Abstract
Evidence suggests that emotional memory plays a role in the pathophysiology of depression/anxiety disorders. Noradrenaline crucially modulates emotional memory. Genetic variants involved in noradrenergic signaling contribute to individual differences in emotional memory and vulnerability to psychopathology. A functional deletion polymorphism in the α-2B adrenoceptor gene (ADRA2B) has been linked to emotional memory and post-traumatic stress disorder. The noradrenaline reuptake inhibitor reboxetine attenuates enhanced memory for negative stimuli in healthy and depressed individuals. We examined whether the effect of reboxetine on emotional memory in healthy individuals would be moderated by ADRA2B genotype. ADRA2B deletion carriers demonstrated enhanced emotional memory for negative stimuli compared with deletion noncarriers, consistent with prior studies. Reboxetine attenuated enhanced memory for negative stimuli in deletion noncarriers but had no significant effect in deletion carriers. This is the first demonstration of genetic variation influencing antidepressant drug effects on emotional processing in healthy humans.
Introduction
Emotional memory, the enhancement of memory for emotional relative to nonemotional experiences, has been repeatedly demonstrated in experiments in healthy human volunteers (Kleinsmith and Kaplan, 1963; Bradley et al., 1992; Cahill and McGaugh, 1995). Normally an adaptive process in healthy individuals, it has also been linked to emotional disorders (Ledoux and Muller, 1997). It has therefore been suggested that emotional memory offers a valuable cognitive “biomarker” against which the therapeutic potential of novel drugs can be evaluated (Harmer et al., 2011). This is supported by studies demonstrating that pharmacological treatments for depression and anxiety attenuate negative, and enhance positive, affective processing in healthy individuals (Harmer et al., 2003, 2004, 2008; Arnone et al., 2009). Furthermore, the noradrenaline reuptake inhibitor reboxetine reverses negative emotional memory biases in depressed patients (Harmer et al., 2009).
Evidence suggests that genetic factors also contribute to individual differences in emotional memory and may therefore also influence vulnerability/resilience to emotional disorders (Todd et al., 2011). For example, the deletion of three residues in the ADRA2B gene, associated with decreased agonist-mediated phosphorylation and desensitization of the α-2B adrenoceptor, is a common polymorphism, occurring in ∼30% of whites (Small et al., 2001). A role for ADRA2B in interindividual differences in emotional memory was hypothesized based on putative effects on noradrenergic signaling. This was confirmed in a seminal study demonstrating enhanced emotional memory in healthy Swiss subjects and increased traumatic memories in Rwandan genocide survivors carrying at least one copy of the deletion variant (de Quervain et al., 2007). Although a recent behavioral study with a smaller sample size failed to replicate the effect on emotional memory (Li et al., 2013), other studies demonstrated that the deletion polymorphism is associated with increased amygdala activation in response to emotionally arousing stimuli (Rasch et al., 2009; Urner et al., 2011) and greater amygdala response to psychological stress (Cousijn et al., 2010). It is possible that such genetic factors may mediate individual differences in response to therapeutic drugs. Yet no studies have investigated the relationship between genetic variants and pharmacological agents influencing emotional processing. We tested the hypothesis that ADRA2B genotype moderates the effect of a single dose of reboxetine on emotional memory in healthy individuals.
Materials and Methods
Participants and procedure.
To avoid confounding of our results by established gender effects on emotional processing (Cahill, 2003), we limited our sample to male participants. Healthy white male volunteers (18–40 years of age) were recruited from staff/student communities at the Universities of Sussex and Brighton and the Brighton and Sussex Medical School. The study was approved by the Brighton and Sussex Medical School Research Governance and Ethics Committee. All participants gave written informed consent and received financial compensation for their participation. Potential participants were screened and excluded on the basis of any self-reported history of heart, liver, kidney, or prostate disease, psychiatric or neurological disorder, alcohol dependence, and regular prescribed or illicit drug use. Cigarette use was recorded, and smokers were asked not to smoke until after testing on the day of the experiment. All participants were asked to abstain from alcoholic beverages for 24 h before the experiment and caffeinated beverages until after testing on the day. The National Adult Reading Test was administered to estimate IQ (Nelson, 1982). Buccal swabs or saliva samples were obtained from participants, and DNA extraction/genotyping was performed by KBioscience as previously reported (Naudts et al., 2012).
Participants received opaque capsules containing reboxetine 4 mg or sugar (placebo) in a double-blind, randomized, between-subjects design. Testing began 2 h later, in keeping with the reboxetine tmax (Dostert et al., 1997). Pulse rate and blood pressure were measured before administering reboxetine or placebo (baseline) and 2 h after treatment. The Positive And Negative Affect Schedule was administered at baseline and after treatment (Watson et al., 1988). Adverse effects were assessed using a visual analog Bodily Symptom Scale (BSS) comprising 9 symptoms (dry mouth, anxiety, sweating, palpitations, nausea, dizziness, irritability, tiredness, and loss of concentration) at baseline and after treatment. Participants were asked to place an “X” on a line to indicate the severity of each symptom ranging from not at all to extremely severe. At the end of the experiment, participants guessed whether they had received reboxetine or placebo for assessment of blinding.
