Abstract
The gonadotropin-releasing hormone (GnRH) neurons are the key cells regulating fertility in all mammalian species. The scattered distribution of these neurons has made investigation of their properties extremely difficult and the key goal of recording their electrical activity in vivo near impossible. The caudal-most extension of the GnRH neuron continuum brings some cells very close to the base of the brain at the level of the anterior hypothalamic area. Taking insight from this, we developed an experimental procedure in anesthetized GnRH-GFP mice that allows the electrical activity of these GnRH neurons to be recorded in vivo. On-cell recordings revealed that the majority of GnRH neurons (86%) were spontaneously active, exhibiting a range of firing patterns, although only a minority (15%) exhibited burst firing. Mean firing frequencies ranged from 0.06 to 3.65 Hz, with the most common interspike interval being ∼500 ms. All GnRH neurons tested were activated by AMPA and kisspeptin. Whereas the GABAA receptor agonist muscimol evoked excitatory, inhibitory, or mixed effects on GnRH neuron firing, the GABAA receptor antagonist picrotoxin resulted in a consistent suppression of firing. These observations represent the first electrical recordings of GnRH neurons in vivo. They reveal that GnRH neurons in vivo exhibit considerable heterogeneity in their firing patterns with both similarities and differences to firing in vitro. These variable patterns of firing in vivo are found to be critically dependent upon ongoing GABAA receptor signaling.
Introduction
The gonadotropin-releasing hormone (GnRH) neurons represent the final output cells of the neuronal network controlling fertility in mammals. Ever since the discovery of GnRH (Schally et al., 1971; Barry et al., 1973), understanding the properties of these cells has been an important goal in the field of reproductive biology. A variety of experimental preparations, including immortalized GnRH-secreting GT1 cells (Weiner et al., 1992), embryonic olfactory placode cultures (Terasawa et al., 1993; Fueshko and Wray, 1994), and transgenic rodent models, have been used to investigate GnRH neurons (Moenter, 2010; Constantin et al., 2012a). Although these experimental approaches have provided considerable insight into the characteristics of embryonic and adult GnRH neurons in vitro, the key goal of recording the electrical activity of GnRH neurons in vivo has remained elusive. Pioneering studies in the 1980s revealed striking correlations between multiunit electrical activity in the mediobasal hypothalamus and pulsatile luteinizing hormone (LH) secretion in vivo (Kawakami et al., 1982; O'Byrne and Knobil, 1993), but the origins of this electrical activity remain unknown.
At present, our understanding of the electrical properties of adult GnRH neurons rests principally upon recordings of GnRH-GFP cells in the acute brain slice preparation. These studies have identified that GnRH neurons exhibit a variety of different firing patterns ranging from bursting to continuous to silent (Kelly and Wagner, 2002; Moenter, 2010). The functional correlates of these different firing patterns are unclear and the degree to which they represent the activity of GnRH neurons in vivo is unknown. The afferent inputs to GnRH neurons, in addition to the long GnRH neuron dendrites themselves (Constantin et al., 2012b), are severed in preparing the brain slice. Accordingly, it has not been surprising to find that action potential-dependent GABA or glutamate release on GnRH neurons is rare in vitro (Christian et al., 2009; Herbison and Moenter, 2011) and that ionotropic amino acid receptor antagonists have little impact on GnRH neuron firing in the slice (Nunemaker et al., 2002; Lee et al., 2012). Unusually, GABAA receptor activation can be excitatory in adult GnRH neurons; and, again, it has been questioned whether this may be attributable to the slice preparation (Herbison and Moenter, 2011).
We recently observed that the caudal-most GnRH neurons, located in the ventral aspect of the anterior hypothalamic area (AHA) of the mouse, were accessible from the uncut ventral surface of the brain in transgenic GnRH-GFP mice (Constantin et al., 2012b). This suggested that it might actually be possible to record from fluorescent GnRH neurons in vivo through a ventral surgical approach. Taking insight from methodologies enabling the visualization of the pituitary gland in vivo in the mouse (Bonnefont et al., 2005; Lafont et al., 2010), we report here the first recordings of GnRH neurons in vivo. We show that GnRH neurons exhibit even greater heterogeneity in their spontaneous firing rates than that found in the brain slice and that GABAA receptor activation by endogenous GABA is essential for GnRH neuron firing in vivo.
Materials and Methods
Animals.
All experimentation was approved by the University of Otago Animal Welfare and Ethics Committee. Adult (2–4 months of age) male and female GnRH-GFP mice (Spergel et al., 1999) were maintained under 12 h light, 12 h dark lighting conditions with food and water ad libitum.
Transpharyngeal exposure of GnRH neurons.
The transpharyngeal exposure of GnRH neurons was adapted from a surgical approach for viewing the median eminence and pituitary gland (Bonnefont et al., 2005; Lafont et al., 2010) (see Fig. 1A). Mice were anesthetized with pentobarbital (0.4 mg/10 g body weight, IP:IM = 5:1), placed on their back, paws taped to the base, and a thread connected to a weight placed behind the teeth to slightly extend the throat (see Fig. 1D). A rectal probe connected to a heat pad was positioned to maintained body temperature between 36°C and 37°C (CT-1000; CWE).
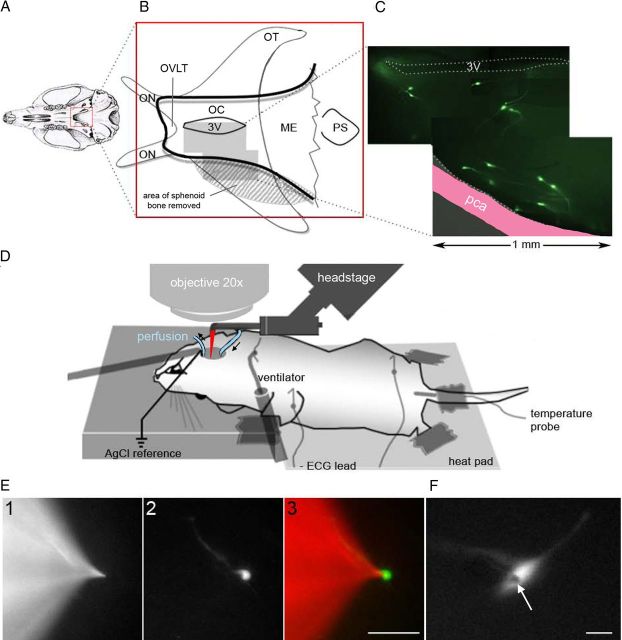
Figure 1.
