Abstract
Cerebral dopamine (DA) transmission is thought to be an important modulator for the development and occurrence of aggressive behavior. However, the link between aggression and DA transmission in humans has not been investigated using molecular imaging and standardized behavioral tasks. We investigated aggression as a function of DA transmission in a group of (N = 21) healthy male volunteers undergoing 6-[18F]-fluoro-l-DOPA (FDOPA)-positron emission tomography (PET) and a modified version of the Point Subtraction Aggression Paradigm (PSAP). This task measures aggressive behavior during a monetary reward-related paradigm, where a putative adversary habitually tries to cheat. The participant can react in three ways (i.e., money substraction of the putative opponent [aggressive punishment], pressing a defense button, or continuing his money-making behavior). FDOPA-PET was analyzed using a steady-state model yielding estimates of the DA-synthesis capacity (K), the turnover of tracer DA formed in living brain (kloss), and the tracer distribution volume (Vd), which is an index of DA storage capacity.
Significant negative correlations between PSAP aggressive responses and the DA-synthesis capacity were present in several regions, most prominently in the midbrain (r = −0.640; p = 0.002). Lower degrees of aggressive responses were associated with higher DA storage capacity in the striatum and midbrain. Additionally, there was a significant positive correlation between the investment into monetary incentive responses on the PSAP and DA-synthesis capacity, notably in the midbrain (r = +0.618, p = 0.003). The results suggest that individuals with low DA transmission capacity are more vulnerable to reactive/impulsive aggression in response to provocation.
Introduction
Aggressive behavior is a prominent and challenging aspect of various mental disorders; furthermore, it is frequently encountered in healthy populations. However, the definition of what constitutes aggression is complex: among other classifications, the distinction between reactive aggression and instrumental aggression is very common. Reactive aggression is prototypically defined as offensive or violent behavior arising in response to frustrating or threatening events, with the primary intention to harm the adversary or oneself. In contrast, instrumental aggression is defined as serving the achievement of personal goals other than primarily inflicting harm (Berkowitz, 1993). In particular, reactive aggression has commonly been linked to increased impulsivity (Vitiello and Stoff, 1997). However, these phenomenological clusters are not linked directly to particular neurobiological pathways and mechanisms; the knowledge about such mechanisms is a matter of ongoing research. Likewise, the psychopharmacological tools for the treatment of pathological manifestations of aggression are insufficient (Rodrigo et al., 2010). Therefore, it is of high relevance to investigate the neurobiological processes involved in physiological forms of aggression. Mainly based on the phenomenon of impulsivity, previous neuropsychiatric research focused on the role of serotonin (Linnoila et al., 1983; Frankle et al., 2005; Witte et al., 2009; Rylands et al., 2012). Nonetheless, it is known that the dopamine (DA) system is highly involved in behavioral control encoding reward expectancies and prediction errors (Schultz, 2002). Aggression frequently arises in contexts where an individual competes with others for rewards or if the expectation of a reward is frustrated or threatened by others. Accordingly, results from animal studies suggest an association between DA and aggression (Ferrari et al., 2003; Couppis and Kennedy, 2008; Anstrom et al., 2009). The few investigations in humans of possible DA/aggression associations point in the same direction (Licata et al., 1993; Soderstrom et al., 2001; Ramirez-Bermudez et al., 2010).
Questionnaires, in particular self-report scales, used in many studies of human aggression are, however, prone to subjectivity and social desirability. Until now, there are no investigations that directly link observable (provoked) aggressive behavior and molecular imaging of the DA system. A frequently used and well validated experimental paradigm for studying aggressive behavior is the Point Subtraction Aggression Paradigm (PSAP; Cherek et al., 1997).
To investigate the relationship between aggression and presynaptic trait parameters of DA transmission we assessed in a series of healthy volunteers the PSAP, and in the same subjects acquired positron emission tomography (PET) scans using the DOPA decarboxylase substrate 6-[18F]-fluoro-l-DOPA (FDOPA). A steady-state kinetic analysis was performed yielding individual estimates of a triad of kinetic terms, i.e., the net blood–brain clearance of FDOPA (K), which indicates DA-synthesis capacity, the washout rate of decarboxylated FDOPA metabolites (kloss), which reflects DA turnover, and the total distribution volume of FDOPA (Vd), which is an index of regional DA storage capacity (Vernaleken et al., 2006, 2008; Kumakura et al., 2007). We used a correlational analysis to test the hypothesis that higher subcortical DA synthesis and turnover is associated with greater aggressive responding on the PSAP.
Materials and Methods
The study was approved by the Ethics Committee of the RWTH University and the German radiation safety authorities in accordance with national and international standards.
Participants.
A group of 22 healthy male subjects was included in the investigation after written informed consent to participate in the study. The age of the subjects ranged from 20 to 39 years (mean: 25.2; SD: 4.8). One participant's data had to be excluded because he obtained insight into the absence of any real human counterpart (v.i.) in the behavioral test of aggression, which requires belief in the deception to be valid. The final sample (n = 21) had a mean age of 25.5 ± 5.5 years (mean ± SD), and consisted of subjects of comparable socioeconomic status (19 participants had a school education of 13 years, whereas 2 had a school education of 10 years); this was intended because the cognitive performance was previously reported to be highly correlated with the DA-synthesis capacity (Vernaleken et al., 2007). Due to the consequently very low variance, age and education were not included as covariates in the final analysis. Physical and mental-state examinations, drug screenings, and blood and urine analyses, as well as electrocardiography and electroencephalography examinations were performed. An experienced psychiatrist screened the participants to ensure the absence of any mental disorder, known family history of mental disorders that required treatment, substance abuse or relevant somatic complaint, any central acting substance for at least 6 weeks, and any medication during the week of the PET scan. A T1-weighted 3D gradient echo magnetic resonance scan was performed to check for possible anatomical abnormalities and for the purposes of anatomical coregistration of the PET data (v.i.). Participants underwent a single FDOPA-PET scan, as described below. The PSAP was performed directly before (n = 16) or after (n = 5) the PET-scan. The respective real-money earnings were paid by cash directly after completion of the paradigm. Furthermore, individual personality traits were characterized using the Temperament and Character Inventory (Cloninger, 1994, 1999).
The PSAP.
The PSAP is a computer-based behavioral paradigm that measures aggressive as well as defensive behavior in participants. The paradigm was programmed using Presentation software (V14.7; Neurobehavioral Systems). The composition of the PSAP was adopted with minor modifications from Cherek et al. (1990, 1997) and Bjork et al. (1999). In brief, the participant was instructed that he can earn real money (by pressing a button ∼100 times) at a rate of 40 cents for each completed sequence. Furthermore, he is told that an unseen opponent has also been instructed to earn money by bar pressing. The putative counterpart is said to be able to interfere with the real participant's game by cheating so as to steal 40 cent sums from the participant's account; this was in fact performed automatically at random intervals of 6–120 s. The participant is provided with the options either to shield (for an uncertain interval) his account against the counterpart's cheating by pressing a second button ∼10 times (defensive response), or alternatively to penalize the fictive counterpart by 100 cents by pressing a third button (aggressive response) 10 times. The participant was explicitly informed that this sum was not credited to his (the participant's) account. To ensure comprehension of the task, the experimenter asked the participants to explain the rules directly before the PSAP was started. If necessary, he then repeated the rules. During the game the earned and lost money was continuously presented to the participant on the display.
