Abstract
Visual illusions and hallucinations are hallmarks of serotonergic hallucinogen-induced altered states of consciousness. Although the serotonergic hallucinogen psilocybin activates multiple serotonin (5-HT) receptors, recent evidence suggests that activation of 5-HT2A receptors may lead to the formation of visual hallucinations by increasing cortical excitability and altering visual-evoked cortical responses. To address this hypothesis, we assessed the effects of psilocybin (215 μg/kg vs placebo) on both α oscillations that regulate cortical excitability and early visual-evoked P1 and N170 potentials in healthy human subjects. To further disentangle the specific contributions of 5-HT2A receptors, subjects were additionally pretreated with the preferential 5-HT2A receptor antagonist ketanserin (50 mg vs placebo). We found that psilocybin strongly decreased prestimulus parieto-occipital α power values, thus precluding a subsequent stimulus-induced α power decrease. Furthermore, psilocybin strongly decreased N170 potentials associated with the appearance of visual perceptual alterations, including visual hallucinations. All of these effects were blocked by pretreatment with the 5-HT2A antagonist ketanserin, indicating that activation of 5-HT2A receptors by psilocybin profoundly modulates the neurophysiological and phenomenological indices of visual processing. Specifically, activation of 5-HT2A receptors may induce a processing mode in which stimulus-driven cortical excitation is overwhelmed by spontaneous neuronal excitation through the modulation of α oscillations. Furthermore, the observed reduction of N170 visual-evoked potentials may be a key mechanism underlying 5-HT2A receptor-mediated visual hallucinations. This change in N170 potentials may be important not only for psilocybin-induced states but also for understanding acute hallucinatory states seen in psychiatric disorders, such as schizophrenia and Parkinson's disease.
Introduction
Recent evidence from molecular, pharmacological, and neuroimaging studies suggests a crucial role for serotonin 2A (5-HT2A) receptors in visual processing and the pathogenesis of visual hallucinations. 5-HT2A receptors are highly expressed in the visual cortex (Gerstl et al., 2008; Watakabe et al., 2009; Moreau et al., 2010) and alterations in their cortical density in both Parkinson's disease (Ballanger et al., 2010; Huot et al., 2010) and schizophrenia (González-Maeso et al., 2008) are associated with the appearance of visual hallucinations. Furthermore, activation of 5-HT2A receptors predominantly mediates the visual hallucinations that are induced by serotonergic hallucinogens, such as psilocybin and LSD (Nichols, 2004; Vollenweider and Kometer, 2010), whereas the 5-HT2A receptor inverse agonist pimavanserin is effective for treatment of visual hallucinations in Parkinson's disease (Meltzer et al., 2010). However, despite the increasingly recognized significance of 5-HT2A receptors in visual perception, the effects of 5-HT2A receptor activation on neurophysiological mechanisms that underlie visual processing and hallucinations remain largely unknown. Therefore, we assessed the effects of 5-HT2A receptor activation on the prestimulus oscillatory state of the visual parieto-occipital network and the subsequent visual stimulus-induced activity. Furthermore, we asked whether these effects are related to the formation of visual hallucinations.
The first evidence to suggest that activation of 5-HT2A receptors increases the excitability of the visual cortex in the absence of externally presented visual stimuli came recently from animal studies (Moreau et al., 2010). In addition, computational models propose that increasing the excitability state of the visual cortex may destabilize spontaneous network activity, which in turn might produce elementary visual hallucinations (Ermentrout and Cowan, 1979; Bressloff et al., 2002; Gutkin et al., 2003; Butler et al., 2012). Parieto-occipital α oscillations are crucial for modulation of visual network excitability, and thereby strongly influence visual perception (Thut et al., 2006; Romei et al., 2008a; Jensen and Mazaheri, 2010; Klimesch, 2011; Mathewson et al., 2011) and visual-evoked potentials (Gruber et al., 2005; Klimesch, 2011; Rajagovindan and Ding, 2011). Therefore, we hypothesized that activating 5-HT2A receptors with psilocybin may modulate α oscillations, which could lead to an altered excitability that would promote the formation of visual hallucinations.
In addition, activation of 5-HT2A receptors may modulate the visual cortex response to external stimuli that are indexed by visual-evoked potentials. In support of this view, the unselective 5-HT2A receptor agonist psilocybin increases medial P1 visual-evoked potentials (Kometer et al., 2011), which reflect early visual gain (Rajagovindan and Ding, 2011). Furthermore, psilocybin-induced visual hallucinations are associated with decreased N1/N170 potentials (Kometer et al., 2011) that underlie mid-level visual organization processes (Herrmann and Bosch, 2001). 5-HT2A receptors likely mediate this psilocybin-induced decrease in N170 potentials because activation of 5-HT2A receptors predominantly mediates psilocybin-induced visual hallucinations (Vollenweider et al., 1998; Vollenweider and Kometer, 2010). However, psilocybin also activates 5-HT1A and 2C receptors (Nichols, 2004), and 5-HT1A receptors are also expressed in the visual cortex (Dyck and Cynader, 1993; Gerstl et al., 2008; Watakabe et al., 2009). Therefore, the specific effects of 5-HT2A receptors activation on visual-evoked potentials remain unknown.
Materials and Methods
Subjects
Seventeen subjects were enrolled. All subjects were deemed healthy through physical examinations, which included detailed blood analyses and electrocardiography. The Mini-International Neuropsychiatric Interview, MINI-SCID (Sheehan et al., 1998), the DIA-X diagnostic expert system (Wittchen and Pfister, 1997), and the Hopkins Symptom Checklist SCL-90-R (Derogatis, 1994) were used to exclude subjects with present or previous psychiatric disorders or a history of major psychiatric disorders in first-degree relatives. Urine drug screens and self-report drug use questionnaires were used to verify the absence of substance dependence.
Subjects received written and oral descriptions of the study procedures as well as the effects and possible risks of psilocybin administration. Subjects provided written informed consent before participation in the study. One of the 17 subjects was excluded for dizziness after the combined administration of psilocybin and ketanserin. The data from another subject were excluded from analyses because the majority of the trials were contaminated by artifacts. A total of 15 subjects were included in the statistical analyses (11 males, 4 females; mean age, 26.6 ± 4.25 years).
Substance and dosing
A double-blind, placebo-controlled, within-subject, randomized design was used. Each subject received on four different experimental days that were separated by at least 2 weeks with four different drug combinations: placebo and ketanserin (50 mg) (pretreatment) followed by placebo and psilocybin (215 μg/kg) (treatment) after 1 h. These specific doses were chosen because they have previously been shown to induce and block the associated changes in conscious states (Carter et al., 2005). The visual stimulation experiments began 90 min after treatment during the known plateau of the peak subjective psilocybin effects (Studerus et al., 2011). The self-report questionnaires were completed 360 min after treatment to retrospectively rate the subjective experience after drug intake.