Electrophysiological setup for recording GnRH neurons in vivo. A, The sphenoid bone was exposed by a transpharyngeal surgical approach. B, Part of the sphenoid bone (edge outlined in black) was removed (stippled area), and a hole was drilled to expose the optic chiasm (OC) beneath. This was removed to visualize the ventral surface of the brain from the organum vasculosum of the lamina terminalis (OVLT) to the median eminence (ME). C, The most superficial GnRH neurons (green fluorescent cells) at the base of the brain lie close to the posterior communicating artery (pca). D, After surgical exposure, the mouse was shifted to the recording platform and a reference electrode and perfusion inflow and outflow positioned. E, The electrode (red in D) was positioned initially by alternating fluorescence wavelengths allowing the visualization of either the pipette (tetra methyl rhodamine, E1) or the targeted neuron (GFP, E2), until they were in proximity (E3; scale bar, 50 μm). When the electrode was close to the GnRH neuron, positive pressure was applied and the pipette shadow (arrow) was used for the final approach (F; scale bar, 10 μm). ON, Optic nerves; OT, optic tracts; PS, pituitary stalk; 3V, third ventricle.
The surgery was commenced under a binocular microscope (M80; Leica) with cold light source (CLS 150X, Leica). The skin was incised longitudinally from the sternal notch to the chin and the salivary glands separated to expose the muscles overlying the trachea. The muscles were lifted anteriorly from the trachea and detached from the hyoid bone. A polyethylene catheter (o.d. 0.96 × i.d. 0.58 mm) was inserted into the trachea above the sternum for ventilation (SAR-830/P; CWE; 100 breaths/min, operated at constant pressured cycles set at 1.4 cm H2O, 0.3 s/inspiration, O2 90–80 ml/min). Subcutaneous electrodes were positioned in the right arm and the left leg (lead II). The heartbeat was digitized by a Digidata (1332A) analogic-to-digital converter (Molecular Devices) and visualized in real time with the pClamp10 module, ClampEx (Molecular Devices). The depth of anesthesia was initially evaluated by toe pinch, then continuously controlled by heart rate monitoring (AC/DC bioamplifier, HR; BMA-200; CWE). Pentobarbital (0.2 mg i.p.) was added as soon as the HR went >5 beats/s. The thyroid gland was then detached from the trachea and flipped on both sides. The trachea above the sternum was lifted anteriorly to expose the esophagus that was then also cut and lifted anteriorly to expose the back of the oral cavity. The trachea and esophagus were cut out simultaneously with the larynx. The digastric muscles were sectioned at the level of their caudal tendons and lifted toward the inferior lip and the hyoid bone cut down the midline.
Retractors were then positioned on the back of the tongue and on the side of the jaw and the soft palate exposed. This was then cut above the nasal cavity to visualize the sphenoid bone (see Fig. 1A). One side of the internal pterygoid process (right) was gently broken to increase the surgery field laterally. The bone was drilled caudally from the transverse sinus of the sphenoid bone (round burr tip, o.d. 0.5 mm; GEBR Brasseler; Ideal Micro-Drill, Stoelting) and progressively obtruded with bone wax (Ethicon Bone Wax W810; Johnson & Johnson). The bone was then carefully thinned between the internal pterygoid processes until the junction of optic nerves and optic chiasm became apparent below the presphenoid bone (see Fig. 1B). The cavity was washed intensively with oxygenated artificial CSF (aCSF) to remove all debris before the thinned bone was then perforated and fragmented. The contralateral side (left) was partially removed to minimize brain movements. In contrast, the surgery side (right) was removed as far as possible until the posterior communicating artery was visible. The cavity (∼2.0 × 0.8 mm) was injected with ice-cold aCSF to stiffen the meninges. The optic chiasm was then removed with a dental vacuum connected to a 23 g needle and the meninges removed with a 30 g needle (see Fig. 1B).
At this point, a reference silver wire coated with chloride (o.d. 0.003”; A-M Systems) and a suction pipette were placed in the rostral part of the cavity. A perfusion tube was positioned in the caudal extent of the cavity to enable continuous perfusion of the base of the cavity with a thin layer of oxygenated aCSF (118 mm NaCl, 3 mm KCl, 2.5 mm CaCl2, 1.2 mm MgCl2,10 mm HEPES, 25 mm NaHCO3, 5.5 mm d-glucose, 2.9 mm sucrose, pH 7.3, 5% CO2 carbogen). The blood loss throughout the procedure, a maximum of 250 μl, was compensated with 200 μl NaCl 0.9% intraperitoneally. Experiments were terminated as soon as electrocardiographic evidence of anoxia was evident.
In vivo electrophysiology.
After the surgery, the animal was repositioned below an upright microscope (BX51, Olympus) (seeFig. 1D) modified to use a long working distance 20× objective (M Plan Apo NA 0.42; Mitutuyo). A headstage holding a pipette bent to an angle of 45 degrees was then placed in the cavity. Glass pipettes (o.d. 1.5 mm; i.d. 1.17), filled with aCSF (in mm): 118 NaCl, 3 KCl, 2.5 CaCl2, 1.2 MgCl2, 10 HEPES, 25 NaHCO3, 5.5 d-glucose, 2.9 sucrose, pH 7.3, 5% CO2 carbogen containing tetramethylrhodamine (TMR dextran 10,000 W, lysine fixable D1868; Invitrogen; 0.38 mg/ml) were visualized under red fluorescence illumination (U-MWIG2, Olympus; excitation wavelength centered at 535 nm with emission wavelength centered at 580 nm) (see Fig. 1E). GnRH neurons were visualized under blue fluorescence illumination (U-MGGFPHQ, Olympus; excitation wavelength centered at 470 nm with high pass emission filtered at 517 nm). The initial positioning of the electrode was undertaken using fluorescence with the final patching of the GnRH neuron undertaken using the pipette shadow under the GFP filter (see Fig. 1F). Metafluor (Molecular Devices) software was used to control the shutter of the Xenon lamp (Lambda DG-4, Sutter Instruments) and acquisition via a CCD camera (ORCA-ER, Hamamatsu).
Electrophysiological recordings were made using a Multiclamp 700A amplifier (Molecular Devices). The electrophysiological signal was filtered from 50 Hz harmonics with a Hum Bug (Quest Scientific Instrument). Both electrocardiographic and electrophysiological signals were digitized by a Digidata (1332A) analog-to-digital converter (Molecular Devices) and visualized in real time with the pClamp10 module, ClampEx (Molecular Devices). Spontaneous electrical activity was recorded using on-cell loose patch recording mode (<30 MOhm seal) in voltage-clamp. Glass pipettes with a resistance of ∼3–5 MOhm were held at 0 mV. Signals were digitized and filtered at 1 kHz and 10 kHz.
In vivo analysis.