When either an aggressive or a defensive response has been completed, any scheduled provocation (point subtraction from the participant's account) is prevented for a provocation free interval (PFI) of 62.5 s (rather than 250 s as in the original version). This interval of 62.5 s is adapted from other studies (New et al., 2009; Greenwald et al., 2011) that also applied only a single session of the PSAP. Thus, aggressive actions of the participant may create the impression that this strategy has an impact on the (fictive) adversary's behavior. After the PFI has ended, new provocations can be scheduled. However, in the case of defensive responding, the PFI is only enabled if at least one provocation has occurred previously. Importantly, the participant is not informed about the PFI. He is informed that in case of a defensive action his account would be protected for an “unknown” amount of time by attacks from the other player. In case of an aggressive response, he is not instructed that a PFI may be the result of this action since a general rule of aggression-associated PFIs would argue against the existence of a real counterpart.
Theoretically, results of the paradigm are dependent on how fast a participant presses the buttons (pressing speed). To correct for interindividual differences in pressing speed, a limitation was implemented, comparable to the one of Cherek et al. (1990): if the time difference between two consecutive button presses was <170 ms, the number of button presses to be made for completion of the respective action (monetary, defensive, aggressive) increased accordingly. This modification served to balance differences in bar-pressing speed between participants. Completion of the PSAP test required 25 min, after which the participants were asked to describe the putative opponent's behavior, and to judge who had succeeded in stealing more money from his respective counterpart. If the answers implied doubts about the existence of a real human counterpart, the deception was evaluated as unsuccessful.
The main outcome variables of the PSAP, i.e., the total completed aggressive responses (TAR), the total completed defensive responses (TDR), and the total completed monetary responses (TMR), were transformed into ratios relative to the sum of all completed actions as follows: (1) aggressive ratio: the ratio of TAR to the total number of completed responses = TAR/(TAR + TMR*10 + TDR), (2) defensive ratio: the ratio of TDR to the total number of completed responses = TDR/(TAR + TMR*10 + TDR), and (3) monetary ratio: the ratio of TMR to the total number of completed responses = 10*TMR/(TAR + TMR*10 + TDR).
In its original version, the PSAP was repeated six times and the scores were averaged across these sessions (Cherek et al., 1997). In recent studies, the PSAP has been reduced to a single session. A report by Golomb et al. (2007) shows reliability of this simplified approach. These studies used shorter PFIs to ensure that participants were confronted with sufficient provocations. The PFI (62.5 s) of the present study was adapted from Greenwald et al. (2011).
Post hoc manipulation of PSAP.
Driven by the results (see below), a second group of 20 subjects of demographics highly comparable to the first was invited to perform the PSAP in a slightly modified version. This version was tested to test whether a more competitive environment might have a significant influence on the aggressive behavior. These subjects did not receive PET scans but were additionally characterized by the Psychopathy Personality Inventory Revised (Hare et al., 1991) and the Proactive–reactive Aggression Questionnaire (RPQ; Raine et al., 2006). Their mean age was 24.7 ± 3.2 years (range 20–32 years). Eighteen of 20 subjects were students, one person was used, and another was seeking employment. All subjects were male, nonsmoking, and free of any mental and relevant somatic complaints. The PSAP was performed with the same parameters and instruction as mentioned before, except that the participants were told that they would receive money only if they defeated the fictive opponent. None of the subjects had previous knowledge of the PSAP.
PET data acquisition.
All PET recordings were obtained using an ECAT HR+ (Siemens) whole-body PET scanner in 3D mode. Decarboxylation of FDOPA in peripheral tissues was reduced by oral administration of carbidopa (Lodosyn; Merck) in a divided dose of 100 mg 1 h before the scan and 50 mg directly before the tracer injection. After a 10 min attenuation scan, a mean of 236 MBq FDOPA (SD: 11 MBq, range: 216–255 MBq) was injected as a slow intravenous bolus into a cubital vein. A sequence of 30 emission frames lasting a total of 124 min was recorded. Frame length increased progressively according to the following schedule: 3 × 20 s 3 × 1 min, 3 × 2 min, 3 × 3 min, 15 × 5 min, and 3 × 10 min. During this recording, arterial blood samples were withdrawn via a catheter placed in a radial artery by an anesthesiologist. Continuous automated blood sampling (Allogg ABSS V3) during the first 10 min was followed by manual sampling at intervals of 5–10 min: a total of 17 2 ml blood samples were withdrawn for measuring the plasma radioactivity curve, and seven additional 5 ml samples were withdrawn for HPLC analysis of FDOPA and 3-O-methyl-[18F]fluorodopa (OMFD) fractions. Upon completion of the PET recording the arterial catheter was removed and a pressure bandage was applied, with monitoring for at least 10 min for any adverse affects.
Image analysis.
Individual emission images were reconstructed using the filtered back projection, using a 4 mm Hanning filter. Framewise motion correction was performed, using an interframe rigid-body transformation as implemented in PMOD (PMOD Technology). For spatial normalization, the PET image was first coregistered to the individual magnetic resonance tomographic (MRT) image, which was then normalized to the ICBM 452 template (Mazziotta et al., 2001) using PMOD (Brain Normalization II routine). Time activity curves (TAC) were calculated for a standard set of volumes of interest (VOI) (Gründer et al., 2008) including the cerebellum (2.38 cm3), the caudate nucleus (NC; 0.52 cm3), the putamen (1.14 cm3), the thalamus (2.50 cm3), and midbrain (0.252 cm3). For four participants with contraindications against MR tomography, PET images were normalized to a standard FDOPA template.
HPLC analysis of FDOPA and metabolite fractions.
HPLC was used to determine the FDOPA and metabolite fractions in plasma extracts from arterial blood collected at seven time points based on previous methods (Cumming et al., 1993). Additional solid phase extractions (Boyes et al., 1986) were performed at 12 additional time points. Continuous FDOPA and OMFD fractions were calculated by bi-exponential fitting of the measured fractions, and the corresponding arterial input curves were calculated as described previously.
FDOPA kinetics.
The kinetic analysis of the FDOPA-TACs was performed by application of the reversible “inlet-outlet-model” (IOM) as described previously (Kumakura et al., 2005, 2006). In brief, this approach comprises two modeling steps. First, an input curve of the radiolabeled metabolite OMFD is calculated using a one-tissue compartment model applied to the cerebellum VOI (Gjedde et al., 1991; Huang et al., 1991). In contrast to several standard procedures, rather than subtracting the entire cerebellar TAC, only the OMFD TAC is subtracted from the telencephalic VOI TACs. In step 2, these OMFD-cleaned TACs are then analyzed using a multilinear two-compartment model to determine the regional outcome parameters. This procedure avoids the usual oversubstraction of precursor pool in cerebellum subtraction methods. Consequently, regions with lesser [18F]FDOPA uptake (midbrain, thalamus) can be readily analyzed in addition to the extended striatum. Furthermore, the IOM enables the calculation of a triad of steady-state FDOPA kinetic parameters during a 2 h scan acquisition: (1) the intrinsic blood–brain clearance of FDOPA (K, ml g−1 min−1), which is an index of DA-synthesis capacity; (2) the washout rate for [18F]fluorodopamine (kloss; min−1), which is an index of dopamine turnover; and (3) the steady-state distribution volume of FDOPA together with its decarboxylated metabolites (Vd; ml g−1), which is an index of DA storage capacity, comparable to the effective distribution volume (ml g−1), as defined by Sossi et al. (2001).