Emotional memory task.
This was an incidental learning task (Knowles and Duka, 2004). Two sets of pictures (A and B), each comprising 72 pictures rated for arousal and valence (24 positive, 24 negative, and 24 neutral), were selected from the International Affective Picture System stimulus set (Lang et al., 1998). Positive and negative pictures were matched for arousal, and sets A and B were matched on content, valence, and arousal. Positive and negative pictures differed significantly on arousal from neutral pictures (p < 0.001) but not from each other (p = 0.998). Either set A or set B was randomly allocated and presented to each participant for encoding, with the unseen set serving as foils for a subsequent recognition memory test (described below). This was to minimize potential bias associated with any possible between-set differences. Pictures were presented for 2.5 s in randomized order on a desktop computer screen using e-prime stimulus presentation software, followed by a consolidation period of 10 s, during which valence and arousal ratings were made using a 9 point Likert-type scale. Thirty minutes later, participants received a surprise recall memory test in which they were asked to spend 10 min writing down a few words to describe each picture. Descriptions were subsequently independently rated for successful recall by two raters with inter-rater reliability 0.94. After recall, participants received a recognition memory test in which all pictures from both sets (one set previously seen and one unseen) were presented in a random order. They were asked to indicate whether each had previously been seen during encoding or not, and rate their confidence on a 9 point Likert scale. Responses and reaction time data were recorded using e-prime software.
Results
Participant characteristics
A total of 119 volunteers were genotyped for the ADRA2B deletion polymorphism. Sixteen were homozygous carriers, 48 were heterozygotes, and 55 were noncarriers, consistent with Hardy–Weinberg Equilibrium (χ(1)2 = 1.4, p = 0.24) (Small et al., 2001). Because of the small number of homozygous carriers, they were combined with heterozygotes, giving two genotype groups of deletion carriers and deletion noncarriers as previously done by ourselves and others (de Quervain et al., 2007; Gibbs et al., 2010). Demographic characteristics are given in Table 1. χ2 testing confirmed independence of genotype and intervention group (χ(1)2 = 1.5, p = 0.22). A 2 × 2 ANOVA with ADRA2B genotype (deletion, no deletion) and intervention group (reboxetine, placebo) as independent variables confirmed that there were no significant between-group differences or interactions for age and IQ. Forty-seven of 62 (75.8%) participants in the placebo group and 39 of 57 (68.4%) in the reboxetine group correctly guessed their group assignment. These data were missing for one participant from the reboxetine group. χ2 analysis indicated that actual group and guess were not independent (χ(1)2 = 23.4, p < 0.001).
Table 1.
Demographic characteristics and genotypes
| Intervention group | Deletion |
No deletion |
||
|---|---|---|---|---|
| PLC | RBX | PLC | RBX | |
| N (%) | 30 (25.0) | 34 (28.6) | 32 (26.9) | 23 (19.3) |
| Age, yr, mean (SD) | 21.7 (3.7) | 20.8 (2.4) | 21.5 (2.4) | 21.2 (3.7) |
| IQ, mean (SD) | 110.8 (6.8) | 109.4 (7.1) | 110.7 (5.7) | 108.1 (7.7) |
Cardiovascular and side effect ratings
The 2 × 2 ANOVAs with post-treatment versus baseline change in score as the dependent variable, ADRA2B genotype (deletion, no deletion) and intervention group (reboxetine, placebo) as independent variables revealed a significant main effect of intervention on pulse rate (F(1,115) = 11.1, p = 0.001), with the reboxetine group exhibiting a significant increase (mean change = 7, SD = 14.2) that was absent in the placebo group (mean change = −0.8, SD = 10.2). There was also a significant main effect of intervention on change in total BSS scores (F(1,115) = 19.3, p < 0.001), with the reboxetine group also demonstrating a significant increase (mean = 17.5, SD = 62.6), whereas there was a significant reduction in the placebo group (mean = −30.4, SD = 52.5). There was a significant genotype × intervention group interaction for specific symptom scores (irritability, anxiety, and dizziness) from BSS (Table 2; Figure 1). There were no significant main effects or interactions for systolic or diastolic blood pressure.
Table 2.
