Abstract
Adaptive decision-making involves interaction between systems regulating Pavlovian and instrumental control of behavior. Here we investigate in humans the role of serotonin in such Pavlovian-instrumental transfer in both the aversive and the appetitive domain using acute tryptophan depletion, known to lower central serotonin levels. Acute tryptophan depletion attenuated the inhibiting effect of aversive Pavlovian cues on instrumental behavior, while leaving unaltered the activating effect of appetitive Pavlovian cues. These data suggest that serotonin is selectively involved in Pavlovian inhibition due to aversive expectations and have implications for our understanding of the mechanisms underlying a range of affective, impulsive, and aggressive neuropsychiatric disorders.
Keywords: aversive, inhibition, instrumental, Pavlovian, serotonin, tryptophan depletion
Introduction
Serotonin is implicated in healthy and disordered functions so wide ranging that elucidating its function is an important scientific puzzle. Best known are its contributions to aversive processing and behavioral inhibition, with evidence showing that a reduction in serotonin disinhibits behavior in the face of expected punishments (Tye et al., 1977; Soubrie, 1986; Graeff et al., 1996; Crockett et al., 2009, 2012). This work provided the basis for a recent proposal that serotonin has a specific role in tying aversive Pavlovian influences to instrumental inhibition (Dayan and Huys, 2008; Boureau and Dayan, 2011; Cools et al., 2011).
This proposal is grounded in a long history of psychological theory according to which there is a dichotomy of Pavlovian versus instrumental control of behavior. Instrumental behavior is elicited by learned associations of stimulus-action pairs with reinforcements, whereas Pavlovian behavior arises as reflexive responses to learned stimulus-associated outcome expectancies (Thorndike, 1911; Pavlov, 1927). Pavlovian and instrumental contingencies may act synergistically or competitively, and anomalous Pavlovian-instrumental interaction might be core to several neuropsychiatric disorders (Dayan et al., 2006; Dayan and Huys, 2008; Boureau and Dayan, 2011). For example, Dayan and Huys (2008, 2009) argue that serotonin deficiency, as seen in depression, leads to a failure to inhibit aversive thoughts and actions. Here we investigate whether and how serotonin regulates the coupling between aversive Pavlovian and instrumental control.
The paradigmatic example of such coupling is aversive Pavlovian-instrumental transfer (PIT), in which an aversive Pavlovian conditioned stimulus (CS) inhibits instrumental behavior (i.e., conditioned suppression; Huys et al., 2011; Geurts et al., 2013). Effects of serotonin on aversive PIT have not been assessed in humans. We fill this gap, while also investigating the valence-specificity of serotonin's PIT effects. Specifically, we examined how aversive and appetitive PIT are affected by acute tryptophan depletion (ATD), a dietary procedure to deplete central serotonin levels in humans (Crockett et al., 2011). The hypothesis that the effect of ATD is particularly pronounced on aversive rather than appetitive PIT concurs with an accumulating body of theory and evidence (Soubrie, 1986; Deakin and Graeff, 1991; Daw et al., 2002; Dayan and Huys, 2008; Boureau and Dayan, 2011; Cools et al., 2011). However, there are also several studies suggesting a potential role in appetitive processing (Cools et al., 2005; Nakamura et al., 2008; Seymour et al., 2012). We aimed to resolve this discrepancy by conducting direct comparison between effects of ATD on aversive and appetitive PIT.
Finally we asked whether such effects are restricted to appetitive instrumental actions, such as approach, or extend to aversive actions, such as withdrawal. We have already shown that the effects of Pavlovian stimuli differ between instrumental approach and withdrawal (Huys et al., 2011). Serotonergic neurons densely innervate structures involved in active defensive behaviors (McNaughton and Corr, 2004; Saulin et al., 2012), raising the possibility that serotonin alters Pavlovian modulation of withdrawal as well as approach actions (Dayan and Huys, 2009; Boureau and Dayan, 2011).
Materials and Methods
Participants.
Fifty-seven healthy right-handed volunteers (18–28 years old; mean age of 23.8 ± 2.8; 22 women) participated in this experiment. The study was approved by the local ethical committee at the Radboud University, Nijmegen. Participants were recruited via local advertisements, and screened during a screening session several days before the experiment for psychiatric and neurological disorders and MRI contra-indications by means of prescreening questionnaires and a (medical) interview by a trained physician. All volunteers gave written informed consent, and were paid for their participation. Exclusion criteria were any history of cardiac, hepatic, renal, pulmonary, neurological, psychiatric, or gastrointestinal disorder, current medication use as well as first-degree family history of mood disorders.
We report data from 45 participants (18–28 years old; mean age of 23.8 ± 2.8), as 12 participants could not be included for the following reasons: five participants did not tolerate the amino acid drink; one participant fainted during venipuncture; one participant did not return for the second session; data from two participants were lost due to technical errors; and one participant reported not following the instructions. Two participants did not meet inclusion criteria for simple query trials during Pavlovian conditioning (see Results).
General procedure.
Participants attended two test sessions at least 6 d apart (maximum 13), and were administered either a tryptophan depleting drink (TRP−) or a balanced amino acid drink (BAL) in a double-blind, placebo-controlled, cross-over design (22 participants received TRP− and 23 received BAL on the first session). Before the test sessions, participants fasted overnight and low-protein food was provided on the test days. Following a resting period of ∼5.5 h after drink intake (mean 5 h 24 m, SD 12 min in the TRP− condition and mean 5 h 26 m, SD 14 min in the balanced condition) to ensure stable and low TRP levels, participants performed a series of tasks. The task presented here was administered after another experiment involving fMRI scanning (reported previously). The current experiment started ∼7 h after the amino acid drink intake (6 h 49 m, SD 14 min in the TRP− condition and 6 h 55 m, SD 20 min in the balanced condition).
Participants were seated comfortably in front of a personal computer with headphones. They used a mouse with their right hand to indicate their choices. Earnings were paid by bank transfer after the second session.
Amino-acid mixtures.
Central TRP was depleted by ingesting an amino-acid load that did not contain TRP but did include other large neutral amino acids (LNAAs; Reilly et al., 1997). The quantities of amino acids in each drink were based on those used by Young et al. (1985), although a 78.2 g mixture was used to minimize nausea. Both amino-acid mixtures (prepared by Nutricia) had the following ingredients: l-alanine, 4.1 g; l-arginine, 3.7 g; l-cystine, 2.0 g; glycine, 2.4 g; l-histidine, 2.4 g; l-isoleucine, 6 g; l-leucine, 10.1 g; l-lysine, 6.7 g; l-methionine, 2.3 g; l-proline, 9.2 g; L-phenylalanine, 4.3 g; l-serine, 5.2 g; l-threonine, 4.9 g; l-tyrosine, 5.2 g; and l-valine, 6.7 g. The balanced amino drink contained additionally l-tryptophan, 3.0 g and the TRP− mixture 3.0 g of micro-crystalline cellulose filler. Female participants received a 20% reduction in quantity to account for lower average body weight. The drinks were prepared by stirring the mixture into ∼200 ml of tap water with a choice of lemon-lime or grapefruit flavoring to compensate for the unpleasant taste. Except for five of the excluded participants, participants reported no side effects apart from transient nausea following ingestion of the drink.