Statistical analyses.
The three IOM FDOPA parameters of interest (K, kloss, and Vd) were calculated for each VOI and then correlated with scores for aggressive, monetary, and defensive responding on the PSAP. Because the majority of the PSAP variables were not normally distributed, the rank-order correlation by Spearman was used. For the same reason the rank-order correlation was the chosen tool for assessing the relationships between personality factors, IOM parameters, and PSAP variables. The significance level for the statistical tests was set to 0.05. A Bonferroni correction was applied for correction of multiple testing. However, the Bonferroni method does not account for high intercorrelations between the respective variables; therefore the proposed α values are overly conservative in the present case.
Results
Twenty-one subjects successfully completed the PET scan and the PSAP. The mean net blood–brain clearance (K) was 0.0076 ± 0.0030 [ml g−1 min−1] in the midbrain, 0.0184 ± 0.0039 [ml g−1 min−1] in the NC, and 0.0213 ± 0.0044 [ml g−1 min−1] in the putamen. The corresponding kloss values were 0.0058 ± 0.0062 [min−1] in midbrain, 0.0034 ± 0.0011 [min−1] in NC, and 0.0042 ± 0.0012 [min−1] in putamen (for details, including Vd-results, see Table 1). The K values were normally distributed in the NC, putamen, and thalamus but right skewed in the midbrain (see Table 1).
Table 1.
Regional FDOPA kinetic results according to the reversible inlet–outlet model
| Region | Parameter | Mean | SD | Skewness |
|
|---|---|---|---|---|---|
| Statistic | SE | ||||
| Midbrain | K | 0.0076 | 0.0030 | 1.43 | 0.50 |
| kloss | 0.0058 | 0.0062 | −2.79 | ||
| Vd | 1.56 | 0.59 | −0.19 | ||
| NC | K | 0.0184 | 0.0039 | 0.12 | |
| kloss | 0.0034 | 0.0011 | 0.29 | ||
| Vd | 6.21 | 1.76 | 0.55 | ||
| Putamen | K | 0.0213 | 0.0044 | 0.52 | |
| kloss | 0.0042 | 0.0012 | 1.37 | ||
| Vd | 5.56 | 1.39 | 0.01 | ||
| Thalamus | K | 0.0098 | 0.0034 | 0.59 | |
| kloss | 0.0139 | 0.0046 | −2.56 | ||
| Vd | 1.07 | 0.23 | 0.34 | ||
K, intrinsic blood–brain clearance of FDOPA [ml·g−1 · min−1]; kloss, rate constant for the elimination of [18F]-fluorodopamine and subsequent metabolites [min−1]; Vd, total distribution volume of FDOPA and its decarboxylated metabolites in brain tissue [ml/g].
The population mean (±SD) of total completed aggressive responses (TARs) was 15.5 ± 19.5. The corresponding population means of completed defensive responses (TDRs) and completed monetary responses (TMR) were 49.6 ± 24.6 and 68.0 ± 5.8, respectively. Details of Aggressive Ratio, Monetary Ratio, and Defensive Ratio results are reported in Table 2. One subject showed fairly high rates of TAR and TMR (outside the 1.5 interquartile range). Since there were no grounds for alleging his insight into the paradigm, there was no reason for exclusion of this subject. Additionally, the rank-order correlation is known to be very robust against skewness. Nevertheless, statistics that might be affected by this outlier were calculated with and without his exclusion.
Table 2.
Results from the Point Subtraction Aggression Paradigm (PSAP)
| Mean | SD | Skewness |
||
|---|---|---|---|---|
| Statistic | SE | |||
| Total_Aggressive_Responding | 15.5 | 19.5 | 2.72 | 0.50 |
| Total_Monetary_Responding | 68.0 | 5.8 | −0.72 | |
| Total_Defensive_Responding | 49.6 | 24.6 | 0.41 | |
| Earnings | 22.8 | 2.5 | 0.33 | |
| Subtractions/Provocations | 10.6 | 3.7 | −0.28 | |
| Ratio_Ag | 0.021 | 0.027 | 2.84 | |
| Ratio_Mo | 0.91 | 0.051 | −2.15 | |
| Ratio_Def | 0.067 | 0.034 | 0.53 | |
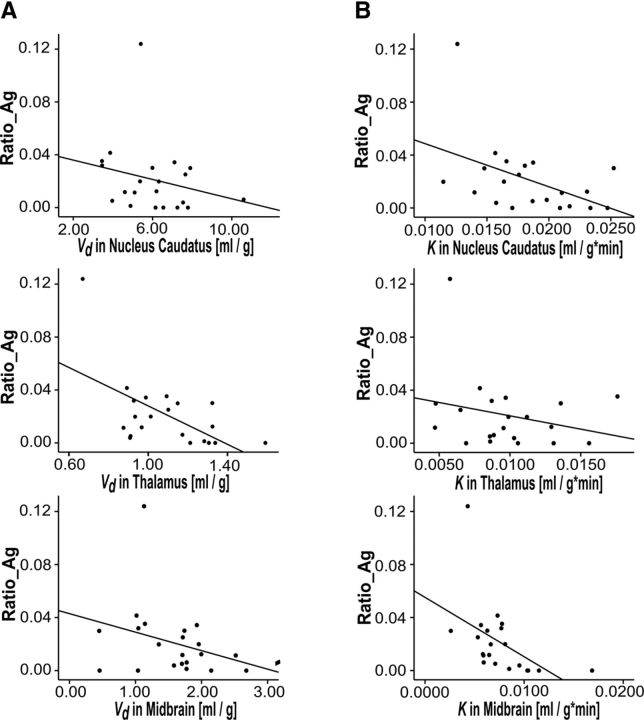
There were significant correlations between Aggressive Ratio and the corresponding individual K values in the midbrain, NC, and putamen. The relationship was strongest in the midbrain (r = −0.640, p = 0.0002; n = 21; after outlier exclusion: r = −0.592, p = 0.006; n = 20; two-tailed rank-order correlation) (see Table 3, Fig. 1). Defensive Ratio, correlated at trend level with K in the midbrain (r = −0.409, p = 0.066; n = 21; two-tailed rank-order correlation). There was a strong and significant positive correlation between Monetary Ratio and regional K values in the midbrain (r = 0.618, p = 0.003; n = 21; after outlier exclusion: r = 0.560, p = 0.010; n = 20; two-tailed rank-order correlation). PSAP parameters did not correlate significantly with regional estimates of kloss and Vd, except for the correlation between Aggressive Ratio and Vd in thalamus (r = −0.523, p = 0.015; n = 21; after outlier exclusion: r = -.417, p = 0.067; n = 20; two-tailed rank-order correlation; Fig. 1). The main TCI variables did not correlate significantly with regional FDOPA parameters.
