Abstract
The development and the ionic nature of bistable behavior in lumbar motoneurons were investigated in rats. One week after birth, almost all (∼80%) ankle extensor motoneurons recorded in whole-cell configuration displayed self-sustained spiking in response to a brief depolarization that emerged when the temperature was raised >30°C. The effect of L-type Ca2+ channel blockers on self-sustained spiking was variable, whereas blockade of the persistent sodium current (INaP) abolished them. When hyperpolarized, bistable motoneurons displayed a characteristic slow afterdepolarization (sADP). The sADPs generated by repeated depolarizing pulses summed to promote a plateau potential. The sADP was tightly associated with the emergence of Ca2+ spikes. Substitution of extracellular Na+ or chelation of intracellular Ca2+ abolished both sADP and the plateau potential without affecting Ca2+ spikes. These data suggest a key role of a Ca2+-activated nonselective cation conductance (ICaN) in generating the plateau potential. In line with this, the blockade of ICaN by flufenamate abolished both sADP and plateau potentials. Furthermore, 2-aminoethoxydiphenyl borate (2-APB), a common activator of thermo-sensitive vanilloid transient receptor potential (TRPV) cation channels, promoted the sADP. Among TRPV channels, only the selective activation of TRPV2 channels by probenecid promoted the sADP to generate a plateau potential. To conclude, bistable behaviors are, to a large extent, determined by the interplay between three currents: L-type ICa, INaP, and a Na+-mediated ICaN flowing through putative TRPV2 channels.
Introduction
Locomotor movements are driven by a central pattern generator (CPG) located in the spinal cord (Grillner and Zangger, 1975). Although the CPG is functional at birth (Cazalets et al., 1990; Fady et al., 1998), rats walk later during the second postnatal week (Altman and Sudarshan, 1975; Westerga and Gramsbergen, 1990). The development of posture is the main limiting factor for locomotion (Brocard et al., 1999; Vinay et al., 2002; Clarac et al., 2004). The gradual acquisition of repetitive firing properties by motoneurons contributes to the development of postural tone (Vinay et al., 2000a).
In spinal motoneurons of adult vertebrates, self-sustained firing appears to arise from a prolonged depolarization known as “plateau potential” (Perrier and Hounsgaard, 2000; Brownstone, 2006; Heckman et al., 2008b). Plateau potentials are triggered by brief excitations and turned off by inhibitory inputs promoting a bistable behavior of the membrane potential (Hultborn et al., 1975; Schwindt and Crill, 1980; Hounsgaard et al., 1984; Crone et al., 1988; Bennett et al., 1998). By means of paired motor unit recordings, correlates of such bistable behaviors have been found in vivo (Eken et al., 1989; Kiehn and Eken, 1997; Gorassini et al., 1998, 1999; Collins et al., 2001). Because these are especially observed in low-threshold motor units (Lee and Heckman, 1998), they are thought to be important in postural functions (Kiehn and Eken, 1998). Plateau potentials are contingent upon a supraspinal monoaminergic control (Conway et al., 1988; Hounsgaard et al., 1988; Hounsgaard and Kiehn, 1989; Perrier and Delgado-Lezama, 2005) and rely on persistent inward currents primarily mediated by dendritic L-type Ca2+ channels (Hounsgaard and Mintz, 1988; Hounsgaard and Kiehn, 1993; Booth and Rinzel, 1995; Carlin et al., 2000a; Heckman et al., 2003; Li and Bennett, 2003; Li et al., 2004; Bui et al., 2006; Carlin et al., 2009). In conjunction with the postnatal development of descending monoaminergic projections (Bregman, 1987; Rajaofetra et al., 1989, 1992; Vinay et al., 2000b) and that of L-type Ca2+ current (Jiang et al., 1999; Carlin et al., 2000a), the emergence of plateaus in motoneurons may parallel the acquisition of a quadrupedal stance in rats.
To test this assumption, the present study investigates the emergence of self-sustained firing in motoneurons of the triceps surae muscle that gradually acquire a tonic EMG activity during the first postnatal week (Brocard et al., 1999). We demonstrate that most motoneurons are bistable as early as birth in neonatal rat. Instead of L-type Ca2+ current, the plateau potentials responsible for self-sustained firing are primarily mediated by a Ca2+-activated nonselective cationic current (ICaN) flowing through putative transient receptor potential (TRPV)2 channels.
Materials and Methods
Experiments were performed on neonatal (0- to 7-d-old) Wistar rats of either sex. We housed rodents in a temperature-controlled animal-care facility with a 12 h light/dark cycle. We made all efforts to minimize animal suffering and the number of animals used. All surgical and experimental procedures conformed to guidelines from the French Ministry for Agriculture and Fisheries, Division of Animal Rights, and the European guidelines for the care and use of laboratory animals (Council Directive 86/6009/EEC).
Labeling of triceps surae motoneurons.
The procedure to label motoneurons before slice preparation was previously described in detail (Sadlaoud et al., 2010). Briefly, in cryoanesthetized rat pups, 5 μl of a solution of fluorescein-conjugated cholera toxin B subunit (Invitrogen, 0.5 mg/ml) was injected bilaterally into the triceps surae muscles to retrogradely label gastrocnemius and soleus motoneuron pools. Animals were returned to their cages and a postinjection survival time of at least 12 h enabled the retrograde transport.
In vitro slice preparation.
Electrophysiological experiments were performed on acute spinal cord slices. Neonatal rats were decapitated and eviscerated, and the lumbar spinal cord was isolated in ice-cold (<4°C) artificial CSF (ACSF) solution composed of the following (in mm): 232 sucrose, 3 KCl, 1.25 KH2PO4, 4 MgSO4, 0.2 CaCl2, 26 NaHCO3, 25 d-glucose, pH 7.4. The lumbar spinal cord was then introduced into a 1% agar solution, quickly cooled, mounted in a vibrating microtome (Leica, VT1000S) and sliced (350 μm) through the L4–5 lumbar segments. Slices were immediately transferred into the holding chamber filled with ACSF solution composed of the following (in mm): 120 NaCl, 3 KCl, 1.25 NaH2PO4, 1.3 MgSO4, 1.2 CaCl2, 25 NaHCO3, 20 d-glucose, pH 7.4, ∼30°C. Following a 1 h resting period, individual slices were transferred to a recording chamber that was continuously perfused (∼4 ml/min) with the same medium heated to ∼34°C or with a HEPES-buffered solution [composed of the following (in mm): 145 NaCl, 3 KCl, 1.3 MgCl2, 1.2 CaCl2, HEPES 10, 20 d-glucose, pH 7.4, ∼34°C) to avoid a precipitate when tranilast, 4αPDD, lanthanum, or the gadolinium (see Drug list, below) were used. The temperature regulation was provided by the CL-100 bipolar temperature controller (Warner Instruments). Slices were visualized with epifluorescence and infrared differential interference contrast microscopy using a Nikon Eclipse E600FN upright microscope coupled with a 40× water-immersion lens. The image was enhanced with a Hitachi KP-200/201 infrared-sensitive CCD camera and displayed on a video monitor. All solutions were oxygenated with 95% O2/5% CO2 except for the HEPES-buffered solution oxygenated with 100% O2. In some experiments, the distal part of lumbar dorsal rootlet was placed into a suction electrode. By means of a Grass stimulator connected to the suction electrode through a stimulus-isolating unit, repetitive electrical pulses (0.4 ms, 10 Hz) were applied to recruit segmental afferent fibers.
Whole-cell patch-clamp recordings.
Whole-cell patch-clamp recordings were made from motoneurons innervating the triceps surae muscle backlabeled with a fluorescein-conjugated cholera toxin (as described previously) or from unidentified motoneurons defined as large cells located in the lateral ventral horn. Whole-cell patch-clamp recordings in current-clamp mode were performed with a Multiclamp 700B amplifier (Molecular Devices). Patch electrodes (3–4 MΩ) were pulled from borosilicate glass capillaries (1.5 mm outer diameter, 1.12 mm inner diameter; World Precision Instruments) on a Sutter P-97 puller (Sutter Instruments) and filled with intracellular solution containing the following (in mm): 140 K+-gluconate, 5 NaCl, 2 MgCl2, 10 HEPES, 0.5 EGTA, 2 ATP, 0.4 GTP, pH 7.3 (280–290 mOsm). In some recordings, 10 mm BAPTA was added in the pipette solution to chelate intracellular free Ca2+. Pipette and neuronal capacitive currents were canceled, and after breakthrough, the series resistance was compensated and monitored. Recordings were digitized on-line and filtered at 10 kHz (Digidata 1322A, Molecular Devices). Access resistance was monitored periodically throughout the experiments by a brief 10 mV or 10 pA hyperpolarizing step during voltage-clamp and current-clamp experiments, respectively.
Data analysis.
Electrophysiological data were analyzed off-line with Clampfit 9 software (Molecular Devices). Only cells exhibiting a stable resting, holding membrane potential, access resistance (no >20% variation) and an action potential amplitude larger than 45 mV were considered. The instantaneous frequency of firing was measured. To investigate the slow afterdepolarization (sADP), a train of spikes was evoked by a 2 s current pulse at holding potential. The peak amplitude of the sADP was measured from the holding potential −60 mV. The sADP area was measured between the end of the stimulus pulse and the return of membrane voltage to control value. Only traces without spiking after the end of the depolarizing pulse were considered for such analysis. Traces with depolarizing current pulses of the same amplitude were chosen to compare the effect of drugs on sADP. If necessary, using bias currents, the prepulse membrane potential was maintained at the holding potential fixed in control condition. Data are presented as means ± SEM. Nonparametric statistical analysis were used with a Wilcoxon matched pairs test when two groups were compared and a Kruskal–Wallis test followed by a Dunns post-test for multiple groups comparisons. Values of p < 0.05 were considered significant (GraphPad Software).
Drug list.
The following pharmacological agents from Sigma-Aldrich were used: apamin (100 nm), BAPTA (10 mm), bicuculline (20 μm), cadmium chloride (100 μm), capaszepine (10 μm), carbenoxolone (50 μm), flufenamic acid (FFA; 50 μm), gadolinium (Gd3+; 100 μm); isopentenyl pyrophosphate (IPP; 200 μm); lanthanum (La3+; 100 μm), mefloquine (50 μm), nifedipine (20 μm), nimodipine (20 μm), riluzole (5 μm), RN-1734 (50 μm), strychnine (1 μm), tetraethylammonium chloride (TEA; 10 mm), tranilast (150 μm), veratridine (40 nm), ω-agatoxin IVA (200 nm), ω-conotoxin-GVIA (1 μm), and 4α-Phorbol 12–13-dicaprinate (4αPDD; 50 μm). In addition, DL-2-amino-5-phosphonopentanoic acid (AP5; 50 μm), 2-aminoethoxydiphenyl borate (50–200 μm), camphor (1–10 μm), capsaicin (5–10 μm), 6-cyano-7-nitroquinoxaline-2,3-dion (CNQX; 10 μm), probenecid (250 μm), SKF 96365 (50 μm), and tetrodotoxin citrate (TTX; 20 nm-1 μm) were purchased from Tocris Bioscience). Nifedipine, nimodipine, tranilast, probenecid, 4α-Phorbol 12–13-dicaprinate, and riluzole were dissolved in dimethylsulphoxide (DMSO) and added to the ACSF (final concentration of DMSO: 0.05–0.1%). Vehicle had no effect on sADP or plateau potentials (data not shown). Ca2+-free solution was made by removing Ca2+ chloride from the recording solution and replacing it with an equimolar concentration of magnesium chloride. Low-Na+ solution was made with substituting equimolar concentrations of Na+ chloride by choline chloride.
