Abstract
Strong reactive cell proliferation occurs in the vestibular nuclei after unilateral vestibular neurectomy (UVN). Most of the newborn cells survive, differentiate into glial cells and neurons with GABAergic phenotype, and have been reported to contribute to recovery of the posturo-locomotor functions in adult cats. Because the GABAergic system modulates vestibular function recovery and the different steps of neurogenesis in mammals, we aimed to examine in our UVN animal model the effect of chronic infusion of GABAA receptor (R) agonist and antagonist in the vestibular nuclei. After UVN and one-month intracerebroventricular infusions of saline, GABAAR agonist (muscimol) or antagonist (gabazine), cell proliferation and differentiation into astrocytes, microglial cells, and neurons were revealed using immunohistochemical methods. We also determined the effects of these drug infusions on the recovery of posturo-locomotor and oculomotor functions through behavioral tests. Our results showed that surprisingly, one month after UVN, newborn cells did not survive in the UVN-muscimol group whereas the number of GABAergic pre-existent neurons increased, and the long-term behavioral recovery of the animals was drastically impaired. Conversely, a significant number of newborn cells survived up to 1 month in the UVN-gabazine group whereas the astroglial population increased, and these animals showed the fastest recovery in behavioral functions. This study reports for the first time that GABA plays multiple roles, ranging from beneficial to detrimental on the different steps of a functional postlesion neurogenesis and further, strongly influences the time course of vestibular function recovery.
Introduction
Unilateral vestibular loss causes a depression of resting activity discharge in deafferented vestibular nuclei (VN) in the brainstem. This leads to transient postural imbalance, circular walking, head and body twisting, spontaneous nystagmus, and vestibulo-ocular reflex impairments in a wide variety of species (Cass and Goshgarian, 1990; Lacour, 2006). Interestingly, in most cases these symptoms progressively disappear and the resting activity of the ipsilateral VN recovers gradually in a process called vestibular compensation (Darlington and Smith, 2000). Because damaged vestibular nerve or receptors do not regenerate after vestibular neurectomy, multiple and parallel plasticity mechanisms take place in the VN and in related central nervous structures, underlying the high plasticity potential of the vestibular system (Lacour and Tighilet, 2010).
Previous studies from our laboratory have shown that unilateral vestibular neurectomy (UVN) in the adult cat induced intense cell proliferation in deafferented VN. Interestingly, most of the reactive newborn cells survived up to 1 month, differentiated into astrocytic cells, microglial cells, and GABAergic neurons (Tighilet et al., 2007), and contributed to the successful recovery of posturo-locomotor function (Dutheil et al., 2009). Such a structural plasticity mechanism after vestibular loss is of particular interest to understand the vestibular compensation process and the factors favoring the expression of reactive neurogenesis with functional contribution in the adult damaged brain.
It is well known that the GABAergic system influences vestibular compensation (Gliddon et al., 2005b) and rebalances the electrical activity between the VN on both sides after vestibular damage (Darlington and Smith, 2000). GABA type A receptors (GABAAR) are expressed in neuroblasts, astrocyte-like cells, and mature neurons, and control different steps of neurogenesis: from proliferation to differentiation, including migration and integration into functional neural networks (Platel et al., 2010; Sernagor et al., 2010).
To better understand the effects of GABA neurotransmission on: (1) the different steps of neurogenesis in the deafferented VN, and (2) the time course of vestibular function recovery, we designed this study with three experimental groups in which adult cats underwent a UVN combined with an infusion in the fourth ventricle of saline (UVN-NaCl group), muscimol (GABAAR agonist, UVN-muscimol group), or gabazine (GABAAR antagonist, UVN-gabazine group). With a cellular approach, we investigated the effects of intracerebroventricular drug infusions on plasticity events occurring in the VN. 5-bromo-2′-deoxyuridine (BrdU) and Ki67 were used to label newborn cells. Then, BrdU-positive(+) cells were double-labeled with either glial fibrillary acidic protein (GFAP) as an astrocyte marker, IBA1 as a microglia marker, NeuN, as postmitotic neuronal marker, and glutamate decarboxylase 67 (GAD67) as an indicator of GABAergic phenotype. This allowed us to determine the lineage of the surviving newly generated cells in the VN. Finally, compensation of the spontaneous vestibular nystagmus and of the posturo-locomotor syndrome (support surface, locomotor equilibrium) was examined with appropriate behavioral tests in all groups of cats as a function of time after vestibular damage.
Materials and Methods
Ethics statement
All experiments were performed in strict accordance with the National Institutes of Health Guide for Care and Use of Laboratory Animals (NIH Publication no. 80-23) revised in 1996 for UK Animals (Scientific Procedures) Act 1986, and associated guidelines, or the Policy on Ethics approved by the Society for Neuroscience in November 1989, and amended in November 1993. Every attempt was made to minimize both the number and the suffering of animals used in this experiment. We selected only the most important post-UVN time delay in light of the findings of our previous studies and to limit the number of cats used. Animals were housed in a large confined space with normal diurnal light variations and free access to water and food.
Surgery and drug protocol
Adult male cats weighing between 4 and 5 kg were anesthetized with ketamine (20 mg/kg, i.m.; Rhône Poulenc), received an analgesic (Tolfedine, 0.5 ml, i.m.; Vetoquinol) and were kept at physiological body temperature using a blanket. The vestibular nerve was sectioned on the left-side at the postganglion level to leave the auditory division intact after mastoidectomy, partial destruction of the bony labyrinth, and surgical exposure of the internal auditory canal (Xerri and Lacour, 1980). Animals were maintained under antibiotics for 7 d and analgesics for 3 d. The classical postural, locomotor, and oculomotor deficits displayed by the animals in the days following nerve transection were used as criteria indicating the effectiveness of the vestibular nerve lesion. Completeness of vestibular nerve section had been assessed by histological procedures in previous studies (Lacour et al., 1976).
For the implantation and use of osmotic minipumps containing GABAA agonist (muscimol) or antagonist (gabazine), or 0.9% sodium chloride (NaCl), first muscimol and gabazine (Sigma-Aldrich) were diluted to the required concentration using artificial CSF (124 mm NaCl, 5 mm KCl, 1.2 mm KH2PO4, and 1.3 mm MgSO4) and tested for pH and adjusted to a pH 7.0, if necessary. As detailed in the study of Gliddon et al. (2005a), we chose concentrations high enough to provide an effect on vestibular compensation without adverse side effects on animals. Then, subcutaneous minipumps (Alzet; flow rate 2.5 μl/h for 30 d) were filled with the solution to infuse as previously done by Dutheil et al., 2009. The air in the system was removed by filling up with saline or GABAergic drugs. So, immediately after the unilateral vestibular neurectomy was done and the skin sutured, as the animal was still under anesthesia, a midline incision was made through the skin and musculature in the back of the neck, the minipump was inserted between the dorsal wall of the brainstem and the ventral face of the cerebellum and then cemented with dental cement to the skull while a stainless steel cannula was implanted into the fourth (IVth) ventricle of the brain.
Study design
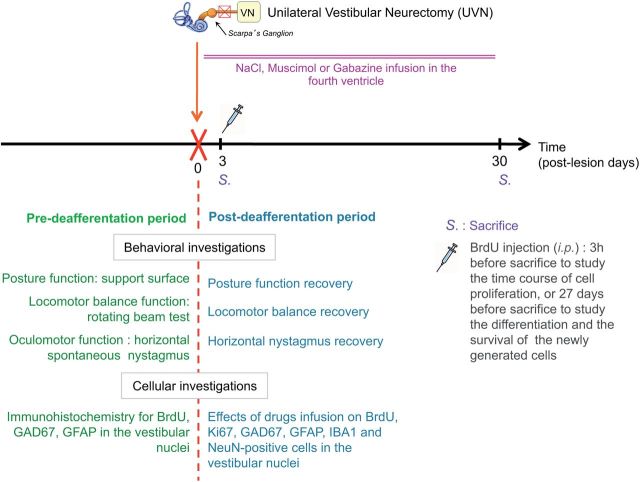
To determine the effects of GABAA agonist or antagonist infusion after UVN on the different steps of reactive neurogenesis at the cellular level and on the time course of the cats' recovery at a behavioral level (Fig. 1), we studied 10 groups of male adult cats: (1) control group, the animals (Group 1, n = 4) were submitted to anesthesia and the same surgical approach as UVN without sectioning the nerve and they immediately received a continuous NaCl infusion. These animals were killed at 30 d later; (2) UVN-NaCl groups, animals underwent UVN with continuous NaCl infusion, then received a BrdU injection (200 mg/kg, i.p.) and were killed at either D3 when cell proliferation reached a peak (Group 2, n = 4) or D30 to study the survival and the differentiation of the proliferating cells (Group 3, n = 4); (3) UVN-muscimol groups, animals underwent UVN with continuous muscimol infusion, received an intraperitoneal BrdU injection and were killed at D3 (Group 4, n = 4) or D30 (Group 5, n = 4); i.v.); UVN-gabazine groups, animals underwent UVN with continuous gabazine infusion, received an intraperitoneal BrdU injection and were killed at D3 (Group 6, n = 4) or D30 (Group 7, n = 4). The two postdeafferentation survival periods (D3 and D30) were selected on the basis of our anterior data (Tighilet et al., 2007).
Figure 1.