Change in BSS symptoms scores (post-treatment minus baseline)
| BSS symptom | Deletion |
No deletion |
ANOVA |
|||||||
|---|---|---|---|---|---|---|---|---|---|---|
| PLC (N = 30) |
RBX (N = 34) |
PLC (N = 32) |
RBX (N = 23) |
ADRA2B × groupa |
||||||
| Mean | SD | Mean | SD | Mean | SD | Mean | SD | F | p | |
| Dizzinessb | 3.80 | 11.18 | 6.59 | 11.91 | 0.63 | 5.43 | 14.00 | 15.83 | 6.60 | 0.01 |
| Dry mouth | −5.22 | 13.35 | −1.17 | 18.85 | −6.2 | 18.53 | 7.18 | 27.78 | 1.36 | 0.24 |
| Anxietyb | −7.23 | 10.63 | 0.76 | 11.96 | −4.59 | 9.08 | −4.70 | 11.58 | 3.87 | 0.05 |
| Sweating | −4.20 | 19.28 | 4.65 | 21.74 | −4.56 | 13.44 | 1.61 | 11.36 | 0.06 | 0.80 |
| Palpitations | −2.00 | 4.40 | 0.74 | 10.23 | −0.91 | 5.06 | 6.39 | 19.50 | 1.86 | 0.17 |
| Nausea | −1.43 | 5.59 | 8.41 | 16.65 | −0.69 | 3.63 | 9.70 | 14.85 | 0.04 | 0.83 |
| Irritabilityb | −4.57 | 10.20 | 0.82 | 15.75 | −0.03 | 6.35 | −4.65 | 14.74 | 4.38 | 0.04 |
| Tiredness | −5.73 | 14.54 | −7.29 | 17.66 | −9.91 | 11.56 | −7.83 | 21.29 | 0.41 | 0.52 |
| Loss of concentration | −4.07 | 19.06 | −1.15 | 14.47 | −4.06 | 8.81 | 0.52 | 15.88 | 0.27 | 0.60 |
aDegrees of freedom = 1, 112.
bp ≤ 0.05.
Figure 1.
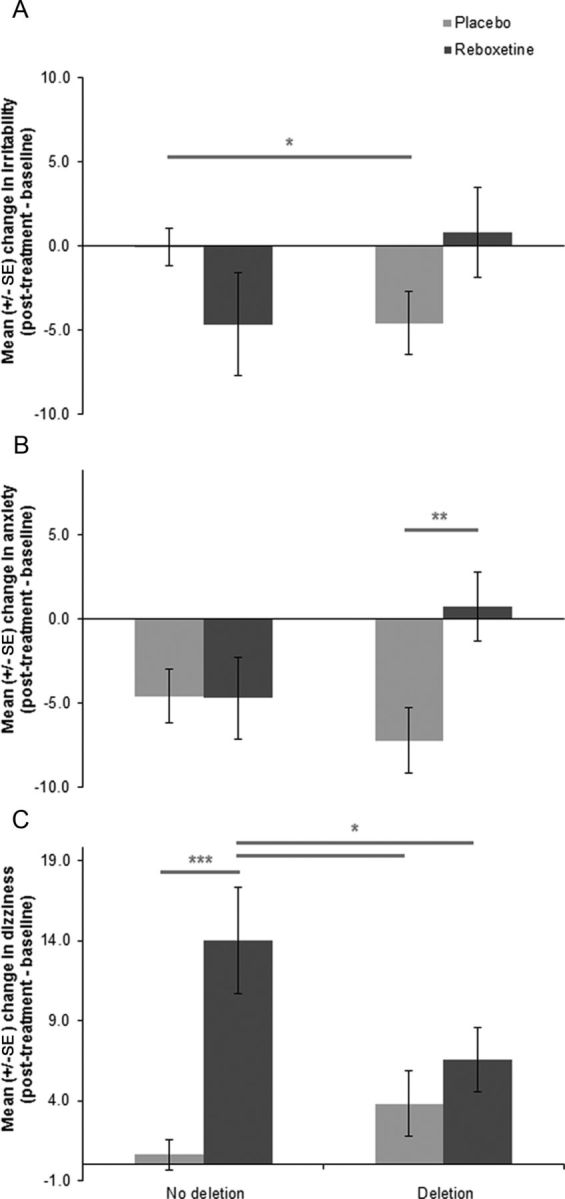
Interaction between reboxetine and ADRA2B genotype influences post-treatment symptoms of irritability, anxiety, and dizziness. A, Significant decrease in post-treatment irritability in ADRA2B deletion carriers under placebo that was absent in nondeletion carriers. B, Significant decrease in anxiety in ADRA2B deletion carriers under placebo compared with reboxetine. C, Significant increase in dizziness in ADRA2B nondeletion carriers under reboxetine compared with placebo. *p < 0.05. **p < 0.01. ***p < 0.001. Bars indicate SEM.
Mood, valence, and arousal ratings
A 2 × 2 ANOVA with post-treatment versus baseline change in score as the dependent variable, ADRA2B genotype (deletion, no deletion), intervention group (reboxetine, placebo) as independent variables, and change in pulse rate and BSS scores as covariates revealed a main effect of change in BSS scores on the change in both positive (F(1,113) = 6.57, p = 0.01) and negative Positive And Negative Affect Schedule scores (F(1,113) = 6.97, p = 0.009), but no main effects or interactions for pulse rate, intervention group, or genotype.