Results
Characterization of plateau potentials in ankle extensor motoneurons
Data were collected from 35 motoneurons innervating the triceps surae muscles that were retrogradely labeled by a fluorescein-conjugated cholera toxin (Fig. 1A). The firing pattern of motoneurons recorded in normal ACSF was classified as either “bistable” (Fig. 1B1–B3) or “non-bistable” (Fig. 1C). Bistable motoneurons were characterized by a steep increase of the firing rate during the depolarizing pulse. From a holding potential of ∼10–15 mV or less below spike threshold, bistable motoneurons displayed a self-sustained firing when the depolarizing pulse was increased in intensity (Fig. 1B1) or duration (Fig. 1B2). The bistability could never be elicited from the resting membrane potential but required a baseline depolarization to be triggered (Fig. 1B3). This long-lasting state of sustained spiking was reliable throughout the recording session and stable (tested up to 5 min) until a hyperpolarizing current was injected. The intracellular dialysis in whole-cell recordings was not responsible for bistable behaviors as they were successfully recorded in the cell-attached configuration in three of four cells tested (data not shown). At hyperpolarized potentials, bistable motoneurons displayed a characteristic slow depolarizing afterpotential that outlasted the stimulus (Fig. 1B3, gray arrow) as previously observed in various CNS neurons (Hasuo et al., 1990; Friedman et al., 1992; Fraser and MacVicar, 1996). We refer to this potential as the sADP (Fig. 1B1–B3, arrows). Instead of a sADP, a pronounced afterhyperpolarization and the absence of an accelerating discharge during the pulse characterized non-bistable motoneurons (Fig. 1C).
Figure 1.

Electrophysiological properties of plateau potentials. A, Confocal image of a transverse section of the lumbar spinal cord shows motoneurons retrogradely labeled with the fluorescein-conjugated cholera toxin B subunit tracer injected into the triceps surae muscle. Scale bar, 100 μm. B, Bistable motoneuron displaying a plateau potential (black traces) when the depolarizing current pulse was increased either in amplitude (B1) or in duration (B2) or when the holding membrane potential was depolarized (B3). Current pulses in B2 and B3 were given from a depolarized membrane potential using bias current. Gray traces show the sADP outlasting the current pulse when the voltage threshold to trigger the plateau potential was not reached. C, Firing properties of a non-bistable motoneuron unable to generate a plateau potential in response to a 2 s depolarizing current pulse. C, Inset, Illustrates with expanded time scale the typical pronounced afterhyperpolarization (AHP) that follows the current pulse (action potentials are truncated). B, C, Instantaneous frequency plots are shown on top of intracellular recordings. D, Proportions of bistable and non-bistable motoneurons at the beginning and the end of the first postnatal week. E, Plateau potential triggered by a brief depolarizing current pulse in the presence of CNQX (10 μm), AP5 (50 μm), strychnine (5 μm) and bicuculline (20 μm). F, Electrical stimulation of the dorsal root (10 Hz, 2 s) induced plateau potential, which was stopped by hyperpolarization. In this and the following figures, bottom traces are the injected current.
Bistable motoneurons increased in proportion during the first postnatal week from ∼60% (12/20) at P0/P1 to 80% (12/15) at P6/P7 (Fig. 1D). Such bistable properties were not caused by local reverberating circuit, because they were observed also when glutamatergic-, GABA-, and glycine-mediated transmissions were blocked (Fig. 1E). However, similar self sustained firing could be induced by dorsal root stimulation in the absence of any pharmacological blockade (Fig. 1F).
The sADPs and plateau potentials are closely related
From this part of the study, 263 recordings were performed in presumed motoneurons targeted as the largest cells in the lateral part of the fourth/fifth lumbar ventral horn (where triceps surae's motoneurons are located; Fig. 1A), during the second half of the first postnatal week (P4/P6). Because the proportion (74%) and the ionic basis of their bistable properties are similar to those of the triceps surae's motoneurons, they have been pooled altogether. In bistable motoneurons, increasing the pulse intensity (n = 8; Fig. 2A1) or duration (n = 8; Fig. 2A2) always enhanced the sADP which could ultimately give rise to a transient spiking activity. The area of the sADP was strongly correlated with the number of spikes evoked during the stimulus (Fig 2A1,A2, insets). To test the impact of the sADP on the generation of plateau potentials a rheobasic current pulse was injected repeatedly. A summation of sADP (windup) was observed and the current pulse, which initially triggered a single spike, then evoked repetitive firing (n = 9; Fig. 2B1). Similar results were obtained when the trials were performed at more depolarized values but the windup led to a plateau potential (Fig. 2B2). To further identify the causal relationship between the two phenomena, a sequence of brief suprathreshold depolarizing pulses was delivered after the initial 2 s square depolarizing pulse (n = 15; Fig. 2C). With this protocol, the initial sADP (Fig. 2C, gray trace) was maintained and initiated a plateau potential (Fig. 2C, black trace). These data indicate that the plateau potential relies on a cumulative depolarization generated by the sADP.
Figure 2.

Electrophysiological properties of the sADP. A, Superimposed voltage traces from a motoneuron showing the sADP in response to current steps of increasing amplitude (A1) or duration (A2). A, Insets, The linear relationship between the area of the sADP and the number of spikes during the stimulus. B, Voltage traces illustrating the wind-up of the sADP in response to repetitive depolarizing current pulses of constant amplitude applied either at resting membrane potential (B1) or at a more depolarized potential producing a plateau potential (B2). C, Superimposed voltage traces from a bistable motoneuron recorded at resting membrane potential illustrating the switch of the sADP (gray trace) into a plateau potential (black trace) when the initial 2 s square pulse was followed by a sequence of suprathreshold depolarizing current pulses.
Self-sustained firing, but neither the sADP nor the plateau potential, relies on persistent inward currents
Considering the critical role of the L-type Ca2+ channels in generating plateau potentials in many preparations (Hounsgaard and Mintz, 1988; Hounsgaard and Kiehn, 1993; Heckman et al., 2003; Li and Bennett, 2003; Carlin et al., 2009), we tested their contribution to self-sustained firing in neonatal motoneurons. The application of nifedipine (20 μm) or nimodipine (20 μm), two specific L-type Ca2+ channel blockers, reversibly abolished bistable behaviors in approximately half of bistable motoneurons (3/7 cells; Fig. 3A1,A2) and did not affect them on the others (4/7 cells; Fig. 3A3). The contribution of the Na+ persistent inward current (Tazerart et al., 2007) was then investigated. The blockade of persistent sodium current (INaP) by riluzole (5 μm) gradually decreased self-sustained firing in duration until it was reversible suppressed in all motoneurons tested (n = 11 cells; Fig. 3B1). The involvement of INaP was confirmed by low concentration of TTX (20 nm) that did not affect spikes (n = 10 cells; Fig. 3B2). By contrast, the alkaloid veratridine, by upregulating INaP in spinal neurons (Tazerart et al., 2008), facilitated the emergence of self-sustained firing (n = 5; Fig. 3C). The addition of TTX specifically blocked the veratridine-induced bistable properties without affecting evoked action potentials (data not shown). Although INaP and to a lesser extent L-type Ca2+ currents appear to be important for self-sustained firing, they are not responsible for plateau potentials as the sADP persisted in the presence of L-type ICa or INaP blockers (Fig. 3A,B).
Figure 3.
Pharmacological profile of plateau potentials. A1–A3: Effect of blocking the L-type Ca2+ channels by nifedipine (20 μm) on plateau potentials. B, Effect of the progressive blockade of the persistent sodium current by 5 μm riluzole (B1) or 10 nm TTX (B2) on plateau potentials. C, Facilitation of plateau potentials by upregulating the persistent sodium current with veratridine (40 nm). Motoneurons (Mns) were recorded at a holding potential between −60 and −55 mV.
The ionic nature of the sADP
The Ca2+ spike is a prerequisite for the sADP to occur
To check whether action potentials are necessary for the sADP, we used TTX at a concentration (1 μm) that blocks both Na+ spikes and INaP. The amplitude of the sADP was decreased as action potentials were suppressed (7.8 ± 0.7 mV vs 2.5 ± 0.3; p < 0.001, Wilcoxon paired test, n = 11 cells; Fig. 4A). However, the sADP was resurrected when a current command mimicking action potentials was added to the 2 s depolarizing pulse (Fig. 4B). These data eliminate the involvement of TTX-sensitive voltage-activated Na+ channels in the sADP and suggest the essential role of TTX-resistant high voltage-activated channels in generating the sADP. In line with this hypothesis, the superfusion of TEA (10 mm), a potassium channel blocker, simultaneously unmasked TTX-resistant spikes and the sADP (Fig. 4C). The removal of Ca2+ from the external solution suppressed spikes and virtually abolished the sADP (13.9 ± 1.4 mV vs 4.2 ± 0.6 mV; p < 0.01, Wilcoxon paired test, n = 8 cells; Fig. 4D). These results indicate that the sADP relies on Ca2+-dependent mechanisms.
Figure 4.