Table 3.
Rank-order correlations between PSAP ratio variables and regional dopamine intrinsic synthesis capacity (K-values)
| Midbrain |
Caudate nucleus |
Putamen |
Thalamus |
|||||
|---|---|---|---|---|---|---|---|---|
| r | p | r | p | r | p | r | p | |
| Ratio_Ag | −0.640** | 0.002 | −0.444* | 0.044 | −0.453* | 0.039 | −0.189 | 0.482 |
| Ratio_Mo | 0.618** | 0.003 | 0.318 | 0.160 | 0.237 | 0.301 | 0.340 | 0.132 |
| Ratio_Def | −0.409 | 0.066 | −0.169 | 0.464 | −0.077 | 0.741 | −0.336 | 0.136 |
*p < 0.05;
**p < 0.01.
Figure 1.
A, Relationship between the aggressive response ratio (Aggressive Ratio) and FDOPA-Vd (ml g−1; index of dopamine storage capacity) in NC, thalamus, and midbrain; correlation in thalamus reached significance (r = −0.497, p = 0.022; n = 21; two-tailed rank-order correlation). B, Relationship between Aggressive Ratio and FDOPA-K (ml g−1 min−1; index of dopamine synthesis capacity). Significant correlations in the NC (r = −0.444, p = 0.044; n = 21; two-tailed rank-order correlation) and midbrain (r = −0.640, p = 0.0002; n = 21; two-tailed rank-order correlation). For illustration, the solid line represents the linear regression line.
Any before mentioned significant correlation between K and the ratios (monetary, aggressive, and defensive) remained significant if including the absolute number (instead of the ratios) of the respective button actions (see Table 4), nor was the correlation between Vd in the thalamus and aggression affected by this consideration/factor (r = −0.519, p = 0.016; n = 21; two-tailed rank-order correlation).
Table 4.
Rank-order correlations between PSAP total completed responses variables and regional dopamine intrinsic synthesis capacity (K values)
| Midbrain |
Caudate nucleus |
Putamen |
Thalamus |
|||||
|---|---|---|---|---|---|---|---|---|
| r | p | r | p | r | p | r | p | |
| TAR | −0.652** | 0.001 | −0.451* | 0.040 | −0.455* | 0.038 | −0.219 | 0.340 |
| TMR | 0.645** | 0.002 | 0.143 | 0.536 | 0.160 | 0.490 | 0.165 | 0.474 |
| TDR | −0.367 | 0.102 | −0.156 | 0.500 | −0.056 | 0.810 | −0.326 | 0.149 |
*p < 0.05;
**p < 0.01.
Intraindividual rank-order correlations between the PSAP variables showed a significant negative association between Monetary Ratio and Defensive Ratio (r = −0.911, p < 0.001; n = 21; after outlier exclusion: r = −0.897, p < 0.001; n = 20; two-tailed rank-order correlation). The correlation between Aggressive Ratio and Monetary Ratio was borderline significant (p = 0.083), whereas there was no association between Aggressive Ratio and Defensive ratio (see Table 5). For correlations with earnings and number of provocations (= “Subtractions”) see Table 5.
Table 5.
Rank-order correlations between PSAP Ratio variables
| Aggressive ratio |
Monetary ratio |
Defensive ratio |
Subtractions |
|||||
|---|---|---|---|---|---|---|---|---|
| r | p | r | p | r | p | r | p | |
| Aggressive ratio | −0.387 | 0.083 | 0.055 | 0.812 | ||||
| Monetary ratio | −0.911** | <0.001 | ||||||
| Earnings | 0.017 | 0.942 | 0.535* | 0.012 | −0.661** | 0.001 | −0.333 | 0.140 |
| Subtractions | −0.697** | <0.001 | 0.112 | 0.630 | 0.168 | 0.466 | ||
*p < 0.05;
**p < 0.01.
Although the regional outcome parameters are apparently not independent from each other, we also conducted a Bonferroni correction for our main results considering four brain regions (NC, putamen, midbrain, and thalamus) and three behavioral outcome values (Aggressive Ratio, Monetary Ratio, and Defensive Ratio). The corresponding corrected α level is p = 0.004. Due to the above-mentioned interrelations of these parameters, this approach is highly conservative. However, even using this overly conservative correction, the correlations between Aggressive Ratio, Monetary Ration, and midbrain K remained significant.
Second PSAP group analysis
The additional group of 20 subjects undergoing the PSAP under competitive conditions showed total completed aggressive responses (TAR) of 33.9 ± 34.3 (mean, SD). The corresponding population means of completed defensive responses (TDR) and completed monetary responses (TMR) were 67.1 ± 31.0 and 60.9 ± 9.3, respectively, giving ratios of 0.05, 0.10, and 0.86, respectively. Compared with the behavior of those individuals who (or study group that) performed the PSAP in the original version, there was a significantly higher Aggressive Ratio response (t = 2.14, p = 0.039, n = 41, t test of independent samples). Furthermore, the RPQ proactive questionnaire correlated positively with the Aggressive Ratio (r = 0.514, p = 0.025; n = 20 two-tailed Pearson correlation), whereas the reactive RPQ did not (r = 0.298, p = 0.215; n = 20 two-tailed Pearson correlation).
Discussion
This investigation focused on the relationship between observable provoked aggressive behavior and in vivo parameters reflecting the functional state of the subcortical dopamine system. The main finding is the negative correlation between aggressive actions (Aggressive Ratio) and the subcortical (particularly the midbrain) DA-synthesis capacity. Conversely, the rate of pressing the money-earning bar correlated positively with the DA-synthesis capacity. Even after correction for multiple testing, these associations remained significant for the midbrain region.
The PSAP was frequently applied before: Greenwald et al. (2011) showed that prenatal cocaine exposure led to increased escape behavior and New et al. (2009) linked aggressive behavior with clinical and brain metabolic dimensions of intermittent explosive disorder. These and similar investigations (Coccaro et al., 1996; Moeller et al., 1996; Bjork et al., 1999; Kouri et al., 1999; Pope et al., 2000) confirm its reliability and construct validity. It provides insight into directly observable behavior in “near real-life conditions.” Questionnaires may show high reliability but suffer from less validity and the problem of social desirability (Henry and Study Research Group TMAC, 2006).
Two issues arise from the strong negative associations: (1) the type of aggression indexed by the present version of the PSAP is uncertain and (2) the present data are difficult to integrate into animal findings of DA/aggression associations, which rather hint toward a positive direction.