Electrophysiological recordings were analyzed post hoc using pClamp10 module, ClampFit, with the number of spikes per 5 s bins collected in Excel (Microsoft). Pharmacological responses were analyzed by comparing the firing rate during the 2 min preceding the onset of the drug application with the firing rate during the 2 min after the onset of the drug application. A silent GnRH neuron was considered responsive if the drug elicited firing. An active cell was considered to have responded if the instantaneous firing rate during the drug application went below or above the averaged firing rate of the control period by 2 SD. Based on the minimal burst parameters required to evoke a single calcium oscillation in GnRH neurons (Lee et al., 2010a), a burst was defined as ≥2 spikes within 1 s, separated by a period of silence for at least 4 s.
In vitro electrophysiology.
Studies in the rat indicate that the anesthetic dose of pentobarbital used in this study (40 mg/kg) results in maximum brain concentrations of pentobarbital ranging from 50 to 160 μm (Crane et al., 1978; Sato et al., 1995; Du et al., 2009). To examine the potential impact of pentobarbital on GnRH neuron firing, we examined the effect of 100 μm pentobarbital on GnRH neurons in the acute brain slice preparation that allows recordings to be made from AHA GnRH neurons (Constantin et al., 2012b). Using methodology reported previously (Constantin et al., 2012b), a single ventral 300- to 400-μm-thick slice was prepared from adult male mice and placed in oxygenated aCSF at 32 C for 2 h (to mirror the time of surgery in vivo) before moving the slice to a recording chamber on an Olympus BX51 microscope for cell-attached recordings. In one group of slices, 100 μm pentobarbital was added to the aCSF for the entire 2 h incubation period as well as to the perfusing aCSF throughout the time the slice was in the recording chamber. The other group of slices received normal aCSF throughout. The spontaneous firing rates and patterns of firing were analyzed as noted previously (Constantin et al., 2012b).
Drugs.
Drugs were diluted into aCSF before the experiments at the final concentration indicated. TTX (1 μm), muscimol (20 μm), α-amino-3-hydroxyl-5-methyl-4-isoxazole-propionate (AMPA) (30 μm), and picrotoxin (100 μm) were purchased from Tocris Bioscience. Pentobarbital was purchased from Provet NZ Pty. Kisspeptin-10 (100 nm; Calbiochem) was diluted in DMSO and stored at −20°C. The final DMSO concentration in aCSF was <0.1% (v/v).
Results
Using this ventral surgical procedure, it was possible to make on-cell recordings from AHA GnRH neurons in vivo. For an experienced investigator, this procedure required ∼1.5 h to perform with recordings possible over the subsequent ∼2 h period. Although the surgical success rate was close to 100% and GFP-tagged GnRH neurons were visible in almost every in vivo preparation (Fig. 1C), cell position and depth, as well as overlying meninges, and distance from the pulsating posterior communicating artery (Fig. 1C) defined cell accessibility for recordings. As such, a recording was only possible in ∼15% of mice. Successful recordings were performed from 29 GnRH neurons in 27 mice (n = 22 females; n = 5 males). Of the GnRH neurons visualized in the in vivo preparation, almost invariably, only one cell was recorded per mouse so that n values represent both numbers of animals and cells. The mean duration of recordings was 10.8 ± 1.8 min.
Spontaneous activity of GnRH neurons in vivo
Of 29 GnRH neurons recorded, four were silent and 25 (86%) were spontaneously active, exhibiting a wide range of irregular and tonic firing patterns (Fig. 2A). Using criteria applied to brain slice experiments (Constantin et al., 2012b), ∼15% of GnRH neurons were classified as exhibiting burst firing. The majority of these spontaneously active recordings were made from female mice (n = 10 diestrus; n = 8 estrus) where individual neurons exhibited mean firing frequencies ranging from 0.06 to 3.65 Hz. The firing frequency of the cells was not correlated with the duration of the recording (Spearman's test, r2 = 0.15). The mean firing rates of GnRH neurons recorded from diestrous and estrous mice were 0.60 ± 0.16 and 1.12 ± 0.40 Hz, respectively (Mann–Whitney's test; p = 0.24).
Figure 2.
Firing patterns of GnRH neurons in vivo. A, GnRH neurons displayed a range of firing activity, from irregular (top), tonic (middle), to phasic (bottom). Action currents exist as large predominantly downward deflections. *Occasional unfiltered high-frequency noise can be seen as deflections of equal upward and downward amplitude. B, The local application of TTX, voltage-gated sodium channel blocker, stopped firing. C, Thirty millimolars of KCl evoked a transient burst of activity from a silent GnRH neuron.
The cumulative distribution of interspike interval (ISI) was used to assess firing patterns in diestrous and estrous mice. For each cell, the distribution was fitted using a Boltzmann model, y = f(x) = [(A1 − A2)/(1 + (x/x0)p̂)] + A2), where y = the proportion of ISI falling between 0 and x the maximal ISI, with A1 = 0 (no spike), and A2 = 1 (all spikes). Two parameters were extracted per cell: x0, the ISI value that splits the ISI population in half; and p, the slope of the linear part of the ISI distributed ∼x0. This distribution was fitted well (r2 >0.99) in 7 diestrous and 8 estrous GnRH neurons where it provides something equivalent to the ISI mode (x0) and an index of the regularity of firing pattern (p). The x0 was 528 ± 41 ms in diestrus and 581 ± 192 ms in estrus, indicating the most common firing rate to be ∼2 Hz in both stages of the cycle with, overall, 50% of the ISIs ≤557 ± 102 ms. The p was 1.74 ± 0.12 in diestrus and p = 1.63 ± 0.19 in estrus, indicating a similar patterning of firing in the two animal groups (Mann–Whitney test, p = 0.23 for x0 and p = 0.61 for p). As the GnRH/LH surge is abolished by pentobarbital, we did not examine GnRH neuron firing rates on proestrus.
In the 4 silent GnRH neurons, electrical activity could be evoked by 30 mm KCl (Fig. 2C) or by 30 μm AMPA. In contrast, 1 μm TTX stopped the firing of three active neurons tested, validating that the action potential firing originated from voltage-gated sodium channels (Fig. 2B).