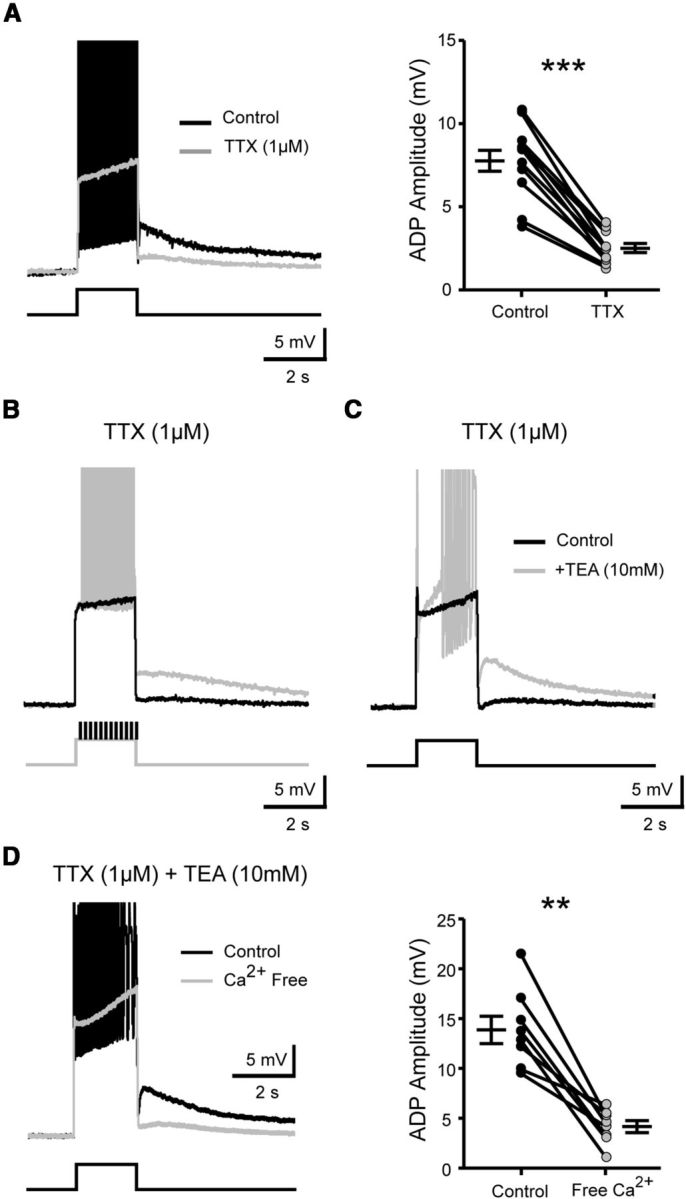
The sADP requires spiking-dependent Ca2+ influx. A, Superimposed voltage traces in response to a 2 s depolarizing pulse before (black trace) and after (gray trace) TTX application (1 μm). The graph shows the mean amplitude of the sADP before and after drug application. B, Superimposed voltage traces collected under TTX, in response to a 2 s depolarizing pulse on which additional brief depolarizing pulses that mimic action potentials were (black trace) or were not (gray trace) applied. C, Superimposed voltage traces in response to a 2 s depolarizing pulse, collected under TTX before and after adding TEA (10 mm). D, Superimposed voltage traces in response to a 2 s depolarizing pulse, collected under TTX and TEA before and after the removal of extracellular Ca2+. Graphs show the mean amplitude of the sADP before and after removing Ca2+. Note that action potentials were truncated for the visualization of the sADP. Holding potential, −60 mV. Error bars indicate SEM. ns, Not significant; **p < 0.01, ***p < 0.001 (Wilcoxon paired test).
Involvement of high-voltage-dependent Ca2+ channels
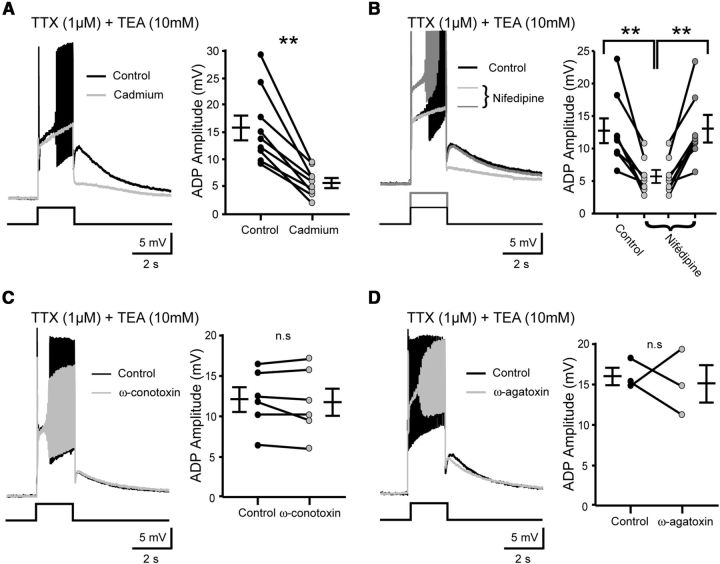
To determine whether voltage-gated Ca2+ channels were required for the sADP, a broad-spectrum Ca2+ channel blocker, cadmium (100 μm), was perfused. In the presence of TTX, the sADP unmasked by TEA was depressed by cadmium (15.8 ± 2.2 mV vs 5.5 ± 0.8 mV; p < 0.01, Wilcoxon paired test, n = 9 cells; Fig. 5A). These results suggest that Ca2+ influx associated with spiking is important in generating the sADP. Because spiking is expected to cause Ca2+ influx through high-voltage-activated Ca2+ channels, we determined which channel subtype was involved. Bath application of nifedipine (20 μm) produced a decrease of the sADP (12.7 ± 1.9 mV vs 5.7 ± 1 mV; p < 0.01, Kruskal–Wallis test, n = 8 cells; Fig. 5B, light gray trace) associated with the abolition of spikes. Notably, both sADP and spikes reappeared when the motoneuron was strongly depolarized (5.7 ± 1 mV vs 13.1 ± 2.1 mV; p < 0.01, Kruskal–Wallis test, n = 8 cells; Fig. 5B, dark gray trace). This result raised the possibility that more than one type of Ca2+ channel was involved. Application of ω-conotoxin-GVIA (1 μm; Fig. 5C), an N-type channel blocker, or ω-agatoxin IVA (200 nm; Fig. 5D), a P/Q-type channel blocker, substantially decreased the peak amplitude of Ca2+ spikes without significant effect on the sADP (12 ± 1.5 mV vs 11.7 ± 1.7 mV and 16 ± 1.1 mV vs 15 ± 2.3 mV, respectively; p > 0.05, Wilcoxon paired test, n = 6 and n = 3 cells, respectively; Figs. 5C,D). Together, these data suggest that high-voltage-activated Ca2+ channels, particularly the L-type ones, take part in generating the sADP.
Figure 5.
The L-type voltage-gated Ca2+ channels are primarily responsible for the spiking-dependent Ca2+ influx necessary to generate the sADP. A–D, Left, Superimposed voltage traces in response to a 2 s depolarizing pulse before (black traces) and after (gray traces) the addition of: (A) cadmium, a broad spectrum voltage-gated Ca2+ channels blocker (200 μm), (B) nifedipine a L-type Ca2+ channels blocker (20 μm), (C) ω-conotoxin GVIA (1 μm) a N-type Ca2+ channels blocker, and (D) ω-agatoxin VIA (200 nm) a P/Q-type Ca2+ channels blocker. Note that in the presence of nifedipine large depolarizing current pulses allow the recovery of both action potentials and sADP (dark gray trace). Right, Graphs show the mean amplitude of the sADP before and after the drug application. All recordings were made under TTX (1 μm) and TEA (10 mm). Holding potential, −60 mV. Error bars indicate SEM. ns, Not significant; *p < 0.05, **p < 0.01 (Wilcoxon paired test in A, C, and D and Kruskal–Wallis test in B).
Plateau potentials depend on a Ca2+-activated current that uses Na+ as the main charge carrier
The Ca2+ dependence of the sADP may reflect two mechanisms: (1) Ca2+ acts as the main charge carrier or, alternatively, (2) the Ca2+ influx acts upstream and triggers a Ca2+-activated current. To identify which of these two mechanisms is involved, we chelated intracellular Ca2+ by adding BAPTA (10 mm) to the internal solution. The sADP gradually decreased as the neuron was progressively loaded with BAPTA, and completely disappeared after ∼5 min of whole-cell recording (11 ± 1 mV vs −1.8 ± 0.8 mV; p < 0.01, Wilcoxon paired test, n = 8 cells; Fig. 6A). This abolition was not due to a nonspecific inhibition of Ca2+ influx because Ca2+ spikes were still observed. Thus, although the Ca2+ influx through voltage-activated Ca2+ channels is a prerequisite for the sADP, it does not underlie the sADP itself. The Ca2+ appeared to activate a conductance responsible for the sADP that uses another ion as the main charge carrier. To characterize the current mediating the sADP we investigated its reversal potential. The current–voltage relationship of the sADP was examined at various holding potentials under voltage-clamp (Fig. 6B). The tail current underlying the sADP was evoked from the holding potential of −60 mV by a 2 s depolarizing pulse that returned to different membrane potentials. To prevent interaction between the Ca2+-dependent potassium channels (IKCa) and a putative Ca2+-activated nonselective cationic current (ICaN), KCa channels were blocked by apamin (100 nm). The protocol induced an inward current that displayed a time course similar to the depolarization observed in current-clamp mode (Fig. 6B, left). The I–V relationship was linear between −60 and −20 mV (Fig. 6B, right, black trace). With the caution of the likely incomplete space clamp of dendrites (Carlin et al., 2009) the reversal potential extrapolated from the I–V curve has been estimated between +20 and +30 mV (n = 5 cells). Such a value suggests that the current is carried by a mixture of cations. The role of Na+ was tested by replacing most of the external Na+ with equimolar amount of choline. Reducing the extracellular Na+ from 152 to 26 mm clearly depressed the inward current (for a holding potential set at −60 mV: 373.4 ± 39.7 pA vs 74.8 ± 26.8 pA; p < 0.05, Wilcoxon paired test, n = 7 cells; Fig. 6C) and made the I–V curve flat (Fig. 6B, right, gray trace). Recorded in current-clamp, the sADP in low Na+ was reversibly decreased (11.9 ± 2.2 mV vs 1.9 ± 0.3 mV; p < 0.01, Wilcoxon paired test, n = 8 cells; Fig. 6D). In agreement with the involvement of the sADP in bistable behaviors, the plateau potential evoked by a sequence of depolarizing current pulses was abolished in low-Na+ solution (Fig. 6E). We conclude that the sADP and the related plateau potential were triggered in lumbar motoneurons by a Ca2+-dependent mechanism activating a Na+ inward current. This is consistent with the properties of an ICaN.
Figure 6.