The Swiss Federal Office of Public Health, Department of Pharmacology and Narcotics, Bern, Switzerland authorized the use of psilocybin in humans. The ethics committee of the University Hospital of Psychiatry, Zurich approved the study.
Self-report questionnaire
The Altered States of Consciousness (5D-ASC) questionnaire (Dittrich, 1998) was used to quantify the subjective psychological effects of drug administration, with a primary focus placed on visual alterations and hallucinations. The 5D-ASC is a visual analog scale of 94 items that measure etiology-independent alterations in consciousness, including changes in perception, mood, cognition, and experience of self and environment. The 5D-ASC comprises five main scales (Dittrich, 1998): Visionary restructuralization, measuring visual illusions and both elementary and complex hallucinations; Oceanic boundlessness, measuring derealization and depersonalization accompanied by an altered sense of time and by positive mood states; Anxious ego dissolution, measuring ego disintegration associated with loss of self-control, thought disorder, arousal, and anxiety; Auditory alterations, comprising auditory illusions and hallucinations; and Reduction of vigilance, measuring changes in vigilance and alertness. In addition, 11 lower-order subscales were recently extracted by confirmatory factor analysis (Studerus et al., 2010). These were here used for a detailed exploratory analysis. The 11 subscales are as follows: Elementary imagery, Complex imagery, Audio-visual synestesiae, Experience of unity, Spiritual experience, Blissful state, Insightfulness, Changed meaning of percepts, Disembodiment, Impaired control and cognition, and Anxiety.
For the purpose of the present study, subscales quantifying visual perceptual alterations were of particular interest. These included the main scale Visionary restructuralization and the 3 lower-order subscales: Elementary imagery, measuring visual hallucinations of regular patterns, colors, or light flashes; Complex imagery, quantifying visual hallucinations of scenes and pictures; and Audio-visual synesthesiae, measuring visual perceptual alterations that are driven by acoustic noise or sound.
Stimulus and procedure
Subjects remained fixated on a central cross during visual stimulus presentations. All stimuli included three “pacman” figures, which were black circles with a 60° section removed from each circle. The removed sectors were either aligned to induce the perception of an illusory triangle (“Kanizsa” condition) or rotated 180° to an alignment that no longer induced the illusory triangle perception (“non-Kanizsa” condition) (Kometer et al., 2011). The visual angle of the stimuli was 5.4° instead of the previously described 3.7° (Kometer et al., 2011) because increases in eccentricity induce higher P1 amplitudes (Murray et al., 2002; Busch et al., 2004). To reduce habituation, the stimuli were randomly presented in four orientations: rotated 0°, 90°, 180°, and 270°. In total, 88 Kanizsa figures and 88 non-Kanizsa figures were presented in a randomized order with a 3 s stimulus onset asynchrony. Participants responded by pressing a button as quickly as possible when they perceived an illusory triangle. Stimuli remained on the monitor for 300 ms after a button press or for a maximum of 2000 ms.
EEG recording
Continuous electroencephalograms (EEGs) were acquired from 64 Ag-AgCl electrodes using the BioSemi ActiveTwo electrode system (BioSemi). Additional electrodes were attached to the outer canthus of each eye and both infraorbitally and supraorbitally to the left eye to record the horizontal and vertical electroocculograms, respectively. Electrophysiological signals were bandpass filtered online between 0.01 and 67 Hz and digitized with a sampling rate of 512 Hz.
EEG analysis
Preprocessing.
The EEG data were recalculated offline against the common average reference, and bad channels (<2%) were interpolated using spherical splines (Perrin et al., 1989). EEG data were epoched from 800 ms before to 600 ms after stimulus onset. To avoid eye movements, periods of high muscle activity, and other noise transients, an automatic artifact rejection excluded epochs in which voltage values of the raw unfiltered data exceeded ± 120 μV in four or more electrodes. The remaining epochs were screened manually to reject epochs with residual eye movement artifacts.
Time-frequency analysis.
To calculate phase and power at 1 Hz frequency steps between 4 and 100 Hz, signals x(t) from each trial were convolved with a family of Morlet wavelets, w(t, fo) = A exp(− t2/2σt2)exp(2iπfot), where fo is the central frequency, t is the time, and i is the imaginary part (i = ). Wavelets were normalized using the factor A(w) = (σt )−1/2. The family ratio, m = fo/σf = 5, was used, where σf is the width of the Gaussian shape in the frequency domain. This ratio was used to optimize the trade-off between the temporal and frequency resolutions of the wavelet convolution.
The power for each trial was computed as the sum of the squares of the real and the imaginary Morlet wavelet components, which was subsequently averaged over single trials separately for all conditions. To quantify stimulus-induced activity (e.g., the change of α power that is induced by the presentation of the visual stimulus), poststimulus power values were baseline-corrected by subtracting the mean power observed during the −400 to −200 ms prestimulus interval (Snyder and Foxe, 2010; Banerjee et al., 2011; Scheeringa et al., 2011; Waldhauser et al., 2012).
The extent of normalized phase variability across trials for each condition was quantified using the phase-locking value (also called intertrial coherence or phase-locking factor) at each time point, t, and frequency, fo. This procedure yielded a phase-locking value measure that ranged from 0 to 1. A value of 0 indicated completely randomized phases across different trials at a specific time point, t, and a value of 1 indicated a consistent phase across trials.
Event-related potential (ERP) analysis.
After bandpass filtering (0.5–40 Hz) of the preprocessed data (see above), ERPs were computed by averaging separately all trials for each condition and subject. Subsequently, the P1 and N170 amplitudes were quantified against the 200 ms baseline as the mean amplitudes observed during a 40 ms time frame that was centered on the time point corresponding to the peak maximum. This resulted in a time frame from 80 to 120 ms for the P1 and 150–190 ms for the N170 amplitudes.
Statistical analysis
Three parieto-occipital ROIs were selected for the statistical analyses of ERPs, α power, and phase values. These three ROIs included the following electrodes: parietal left (P3, P5, P7, PO3, PO7), parietal medial (POz, Oz, O1, O2, Iz), and parietal right (P4, P6, P8, PO4, PO8). These ROIs have previously been used to quantify α power and phase values (Zanto et al., 2011) and covered the maximal P1 and N170 amplitudes in the current study.