Study design. Experimental protocol elaborated for studying the effects of continuous infusion in the fourth ventricle of GABAAR agonist or antagonist on cell proliferation, survival and on the lineage of the surviving newly generated cells in the vestibular nuclei at different post-UVN days. In addition, behavioral analyses (oculomotor and posturo-locomotor tests) were done to evaluate the time course of functional recovery in all groups (n = 4 animals per group).
Concerning the behavioral investigations, 12 UVN cats were used for this study, they received continuous infusion of NaCl (Group 8, n = 4), muscimol (Group 9, n = 4), or gabazine (Group 10, n = 4) and were killed when vestibular compensation was complete or after 180 d for the UVN-muscimol Group 9.
Behavioral investigations
Spontaneous nystagmus recovery.
For nystagmus recording, 1 d after surgery, the cat was placed on an apparatus with its head fixed, thus maintaining the horizontal semicircular canals in the horizontal plane. The frequency of the horizontal spontaneous nystagmus was measured in the light as the number of quick phase beats toward the contralateral side relative to UVN in 10 s (five repeated measures per animal per sampling time) with a video camera (Sony HDV; Tighilet et al., 2006).
Posture recovery.
The support surface measure serves to evaluate the postural stability of the animal. Posture deficits and recovery were evaluated by measuring the surface delimited by the four legs of the cat while standing erect at rest, without walking. Support surface is considered a good estimate of postural control because it reflects the cat's behavioral adaptation compensating the static vestibulospinal deficits induced by the vestibular lesion (Tighilet et al., 1995). As a rule, the surface was very small in the normal cat (∼50–100 cm2) and greatly increased in the days following unilateral vestibular lesion. To quantify the support surface, cats were placed in a device with a graduated transparent floor that allowed them to be photographed from underneath. Five repeated measurements were done for each cat tested at each postoperative time, and an average was calculated for each experimental session. The support surface was measured as the surface delimited by the four legs by an image analysis system (Canvas 9, Deneba Software). Data recorded after vestibular lesion were compared with prelesion values by using individual references, permitting each animal to act as its own control.
Equilibrium function recovery.
Locomotor balance function was quantified using an adapted rotating beam experimental device (Xerri and Lacour, 1980). Two compartments (0.5 × 0.6 × 0.5 m) were connected by a horizontal beam (length: 2 m; diameter: 0.12 m). The beam, placed 1.2 m off the ground, could be rotated along its longitudinal axis with a constant angular velocity ranging from 0 to 588.4 deg/s (∼1.5 turn/s). Behavioral training on the rotating beam consisted in depriving the animals of food overnight before the first training session. Animals were conditioned to cross over the beam and were rewarded by a small piece of fish (or meat) placed in a small bowl in the target compartment. First crossings were made on the immobile beam and, thereafter, on the rotating beam. As a rule, rotation velocity of the beam was progressively increased after four consecutive trials without fall. Equilibrium function was thus quantified by measuring the highest speed of beam rotation that did not induce a fall. This maximal rotation speed determined the maximal locomotor balance performance (Max P). Preoperative training on the rotating beam necessitated 6–10 periods depending on the cats. Training was stopped when the cats' Max P was reached and stabilized at its highest level, which was found to be remarkably similar from one cat to another in each group.
Statistical analysis.
Statistical analysis was performed using Statview II software (SAS Institute). A two-way ANOVA was used to test for changes in the spontaneous nystagmus, the support surface, and the maximal equilibrium performance at the different postlesion delays. Results were considered statistically significant at p < 0.05 (Table 1).
Table 1.
Statistical analysis of chronic intracerebroventricular (fourth ventricle) infusions of saline, GABAAR agonist (muscimol), and antagonist (gabazine) on the horizontal spontaneous nystagmus, posture, and equilibrium function in unilateral vestibular neurectomized cats
| Source of variation | df | F | P* |
|---|---|---|---|
| Horizontal nystagmus | |||
| Group | 2 | 303.87 | 0.0001 |
| Postoperative time | 12 | 1024.04 | 0.0001 |
| Group × postoperative time | 24 | 74.05 | 0.0001 |
| Posture | |||
| Group | 2 | 12262.57 | 0.0001 |
| Postoperative time | 34 | 18083.34 | 0.0001 |
| Group × postoperative time | 68 | 63.63 | 0.0001 |
| Equilibrium function | |||
| Group | 2 | 60213.30 | 0.0001 |
| Postoperative time | 51 | 11723.15 | 0.0001 |
| Group × postoperative time | 102 | 2377.64 | 0.0001 |
*Repeated-measure ANOVA of the horizontal spontaneous nystagmus, posture recovery, and equilibrium function recovery. Group (unilateral vestibular neurectomized cats infused with muscimol, gabazine, or NaCl) and survival period are the main fixed effects providing the sources of variation among cats, as also illustrated by the significant interaction between these two variables. df, Degree of freedom; F, Scheffé's test; P, probability level.
Cellular investigations
Tissue preparation.
BrdU (10 mg/ml, Sigma-Aldrich) was dissolved in a solution of sodium chloride (NaCl) 0.9% heated to 56°C and injected into animals (200 mg/kg). Cameron and McKay (2001) showed in adult rat dentate gyrus that a single dose of BrdU 100, 50, or 25 mg/kg (body weight, i.p.) labeled 60, 45, and 8% of S-phase cells, respectively. At 300 mg/kg, BrdU labeled most S-phase cells and had no physiological side effects. So, in line with the conclusions of Taupin (2007), we considered 200 mg/kg as a saturating concentration of BrdU for studying adult neurogenesis (Taupin, 2007). BrdU doses were not likely to generate side effects, but were sufficient to mark the cells in S-phase synthesizing DNA. Before BrdU administration, the cats of each group were deeply anesthetized with ketamine dihydrochoride (20 mg/kg, i.m., Merial) and killed by 0.9% NaCl (1 L per animal) then paraformaldehyde 4% (2 L per animal) transcardiac perfusion either 3 h or 27 d later according to their experimental group. After removal from the skull, brains were cut into several blocks containing the VN. The blocks were rapidly frozen with dry ice and stored at −80°C. Coronal sections (40-μm-thick) were cut in a cryostat (Leica) for immunochemistry.
Immunochemistry.
Immunochemical labeling was performed according to previously validated protocols (Brezun and Daszuta, 2000; Tighilet et al., 2007). Ki67 marker was used in addition to BrdU to confirm that BrdU had been incorporated into mitotic cells and did not correspond to dying cells or DNA repair mechanism. For BrdU immunostaining, free-floating sections were first rinsed in 0.1 m PBS and incubated with 2N HCl and 0.5% Triton X-100 in PBS (30 min, 37°C) for DNA hydrolysis. Then sections were rinsed in 0.1 m sodium tetraborate buffer, pH 8.5 before overnight incubation with the primary antibody at 4°C, followed by incubation with the secondary antibody for 1.5 h at room temperature, and visualized using horseradish peroxidase avidin-D (Vector Laboratories). GFAP and GAD67 immunoreactivity assays were performed according to Tighilet et al., 2007. After several rinses, sections were mounted on gelatin-coated slides, dehydrated, and coverslipped in Depex mounting medium for peroxidase staining. The differentiation of the newly generated cells was analyzed in the UVN-NaCl and UVN-gabazine group of cats injected with BrdU 3 d after UVN and killed after 1 month. We used double-immunofluorescent stained sections incubated with BrdU and one of four antibodies: NeuN, a postmitotic neuronal nuclei marker expressed in most neurons; GFAP, a specific type of intermediate filament protein used as astrocyte marker; IBA1, an ionized calcium binding adapter molecule 1, specific to microglia and macrophages but not cross-reactive with neurons and astrocytes; and GAD 67, the enzyme that catalyzes the decarboxylation of glutamate to GABA and expressed in GABAergic neurons. Each antibody was processed sequentially, the differentiation marker detection first and then the BrdU labeling. For fluorescent labeling, sections were incubated with a secondary antibody coverslipped in Mowiol. The optimal antibody dilutions and staining procedures are described in Table 2. Differentiation of the newly generated cells was analyzed with double-labeling analysis performed using confocal imaging with a Zeiss LM 710 NLO laser scanning microscope equipped with a 63×/1.32 NA oil-immersion lens. The fields of view were then examined by confocal microscopy, and 1 μm-step z-series were obtained.
Table 2.
Antibodies and methods of detection
| Marker | Primary antibody | Secondary antibody | Technique, coloration |
|---|---|---|---|
| BrdU | Mouse 1/100, Dako | Horse anti-mouse 1/200, Vector | DAB, brown |
| GFAP | Rabbit 1/200, Dako | Goat anti-rabbit 1/200, Vector | DAB, brown |
| GAD67 | Mouse 1/1,000, Chemicon | Horse anti-mouse 1/200, Vector | DAB, brown |
| Ki67 | Mouse 1/100, Dako | Horse anti-mouse 1/200, Vector | DAB, brown |
| BrdU | Rat 1/100, Oxford Biot | Rabbit anti-rat 1/200, Interchim | Alexa Fluor 594, red |
| GFAP | Rabbit 1/200, Dako | Goat anti-rabbit 1/200, Interchim | Alexa Fluor 488, green |
| GAD67 | Mouse 1/100, Chemicon | Rabbit anti-mouse 1/200, Interchim | Alexa Fluor 488, green |
| NeuN | Mouse 1/100, Chemicon | Rabbit anti-mouse 1/200, Interchim | Alexa Fluor 488, green |
| IBA1 | Rabbit 1/2000, Wako | Goat anti-rabbit 1/200, Interchim | Alexa Fluor 488, green |
Combination and sequential processing of primary and secondary antibodies used for immunohistochemical and dual immunofluorescent stainings for Ki 67, BrdU, GFAP, GAD67, NeuN, or IBA1.