The sADP and plateau potentials are dependent on ICaN that uses Na+ as the main charge carrier, not a Na+/Ca2+ exchanger. A, Superimposed voltage traces in response to a 2 s depolarizing pulse before (black traces) and after (gray traces) chelating the intracellular Ca2+ with BAPTA (10 mm) in the pipette solution. B, Left, Superimposed current traces from a motoneuron recorded under TEA (10 mm), TTX (1 μm), and apamin (100 nm), held at −60 mV, step-depolarized to +10 mV and then returned to potentials between −60 and 0 mV. On the right, I–V relationship of the peak inward tail current plotted against the return potential before (black trace) and after (gray trace) lowering the extracellular concentration of Na+. C, D, Superimposed current (C) and voltage (D) traces in response to a 2 s depolarizing pulse before (black traces) and after (gray traces) lowering the extracellular concentration of Na+. E, Superimposed voltage traces from a bistable motoneuron recorded at resting membrane potential in response to a 2 s square pulse followed by a series of brief depolarizing current pulses before (black trace) and after (gray trace) lowering the concentration of Na+. F, Superimposed voltage traces in response to a 2 s depolarizing pulse before (black traces) and after (gray traces) the blockade of the Na+/Ca2+ exchanger by lithium. A, C, D, F, Right, Graphs show the mean amplitude of the sADP before and after the drug application. Recordings were made under TTX (1 μm) and TEA (10 mm). The apamin (100 nm) was superfused for voltage-clamp recordings. Holding potential, −60 mV. Error bars indicate SEM. ns, Not significant; *p < 0.05, **p < 0.01 (Wilcoxon paired test).
The Na+/Ca2+ exchanger is not responsible for the sADP
Aside from a contribution of ICaN, we had to consider the Na+/Ca2+ exchanger as a putative mechanism underlying the sADP (Friedman et al., 1992). Such exchanger evokes a depolarizing current by intruding three Na+ ions in exchange of one Ca2+ ion. The involvement of a Na+/Ca2+ exchanger was tested in two ways. First, we replaced extracellular Na+ with equimolar Li+ that inhibits the Na+/Ca2+ exchanger (Reuter and Porzig, 1995) but permeates Ca2+-activated nonselective cation channels (Yellen, 1982). This condition did not affect the amplitude of the sADP (11.8 ± 1.8 mV vs 11.6 ± 1.3 mV; p > 0.05, Wilcoxon paired test, n = 6 cells; Fig. 6F). In an additional series of experiments, we used the isothiourea derivative KB-R7943 (50 μm), which selectively inhibits the Na+/Ca2+ exchanger. This blocker did not alter the sADP (7 ± 2.4 mV vs 7 ± 2.4 mV; p > 0.05, Wilcoxon paired test, n = 4 cells; data not shown). These findings imply that the Na+/Ca2+ exchanger plays a minor role, if any, in the genesis of the sADP.
TRPV cation channels upregulate the sADP
The involvement of ICaN in the sADP was investigated further using either flufenamate (50 μm; n = 9 cells; Fig. 7A), ruthenium red (10 μm; data not shown; n = 4 cells), or trivalent cations, such as La3+ (100 μm; n = 3 cells; Fig. 7B) or Gd3+(100 μm, data not shown; n = 2 cells), routinely used to block ICaN (Table 1). Whatever the blocker used, Ca2+ spikes were abolished and the sADP was significantly lowered (13.1 ± 1.6 mV vs 2.9 ± 0.4 mV; p < 0.001, Wilcoxon paired test, n = 18 cells; Fig. 7A,B). The blockade of Ca2+ spikes emphasizes the side effects of such blockers on voltage-gated Ca2+ channels (Reichling and MacDermott, 1991; Guinamard et al., 2013). Channels responsible for ICaN are sorted into six subfamilies (for review, see Montell, 2005). To date, only TRPM (melestatin), TRPC (canonical), and TRPV channels are expressed in the CNS (Moran et al., 2004). A blocker of some TRPC channels (SKF 96365, 50 μm; Table 1), did not affect the sADP (p > 0.05, Wilcoxon paired test, n = 4 cells; Fig. 7C). Another compound, 2-APB (50 μm), was used to block at least partially TRPC and TRPM channels (Table 1). Instead of a shortening, 2-APB caused a significant increase of both the amplitude (11.7 ± 1.7 mV vs 18.1 ± 1.4 mV; p < 0.001, Wilcoxon paired test, n = 11 cells; Fig. 7D) and the duration of the sADP (from 9.8 ± 1.6 s to 19.2 ± 0.4 s; p < 0.01, Wilcoxon paired test, n = 11 cells; Fig. 7D). Aside from its broad spectrum inhibition of TRPC and TRPM channels, 2-APB was recently identified as a chemical activator of TRPV1, TRPV2, and TRPV3 channels (Table 1).
Figure 7.
The sADP is upregulated by thermosensitive TRPV cation channels. A–D, Superimposed voltage traces in response to a 2 s depolarizing pulse before (black traces) and after (gray traces) the addition of: (A) flufenamate (75 μm) or (B) lanthanum (100 μm), two broad spectrum TRP channel blockers; (C) SKF 96365, a nonselective TRPC channel blocker (20 μm); and (D) 2-APB, a common activator of TRPV channels. Right, Graphs show the mean amplitude of the sADP before and after the drug application. Recordings were made under TTX and TEA. Holding potential, −60 mV. Error bars indicate SEM. ns, Not significant; *p < 0.05, **p < 0.01, ***p < 0.001 (Wilcoxon paired test).
Table 1.
Agonists and antagonists of TRPV
| TRPV1 |
TRPV2 |
TRPV3 |
TRPV4 |
Other TRPs |
References | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Agonist | Antagonist | Agonist | Antagonist | Agonist | Antagonist | Agonist | Antagonist | Agonist | Antagonist | ||
| Lanthanum | — | + | — | + | — | + | — | + | — | + | (Wu et al., 2010) |
| Gadolinium | — | + | — | + | — | + | — | + | — | + | |
| Ruthenium red | — | + | — | + | — | + | — | + | — | + | |
| FFA | — | + | — | + | — | + | — | + | — | + | (Guinamard et al., 2013) |
| 2APB | + | — | + | — | + | — | — | — | — | + | (Hu et al., 2004; Harteneck and Gollasch, 2011) |
| SKF 96365 | — | — | — | — | — | — | — | — | — | + | (Wu et al., 2010) |
| Capsaicin | + | — | — | — | — | — | — | — | — | — | (Caterina et al., 1997) |
| Capsazepine | — | + | — | — | — | — | — | — | — | — | (Caterina et al., 1997) |
| Probenecid | — | — | + | — | — | — | — | — | — | — | (Bang et al., 2007) |
| Tranilast | — | — | — | + | — | — | — | — | — | — | (Hisanaga et al., 2009) |
| Camphor | — | — | — | — | + | — | — | — | — | — | (Moqrich et al., 2005) |
| IPP | — | — | — | — | — | + | — | — | — | — | (Bang et al., 2011) |
| 4αPDD | — | — | — | — | — | — | + | — | — | — | (Watanabe et al., 2002) |
| RN 1734 | — | — | — | — | — | — | — | + | — | — | (Vincent et al., 2009) |
Activation of TRPV2 channels upregulates the sADP
We further tried to identify which of the TRPV channels was involved in the upregulation of the sADP. Capsaicin (10 μm; Fig. 8A1) and capsazepine (10 μm; Fig. 8A2), a potent activator and a blocker of TRPV1 channels (Table 1), respectively, did not affect the sADP (for capsaicin: 7.7 ± 2.6 mV vs 7.6 ± 2.6 mV, n = 4 cells; for capsazepine: 10.6 ± 0.8 mV vs 10.6 ± 0,9 mV, n = 4 cells; p > 0.05, Wilcoxon paired test). These results corroborate anatomical evidence that motoneurons do not express TRPV1 channels (Mandadi et al., 2009). In contrast to TRPV1, TRPV2 and TRPV3 channels are expressed in lumbar motoneurons (Lewinter et al., 2004; Facer et al., 2007). The specific recruitment of TRPV2 channels by probenecid (250 μm; Table 1) had a pronounced effect and, similar to 2-APB, increased the sADP amplitude (13.7 ± 2.5 mV vs 17.1 ± 2.6 mV; p < 0.01, Wilcoxon paired test, n = 8 cells; Fig. 8B1) and duration (from 9.3 ± 1.6 s to 18.9 ± 0.8 s; p < 0.01, Wilcoxon paired test, n = 8 cells). In voltage-clamp recordings, inward currents sustaining the sADP were significantly increased (for holding potential at −60 mV: 253 ± 28 pA vs 373 ± 40 pA; p < 0.01, Wilcoxon paired test, n = 6 cells; Fig. 8B2). Surprisingly, tranilast (150 μm), a presumed specific blocker of TRPV2 channels (Table 1), did not affect the sADP (12.6 ± 2.8 mV vs 12.7 ± 2.9 mV; p > 0.05, Wilcoxon paired test, n = 5 cells; Fig. 8B3). Because of potential side effects of probenecid on pannexin-1 (Ma et al., 2009), the effect of probenecid on the sADP was re-evaluated in the presence of a broad spectrum inhibitor of pannexins, carbenoxolone (50 μm, data not shown), and a more specific blocker of pannexin-1 channels, mefloquine (50 μm). The mean amplitude of the sADP recorded under carbenoxolone and mefloquine did not differ from that recorded under control conditions (control: 12.41 ± 0.51 mV, n = 126 vs pannexin-1 inhibitors: 10.57 ± 0.91 mV, n = 12; p > 0.05, Mann–Whitney test). Furthermore, the two inhibitors did not prevent the probenecid-induced potentiation of the sADP (10.57 ± 0.91 mV vs 15.60 ± 1.45 mV; p < 0.01, Wilcoxon paired test, n = 12 cells; Fig. 8B4). These data do not support a role of pannexin hemichannels in the generation/modulation of the sADP. Camphor (10 μm), a TRPV3 agonist (Table 1), slightly but significantly increased the amplitude of the sADP (12.3 ± 0.9 mV vs 14.7 ± 1.6 mV; p < 0.05, Wilcoxon paired test, n = 6 cells; Fig. 8C1). However, IPP (200 μm), a TRPV3 blocker (Table 1), did not affect the sADP (13.4 ± 3 mV vs 13.5 ± 3.2 mV; p > 0.05, Wilcoxon paired test, n = 4 cells; Fig. 8C2). Finally, the fourth member of the TRPV family does not seem to be involved in the generation/modulation of the sADP as neither 4αPDD (10 μm; Fig. 8D1) nor RN-1734 (50 μm; Fig. 8D2), agonist and antagonist of TRPV4 channels respectively (Table 1), altered the sADP (for 4αPDD: 13.2 ± 4.4 mV vs 13.2 ± 4.6 mV, n = 6 cells; for RN 1734: 10.3 ± 1.9 mV vs 10.3 ± 1.8 mV, n = 3 cells; p > 0.05, Wilcoxon paired test).
Figure 8.