Only the time frame between −600 and 400 ms was considered in the analyses of power and phase values to ensure that edge effects did not contaminate the statistical analyses (Cohen et al., 2007). Prestimulus α (8–12 Hz) power was collapsed over the −600 to −200 ms time frame. Stimulus-induced α power was collapsed into two time frames (0–200 ms and 200–400 ms) because visual inspection of the data indicated that there was a stimulus-induced increase during the first time frame (0–200 ms), but a stimulus-induced decrease during the second time frame (200–400 ms).
All dependent variables were subjected to repeated-measures ANOVA (specific factors are defined in Results), and the Greenhouse-Geisser correction was applied. Bonferroni-corrected post hoc comparisons were performed based on significant main effects and interactions. Pearson's product moment correlations were computed to assess hypothesis-driven relationships between physiological parameters and self-reported visual perceptual alterations. First, the relationship between psilocybin-induced decreases of N170 potentials and perceptual alterations was replicated (Kometer et al., 2011) and specified. Second, whether increased excitability that is indexed by decreased α oscillations (Thut et al., 2006; Romei et al., 2008a; Jensen and Mazaheri, 2010; Klimesch, 2011; Mathewson et al., 2011) resulted in elementary hallucinations (Ermentrout and Cowan, 1979; Bressloff et al., 2002; Gutkin et al., 2003; Butler et al., 2012) was tested by correlating α power values with the elementary hallucination subscale of the 5D-ASC. Finally, psilocybin-induced changes in prestimulus α power values were correlated with the psilocybin-induced changes in P1 potentials because prestimulus α power values are closely associated with P1 potentials (Klimesch, 2011; Rajagovindan and Ding, 2011).
Results
Behavioral data
Psychometrics
A repeated-measures ANOVA on the three primary dimensions of the 5D-ASC rating scale using pretreatment, treatment, and dimensions as within factors revealed a main effect of treatment (F(1,14) = 75.85, p < 0.000001) and a treatment × pretreatment interaction (F(1,14) = 85.05, p < 0.000001). Bonferroni-corrected post hoc analyses of this interaction indicated that treatment with psilocybin generally increased 5D-ASC scores when administered after placebo pretreatment (p < 0.0000001), but not after ketanserin pretreatment (p = 1). The triple interaction between pretreatment × treatment × factor (F(2,28) = 30.62, p < 0.00001) demonstrated a similar significant relationship for the Visionary restructuralization scale. Bonferroni-corrected post hoc analysis indicated that the strong psilocybin-induced increase in the Visionary restructuralization scores (p < 0.0001) was absent after ketanserin pretreatment (p = 1). The scores of the recently formed 11 subscales (see Materials and Methods) were submitted to separate repeated-measures ANOVAs. The triple interaction between treatment × pretreatment × factor (F(10,140) = 9.44, p < 0.00001) indicated that psilocybin robustly induced several visual perceptual alterations after placebo pretreatment, including complex visual hallucinations of scenes and pictures (p < 0.0000001), visual elementary hallucinations of regular patterns, colors, light and light-flashes (p < 0.0000001), and an alteration of visual percepts by auditory stimuli (p < 0.0001). However, no subscales were altered after ketanserin pretreatment (all p values = 1).
Reaction times (RT) and error rates
Neither RTs for correct responses nor error rates were significantly modulated by psilocybin treatment (RTs: F(1,14) = 2.16, p = 0.16, error rates: F(1,14) = 0.21, p = 0.65) or ketanserin pretreatment (RTs: F(1,14) = 0.19, p = 0.090, error rates: F(1,14) = 3.73, p = 0.074). Although an interaction between treatment and stimulus (F(1,14) = 5.124, p < 0.05) indicated that psilocybin increased the error rates for Kanizsa stimuli (p < 0.05) but not those for non-Kanizsa stimuli (p = 1), the error rates remained very low under all conditions (between 1.1% and 3.4%). Therefore, we are confident that participants could do the task under all drug conditions.
ERP analyses
P1
A repeated-measures ANOVA on P1 amplitudes (using pretreatment, treatment, stimulus, and ROI as within factors) revealed a significant interaction between treatment and ROI (F(2,28) = 8.15, p < 0.01) and between pretreatment and ROI (F(2,28) = 19.88, p < 0.0001). Post hoc analyses of these interactions indicated that psilocybin treatment selectively increased P1 amplitudes over the medial (p < 0.05), but not the lateral, parieto-occipital ROIs. By contrast, P1 amplitudes over the medial parieto-occipital ROI were selectively decreased by ketanserin pretreatment (p < 0.00001) (Figs. 1 and 2).
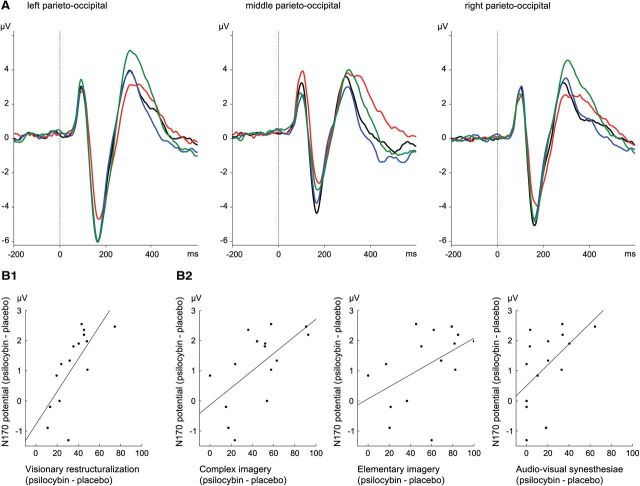
Figure 1.
A, Effects of psilocybin and ketanserin on group-averaged event-related potential waveforms for the three parieto-occipital ROIs. Placebo + placebo (black), placebo + psilocybin (red), ketanserin + placebo (blue), and ketanserin + psilocybin (green). B, Correlation between the psilocybin-induced reduction of N170 amplitudes and the psilocybin-induced perceptual visual alterations as measured by the main scales (B1) and subscales (B2) of the 5D-ASC questionnaire.
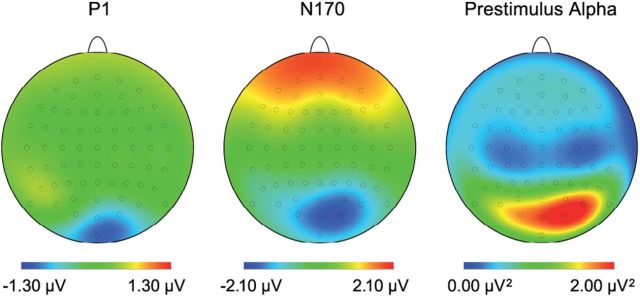
Figure 2.