Cell counts and statistical analysis.
Cell counts were performed according to a previously validated protocol (Dutheil et al., 2009, 2011). Great care was taken not to count blood cells as BrdU+ cells. The VN were identified through Berman's stereotaxic atlas (Berman, 1968). BrdU+, GFAP+, and GAD67+ were quantified for each VN (medial vestibular nuclei, MVN; inferior vestibular nuclei, IVN; superior vestibular nuclei, SVN; and lateral vestibular nuclei, LVN) from selected serial frontal sections collected from the dorsal (5.2) to the caudal (12.1) part of the brainstem and depending on the size and the rostrocaudal length of each VN. BrdU+ cells, Ki67+cells, GFAP+ cells, and GAD67+ neurons were analyzed in each VN on both sides (left/right: sham-operated cats; ipsilateral/contralateral: UVN-lesioned cats). We decided to analyze the VN subdivisions instead of analyzing the whole set of nuclei because (1) these markers are expressed differentially in the VN after UVN and (2) the VN are involved differentially in the vestibular compensation processes. The SVN, which is the structure most involved in oculomotor function, does not exhibit neurogenesis. Conversely, a large number of newborn neurons were observed in the MVN, LVN, and IVN, which are mainly associated with static and dynamic postural functions. Although it is usually straightforward to distinguish large- and middle-sized neurons from glial cells, the distinction between small neurons and large glial cells can be challenging. Thus, the following criteria were used as characteristic to distinguish small GAD 67+ neurons from GFAP+ glial cells at D30: a centrally located nucleolus, a distinctive nucleus, visible cytoplasm, presence of dendritic processes, and larger cell body size. Hence, glial cells were identified by sparse cytoplasm and smaller cell body size (Christensen et al., 2007). The cell count was done with a Nikon microscope (Eclipse 80i) equipped with a motorized x-y-z-sensitive stage and a video camera connected to a computerized image analysis system (Mercator, Explora Nova). The total number of immunolabeled cells was estimated using the optical fractionator method (West et al., 1991). BrdU+, Ki67+, GFAP+, NeuN+, IBA1+, and GAD67+ were counted and sampled according to the so-called fractionator principles, that is, a combination of the optical disector, a three-dimensional probe used for counting, and fractionator sampling, a scheme involving the probing of a known fraction of the tissue (West, 1993). This cell-counting method has been described and validated in previous publications (Dutheil et al., 2009, 2011). Accordingly, the statistical analyses were evaluated by ANOVA to test the effects of the group (UVN-NaCl, -musimol, or -gabazine), the structure (MVN, IVN, LVN, SVN), and the postoperative time on Ki 67+, BrdU+, GFAP+, and GAD67+ cells and to determine whether there were any interactions between these variables (Table 3). ANOVA was followed by post hoc analysis with the Scheffé test (StatView II, SAS Institute; Table 3). The values of the coefficient of error of the estimated number of BrdU+, Ki67+, GAD67+, and GFAP+ cells in the ipsilateral/deafferented VN of the experimental and the sham groups for each survival period tested were within acceptable ranges, as described in an earlier study (West, 1999; Dutheil et al., 2011).
Table 3.
Statistical analysis of the effects of chronic intracerebroventricular (fourth ventricle) infusions of saline, GABAAR agonist (muscimol), and antagonist (gabazine) on the Ki67, BrdU, GFAP, and GAD67 immunoreactivity in the vestibular nuclei of control and unilateral vestibular neurectomized cats
| Source of variation | df | F | P* |
|---|---|---|---|
| Ki67-positive cells | |||
| Group | 2 | 3078.12 | 0.0001 |
| Vestibular nuclei | 3 | 254.65 | 0.0001 |
| Group × vestibular nuclei | 6 | 87.51 | 0.0001 |
| BrdU-positive cells | |||
| Group | 2 | 1553.91 | 0.0001 |
| Vestibular nuclei | 3 | 830.66 | 0.0001 |
| Group × vestibular nuclei | 6 | 322.53 | 0.0001 |
| Postoperative time | 1 | 25517.95 | 0.0001 |
| Group × postoperative time | 2 | 8095.05 | 0.0001 |
| Nuclei × postoperative time | 3 | 342.06 | 0.0001 |
| Group × vestibular nuclei × postoperative time | 6 | 142.66 | 0.0001 |
| GAD67-positive cells | |||
| Group | 3 | 2699.69 | 0.0001 |
| Vestibular nuclei | 3 | 2125.73 | 0.0001 |
| Group × vestibular nuclei | 9 | 344.42 | 0.0001 |
| GFAP-positive cells | 3 | ||
| Group | 3 | 2189.05 | 0.0001 |
| Vestibular nuclei | 9 | 1383.88 | 0.0001 |
| Group × vestibular nuclei | 158.22 | 0.0001 |
*Repeated-measure analysis of variance of the number of Ki67-, BrdU-, GFAP-, and GAD67-positive cells in vestibular nuclei. Group (control, unilateral vestibular neurectomized cats infused with muscimol, gabazine, or NaCl), vestibular nuclei (MVN, IVN, LVN, and SVN) and postoperative time (3 d post UVN vs 30 d post UVN) are the main fixed effects providing the sources of variation among cats. df, Degree of freedom; F, Scheffé's test; P, probability level.
Results
Proliferation and survival of newborn cells in deafferented vestibular nuclei are altered by infusion of GABAergic agents
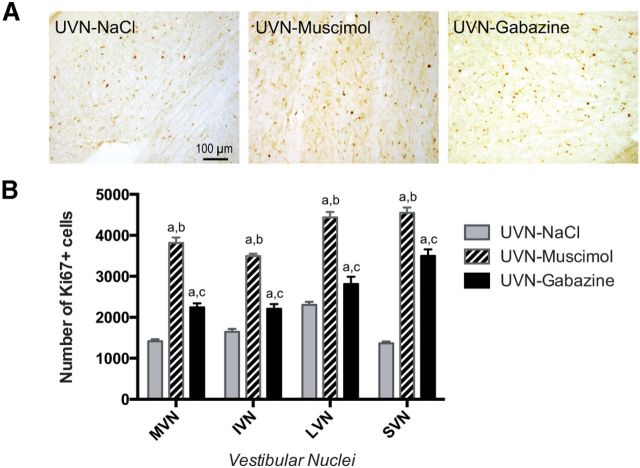
To investigate whether GABAAR agonist and antagonist infusions alter cell proliferation, survival, and differentiation in the VN after UVN, we used immunohistochemistry methods. Cell proliferation was examined by injecting BrdU 3 d after UVN, when the number of proliferative cells is maximal in the deafferented VN, and then animals were killed 3 h later (Tighilet et al., 2007; Dutheil et al., 2011). As in our previous studies, in control nonlesioned cats, BrdU-labeled cells were absent in the VN at D3 and even D30 postsurgery, whereas in the group of cats submitted to a UVN and infused with saline (NaCl), a large number of BrdU+ cells was observed in the deafferented VN at D3 (Tighilet et al., 2007; Dutheil et al., 2009). The number of BrdU+ cells in the VN complex was comparable to that for the same time point in adult cats submitted to a UVN without saline infusion (Tighilet et al., 2007; Dutheil et al., 2009, 2011). Interestingly, in the UVN-gabazine group, the number of BrdU+ cells was significantly higher at the third postlesion day (+39.6% in the MVN, +19.4% in the IVN, +17.3% in the LVN, and +126.1% in the SVN, p < 0.0001 compared with UVN-NaCl, n = 4) and even further enhanced in the UVN-muscimol group (+196% in the MVN, +80% in the IVN, +97.5% in the LVN, and +297% in the SVN, p < 0.0001 compared with UVN-NaCl, n = 4; Fig. 2A,B). This ratio was confirmed by Ki67 immunostainings (Fig. 3B).
Figure 2.