Frequently two types of aggression were defined (instrumental/proactive vs impulsive/reactive; Atkins et al., 1993; Berkowitz, 1993; Vitiello and Stoff, 1997). Thus, lower aggression scores could either reflect a weaker drive for goal-directed attacks (instrumental), or a higher resilience against frustrations (less vulnerability to impulsive aggression). In the latter case, subjects with higher DA-synthesis capacity would maintain money-earning behavior and resist the impulse of delivering punishment after weighing the monetary effect of the possible actions. The participant's reward expectancy should initially be focused on money bar pressing. Importantly, the participant is not instructed that aggressive actions are beneficial since the knowledge about automated aggression associated PFIs would act against a convincing illusion of a real counterpart. At least at the beginning of the test, the participant could not predict the effect of his actions; in fact, there was not the slightest correlation between final earnings and Ratio Aggression; i.e., aggressive behavior was not actually rewarded or detrimental. Given that the participant could not expect a straightforward beneficial effect of aggressive actions, the PSAP in the present version reflects more reactive sources of aggression. Thus, subjects with higher DA-synthesis capacity in subcortical structures–subserving higher reward expectancy–might refrain from being distracted by provocations and time-consuming defense strategies. This distraction does not necessarily lead to aggressive but also to defensive strategies. Accordingly, we found a negative correlation between the midbrain K and Defensive Ratio. Subjects with higher DA-synthesis capacity, however, show higher resilience in maintaining their original money-earning behavior. Therefore, the PSAP results should be highly sensitive to the instructions and rules of the paradigm.
To test this sensitivity, a slightly modified PSAP paradigm was tested post hoc in a second group of subjects, without PET acquisition: a small change in the instructions (money will only be paid if the enemy is defeated) caused increased aggression, although the social background and mental state was similar in the two samples. Direct competition subserved utilization of proactive aggressive behavior (positive association between Aggressive Ratio and RPQ proactive rating). Furthermore, previous investigations have shown that harm or misfortune befalling another person is experienced as more rewarding if the observer considered himself to be in direct competition with the unfortunate counterpart (de Bruijn et al., 2009; Takahashi et al., 2009). In the PET participants, however, high DA-synthesis capacity was associated with the expectancy of monetary incentives to be obtained by nonaggressive actions, without resorting to more aggressive and socially less accepted patterns of behavior. It might be hypothesized that with a higher degree of direct competition, aggression could be under positive dopaminergic control.
Furthermore, why has research in animals found overt aggression (Ferrari et al., 2003; Couppis and Kennedy, 2008; Anstrom et al., 2009) to be associated with increased DA transmission? For rodents, food and sex are the most important incentives, access to which depends on the individual's position in a group hierarchy, which is organized by aggression or dominant stance. Prodopaminergic parameters appear to be positively associated with hierarchic position among nonhuman primates (Morgan et al., 2002). Furthermore, the linkage between aggression and reward expectancy appears to be more straightforward in the repertoire of animals than in humans.
Aggression in humans is one of the possible approaches to achieve aspired goals (Blanchard and Blanchard, 2003); it is broadened by culturally defined (and potentially more effective) behavioral alternatives. The latter condition might be reflected by the bar-pressing behavior (work) for monetary payoffs. This flexibility of diverse behavioral choices has an important impact on the participant's decision making during the PSAP. Increasing its competitive character appears to facilitate more aggressive strategies, which might be usually suppressed in normally socialized subjects (in absence of plain rewards). Furthermore, many animal investigations focus on DA release during aggressive actions, whereas our PET scans were not acquired during PSAP performance, which is presumed to reveal trait markers rather than state markers of aggression; we cannot make any claims about phasic DA release during punishment in the PSAP. Finally, monetary reward is a non-natural incentive. Nonetheless, its effect on reward systems (Ernst et al., 2004; Rademacher et al., 2010) is comparable to that evoked by food-related expectancies (O'Doherty et al., 2002) or sexual incentives (Oei et al., 2012).
Dopaminergic transmission impacts impulsivity, which can affect aggressive actions. In animals, stimulants seem to increase premature responses but decrease signaled delay-discounting impulsivity, whereas such drugs have minimal effect on motor impulsivity (Dalley and Roiser, 2012). Similarly, on a delay-discounting task in humans, increasing cortical DA levels by treatment with a COMT inhibitor decreases impulsivity and increases task-dependent blood oxygenation level-dependent activity in the left ventral striatum and the insula (Kayser et al., 2012). Studies using only questionnaires for behavioral characterization, however, do not show an unequivocal pattern: whereas Buckholtz et al. (2010) found amphetamine-associated DA release to be positively correlated with the Barrett Impulsiveness Scale and Oswald et al. (2007) found DA release to be negatively correlated with impulsivity (NEO PI-R).
Several PET investigations reported increased DA transmission during reward expectancy (gambling) in various populations, in a manner depending on the win/loss relationship (Linnet et al., 2011; Joutsa et al., 2012). With regard to coping with uncertainties, some studies have proposed a positive association between DA transmission and risk seeking (Cocker et al., 2012; Linnet et al., 2012). The PSAP is no gambling or delay-discounting task. Nevertheless, the counterpart's cheating can be interpreted as uncertainty, whereas the requirement of repeated bar pressing can be regarded as delayed reward. Thus, the present data are consistent with DA-driven decreased impulsivity or rather that DA imparts greater disposition to deal with uncertainties (resilience against provocations). In ADHD, a disorder with decreased subcortical DA-synthesis capacity (Ludolph et al., 2008), deficits in DA signals during reinforcement learning might interfere with delay discounting (Tripp and Wickens, 2008). These considerations are necessarily simplistic and neglect any possible rewarding effects of punishment and any influence of DA on emotional processing, defensive and aggressive actions.
There are methodological limitations of our study: PSAPs were in 16/21 cases administered directly before PET scans, which may have theoretically perturbed DA transmission measured 1 h later. However, DA-synthesis capacity is a very stable parameter, and task-dependent changes in the magnitude of K were never reported. In previous investigations, DA-synthesis capacity correlated with stable trait parameters of healthy subjects, whereas strong pharmacological challenges are necessary to cause significant changes in K (Vernaleken et al., 2008). For kloss, an order effect cannot fully be excluded. In general, FDOPA kinetics is difficult to interpret since DOPA decarboxylase is not the rate-limiting enzyme of DA synthesis. Nevertheless, its activity determines the fraction of endogenous l-DOPA trapped as DA (Cumming and Gjedde, 1998). K represents the capacity to use FDOPA in brain, whereas kloss and Vd are sensitive to pharmacological challenges (Schabram et al., 2013). In the present study, these more state-dependent kinetic parameters scarcely correlated with the PSAP results, suggesting that behavioral traits in the spectrum of aggression were almost exclusively linked to trait DA-synthesis capacity.
The present group size is larger than in many PET studies, but is small for the usual requirements of behavioral testing. A power analysis reveals a probability of 61% to reject a true correlation with ρ ≥ 0.5. Nevertheless, the correlational analyses indicated that ∼40% of variability in provoked aggressive behavior could be attributed to FDOPA-PET results. This might be due to the homogenous composition of the study group. Age and education ranges were restricted to decrease respective confounds. Another limitation is the inclusion of smokers (6/21) (Salokangas et al., 2000). However, a split analysis of smokers and nonsmokers did not indicate group differences in any parameter.
This investigation constitutes one of the first studies showing a relationship between neurobiological in vivo parameters and the results of a complex behavioral task, with particular implications for the neurochemical basis of human aggression.
Footnotes
This work is funded by the Deutsche Forschungsgemeinschaft (GR1399/7-1); the Project House “Interdisciplinary Management Practice,” Aachen (IMP-12); and the Interdisciplinary Centre for Clinical Research within the faculty of Medicine at the RWTH Aachen University, Germany (VBAI N4-5). We thank the Brain Imaging Facility of the Interdisciplinary Centre for Clinical Research within the Faculty of Medicine at the RWTH Aachen University, Germany, for support.