Among TRPV cationic channels, TRPV2 upregulates the sADP. A–D, Superimposed voltage traces in response to a 2 s depolarizing pulse before (black traces) and after (gray traces) the addition of agonists (left in A1, B1, C1, and D1) or antagonists (right in A2, B2, C2, and D2) of specific TRPV1 (A), TRPV2 (B), TRPV3 (C), or TRPV4 (D) channels. B2, Left, Superimposed current traces in response to a 2 s depolarizing pulse before (black traces) and after (gray traces) applying probenecid (250 μm). Right, I–V relationship of the peak inward tail current plotted against the return potential. B4, Superimposed current traces in response to a 2 s depolarizing pulse recorded under mefloquine (50 μM) before (black traces) and after (gray traces) applying probenecid (250 μM). Recordings were made under TTX and TEA. Holding potential, −60 mV. Error bars indicate SEM. ns, Not significant; *p < 0.05, **p < 0.01 (Wilcoxon paired test).
Activation of TRPV2 channels generates self-sustained firing
In contrast to the pharmacological activation of TRPV3 channels, that of TRPV2 triggered self-sustained firing in motoneurons recorded in normal conditions (in the absence of TTX and TEA; n = 5 cells; Fig. 9A). Furthermore, when intracellular Ca2+ ions were buffered by means of BAPTA (10 mm), bistable behaviors were gradually suppressed (n = 5; Fig. 9B). This effect did not result from the indirect blockade of IKCa as apamin did not abolish self-sustained firing (n = 4; Fig. 9C). In addition to its dependence on intracellular Ca2+ concentration, TRPV2 channels, so called thermo-TRPs, are activated by heating (Benham et al., 2003). To further test the functional contribution of TRPV2 channels, we examined whether changes in the temperature were able to affect the generation of bistable behaviors. A decrease of 10°C, from 34 to 24°C, induced a reversible loss of self sustained firing in all bistable motoneurons tested (n = 10 cells; Fig. 9D). The threshold temperature at which self-sustained firing disappeared was 29.5 ± 0.6°C (n = 10). Altogether, these data suggest that TRPV2 channels generate and/or modulate the ICaN underlying plateau potentials in lumbar motoneurons.
Figure 9.

Activation of TRPV2 channels generates self-sustained firing. A–D, Superimposed voltage traces recorded in normal saline (without TTX and TEA) in response to a 2 s depolarizing pulse before (black traces) and after (gray traces) bath applying probenecid (A), chelating intracellular Ca2+ with BAPTA (B), blocking IKCa with apamine (C), or reducing the temperature of the bath from 34°C to 28°C (D). C, Instantaneous frequency plots are shown on top of intracellular recordings.
Discussion
New insights into mechanisms underlying plateau potentials in spinal motoneurons can be drawn: (1) Bistable properties in motoneurons innervating the triceps surae muscles appear before the acquisition of a quadrupedal stance. (2) The mechanism underlying self-sustained firing involves the interplay of three conductances: L-type ICa, INaP, and ICaN. (3) Plateau potentials use Na+ as the main charge carrier flowing through putative cationic TRPV2 channels.
Plateau potentials rely on ICaN
In a wide range of invertebrate (Kiehn and Harris-Warrick, 1992; Cattaert et al., 1994; Angstadt and Choo, 1996) and vertebrate neurons (Kiehn and Harris-Warrick, 1992; Fraser and MacVicar, 1996; Russo and Hounsgaard, 1996; Di Prisco et al., 1997; Morisset and Nagy, 1999; Derjean et al., 2005), plateau potentials are generated by noninactivating conductances mediating “persistent inward currents” (PICs). The PICs in motoneurons are composed of INaP (Nishimura et al., 1989; Hsiao et al., 1998; Lee and Heckman, 2001; Li and Bennett, 2003; Powers and Binder, 2003; Tazerart et al., 2007) and calcium components (Schwindt and Crill, 1980; Hounsgaard and Kiehn, 1989; Heckman et al., 2008a; Carlin et al., 2009). PICs generating plateaus in motoneurons of adult cats (Hounsgaard et al., 1984), mice (Carlin et al., 2000b), rats (Li and Bennett, 2003), turtles (Hounsgaard and Mintz, 1988), and frogs (Perrier and Tresch, 2005) are predominantly mediated by L-type calcium channels and to a lesser extent by INaP (Li et al., 2004; Harvey et al., 2006). Although PICs are present in neonatal rodent motoneurons (Gao and Ziskind-Conhaim, 1998; Tazerart et al., 2007; Pambo-Pambo et al., 2009), plateau potentials had never been revealed during the first postnatal week. Herein, we unmasked such bistability by raising the temperature. Several pieces of evidence support that L-type Ca2+ channels do not provide the main charge carrier for plateau potential. First, dihydropyridines L-type Ca2+ channel blockers did not block plateau potentials reliably. Second, after the intracellular Ca2+ was chelated, Ca2+ spikes evoked by depolarizing current pulses persisted, whereas the sADP and plateau potentials were abolished. Third, removing Na+ from the extracellular solution abolished plateau potentials. These results raise INaP as a putative candidate for bistability. However, if INaP appears essential for self-sustained firing (Fig. 3B; Kuo et al., 2006), it does not drive plateau potentials because sADP and plateau potentials are TTX-insensitive (Fig. 6D).
Results from TTX application, intracellular Ca2+ chelation, and Na+ substitution suggest a tonic Na+ influx through nonselective cationic channels at the source of plateau potentials. Results with FFA and trivalent ions support this assumption but should be interpreted with caution as these agents are broad-spectrum ion channel modulators (Reichling and MacDermott, 1991; Guinamard et al., 2013). More convincing evidence is provided by the increase of the sADP by 2-APB. This response profile points to the activation of TRPV channels (Hu et al., 2004). Several features of TRPV channels are compatible with plateau potentials. First, activation of TRPV channels requires intracellular Ca2+ (Partridge and Swandulla, 1988). Second, TRPV channels are thermosensitive and mediate cell depolarization in response to temperature increases (Benham et al., 2003), and the likelihood to record self-sustained firing increases from 28 to 34°C (Fig. 8). Third, as for the windup characteristic of bistable motoneurons, TRPV2- and TRPV3-mediated currents display marked sensitization (gradual increase in amplitude) with repeated stimuli (Moqrich et al., 2005; Leffler et al., 2007). Fourth, TRPV2 and TRPV3 channels are expressed in spinal motoneurons (Lewinter et al., 2004; Facer et al., 2007). Fifth, from the application of different TRPV channel openers it appeared that TRPV2 channels are able to trigger plateau potentials. All these properties therefore support the involvement of TRPV2 channels in the generation/modulation of plateau potentials in developing motoneurons. However, tranilast did not alter the sADP (Fig. 8B3). The assumed specificity of the blockade of TRPV2 channels by this compound relies on very little and indirect evidence. Hisanaga et al. (2009) showed that upon stimulation of pancreatic β-cells with glucose or insulin, TRPV2 translocates to the cell membrane and that this translocation is blocked by tranilast. The translocation of TRPV2 channels can be regulated at many levels and, for instance, a similar blockade of the translocation was also obtained with an inhibitor of phosphatidylinositol 3-kinase or a blocker of the L-type voltage calcium channel. Due to the lack of reliable pharmacological tools, future investigations using genetic tools will be required to determine the precise role of TRPV2 channels in the generation/modulation of motoneuronal bistability.
The cascade of events underlying plateau firing mode
In sum, plateau potentials are to a large extent determined by a “ménage à trois” of conductances ICaN, INaP, and L-type ICa. Based on the physiological and pharmacological properties of plateaus, we suggest the following scenario (Fig. 10): (1) Brief membrane depolarization (and subsequent recruitment of Na+-dependent spikes) activates nifedipine-sensitive L-type Ca2+ channels. (2) The resulting Ca2+ entry activates ICaN likely through a process involving a Ca2+-induced Ca2+ release (Mejia-Gervacio et al., 2004). (3) After the cation channel has brought the initial sADP close to the threshold of INaP, this steady-state inward current promotes repetitive spiking activity. (4) When spikes occur close to each other, the summed sADPs generate a persistent plateau potential that further sustains firing by bringing the membrane potential closer to action potential threshold and so on. (5) This mechanism ends when an inhibitory input is large enough to break the ménage à trois.
Figure 10.
A schematic ménage à trois relationship between currents underlying the different phases of the plateau firing mode. The Ca2+ entry via voltage-dependent Ca2+ current occurs during the train of action potential (step 1 and 2 in A and B). This increase in intracellular Ca2+ triggers a voltage-independent cation current that depolarizes the membrane and mediates the sADP (step 3 in A and B). The positive change in membrane potential opens voltage-dependent persistent Na+ current to maintain a train of action potential (step 4 in A and B). The repetitive spiking activity will then induce Ca2+ entry via voltage-dependent Ca2+ current and so forth.
ICaN and bistable properties in the CNS
ICaN generates plateau potentials in numerous classes of neurons (Zhang et al., 1995; Fraser and MacVicar, 1996; Beurrier et al., 2000; Di Prisco et al., 2000; Lee and Tepper, 2007; Mrejeru et al., 2011). This current has been reported in spinal motoneurons of turtles but, likely due to species differences, plays a minor role in sustaining the inward current during the plateau (Perrier and Hounsgaard, 1999). In the spinal cord, the presence of a plateau potential and ICaN is not specific to motoneurons. Neurons in the dorsal horn (Morisset and Nagy, 1999) and the lateral intermediate gray matter (Derjean et al., 2005) exhibit ICaN-dependent plateau potentials. Likewise, in motoneurons of the nucleus ambiguous, Rekling and Feldman (1997) provided evidence that ICaN contributes to the plateau depolarization. The ICaN has been shown to underlie neuronal burst firing in various areas of the CNS (Hasuo et al., 1990; Raggenbass et al., 1997; Beurrier et al., 2000; Thoby-Brisson and Ramirez, 2001; Chang and Kim, 2004; Zhu et al., 2004; Del Negro et al., 2005; Jasinski et al., 2013; Tsuruyama et al., 2013).
Comparative aspects
This report provides evidence that motoneurons innervating the triceps surae muscle exhibit self-sustained firing early after birth in rodents. Differences in recording conditions likely account for the fact that plateau potentials have not been reported earlier in neonatal rodents. Most studies have worked at room temperature or <28°C, conditions that prevent the expression of plateau potentials.
Given the strong dependence of plateau potentials on L-type Ca2+ channels in adults, it is possible that the ICaN-dependent plateau potential is a transient characteristic at a time when all membrane conductances are not fully functional. Indeed, L-type Ca2+ channels are responsible for a small component of Ca2+ currents in neonatal rat motoneurons (Gao and Ziskind-Conhaim, 1998) and start to make noticeable contributions to motoneuronal firing only at P7 (Jiang et al., 1999). Alternatively, the ménage à trois we revealed in neonates may be transposed in adults. Blockade of L-type Ca2+ channels in adults may abolish the ICaN upstream. In addition, the interpretation that plateau potentials rely on L-type Ca2+ channels in adults may be biased by experimental conditions. Most, if not all, studies investigating the contribution of L-type Ca2+ channels to plateau potentials were done in high concentrations of extracellular Ca2+ (≥2.75 mm), far from the physiological value (1.2 mm) in the CSF (Nicholson et al., 1977; Jones and Keep, 1988; Brocard et al., 2013). In this condition, Ca2+ currents are strongly potentiated (Carlin et al., 2000b, 2009), wheras INaP is inhibited (Tazerart et al., 2008). Therefore, the real contribution of L-type Ca2+ and TRPV2 channels in generating plateau potentials should be reevaluated in adult mammalian motoneurons using physiological concentrations of Ca2+.