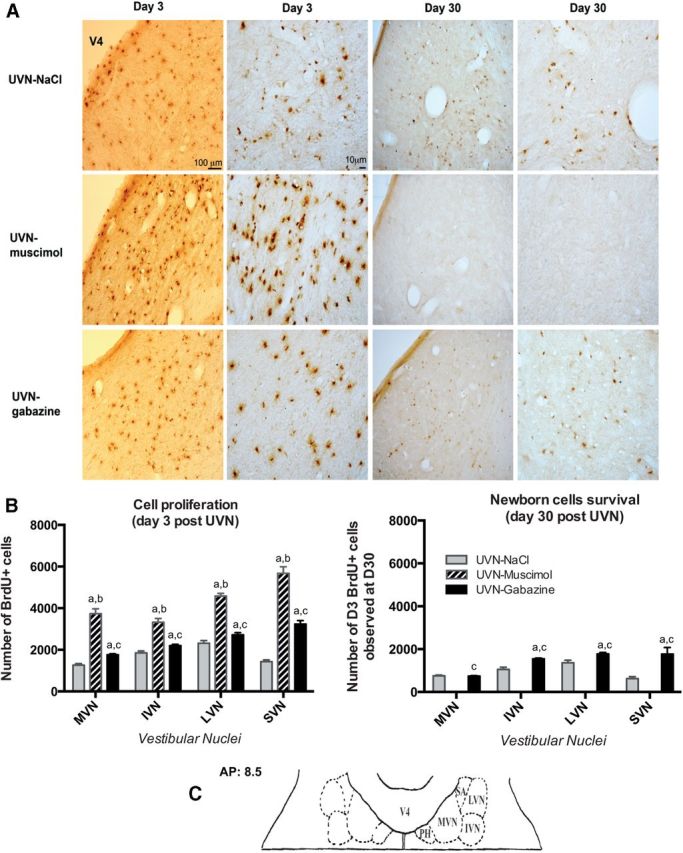
Reactive cell proliferation and survival are altered in the deafferented vestibular nuclei under continuous GABAAR agonist and antagonist infusions following unilateral vestibular neurectomy. A, Photomicrographs showing BrdU immunostainings in the deafferented MVN in cats submitted to UVN and drug infusion in the fourth ventricle (NaCl, muscimol, or gabazine). Animals received BrdU injection (i.p. 200 mg/kg body weight) 3 d after UVN and then were perfused with paraformaldehyde either 3 h later to study cell proliferation since it has been described to be the strongest at this time point, or 27 d later to study survival of these newborn cells. Scale bars: 100 and 10 μm for lower and higher-magnification respectively at both D3 and D30. B, Quantitative evaluation. Histograms comparing the mean values (±SEM) of the number of BrdU-immunopositive cells in the deafferented VN 3 and 30 d after UVN in cats submitted to NaCl, muscimol, or gabazine infusion. Different letters indicate significant differences between groups of animals assessed by ANOVA followed by Scheffé test, p < 0.0001; Table 3. a, Significantly different from NaCl group; b, significantly different from gabazine group; and c, significantly different from muscimol group. Only values recorded on the lesioned side are illustrated; n = 4 animals per group. C, Schematic representation illustrating the localization of the anteroposterior (AP) axis adapted from Berman's strereotaxis atlas where the photomicrographs of the medial vestibular nuclei were taken. PH, Praepositus hypoglossi; SA, stria acustica; V4, fourth ventricle.
Figure 3.
Ki67 immunostaining was used to confirm cell proliferation. A, Photomicrographs showing Ki67 immunostainings in the deafferented MVN in cats receiving NaCl, muscimol, or gabazine continuous infusion immediately after UVN and killed at postlesion day third. Scale bar, 100 μm. B, Quantitative evaluation. Histograms comparing the mean values (±SEM) of the number of Ki67-immunopositive cells in the deafferented VN 3 d after UVN in cats submitted to NaCl, muscimol, or gabazine infusion using ANOVA followed by Scheffé test (p < 0.0001; Table 3). Different letters indicate significant differences between groups of animals: a, significantly different from NaCl group; b, significantly different from gabazine group; and c, significantly different from muscimol group. Only values recorded on the lesioned side are illustrated; n = 4 animals per group.
To study the survival of the newly generated cells stained with BrdU at D3, three subgroups of animals were killed 27 d after BrdU injection. We found that ∼60% of BrdU+ cells survived in the UVN-gabazine group (42% in the MVN, 70% in the IVN, 65% in the LVN, and 55% in the SVN compared with the number of BrdU+ cells at D3) and ∼50% survived in the UVN-NaCl group (59% in the MVN, 57% in the IVN, 58% in the LVN, and 44% in the SVN). The data between these two groups were significantly different except for the MVN (UVN-NaCl versus UVN-gabazine). Strikingly, no BrdU+ cells were found in the UVN-muscimol group 30 d after UVN (Fig. 2B).
Fate of newly generated cells in deafferented vestibular nuclei
The BrdU+ surviving cells differentiated and acquired a neurochemical phenotype ∼20 d after birth; cell differentiation was investigated by double-immunohistochemical labeling using BrdU combined with the four specific cell type markers: GFAP, NeuN, IBA1, and GAD67.
Double immunohistochemistry against BrdU and GFAP at D30 showed that the UVN-NaCl and UVN-gabazine groups displayed numerous cells with costaining, whereas no surviving BrdU+ cells were observed at this delay in the UVN-muscimol group, suggesting that newborn cells that had incorporated BrdU at the third post-UVN day did not survive until 30 d postinjection (data not shown). The double labeling has been quantified only at D30 in the UVN-NaCl and UVN-gabazine groups since colabeled cells were not observed at the early time point D3.
The photomicrographs in Figure 4A show the colocalization of BrdU+ with GFAP+, NeuN+, IBA1+, and GFAP+ cells observed in the deafferented MVN at the 30th postlesion day in the UVN-NaCl group. The fate of the newly generated cells varied depending on the VN (Fig. 4B). The results are expressed in percentage defined as the ratio between the mean number of immunopostive-elements colocalizing a cell type marker (GFAP, IBA1, NeuN, or GAD 67) and BrdU relative to the total mean number of BrdU+ nuclei counted in the areas of quantification. For the glial (GFAP and IBA1) and neuronal marker (NeuN), the newly generated cells differentiated approximately in similar proportion in the MVN (15.58, 16.88, and 21.82%), IVN (11.79, 13.26, and 14.74%), and the LVN (11.50, 12.25, and 14.92%, respectively). In the SVN, by contrast, GFAP and IBA1 labeling were higher (30.71 and 20%) than NeuN labeling (5.71%). The mean number of newly generated GAD67+ neurons varied depending on the VN (Fig. 4B). The highest proportion was observed in the MVN (16.62%), a lower proportion was observed in the IVN and LVN (8.42 and 8.08%). The weakest number was observed in the SVN (4.29%).
Figure 4.
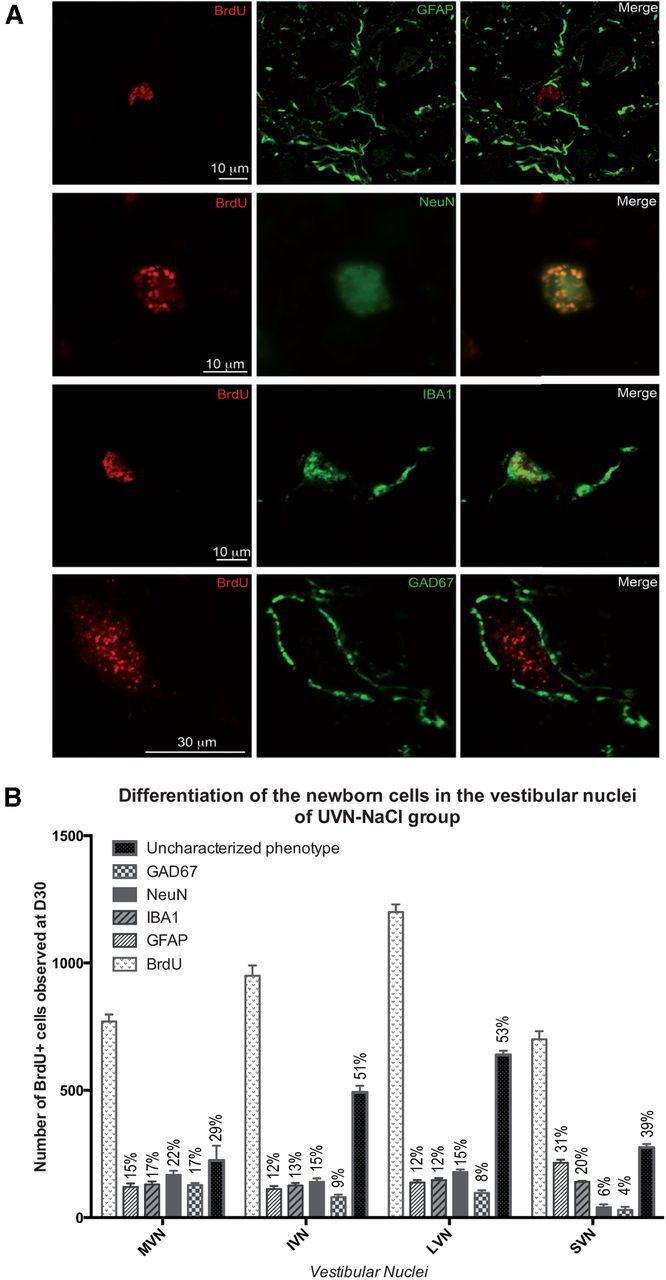
A, Confocal analysis of differentiated newly generated cells by double immunostainings processed on consecutive serial sections. The BrdU+ nuclei are in red and the other markers of differentiation: GFAP+, IBA1+, NeuN+, and GAD 67+ in green. The representative illustrations of the differentiation were made in the deafferented MVN of cats infused with NaCl from day 0 to day 30 after unilateral vestibular neurectomy. B, Histograms illustrating the percentage of GFAP, IBA1, NeuN, and GAD67-positive cells among the BrdU-positive cells in the vestibular nuclei 30 d after unilateral vestibular neurectomy and chronic NaCl infusion in the fourth ventricle.
In the UVN-gabazine group, the MVN had a higher proportion of GFAP+, with nearly similar proportions of IBA1+ and NeuN+ cells (Fig. 5A,B) and the proportion of newly generated GAD67+ neurons were reduced by half compared with the UVN-NaCl group in all the VN (Fig. 5B). In both groups, regardless of the VN examined, ∼50% of the phenotype of the newly generated BrdU+ cells did not colocalize with GFAP, IBA1, GAD67, and NeuN markers.