Response to kisspeptin-10
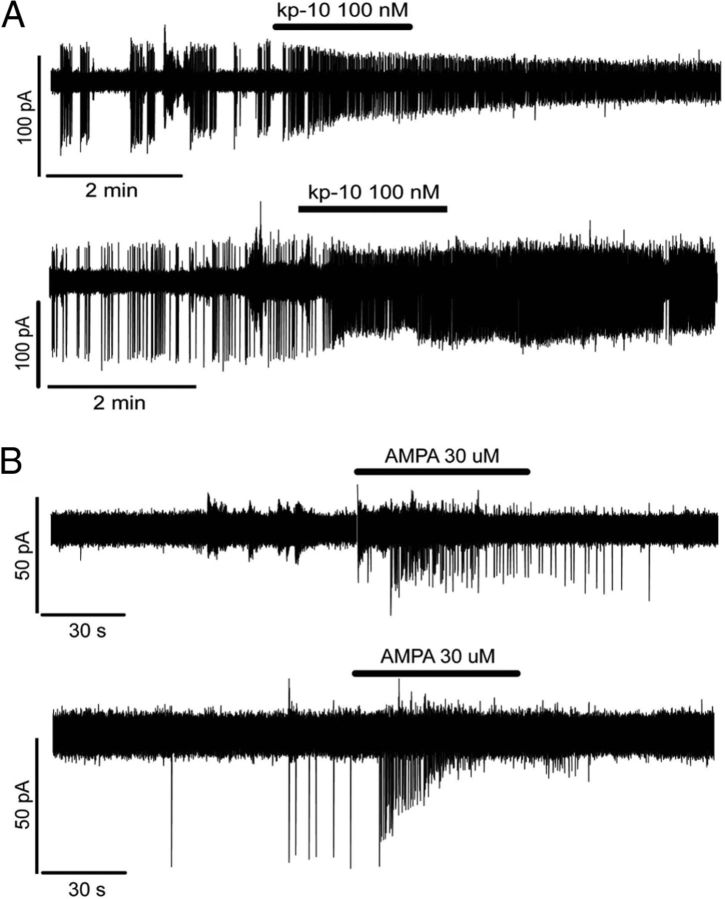
Kisspeptin is the most potent stimulator of GnRH neuronal activity presently known in vitro (Han et al., 2005), and GnRH neurons located in the AHA express the kisspeptin receptor (Herbison et al., 2010). As we expected, GnRH neurons to be activated by kisspeptin, those GnRH neurons that were silent, or those that showed low firing rates were tested with kisspeptin, saving more highly firing cells for other pharmacological experiments. The response of GnRH neurons (4 female, 1 male) to the addition of 100 nm kisspeptin-10 to the perfusing aCSF for 2 min was evaluated in vivo. All cells increased their firing from a mean of 0.77 ± 0.21 to 2.06 ± 0.47 Hz (n = 5; paired t test, p = 0.047). Whereas one cell went from being almost silent to active (26-fold increase), the other 4 showed an ∼3-fold increase (Fig. 3A). These cells displayed enhanced activity until the end of the recording period (Fig. 3A).
Figure 3.
In vivo activation of GnRH neuron firing by kisspeptin-10 (kp-10) and AMPA. A, A 2 min application of kisspeptin-10 (kp-10) evoked a potent and sustained increase in GnRH neuronal firing rate. B, In contrast, a 1 min application of the glutamatergic receptor agonist, AMPA, induced only a transient activation of GnRH neurons.
Response to glutamatergic agonist, AMPA
AMPA reliably activates virtually all GnRH neurons in vitro (Suter, 2004; Constantin et al., 2012b). The response of GnRH neurons to AMPA was evaluated in vivo. Five cells were challenged with a 1 min application of 30 μm AMPA to the aCSF with all increasing their firing frequency from a mean of 0.11 ± 0.06 to 0.33 ± 0.12 Hz (n = 5; paired t test, p = 0.059; Fig. 3B) during the exposure period. In two cells, AMPA was tested twice with the excitation found to be repeatable.
Response to GABAA receptor agonist, muscimol
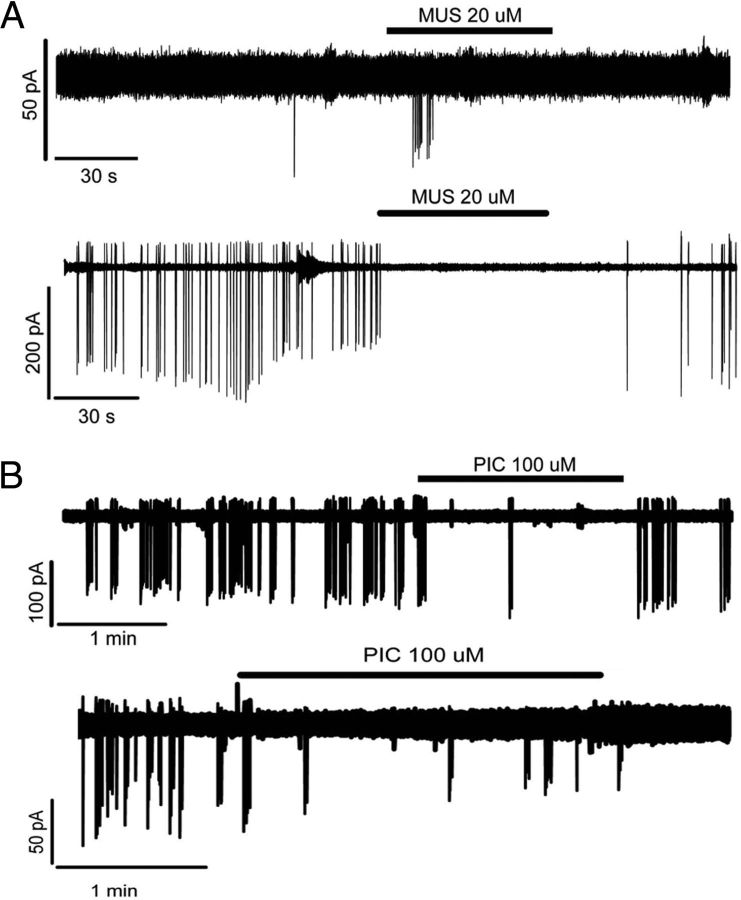
The GABAA receptor agonist, muscimol, can evoke a transient increase in firing from GnRH neurons in vitro (DeFazio et al., 2002; Constantin et al., 2012b). The response of female GnRH neurons to 20 μm muscimol was evaluated in vivo with a 1 min application to the aCSF. Eight cells were tested, and three types of responses were observed independent of estrous cycle stage: 2 silent cells showed a transient repeatable activation (0.00 ± 0.00 Hz to 0.07 ± 0.03 Hz; Fig. 4A), 4 active cells showed an abrupt arrest of firing (1.08 ± 0.41 Hz to 0.10 ± 0.03 Hz; Fig. 4A), whereas the remaining 2 cells (2.51 ± 0.16 Hz) exhibited a transient activation before becoming silent. The differences in basal firing rate or response were not attributable to different levels of anesthesia as heart rate (3.4 ± 0.4 Hz), a reliable index of anesthesia depth (Musizza et al., 2007), showed no correlation with either variable (Spearman's test, r2 = 0.02).
