Abstract
After spatial exploration in rats, Arc mRNA is expressed in ∼2% of dentate gyrus (DG) granule cells, and this proportion of Arc-positive neurons remains stable for ∼8 h. This long-term presence of Arc mRNA following behavior is not observed in hippocampal CA1 pyramidal cells. We report here that in rats ∼50% of granule cells with cytoplasmic Arc mRNA, induced some hours previously during exploration, also show Arc expression in the nucleus. This suggests that recent transcription can occur long after the exploration behavior that elicited it. To confirm that the delayed nuclear Arc expression was indeed recent transcription, Actinomycin D was administered immediately after exploration. This treatment resulted in inhibition of recent Arc expression both when evaluated shortly after exploratory behavior as well as after longer time intervals. Together, these data demonstrate a unique kinetic profile for Arc transcription in hippocampal granule neurons following behavior that is not observed in other cell types. Among a number of possibilities, this sustained transcription may provide a mechanism that ensures that the synaptic connection weights in the sparse population of granule cells recruited during a given behavioral event are able to be modified.
Introduction
Memory consolidation requires the regulation of existing proteins (Holahan and Routtenberg, 2007) and de novo synthesis of others (Bekinschtein et al., 2008; Bingol and Sheng, 2011; Martínez-Moreno et al., 2011). These new proteins may result from new transcripts (Morgan and Curran., 1989; Guzowski et al., 1999; Hopkins and Buci, 2010) such as the immediate early genes (IEGs), which are transiently and rapidly induced in response to stimulation and are functionally defined by their ability to be transcribed in the presence of protein synthesis inhibitors (Cole et al., 1989; Lanahan and Worley, 1998).
The IEGs Arc/Arg3.1 or activity-regulated cytoskeleton protein is known to play a key role in the cellular mechanisms underlying long-term synaptic plasticity (Link et al., 1995; Lyford et al., 1995; Miyashita et al., 2008; Bramham et al., 2010; Shepherd and Bear, 2011). Its transcription in hippocampal and cortical pyramidal neurons is transitory (Guzowski et al., 1999; Vazdarjanova et al., 2002; Ramírez-Amaya et al., 2005). Arc mRNA accumulates in the nucleus ∼5 min after strong neural activity (such as high-frequency spiking), translocates into the cytoplasm ∼30 min later, and is then rapidly degraded (Vazdarjanova et al., 2002). Arc protein is present in these neurons for ∼1 h (Ramírez-Amaya et al., 2005). However, it has been suggested that to accomplish its role during synaptic plasticity (Okuno et al., 2012), Arc protein must remain for a longer period of time (Bramham and Messaoudi, 2005; Messaoudi et al., 2007). After spatial exploration, hippocampal granule cells show sparse neural activity (Jung and McNaughton, 1993; Leutgeb et al., 2007; Alme et al., 2010) and also sparse Arc mRNA expression (Chawla et al., 2005; Alme et al., 2010), but these few neurons express Arc mRNA and its protein for ∼8 h (Ramírez-Amaya et al., 2005; Marrone et al., 2012). Sustained Arc expression in the dentate gyrus (DG) has been suggested to underlie long-term potentiation (LTP) stabilization (Messaoudi et al., 2007), suggesting that sustained Arc synthesis in granule cells may be important for synaptic plasticity and memory consolidation.
The sustained presence of Arc in hippocampal granule cells after stimulation may result from either an elevated half-life of Arc mRNA in these cells or from sustained transcription of Arc mRNA itself (Ramírez-Amaya et al., 2005). The current study investigates these possibilities with two separate experiments: first, by separately detecting presplicing Arc mRNA (pre-mRNA) observed only in the nucleus and Arc mRNA observed in both the cytoplasm and the nucleus of DG granule cells (Guzowski et al., 1999; Rosi et al., 2009) after animals were killed at different time points after a single spatial exploration session and second, by studying the effect of the transcription inhibitor Actinomycin D, administered immediately after spatial exploration, on the expression of Arc (pre-mRNA/mRNA) detected at different time points after exploration.
Our results demonstrate that, compared with controls, spatial exploration induces sustained transcription of Arc for ∼8 h in a sparse population of neurons; the sustained presence of Arc may have a number of implications for Arc-dependent synaptic plasticity in granule cells.
Materials and Methods
Animals.