Figure 5.
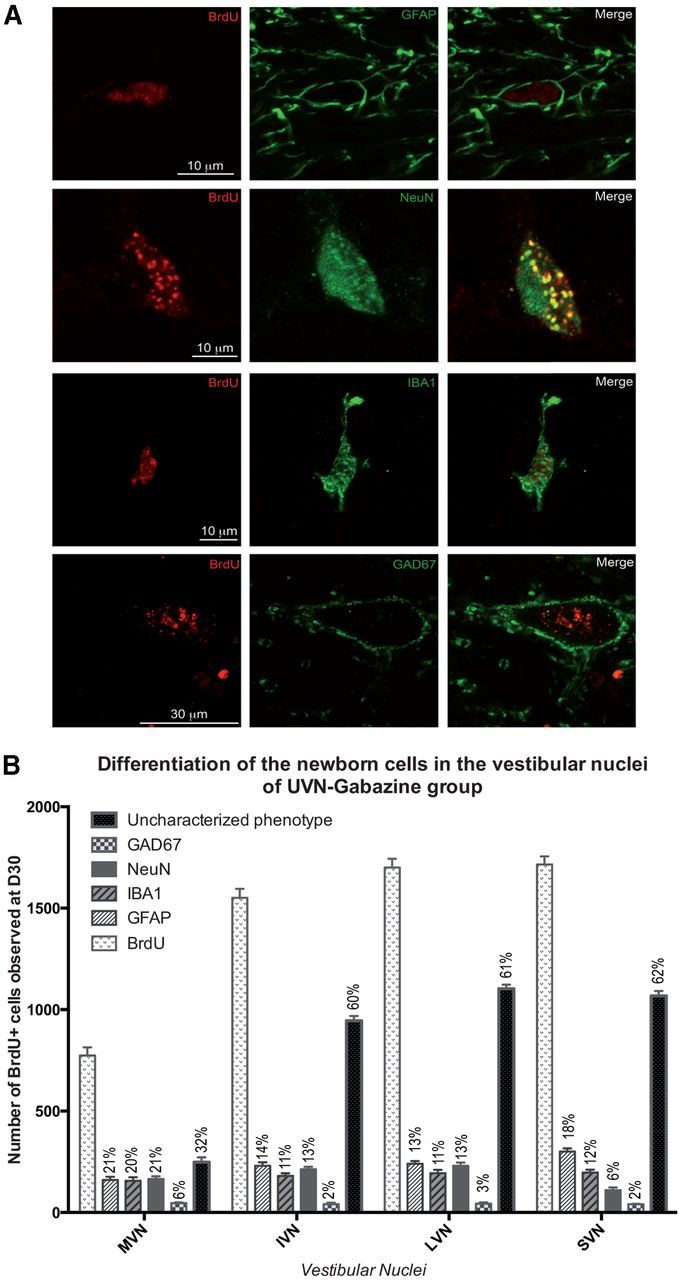
A, Confocal analysis of differentiated newly generated cells by double immunostainings processed on consecutive serial sections. The BrdU+ nuclei are in red and the other markers of differentiation: GFAP+, IBA1+, NeuN+, and GAD 67+ in green. Illustrations of the differentiation were made in the deafferented MVN of cats infused with gabazine from day 0 to day 30 after unilateral vestibular neurectomy. B, Histograms illustrating the percentage of GFAP, IBA1, NeuN, and GAD67-positive cells among the BrdU-positive cells observed in the vestibular nuclei 30 d after unilateral vestibular neurectomy and chronic gabazine infusion in the fourth ventricle.
Astrocytic and GABAergic populations are altered in the deafferented vestibular nuclei
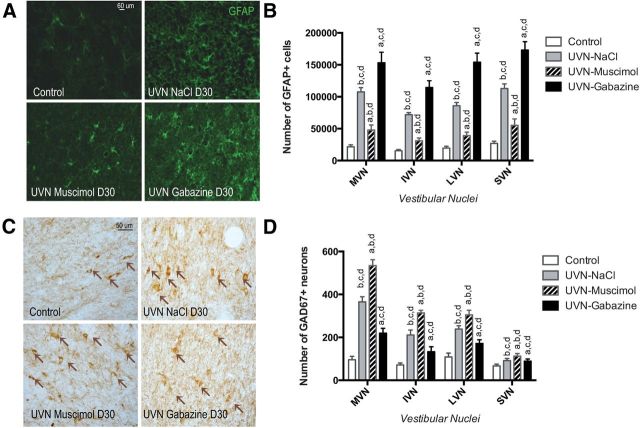
The immunohistochemistry results with GAD67 and GFAP antibodies served to quantitatively analyze the number of GABAergic neurons and astrocytes respectively. In line with our previous reports (Tighilet et al., 2007; Dutheil et al., 2009, 2011), in the UVN-NaCl group, there was a significant astroglial reaction at the 30th day post-UVN in the deafferented VN (Fig. 6A,B). Further, the UVN-gabazine group displayed more GFAP+ cells in the deafferented VN than the UVN-NaCl group (+42.5% in the MVN, +59.5% in the IVN, +79.4% in the LVN, and +53.2% in the SVN, n = 4). In contrast, the number of GFAP+ cells decreased significantly in the UVN-muscimol group (−55.4% in the MVN, −56.7% in the IVN, −54.4% in the LVN, and −51% in the SVN compared with the UVN-NaCl group, n = 4; p < 0.0001 (Fig. 6B).
Figure 6.
One month after UVN, GFAP, and GAD67 immunoreactivity are differentially expressed in the vestibular nuclei of unilateral vestibular neurectomized cats according to the drug infused (NaCl, Muscimol, or gabazine). A, Illustrations of GFAP immunoreactivity in the MVN of control, UVN-NaCl, UVN-muscimol, and UVN-gabazine animals 27 d after BrdU injection performed the third postlesion day. Scale bar, 60 μm and n = 4 animals per group. B, Histograms showing the effects of the drug infusion (NaCl, muscimol, or gabazine) on the number of GFAP-immunopositive cells in the deafferented vestibular nuclei. C, Illustrations of GAD67 immunoreactivity in the MVN of control, UVN-NaCl, UVN-muscimol, and UVN-gabazine animals at D30. Scale bar, 50 μm. n = 4 animals per group. D, Histograms showing the effects of NaCl, Muscimol, or gabazine continuous infusion on the number of GAD67-immunopositive neurons in the deafferented vestibular nuclei. Only values recorded on the lesioned side are illustrated and data from both sides of control cats were pooled for direct comparison with the subgroups of vestibular deafferented cats. Different letters indicate significant differences between groups of animals: a, significantly different from NaCl group; b, significantly different from gabazine group; c, significantly different from muscimol group; and d, significantly different from control group. Analyzes were assessed by ANOVA followed by Scheffé test for all the VN and the groups (p < 0.0001; Table 3).
In the UVN-muscimol group, despite the nonsurvival of the newborn cells, as revealed by the lack of BrdU+ cells at D30 (Fig. 2), we observed a strong increase in the number of GAD67+ neurons in the deafferented VN (Fig. 6C,D; +46.3% in the MVN, +48.4% in the IVN, +27.5% in the LVN, and +22.7% in the SVN compared with the UVN-NaCl group, p < 0.0001). This suggests an increased GAD67 expression by mature pre-existent neurons. In addition, the number of GAD67+ cells was lower in the UVN-gabazine group than in the UVN-NaCl group (Fig. 6D; −39.7% in the MVN, −36.9% in the IVN, −28% in the LVN, and −2.8% in the SVN).
Vestibular functional recovery
It is well known that unilateral vestibular damage leads to a vestibular syndrome characterized by a spontaneous horizontal nystagmus with its slow phase directed toward the lesioned side, postural imbalance, yaw, and head tilt, circling and rolling toward the damaged side, and extension of the contralateral forelimb. Over time, these symptoms decrease and even disappear.
Nystagmus
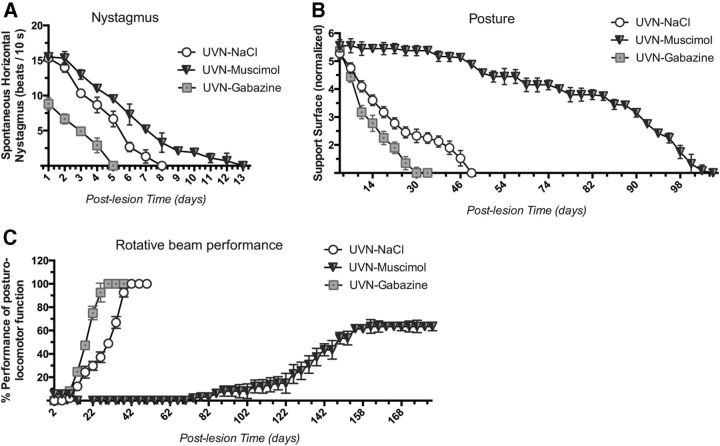
At the first post-UVN day, the frequency of the spontaneous nystagmus was 15.3 beats/10 s in the UVN-NaCl, 15.5 in the UVN-muscimol, and 8.8 in the UVN-gabazine groups, respectively. The number of eye beats declined significantly in all experimental groups to reach control values at D5 in the UVN-gabazine group, at D8 in the UVN-NaCl group, and at D13 in the UVN-muscimol group (p < 0.0001; Fig. 7A).