The scalp topographic maps depict the difference between the placebo + placebo condition and the placebo + psilocybin condition in the P1 amplitude (80 to 120 ms), in the N170 amplitude (150 to 190 ms) and in the prestimulus α power values (−600 to −200 ms).
N170
A repeated-measures ANOVA on N170 amplitudes (using pretreatment, treatment, stimulus, and ROI as within factors) revealed a significant main effect of treatment (F(1,14) = 5.34, p < 0.05), which replicated a previous report of psilocybin-induced decrease of N170 amplitudes (Kometer et al., 2011). In addition, the significant interaction between treatment and pretreatment (F(1,14) = 7.55, p < 0.05) and the subsequent Bonferroni-corrected post hoc analyses indicated that psilocybin treatment decreased the N170 component after placebo (p < 0.01) but not after ketanserin pretreatment (p = 1) (Fig. 1). Correlation analysis with the original main five factors of the 5D-ASC revealed that this psilocybin-induced N170 decrease correlated only with the psilocybin-induced increase in Visionary restructuralization (r = 0.73, p < 0.01), but not with Auditory alteration (r = 0.37, p = 0.169), Oceanic boundlessness (r = 0.40, p = 0.135), Anxious ego dissolution (r = 0.07, p = 0.801), or the Reduction of vigilance (r = 0.35, p = 0.203). Thus, the N170 decrease seems to be selectively associated with visual perceptual alterations. In support of this view, correlation analysis between the 11 newly constructed lower-order subscales of the 5D-ASC scales and the N170 decrease revealed that only the three subscales comprising visual perceptual alterations were associated with the N170 decrease (Complex imagery, r = 0.61, p < 0.05; Elementary imagery, r = 0.49, p = 0.067; Audiovisual synesthesiae, r = 0.54, p < 0.05) (Fig. 1).
Time-frequency analyses
Prestimulus α power
The α power values (8–12 Hz) observed during the prestimulus time frame (−600 to −200 ms) were subjected to a repeated-measures ANOVA (using pretreatment, treatment, stimulus, and ROI as within factors). A main effect of ROI (F(2,28) = 3.66, p < 0.05) indicated that the α power values were most pronounced over the right parieto-occipital region. Psilocybin strongly decreased the prestimulus α power values (F(1,14) = 10.05, p < 0.01). This decrease was dependent on the pretreatment condition (F(1,14) = 8.55, p < 0.05) as the psilocybin-induced decrease in α power values was significant after pretreatment with placebo (p < 0.01) but not ketanserin (p = 1) (Fig. 3). Correlational analyses revealed that the psilocybin-induced decrease in α power values correlated significantly with the psilocybin-induced increase in the medial P1 potential (r = −0.634, p < 0.05).
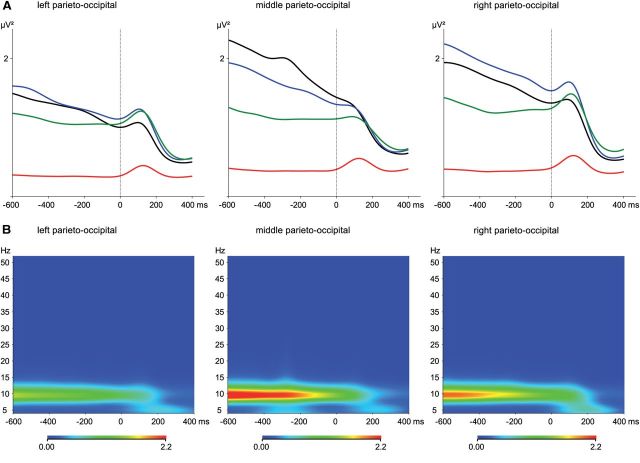
Figure 3.
Effects of psilocybin treatment and ketanserin pretreatment on α power values in three parieto-occipital ROIs. A, Time course for α power values (8–12 Hz) as a function of placebo + placebo (black), placebo + psilocybin (red), ketanserin + placebo (blue), and ketanserin + psilocybin (green). B, Time course for the difference in power values between placebo + psilocybin and placebo + placebo for all frequencies (4–52 Hz).
Stimulus-induced α power
A repeated-measures ANOVA on the stimulus-induced α power values (using pretreatment, treatment, stimulus, time, and ROI as within factors) revealed a main effect of time (F(1,14) = 32.80, p < 0.0001), indicating a decrease in α power from baseline level, during the 200–400 ms time frame (Fig. 3). The significant interaction between time × treatment (F(1,14) = 16.58, p < 0.01) revealed that this stimulus-induced α power decrease was reduced by psilocybin, particularly during the 200–400 ms time frame (p < 0.0000001). The three-way interaction between treatment × pretreatment × time (F(1,14) = 7.24, p < 0.05) further indicated that the reduction of stimulus-induced α power decreases by psilocybin during the 200–400 ms time frame was reversed by ketanserin pretreatment, as the stimulus-induced decrease of α power was significantly higher in the ketanserin + psilocybin condition than in the placebo + psilocybin condition (p < 0.0001).
Relationship between prestimulus and stimulus-induced α power
Because prestimulus α power values were strongly reduced by psilocybin administration, it is conceivable that this reduction was responsible for the subsequent lack of stimulus-induced α power decrease in the psilocybin condition. To test this hypothesis, we restricted the analysis of the stimulus-induced α power to the small subsets of trials that had low prestimulus α power values equal to those in the placebo + psilocybin condition. Thus, from all pharmacological conditions, only trials with a prestimulus power value between 0 and 0.5 μV2 were subjected to a repeated-measures ANOVA using pretreatment, treatment, time, and ROI as within factors. In contrast to the analysis, including all trials, this analysis did not reveal a significant interaction between time and treatment (F(1,14) = 0.09, p = 0.76) or between time × treatment × pretreatment (F(1,14) = 1.02, p = 0.33), which indicates that the lack of stimulus-induced α power decrease is a consequence of the strong psilocybin-induced decrease in prestimulus α power.
Phase-Locking Index (PLI)
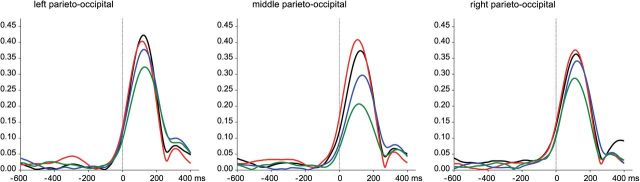
A repeated-measures ANOVA (using pretreatment, treatment, time, stimulus, and ROI as within factors) on the PLI revealed a main effect of time (F(1,14) = 30.74, p < 0.0001), indicating particularly high PLI values during the 0–200 ms time frame. The significant interaction between pretreatment and time (F(1,14) = 37.60, p < 0.0001) indicated that ketanserin pretreatment decreased PLI during the 0–200 ms time frame (p < 0.0001) but not during the 200–400 ms time frame (p = 0.439). This decrease was most pronounced over the medial parieto-occipital ROI (p < 0.0000001), followed by the left (p < 0.001), and then the right parieto-occipital ROIs (p < 0.01; pretreatment × time × ROI: F(2,28) = 5.56, p < 0.05) (Fig. 4).