Male Wistar rats (5–7 months old), obtained from the Animal Care and Reproduction facilities at the Instituto de Neurobiología, were housed individually and maintained on an inverted 12 h light/dark cycle, with lights on at 9:00 P.M. Food and water were provided ad libitum. Animals were handled for 10 d before spatial exploration experiments to minimize stress during the behavioral procedures. The Bioethics Committee from the Instituto de Neurobiología from the Universidad Nacional Autónoma de México, approved all the animal protocols and experimental procedures used in the present study, and they were performed in accordance with international ethical guidelines for animal care and handling.
Spatial exploration procedure.
The exploration environment was an open square box, 70 × 70 cm, with 20 cm high walls made of translucent acrylic. All the walls were covered from the outside with orange foam paper sheets, and the floor was partitioned into nine grids using black foam paper sheet strips. The box was located in a room with constant distal cues and dim lighting conditions (as described previously Sandoval et al., 2011). All behavioral testing was conducted during the dark half of the dark/light cycle, which is the active period for rodents. Each rat was fully covered with a white towel and then individually transported to the behavioral room (5 m from the vivarium). Subsequently, the rat was placed into the exploration environment, right in the center of one of the grids, and moved to the center of a different grid every 15 s. While no differences are observed in the proportion of cells that express Arc between ad libitum exploration or assisted exploration treatments (Vazdarjanova and Guzowski, 2004; Ramírez-Amaya et al., 2005), assisted exploration ensures that each of the grids is visited two or three times during the 5 min exploration session. This assures that Arc is expressed with lower variability between animals than would be the case with ad libitum exploration, where some animals may not explore certain portions of an environment (Vazdarjanova and Guzowski, 2004). For that reason we will refer to this behavioral treatment hereafter as spatial exploration.
Surgical procedures for lateral ventricle cannulation.
To infuse the transcription inhibitor Actinomycin D intracerebroventricularly (ICV), a single cannula was placed in the right lateral ventricle 20 d before the behavioral experiment. Animals where anesthetized with ketamine (70 mg/kg) and xylazine (6 mg/kg) and placed in a stereotaxic instrument with an incisor bar below the ear bars (i.e., flat skull). The scalp was then incised and retracted. An indwelling cannula, 12 mm long and 25 gauge (Small Parts) was used to insert a modified dental needle (BD). Coordinates for implantation of the cannula were as follows: −0.92 mm anteroposterior, −1.5 mm lateral, −4 mm dorsoventral from bregma. Fifteen days after the implant surgery, the Actinomycin D (Sigma) administration and behavioral procedures were performed while the animals were awake, either before (Experiment 2) or immediately after (Experiment 3) the 5 min exploration. A solution of Actinomycin D (6.8 μg/μl) was prepared in 0.9% NaCl and adjusted to pH 7.4. Each animal received a total 10 μl of this solution (68 μg of Actinomycin D), and the injection was performed over a 5 min period at a rate of 2 μl/min. Vehicle control animals received 10 μl of NaCl 0.9%, pH 7.4, delivered at the same rate.
Brain extraction and dissection.
At different time points after exploration, specified below, rats were decapitated with a guillotine and their brains were rapidly removed (∼2 min) and immediately frozen in isopentane immersed in a dry ice/ethanol slurry (Guzowski et al., 1999; Vazdarjanova et al., 2002). After freezing, (all) the brains were stored at –70°C until cryosectioning for in situ hybridization. Using a stainless steel matrix (Electron Microscopy Sciences), brain hemisections (we used the left hemisphere) that included the whole hippocampi were obtained. Eight to 10 brain hemisections were molded into a block with Tissue-Tek OCT compound (Sakura Finetek), such that each block contained brains from all different groups, and the position of each group differed in each block. Each block was coronally cryosectioned at 20 μm, at −21°C throughout the dorsal hippocampus between ∼ −3.2 to −3.8 mm from bregma (Paxinos and Watson, 1998). This procedure allowed us to obtain slides containing brains from each group of animals (at least one caged control hemibrain per block), using a CM1850 Leica cryostat; the sections were captured on SuperFrost slides (VWR).
Fluorescence in situ hybridization.
To unambiguously identify recent Arc transcription visualized as two transcription foci of Arc pre-mRNA and color distinguish it from earlier transcription visualized as cytoplasmic Arc mRNA surrounding the nucleus, we used the Arc intron (intron-1) riboprobe (∼700 bp) labeled with digoxigenin (DIG)-UTP and the Arc full-length riboprobe (∼3000 bp) labeled with fluorescein-UTP (Roche Molecular Biochemicals). Each riboprobe was visualized with a different fluorophore (Cy3 for the intron probe and FITC for the full-length probe). This allowed visualization of the earlier Arc transcription that had already been translocated to the cytoplasm with fluorescein (FITC; PerkinElmer) and the more recent transcription as intranuclear Arc pre-mRNA foci with cyanine-3 (Cy3, PerkinElmer). Anti-FITC antibody 1:250 (Jackson ImmunoResearch Laboratories) or Anti-DIG antibody 1:500 (Roche Molecular Biochemicals), respectively, was used as secondary antibody for each detection, respectively (Guzowski et al., 2006; Rosi et al., 2009).