Blood sample analyses.
Blood samples were taken twice, once before amino acid intake, and once before testing (9 min ±5 m), to establish the efficacy of the ATD procedure. Venous samples were taken in EDTA tubes, centrifuged at 2650 gmax during 20 min, and then pipetted into heparin aliquots. These were stored for a maximum of 3 weeks at −20°C before moving them to an −80°C environment. From there, they were sent to an external laboratory. Quantitative amino acid analysis was performed by high-performance liquid chromatography as described previously (Fekkes et al., 1995). The ratio total TRP/LNAA was calculated as 100 times the concentration of TRP divided by the summed concentrations of the LNAAs (TRP/ΣLNAA; Fernstrom and Wurtman, 1972).
Task description.
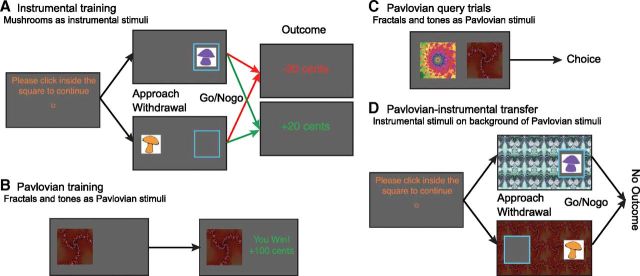
We used the task as previously described by Huys et al., (2011). In short, the task was divided into two blocks (approach and withdrawal), each consisting of an instrumental training, a Pavlovian training and a PIT stage (Fig. 1).
Figure 1.
A, Instrumental training. To center the cursor, participants clicked in a central square. The experiment consisted of a block with exclusively instrumental approach trials (n = 120) and a block with exclusively withdrawal trials (n = 120). In approach trials (top), participants chose whether to move the cursor toward the mushroom and click inside the blue frame onto the mushroom (go), or do nothing (nogo). In withdrawal trials, they instead moved the cursor away from the mushroom and clicked in the empty blue frame (go) or did nothing (nogo). Outcomes were presented immediately after go actions, or after 1.5 s. Per block, there were three “good” and three “bad” instrumental stimuli. Participants played each block ones per testing day. Instrumental stimuli were different for both blocks, but the same for both days. B, Pavlovian conditioning. Participants passively viewed stimuli and heard auditory tones, followed by wins and losses. There were five fractal/tone combinations. Each combination was displayed 12 times in the first block and another six times in the second block. C, On Pavlovian query trials, participants chose between two Pavlovian stimuli. No outcomes were presented, but they were counted and added to the total presented at the end of the experiment. Query trials were administered after every five Pavlovian conditioning trials. D, PIT participants responded to the instrumental stimuli trained during the instrumental training stage, with Pavlovian stimuli tiling the background. No outcomes were presented, but participants were instructed that their choices counted toward the final total. No explicit instructions about the contribution of Pavlovian stimuli towards the final total were given.
Instrumental training.
The instrumental task (Fig. 1A) was an approach or withdrawal go/nogo task, framed in terms of collecting or discarding mushrooms. In the approach block, participants chose whether to collect the mushroom by moving the mouse toward and clicking on the stimulus (approach-go) within a response-window of 1.5 s, or not collect the mushroom by abstaining from a response for 1.5 s (approach-nogo). In the withdrawal block, participants chose whether to discard mushrooms by clicking in a blue frame located on the opposite side of the stimulus (withdrawal-go) or do nothing (withdrawal-nogo). The outcome (±20 Euro cents) was then presented in the middle of the screen. Reinforcements were probabilistic, with the “correct” response for each mushroom leading to gain or avoidance of loss on 75% of the trials. Correct trials were those on which participants discarded a “bad” or kept a “good” mushroom, and those on which they collected a good or refrained from collecting a bad mushroom. Participants had to learn the better response for each stimulus from the noisy reinforcement feedback. There were 3 good and 3 bad mushrooms in each context, meaning that all actions (i.e., approach-go, approach-nogo, withdrawal-go, and withdrawal-nogo) could be followed by both rewards and punishments (Table 1). Thus, the expected value of correct approach and withdrawal actions were equal and positive on average.
Table 1.
Action outcome contingencies for the different instrumental stimuli
| Block | Type of instrumental stimulus (mushroom) | If the following action: | Then the following outcome (75%/25%): | |
|---|---|---|---|---|
| Approach | Go (good) | Go | (collect) | +20/−20 |
| Nogo | (avoid) | −20/+20 | ||
| Nogo (bad) | Go | (collect) | −20/+20 | |
| Nogo | (avoid) | +20/−20 | ||
| Withdrawal | Go (bad) | Go | (throw away) | +20/−20 |
| Nogo | (collect) | −20/+20 | ||
| Nogo (good) | Go | (throw away) | −20/+20 | |
| Nogo | (collect) | +20/−20 | ||
Pavlovian training.
The second part of the task consisted of a separate classical conditioning procedure. Five compound Pavlovian stimuli (CS), consisting of a fractal visual stimulus (Fig. 1B) and a tone were deterministically paired with outcomes. The best (S++P) and worst (S−P) CS predicted a gain/loss of 100 cents, whereas the intermediate CSs (S+P,S0P,S−P) were followed, respectively, by +10 0–10 cents. To ensure that participants paid attention, a query trial was presented on every fifth trial. Participants then had to choose between two different Pavlovian stimuli (Fig. 1C) in extinction.
PIT.
This was the main part of interest. Subjects chose whether to collect or discard mushrooms while the Pavlovian stimuli tiled the entire background (Fig. 1D). Critically, no outcomes were presented. Participants were instructed to continue performing the instrumental task; that choices were still earning them the same outcomes and were being counted; but that they would not be told about the outcomes.
Psychometric measurements.
Participants completed the following questionnaires during the screening session: Barratt Impulsiveness Scale (Patton et al., 1995), Behavioral Inhibition/Behavioral Activation Scale (Carver and White, 1994), Beck Depression Inventory (Beck et al., 1996), Eysenck Personality Questionnaire (Eysenck and Eysenck, 1975), Hamilton Rating Scale for Depression (Hamilton, 1960), Spielberger Trait Anxiety Inventory (Spielberger et al., 1983), Kirby Questionnaire (Kirby and Maraković, 1996), Sensitivity to Punishment and Sensitivity to Reward Questionnaire (Torrubia et al., 2001), and the Dutch reading test (Schmand et al., 1991). Scores are reported in Table 2. In addition, participants completed the Positive Affect Negative Affect Schedule (PANAS; Watson et al., 1988) and the Bond and Lader Visual Analog Scales (BLV; Bond and Lader, 1974) just before the PIT experiment. Finally, a neuropsychological test battery was administered at the end of each testing day (∼15 min after the end of the PIT experiment) consisting of a number cancellation task, a box completion test, and a digit span (Table 2).