Functional significance
We show that bistability of motoneurons is a powerful cellular mechanism for the transformation of sensory inputs into a motor command. Hounsgaard et al. (1988) emphasized the potential importance of self-sustained firing of motoneurons for the maintenance of posture. Although plateau potentials become dominant in triceps surae motoneurons at birth, animals are unable to weight-bear before the second postnatal week (Westerga and Gramsbergen, 1990). This discrepancy suggests that the expression of plateau properties is not the limiting factor in the development of posture. However, plateau potentials emerged only at temperatures >30°C. During the first postnatal week, the body temperature of a pup isolated from its mother does not exceed 30°C (Fowler and Kellogg, 1975). A steep increase of the body temperature in the same condition is observed at the beginning of the second postnatal week to reach 34°C at P9–P10. Therefore, by regulating the expression of plateaus, the development of thermoregulatory mechanisms may contribute to the late emergence of posture in rodents.
Footnotes
This work and S.T. were supported by the French Institut pour la Recherche sur la Moelle Epinière et l'Encéphale (IRME to F.B. and L.V.), and the Agence Nationale pour la Recherche. S.T. received a grant from the Fondation pour la Recherche Médicale (FRM) and the Fondation Aix-Marseille Université.
The authors declare no competing financial interests.
References
- Altman J, Sudarshan K. Postnatal development of locomotion in the laboratory rat. Anim Behav. 1975;23:896–920. doi: 10.1016/0003-3472(75)90114-1. [DOI] [PubMed] [Google Scholar]
- Angstadt JD, Choo JJ. Sodium-dependent plateau potentials in cultured Retzius cells of the medicinal leech. J Neurophysiol. 1996;76:1491–1502. doi: 10.1152/jn.1996.76.3.1491. [DOI] [PubMed] [Google Scholar]
- Bang S, Kim KY, Yoo S, Lee SH, Hwang SW. Transient receptor potential V2 expressed in sensory neurons is activated by probenecid. Neurosci Lett. 2007;425:120–125. doi: 10.1016/j.neulet.2007.08.035. [DOI] [PubMed] [Google Scholar]
- Bang S, Yoo S, Yang TJ, Cho H, Hwang SW. Isopentenyl pyrophosphate is a novel antinociceptive substance that inhibits TRPV3 and TRPA1 ion channels. Pain. 2011;152:1156–1164. doi: 10.1016/j.pain.2011.01.044. [DOI] [PubMed] [Google Scholar]
- Benham CD, Gunthorpe MJ, Davis JB. TRPV channels as temperature sensors. Cell Calcium. 2003;33:479–487. doi: 10.1016/S0143-4160(03)00063-0. [DOI] [PubMed] [Google Scholar]
- Bennett DJ, Hultborn H, Fedirchuk B, Gorassini M. Short-term plasticity in hindlimb motoneurons of decerebrate cats. J Neurophysiol. 1998;80:2038–2045. doi: 10.1152/jn.1998.80.4.2038. [DOI] [PubMed] [Google Scholar]
- Beurrier C, Bioulac B, Hammond C. Slowly inactivating sodium current (INaP) underlies single-spike activity in rat subthalamic neurons. J Neurophysiol. 2000;83:1951–1957. doi: 10.1152/jn.2000.83.4.1951. [DOI] [PubMed] [Google Scholar]
- Booth V, Rinzel J. A minimal, compartmental model for a dendritic origin of bistability of motoneuron firing patterns. J Comput Neurosci. 1995;2:299–312. doi: 10.1007/BF00961442. [DOI] [PubMed] [Google Scholar]
- Bregman BS. Development of serotonin immunoreactivity in the rat spinal cord and its plasticity after neonatal spinal cord lesions. Brain Res. 1987;431:245–263. doi: 10.1016/0165-3806(87)90213-6. [DOI] [PubMed] [Google Scholar]
- Brocard F, Vinay L, Clarac F. Development of hindlimb postural control during the first postnatal week in the rat. Brain Res Dev Brain Res. 1999;117:81–89. doi: 10.1016/S0165-3806(99)00101-7. [DOI] [PubMed] [Google Scholar]
- Brocard F, Shevtsova NA, Bouhadfane M, Tazerart S, Heinemann U, Rybak IA, Vinay L. Activity-dependent changes in extracellular Ca2+ and K+ reveal pacemakers in the spinal locomotor-related network. Neuron. 2013;77:1047–1054. doi: 10.1016/j.neuron.2013.01.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brownstone RM. Beginning at the end: repetitive firing properties in the final common pathway. Prog Neurobiol. 2006;78:156–172. doi: 10.1016/j.pneurobio.2006.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bui TV, Ter-Mikaelian M, Bedrossian D, Rose PK. Computational estimation of the distribution of L-type Ca2+ channels in motoneurons based on variable threshold of activation of persistent inward currents. J Neurophysiol. 2006;95:225–241. doi: 10.1152/jn.00646.2005. [DOI] [PubMed] [Google Scholar]
- Carlin KP, Jiang Z, Brownstone RM. Characterization of calcium currents in functionally mature mouse spinal motoneurons. Eur J Neurosci. 2000a;12:1624–1634. doi: 10.1046/j.1460-9568.2000.00050.x. [DOI] [PubMed] [Google Scholar]
- Carlin KP, Jones KE, Jiang Z, Jordan LM, Brownstone RM. Dendritic L-type calcium currents in mouse spinal motoneurons: implications for bistability. Eur J Neurosci. 2000b;12:1635–1646. doi: 10.1046/j.1460-9568.2000.00055.x. [DOI] [PubMed] [Google Scholar]
- Carlin KP, Bui TV, Dai Y, Brownstone RM. Staircase currents in motoneurons: insight into the spatial arrangement of calcium channels in the dendritic tree. J Neurosci. 2009;29:5343–5353. doi: 10.1523/JNEUROSCI.5458-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caterina MJ, Schumacher MA, Tominaga M, Rosen TA, Levine JD, Julius D. The capsaicin receptor: a heat-activated ion channel in the pain pathway. Nature. 1997;389:816–824. doi: 10.1038/39807. [DOI] [PubMed] [Google Scholar]
- Cattaert D, Araque A, Buno W, Clarac F. Motor neurons of the crayfish walking system possess TEA-revealed regenerative electrical properties. J Exp Biol. 1994;188:339–345. doi: 10.1242/jeb.188.1.339. [DOI] [PubMed] [Google Scholar]
- Cazalets JR, Menard I, Crémieux J, Clarac F. Variability as a characteristic of immature motor systems: an electromyographic study of swimming in the newborn rat. Behav Brain Res. 1990;40:215–225. doi: 10.1016/0166-4328(90)90078-S. [DOI] [PubMed] [Google Scholar]
- Chang SY, Kim U. Ionic mechanism of long-lasting discharges of action potentials triggered by membrane hyperpolarization in the medial lateral habenula. J Neurosci. 2004;24:2172–2181. doi: 10.1523/JNEUROSCI.4891-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarac F, Brocard F, Vinay L. The maturation of locomotor networks. Prog Brain Res. 2004;143:57–66. doi: 10.1016/S0079-6123(03)43006-9. [DOI] [PubMed] [Google Scholar]
- Collins DF, Burke D, Gandevia SC. Large involuntary forces consistent with plateau-like behavior of human motoneurons. J Neurosci. 2001;21:4059–4065. doi: 10.1523/JNEUROSCI.21-11-04059.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conway BA, Hultborn H, Kiehn O, Mintz I. Plateau potentials in alpha-motoneurones induced by intravenous injection of L-dopa and clonidine in the spinal cat. J Physiol. 1988;405:369–384. doi: 10.1113/jphysiol.1988.sp017337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crone C, Hultborn H, Kiehn O, Mazieres L, Wigström H. Maintained changes in motoneuronal excitability by short-lasting synaptic inputs in the decerebrate cat. J Physiol. 1988;405:321–343. doi: 10.1113/jphysiol.1988.sp017335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Del Negro CA, Morgado-Valle C, Hayes JA, Mackay DD, Pace RW, Crowder EA, Feldman JL. Sodium and calcium current-mediated pacemaker neurons and respiratory rhythm generation. J Neurosci. 2005;25:446–453. doi: 10.1523/JNEUROSCI.2237-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Derjean D, Bertrand S, Nagy F, Shefchyk SJ. Plateau potentials and membrane oscillations in parasympathetic preganglionic neurones and intermediolateral neurones in the rat lumbosacral spinal cord. J Physiol. 2005;563:583–596. doi: 10.1113/jphysiol.2004.076802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Prisco GV, Pearlstein E, Robitaille R, Dubuc R. Role of sensory-evoked NMDA plateau potentials in the initiation of locomotion. Science. 1997;278:1122–1125. doi: 10.1126/science.278.5340.1122. [DOI] [PubMed] [Google Scholar]
- Di Prisco GV, Pearlstein E, Le Ray D, Robitaille R, Dubuc R. A cellular mechanism for the transformation of a sensory input into a motor command. J Neurosci. 2000;20:8169–8176. doi: 10.1523/JNEUROSCI.20-21-08169.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eken T, Hultborn H, Kiehn O. Possible functions of transmitter-controlled plateau potentials in alpha motoneurones. Prog Brain Res. 1989;80:257–267. doi: 10.1016/S0079-6123(08)62219-0. discussion 239–242. [DOI] [PubMed] [Google Scholar]
- Facer P, Casula MA, Smith GD, Benham CD, Chessell IP, Bountra C, Sinisi M, Birch R, Anand P. Differential expression of the capsaicin receptor TRPV1 and related novel receptors TRPV3, TRPV4 and TRPM8 in normal human tissues and changes in traumatic and diabetic neuropathy. BMC Neurol. 2007;7:11. doi: 10.1186/1471-2377-7-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fady JC, Jamon M, Clarac F. Early olfactory-induced rhythmic limb activity in the newborn rat. Brain Res Dev Brain Res. 1998;108:111–123. doi: 10.1016/S0165-3806(98)00040-6. [DOI] [PubMed] [Google Scholar]
- Fowler SJ, Kellogg C. Ontogeny of thermoregulatory mechanisms in the rat. J Comp Physiol Psychol. 1975;89:738–746. doi: 10.1037/h0077037. [DOI] [PubMed] [Google Scholar]