Valence and arousal ratings were missing for one participant. A repeated-measures 3 × 2 × 2 ANOVA with emotional category (neutral, positive, negative) as the within-subjects variable and ADRA2B genotype (deletion, no deletion) and intervention group (reboxetine, placebo) as the between-subjects variables revealed a highly significant main effect of emotional category on valence ratings (F(1.5,171.5) = 866.6, p < 0.001, Greenhouse-Geisser corrected) evident for neutral versus positive (F(1,114) = 388.9, p < 0.001), neutral vs negative (F(1,114) = 799.6, p < 0.001), and positive versus negative (F(1,114) = 1088.7, p < 0.001) pairwise comparisons, but no main effect or interactions for genotype or intervention group. Similarly, there was a highly significant main effect of emotional category on arousal ratings (F(1.8,210.3) = 203.6, p < 0.001, Greenhouse-Geisser corrected), also evident for neutral versus positive (F(1,114) = 334.3, p < 0.001), neutral versus negative (F(1,114) = 298.6, p < 0.001), and positive versus negative (F(1,114) = 11.0, p = 0.001) pairwise comparisons. There was also a significant interaction between emotional category and ADRA2B genotype (F(1.8,210.3) = 4.2, p = 0.02, Greenhouse-Geisser corrected) with pairwise comparisons indicating that this interaction was significant when comparing negative versus neutral (F(1,114) = 6.9, p = 0.009) but not positive versus neutral pictures (F(1,110) = 2.9, p = 0.09) or positive versus negative (F(1,110) = 1.9, p = 0.17). In effect, deletion carriers gave negative pictures higher arousal ratings relative to neutral or positive pictures than nondeletion carriers (Table 3). There were no main effects or interactions for intervention group.
Table 3.
Valence, arousal ratings, and percentage recall of pictures
| Deletion |
No deletion |
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| PLC (N = 29) |
RBX (N = 34) |
All (N = 63) |
PLC (N = 32) |
RBX (N = 23) |
All (N = 55) |
|||||||
| Mean | SD | Mean | SD | Mean | SD | Mean | SD | Mean | SD | Mean | SD | |
| Valence | ||||||||||||
| Neutral | 5.39 | 0.52 | 5.30 | 0.60 | 5.34 | 0.56 | 5.36 | 0.63 | 4.93 | 0.81 | 5.18 | 0.73 |
| Positive | 6.50 | 0.74 | 6.64 | 0.79 | 6.57 | 0.76 | 6.63 | 0.97 | 6.32 | 1.08 | 6.45 | 1.02 |
| Negative | 3.16 | 0.45 | 3.28 | 0.68 | 3.23 | 0.58 | 3.10 | 0.67 | 2.89 | 1.02 | 3.01 | 0.83 |
| Arousal | ||||||||||||
| Neutral | 3.17 | 1.18 | 3.73 | 1.09 | 3.47 | 1.16 | 3.88 | 1.09 | 3.78 | 1.32 | 3.84 | 1.78 |
| Positive | 4.92 | 1.36 | 5.43 | 1.31 | 5.20 | 1.35 | 4.41 | 1.46 | 5.13 | 1.33 | 5.29 | 1.40 |
| Negative | 5.44 | 1.46 | 5.92 | 1.20 | 5.70 | 1.34 | 5.33 | 1.73 | 5.62 | 1.71 | 5.45 | 1.72 |
| Recall | ||||||||||||
| Neutral | 29.0 | 12.3 | 28.7 | 12.3 | 28.8 | 12.2 | 26.4 | 13.2 | 26.7 | 13.3 | 26.5 | 13.1 |
| Positive | 33.2 | 9.8 | 32.0 | 13.2 | 32.6 | 11.7 | 28.0 | 13.2 | 26.5 | 12.2 | 27.4 | 12.7 |
| Negative | 45.2 | 10.9 | 48.8 | 11.3 | 47.1 | 11.2 | 40.1 | 15.9 | 33.1 | 14.5 | 37.1 | 15.5 |
Emotional memory
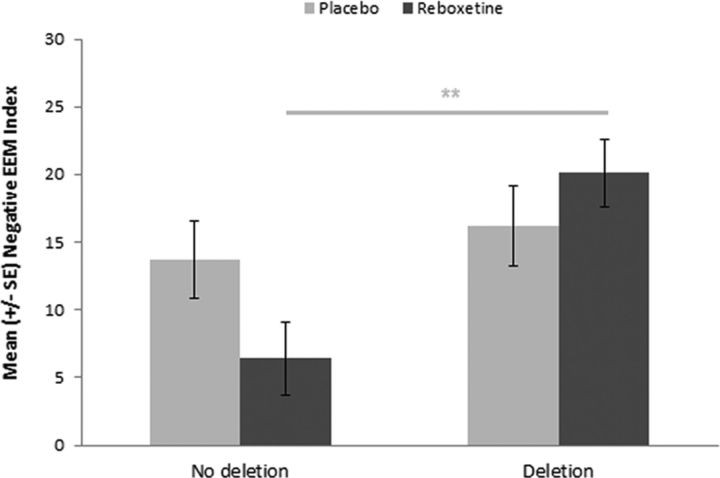
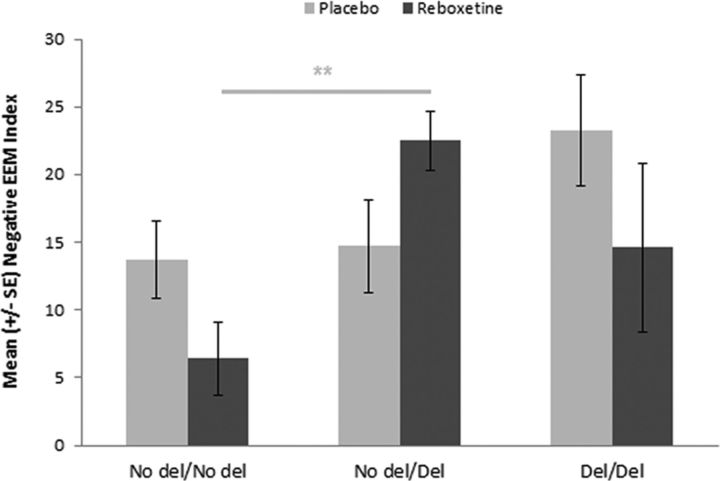
All groups performed the recognition memory task at ceiling; therefore, these data are not presented. Three participants from the reboxetine group and two from the placebo group were excluded because of failure to complete the recall task according to instructions. See Table 3 for mean recall rates. We calculated the percentage increase in recall of emotional, relative to neutral pictures as an index of emotional enhancement of memory (EEM) for negative and positive pictures. A 2 × 2 ANOVA with negative and positive EEM indices as dependent variables and ADRA2B genotype (deletion, no deletion) and intervention group (reboxetine, placebo) as independent variables was conducted. We included the accuracy of estimation of group assignment as a covariate given the apparent inadequacy of blinding. This revealed a significant main effect of ADRA2B genotype on the negative (F(1,109) = 8.07, p = 0.005), but not positive, EEM index (F(1,109) = 1.24, p = 0.27). There was also a significant interaction between ADRA2B genotype and intervention group on the negative (F(1,109) = 3.87, p = 0.05), but not positive, EEM index (F(1,110) = 0.02, p = 0.88) (Figure 2). Post hoc t tests showed that, under reboxetine (t(53) = 3.66, p = 0.001), but not placebo (t(57) = 0.61, p = 0.54), the enhanced recall of negative pictures was significantly attenuated in deletion noncarriers compared with carriers. Despite the small