Table 2.
Trait characteristics and data from neuropsychological background tests as a function of order (BAL1st/TRP−1st; SEM)
| Questionnaire | BAL1st | TRP−1st |
|---|---|---|
| Barratt-total | 59.4 (3.1) | 54.3 (4.1) |
| Barratt-attention | 16.1 (1.0) | 14.4 (1.1) |
| Barratt-motor | 18.9 (1.1) | 17.6 (1.5) |
| Barratt-nonplanning | 24.4 (1.3) | 22.2 (1.7) |
| BIS | 18.3 (0.8) | 17.0 (0.9) |
| BAS-total | 24.7 (1.1) | 28.1 (3.1) |
| BAS-reward | 8.9 (0.5) | 9.1 (0.5) |
| BAS-drive | 7.6 (0.5) | 7.5 (0.5) |
| BAS-fun | 8.2 (0.3) | 8.4 (0.5) |
| BDI | 1.1 (0.35) | 1.4 (0.36) |
| EPQ-psychoticism | 2.1 (0.4) | 1.2 (0.2) |
| EPQ-extraversion | 10.0 (0.4) | 9.3 (0.6) |
| EPQ-neuroticism | 2.2 (0.4) | 1.9 (0.3) |
| EPQ-lie | 6.2 (0.6) | 6.6 (0.7) |
| HRSD | 0.9 (0.2) | 0.9 (0.3) |
| STAI | 30.2 (1.3) | 30.5 (1.2) |
| Kirby-small | 0.04 (0.01) | 0.05 (0.02) |
| Kirby-medium | 0.03 (0.01) | 0.03 (0.02) |
| Kirby-large | 0.02 (0.01) | 0.03 (0.02) |
| SPSRQ-punishment | 4.9 (0.7) | 4.4 (0.6) |
| SPSRQ-reward | 11.7 (0.8) | 10.8 (0.9) |
| NLV | 85.8 (1.4) | 85.6 (1.8) |
| Number cancelation | 227.7 (6.2) | 207.5 (5.7) |
| Box completion | 79.7 (3.0) | 73.5 (3.6) |
| Digit span | 16.2 (0.6) | 18.1 (0.6) |
Barratt, Barratt Impulsivity Scale; BAS, behavioral activation system score; BDI, Beck Depression Inventory; BIS, behavioral inhibition system score from the BIS/BAS scale; EPQ, Eysenck Personality Questionnaire; Kirby, Kirby Questionnaire; NLV, Dutch reading test; SPSRQ, Sensitivity to Punishment and Sensitivity to Reward Questionnaire; STAI, Spielberger Trait Anxiety Inventory; HRSD, Hamilton Rating Scale for Depression.
Data analysis.
Data were analyzed using the statistic software SPSS 16.0. Where appropriate, we performed repeated-measures (rm) ANOVA. Factors included drink (two levels: BAL/TRP−, within-subject), order (two levels: TRP− first or BAL first, between-subjects) and other factors defined below.
Serum levels.
The TRP/ ΣLNAA ratio was used as the dependent variable in an rmANOVA with time (two levels: before/after, within-subject) × drink × order. This was followed by simple effects analyses: an rmANOVA with factor Time for each drink separately, and an rmANOVA with factor Drink only to comparing TRP/ΣLNAA after ATD and BAL.
Pavlovian conditioning.
The threshold for performing above chance at the query trials (Fig. 1C) was set to at least 14 (of 18) correct (based on a sign test). Proportion correct choices were also submitted to a drink × order rmANOVA.
Instrumental training.
There were four trial types, consisting of stimulus for which the correct response was as follows: (1) go-approach, (2) nogo-approach, (3) go-withdrawal, and (4) nogo-withdrawal. We calculated the proportion of correct responses [p(correct)] for the first and last 10 trials of each trial type, both for the instrumental training and for the PIT stage. To assess whether participants learned to make the correct choice during instrumental training, we used a rmANOVA with time (two levels: first/last trial bin), action context (two levels: approach/withdrawal), correct choice (two levels: go/nogo), and drink and order.
To assess whether the learned behavior generalized to the PIT stage, the two level factor Time was changed to include three levels (henceforth “extended time factor”): the last instrumental, the first PIT, and the last PIT trial bin. Adequate generalization to the PIT stage implies an absence of any effect of, or interaction with, the factor time.
PIT stage.
The primary effect of interest was that of the Pavlovian CSs on instrumental responding. This was assessed in terms of choice [proportion of go responses; p(go)] as a function of CS valence and action context. We analyzed this using an rmANOVA with drink, action context, and CS valence (five levels: S++P /S+P /SnP /S−−P /S−P, within-subject). We modeled CS Valence as a linear contrast (+2/+1/0/−1/−2).
Planned contrasts were targeted at the most aversive and most appetitive Pavlovian stimuli (i.e., SP++ and S−P). For this analysis, the five-level factor Pavlovian valence in the omnibus rmANOVA was replaced by a Pavlovian valence factor with 2 levels: S++P and SP−.
To account for variability of no interest introduced by the cross-sectional design (2 d) and the blocked design of the PIT task (two blocks) we added the following between-subject factors to the rmANOVAs described above. First, to capture variance due to test-retest effects from day 1 to day 2 we added a between-subject factor order [started with BAL on day 1 (BAL1st)/started with TRP− on day 1 (TRP− first)]. Note that the interaction between order and the within-subject factor drink (BAL/TRP−) is statistically similar to a main effect of day (day1/day2). Likewise, to capture variability of no interest in PIT task performance that might be caused by block order (i.e., better performance on the second compared with the first block) we added a between-subject factor first block [approach as first block (appr1st)/withdrawal as first block (wthd1st)]. An interaction between block order and the within-subject factor action context (approach/withdrawal) represents a main effect of block (block1/block2).
Results
Blood plasma analysis
ATD resulted in decreased TRP/ΣLNAA ratio as evidenced by a significant drink × time interaction (F(1,41) = 492.9, p < 0.001; Table 3). This was due to a 92.8% decrease in the TRP/ΣLNAA ratio following TRP−. The TRP/ΣLNAA ratio was lower for the TRP− than the BAL condition at the start of the PIT experiment (F(1,41) = 866.4, p < 0.001).
Table 3.