- Fraser DD, MacVicar BA. Cholinergic-dependent plateau potential in hippocampal CA1 pyramidal neurons. J Neurosci. 1996;16:4113–4128. doi: 10.1523/JNEUROSCI.16-13-04113.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedman A, Arens J, Heinemann U, Gutnick MJ. Slow depolarizing afterpotentials in neocortical neurons are sodium and calcium-dependent. Neurosci Lett. 1992;135:13–17. doi: 10.1016/0304-3940(92)90125-Q. [DOI] [PubMed] [Google Scholar]
- Gao BX, Ziskind-Conhaim L. Development of ionic currents underlying changes in action potential waveforms in rat spinal motoneurons. J Neurophysiol. 1998;80:3047–3061. doi: 10.1152/jn.1998.80.6.3047. [DOI] [PubMed] [Google Scholar]
- Gorassini MA, Bennett DJ, Yang JF. Self-sustained firing of human motor units. Neurosci Lett. 1998;247:13–16. doi: 10.1016/S0304-3940(98)00277-8. [DOI] [PubMed] [Google Scholar]
- Gorassini M, Bennett DJ, Kiehn O, Eken T, Hultborn H. Activation patterns of hindlimb motor units in the awake rat and their relation to motoneuron intrinsic properties. J Neurophysiol. 1999;82:709–717. doi: 10.1152/jn.1999.82.2.709. [DOI] [PubMed] [Google Scholar]
- Grillner S, Zangger P. How detailed is the central pattern generation for locomotion? Brain Res. 1975;88:367–371. doi: 10.1016/0006-8993(75)90401-1. [DOI] [PubMed] [Google Scholar]
- Guinamard R, Simard C, Del Negro C. Flufenamic acid as an ion channel modulator. Pharmacol Ther. 2013;138:272–284. doi: 10.1016/j.pharmthera.2013.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harteneck C, Gollasch M. Pharmacological modulation of diacylglycerol-sensitive TRPC3/6/7 channels. Curr Pharm Biotechnol. 2011;12:35–41. doi: 10.2174/138920111793937943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harvey PJ, Li X, Li Y, Bennett DJ. 5-HT2 receptor activation facilitates a persistent sodium current and repetitive firing in spinal motoneurons of rats with and without chronic spinal cord injury. J Neurophysiol. 2006;96:1158–1170. doi: 10.1152/jn.01088.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasuo H, Phelan KD, Twery MJ, Gallagher JP. A calcium-dependent slow afterdepolarization recorded in rat dorsolateral septal nucleus neurons in vitro. J Neurophysiol. 1990;64:1838–1846. doi: 10.1152/jn.1990.64.6.1838. [DOI] [PubMed] [Google Scholar]
- Heckman CJ, Lee RH, Brownstone RM. Hyperexcitable dendrites in motoneurons and their neuromodulatory control during motor behavior. Trends Neurosci. 2003;26:688–695. doi: 10.1016/j.tins.2003.10.002. [DOI] [PubMed] [Google Scholar]
- Heckman CJ, Hyngstrom AS, Johnson MD. Active properties of motoneurone dendrites: diffuse descending neuromodulation, focused local inhibition. J Physiol. 2008a;586:1225–1231. doi: 10.1113/jphysiol.2007.145078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heckman CJ, Johnson M, Mottram C, Schuster J. Persistent inward currents in spinal motoneurons and their influence on human motoneuron firing patterns. Neuroscientist. 2008b;14:264–275. doi: 10.1177/1073858408314986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hisanaga E, Nagasawa M, Ueki K, Kulkarni RN, Mori M, Kojima I. Regulation of calcium-permeable TRPV2 channel by insulin in pancreatic beta-cells. Diabetes. 2009;58:174–184. doi: 10.2337/db08-0862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hounsgaard J, Kiehn O. Serotonin-induced bistability of turtle motoneurones caused by a nifedipine-sensitive calcium plateau potential. J Physiol. 1989;414:265–282. doi: 10.1113/jphysiol.1989.sp017687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hounsgaard J, Kiehn O. Calcium spikes and calcium plateaux evoked by differential polarization in dendrites of turtle motoneurones in vitro. J Physiol. 1993;468:245–259. doi: 10.1113/jphysiol.1993.sp019769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hounsgaard J, Mintz I. Calcium conductance and firing properties of spinal motoneurones in the turtle. J Physiol. 1988;398:591–603. doi: 10.1113/jphysiol.1988.sp017059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hounsgaard J, Hultborn H, Jespersen B, Kiehn O. Intrinsic membrane properties causing a bistable behaviour of alpha-motoneurones. Exp Brain Res. 1984;55:391–394. doi: 10.1007/BF00237290. [DOI] [PubMed] [Google Scholar]
- Hounsgaard J, Hultborn H, Jespersen B, Kiehn O. Bistability of alpha-motoneurones in the decerebrate cat and in the acute spinal cat after intravenous 5-hydroxytryptophan. J Physiol. 1988;405:345–367. doi: 10.1113/jphysiol.1988.sp017336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsiao CF, Del Negro CA, Trueblood PR, Chandler SH. Ionic basis for serotonin-induced bistable membrane properties in guinea pig trigeminal motoneurons. J Neurophysiol. 1998;79:2847–2856. doi: 10.1152/jn.1998.79.6.2847. [DOI] [PubMed] [Google Scholar]
- Hu HZ, Gu Q, Wang C, Colton CK, Tang J, Kinoshita-Kawada M, Lee LY, Wood JD, Zhu MX. 2-aminoethoxydiphenyl borate is a common activator of TRPV1, TRPV2, and TRPV3. J Biol Chem. 2004;279:35741–35748. doi: 10.1074/jbc.M404164200. [DOI] [PubMed] [Google Scholar]
- Hultborn H, Wigström H, Wängberg B. Prolonged activation of soleus motoneurones following a conditioning train in soleus Ia afferents: a case for a reverberating loop? Neurosci Lett. 1975;1:147–152. doi: 10.1016/0304-3940(75)90030-0. [DOI] [PubMed] [Google Scholar]
- Jasinski PE, Molkov YI, Shevtsova NA, Smith JC, Rybak IA. Sodium and calcium mechanisms of rhythmic bursting in excitatory neural networks of the pre-Bötzinger complex: a computational modelling study. Eur J Neurosci. 2013;37:212–230. doi: 10.1111/ejn.12042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang Z, Rempel J, Li J, Sawchuk MA, Carlin KP, Brownstone RM. Development of L-type calcium channels and a nifedipine-sensitive motor activity in the postnatal mouse spinal cord. Eur J Neurosci. 1999;11:3481–3487. doi: 10.1046/j.1460-9568.1999.00765.x. [DOI] [PubMed] [Google Scholar]
- Jones HC, Keep RF. Brain fluid calcium concentration and response to acute hypercalcaemia during development in the rat. J Physiol. 1988;402:579–593. doi: 10.1113/jphysiol.1988.sp017223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiehn O, Eken T. Prolonged firing in motor units: evidence of plateau potentials in human motoneurons? J Neurophysiol. 1997;78:3061–3068. doi: 10.1152/jn.1997.78.6.3061. [DOI] [PubMed] [Google Scholar]
- Kiehn O, Eken T. Functional role of plateau potentials in vertebrate motor neurons. Curr Opin Neurobiol. 1998;8:746–752. doi: 10.1016/S0959-4388(98)80117-7. [DOI] [PubMed] [Google Scholar]
- Kiehn O, Harris-Warrick RM. Serotonergic stretch receptors induce plateau properties in a crustacean motor neuron by a dual-conductance mechanism. J Neurophysiol. 1992;68:485–495. doi: 10.1152/jn.1992.68.2.485. [DOI] [PubMed] [Google Scholar]
- Kuo JJ, Lee RH, Zhang L, Heckman CJ. Essential role of the persistent sodium current in spike initiation during slowly rising inputs in mouse spinal neurones. J Physiol. 2006;574:819–834. doi: 10.1113/jphysiol.2006.107094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee CR, Tepper JM. A calcium-activated nonselective cation conductance underlies the plateau potential in rat substantia nigra GABAergic neurons. J Neurosci. 2007;27:6531–6541. doi: 10.1523/JNEUROSCI.1678-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee RH, Heckman CJ. Bistability in spinal motoneurons in vivo: systematic variations in persistent inward currents. J Neurophysiol. 1998;80:583–593. doi: 10.1152/jn.1998.80.2.583. [DOI] [PubMed] [Google Scholar]
- Lee RH, Heckman CJ. Essential role of a fast persistent inward current in action potential initiation and control of rhythmic firing. J Neurophysiol. 2001;85:472–475. doi: 10.1152/jn.2001.85.1.472. [DOI] [PubMed] [Google Scholar]
- Leffler A, Linte RM, Nau C, Reeh P, Babes A. A high-threshold heat-activated channel in cultured rat dorsal root ganglion neurons resembles TRPV2 and is blocked by gadolinium. Eur J Neurosci. 2007;26:12–22. doi: 10.1111/j.1460-9568.2007.05643.x. [DOI] [PubMed] [Google Scholar]
- Lewinter RD, Skinner K, Julius D, Basbaum AI. Immunoreactive TRPV-2 (VRL-1), a capsaicin receptor homolog, in the spinal cord of the rat. J Comp Neurol. 2004;470:400–408. doi: 10.1002/cne.20024. [DOI] [PubMed] [Google Scholar]
- Li Y, Bennett DJ. Persistent sodium and calcium currents cause plateau potentials in motoneurons of chronic spinal rats. J Neurophysiol. 2003;90:857–869. doi: 10.1152/jn.00236.2003. [DOI] [PubMed] [Google Scholar]
- Li Y, Gorassini MA, Bennett DJ. Role of persistent sodium and calcium currents in motoneuron firing and spasticity in chronic spinal rats. J Neurophysiol. 2004;91:767–783. doi: 10.1152/jn.00788.2003. [DOI] [PubMed] [Google Scholar]
- Ma W, Hui H, Pelegrin P, Surprenant A. Pharmacological characterization of pannexin-1 currents expressed in mammalian cells. J Pharmacol Exp Ther. 2009;328:409–418. doi: 10.1124/jpet.108.146365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mandadi S, Nakanishi ST, Takashima Y, Dhaka A, Patapoutian A, McKemy DD, Whelan PJ. Locomotor networks are targets of modulation by sensory transient receptor potential vanilloid 1 and transient receptor potential melastatin 8 channels. Neuroscience. 2009;162:1377–1397. doi: 10.1016/j.neuroscience.2009.05.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mejia-Gervacio S, Hounsgaard J, Diaz-Muñoz M. Roles of ryanodine and inositol triphosphate receptors in regulation of plateau potentials in turtle spinal motoneurons. Neuroscience. 2004;123:123–130. doi: 10.1016/j.neuroscience.2003.08.049. [DOI] [PubMed] [Google Scholar]