The authors declare no competing financial interests.
References
- Anstrom KK, Miczek KA, Budygin EA. Increased phasic dopamine signaling in the mesolimbic pathway during social defeat in rats. Neuroscience. 2009;161:3–12. doi: 10.1016/j.neuroscience.2009.03.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atkins MS, Stoff DM, Osborne ML, Brown K. Distinguishing instrumental and hostile aggression: does it make a difference? J Abnorm Child Psychol. 1993;21:355–365. doi: 10.1007/BF01261598. [DOI] [PubMed] [Google Scholar]
- Berkowitz L. Pain and aggression: some findings and implications. Motiv Emot. 1993;17:277–293. doi: 10.1007/BF00992223. [DOI] [Google Scholar]
- Bjork JM, Dougherty DM, Moeller FG, Cherek DR, Swann AC. The effects of tryptophan depletion and loading on laboratory aggression in men: time course and a food-restricted control. Psychopharmacology. 1999;142:24–30. doi: 10.1007/s002130050858. [DOI] [PubMed] [Google Scholar]
- Blanchard DC, Blanchard RJ. What can animal aggression research tell us about human aggression? Horm Behav. 2003;44:171–177. doi: 10.1016/S0018-506X(03)00133-8. [DOI] [PubMed] [Google Scholar]
- Boyes BE, Cumming P, Martin WR, McGeer EG. Determination of plasma [18F]-6-fluorodopa during positron emission tomography: elimination and metabolism in carbidopa treated subjects. Life Sci. 1986;39:2243–2252. doi: 10.1016/0024-3205(86)90403-0. [DOI] [PubMed] [Google Scholar]
- Buckholtz JW, Treadway MT, Cowan RL, Woodward ND, Li R, Ansari MS, Baldwin RM, Schwartzman AN, Shelby ES, Smith CE, Kessler RM, Zald DH. Dopaminergic network differences in human impulsivity. Science. 2010;329:532. doi: 10.1126/science.1185778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cherek DR, Spiga R, Steinberg JL, Kelly TH. Human aggressive responses maintained by avoidance or escape from point loss. J Exp Anal Behav. 1990;53:293–303. doi: 10.1901/jeab.1990.53-293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cherek DR, Moeller FG, Schnapp W, Dougherty DM. Studies of violent and nonviolent male parolees: I. Laboratory and psychometric measurements of aggression. Biol Psychiatry. 1997;41:514–522. doi: 10.1016/S0006-3223(96)00059-5. [DOI] [PubMed] [Google Scholar]
- Cloninger CR. Temperament and personality. Curr Opin Neurobiol. 1994;4:266–273. doi: 10.1016/0959-4388(94)90083-3. [DOI] [PubMed] [Google Scholar]
- Cloninger CR, Pryzbeck TR, Svrakic DM, Wetzel RD. Das Temperament- und Charakter-Inventar (TCI) Frankfurt: Swets and Zeitlinger; 1999. [Google Scholar]
- Coccaro EF, Berman ME, Kavoussi RJ, Hauger RL. Relationship of prolactin response to d-fenfluramine to behavioral and questionnaire assessments of aggression in personality-disordered men. Biol Psychiatry. 1996;40:157–164. doi: 10.1016/0006-3223(95)00398-3. [DOI] [PubMed] [Google Scholar]
- Cocker PJ, Dinelle K, Kornelson R, Sossi V, Winstanley CA. Irrational choice under uncertainty correlates with lower striatal D2/3 receptor binding in rats. J Neurosci. 2012;32:15450–15457. doi: 10.1523/JNEUROSCI.0626-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Couppis MH, Kennedy CH. The rewarding effect of aggression is reduced by nucleus accumbens dopamine receptor antagonism in mice. Psychopharmacology. 2008;197:449–456. doi: 10.1007/s00213-007-1054-y. [DOI] [PubMed] [Google Scholar]
- Cumming P, Gjedde A. Compartmental analysis of dopa decarboxylation in living brain from dynamic positron emission tomograms. Synapse. 1998;29:37–61. doi: 10.1002/(SICI)1098-2396(199805)29:1<37::AID-SYN4>3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]
- Cumming P, Léger GC, Kuwabara H, Gjedde A. Pharmacokinetics of plasma 6-[18F]fluoro-L-3,4-dihydroxyphenylalanine ([18F]Fdopa) in humans. J Cereb Blood Flow Metab. 1993;13:668–675. doi: 10.1038/jcbfm.1993.85. [DOI] [PubMed] [Google Scholar]
- Dalley JW, Roiser JP. Dopamine, serotonin and impulsivity. Neuroscience. 2012;215:42–58. doi: 10.1016/j.neuroscience.2012.03.065. [DOI] [PubMed] [Google Scholar]
- de Bruijn ER, de Lange FP, von Cramon DY, Ullsperger M. When errors are rewarding. J Neurosci. 2009;29:12183–12186. doi: 10.1523/JNEUROSCI.1751-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ernst M, Nelson EE, McClure EB, Monk CS, Munson S, Eshel N, Zarahn E, Leibenluft E, Zametkin A, Towbin K, Blair J, Charney D, Pine DS. Choice selection and reward anticipation: an fMRI study. Neuropsychologia. 2004;42:1585–1597. doi: 10.1016/j.neuropsychologia.2004.05.011. [DOI] [PubMed] [Google Scholar]
- Ferrari PF, van Erp AM, Tornatzky W, Miczek KA. Accumbal dopamine and serotonin in anticipation of the next aggressive episode in rats. Eur J Neurosci. 2003;17:371–378. doi: 10.1046/j.1460-9568.2003.02447.x. [DOI] [PubMed] [Google Scholar]
- Frankle WG, Lombardo I, New AS, Goodman M, Talbot PS, Huang Y, Hwang DR, Slifstein M, Curry S, Abi-Dargham A, Laruelle M, Siever LJ. Brain serotonin transporter distribution in subjects with impulsive aggressivity: a positron emission study with [11C]McN 5652. Am J Psychiatry. 2005;162:915–923. doi: 10.1176/appi.ajp.162.5.915. [DOI] [PubMed] [Google Scholar]
- Gjedde A, Reith J, Dyve S, Léger G, Guttman M, Diksic M, Evans A, Kuwabara H. Dopa decarboxylase activity of the living human brain. Proc Natl Acad Sci U S A. 1991;88:2721–2725. doi: 10.1073/pnas.88.7.2721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golomb BA, Cortez-Perez M, Jaworski BA, Mednick S, Dimsdale J. Point subtraction aggression paradigm: validity of a brief schedule of use. Violence Vict. 2007;22:95–103. doi: 10.1891/vv-v22i1a006. [DOI] [PubMed] [Google Scholar]