numbers of homozygous deletion carriers, we conducted a secondary exploratory analysis to examine whether there might be dose-dependent effects of this variant in relation to the effect of reboxetine on the negative EEM index (i.e., whether heterozygotes displayed an intermediate phenotype). This 3 × 2 ANOVA also revealed a significant main effect of ADRA2B genotype (F(2,108) = 3.67, p = 0.03). A follow-up 2 × 2 ANOVA in the reboxetine group revealed a significant main effect of genotype, with post hoc Tukey HSD tests confirming a reduced EEM index in homozygous noncarriers compared with heterozygous carriers (p = 0.001), but with no significant differences between either of these groups and homozygous deletion carriers. The 2 × 2 ANOVA in the placebo group did not reveal any significant between group differences (Figure 3).
Figure 2.
Effect of reboxetine on enhanced memory for negative pictures is dependent on ADRA2B genotype. Under reboxetine, EEM for negative pictures is attenuated with nondeletion carriers (N = 22) compared with under placebo (N = 30); however, there was no difference in EEM in deletion carriers under reboxetine (N = 33) compared with placebo (N = 29). **p < 0.01. Negative EEM Index = percentage increased recall of negative versus neutral pictures.
Figure 3.
Exploration of deletion variant dose-dependent effects of reboxetine on enhanced memory for negative pictures and gene dose-dependent effects. 0 indicates noncarriers (N = 52); 1, heterozygous carriers (N = 47); 3, homozygous carriers (N = 15). **p < 0.01. Negative EEM Index = percentage increased recall of negative versus neutral pictures.
Discussion
The key novel finding from this study is that reboxetine attenuated emotional memory for negative pictures in healthy male volunteers who do not have a copy of the ADRA2B deletion allele but had no effect in deletion carriers. Additionally, this is the first replication of the findings of enhanced emotional memory associated with the ADRA2B deletion in healthy Swiss subjects (de Quervain et al., 2007), in healthy male United Kingdom participants.
Noradrenergic gene and drug effects on emotional memory
We did not find a dose–response relationship between the number of deletion alleles and emotional memory; however, this is likely because of the small number of homozygous deletion carriers. Since the seminal report of de Quervain et al. (2007), a number of smaller neuroimaging studies have demonstrated a relationship between the neural correlates of emotional memory and the ADRA2B deletion allele (Rasch et al., 2009; Urner et al., 2011). Although these studies did not find enhanced emotional memory in deletion carriers, ostensibly because of inadequate power to detect behavioral differences, they provided converging evidence suggesting that the ADRA2B deletion allele contributes to enhanced emotional memory via its effects on amygdala activation and that this may be mediated via its effects on arousal (Cousijn et al., 2010). This may also explain the absence of an effect of ADRA2B genotype on memory enhancement for positive pictures in our study. Negative stimuli typically have greater arousal properties than positive stimuli (Bradley et al., 2001), as reflected by the fact that our participants rated negative pictures as significantly more arousing than positive ones, despite careful matching on this dimension. Our findings of increased arousal ratings for negative versus neutral stimuli in deletion carriers also support the notion that increased arousal may be a mechanism linking the deletion variant to enhanced emotional memory, although we did not specifically test this hypothesis in the present study. This could also explain the absence of a main effect of reboxetine on emotional memory in our study and others (Papps et al., 2002), compared with others studies that have examined the differential effects of reboxetine recall on positively versus negatively valenced stimuli, independently of arousal (Harmer et al., 2003, 2004; Miskowiak et al., 2007). While increased norepinephrine availability has been proposed as an additional mediating factor in the relationship between arousal, ADRA2B genotype, and enhanced memory, this also has not been previously empirically demonstrated. Ours is therefore the first study empirically linking the effects of ADRA2B on emotional memory to pharmacological manipulation of norepinephrine availability, providing further evidence for its role in the cognitive effects of ADRA2B.