This method allowed separate visualization of the cytoplasmic Arc mRNA and the recently transcribed intranuclear foci of Arc pre-mRNA (Guzowski et al., 2006; Rosi et al., 2009) in the confocal images. This approach improves reliability and counting efficacy since the larger amount of Arc mRNA found in DG granule cell cytoplasm tends to impair the ability to distinguish recent intranuclear Arc foci.
Briefly, to determine yield and integrity of the riboprobes, they were each purified on a G-50 spin column (Boehringer Mannheim), and a small aliquot was subjected to gel electrophoresis before use. Slides were fixed with freshly prepared, buffered 4% paraformaldehyde, treated with 0.5% acetic anhydride/1.5% triethanolamine, incubated in methanol/acetone (1:1 v/v) for 5 min, and equilibrated in 2× SSC buffer. Sections were then incubated with 100 μl 1× prehybridization buffer (Sigma) for 30 min at room temperature. Approximately 100 ng of riboprobe was diluted in 1× enhanced hybridization buffer (GE Healthcare) and applied to each section for overnight incubation at 56°C. Posthybridization washes with 2× SSC were followed by RNase A (10 μg/ml, 37°C, 15 min) treatment to degrade any single-stranded RNA. After quenching endogenous peroxidases with 2% H2O2, slides were incubated overnight at 4°C with an anti-DIG or antifluorescein antibody conjugated with horseradish peroxidase (HRP; Roche Molecular Biochemicals). Slides were then washed with Tris-buffered saline, and the HRP-antibody conjugate was detected using the CY3 or FITC signal amplification kit. Nuclei were counterstained with DAPI (Invitrogen).
Confocal microscopy and analysis.
Based on previous studies showing that the behaviorally induced Arc expression occurs primarily in the DG upper blade (Chawla et al., 2005; Ramírez-Amaya et al., 2005; Alme et al., 2010; Marrone et al., 2012), here we analyzed only this DG subregion. Images from the DG and CA1 were taken using a Zeiss LSM 510 Meta confocal microscope with a Plan-Apochromat 40×/1.3 NA oil-immersion objective and StichArt module. Confocal settings, such as detector gain and offset, were established with the caged control section on each slide and kept constant for the rest of the tissue sections on the same slide. Three to four z-stack (0.55 μm optical thickness/plane) sample images from CA1 from each slide were taken and a total of four slides were imaged per block, which gave us a total of 12–16 CA1 images per animal. For the DG, image z-stacks were taken from the DG granular upper blade layer systematically covering the whole blade layer; this represents 6–12 images per DG with ∼5% area overlap to build a mosaic image in which the whole DG upper blade was reconstructed. A total of 7–9 whole DG mosaic images were used as the reference image for this analysis.
Off-line image analysis was performed using the MetaMorph imaging software (Universal Image Corporation) by an experimenter blind to the experimental treatments, as described previously (Rosi et al., 2009). To calculate the total number of neurons for the enclosed granule cell layer in the DG containing the Arc-positive neurons, the area of the DG layer was measured, and to determine the volume the useful planes in the stack were counted as reported previously (Ramírez-Amaya et al., 2005; Rosi et al., 2005). The neuronal nuclei were classified as follows: negative (no staining), Arc pre-mRNA foci positive (simplified as “Arc-foci,” containing only Arc intranuclear foci staining detected with the Arc intron probe), Arc mRNA cytoplasmic positive (simplified as “Arc-cyto,” containing only cytoplasmic staining detected with the full-length probe), or as double-labeled for Arc pre-mRNA foci and Arc mRNA cytoplasmic (simplified as “Arc-double,” containing both intranuclear Arc pre-mRNA foci and cytoplasmic Arc mRNA transcripts in two different colors). When a count included all classifications we refer to it as an Arc-positive neuron (this includes the total number of the three above mentioned classifications). The percentages of Arc-foci, Arc-cyto, or Arc-double were calculated relative to the total number of granule cells included in the analysis (Ramírez-Amaya et al., 2005). Cell counts for CA1 were conducted as previously reported (Guzowski et al., 1999; Vazdarjanova et al., 2002; Ramírez-Amaya et al., 2005; Rosi et al., 2009).