Figure 4.
In vivo modulation of GnRH neuron firing activity by GABAA receptor agonists and antagonists. A, The response to muscimol (MUS), a GABAA receptor agonist, was variable, inducing either a transient activation (top) or a sustained inhibition (bottom) of GnRH neuron firing. B, However, blocking endogenous GABAA receptor signaling with picrotoxin (PIC) was consistently inhibitory.
Response to GABAA receptor antagonist, picrotoxin
The effect of endogenous GABAA receptor signaling on GnRH neurons was examined using a 2 min application of the GABAA receptor antagonist, 100 μm picrotoxin to the aCSF. In contrast to muscimol, the response of spontaneously active GnRH neurons to picrotoxin was consistent, with all 5 cells showing a reduction in firing rate that was reversible in 3 cases (Fig. 4B). Although the ability to exhibit burst firing was not completely abolished, the interburst interval was greatly increased (Fig. 4B). Mean firing rates reduced by >60% from 1.45 ± 0.87 Hz to 0.63 ± 0.50 Hz (Fig. 4B; paired t test, p = 0.020). As for muscimol, no correlation was found between the initial heart rate and magnitude of the inhibition by picrotoxin (Spearman's test, r2 = 0.23).
Effects of pentobarbital on GnRH neuron firing in vitro
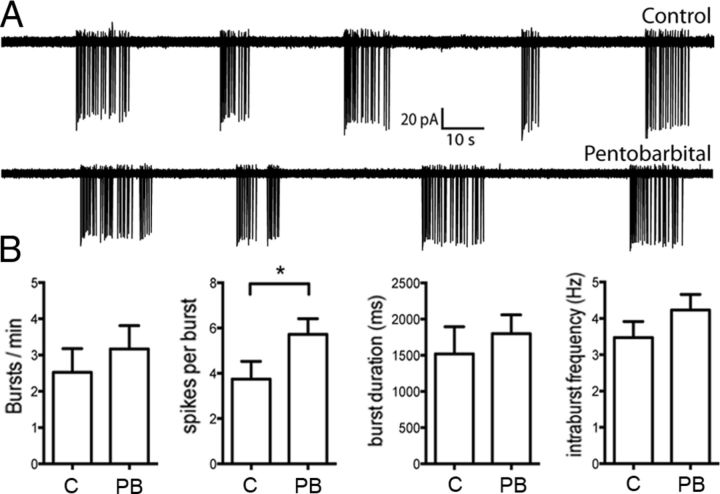
In control brain slices, cell-attached recordings were made from 12 AHA GnRH neurons with 9 cells exhibiting clear burst firing (75%; Fig. 5A), 2 irregular firing (17%), and 1 being silent. In slices incubated in 100 μm pentobarbital, recordings from 20 GnRH neurons found that 15 exhibited burst firing (75%; Fig. 5A), whereas the other 5 cells were silent. The average firing frequency of GnRH neurons from control brain slices was 0.20 ± 0.05 Hz (n = 12) and was not significantly different from pentobarbital-treated slices (0.30 ± 0.008 Hz, n = 20, Mann–Whitney's test, p > 0.05). Analysis of bursting characteristics in control and pentobarbital-treated slices revealed that the number of bursts per minute, burst duration, and intraburst frequency were not different between the two groups (Mann–Whitney's test, p > 0.05, Fig. 5B). We did however find that pentobarbital-treated neurons exhibited significantly more spikes per burst compared with control (control = 3.75 ± 0.78, pentobarbital = 5.73 ± 0.69, Mann–Whitney's test, p = 0.012). Together, these results demonstrate that anesthetic level concentrations of pentobarbital only have a minor impact on the firing characteristics of GnRH neurons in vitro.
Figure 5.
Effects of pentobarbital on GnRH neuron firing activity in vitro. A, Traces showing the firing pattern of GnRH neurons from a control (top) and pentobarbital (bottom)-treated brain slice. B, Summary data (mean ± SEM), illustrating the effect of 100 μm pentobarbital (PB, n = 15) on burst firing characteristics of GnRH neurons. C, Control cells (n = 9). *p < 0.05.
Discussion
By developing a transpharyngeal surgical approach, we provide here the first electrical recordings of GnRH neurons in vivo. This has been made possible by the observation that AHA GnRH neurons are accessible from the ventral surface of the mouse brain. We have recently demonstrated that ∼75% of AHA GnRH neurons likely project to the median eminence and that ∼40% of these cells take part in the induction of the preovulatory GnRH/LH surge (Constantin et al., 2012b). This indicates that caudal-most AHA cells are an appropriate subpopulation of GnRH neurons to investigate. Although the surgical approach proves to be highly effective at visualizing AHA GnRH neurons in vivo, the successful patching of a GnRH neuron is challenging, with only an ∼15% success rate.
Although this procedure enables GnRH neurons to be recorded in vivo, it remains that the ventral approach can only be undertaken in anesthetized animals. In the present study, we used pentobarbital after finding that the neurosteroid-based anesthetic Althesin, reported to maintain the GnRH surge in vivo in rats (Sarkar et al., 1976), does not exist any longer under its initial formulation (alphaxalone/alphadolone; 1:1). A similar formulation Alfaxan (alphaxalone alone) did not provide the duration and the depth of anesthesia sufficient for the ventral surgical procedure in mice. To address the potential impact of pentobarbital on GnRH neurons, we compared the firing rates and patterns of GnRH neurons in the presence and absence of 100 μm pentobarbital in the acute brain slice preparation. We found that the patterns of firing displayed by GnRH neurons were very similar, with the only significant difference being that the number of spikes per burst was enhanced in the presence of pentobarbital. This suggests that the firing patterns and dynamics recorded here from GnRH neurons in vivo are not impacted substantially by pentobarbital anesthesia. Of note, the pentobarbital suppression of pulsatile LH secretion in vivo in rats results from the ensuing hypothermia rather than pentobarbital anesthesia itself (Strutton and Coen, 1996).
Technical advances over recent years have enabled recordings to be made in vivo from neurons located near the dorsal surface of the brain (Murayama and Larkum, 2009; Cheng et al., 2011; Petreanu et al., 2012). We describe here a surgical approach that allows investigators to make electrical recordings from neurons located along the ventral surface of the brain in vivo. The present preparation, adapted from that used to visualize the median eminence and pituitary gland of the mouse (Bonnefont et al., 2005; Lafont et al., 2010), may prove useful for making in vivo electrical recordings from other neuroendocrine and hypothalamic cell populations in GFP reporter mouse lines.