Values present TRP/ΣLNAA ratio before and after drink ingestion on days 1 and 2 (SEM)
| TRP− |
BAL |
|||
|---|---|---|---|---|
| Before | After | Before | After | |
| Day 1 | 9.44 (0.33) | 0.63 (0.08) | 9.04 (0.27) | 5.80 (0.18) |
| Day 2 | 9.53 (0.31) | 0.73 (0.08) | 9.42 (0.28) | 6.83 (0.37) |
Pavlovian conditioning
Participants performed highly accurately on the query trials during the Pavlovian stage evidencing successful Pavlovian conditioning [mean p(correct) = 0.97 correct, SD = 0.04]. Two participants performed at chance level and were removed from further analysis. There was no significant effect of ATD on accuracy on the query trials [mean p(correct)BAL = 0.97, mean p(correct)TRP− = 0.97, F(1,41) = 0.54, p = 0.82].
Instrumental responding
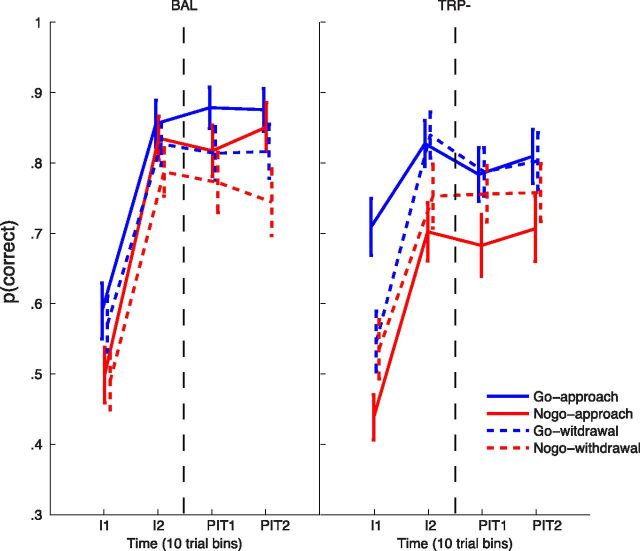
Participants showed robust acquisition of the instrumental contingencies (Fig. 2; main effect of time, F(1,41) = 242.4, p < 0.001) and this effect was maintained throughout the PIT stage (no main effect of, or interaction with, the extended time factor; all (F(1,40) /(2,80) < 3.0, p > 0.091). ATD impaired instrumental learning [drink × time interaction F(1,41) = 4.9, p = 0.033; mean p(correct) at the end of the instrumental training: TRP−, 0.77; BAL, 0.82; mean improvement in p(correct) between first and last stage of instrumental learning: TRP−, 0.22; BAL, 0.28]. This effect was maintained in the PIT stage (main effect of drink: F(1,40) = 6.7, p = 0.014; no interaction with extended time factor).
Figure 2.
Instrumental learning and generalization to the PIT stage after tryptophan depletion (right graph, TRP−) and after the balanced amino acid drink (left graph, BAL). The proportion of correct choices [p(correct)] are divided over the four different types of instrumental stimuli: go-approach, nogo-approach, go-withdrawal, and nogo-withdrawal. Time is represented by bins of 10 trials for each type of stimulus at the beginning (I1) and the end (I2) of the instrumental training and at the beginning (PIT1) and the end (PIT2) of the PIT stage. Error bars represent SEM.
ATD also specifically impaired nogo-approach actions (Fig. 2); there was a significant three-way drink × action context × correct choice interaction (F(1,41) = 5.7, p = 0.022) which was driven by a main effect of drink on nogo-approach stimuli (F(1,41) = 10.7, p = 0.002) with the effect of ATD on all other actions failing to reach significance (F(1,41) < 1.2). Thus, ATD impaired the ability to make nogo responses to passively avoid bad mushrooms.
PIT
Consistent with our primary hypothesis, ATD altered the effects of Pavlovian stimuli on instrumental behavior. Specifically, the inhibitory effect of aversive Pavlovian CSs on instrumental responding seen at baseline was reversed by ATD [drink × CS valence (five levels, S++P /S+P /SnP /S−−P /S−P) linear contrast, F(1,41) = 4.3, p = 0.045; planned contrast drink × CS valence (two levels, S−−P /S++P), F(1,41) = 5.5, p = 0.023]. This effect was driven by an effect of ATD on the aversive CS. For the aversive CSs, the proportion of go choices was larger after TRP− than after BAL (main effect of drink for S−P only, F(1,41) = 6.8, p = 0.013). Responding to the appetitive CSs was unaltered by ATD (F(1,41) = 0.1, p = 0.74). Thus, ATD abolished the inhibitory effect of aversive Pavlovian CS on instrumental responding.
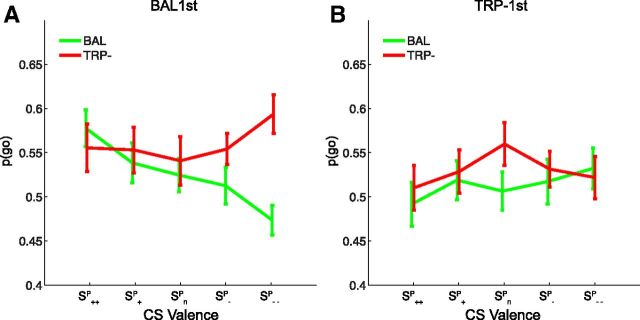
The order of drink sessions was counterbalanced, so that 22 participants received TRP− on the first session, and 23 received TRP− on the second session. Moreover, the analyses reported above were conducted with testing order as a between-subjects factor to account for variability of no interest. Contrary to our expectation, this factor interacted with the effect of interest. There was a significant three-way interaction between drink × CS valence (S++P /S−−P) × order (F(1,41) = 11.7, p = 0.001; Fig. 3). Breakdown of this three-way interaction effect by group (BAL1st vs TRP− first) revealed that our effect of interest, i.e., aversive disinhibition after ATD, was present in participants who received BAL, but not in those who received TRP− on day 1 (drink × CS valence for BAL1st, F(1,21) = 18.8, p < 0.001; for TRP− first, F(1,20) = 0.5, p = 0.49; Fig. 3). Furthermore, alternative break down of the interaction effect by day (day 1 vs day 2) revealed that it was present on the first, but not the second day (day 1, drink × CS valence, F(1,41) = 5.9, p = 0.02; day 2, F(1,41) <0.1, p = 0.88).
Figure 3.
Behavioral data from the PIT stage as a function of group. Shown are choice data as a function of CS Valence (SP++ / SP+ / SPn / SP−/ SP−−) after acute tryptophan depletion (TRP−, red line) and after the balanced amino acid drink (BAL, green line). A, Participants who started with BAL on day 1. B, Participants who started with TRP− on day 1. Error bars represent SEM.
As in the overall group, these effects in the BAL first group were also driven by the aversive CS rather than the appetitive CS. Thus, in this BAL first group, for the aversive CS, the proportion of go choices was larger after TRP− than after BAL (main effect of drink for S−P only, F(1,21) = 13.9, p = 0.001), whereas responding to the appetitive CSs was unaltered by ATD (F(1,21) = 1.7, p = 0.21). Moreover, there was also a significant interaction between drink, CS valence in this BAL first group when comparing the aversive with the neutral CS, (F(1,21) = 6.7, p = 0.017), but not when comparing the appetitive with the neutral CS (F(1,21) = 2.1, p = 0.16). This interaction is depicted in Figure 3 and confirms a specific effect of ATD on aversive PIT.