Figure 7.
Behavioral recovery time course can be delayed or accelerated according to the GABAergic drug infused after unilateral vestibular neurectomy. A, Curves illustrating the time course (on the abscissae) of disappearance of horizontal spontaneous nystagmus (HSN) frequency (on the ordinates) for each group of vestibular deafferented cats at different postoperative days. Each data point represents the mean number of HSN quick phase movements in 10 s for four animals (five repeated measures per animal per sampling). Error bars represent SEM. B, Curves indicating the mean postoperative recovery of the support surface in the three experimental groups of cats (UVN-NaCl, UVN-muscimol, and UVN-gabazine). Data recorded after vestibular deafferentation were related to individual references and normalized with respect to the preoperative values referred to unity (one being close to 50 cm2). SEs of the mean are reported as vertical lines. C, The Max P is defined as the highest beam rotation speed that did not lead to a fall on four consecutive crossings. The curves are expressed in percentage of the preoperative maximal performance (in the ordinates) as a function of the postoperative time in days (on the abscissae). SEMs are reported as vertical lines. Note the time of recovery in the UVN-gabazine group compared with the other groups: 30 d instead of 42 for UVN-NaCl, interestingly, at the 180 postlesion day UVN-muscimol cats were still below their Max P (63.49%).
Posture function recovery
In four-footed animals standing erect, vestibular syndrome leads to an increased support surface delimited by the four paw pads. This parameter provides a good estimation of postural stability and recovery. It displays the tonic asymmetries of extensor and flexor muscles of the anterior and posterior paws that are induced by the vestibular deafferentation. Return to preoperative control values was faster for the UVN-gabazine group (34 d) than the UVN-NaCl group (48 d) and the UVN-muscimol group (104 d; p < 0.0001; Fig. 7B). ANOVA established significant effects depending on the groups (p < 0.0001), the postoperative time (p < 0.0001), and the interaction between these two factors.
Locomotor balance recovery
In line with data of the posture function and the nystagmus, animals of the UVN-gabazine group more quickly recovered their dynamic locomotor balance and crossed the rotating beam at their Max P at the 30th day after deafferentation. The cats of the UVN-NaCl group reached their Max P 42 d after deafferentation (Fig. 7C). Interestingly, the UVN cats infused with muscimol were not able to walk on the beam rotating at the lowest speed until 62 d after surgery and finally reached a plateau of 63.49% of their Max P at D180. ANOVA of the locomotor balance function recovery demonstrated significant effects depending on the groups (p < 0.0001), the postoperative time (p < 0.0001), and the interaction between these two factors (p < 0.0001).
Discussion
It is now established that CNS injuries can lead to local cell proliferation increase (Moyse et al., 2008). However, in most cases, the newborn cells either disappear or adopt a glial phenotype as described, for example, in striatum after stroke (Arvidsson et al., 2002; Parent et al., 2002). Previous work in our laboratory already described the occurrence of the vestibular cell proliferation after UVN (Tighilet et al., 2007; Dutheil et al., 2009, 2011), hence highlighting the reliability of this neuroplasticity event in response to nerve injury. The originality of our model is to provide a new tool to analyze lesion-dependent neurogenesis which also contributes to the behavioral recovery of vestibular neurectomized cats (Tighilet et al., 2007). In the deafferented VN, newborn cells survive up to 2 months and have been demonstrated to be involved in behavioral recovery (Dutheil et al., 2009). These results are in accordance with our previous findings, suggesting that hampering newborn cell proliferation and/or survival in the VN after vestibular neurectomy impedes the recovery of posturo-locomotor functions: a process that appears to be modulated through GABAAR activity.
Chronic muscimol or gabazine infusion increase cell proliferation in the deafferented vestibular nuclei
Newly generated cells have been observed in vagal dorsal complex of the brainstem rat after axotomy of the vagus nerve (Bauer et al., 2005; Moyse et al., 2006). Likewise, in the vicinity of these nuclei, our laboratory previously demonstrated a strong induction of cell proliferation in the deafferented VN following vestibular nerve section (Tighilet et al., 2007). In the current study, we now demonstrate that with intracerebroventricular infusion of either gabazine or muscimol for 1 month, cell proliferation was significantly enhanced on the lesioned side of the VN. Because we found comparable findings with Ki67 immunostaining, we can exclude that BrdU+ cells underwent cell death or DNA repair. In support of our data, some authors also described an increased number of proliferative cells after GABAAR alterations either in vitro or in vivo (LoTurco et al., 1995; Fiszman et al., 1999; Liu et al., 2005; Fernando et al., 2011). More precisely, Chan et al. (2008) reported that hippocampal cell proliferation was modulated by GABAAR blockade with injections of Picrotoxin for three consecutive days in wild-type (WT) or BDNF-deficient mice. Although no significant effect was found in BDNF mutants, cell proliferation was increased in the dentate gyrus of WT mice with GABAAR antagonist administration. Our findings fall in line with such finding since we observed an increase in newly generated cells in the VN with GABAAR antagonist infusion as well. In addition, we previously observed increased growth factors expression (brain derived neurotrophic factor, BDNF and nerve growth factor, NGF) the first few days after UVN (Tighilet, 2004) which suggests that GABA have trophic effects on cell proliferation, possibly through growth factors signaling implemented in cell growth and survival (e.g., BDNF and NGF).
Gabazine infusion favors newborn cell survival and astrocytic population increase in the vestibular nuclei whereas muscimol has opposite effects
GABAAR modulation can affect proliferation, migration, maturation and survival of neural precursors (Bordey, 2007; Salazar et al., 2008; Song et al., 2012). Using the model of UVN, we show the influence of these receptors in these different steps in the context of injury-induced adult neurogenesis. Reactive cell proliferation in the VN does not seem to share the same properties as ongoing neurogenesis in the adult subventricular and subgranular zones. Despite the cell proliferation peak occurring 3 d after injury, we did not observe colocalization of BrdU with astrocytic and microglial markers, suggesting that in the cat brainstem, newborn cells may require longer time to express the markers that we examined in this study. Interestingly, although cell proliferation increased in the VN at D3, this was actually transitory with muscimol infusion because 1 month later newborn cells were no longer observed. This phenomenon is quite similar to what happens during development and could be related to the cell death of newly generated cells induced by GABAA activation (Honegger et al., 1998; Nuñez et al., 2003). This effect seems to occur after the peak of proliferation occurring at D3 (Tighilet et al., 2007) and can be regulated by distinct mechanisms. Indeed, as muscimol potentiates GABA inhibitory actions and confer depressant properties, the hampering of newborn cell survival and maturation may be related to the lack of depolarizing activity required for their differentiation and integration into pre-existing network (Overstreet-Wadiche et al., 2006). Another possible mechanism could involve the decrease in astroglial cells and their consecutive lack of trophic support, which could in part explain the disappearance of newborn cells between our snapshot observations (i.e., D3 and D30) and necessitate further investigations.
Another important point of the study is the highest percentage of surviving newborn cells observed following gabazine chronic intracerebroventricular infusion, associated with an increased number of astrocytic cells. Given the beneficial role of astrocytes in supporting new neurons, it is not surprising that an increased astrocyte population was coupled with a high newborn cell survival ratio. In addition, gabazine infusion led to an increased number of GABAergic neurons, which could result from either an enhanced expression of GAD67 from GABAergic neurons that were previously secreting GABA levels below the detection threshold, or a phenotypic change (a non-GABAergic neuron becoming GABAergic) as already postulated in a previous study (Tighilet and Lacour, 2001). In addition, although the percentage of BrdU/NeuN+ was not affected by gabazine, the percentage of BrdU/GAD67+ was decreased by half. Hence, it is possible that a subset of these newly generated neurons could have an excitatory phenotype favoring the recovery of intrinsic excitability within the deafferented VN. Also, it has been demonstrated that when the majority of newborn cells disappear a few days after production (Tashiro et al., 2006; Tronel et al., 2010), experience and learning can facilitate maturation of newborn neurons and enhance their survival in the adult rodent (Overstreet-Wadiche et al., 2006). Here a hypothesis can be that the survival effects of gabazine on both neurons and glial cells would be mediated through neuronal networks disinhibition. Thus, such mechanisms would support direct and indirect intrinsic network depolarization and promote the rebalance of electrical activity between the VN. Further studies would allow us to distinguish between these possibilities.
Together, our results thus show that these two pharmacological treatments trigger contrasting effects on GABAergic, astroglial and newborn cell populations in the VN after vestibular damage, affecting not only the number of cells but also the architecture and the function of local neuronal networks.
Gabazine accelerates vestibular function recovery but muscimol drastically delays it
After UVN, depression of resting activity discharge of the ipsilateral second-order vestibular neurons is responsible for the appearance of vestibular symptoms. Interestingly, pharmacological treatments can accelerate or delay the behavioral recovery by acting on the neuronal spontaneous activity between homologous VN. Notably, both GABAA and GABAB receptors participate in vestibular reflex pathways and vestibular compensation (de Waele et al., 1995; Gliddon et al., 2005a,b). Our data show that gabazine accelerated, while Muscimol delayed spontaneous nystagmus disappearance. GABAAR agonists and antagonists, such as diazepan and picrotoxin have been demonstrated to affect spontaneous nystagmus frequency in animals and vertigo sensation in patients (McCabe et al., 1973; Ehrenberger et al., 1982; Ehrenberger and Felix, 1996).