The acute brain slice procedure unavoidably damages the GnRH neuron dendritic tree and severs many of the synaptic inputs to GnRH neurons. The degree to which this damage impacts upon the spontaneous firing patterns of adult GnRH neurons in vitro has been of some concern (Herbison and Moenter, 2011; Constantin et al., 2012b). Interestingly, we observe here that undamaged GnRH neurons in their complete synaptic environment in vivo can also be silent and exhibit irregular or bursting patterns of firing similar to that observed in the acute brain slice (Moenter, 2010). However, this behavior is not exactly the same. For example, whereas 75% of AHA GnRH neurons exhibited clear burst firing in vitro, this was uncommon in vivo (∼15%). In addition, GnRH neurons in vivo display considerably greater heterogeneity in their firing patterns.
Where it has been possible to make the comparison, other neuronal cell populations have also been found to exhibit more heterogeneous firing patterns in vivo compared with brain slice experiments. For example, neocortical neurons exhibit much more variable firing patterns in vivo than in vitro, and this has been attributed to reduced connectivity within the brain slice and consequent increased input resistance (Holt et al., 1996; Pare et al., 1998; Destexhe et al., 2003). Studies of the hypothalamic magnocellular neurons have shown that burst firing is enhanced but more variable in vivo than in vitro (Sabatier et al., 2004) and that the frequency of postsynaptic potentials in these cells is dramatically reduced in vitro compared with in vivo (Bourque and Renaud, 1991; Dyball et al., 1991). These studies make it likely that the less variable firing patterns exhibited by GnRH neurons in vitro reflect the absence of their normal synaptic inputs.
There is wide agreement that kisspeptin is a potent activator of GnRH neurons in the acute brain slice as well as in vivo (Colledge, 2009). We provide here direct evidence for a robust increase in GnRH neuron firing by kisspeptin-10 in vivo. One of the hallmarks of brief kisspeptin exposure in vitro is its ability to activate the GnRH neuron for prolonged periods sometimes lasting over an hour. Interestingly, we find that the prolonged duration of kisspeptin activation of GnRH neurons persists in vivo.
The fast-acting amino acid transmitters play a major role in regulating the activity of GnRH neurons (for review, see Iremonger et al., 2010; Herbison and Moenter, 2011). We show here that, as expected from in vitro studies (Spergel et al., 1999; Suter, 2004; Constantin et al., 2012b), activation of AMPA receptors initiates a rapid increase in firing rate in all GnRH neurons in vivo. The role of GABAA receptor activation in the regulation of GnRH neuron excitability has been more difficult to ascertain, with in vitro studies reporting both stimulatory and inhibitory actions of GABAA receptor agonists on GnRH neurons, whereas in vivo experiments have shown perplexing inhibitory effects of either GABAA receptor activation or antagonism on LH secretion (for review, see Herbison and Moenter, 2011). Furthermore, the near-complete abolition of GABAA receptor signaling at the GnRH neuron was found to have no major effect on fertility in vivo (Lee et al., 2010b). A variety of technical reasons may explain these findings, and it has been suggested that the resolution of the “GABAA receptor controversy” might come from recordings of GnRH neurons in vivo (Herbison and Moenter, 2011).
We found that exogenous GABAA receptor activation has variable effects on GnRH neurons in vivo, with individual cells exhibiting excitation, inhibition, or both responses. The reasons for this range of effects are not clear and may reflect different combinations of direct and indirect GABAA receptor activation, variable resting membrane potentials, or heterogeneous chloride ion homeostasis in GnRH neurons (DeFazio et al., 2002; Leupen et al., 2003). The most important observation, however, comes from antagonist studies where blockade of endogenous GABAA receptor signaling suppressed the electrical activity of all GnRH neurons recorded in vivo, regardless of their prior level or pattern of electrical activity. Although we are not sure of the reasons behind the discordant effects of muscimol (inhibition, excitation, no effect) and picrotoxin (only inhibition), the acute pharmacological activation of all GABAA receptors is very different from the reduction of ongoing endogenous GABAA receptor signaling in vivo. As activation of extrasynaptic GABAA receptors appears to be inhibitory (Bhattarai et al., 2011) while synaptic GABAA receptors are excitatory (Herbison and Moenter, 2011), it is possible that the variable effects of muscimol result from differential activation of synaptic versus extrasynaptic GABAA receptors on GnRH neurons. If tonic extrasynaptic GABAA receptor activation was low in vivo, then picotoxin would predominantly block excitatory synaptic GABAA receptors. As noted above, this possibility excludes indirect actions of these compounds; and, undoubtedly, further in vivo work will be needed to clarify this situation. Regardless of the exact mechanism, however, the in vivo results are clearly different from the situation in vitro where GABAA receptor antagonism has little effect on burst firing or longer rhythms in GnRH neuron activity (Nunemaker et al., 2002; Lee et al., 2012). With all of their afferent inputs intact, GnRH neurons likely receive a different profile of neurotransmitter input, including action potential-dependent GABA inputs along the full length of their dendrite (Hemond et al., 2012).
In conclusion, we report a novel ventral surgical approach that has allowed the spontaneous firing patterns of the caudal-most GnRH neurons to be recorded in vivo in the anesthetized mouse. This approach can likely be adapted to explore the firing of other neuroendocrine and hypothalamic cell populations in vivo. The in vivo recordings exhibit important differences and similarities with data accrued over recent years from in vitro recordings of GnRH neurons. Importantly, they show that caudal GnRH neurons in vivo are, indeed, truly heterogeneous and that the variable firing patterns observed in the brain slice are not simply an artifact of that preparation. This suggests that the GnRH neuron population is one of intermingled, functionally distinct GnRH neurons. It is also evident that the absence of normal synaptic inputs to GnRH neurons in the acute brain slice enables a much more stereotyped pattern of predominantly burst firing that is not common in vivo. A further major difference between in vitro and in vivo recordings has been the observation that ongoing GABAA receptor-mediated neurotransmission is critical to the functional integrity of the GnRH neuron in vivo. Together, these observations underscore the importance of elucidating the firing properties of forebrain neurons in vivo.
Footnotes
This work was supported by New Zealand Health Research Council and by the Department of Physiology. We thank Leonardus Van Rens and James Wood (EMTech Unit, University of Otago) for expert assistance in manufacturing parts of the in vivo rig and Rob Porteous (Department of Physiology) and Dr. Vincent Bennani (Department of Oral Rehabilitation) for technical assistance.
The authors declare no competing financial interests.