In supplemental analyses, we assessed whether this aversive disinhibition in the BAL first group was due to increased proportion of go choices when go was correct, when go was an error, or some combination of both. To this end we assessed the probability of correct responses in the PIT stage during the aversive trials only using an ANOVA with an additional within-subject factor type of stimulus. There were two types of stimuli, one that required a go and one that required a nogo response to be correct. This analysis revealed a significant drink × type of stimulus interaction for the aversive CS (F(1,20) = 8.4, p = 0.009), which was due to increased proportion of correct go responses after TRP− versus BAL (F(1,21) = 9.2, p = 0.007). There was no effect of ATD on the proportion of correct nogo responses (F(1,21) = 0.9, p = 0.773). Thus, the aversive disinhibition induced by serotonin depletion was driven by increased proportion of go choices when go was correct and not when go was an error.
With respect to the proportion of go choices, we did not find a significant interaction between action context (approach versus withdrawal) and CS valence (cf. Huys et al., 2011) across sessions (F(1,41) = 0.6, p = 0.43) or after BAL only (F(1,41) < 0.1, p = 0.95) and no modulation of this interaction by drink (F(1,41) = 1.3, p = 0.27; Table 4).
Table 4.
Values represent proportion of go actions as a function of order (BAL1st/TRP−1st), drink (BAL/TRP−), action context (approach/withdrawal), and CS valence [very appetitive (S++P) to very aversive (S−−P)] during the Pavlovian-instrumental transfer stage (SEM)
| BAL1st |
TRP−1st |
|||||||
|---|---|---|---|---|---|---|---|---|
| BAL |
TRP− |
BAL |
TRP− |
|||||
| Appr | Wthd | Appr | Wthd | Appr | Wthd | Appr | Wthd | |
| S++P | 0.581 (0.162) | 0.572 (0.163) | 0.590 (0.124) | 0.521 (0.157) | 0.453 (0.151) | 0.525 (0.141) | 0.538 (0.175) | 0.477 (0.175) |
| S+P | 0.550 (0.156) | 0.528 (0.157) | 0.576 (0.116) | 0.530 (0.168) | 0.515 (0.139) | 0.518 (0.141) | 0.537 (0.170) | 0.516 (0.160) |
| SnP | 0.560 (0.126) | 0.491 (0.204) | 0.609 (0.141) | 0.473 (0.139) | 0.435 (0.130) | 0.573 (0.116) | 0.553 (0.172) | 0.561 (0.165) |
| S−P | 0.543 (0.172) | 0.485 (0.161) | 0.559 (0.151) | 0.549 (0.170) | 0.482 (0.164) | 0.548 (0.108) | 0.513 (0.115) | 0.546 (0.151) |
| S−−P | 0.492 (0.164) | 0.459 (0.179) | 0.606 (0.159) | 0.579 (0.146) | 0.490 (0.152) | 0.570 (0.119) | 0.511 (0.159) | 0.528 (0.194) |
Order effects
The order effect might raise the concern that random assignment of participants to groups failed, resulting in differences between groups (BAL1st vs TRP− first) in vulnerability to ATD. Therefore we investigated whether there was evidence for any differences between the groups with respect to screening questionnaires and background neuropsychological tests (Table 2). The only measure that differed between the groups and was not affected by drink or day was the digit span test: participants who received BAL on day 1 performed more poorly on the digit span task across both sessions than participants who received TRP− on day 1 (main effect of order, F(1,41) = 6.4, p = 0.015). However, adding this measure as a covariate in the omnibus rmANOVA did not reduce significance of the interaction between order, drink, and CS valence [order × drink × CS valence (S++P/S−−P), F(1,40) = 7.7, p = 0.008], and it also did not interact with our main finding of aversive disinhibition (digit span × drink × CS valence, F(1,40) = 0.4, p = 0.511).
Mood ratings
Positive affect as measured with the PANAS immediately before the PIT experiment was significantly affected by ATD (F(1,37) = 9.7, p = 0.004; Table 5). Critically, this effect was not related to our main finding, i.e., no correlation existed between the effects of ATD on positive affect and the effects of ATD on the inhibiting effect of the aversive Pavlovian cue (Pearson r(41) = −0.11, p = 0.51). In addition, we did not find any other main effect of or interaction with ATD on the other mood ratings (BLV subscales, F(1,43) < 1, p > 0.52, PANAS negative affect: F(1,37) = 0.3, p = 0.58). Thus, the finding that ATD modulates the inhibitory impact of an aversive Pavlovian stimulus is unlikely to be mediated by ATD-related changes in mood.
Table 5.
Mood ratings as a function of Drink (BAL/TRP−; SEM)
| Rating | BAL | TRP− |
|---|---|---|
| PANAS-positive | 25.4 (0.8) | 27.7 (1.1) |
| PANAS-negative | 12.4 (0.9) | 11.8 (0.4) |
| BLV-alertness | 45.1 (1.0) | 44.8 (1.0) |
| BLV-contentedness | 48.1 (1.8) | 47.0 (1.2) |
| BLV-calmness | 55.2 (0.7) | 54.5 (1.0) |
BLV, Bond and Lader visual analogue scale; PANAS, Positive Affect Negative Affect Schedule.
Discussion
Results show that serotonin depletion attenuates aversive PIT without affecting appetitive PIT, thus providing evidence for a selective role of serotonin in tying aversive expectations to behavioral inhibition. This concurs with current theories according to which serotonin serves as a motivational opponent to dopamine (Daw et al., 2002; Boureau and Dayan, 2011; Cools et al., 2011). According to these theories, both serotonin and dopamine have coordinated effects that serve to couple a motivational axis (appetitive versus aversive processing), and an activational axis (energizing vs inhibiting behavior). In contrast to dopamine, which is well established to promote behavioral activation to seek rewards, serotonin was hypothesized to inhibit actions when punishment may occur. Data from the PIT phase of the current study concur with this hypothesis. The supplemental finding that withholding an action in the approach context is compromised by ATD also fits with this framework. Moreover it generally concurs with rodent work showing that performance on passive avoidance tasks is particularly vulnerable to manipulations that lower serotonin transmission, while leaving active avoidance unaltered (Soubrie, 1986).