Furthermore, here we found that animals treated with gabazine were able to cross the rotating beam earlier and recovered their posturo-locomotor functions faster. In line with our observations, previous reports showed that systemic injection of GABAA antagonists accelerated posture recovery in rats (Dutia et al., 1992; Vibert et al., 1995). Remarkably, this draws a coherent picture with what we observed at the cellular level in the VN: as vestibular compensation is accelerated, gabazine treatment promotes both cell proliferation and survival of new neurons, suggesting some clues about the functional contribution of these new cells in the VN.
Another notable finding of this study is the drastically delayed posturo-locomotor function recovery with muscimol treatment. Indeed, 6 months after UVN, animals had still not recovered their preoperative whereas gabazine- and saline-lesioned animals reached their preoperative Max P at 30 and 42 d respectively. Similarly, Pompeiano et al. (1993) reported that acute injection of muscimol into the lateral vestibular nucleus gave rise to hypotonia of the ipsilateral forelimbs and hypertonia of the contralateral forelimbs. It has also been described that GABAAR agonists decompensated vestibular symptoms (i.e., led to acute symptoms) of animals that had already compensated, acting on the resting activity mediated by the vestibular commissures (Furuya et al., 1991; Furuya and Koizumi, 1998). Therefore, GABAAR agonists may not to be beneficial for posturo-locomotor functions recovery after vestibular damage and it is noteworthy to consider that cell proliferation was only transitory after muscimol treatment. Because it has been showed that blockade of cell proliferation in the dentate gyrus can lead to neuronal activity disturbances (Kitamura et al., 2009; Lacefield et al., 2012), a possible explanation would be that newborn cells loss induced by muscimol could accentuate acute pathological conditions and result in comparable neuronal activity disorganizations, thus impairing cell survival and behavioral recovery in the context of vestibular injury.
In addition, previous studies from our laboratory have described that when infused continuously with an antimitotic agent (i.e., cytosine-β-d-arabinofuranoside, AraC) in the IVth ventricle, blockade of reactive cell proliferation threefold delayed posturo-locomotor function compared with saline-treated animals while astrocyte population was also decreased (Dutheil et al., 2009). Our new study supports these earlier results confirming that newborn neurons and astrocytes play an important role during postvestibular lesion processes, especially for fine compensation of postural deficits.
In conclusion, this study is the first to report that after UVN, GABA plays multiple roles ranging from beneficial to detrimental, on different steps of reactive adult neurogenesis in the VN and on the time course of vestibular function recovery in the adult cat. This lesion model opens new landscapes that would help to understand why some CNS microenvironments allow functional neurogenesis to occur or not, and offers easily observable behavioral correlates in the adult mammal.
Footnotes
This research was supported by grants from the Ministère de l'enseignement supérieur et de la recherche and CNRS (UMR 7260 Aix-Marseille Université). We thank Angélique Bordey and Neil Fournier for reading of the paper, Valérie Gilbert and Elodie Mansour for taking care of the animals, Alain Tonetto and Céline Hecquet from the “PRATIM” for technical assistance in confocal imaging, and Abdessadek El Ahmadi for expertise in statistical analysis.
The authors declare no competing financial interests.
References
- Arvidsson A, Collin T, Kirik D, Kokaia Z, Lindvall O. Neuronal replacement from endogenous precursors in the adult brain after stroke. Nat Med. 2002;8:963–970. doi: 10.1038/nm747. [DOI] [PubMed] [Google Scholar]
- Bauer S, Hay M, Amilhon B, Jean A, Moyse E. In vivo neurogenesis in the dorsal vagal complex of the adult rat brainstem. Neuroscience. 2005;130:75–90. doi: 10.1016/j.neuroscience.2004.08.047. [DOI] [PubMed] [Google Scholar]
- Berman AL. The brain stem of the cats, a cytoarchitechtonic atlas with stereotaxic coordinates. Madison, WI: Wisconsin UP; 1968. [Google Scholar]
- Bordey A. Enigmatic GABAergic networks in adult neurogenic zones. Brain Res Rev. 2007;53:124–134. doi: 10.1016/j.brainresrev.2006.07.004. [DOI] [PubMed] [Google Scholar]
- Brezun JM, Daszuta A. Serotonin may stimulate granule cell proliferation in the adult hippocampus, as observed in rats grafted with foetal raphe neurons. Eur J Neurosci. 2000;12:391–396. doi: 10.1046/j.1460-9568.2000.00932.x. [DOI] [PubMed] [Google Scholar]
- Cameron HA, McKay RD. Adult neurogenesis produces a large pool of new granule cells in the dentate gyrus. J Comp Neurol. 2001;435:406–417. doi: 10.1002/cne.1040. [DOI] [PubMed] [Google Scholar]
- Cass SP, Goshgarian HG. Increased glial fibrillary acidic protein immunoreactivity in astrocytes within the lateral vestibular nucleus of the cat following labyrinthectomy and vestibular neurectomy. Ann Otol Rhinol Laryngol. 1990;99:221–227. [PubMed] [Google Scholar]
- Chan JP, Cordeira J, Calderon GA, Iyer LK, Rios M. Depletion of central BDNF in mice impedes terminal differentiation of new granule neurons in the adult hippocampus. Mol Cell Neurosci. 2008;39:372–383. doi: 10.1016/j.mcn.2008.07.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christensen JR, Larsen KB, Lisanby SH, Scalia J, Arango V, Dwork AJ, Pakkenberg B. Neocortical and hippocampal neuron and glial cell numbers in the rhesus monkey. Anat Rec (Hoboken) 2007;290:330–340. doi: 10.1002/ar.20504. [DOI] [PubMed] [Google Scholar]
- Darlington CL, Smith PF. Molecular mechanisms of recovery from vestibular damage in mammals: recent advances. Prog Neurobiol. 2000;62:313–325. doi: 10.1016/S0301-0082(00)00002-2. [DOI] [PubMed] [Google Scholar]
- de Waele C, Mühlethaler M, Vidal PP. Neurochemistry of the central vestibular pathways. Brain Res Brain Res Rev. 1995;20:24–46. doi: 10.1016/0165-0173(94)00004-9. [DOI] [PubMed] [Google Scholar]
- Dutheil S, Brezun JM, Leonard J, Lacour M, Tighilet B. Neurogenesis and astrogenesis contribution to recovery of vestibular functions in the adult cat following unilateral vestibular neurectomy: cellular and behavioral evidence. Neuroscience. 2009;164:1444–1456. doi: 10.1016/j.neuroscience.2009.09.048. [DOI] [PubMed] [Google Scholar]
- Dutheil S, Lacour M, Tighilet B. Neurogenic potential of the vestibular nuclei and behavioural recovery time course in the adult cat are governed by the nature of the vestibular damage. PLoS ONE. 2011;6:e22262. doi: 10.1371/journal.pone.0022262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dutia MB, Johnston AR, McQueen DS. Tonic activity of rat medial vestibular nucleus neurones in vitro and its inhibition by GABA. Exp Brain Res. 1992;88:466–472. doi: 10.1007/BF00228176. [DOI] [PubMed] [Google Scholar]
- Ehrenberger K, Felix D. Receptorpharmacological models for the therapy of labyrinthine vertigo. Acta Otolaryngol. 1996;116:189–191. doi: 10.3109/00016489609137820. [DOI] [PubMed] [Google Scholar]
- Ehrenberger K, Benkoe E, Felix D. Suppressive action of picrotoxin, a GABA antagonist, on labyrinthine spontaneous nystagmus and vertigo in man. Acta Otolaryngol. 1982;93:269–273. doi: 10.3109/00016488209130882. [DOI] [PubMed] [Google Scholar]
- Fernando RN, Eleuteri B, Abdelhady S, Nussenzweig A, Andäng M, Ernfors P. Cell cycle restriction by histone H2AX limits proliferation of adult neural stem cells. Proc Natl Acad Sci U S A. 2011;108:5837–5842. doi: 10.1073/pnas.1014993108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiszman ML, Borodinsky LN, Neale JH. GABA induces proliferation of immature cerebellar granule cells grown in vitro. Brain Res Dev Brain Res. 1999;115:1–8. doi: 10.1016/S0165-3806(99)00035-8. [DOI] [PubMed] [Google Scholar]
- Furuya N, Koizumi T. Neurotransmitters of vestibular commissural inhibition in the cat. Acta Otolaryngol. 1998;118:64–69. doi: 10.1080/00016489850155143. [DOI] [PubMed] [Google Scholar]
- Furuya N, Yabe T, Koizumi T. Neurotransmitters regulating vestibular commissural inhibition in the cat. Acta Otolaryngol Suppl. 1991;481:205–208. doi: 10.3109/00016489109131381. [DOI] [PubMed] [Google Scholar]