Figure 4.
Effects of psilocybin and ketanserin on the phase-locking index for the three parieto-occipital ROIs. Placebo + placebo (black), placebo + psilocybin (red), ketanserin + placebo (blue), and ketanserin + psilocybin (green).
Discussion
The data presented in the current study demonstrate that psilocybin strongly decreased prestimulus α power values and, consequently, precluded the subsequent stimulus-induced α power decrease. Moreover, psilocybin decreased N170 visual-evoked potentials that were associated with the appearance of visual hallucinations. These psilocybin-induced effects were normalized by pretreatment with the 5-HT2A receptor antagonist ketanserin, indicating that activation of 5-HT2A receptors is crucial for mediating these effects.
Alpha oscillations (8–12 Hz) have been identified as major neural mechanisms that regulate the excitability levels of cortical sensory networks through inhibition (Thut et al., 2006; Romei et al., 2008b; Klimesch, 2011). In the current study, high parieto-occipital α power levels were observed with placebo conditions during the prestimulus time range, which suggests that a high level of inhibition reduced the excitability of the visual network in the absence of task-relevant visual inputs (Klimesch, 2011; Palva and Palva, 2011). This high level of prestimulus α power was strongly attenuated by psilocybin. Furthermore, this effect of psilocybin was in turn reversed by ketanserin pretreatment, suggesting that activation of 5-HT2A receptors increases the excitability of the visual network in the absence of externally presented stimuli by decreasing ongoing α oscillation. As a consequence of this strong α power decrease during the prestimulus time-frame, the generation of the subsequent stimulus-induced decrease in α power was precluded, indicating a lack of a stimulus-induced increase in excitability (Hanslmayr et al., 2009; Klimesch, 2011). Thus, the high excitability levels in the absence of externally presented stimuli may have restricted the capacity of the visual system to increase, by a modulation of α power, the excitability for processing incoming stimuli.
Together, the results indicate that 5-HT2A receptor activation induces primarily by attenuating ongoing α oscillation a dysbalance between the excitability observed in the absence of an external stimulus and the excitability that is induced by the presentation of the stimulus. The dysbalance may reflect a shift away from external stimulus-driven toward internal-driven information processing and therefore may be a determining factor for the formation of visual hallucinations. In line with this view, several computational models postulate that an increase in the excitability of the visual network in the absence of visual input destabilizes spontaneous neuronal activity, which results in the formation of elementary visual hallucinations (Ermentrout and Cowan, 1979; Bressloff et al., 2002; Gutkin et al., 2003; Butler et al., 2012) that resemble the visual hallucinations produced by classic hallucinogens (Klüver, 1966; Siegel et al., 1975). In addition, increased stimulus-independent excitability that is associated with low α power values is implicated in TMS-induced phosphenes because the likelihood of experiencing these percepts is higher during low α power values relative to high α power values (Romei et al., 2008b). Psilocybin both strongly increased excitability in the absence of externally presented stimuli by decreasing α power values and robustly induced elementary hallucinations in almost all of the subjects. Together, these results suggest that these α effects may be involved in the formation of visual hallucinations. However, the correlation between the psilocybin-induced decrease in α power values and the subject-reported intensity of visual hallucinations did not reach statistical significance, indicating that the α power decrease induced by psilocybin may not be sufficient to generate a subjectively experienced visual hallucination.
The strong effects of activation of 5-HT2A receptors on α power values found in this study are consistent with the known anatomical and functional properties of 5-HT2A receptors in the visual cortex of animals. The 5-HT2A receptors in the visual cortex are primarily expressed in layers IV-VI (Watakabe et al., 2009; Moreau et al., 2010), which are crucially implicated in the generation of α rhythms (Steriade et al., 1990; Bollimunta et al., 2008, 2011). Furthermore, activation of 5-HT2A receptors in the visual cortex alters the excitatory-inhibitory balance toward excitation of neurons in layer V (Moreau et al., 2010). Excitatory synaptic inputs in layer V modulate α oscillations (Jones et al., 2000; Sun and Dan, 2009), which suggests that 5-HT2A receptor-induced excitatory effects observed in previous animal studies in layer V are closely associated with the modulation of α oscillations. Finally, assuming that the presentation of external stimuli predominantly increases the firing rate of visual neurons from their prestimulus level (Quiroga et al., 2005; Montemurro et al., 2008), the opposing effects of 5-HT2A receptors on the excitability during the absence of sensory stimuli and in response to sensory stimuli are consistent with both the suppression effects of 5-HT2A receptor activation on visual neurons with high firing rates and the facilitation effects on neurons with low firing rate (Watakabe et al., 2009).
In addition to the profound effects of activation of 5-HT2A receptors on α oscillations, we also found that activation of 5-HT2A receptors strongly modulated the visual cortex response that is indexed by visual-evoked P1 and N170 potentials. Specifically, psilocybin increased the medial P1 potential, and the preferential 5-HT2A receptor antagonist ketanserin decreased the P1 potential at the same electrode sites. These results suggest opposing influences of 5-HT2A agonism and antagonism on medial P1 potentials. Furthermore, these opposing effects seem to be associated with either α power values or phase locking, which supports previous findings that P1 potentials are related to α oscillations (Gruber et al., 2005; Fellinger et al., 2011; Klimesch, 2011; Rajagovindan and Ding, 2011). Specifically, the individual decrease in parieto-occipital prestimulus α power by psilocybin was significantly correlated with the psilocybin-induced increase in medial P1 potentials in this study. Assuming that the initial visual gain is modulated by parieto-occipital prestimulus α power values (Rajagovindan and Ding, 2011) and that the psilocybin-induced increase in medial P1 potential reflects increased activity in early visual structures (Kometer et al., 2011), the psilocybin-induced increase in prestimulus excitability may have amplified the initial visual gain in early visual areas. By contrast, the decrease in the medial P1 that was observed after the administration of the 5-HT2A antagonist ketanserin was associated with a concomitant decrease in α phase locking, which was most pronounced over the medial parieto-occipital ROI. This result supports the notion that PLI and P1 potentials are closely associated (Gruber et al., 2005; Fellinger et al., 2011) and that attenuation of P1 potentials may be driven by 5-HT2A receptor antagonism-induced disruption of α phase resetting.