- Montell C. The TRP superfamily of cation channels. Sci STKE. 2005;2005:re3. doi: 10.1126/stke.2722005re3. [DOI] [PubMed] [Google Scholar]
- Moqrich A, Hwang SW, Earley TJ, Petrus MJ, Murray AN, Spencer KS, Andahazy M, Story GM, Patapoutian A. Impaired thermosensation in mice lacking TRPV3, a heat and camphor sensor in the skin. Science. 2005;307:1468–1472. doi: 10.1126/science.1108609. [DOI] [PubMed] [Google Scholar]
- Moran MM, Xu H, Clapham DE. TRP ion channels in the nervous system. Curr Opin Neurobiol. 2004;14:362–369. doi: 10.1016/j.conb.2004.05.003. [DOI] [PubMed] [Google Scholar]
- Morisset V, Nagy F. Ionic basis for plateau potentials in deep dorsal horn neurons of the rat spinal cord. J Neurosci. 1999;19:7309–7316. doi: 10.1523/JNEUROSCI.19-17-07309.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mrejeru A, Wei A, Ramirez JM. Calcium-activated nonselective cation currents are involved in generation of tonic and bursting activity in dopamine neurons of the substantia nigra pars compacta. J Physiol. 2011;589:2497–2514. doi: 10.1113/jphysiol.2011.206631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicholson C, Bruggencate GT, Steinberg R, Stöckle H. Calcium modulation in brain extracellular microenvironment demonstrated with ion-selective micropipette. Proc Natl Acad Sci U S A. 1977;74:1287–1290. doi: 10.1073/pnas.74.3.1287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishimura Y, Schwindt PC, Crill WE. Electrical properties of facial motoneurons in brainstem slices from guinea pig. Brain Res. 1989;502:127–142. doi: 10.1016/0006-8993(89)90468-X. [DOI] [PubMed] [Google Scholar]
- Pambo-Pambo A, Durand J, Gueritaud JP. Early excitability changes in lumbar motoneurons of transgenic SOD1G85R and SOD1G(93A-low) mice. J Neurophysiol. 2009;102:3627–3642. doi: 10.1152/jn.00482.2009. [DOI] [PubMed] [Google Scholar]
- Partridge LD, Swandulla D. Calcium-activated nonspecific cation channels. Trends Neurosci. 1988;11:69–72. doi: 10.1016/0166-2236(88)90167-1. [DOI] [PubMed] [Google Scholar]
- Perrier JF, Delgado-Lezama R. Synaptic release of serotonin induced by stimulation of the raphe nucleus promotes plateau potentials in spinal motoneurons of the adult turtle. J Neurosci. 2005;25:7993–7999. doi: 10.1523/JNEUROSCI.1957-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perrier JF, Hounsgaard J. Ca(2+)-activated nonselective cationic current (I(CAN)) in turtle motoneurons. J Neurophysiol. 1999;82:730–735. doi: 10.1152/jn.1999.82.2.730. [DOI] [PubMed] [Google Scholar]
- Perrier JF, Hounsgaard J. Development and regulation of response properties in spinal cord motoneurons. Brain Res Bull. 2000;53:529–535. doi: 10.1016/S0361-9230(00)00386-5. [DOI] [PubMed] [Google Scholar]
- Perrier JF, Tresch MC. Recruitment of motor neuronal persistent inward currents shapes withdrawal reflexes in the frog. J Physiol. 2005;562:507–520. doi: 10.1113/jphysiol.2004.072769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Powers RK, Binder MD. Persistent sodium and calcium currents in rat hypoglossal motoneurons. J Neurophysiol. 2003;89:615–624. doi: 10.1152/jn.00241.2002. [DOI] [PubMed] [Google Scholar]
- Raggenbass M, Pierson P, Metzger D, Alberi S. Action of a metabotropic glutamate receptor agonist in rat lateral septum: induction of a sodium-dependent inward aftercurrent. Brain Res. 1997;776:75–87. doi: 10.1016/S0006-8993(97)00945-1. [DOI] [PubMed] [Google Scholar]
- Rajaofetra N, Sandillon F, Geffard M, Privat A. Pre- and post-natal ontogeny of serotonergic projections to the rat spinal cord. J Neurosci Res. 1989;22:305–321. doi: 10.1002/jnr.490220311. [DOI] [PubMed] [Google Scholar]
- Rajaofetra N, Poulat P, Marlier L, Geffard M, Privat A. Pre- and postnatal development of noradrenergic projections to the rat spinal cord: an immunocytochemical study. Brain Res Dev Brain Res. 1992;67:237–246. doi: 10.1016/0165-3806(92)90224-K. [DOI] [PubMed] [Google Scholar]
- Reichling DB, MacDermott AB. Lanthanum actions on excitatory amino acid-gated currents and voltage-gated calcium currents in rat dorsal horn neurons. J Physiol. 1991;441:199–218. doi: 10.1113/jphysiol.1991.sp018746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rekling JC, Feldman JL. Calcium-dependent plateau potentials in rostral ambiguus neurons in the newborn mouse brain stem in vitro. J Neurophysiol. 1997;78:2483–2492. doi: 10.1152/jn.1997.78.5.2483. [DOI] [PubMed] [Google Scholar]
- Reuter H, Porzig H. Localization and functional significance of the Na+/Ca2+ exchanger in presynaptic boutons of hippocampal cells in culture. Neuron. 1995;15:1077–1084. doi: 10.1016/0896-6273(95)90096-9. [DOI] [PubMed] [Google Scholar]
- Russo RE, Hounsgaard J. Plateau-generating neurones in the dorsal horn in an in vitro preparation of the turtle spinal cord. J Physiol. 1996;493:39–54. doi: 10.1113/jphysiol.1996.sp021363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sadlaoud K, Tazerart S, Brocard C, Jean-Xavier C, Portalier P, Brocard F, Vinay L, Bras H. Differential plasticity of the GABAergic and glycinergic synaptic transmission to rat lumbar motoneurons after spinal cord injury. J Neurosci. 2010;30:3358–3369. doi: 10.1523/JNEUROSCI.6310-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwindt PC, Crill WE. Properties of a persistent inward current in normal and TEA-injected motoneurons. J Neurophysiol. 1980;43:1700–1724. doi: 10.1152/jn.1980.43.6.1700. [DOI] [PubMed] [Google Scholar]
- Tazerart S, Viemari JC, Darbon P, Vinay L, Brocard F. Contribution of persistent sodium current to locomotor pattern generation in neonatal rats. J Neurophysiol. 2007;98:613–628. doi: 10.1152/jn.00316.2007. [DOI] [PubMed] [Google Scholar]
- Tazerart S, Vinay L, Brocard F. The persistent sodium current generates pacemaker activities in the central pattern generator for locomotion and regulates the locomotor rhythm. J Neurosci. 2008;28:8577–8589. doi: 10.1523/JNEUROSCI.1437-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thoby-Brisson M, Ramirez JM. Identification of two types of inspiratory pacemaker neurons in the isolated respiratory neural network of mice. J Neurophysiol. 2001;86:104–112. doi: 10.1152/jn.2001.86.1.104. [DOI] [PubMed] [Google Scholar]
- Tsuruyama K, Hsiao CF, Chandler SH. Participation of a persistent sodium current and calcium-activated non-specific cationic current to burst generation in trigeminal principal sensory neurons. J Neurophysiol. 2013 doi: 10.1152/jn.00410.2013. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vinay L, Brocard F, Clarac F. Differential maturation of motoneurons innervating ankle flexor and extensor muscles in the neonatal rat. Eur J Neurosci. 2000a;12:4562–4566. doi: 10.1046/j.0953-816X.2000.01321.x. [DOI] [PubMed] [Google Scholar]
- Vinay L, Brocard F, Pflieger JF, Simeoni-Alias J, Clarac F. Perinatal development of lumbar motoneurons and their inputs in the rat. Brain Res Bull. 2000b;53:635–647. doi: 10.1016/S0361-9230(00)00397-X. [DOI] [PubMed] [Google Scholar]
- Vinay L, Brocard F, Clarac F, Norreel JC, Pearlstein E, Pflieger JF. Development of posture and locomotion: an interplay of endogenously generated activities and neurotrophic actions by descending pathways. Brain Res Rev. 2002;40:118–129. doi: 10.1016/S0165-0173(02)00195-9. [DOI] [PubMed] [Google Scholar]
- Vincent F, Acevedo A, Nguyen MT, Dourado M, DeFalco J, Gustafson A, Spiro P, Emerling DE, Kelly MG, Duncton MA. Identification and characterization of novel TRPV4 modulators. Biochem Biophys Res Commun. 2009;389:490–494. doi: 10.1016/j.bbrc.2009.09.007. [DOI] [PubMed] [Google Scholar]
- Watanabe H, Davis JB, Smart D, Jerman JC, Smith GD, Hayes P, Vriens J, Cairns W, Wissenbach U, Prenen J, Flockerzi V, Droogmans G, Benham CD, Nilius B. Activation of TRPV4 channels (hVRL-2/mTRP12) by phorbol derivatives. J Biol Chem. 2002;277:13569–13577. doi: 10.1074/jbc.M200062200. [DOI] [PubMed] [Google Scholar]
- Westerga J, Gramsbergen A. The development of locomotion in the rat. Brain Res Dev Brain Res. 1990;57:163–174. doi: 10.1016/0165-3806(90)90042-W. [DOI] [PubMed] [Google Scholar]
- Wu LJ, Sweet TB, Clapham DE. International union of basic and clinical pharmacology: LXXVI. Current progress in the mammalian TRP ion channel family. Pharmacol Rev. 2010;62:381–404. doi: 10.1124/pr.110.002725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yellen G. Single Ca2+-activated nonselective cation channels in neuroblastoma. Nature. 1982;296:357–359. doi: 10.1038/296357a0. [DOI] [PubMed] [Google Scholar]
- Zhang B, Wootton JF, Harris-Warrick RM. Calcium-dependent plateau potentials in a crab stomatogastric ganglion motor neuron: II. Calcium-activated slow inward current. J Neurophysiol. 1995;74:1938–1946. doi: 10.1152/jn.1995.74.5.1938. [DOI] [PubMed] [Google Scholar]
- Zhu ZT, Munhall A, Shen KZ, Johnson SW. Calcium-dependent subthreshold oscillations determine bursting activity induced by N-methyl-d-aspartate in rat subthalamic neurons in vitro. Eur J Neurosci. 2004;19:1296–1304. doi: 10.1111/j.1460-9568.2004.03240.x. [DOI] [PubMed] [Google Scholar]