References
- Barry J, Dubois MP, Poulain P. LRF producing cells of the mammalian hypothalamus. Z Zellforsch. 1973;146:351–366. doi: 10.1007/BF02346227. [DOI] [PubMed] [Google Scholar]
- Bhattarai JP, Park SA, Park JB, Lee SY, Herbison AE, Ryu PD, Han SK. Tonic extrasynaptic GABA(A) receptor currents control gonadotropin-releasing hormone neuron excitability in the mouse. Endocrinology. 2011;152:1551–1561. doi: 10.1210/en.2010-1191. [DOI] [PubMed] [Google Scholar]
- Bonnefont X, Lacampagne A, Sanchez-Hormigo A, Fino E, Creff A, Mathieu MN, Smallwood S, Carmignac D, Fontanaud P, Travo P, Alonso G, Courtois-Coutry N, Pincus SM, Robinson IC, Mollard P. Revealing the large-scale network organization of growth hormone-secreting cells. Proc Natl Acad Sci U S A. 2005;102:16880–16885. doi: 10.1073/pnas.0508202102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bourque CW, Renaud LP. Membrane properties of rat magnocellular neuroendocrine cells in vivo. Brain Res. 1991;540:349–352. doi: 10.1016/0006-8993(91)90535-4. [DOI] [PubMed] [Google Scholar]
- Cheng A, Gonçalves JT, Golshani P, Arisaka K, Portera-Cailliau C. Simultaneous two-photon calcium imaging at different depths with spatiotemporal multiplexing. Nat Methods. 2011;8:139–142. doi: 10.1038/nmeth.1552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christian CA, Pielecka-Fortuna J, Moenter SM. Estradiol suppresses glutamatergic transmission to gonadotropin-releasing hormone neurons in a model of negative feedback in mice. Biol Reprod. 2009;80:1128–1135. doi: 10.1095/biolreprod.108.075077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colledge WH. Kisspeptins and GnRH neuronal signalling. Trends Endocrinol Metab. 2009;20:115–121. doi: 10.1016/j.tem.2008.10.005. [DOI] [PubMed] [Google Scholar]
- Constantin S, Jasoni C, Romanò N, Lee K, Herbison AE. Understanding calcium homeostasis in postnatal gonadotropin-releasing hormone neurons using cell-specific Pericam transgenics. Cell Calcium. 2012a;51:267–276. doi: 10.1016/j.ceca.2011.11.005. [DOI] [PubMed] [Google Scholar]
- Constantin S, Piet R, Iremonger K, Hwa Yeo S, Clarkson J, Porteous R, Herbison AE. GnRH neuron firing and response to GABA in vitro depend on acute brain slice thickness and orientation. Endocrinology. 2012b;153:3758–3769. doi: 10.1210/en.2012-1126. [DOI] [PubMed] [Google Scholar]
- Crane PD, Braun LD, Cornford EM, Cremer JE, Glass JM, Oldendorf WH. Dose dependent reduction of glucose utilization by pentobarbital in rat brain. Stroke. 1978;9:12–18. doi: 10.1161/01.STR.9.1.12. [DOI] [PubMed] [Google Scholar]
- DeFazio RA, Heger S, Ojeda SR, Moenter SM. Activation of A-type γ-aminobutyric acid receptors excites gonadotropin-releasing hormone neurons. Mol Endocrinol. 2002;16:2872–2891. doi: 10.1210/me.2002-0163. [DOI] [PubMed] [Google Scholar]
- Destexhe A, Rudolph M, Paré D. The high-conductance state of neocortical neurons in vivo. Nat Rev Neurosci. 2003;4:739–751. doi: 10.1038/nrn1198. [DOI] [PubMed] [Google Scholar]
- Du F, Zhang Y, Iltis I, Marjanska M, Zhu XH, Henry PG, Chen W. In vivo proton MRS to quantify anesthetic effects of pentobarbital on cerebral metabolism and brain activity in rat. Magn Reson Med. 2009;62:1385–1393. doi: 10.1002/mrm.22146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dyball RE, Tasker JG, Wuarin JP, Dudek FE. In vivo intracellular recording of neurons in the supraoptic nucleus of the rat hypothalamus. J Neuroendocrinol. 1991;3:383–386. doi: 10.1111/j.1365-2826.1991.tb00291.x. [DOI] [PubMed] [Google Scholar]
- Fueshko S, Wray S. LHRH cells migrate on peripherin fibers in embryonic olfactory explant cultures: an in vitro model for neurophilic neuronal migration. Dev Biol. 1994;166:331–348. doi: 10.1006/dbio.1994.1319. [DOI] [PubMed] [Google Scholar]
- Han SK, Gottsch ML, Lee KJ, Popa SM, Smith JT, Jakawich SK, Clifton DK, Steiner RA, Herbison AE. Activation of gonadotropin-releasing hormone (GnRH) neurons by kisspeptin as a neuroendocrine switch for the onset of puberty. J Neurosci. 2005;25:11349–11356. doi: 10.1523/JNEUROSCI.3328-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hemond PJ, O'Boyle MP, Roberts CB, Delgado-Reyes A, Hemond Z, Suter KJ. Simulated GABA synaptic input and L-type calcium channels form functional microdomains in hypothalamic gonadotropin-releasing hormone neurons. J Neurosci. 2012;32:8756–8766. doi: 10.1523/JNEUROSCI.4188-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herbison AE, Moenter SM. Depolarising and hyperpolarising actions of GABA(A) receptor activation on gonadotrophin-releasing hormone neurones: towards an emerging consensus. J Neuroendocrinol. 2011;23:557–569. doi: 10.1111/j.1365-2826.2011.02145.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herbison AE, de Tassigny Xd, Doran J, Colledge WH. Distribution and postnatal development of Gpr54 gene expression in mouse brain and gonadotropin-releasing hormone neurons. Endocrinology. 2010;151:312–321. doi: 10.1210/en.2009-0552. [DOI] [PubMed] [Google Scholar]
- Holt GR, Softky WR, Koch C, Douglas RJ. Comparison of discharge variability in vitro and in vivo in cat visual cortex neurons. J Neurophysiol. 1996;75:1806–1814. doi: 10.1152/jn.1996.75.5.1806. [DOI] [PubMed] [Google Scholar]
- Iremonger KJ, Constantin S, Liu X, Herbison AE. Glutamate regulation of GnRH neuron excitability. Brain Res. 2010;1364:35–43. doi: 10.1016/j.brainres.2010.08.071. [DOI] [PubMed] [Google Scholar]
- Kawakami M, Uemura T, Hayashi R. Electrophysiological correlates of pulsatile gonadotropin release in rats. Neuroendocrinology. 1982;35:63–67. doi: 10.1159/000123356. [DOI] [PubMed] [Google Scholar]