To appreciate the relevance of PIT it is important to recognize that instrumental learning always involves both instrumental as well as Pavlovian contingencies (Yin et al., 2008). Therefore, PIT might influence the majority of instrumental responses, and these influences might be core to a wide range of adaptive and maladaptive behaviors (Dayan et al., 2006; Guitart-Masip et al., 2012; Huys et al., 2012). Consider the specific cases of depression and impulse control disorders. Both implicate low serotonin, an observation that appears paradoxical given that depression has been primarily associated with aversive abnormalities, whereas impulsivity has been associated primarily with behavioral disinhibition (Cools et al., 2008a). The present data strengthen the hypothesis that serotonin does not control aversive processing per se or behavioral inhibition per se, but rather facilitates the coupling between aversive processing and behavioral inhibition. Accordingly, serotonin deficiency, as seen in depression and impulsivity, is accompanied not by enhanced impact of aversive stimuli per se or by reduced inhibition per se, but rather by reduced impact of aversive stimuli on the inhibition of behavior (as well as thoughts; Dayan and Huys, 2008; Crockett et al., 2009; Boureau and Dayan, 2011; Cools et al., 2011; Huys et al., 2012; Robinson et al., 2012).
The first empirical evidence in humans for this hypothesis came from work by Crockett et al. (2012) who used a reinforced categorization task rather than a PIT task to show that ATD abolishes slowing of responding in the presence of punishment-predicting stimuli. The present study extends this work, not least by enabling direct comparison of aversive with appetitive Pavlovian influences. We show that the effects of ATD are valence-specific, and are restricted to the aversive domain. This observation concurs with some classic accounts of serotonin, according to which it is involved in aversive rather than appetitive processing (Deakin, 1983; Deakin and Graeff, 1991; but see Kranz et al., 2010). Moreover, it fits with formal theories, according to which serotonin is involved in the aversive side of model-free learning (Daw et al., 2002). Third, it is consistent with our previous findings, showing that ATD altered performance on a punishment, but not reward prediction learning task (Cools et al., 2008b; Robinson et al., 2012). Specifically, we have shown that ATD enhanced the ability to predict punishment while leaving reward prediction unaffected (Cools et al., 2008b). Initially, we interpreted this effect to reflect enhanced punishment prediction learning (Cools et al., 2008b). However, the present finding suggests that these prior observations might reflect disinhibition of responding in anticipation of punishment rather than enhanced punishment learning (cf. Dayan and Huys, 2009; Robinson et al., 2012).
The observation that our effects were restricted to the aversive domain might not seem consistent with electrophysiological data, revealing reward-responsive neurons in the dorsal raphe nucleus, the primary source of serotonergic input into the brain (Nakamura et al., 2008; Bromberg-Martin et al., 2010; Okada et al., 2011). However, it should be recognized that the dorsal raphe nucleus contains a number of different types of nonserotonergic units that are also likely to be recorded. Thus, the serotonergic identity of these neurons is not known. In addition, there are also serotonin depletion studies in marmosets and humans emphasizing effects in the reward domain (Rogers et al., 2003; Cools et al., 2005; Man et al., 2011; may be reflecting interactions with dopamine). For example, we have shown that ATD abolished speeding of responding with increasing feedback likelihood in a monetary incentive delay like task (Cools et al., 2005). The findings of the latter study may be reconciled with the present observation by recognizing that the ATD-induced abolition of speeding in that study might have resulted not just from reduced sensitivity to reward, but also from enhanced sensitivity to punishment. Of course, we acknowledge that our findings do not exclude effects of serotonin on reward processing outside the domain of PIT, for example in the domain of delayed discounting (Miyazaki et al., 2012).
The effects on aversive PIT are unlikely to reflect attenuation by ATD of Pavlovian conditioning per se or instrumental conditioning per se. First, we did not observe any effects on the query trials during the Pavlovian stage, although we acknowledge that this might not be the most sensitive measure. Second, the pattern of performance on the PIT stage is not consistent with an attenuation of Pavlovian conditioning. Attenuation of Pavlovian conditioning would have led to a flattening rather than a reversal of PIT effects. Third, effects on the PIT stage are also not confounded by effects during the instrumental learning stage. We did find effects of ATD during instrumental learning, with general declines of learning as well as a specific passive avoidance deficit after the depleting drink. However, these effects cannot account for the PIT effect, because the latter was restricted to the aversive domain, not extending to the appetitive domain.
One caveat of the present study is that the effect of interest was present only in the group of participants that received the balanced drink (BAL) on day 1 (Fig. 3; although it was significant when both groups were collapsed). Those who received the tryptophan depleting drink on day 1 did not show an effect of ATD. In fact, these participants, who also exhibited greater working memory capacity, did not show any PIT effect at all, even when tested after BAL. Thus an unexpected result was the absence of PIT after BAL in half of our participants, who incidentally also had greater working memory capacity (as measured with the digit span). We consider two possibilities. First participants with greater working memory capacity might be less vulnerable to Pavlovian response biases. This account is less plausible given the lack of a continuous association between working memory capacity and our effect of interest. Alternatively, the combined administration of the ATD and an affective manipulation that induces a certain cognitive/affective state (e.g., a PIT task) might lead to transfer or reinstatement of that state from a first testing session to subsequent testing sessions, even though the subsequent testing sessions were not done under ATD. According to this account, the abolition of PIT after BAL on day 2 may reflect formation of an association between abolished PIT and reduced serotonin states during the first visit. This alternative account concurs generally with the associative hypothesis of recurrence in depression (Robinson and Sahakian, 2008) and with empirical data from Robinson and Sahakian (2009). They showed that negative mood induction under ATD led to negative mood after ATD on a second day. Critically on this second day, there was no mood induction and the effect was not found for participants who received BAL on the first day.
A final point is that we had expected, based on Huys et al. (2011) that the aversive Pavlovian stimuli would influence instrumental responses in an action-specific manner, inhibiting approach-go actions and promoting withdrawal-go actions. We did not replicate these effects and consider the following accounts: the key difference with the paradigm of Huys et al. (2011) is that we explicitly modulated monoamines in our participants and that food intake was restricted during several hours before the experiment. This resulted in a drop in the TRP/ΣLNAA ratio even after the balanced amino acid drink (30%). It might well be that this relatively small drop in TRP/ΣLNAA might have been sufficient to disrupt action-specificity of PIT. This speculation concurs with the abolition of action-specificity in pathologies associated with serotonergic dysfunction, such as depression (Q.J.M.H. et al., unpublished data).
In conclusion, these data suggest that serotonin is selectively involved in Pavlovian inhibition due to aversive expectations. These findings might have implications for our understanding of the mechanisms underlying a range of affective, impulsive, and aggressive neuropsychiatric disorders, which have been associated with abnormal serotonin transmission. An obvious next step would be to assess the putatively aberrant Pavlovian biases on instrumental behavior in these patient groups, to advance our understanding of the neurochemical and cognitive mechanisms underlying these disorders.