Translational significance
Our findings have a number of potential translational implications. A recent study with an acute dose of reboxetine suggests that this modulation of emotional memory also occurs in depressed patients early on in treatment, before changes in mood symptoms, and may constitute an important component of its therapeutic effects (Harmer et al., 2009). It has been suggested that this may similarly be the case in the treatment of post-traumatic stress disorder (Hierholzer, 2010). Our findings indicate that this cognitive mechanism of action of reboxetine may be linked to the ADRA2B genotype. Thus, individuals with the ADRA2B deletion may not only be more susceptible to traumatic memories after adverse emotional events (de Quervain et al., 2007), but they could also be more resistant to treatment. However it is worth noting that a recent study found elevated diurnal cortisol levels in Holocaust survivors without the deletion variant compared with deletion carriers (Fridman et al., 2012). The authors suggest that increased longer-term distress associated with traumatic memories in the latter group may lead them to seek treatment, resulting in HPA axis normalization. Further work is required to clarify mechanisms involved; however, information regarding ADRA2B genotype may assist in predicting response to pharmacotherapy.
In addition, ADRA2B genotype may impact other outcomes, such as tolerability. We recently reported a significant interaction between ADRA2B genotype and the severity of post-treatment adverse effects (total BSS score) based on an interim analysis of our data (Gibbs et al., 2013). Our analysis of the full dataset reported here did not reveal a significant interaction for total symptoms scores. Instead, we found a pattern of significant interactions in specific symptom domains. These findings are limited by the subjective nature of the visual analog scales used; therefore, a detailed discussion on putative mechanisms would be speculative and beyond the scope of the present report. However, these data suggest that further investigation of the contribution of adrenergic genetic polymorphisms to adverse effects associated with noradrenergic drugs is warranted.
Limitations and future directions
We examined the effect of a single dose of reboxetine in healthy individuals; therefore, our predictions regarding clinical populations remain speculative. Our reliance on self-reported responses to screening questions to establish healthy volunteer status and self-reported ethnicity to minimize population stratification effects represent further limitations. Additionally, this was a relatively small study, and we did not correct for multiple comparisons in our post hoc analyses. The total sample size was similar to the number of male Swiss participants (n = 113) included in the study by de Quervain et al. (2007); however, to minimize gender biases, we included only male participants, limiting the generalizability of our findings. Finally, despite the double-blind design, participants were better than chance at identifying their intervention allocation, although this is unlikely to have been a major source of bias given the objective nature of the key outcome measure, emotional memory performance.
In keeping with prior studies investigating genetic variation in noradrenergic signaling and emotional memory, we examined a single polymorphism in the ADRA2B gene. However, other genetic mechanisms may be involved. Studies by other groups suggest that other functional polymorphisms within the ADRA2B gene (Cayla et al., 2004; Crassous et al., 2010) and other genes encoding other α-2 adrenoceptor subtypes (Feng et al., 2001; Small and Liggett, 2001) may influence noradrenergic transmission, and some of these may have also be implicated in cognitive and emotional processing (Lei et al., 2012).
In conclusion, despite these limitations, our findings represent an important opportunity to better understand individual responses to pharmacological agents and may contribute to the further development of personalized approaches to pharmacotherapy. Further investigation, replication, and confirmation in clinical populations are therefore warranted.
Footnotes
This work was supported by Brighton and Sussex Medical School.
The authors declare no competing financial interests.
