhou W, Hume RI, Kuwada JY, Saint-Amant L. touché is required for touch-evoked generator potentials within vertebrate sensory neurons. J Neurosci. 2010;30:9359–9367. doi: 10.1523/JNEUROSCI.1639-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lumpkin EA, Marshall KL, Nelson AM. The cell biology of touch. J Cell Biol. 2010;191:237–248. doi: 10.1083/jcb.201006074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Metcalfe WK, Myers PZ, Trevarrow B, Bass MB, Kimmel CB. Primary neurons that express the L2/HNK-1 carbohydrate during early development in the zebrafish. Development (Cambridge, England) 1990;110:491–504. doi: 10.1242/dev.110.2.491. [DOI] [PubMed] [Google Scholar]
- Nakano Y, Fujita M, Ogino K, Saint-Amant L, Kinoshita T, Oda Y, Hirata H. Biogenesis of GPI-anchored proteins is essential for surface expression of sodium channels in zebrafish Rohon-Beard neurons to respond to mechanosensory stimulation. Development (Cambridge, England) 2010;137:1689–1698. doi: 10.1242/dev.047464. [DOI] [PubMed] [Google Scholar]
- Nilius B, Honoré E. Sensing pressure with ion channels. Trends Neurosci. 2012;35:477–486. doi: 10.1016/j.tins.2012.04.002. [DOI] [PubMed] [Google Scholar]
- Palanca AM, Lee SL, Yee LE, Joe-Wong C, Trinh le A, Hiroyasu E, Husain M, Fraser SE, Pellegrini M, Sagasti A. New transgenic reporters identify somatosensory neuron subtypes in larval zebrafish. Dev Neurobiol. 2013;73:152–167. doi: 10.1002/dneu.22049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pietri T, Manalo E, Ryan J, Saint-Amant L, Washbourne P. Glutamate drives the touch response through a rostral loop in the spinal cord of zebrafish embryos. Dev Neurobiol. 2009;69:780–795. doi: 10.1002/dneu.20741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prober DA, Zimmerman S, Myers BR, McDermott BM, Jr, Kim SH, Caron S, Rihel J, Solnica-Krezel L, Julius D, Hudspeth AJ, Schier AF. Zebrafish TRPA1 channels are required for chemosensation but not for thermosensation or mechanosensory hair cell function. J Neurosci. 2008;28:10102–10110. doi: 10.1523/JNEUROSCI.2740-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reyes R, Haendel M, Grant D, Melancon E, Eisen JS. Slow degeneration of zebrafish Rohon-Beard neurons during programmed cell death. Dev Dyn. 2004;229:30–41. doi: 10.1002/dvdy.10488. [DOI] [PubMed] [Google Scholar]
- Rossi CC, Kaji T, Artinger KB. Transcriptional control of Rohon-Beard sensory neuron development at the neural plate border. Dev Dyn. 2009;238:931–943. doi: 10.1002/dvdy.21915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao R, Xu XZ. Mechanosensitive channels: in touch with Piezo. Curr Biol. 2010;20:R936–R938. doi: 10.1016/j.cub.2010.09.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao C, He X, Tian C, Meng A. Two GC-rich boxes in huC promoter play distinct roles in controlling its neuronal specific expression in zebrafish embryos. Biochemical and biophysical research communications. 2006;342:214–220. doi: 10.1016/j.bbrc.2006.01.134. [DOI] [PubMed] [Google Scholar]