Footnotes
This work was supported by a Human Frontiers Science Program Grant to Kae Nakamura, Nathaniel Daw, and R.C., as well as a VIDI grant from the Innovational Research Incentives Scheme of the Netherlands Organization for Scientific Research (NWO) and a James McDonnell scholar award. R.C. has been a consultant to Abbott Laboratories, but is not an employee or a stock shareholder. H.E.M.d.O. was supported by a VENI grant from NWO. Q.J.M.H. was supported by the German Research Foundation (Deutsche Forschungsgemeinschaft, DFG, FOR 1617, Grant RA1047/2-1). We thank the collaborators of the Psychiatric Laboratory of Erasmus University Medical Center Rotterdam for expert technical assistance.
References
- Beck AT, Steer RA, Ball R, Ranieri W. Comparison of beck depression inventories -IA and -II in psychiatric outpatients. J Pers Assess. 1996;67:588–597. doi: 10.1207/s15327752jpa6703_13. [DOI] [PubMed] [Google Scholar]
- Bond A, Lader M. The use of analogue scales in rating subjective feelings. Br J Med Psychol. 1974;47:211–218. doi: 10.1111/j.2044-8341.1974.tb02285.x. [DOI] [Google Scholar]
- Boureau YL, Dayan P. Opponency revisited: competition and cooperation between dopamine and serotonin. Neuropsychopharmacology. 2011;36:74–97. doi: 10.1038/npp.2010.151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bromberg-Martin ES, Hikosaka O, Nakamura K. Coding of task reward value in the dorsal raphe nucleus. J Neurosci. 2010;30:6262–6272. doi: 10.1523/JNEUROSCI.0015-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carver CS, White TL. Behavioral inhibition, behavioral activation, and affective responses to impending reward and punishment: the BIS/BAS scales. J Pers Soc Psychol. 1994;67:319. doi: 10.1037/0022-3514.67.2.319. [DOI] [Google Scholar]
- Cools R, Blackwell A, Clark L, Menzies L, Cox S, Robbins TW. Tryptophan depletion disrupts the motivational guidance of goal-directed behavior as a function of trait impulsivity. Neuropsychopharmacology. 2005;30:1362–1373. doi: 10.1038/sj.npp.1300704. [DOI] [PubMed] [Google Scholar]
- Cools R, Roberts AC, Robbins TW. Serotoninergic regulation of emotional and behavioral control processes. Trends Cogn Sci. 2008a;12:31–40. doi: 10.1016/j.tics.2007.10.011. [DOI] [PubMed] [Google Scholar]
- Cools R, Robinson OJ, Sahakian B. Acute tryptophan depletion in healthy volunteers enhances punishment prediction but does not affect reward prediction. Neuropsychopharmacology. 2008;33:2291–2299. doi: 10.1038/sj.npp.1301598. [DOI] [PubMed] [Google Scholar]
- Cools R, Nakamura K, Daw ND. Serotonin and dopamine: unifying affective, activational, and decision functions. Neuropsychopharmacology. 2011;36:98–113. doi: 10.1038/npp.2010.121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crockett MJ, Clark L, Robbins TW. Reconciling the role of serotonin in behavioral inhibition and aversion: acute tryptophan depletion abolishes punishment-induced inhibition in humans. J Neurosci. 2009;29:11993–11999. doi: 10.1523/JNEUROSCI.2513-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crockett MJ, Clark L, Roiser JP, Robinson OJ, Cools R, Chase HW, Ouden Hd, Apergis-Schoute A, Campbell-Meiklejohn D, Campbell-Meikeljohn D, Seymour B, Sahakian BJ, Rogers RD, Robbins TW. Converging evidence for central 5-HT effects in acute tryptophan depletion. Mol Psychiatry. 2012;17:121–123. doi: 10.1038/mp.2011.106. [DOI] [PubMed] [Google Scholar]
- Crockett MJ, Clark L, Apergis-Schoute AM, Morein-Zamir S, Robbins TW. Serotonin modulates the effects of Pavlovian aversive predictions on response vigor. Neuropsychopharmacology. 2012;37:2244–2252. doi: 10.1038/npp.2012.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daw ND, Kakade S, Dayan P. Opponent interactions between serotonin and dopamine. Neural Netw. 2002;15:603–616. doi: 10.1016/S0893-6080(02)00052-7. [DOI] [PubMed] [Google Scholar]
- Dayan P, Huys QJ. Serotonin, inhibition, and negative mood. PLoS Comput Biol. 2008;4:e4. doi: 10.1371/journal.pcbi.0040004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dayan P, Huys QJ. Serotonin in affective control. Annu Rev Neurosci. 2009;32:95–126. doi: 10.1146/annurev.neuro.051508.135607. [DOI] [PubMed] [Google Scholar]
- Dayan P, Niv Y, Seymour B, Daw ND. The misbehavior of value and the discipline of the will. Neural Netw. 2006;19:1153–1160. doi: 10.1016/j.neunet.2006.03.002. [DOI] [PubMed] [Google Scholar]
- Deakin JF. Roles of serotonergic systems in escape, avoidance and other behaviors. In: S C, editor. Theory in Psychopharmacology. London and New York: Academic; 1983. [Google Scholar]
- Deakin JF, Graeff FG. 5-HT and mechanisms of defence. J Psychopharmacol. 1991;5:305–315. doi: 10.1177/026988119100500414. [DOI] [PubMed] [Google Scholar]
- Eysenck HJ, Eysenck SBG. Manual of the Eysenck Personality Questionnaire. London: Hodder and Stoughton; 1975. [Google Scholar]
- Fekkes D, van Dalen A, Edelman M, Voskuilen A. Validation of the determination of amino acids in plasma by high-performance liquid chromatography using automated pre-column derivatization with o-phthaldialdehyde. J Chromatogr B Biomed Appl. 1995;669:177–186. doi: 10.1016/0378-4347(95)00111-U. [DOI] [PubMed] [Google Scholar]
- Fernstrom JD, Wurtman RJ. Brain serotonin content: physiological regulation by plasma neutral amino acids. Science. 1972;178:414–416. doi: 10.1126/science.178.4059.414. [DOI] [PubMed] [Google Scholar]
- Geurts DEM, Huys QJM, Ouden den HEM, Cools R. Aversive Pavlovian control of instrumental behavior in humans. J Cogn Neurosci. 2013;25:1428–1441. doi: 10.1162/jocn_a_00425. [DOI] [PubMed] [Google Scholar]
- Graeff FG, Guimarães FS, De Andrade TG, Deakin JF. Role of 5-HT in stress, anxiety, and depression. Pharmacol Biochem Behav. 1996;54:129–141. doi: 10.1016/0091-3057(95)02135-3. [DOI] [PubMed] [Google Scholar]