References
- Arnone D, Horder J, Cowen PJ, Harmer CJ. Early effects of mirtazapine on emotional processing. Psychopharmacology. 2009;203:685–691. doi: 10.1007/s00213-008-1410-6. [DOI] [PubMed] [Google Scholar]
- Bradley MM, Greenwald MK, Petry MC, Lang PJ. Remembering pictures: pleasure and arousal in memory. J Exp Psychol. 1992;18:379–390. doi: 10.1037/0278-7393.18.2.379. [DOI] [PubMed] [Google Scholar]
- Bradley MM, Codispoti M, Cuthbert BN, Lang PJ. Emotion and motivation: I. Defensive and appetitive reactions in picture processing. Emotion. 2001;1:276–298. doi: 10.1037/1528-3542.1.3.276. [DOI] [PubMed] [Google Scholar]
- Cahill L. Sex-related influences on the neurobiology of emotionally influenced memory. Ann N Y Acad Sci. 2003;985:163–173. doi: 10.1111/j.1749-6632.2003.tb07080.x. [DOI] [PubMed] [Google Scholar]
- Cahill L, McGaugh JL. A novel demonstration of enhanced memory associated with emotional arousal. Conscious Cogn. 1995;4:410–421. doi: 10.1006/ccog.1995.1048. [DOI] [PubMed] [Google Scholar]
- Cayla C, Heinonen P, Viikari L, Schaak S, Snapir A, Bouloumié A, Karvonen MK, Pesonen U, Scheinin M, Paris H. Cloning, characterisation and identification of several polymorphisms in the promoter region of the human α2B-adrenergic receptor gene. Biochem Pharmacol. 2004;67:469–478. doi: 10.1016/j.bcp.2003.09.029. [DOI] [PubMed] [Google Scholar]
- Cousijn H, Rijpkema M, Qin S, van Marle HJ, Franke B, Hermans EJ, van Wingen G, Fernández G. Acute stress modulates genotype effects on amygdala processing in humans. Proc Natl Acad Sci U S A. 2010;107:9867–9872. doi: 10.1073/pnas.1003514107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crassous PA, Blaise R, Marquette A, Snapir A, Scheinin M, Paris H, Schaak S. Identification of a novel 12-nucleotide insertion polymorphism in the promoter region of ADRA2B: full linkage with the 9-nucleotide deletion in the coding region and influence on transcriptional activity. Biochem Pharmacol. 2010;79:407–412. doi: 10.1016/j.bcp.2009.08.024. [DOI] [PubMed] [Google Scholar]
- de Quervain DJ, Kolassa IT, Ertl V, Onyut PL, Neuner F, Elbert T, Papassotiropoulos A. A deletion variant of the α2b-adrenoceptor is related to emotional memory in Europeans and Africans. Nat Neurosci. 2007;10:1137–1139. doi: 10.1038/nn1945. [DOI] [PubMed] [Google Scholar]
- Dostert P, Benedetti MS, Poggesi I. Review of the pharmacokinetics and metabolism of reboxetine, a selective noradrenaline reuptake inhibitor. Eur Neuropsychopharmacol. 1997;7:S23–S35. doi: 10.1016/S0924-977X(97)00417-3. discussion S71–S73. [DOI] [PubMed] [Google Scholar]
- Feng J, Zheng J, Gelernter J, Kranzler H, Cook E, Goldman D, Jones IR, Craddock N, Heston LL, Delisi L, Peltonen L, Bennett WP, Sommer SS. An in-frame deletion in the α2C adrenergic receptor is common in African-Americans. Mol Psychiatry. 2001;6:168–172. doi: 10.1038/sj.mp.4000817. [DOI] [PubMed] [Google Scholar]
- Fridman A, van IJzendoorn MH, Sagi-Schwartz A, Bakermans-Kranenburg MJ. Genetic moderation of cortisol secretion in Holocaust survivors: a pilot study on the role of ADRA2B. Int J Behav Dev. 2012;36:79–84. doi: 10.1177/0165025411406859. [DOI] [Google Scholar]
- Gibbs AA, Naudts KH, Azevedo RT, David AS. Deletion variant of α2b-adrenergic receptor gene moderates the effect of COMT val158met polymorphism on episodic memory performance. Eur Neuropsychopharmacol. 2010;20:272–275. doi: 10.1016/j.euroneuro.2009.12.007. [DOI] [PubMed] [Google Scholar]
- Gibbs AA, Somal S, Chan K, Bautista C, Mowlem F, Naudts K, et al. Adrenergic receptor gene variation and selective norepinephrine reuptake inhibitors. Am J Psychiatry. 2013;170:446–447. doi: 10.1176/appi.ajp.2013.12121544. [DOI] [PubMed] [Google Scholar]
- Harmer CJ, Hill SA, Taylor MJ, Cowen PJ, Goodwin GM. Toward a neuropsychological theory of antidepressant drug action: increase in positive emotional bias after potentiation of norepinephrine activity. Am J Psychiatry. 2003;160:990–992. doi: 10.1176/appi.ajp.160.5.990. [DOI] [PubMed] [Google Scholar]
- Harmer CJ, Shelley NC, Cowen PJ, Goodwin GM. Increased positive versus negative affective perception and memory in healthy volunteers following selective serotonin and norepinephrine reuptake inhibition. Am J Psychiatry. 2004;161:1256–1263. doi: 10.1176/appi.ajp.161.7.1256. [DOI] [PubMed] [Google Scholar]
- Harmer CJ, O'Sullivan U, Favaron E, Massey-Chase R, Ayres R, Reinecke A, Goodwin GM, Cowen PJ. Effect of acute antidepressant administration on negative affective bias in depressed patients. Am J Psychiatry. 2009;166:1178–1184. doi: 10.1176/appi.ajp.2009.09020149. [DOI] [PubMed] [Google Scholar]