In contrast to the increase of the medial P1 potentials by psilocybin, the N170 potentials were strongly decreased by psilocybin and were associated with perceptual alterations, including visual hallucinations and audio-visual synesthesia. Both the N170 potentials and the perceptual alterations were blocked by pretreatment with ketanserin. This result not only supports the notion that activation of 5-HT2A rather than 5-HT1A receptors is a key mechanism underlying psilocybin-induced visual hallucinations and audio-visual synesthesia, but also suggests that the decrease in the N170 potentials is a crucial mechanism underlying 5-HT2A receptor-mediated alterations of visual perceptions. This mechanism could also constitute a target for the treatment of visual hallucinations in schizophrenia and Parkinson's Disease, because visual hallucinations in these disorders are associated with altered 5-HT2A receptor expression (González-Maeso et al., 2008; Ballanger et al., 2010; Huot et al., 2010) and are effectively treated by compounds that antagonize 5-HT2A receptors (Meltzer et al., 2010). In line with this view, schizophrenic patients that experience visual hallucinations show not only increased 5-HT2A receptor densities (González-Maeso et al., 2008) but also attenuated N170 potentials in response to visual stimuli (Spencer et al., 2004). Accordingly, it is conceivable that the normalization of N170 potentials, which was linked to 5-HT2A antagonism and associated with the disappearance of visual hallucinations in the present study, may critically contribute to the therapeutic effect of 5-HT2a antagonists such as atypical antipsychotics.
The decrease of N170 potentials by 5-HT2A receptor activation might be associated with visual perceptual distortions because N170 potentials are crucial for perceiving coherent and meaningful structures in natural images. Natural visual scenes often provide ambiguous and incomplete retinal information about objects; therefore, perceptual organization mechanisms are required for integrating local edge information into complex object representations and for interpolating missing parts of objects. These visual organization processes are associated with N170 potentials (Herrmann and Bosch, 2001; Murray et al., 2002), which are localized to the 5-HT2A receptor-rich LOC and V2 visual areas (Murray et al., 2002; Kometer et al., 2011; Savli et al., 2012). Thus, 5-HT2A receptor activation within the LOC and V2 visual area is likely to decrease N170 potentials, leading to incoherent object representation and arbitrary filling-in processes. On the one hand, these processes may be additionally aggravated by the 5-HT2A receptor-mediated increase in stimulus-independent excitability of the visual network observed in this study, which would in turn lead to the formation of spontaneous patterns that compete for neuronal representation. On the other hand, the activation of 5-HT2A receptors decreases attentional resources (Kometer et al., 2012; Quednow et al., 2012), which are crucial for visual organization processes (Zhang and von der Heydt, 2010; Zaretskaya et al., 2013). Therefore, further studies should address how these two interacting mechanisms contribute to the 5-HT2A receptor-mediated decrease of N170 potentials.
Footnotes
This work was supported by the Swiss Neuromatrix Foundation (M.K., A.S., F.X.V.) and the Heffter Research Center Zurich (F.X.V.). We thank Chris Pryce for critical comments on the manuscript.
The authors declare no competing financial interests.
References
- Ballanger B, Strafella AP, van Eimeren T, Zurowski M, Rusjan PM, Houle S, Fox SH. Serotonin 2A receptors and visual hallucinations in Parkinson disease. Arch Neurol. 2010;67:416–421. doi: 10.1001/archneurol.2010.35. [DOI] [PubMed] [Google Scholar]
- Banerjee S, Snyder AC, Molholm S, Foxe JJ. Oscillatory α-band mechanisms and the deployment of spatial attention to anticipated auditory and visual target locations: supramodal or sensory-specific control mechanisms? J Neurosci. 2011;31:9923–9932. doi: 10.1523/JNEUROSCI.4660-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bollimunta A, Chen Y, Schroeder CE, Ding M. Neuronal mechanisms of cortical α oscillations in awake-behaving macaques. J Neurosci. 2008;28:9976–9988. doi: 10.1523/JNEUROSCI.2699-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bollimunta A, Mo J, Schroeder CE, Ding M. Neuronal mechanisms and attentional modulation of corticothalamic α oscillations. J Neurosci. 2011;31:4935–4943. doi: 10.1523/JNEUROSCI.5580-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bressloff PC, Cowan JD, Golubitsky M, Thomas PJ, Wiener MC. What geometric visual hallucinations tell us about the visual cortex. Neural Comput. 2002;14:473–491. doi: 10.1162/089976602317250861. [DOI] [PubMed] [Google Scholar]
- Busch NA, Debener S, Kranczioch C, Engel AK, Herrmann CS. Size matters: effects of stimulus size, duration and eccentricity on the visual γ-band response. Clin Neurophysiol. 2004;115:1810–1820. doi: 10.1016/j.clinph.2004.03.015. [DOI] [PubMed] [Google Scholar]
- Butler TC, Benayoun M, Wallace E, van Drongelen W, Goldenfeld N, Cowan J. Evolutionary constraints on visual cortex architecture from the dynamics of hallucinations. Proc Natl Acad Sci U S A. 2012;109:606–609. doi: 10.1073/pnas.1118672109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter OL, Burr DC, Pettigrew JD, Wallis GM, Hasler F, Vollenweider FX. Using psilocybin to investigate the relationship between attention, working memory, and the serotonin 1A and 2A receptors. J Cogn Neurosci. 2005;17:1497–1508. doi: 10.1162/089892905774597191. [DOI] [PubMed] [Google Scholar]
- Cohen MX, Elger CE, Ranganath C. Reward expectation modulates feedback-related negativity and EEG spectra. Neuroimage. 2007;35:968–978. doi: 10.1016/j.neuroimage.2006.11.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Derogatis L. SCL-90-R: Symptom Checklist-90-R. Administration, scoring and procedures manual. Minneapolis: National Computer Systems; 1994. [Google Scholar]