- Guitart-Masip M, Huys QJ, Fuentemilla L, Dayan P, Duzel E, Dolan RJ. Go and no-go learning in reward and punishment: interactions between affect and effect. Neuroimage. 2012;62:154–166. doi: 10.1016/j.neuroimage.2012.04.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamilton M. A rating scale for depression. J Neurol Neurosurg Psychiatry. 1960;23:56–62. doi: 10.1136/jnnp.23.1.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huys QJ, Cools R, Gölzer M, Friedel E, Heinz A, Dolan RJ, Dayan P. Disentangling the roles of approach, activation and valence in instrumental and Pavlovian responding. PLoS Comput Biol. 2011;7:e1002028. doi: 10.1371/journal.pcbi.1002028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huys QJ, Eshel N, O'Nions E, Sheridan L, Dayan P, Roiser JP. Bonsai trees in your head: how the Pavlovian system sculpts goal-directed choices by pruning decision trees. PLoS Comput Biol. 2012;8:e1002410. doi: 10.1371/journal.pcbi.1002410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirby KN, Maraković NN. Delay-discounting probabilistic rewards: rates decrease as amounts increase. Psychonomic Bull Rev. 1996;3:100–104. doi: 10.3758/BF03210748. [DOI] [PubMed] [Google Scholar]
- Kranz GS, Kasper S, Lanzenberger R. Reward and the serotonergic system. Neuroscience. 2010;166:1023–1035. doi: 10.1016/j.neuroscience.2010.01.036. [DOI] [PubMed] [Google Scholar]
- Man MS, Mikheenko Y, Braesicke K, Cockcroft G, Roberts AC. Serotonin at the level of the amygdala and orbitofrontal cortex modulates distinct aspects of positive emotion in primates. Int J Neuropsychopharmacol. 2011;15:91–105. doi: 10.1017/S1461145711000587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNaughton N, Corr PJ. A two-dimensional neuropsychology of defense: fear/anxiety and defensive distance. Neurosci Biobehav Rev. 2004;28:285–305. doi: 10.1016/j.neubiorev.2004.03.005. [DOI] [PubMed] [Google Scholar]
- Miyazaki K, Miyazaki KW, Doya K. The role of serotonin in the regulation of patience and impulsivity. Mol Neurobiol. 2012;45:213–224. doi: 10.1007/s12035-012-8232-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura K, Matsumoto M, Hikosaka O. Reward-dependent modulation of neuronal activity in the primate dorsal raphe nucleus. J Neurosci. 2008;28:5331–5343. doi: 10.1523/JNEUROSCI.0021-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okada KI, Nakamura K, Kobayashi Y. A neural correlate of predicted and actual reward-value information in monkey pedunculopontine tegmental and dorsal raphe nucleus during saccade tasks. Neural Plast. 2011;2011 doi: 10.1155/2011/579840. 2011:579840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patton JH, Stanford MS, Barratt ES. Factor structure of the Barratt impulsiveness scale. J Clin Psychol. 1995;51:768–774. doi: 10.1002/1097-4679(199511)51:6<768::AID-JCLP2270510607>3.0.CO;2-1. [DOI] [PubMed] [Google Scholar]
- Pavlov IP. Conditioned reflexes: an investigation of the physiological activity of the cerebral cortex. London: Oxford UP; 1927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reilly JG, McTavish SF, Young AH. Rapid depletion of plasma tryptophan: a review of studies and experimental methodology. J Psychopharmacol. 1997;11:381–392. doi: 10.1177/026988119701100416. [DOI] [PubMed] [Google Scholar]
- Robinson OJ, Sahakian BJ. Recurrence in major depressive disorder: a neurocognitive perspective. Psychol Med. 2008;38:315–318. doi: 10.1017/S0033291707001249. [DOI] [PubMed] [Google Scholar]
- Robinson OJ, Sahakian BJ. Acute tryptophan depletion evokes negative mood in healthy females who have previously experienced concurrent negative mood and tryptophan depletion. Psychopharmacology. 2009;205:227–235. doi: 10.1007/s00213-009-1533-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson OJ, Cools R, Sahakian BJ. Tryptophan depletion disinhibits punishment but not reward prediction: implications for resilience. Psychopharmacology. 2012;219:599–605. doi: 10.1007/s00213-011-2410-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers RD, Tunbridge EM, Bhagwagar Z, Drevets WC, Sahakian BJ, Carter CS. Tryptophan depletion alters the decision-making of healthy volunteers through altered processing of reward cues. Neuropsychopharmacology. 2003;28:153–162. doi: 10.1038/sj.npp.1300001. [DOI] [PubMed] [Google Scholar]
- Saulin A, Savli M, Lanzenberger R. Serotonin and molecular neuroimaging in humans using PET. Amino Acids. 2012;42:2039–2057. doi: 10.1007/s00726-011-1078-9. [DOI] [PubMed] [Google Scholar]
- Schmand B, Bakker D, Saan R, Louman J. The Dutch reading test for adults: a measure of premorbid intelligence level. Tijdschrift voor Gerontologie en Geriatrie. 1991;22:15–19. [PubMed] [Google Scholar]
- Seymour B, Daw ND, Roiser JP, Dayan P, Dolan R. Serotonin selectively modulates reward value in human decision-making. J Neurosci. 2012;32:5833–5842. doi: 10.1523/JNEUROSCI.0053-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soubrie P. Reconciling the role of central serotonin neurons in human and animal behavior. Behav Brain Sci. 1986;9:319–335. doi: 10.1017/S0140525X00022871. [DOI] [Google Scholar]
- Spielberger CD, Gorsuch RL, Lushene R, Vagg PR, Jacobs GA. Manual for the state-trait anxiety inventory. Palo Alto, CA: Consluting Pscyhologists; 1983. [Google Scholar]
- Thorndike EL. Animal intelligence. New York: Macmillan; 1911. [Google Scholar]
- Torrubia R, Ávila C, Moltó J, Caseras X. The sensitivity to punishment and sensitivity to reward questionnaire (SPSRQ) as a measure of gray's anxiety and impulsivity dimensions. Pers Individ Diff. 2001;31:837–862. doi: 10.1016/S0191-8869(00)00183-5. [DOI] [Google Scholar]
- Tye NC, Everitt BJ, Iversen SD. 5-hydroxytryptamine and punishment. Nature. 1977;268:741–743. doi: 10.1038/268741a0. [DOI] [PubMed] [Google Scholar]
- Watson D, Clark LA, Tellegen A. Development and validation of brief measures of positive and negative affect: the PANAS scales. J Pers Soc Psychol. 1988;54:1063–1070. doi: 10.1037/0022-3514.54.6.1063. [DOI] [PubMed] [Google Scholar]
- Yin HH, Ostlund SB, Balleine BW. Reward-guided learning beyond dopamine in the nucleus accumbens: the integrative functions of cortico-basal ganglia networks. Eur J Neurosci. 2008;28:1437–1448. doi: 10.1111/j.1460-9568.2008.06422.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young SN, Smith SE, Pihl RO, Ervin FR. Tryptophan depletion causes a rapid lowering of mood in normal males. Psychopharmacology. 1985;87:173–177. doi: 10.1007/BF00431803. [DOI] [PubMed] [Google Scholar]