- Greenwald MK, Chiodo LM, Hannigan JH, Sokol RJ, Janisse J, Delaney-Black V. Teens with heavy prenatal cocaine exposure respond to experimental social provocation with escape not aggression. Neurotoxicol Teratol. 2011;33:198–204. doi: 10.1016/j.ntt.2010.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gründer G, Fellows C, Janouschek H, Veselinovic T, Boy C, Bröcheler A, Kirschbaum KM, Hellmann S, Spreckelmeyer KM, Hiemke C, Rösch F, Schaefer WM, Vernaleken I. Brain and plasma pharmacokinetics of aripiprazole in patients with schizophrenia: an [18F]fallypride PET study. Am J Psychiatry. 2008;165:988–995. doi: 10.1176/appi.ajp.2008.07101574. [DOI] [PubMed] [Google Scholar]
- Hare RD, Hart SD, Harpur TJ. Psychopathy and the DSM-IV criteria for antisocial personality disorder. J Abnorm Psychol. 1991;100:391–398. doi: 10.1037/0021-843X.100.3.391. [DOI] [PubMed] [Google Scholar]
- Henry DB Study Research Group TMAC. Associations between peer nominations, teacher ratings, self-reports, and observations of malicious and disruptive behavior. Assessment. 2006;13:241–252. doi: 10.1177/1073191106287668. [DOI] [PubMed] [Google Scholar]
- Huang SC, Yu DC, Barrio JR, Grafton S, Melega WP, Hoffman JM, Satyamurthy N, Mazziotta JC, Phelps ME. Kinetics and modeling of L-6-[18F]fluoro-dopa in human positron emission tomographic studies. J Cereb Blood Flow Metab. 1991;11:898–913. doi: 10.1038/jcbfm.1991.155. [DOI] [PubMed] [Google Scholar]
- Joutsa J, Johansson J, Niemelä S, Ollikainen A, Hirvonen MM, Piepponen P, Arponen E, Alho H, Voon V, Rinne JO, Hietala J, Kaasinen V. Mesolimbic dopamine release is linked to symptom severity in pathological gambling. Neuroimage. 2012;60:1992–1999. doi: 10.1016/j.neuroimage.2012.02.006. [DOI] [PubMed] [Google Scholar]
- Kayser AS, Allen DC, Navarro-Cebrian A, Mitchell JM, Fields HL. Dopamine, corticostriatal connectivity, and intertemporal choice. J Neurosci. 2012;32:9402–9409. doi: 10.1523/JNEUROSCI.1180-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kouri EM, Pope HG, Jr, Lukas SE. Changes in aggressive behavior during withdrawal from long-term marijuana use. Psychopharmacology. 1999;143:302–308. doi: 10.1007/s002130050951. [DOI] [PubMed] [Google Scholar]
- Kumakura Y, Vernaleken I, Gründer G, Bartenstein P, Gjedde A, Cumming P. PET studies of net blood–brain clearance of FDOPA to human brain: age-dependent decline of [18F]fluorodopamine storage capacity. J Cereb Blood Flow Metab. 2005;25:807–819. doi: 10.1038/sj.jcbfm.9600079. [DOI] [PubMed] [Google Scholar]
- Kumakura Y, Gjedde A, Danielsen EH, Christensen S, Cumming P. Dopamine storage capacity in caudate and putamen of patients with early Parkinson's disease: correlation with asymmetry of motor symptoms. J Cereb Blood Flow Metab. 2006;26:358–370. doi: 10.1038/sj.jcbfm.9600202. [DOI] [PubMed] [Google Scholar]
- Kumakura Y, Cumming P, Vernaleken I, Buchholz HG, Siessmeier T, Heinz A, Kienast T, Bartenstein P, Gründer G. Elevated [18F]fluorodopamine turnover in brain of patients with schizophrenia: an [18F]fluorodopa/positron emission tomography study. J Neurosci. 2007;27:8080–8087. doi: 10.1523/JNEUROSCI.0805-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Licata A, Taylor S, Berman M, Cranston J. Effects of cocaine on human aggression. Pharmacol Biochem Behav. 1993;45:549–552. doi: 10.1016/0091-3057(93)90504-M. [DOI] [PubMed] [Google Scholar]
- Linnet J, Møller A, Peterson E, Gjedde A, Doudet D. Dopamine release in ventral striatum during Iowa Gambling Task performance is associated with increased excitement levels in pathological gambling. Addiction. 2011;106:383–390. doi: 10.1111/j.1360-0443.2010.03126.x. [DOI] [PubMed] [Google Scholar]
- Linnet J, Mouridsen K, Peterson E, Møller A, Doudet DJ, Gjedde A. Striatal dopamine release codes uncertainty in pathological gambling. Psychiatry Res. 2012;204:55–60. doi: 10.1016/j.pscychresns.2012.04.012. [DOI] [PubMed] [Google Scholar]
- Linnoila M, Virkkunen M, Scheinin M, Nuutila A, Rimon R, Goodwin FK. Low cerebrospinal fluid 5-hydroxyindoleacetic acid concentration differentiates impulsive from nonimpulsive violent behavior. Life Sci. 1983;33:2609–2614. doi: 10.1016/0024-3205(83)90344-2. [DOI] [PubMed] [Google Scholar]
- Ludolph AG, Kassubek J, Schmeck K, Glaser C, Wunderlich A, Buck AK, Reske SN, Fegert JM, Mottaghy FM. Dopaminergic dysfunction in attention deficit hyperactivity disorder (ADHD), differences between pharmacologically treated and never treated young adults: a 3,4-dihdroxy-6-[18F]fluorophenyl-l-alanine PET study. Neuroimage. 2008;41:718–727. doi: 10.1016/j.neuroimage.2008.02.025. [DOI] [PubMed] [Google Scholar]
- Mazziotta J, Toga A, Evans A, Fox P, Lancaster J, Zilles K, Woods R, Paus T, Simpson G, Pike B, Holmes C, Collins L, Thompson P, MacDonald D, Iacoboni M, Schormann T, Amunts K, Palomero-Gallagher N, Geyer S, Parsons L, et al. A probabilistic atlas and reference system for the human brain: International Consortium for Brain Mapping (ICBM) Philos Trans R Soc Lond B Biol Sci. 2001;356:1293–1322. doi: 10.1098/rstb.2001.0915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moeller FG, Dougherty DM, Swann AC, Collins D, Davis CM, Cherek DR. Tryptophan depletion and aggressive responding in healthy males. Psychopharmacology. 1996;126:97–103. doi: 10.1007/BF02246343. [DOI] [PubMed] [Google Scholar]
- Morgan D, Grant KA, Gage HD, Mach RH, Kaplan JR, Prioleau O, Nader SH, Buchheimer N, Ehrenkaufer RL, Nader MA. Social dominance in monkeys: dopamine D2 receptors and cocaine self-administration. Nat Neurosci. 2002;5:169–174. doi: 10.1038/nn798. [DOI] [PubMed] [Google Scholar]
- New AS, Hazlett EA, Newmark RE, Zhang J, Triebwasser J, Meyerson D, Lazarus S, Trisdorfer R, Goldstein KE, Goodman M, Koenigsberg HW, Flory JD, Siever LJ, Buchsbaum MS. Laboratory induced aggression: a positron emission tomography study of aggressive individuals with borderline personality disorder. Biol Psychiatry. 2009;66:1107–1114. doi: 10.1016/j.biopsych.2009.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Doherty JP, Deichmann R, Critchley HD, Dolan RJ. Neural responses during anticipation of a primary taste reward. Neuron. 2002;33:815–826. doi: 10.1016/S0896-6273(02)00603-7. [DOI] [PubMed] [Google Scholar]