- Gliddon CM, Darlington CL, Smith PF. Effects of chronic infusion of a GABAA receptor agonist or antagonist into the vestibular nuclear complex on vestibular compensation in the guinea pig. J Pharmacol Exp Ther. 2005a;313:1126–1135. doi: 10.1124/jpet.104.082172. [DOI] [PubMed] [Google Scholar]
- Gliddon CM, Darlington CL, Smith PF. GABAergic systems in the vestibular nucleus and their contribution to vestibular compensation. Prog Neurobiol. 2005b;75:53–81. doi: 10.1016/j.pneurobio.2004.11.001. [DOI] [PubMed] [Google Scholar]
- Honegger P, Pardo B, Monnet-Tschudi F. Muscimol-induced death of GABAergic neurons in rat brain aggregating cell cultures. Brain Res Dev Brain Res. 1998;105:219–225. doi: 10.1016/S0165-3806(97)00194-6. [DOI] [PubMed] [Google Scholar]
- Kitamura T, Saitoh Y, Takashima N, Murayama A, Niibori Y, Ageta H, Sekiguchi M, Sugiyama H, Inokuchi K. Adult neurogenesis modulates the hippocampus-dependent period of associative fear memory. Cell. 2009;139:814–827. doi: 10.1016/j.cell.2009.10.020. [DOI] [PubMed] [Google Scholar]
- Lacefield CO, Itskov V, Reardon T, Hen R, Gordon JA. Effects of adult-generated granule cells on coordinated network activity in the dentate gyrus. Hippocampus. 2012;22:106–116. doi: 10.1002/hipo.20860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lacour M. Restoration of vestibular function: basic aspects and practical advances for rehabilitation. Curr Med Res Opin. 2006;22:1651–1659. doi: 10.1185/030079906X115694. [DOI] [PubMed] [Google Scholar]
- Lacour M, Tighilet B. Plastic events in the vestibular nuclei during vestibular compensation: the brain orchestration of a “deafferentation” code. Restor Neurol Neurosci. 2010;28:19–35. doi: 10.3233/RNN-2010-0509. [DOI] [PubMed] [Google Scholar]
- Lacour M, Roll JP, Appaix M. Modifications and development of spinal reflexes in the alert baboon (Papio papio) following an unilateral vestibular neurotomy. Brain Res. 1976;113:255–269. doi: 10.1016/0006-8993(76)90940-9. [DOI] [PubMed] [Google Scholar]
- Liu X, Wang Q, Haydar TF, Bordey A. Nonsynaptic GABA signaling in postnatal subventricular zone controls proliferation of GFAP-expressing progenitors. Nat Neurosci. 2005;8:1179–1187. doi: 10.1038/nn1522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LoTurco JJ, Owens DF, Heath MJ, Davis MB, Kriegstein AR. GABA and glutamate depolarize cortical progenitor cells and inhibit DNA synthesis. Neuron. 1995;15:1287–1298. doi: 10.1016/0896-6273(95)90008-X. [DOI] [PubMed] [Google Scholar]
- McCabe BF, Sekitani T, Ryu JH. Drug effects on postlabyrinthectomy nystagmus. Arch Otolaryngol. 1973;98:310–313. doi: 10.1001/archotol.1973.00780020322006. [DOI] [PubMed] [Google Scholar]
- Moyse E, Bauer S, Charrier C, Coronas V, Krantic S, Jean A. Neurogenesis and neural stem cells in the dorsal vagal complex of adult rat brain: new vistas about autonomic regulations—a review. Auton Neurosci. 2006;126–127:50–58. doi: 10.1016/j.autneu.2006.03.006. [DOI] [PubMed] [Google Scholar]
- Moyse E, Segura S, Liard O, Mahaut S, Mechawar N. Microenvironmental determinants of adult neural stem cell proliferation and lineage commitment in the healthy and injured central nervous system. Curr Stem Cell Res Ther. 2008;3:163–184. doi: 10.2174/157488808785740334. [DOI] [PubMed] [Google Scholar]
- Nuñez JL, Alt JJ, McCarthy MM. A new model for prenatal brain damage: I. GABAA receptor activation induces cell death in developing rat hippocampus. Exp Neurol. 2003;181:258–269. doi: 10.1016/S0014-4886(03)00053-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Overstreet-Wadiche LS, Bensen AL, Westbrook GL. Delayed development of adult-generated granule cells in dentate gyrus. J Neurosci. 2006;26:2326–2334. doi: 10.1523/JNEUROSCI.4111-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parent JM, Vexler ZS, Gong C, Derugin N, Ferriero DM. Rat forebrain neurogenesis and striatal neuron replacement after focal stroke. Ann Neurol. 2002;52:802–813. doi: 10.1002/ana.10393. [DOI] [PubMed] [Google Scholar]
- Platel JC, Stamboulian S, Nguyen I, Bordey A. Neurotransmitter signaling in postnatal neurogenesis: the first leg. Brain Res Rev. 2010;63:60–71. doi: 10.1016/j.brainresrev.2010.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pompeiano O, Luccarini P, Gahery Y, Blanchet G. Somatotopical effects of local microinjection of GABAergic agents in Deiters nucleus on the posturokinetic responses to cortical stimulation. J Vestib Res. 1993;3:391–407. [PubMed] [Google Scholar]
- Salazar P, Velasco-Velázquez MA, Velasco I. GABA effects during neuronal differentiation of stem cells. Neurochem Res. 2008;33:1546–1557. doi: 10.1007/s11064-008-9642-8. [DOI] [PubMed] [Google Scholar]
- Sernagor E, Chabrol F, Bony G, Cancedda L. GABAergic control of neurite outgrowth and remodeling during development and adult neurogenesis: general rules and differences in diverse systems. Front Cell Neurosci. 2010;4:11. doi: 10.3389/fncel.2010.00011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song J, Zhong C, Bonaguidi MA, Sun GJ, Hsu D, Gu Y, Meletis K, Huang ZJ, Ge S, Enikolopov G, Deisseroth K, Luscher B, Christian KM, Ming GL, Song H. Neuronal circuitry mechanism regulating adult quiescent neural stem-cell fate decision. Nature. 2012;489:150–154. doi: 10.1038/nature11306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tashiro A, Sandler VM, Toni N, Zhao C, Gage FH. NMDA-receptor-mediated, cell-specific integration of new neurons in adult dentate gyrus. Nature. 2006;442:929–933. doi: 10.1038/nature05028. [DOI] [PubMed] [Google Scholar]
- Taupin P. BrdU immunohistochemistry for studying adult neurogenesis: paradigms, pitfalls, limitations, and validation. Brain Res Rev. 2007;53:198–214. doi: 10.1016/j.brainresrev.2006.08.002. [DOI] [PubMed] [Google Scholar]
- Tighilet B. Role of the neurotrophins in vestibular compensation in the cat. J Vestib Res; Abstracts of the Bárány society XXIII international congress; Paris, France. 2004. pp. 95–303. [PubMed] [Google Scholar]
- Tighilet B, Lacour M. Gamma amino butyric acid (GABA) immunoreactivity in the vestibular nuclei of normal and unilateral vestibular neurectomized cats. Eur J Neurosci. 2001;13:2255–2267. doi: 10.1046/j.0953-816x.2001.01622.x. [DOI] [PubMed] [Google Scholar]
- Tighilet B, Leonard J, Lacour M. Betahistine dihydrochloride treatment facilitates vestibular compensation in the cat. J Vestib Res. 1995;5:53–66. doi: 10.1016/0957-4271(94)00023-U. [DOI] [PubMed] [Google Scholar]
- Tighilet B, Trottier S, Mourre C, Lacour M. Changes in the histaminergic system during vestibular compensation in the cat. J Physiol. 2006;573:723–739. doi: 10.1113/jphysiol.2006.107805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tighilet B, Brezun JM, Dit Duflo Sylvie GD, Gaubert C, Lacour M. New neurons in the vestibular nuclei complex after unilateral vestibular neurectomy in the adult cat. Eur J Neurosci. 2007;25:47–58. doi: 10.1111/j.1460-9568.2006.05267.x. [DOI] [PubMed] [Google Scholar]
- Tronel S, Fabre A, Charrier V, Oliet SH, Gage FH, Abrous DN. Spatial learning sculpts the dendritic arbor of adult-born hippocampal neurons. Proc Natl Acad Sci U S A. 2010;107:7963–7968. doi: 10.1073/pnas.0914613107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vibert N, Serafin M, Vidal PP, Mühlethaler M. Direct and indirect effects of muscimol on medial vestibular nucleus neurones in guinea-pig brainstem slices. Exp Brain Res. 1995;104:351–356. doi: 10.1007/BF00242021. [DOI] [PubMed] [Google Scholar]
- West MJ. New stereological methods for counting neurons. Neurobiol Aging. 1993;14:275–285. doi: 10.1016/0197-4580(93)90112-O. [DOI] [PubMed] [Google Scholar]
- West MJ. Stereological methods for estimating the total number of neurons and synapses: issues of precision and bias. Trends Neurosci. 1999;22:51–61. doi: 10.1016/S0166-2236(98)01362-9. [DOI] [PubMed] [Google Scholar]
- West MJ, Slomianka L, Gundersen HJ. Unbiased stereological estimation of the total number of neurons in the subdivisions of the rat hippocampus using the optical fractionator. Anat Rec. 1991;231:482–497. doi: 10.1002/ar.1092310411. [DOI] [PubMed] [Google Scholar]
- Xerri C, Lacour M. Compensation deficits in posture and kinetics following unilateral vestibular neurectomy in cats. The role of sensorimotor activity. Acta Otolaryngol. 1980;90:414–424. doi: 10.3109/00016488009131743. [DOI] [PubMed] [Google Scholar]