- Harmer CJ, Cowen PJ, Goodwin GM. Efficacy markers in depression. J Psychopharmacol. 2011;25:1148–1158. doi: 10.1177/0269881110367722. [DOI] [PubMed] [Google Scholar]
- Harmer CJ, Heinzen J, O'Sullivan U, Ayres RA, Cowen PJ. Dissociable effects of acute antidepressant drug administration on subjective and emotional processing measures in healthy volunteers. Psychopharmacology. 2008;199:495–502. doi: 10.1007/s00213-007-1058-7. [DOI] [PubMed] [Google Scholar]
- Hierholzer R. Do antidepressants alter emotional processing in PTSD? Am J Psychiatry. 2010;167:599. doi: 10.1176/appi.ajp.2010.09121733. author reply 599–600. [DOI] [PubMed] [Google Scholar]
- Kleinsmith LJ, Kaplan S. Paired-associate learning as a function of arousal and interpolated interval. J Exp Psychol. 1963;65:190–193. doi: 10.1037/h0040288. [DOI] [PubMed] [Google Scholar]
- Knowles SK, Duka T. Does alcohol affect memory for emotional and non-emotional experiences in different ways? Behav Pharmacol. 2004;15:111–121. doi: 10.1097/00008877-200403000-00003. [DOI] [PubMed] [Google Scholar]
- Lang PJ, Bradley MM, Cuthbert BN. Gainesville, FL: University of Florida, Center for Research in Psychophysiology; 1998. International affective picture system (IAPS): technical manual and affective ratings. [Google Scholar]
- Ledoux JE, Muller J. Emotional memory and psychopathology. Philos Trans R Soc Lond B Biol Sci. 1997;352:1719–1726. doi: 10.1098/rstb.1997.0154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lei X, Chen C, He Q, Moyzis R, Xue G, Chen C, Cao Z, Li J, Li H, Zhu B, Zhang M, Li J, Dong Q. Haplotype polymorphism in the alpha-2B-adrenergic receptor gene influences response inhibition in a large Chinese sample. Neuropsychopharmacology. 2012;37:1115–1121. doi: 10.1038/npp.2011.266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li S, Weerda R, Guenzel F, Wolf OT, Thiel CM. ADRA2B genotype modulates effects of acute psychosocial stress on emotional memory retrieval in healthy young men. Neurobiol Learn Mem. 2013;103:11–18. doi: 10.1016/j.nlm.2013.03.006. [DOI] [PubMed] [Google Scholar]
- Miskowiak K, Papadatou-Pastou M, Cowen PJ, Goodwin GM, Norbury R, Harmer CJ. Single dose antidepressant administration modulates the neural processing of self-referent personality trait words. Neuroimage. 2007;37:904–911. doi: 10.1016/j.neuroimage.2007.05.036. [DOI] [PubMed] [Google Scholar]
- Naudts KH, Azevedo RT, David AS, van Heeringen K, Gibbs AA. Epistasis between 5-HTTLPR and ADRA2B polymorphisms influences attentional bias for emotional information in healthy volunteers. Int J Neuropsychopharmacol. 2012;15:1027–1036. doi: 10.1017/S1461145711001295. [DOI] [PubMed] [Google Scholar]
- Nelson H. The National Adult Reading Test (NART) Windsor: NEFR-Nelson; 1982. [Google Scholar]
- Papps BP, Shajahan PM, Ebmeier KP, O'Carroll RE. The effects of noradrenergic re-uptake inhibition on memory encoding in man. Psychopharmacology (Berl) 2002;159:311–318. doi: 10.1007/s00213-001-0924-y. [DOI] [PubMed] [Google Scholar]
- Rasch B, Spalek K, Buholzer S, Luechinger R, Boesiger P, Papassotiropoulos A, de Quervain DJ. A genetic variation of the noradrenergic system is related to differential amygdala activation during encoding of emotional memories. Proc Natl Acad Sci U S A. 2009;106:19191–19196. doi: 10.1073/pnas.0907425106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Small KM, Liggett SB. Identification and functional characterization of alpha(2)-adrenoceptor polymorphisms. Trends Pharmacol Sci. 2001;22:471–477. doi: 10.1016/S0165-6147(00)01758-2. [DOI] [PubMed] [Google Scholar]
- Small KM, Brown KM, Forbes SL, Liggett SB. Polymorphic deletion of three intracellular acidic residues of the alpha 2B-adrenergic receptor decreases G protein-coupled receptor kinase-mediated phosphorylation and desensitization. J Biol Chem. 2001;276:4917–4922. doi: 10.1074/jbc.M008118200. [DOI] [PubMed] [Google Scholar]
- Todd RM, Palombo DJ, Levine B, Anderson AK. Genetic differences in emotionally enhanced memory. Neuropsychologia. 2011;49:734–744. doi: 10.1016/j.neuropsychologia.2010.11.010. [DOI] [PubMed] [Google Scholar]
- Urner M, van Wingen G, Franke B, Rijpkema M, Fernández G, Tendolkar I. Genetic variation of the α2b-adrenoceptor affects neural correlates of successful emotional memory formation. Hum Brain Mapp. 2011;32:2096–2103. doi: 10.1002/hbm.21171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson D, Clark LA, Tellegen A. Development and validation of brief measures of positive and negative affect: the PANAS scales. J Pers Soc Psychol. 1988;54:1063–1070. doi: 10.1037/0022-3514.54.6.1063. [DOI] [PubMed] [Google Scholar]