- Oei NY, Rombouts SA, Soeter RP, van Gerven JM, Both S. Dopamine modulates reward system activity during subconscious processing of sexual stimuli. Neuropsychopharmacology. 2012;37:1729–1737. doi: 10.1038/npp.2012.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oswald LM, Wong DF, Zhou Y, Kumar A, Brasic J, Alexander M, Ye W, Kuwabara H, Hilton J, Wand GS. Impulsivity and chronic stress are associated with amphetamine-induced striatal dopamine release. Neuroimage. 2007;36:153–166. doi: 10.1016/j.neuroimage.2007.01.055. [DOI] [PubMed] [Google Scholar]
- Pope HG, Jr, Kouri EM, Hudson JI. Effects of supraphysiologic doses of testosterone on mood and aggression in normal men: a randomized controlled trial. Arch Gen Psychiatry. 2000;57:133–140. doi: 10.1001/archpsyc.57.2.133. discussion 155–156. [DOI] [PubMed] [Google Scholar]
- Rademacher L, Krach S, Kohls G, Irmak A, Gründer G, Spreckelmeyer KN. Dissociation of neural networks for anticipation and consumption of monetary and social rewards. Neuroimage. 2010;49:3276–3285. doi: 10.1016/j.neuroimage.2009.10.089. [DOI] [PubMed] [Google Scholar]
- Raine A, Dodge K, Loeber R, Gatzke-Kopp L, Lynam D, Reynolds C, Stouthamer-Loeber M, Liu J. The reactive–proactive aggression questionnaire: differential correlates of reactive and proactive aggression in adolescent boys. Aggress Behav. 2006;32:159–171. doi: 10.1002/ab.20115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramírez-Bermudez J, Perez-Neri I, Montes S, Ramirez-Abascal M, Nente F, Abundes-Corona A, Soto-Hernandez JL, Rios C. Imbalance between nitric oxide and dopamine may underly aggression in acute neurological patients. Neurochem Res. 2010;35:1659–1665. doi: 10.1007/s11064-010-0227-y. [DOI] [PubMed] [Google Scholar]
- Rodrigo C, Rajapakse S, Jayananda G. The ‘antisocial’ person: an insight in to biology, classification and current evidence on treatment. Ann Gen Psychiatry. 2010;9:31. doi: 10.1186/1744-859X-9-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rylands AJ, Hinz R, Jones M, Holmes SE, Feldmann M, Brown G, McMahon AW, Talbot PS. Pre- and postsynaptic serotonergic differences in males with extreme levels of impulsive aggression without callous unemotional traits: a positron emission tomography study using 11C-DASB and 11C-MDL100907. Biol Psychiatry. 2012;72:1004–1011. doi: 10.1016/j.biopsych.2012.06.024. [DOI] [PubMed] [Google Scholar]
- Salokangas RK, Vilkman H, Ilonen T, Taiminen T, Bergman J, Haaparanta M, Solin O, Alanen A, Syvälahti E, Hietala J. High levels of dopamine activity in the basal ganglia of cigarette smokers. Am J Psychiatry. 2000;157:632–634. doi: 10.1176/appi.ajp.157.4.632. [DOI] [PubMed] [Google Scholar]
- Schabram I, Prinz S, Henkel K, Dietrich CA, Felzen M, Winz O, Shali SM, Gründer G, Mottaghy FM, Vernaleken I. Impact of acute methylphenidate challenge on presynaptic dopamine metabolism: an [18F]FDOPA PET study. International Society for Cerebral Blood Flow and Metabolism; May 20–23; Shanghai, China. 2013. [Google Scholar]
- Schultz W. Getting formal with dopamine and reward. Neuron. 2002;36:241–263. doi: 10.1016/S0896-6273(02)00967-4. [DOI] [PubMed] [Google Scholar]
- Soderstrom H, Blennow K, Manhem A, Forsman A. CSF studies in violent offenders. I. 5-HIAA as a negative and HVA as a positive predictor of psychopathy. J Neural Transm. 2001;108:869–878. doi: 10.1007/s007020170036. [DOI] [PubMed] [Google Scholar]
- Sossi V, Doudet DJ, Holden JE. A reversible tracer analysis approach to the study of effective dopamine turnover. J Cereb Blood Flow Metab. 2001;21:469–476. doi: 10.1097/00004647-200104000-00015. [DOI] [PubMed] [Google Scholar]
- Takahashi H, Kato M, Matsuura M, Mobbs D, Suhara T, Okubo Y. When your gain is my pain and your pain is my gain: neural correlates of envy and schadenfreude. Science. 2009;323:937–939. doi: 10.1126/science.1165604. [DOI] [PubMed] [Google Scholar]
- Tripp G, Wickens JR. Research review: dopamine transfer deficit: a neurobiological theory of altered reinforcement mechanisms in ADHD. J Child Psychol Psychiatry. 2008;49:691–704. doi: 10.1111/j.1469-7610.2007.01851.x. [DOI] [PubMed] [Google Scholar]
- Vernaleken I, Kumakura Y, Cumming P, Buchholz HG, Siessmeier T, Stoeter P, Müller MJ, Bartenstein P, Gründer G. Modulation of [18F]fluorodopa (FDOPA) kinetics in the brain of healthy volunteers after acute haloperidol challenge. Neuroimage. 2006;30:1332–1339. doi: 10.1016/j.neuroimage.2005.11.014. [DOI] [PubMed] [Google Scholar]
- Vernaleken I, Buchholz HG, Kumakura Y, Siessmeier T, Stoeter P, Bartenstein P, Cumming P, Gründer G. ‘Prefrontal’ cognitive performance of healthy subjects positively correlates with cerebral FDOPA influx: an exploratory [18F]-fluoro-L-DOPA-PET investigation. Hum Brain Mapp. 2007;28:931–939. doi: 10.1002/hbm.20325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vernaleken I, Kumakura Y, Buchholz HG, Siessmeier T, Hilgers RD, Bartenstein P, Cumming P, Gründer G. Baseline [18F]-FDOPA kinetics are predictive of haloperidol-induced changes in dopamine turnover and cognitive performance: a positron emission tomography study in healthy subjects. Neuroimage. 2008;40:1222–1231. doi: 10.1016/j.neuroimage.2007.12.045. [DOI] [PubMed] [Google Scholar]
- Vitiello B, Stoff DM. Subtypes of aggression and their relevance to child psychiatry. J Am Acad Child Adolesc Psychiatry. 1997;36:307–315. doi: 10.1097/00004583-199703000-00008. [DOI] [PubMed] [Google Scholar]
- Witte AV, Flöel A, Stein P, Savli M, Mien LK, Wadsak W, Spindelegger C, Moser U, Fink M, Hahn A, Mitterhauser M, Kletter K, Kasper S, Lanzenberger R. Aggression is related to frontal serotonin-1A receptor distribution as revealed by PET in healthy subjects. Hum Brain Mapp. 2009;30:2558–2570. doi: 10.1002/hbm.20687. [DOI] [PMC free article] [PubMed] [Google Scholar]