- Kelly MJ, Wagner EJ. GnRH neurons and episodic bursting activity. Trends Endocrinol Metab. 2002;13:409–410. doi: 10.1016/S1043-2760(02)00698-7. [DOI] [PubMed] [Google Scholar]
- Lafont C, Desarménien MG, Cassou M, Molino F, Lecoq J, Hodson D, Lacampagne A, Mennessier G, El Yandouzi T, Carmignac D, Fontanaud P, Christian H, Coutry N, Fernandez-Fuente M, Charpak S, Le Tissier P, Robinson IC, Mollard P. Cellular in vivo imaging reveals coordinated regulation of pituitary microcirculation and GH cell network function. Proc Natl Acad Sci U S A. 2010;107:4465–4470. doi: 10.1073/pnas.0902599107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee K, Duan W, Sneyd J, Herbison AE. Two slow calcium-activated afterhyperpolarization currents control burst firing dynamics in gonadotropin-releasing hormone neurons. J Neurosci. 2010a;30:6214–6224. doi: 10.1523/JNEUROSCI.6156-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee K, Porteous R, Campbell RE, Lüscher B, Herbison AE. Knockdown of GABA(A) receptor signaling in GnRH neurons has minimal effects upon fertility. Endocrinology. 2010b;151:4428–4436. doi: 10.1210/en.2010-0314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee K, Liu X, Herbison AE. Burst firing in gonadotrophin-releasing hormone neurones does not require ionotrophic GABA or glutamate receptor activation. J Neuroendocrinol. 2012;24:1476–1483. doi: 10.1111/j.1365-2826.2012.02360.x. [DOI] [PubMed] [Google Scholar]
- Leupen SM, Tobet SA, Crowley WF, Jr, Kaila K. Heterogeneous expression of the potassium-chloride cotransporter KCC2 in gonadotropin-releasing hormone neurons of the adult mouse. Endocrinology. 2003;144:3031–3036. doi: 10.1210/en.2002-220995. [DOI] [PubMed] [Google Scholar]
- Moenter SM. Identified GnRH neuron electrophysiology: a decade of study. Brain Res. 2010;1364:10–24. doi: 10.1016/j.brainres.2010.09.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murayama M, Larkum ME. In vivo dendritic calcium imaging with a fiberoptic periscope system. Nat Protoc. 2009;4:1551–1559. doi: 10.1038/nprot.2009.142. [DOI] [PubMed] [Google Scholar]
- Musizza B, Stefanovska A, McClintock PV, Palus M, Petrovcic J, Ribaric S, Bajrovic FF. Interactions between cardiac, respiratory and EEG-δ oscillations in rats during anaesthesia. J Physiol. 2007;580:315–326. doi: 10.1113/jphysiol.2006.126748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nunemaker CS, DeFazio RA, Moenter SM. Estradiol-sensitive afferents modulate long-term episodic firing patterns of GnRH neurons. Endocrinology. 2002;143:2284–2292. doi: 10.1210/en.143.6.2284. [DOI] [PubMed] [Google Scholar]
- O'Byrne KT, Knobil E. Electrophysiological approaches to gonadotrophin releasing hormone pulse generator activity in the rhesus monkey. Hum Reprod. 1993;8(Suppl 2):37–40. doi: 10.1093/humrep/8.suppl_2.37. [DOI] [PubMed] [Google Scholar]
- Paré D, Shink E, Gaudreau H, Destexhe A, Lang EJ. Impact of spontaneous synaptic activity on the resting properties of cat neocortical pyramidal neurons in vivo. J Neurophysiol. 1998;79:1450–1460. doi: 10.1152/jn.1998.79.3.1450. [DOI] [PubMed] [Google Scholar]
- Petreanu L, Gutnisky DA, Huber D, Xu NL, O'Connor DH, Tian L, Looger L, Svoboda K. Activity in motor-sensory projections reveals distributed coding in somatosensation. Nature. 2012;489:299–303. doi: 10.1038/nature11321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sabatier N, Brown CH, Ludwig M, Leng G. Phasic spike patterning in rat supraoptic neurones in vivo and in vitro. J Physiol. 2004;558:161–180. doi: 10.1113/jphysiol.2004.063982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarkar DK, Chiappa SA, Fink G, Sherwood NM. Gonadotropin-releasing hormone surge in pro-oestrous rats. Nature. 1976;264:461–463. doi: 10.1038/264461a0. [DOI] [PubMed] [Google Scholar]
- Sato S, Koshiro A, Kakemi M, Fukasawa Y, Katayama K, Koizumi T. Pharmacokinetic and pharmacodynamic studies of centrally acting drugs in rat: effect of pentobarbital and chlorpromazine on electroencephalogram in rat. Biol Pharm Bull. 1995;18:1094–1103. doi: 10.1248/bpb.18.1094. [DOI] [PubMed] [Google Scholar]
- Schally AV, Arimura A, Baba Y, Nair RM, Matsuo H, Redding TW, Debeljuk L. Isolation and properties of the FSH and LH-releasing hormone. Biochem Biophys Res Commun. 1971;43:393–399. doi: 10.1016/0006-291X(71)90766-2. [DOI] [PubMed] [Google Scholar]
- Spergel DJ, Krüth U, Hanley DF, Sprengel R, Seeburg PH. GABA-and glutamate-activated channels in green fluorescent protein-tagged gonadotropin-releasing hormone neurone in transgenic mice. J Neurosci. 1999;19:2037–2050. doi: 10.1523/JNEUROSCI.19-06-02037.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strutton PH, Coen CW. Sodium pentobarbitone and the suppression of luteinizing hormone pulses in the female rat: the role of hypothermia. J Neuroendocrinol. 1996;8:941–946. doi: 10.1111/j.1365-2826.1996.tb00825.x. [DOI] [PubMed] [Google Scholar]
- Suter KJ. Control of firing by small (S)-alpha-amino-3-hydroxy-5-methyl-isoxazolepropionic acid-like inputs in hypothalamic gonadotropin releasing-hormone (GnRH) neurons. Neuroscience. 2004;128:443–450. doi: 10.1016/j.neuroscience.2004.06.044. [DOI] [PubMed] [Google Scholar]
- Terasawa E, Quanbeck CD, Schulz CA, Burich AJ, Luchansky LL, Claude P. A primary cell culture system of luteinizing hormone releasing hormone neurons derived from embryonic olfactory placode in the rhesus monkey. Endocrinology. 1993;133:2379–2390. doi: 10.1210/en.133.5.2379. [DOI] [PubMed] [Google Scholar]
- Weiner RI, Wetsel W, Goldsmith P, Martinez de la Escalera G, Windle J, Padula C, Choi A, Negro-Vilar A, Mellon P. Gonadotropin-releasing hormone neuronal cell lines. Front Neuroendocrinol. 1992;13:95–119. [PubMed] [Google Scholar]