- Dittrich A. The standardized psychometric assessment of altered states of consciousness (ASCs) in humans. Pharmacopsychiatry. 1998;31:80–84. doi: 10.1055/s-2007-979351. [DOI] [PubMed] [Google Scholar]
- Dyck RH, Cynader MS. Autoradiographic localization of serotonin receptor subtypes in cat visual cortex: transient regional, laminar, and columnar distributions during postnatal development. J Neurosci. 1993;13:4316–4338. doi: 10.1523/JNEUROSCI.13-10-04316.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ermentrout GB, Cowan JD. A mathematical theory of visual hallucination patterns. Biol Cybern. 1979;34:137–150. doi: 10.1007/BF00336965. [DOI] [PubMed] [Google Scholar]
- Fellinger R, Klimesch W, Gruber W, Freunberger R, Doppelmayr M. Pre-stimulus α phase-alignment predicts P1-amplitude. Brain Res Bull. 2011;85:417–423. doi: 10.1016/j.brainresbull.2011.03.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerstl F, Windischberger C, Mitterhauser M, Wadsak W, Holik A, Kletter K, Moser E, Kasper S, Lanzenberger R. Multimodal imaging of human early visual cortex by combining functional and molecular measurements with fMRI and PET. Neuroimage. 2008;41:204–211. doi: 10.1016/j.neuroimage.2008.02.044. [DOI] [PubMed] [Google Scholar]
- González-Maeso J, Ang RL, Yuen T, Chan P, Weisstaub NV, López-Giménez JF, Zhou M, Okawa Y, Callado LF, Milligan G, Gingrich JA, Filizola M, Meana JJ, Sealfon SC. Identification of a serotonin/glutamate receptor complex implicated in psychosis. Nature. 2008;452:93–97. doi: 10.1038/nature06612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gruber WR, Klimesch W, Sauseng P, Doppelmayr M. Alpha phase synchronization predicts P1 and N1 latency and amplitude size. Cereb Cortex. 2005;15:371–377. doi: 10.1093/cercor/bhh139. [DOI] [PubMed] [Google Scholar]
- Gutkin B, Pinto D, Ermentrout B. Mathematical neuroscience: from neurons to circuits to systems. J Physiol Paris. 2003;97:209–219. doi: 10.1016/j.jphysparis.2003.09.005. [DOI] [PubMed] [Google Scholar]
- Hanslmayr S, Spitzer B, Bäuml KH. Brain oscillations dissociate between semantic and nonsemantic encoding of episodic memories. Cereb Cortex. 2009;19:1631–1640. doi: 10.1093/cercor/bhn197. [DOI] [PubMed] [Google Scholar]
- Herrmann CS, Bosch V. Gestalt perception modulates early visual processing. Neuroreport. 2001;12:901–904. doi: 10.1097/00001756-200104170-00007. [DOI] [PubMed] [Google Scholar]
- Huot P, Johnston TH, Darr T, Hazrati LN, Visanji NP, Pires D, Brotchie JM, Fox SH. Increased 5-HT2A receptors in the temporal cortex of parkinsonian patients with visual hallucinations. Mov Disord. 2010;25:1399–1408. doi: 10.1002/mds.23083. [DOI] [PubMed] [Google Scholar]
- Jensen O, Mazaheri A. Shaping functional architecture by oscillatory α activity: gating by inhibition. Front Hum Neurosci. 2010;4:186. doi: 10.3389/fnhum.2010.00186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones SR, Pinto DJ, Kaper TJ, Kopell N. Alpha-frequency rhythms desynchronize over long cortical distances: a modeling study. J Comput Neurosci. 2000;9:271–291. doi: 10.1023/A:1026539805445. [DOI] [PubMed] [Google Scholar]
- Klimesch W. Evoked α and early access to the knowledge system: the P1 inhibition timing hypothesis. Brain Res. 2011;1408:52–71. doi: 10.1016/j.brainres.2011.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klüver H. Mescal and mechanisms of hallucinations. Chicago: University of Chicago; 1966. [Google Scholar]
- Kometer M, Cahn BR, Andel D, Carter OL, Vollenweider FX. The 5-HT2A/1A agonist psilocybin disrupts modal object completion associated with visual hallucinations. Biol Psychiatry. 2011;69:399–406. doi: 10.1016/j.biopsych.2010.10.002. [DOI] [PubMed] [Google Scholar]
- Kometer M, Schmidt A, Bachmann R, Studerus E, Seifritz E, Vollenweider FX. Psilocybin biases facial recognition, goal-directed behavior, and mood state toward positive relative to negative emotions through different serotonergic subreceptors. Biol Psychiatry. 2012;72:898–906. doi: 10.1016/j.biopsych.2012.04.005. [DOI] [PubMed] [Google Scholar]
- Mathewson KE, Lleras A, Beck DM, Fabiani M, Ro T, Gratton G. Pulsed out of awareness: EEG α oscillations represent a pulsed-inhibition of ongoing cortical processing. Front Psychol. 2011;2:99. doi: 10.3389/fpsyg.2011.00099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meltzer HY, Mills R, Revell S, Williams H, Johnson A, Bahr D, Friedman JH. Pimavanserin, a serotonin(2A) receptor inverse agonist, for the treatment of parkinson's disease psychosis. Neuropsychopharmacology. 2010;35:881–892. doi: 10.1038/npp.2009.176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montemurro MA, Rasch MJ, Murayama Y, Logothetis NK, Panzeri S. Phase-of-firing coding of natural visual stimuli in primary visual cortex. Curr Biol. 2008;18:375–380. doi: 10.1016/j.cub.2008.02.023. [DOI] [PubMed] [Google Scholar]
- Moreau AW, Amar M, Le Roux N, Morel N, Fossier P. Serotoninergic fine-tuning of the excitation-inhibition balance in rat visual cortical networks. Cereb Cortex. 2010;20:456–467. doi: 10.1093/cercor/bhp114. [DOI] [PubMed] [Google Scholar]
- Murray MM, Wylie GR, Higgins BA, Javitt DC, Schroeder CE, Foxe JJ. The spatiotemporal dynamics of illusory contour processing: combined high-density electrical mapping, source analysis, and functional magnetic resonance imaging. J Neurosci. 2002;22:5055–5073. doi: 10.1523/JNEUROSCI.22-12-05055.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nichols DE. Hallucinogens. Pharmacol Ther. 2004;101:131–181. doi: 10.1016/j.pharmthera.2003.11.002. [DOI] [PubMed] [Google Scholar]
- Palva S, Palva JM. Functional roles of α-band phase synchronization in local and large-scale cortical networks. Front Psychol. 2011;2:204. doi: 10.3389/fpsyg.2011.00204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perrin F, Pernier J, Bertrand O, Echallier JF. Spherical splines for scalp potential and current density mapping. Electroencephalogr Clin Neurophysiol. 1989;72:184–187. doi: 10.1016/0013-4694(89)90180-6. [DOI] [PubMed] [Google Scholar]
- Quednow BB, Kometer M, Geyer MA, Vollenweider FX. Psilocybin-induced deficits in automatic and controlled inhibition are attenuated by ketanserin in healthy human volunteers. Neuropsychopharmacology. 2012;37:630–640. doi: 10.1038/npp.2011.228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quiroga RQ, Reddy L, Kreiman G, Koch C, Fried I. Invariant visual representation by single neurons in the human brain. Nature. 2005;435:1102–1107. doi: 10.1038/nature03687. [DOI] [PubMed] [Google Scholar]
- Rajagovindan R, Ding M. From prestimulus α oscillation to visual-evoked response: an inverted-U function and its attentional modulation. J Cogn Neurosci. 2011;23:1379–1394. doi: 10.1162/jocn.2010.21478. [DOI] [PubMed] [Google Scholar]
- Romei V, Rihs T, Brodbeck V, Thut G. Resting electroencephalogram α-power over posterior sites indexes baseline visual cortex excitability. Neuroreport. 2008a;19:203–208. doi: 10.1097/WNR.0b013e3282f454c4. [DOI] [PubMed] [Google Scholar]
- Romei V, Brodbeck V, Michel C, Amedi A, Pascual-Leone A, Thut G. Spontaneous fluctuations in posterior α-band EEG activity reflect variability in excitability of human visual areas. Cereb Cortex. 2008b;18:2010–2018. doi: 10.1093/cercor/bhm229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savli M, Bauer A, Mitterhauser M, Ding YS, Hahn A, Kroll T, Neumeister A, Haeusler D, Ungersboeck J, Henry S, Isfahani SA, Rattay F, Wadsak W, Kasper S, Lanzenberger R. Normative database of the serotonergic system in healthy subjects using multi-tracer PET. Neuroimage. 2012;63:447–459. doi: 10.1016/j.neuroimage.2012.07.001. [DOI] [PubMed] [Google Scholar]
- Scheeringa R, Mazaheri A, Bojak I, Norris DG, Kleinschmidt A. Modulation of visually evoked cortical FMRI responses by phase of ongoing occipital α oscillations. J Neurosci. 2011;31:3813–3820. doi: 10.1523/JNEUROSCI.4697-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheehan D, Lecrubier Y, Sheehan K, Amorim P, Janavs J, Weiller E, Hergueta T, Baker R, Dunbar G. The Mini-International Neuropsychiatric Interview (MINI): the development and validation of a structured diagnostic psychiatric interview for DSM-IV and ICD-10. J Clin Psychiatry. 1998;59:22–33. [PubMed] [Google Scholar]
- Siegel RK, Gusewelle BE, Jarvik ME. Naloxone-induced jumping in morphine dependent mice: stimulus control and motivation. Int Pharmacopsychiatry. 1975;10:17–23. doi: 10.1159/000468164. [DOI] [PubMed] [Google Scholar]
- Snyder AC, Foxe JJ. Anticipatory attentional suppression of visual features indexed by oscillatory α-band power increases: a high-density electrical mapping study. J Neurosci. 2010;30:4024–4032. doi: 10.1523/JNEUROSCI.5684-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spencer KM, Nestor PG, Perlmutter R, Niznikiewicz MA, Klump MC, Frumin M, Shenton ME, McCarley RW. Neural synchrony indexes disordered perception and cognition in schizophrenia. Proc Natl Acad Sci U S A. 2004;101:17288–17293. doi: 10.1073/pnas.0406074101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steriade M, Gloor P, Llinás RR, Lopes de Silva FH, Mesulam MM. Report of IFCN Committee on Basic Mechanisms: basic mechanisms of cerebral rhythmic activities. Electroencephalogr Clin Neurophysiol. 1990;76:481–508. doi: 10.1016/0013-4694(90)90001-Z. [DOI] [PubMed] [Google Scholar]
- Studerus E, Gamma A, Vollenweider FX. Psychometric evaluation of the altered states of consciousness rating scale (OAV) PLoS One. 2010;5:e12412. doi: 10.1371/journal.pone.0012412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Studerus E, Kometer M, Hasler F, Vollenweider FX. Acute, subacute and long-term subjective effects of psilocybin in healthy humans: a pooled analysis of experimental studies. J Psychopharmacol. 2011;25:1434–1452. doi: 10.1177/0269881110382466. [DOI] [PubMed] [Google Scholar]
- Sun W, Dan Y. Layer-specific network oscillation and spatiotemporal receptive field in the visual cortex. Proc Natl Acad Sci U S A. 2009;106:17986–17991. doi: 10.1073/pnas.0903962106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thut G, Nietzel A, Brandt SA, Pascual-Leone A. Alpha-band electroencephalographic activity over occipital cortex indexes visuospatial attention bias and predicts visual target detection. J Neurosci. 2006;26:9494–9502. doi: 10.1523/JNEUROSCI.0875-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vollenweider FX, Kometer M. The neurobiology of psychedelic drugs: implications for the treatment of mood disorders. Nat Rev Neurosci. 2010;11:642–651. doi: 10.1038/nrn2884. [DOI] [PubMed] [Google Scholar]
- Vollenweider FX, Vollenweider-Scherpenhuyzen MF, Bäbler A, Vogel H, Hell D. Psilocybin induces schizophrenia-like psychosis in humans via a serotonin-2 agonist action. Neuroreport. 1998;9:3897–3902. doi: 10.1097/00001756-199812010-00024. [DOI] [PubMed] [Google Scholar]
- Waldhauser GT, Johansson M, Hanslmayr S. α/β oscillations indicate inhibition of interfering visual memories. J Neurosci. 2012;32:1953–1961. doi: 10.1523/JNEUROSCI.4201-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watakabe A, Komatsu Y, Sadakane O, Shimegi S, Takahata T, Higo N, Tochitani S, Hashikawa T, Naito T, Osaki H, Sakamoto H, Okamoto M, Ishikawa A, Hara S, Akasaki T, Sato H, Yamamori T. Enriched expression of serotonin 1B and 2A receptor genes in macaque visual cortex and their bidirectional modulatory effects on neuronal responses. Cereb Cortex. 2009;19:1915–1928. doi: 10.1093/cercor/bhn219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wittchen HU, Pfister H. DIA-X-Interviews: Manual für Screening-Verfahren und Interview. Frankfurt: Swets and Zeitlinger; 1997. [Google Scholar]
- Zanto TP, Rubens MT, Thangavel A, Gazzaley A. Causal role of the prefrontal cortex in top-down modulation of visual processing and working memory. Nat Neurosci. 2011;14:656–661. doi: 10.1038/nn.2773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaretskaya N, Anstis S, Bartels A. Parietal cortex mediates conscious perception of illusory gestalt. J Neurosci. 2013;33:523–531. doi: 10.1523/JNEUROSCI.2905-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang NR, von der Heydt R. Analysis of the context integration mechanisms underlying figure-ground organization in the visual cortex. J Neurosci. 2010;30:6482–6496. doi: 10.1523/JNEUROSCI.5168-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]