Statistical analysis.
StatView software was used to perform all the statistical analyses. For each animal the total number of granule cells and the number of cells classified as Arc-foci, Arc-cyto, or Arc-double were computed for both the DG and CA1 regions. The number of cells in each classification was converted to the proportion of cells, as a relative number to the total number of cells included in the analysis. We used the proportion of neurons in each of these classifications as the dependent variables for analysis. The average proportion was calculated for each different group and group differences for the time and treatment were established with ANOVA for each classification. When the overall ANOVA was significant (p < 0.05), further comparison between groups was done using Bonferroni post hoc tests. For comparing the proportion of cells for each classification, a Student's t test was used. For all tests, the null hypothesis was rejected at the level p < 0.05.
Experiment 1: Detection of Arc (pre-mRNA/mRNA) expression in the DG at different time points after spatial exploration.
By using the intron-only sequence Arc probe, we detected recent Arc pre-mRNA transcription, and its color distinguished it from the Arc full probe, which is the only one seen in the cytoplasm surrounding the nucleus and it is characterized as Arc-cyto. This allowed us to evaluate recent transcription in the DG granule cells at different time points following the 5 min exploration session (Ramírez-Amaya et al., 2005). All animals (n = 28) were killed after exploration, either immediately (5 min, n = 3) or after 30 min (n = 3), 2 h (n = 3), 6 h (n = 4), 8 h (n = 4), 12 h (n = 4), or 24 h (n = 3). Control animals (n = 4) were taken directly from their home cages and killed at the different times on the day of the study (caged controls). To confirm that the intronic Arc pre-mRNA present in DG neurons as long as 8 h indicates a sustained transcription mechanism, we designed a pharmacological experiment to evaluate the effect of Actinomycin D on the sustained presence of Arc in DG neurons after spatial exploration.
Experiment 2: Behaviorally induced Arc mRNA transcription in the presence of Actinomycin D.
The length of time that Arc transcription was inhibited by the Actinomycin D administration was determined by using a second cohort of animals (n = 19) that received an intraventricular injection of Actinomycin D at different time points before the 5 min behavioral exploration (0.15 min, 1 h, 2 h, 8 h: n = 3 each group). Vehicle-control animals received an intraventricular injection of the vehicle where Actinomycin D was diluted and the rats were killed 15 min later (n = 3). Caged control animals remained undisturbed in their home cage (n = 4). All animals (except caged controls) were killed immediately after spatial exploration, and intranuclear foci of Arc mRNA were detected and analyzed in CA1 and DG neurons.
Experiment 3: Time course of Arc expression with Actinomycin D added after exploration.
To determine whether Arc mRNA expression in DG granule cells observed at long time points after exploration was indeed due to sustained transcription, we analyzed the effect of Actinomycin D on the sustained presence of Arc in the DG granule cells after exploration. For this purpose, a third cohort of animals (n = 25) received an intraventricular injection (through an implanted cannula) of Actinomycin D immediately after a 5 min behavioral exploration and groups were killed 0.5 h, 2 h, or 8 h later (n = 4 each). Control groups received vehicle only and were killed at the same times (0.5 h n = 3; 2 h n = 4; 8 h n = 3). Three animals were killed directly from their home cage (caged), without receiving Actinomycin D treatment or exploration, to measure basal Arc expression.
Results
Persistent detection of intronic Arc pre-mRNA in granule cells activated by spatial exploration
The proportion of DG granule neurons classified as Arc-positive (Arc-foci, Arc-cyto, and Arc-double) in animals killed immediately after a 5 min exploration session is ∼2%, and this proportion is significantly higher than the 0.7% observed in caged control animals (Fig. 1C). ANOVA revealed significant differences among groups (F(7,20) = 9.89, p < 0.01), and the Bonferroni post hoc analysis showed significant differences between caged controls and the groups examined 5 min after exploration, indicating a clear Arc-expression response in the DG after spatial exploration. The proportion of Arc-positive neurons (all classifications) was similar to that in the 5 min group when examined 30 min, 2 h, 6 h, and 8 h after a single behavioral exploration experience and the Bonferroni analysis yielded significant differences in the proportion of Arc-expressing cells compared with caged controls at these time points (p < 0.01). Twelve hours after exploration, the number of Arc-expressing cells starts to go down to normal levels, and the Bonferroni test showed significant differences between the 12 h and the 5 min groups (p = 0.003), and no differences between the group of animals killed 12 h after exploration and the caged control group (p = 0.07), suggesting that for most granule cells, the sustained Arc expression after exploration lasts for at least 8 h but <12 h.
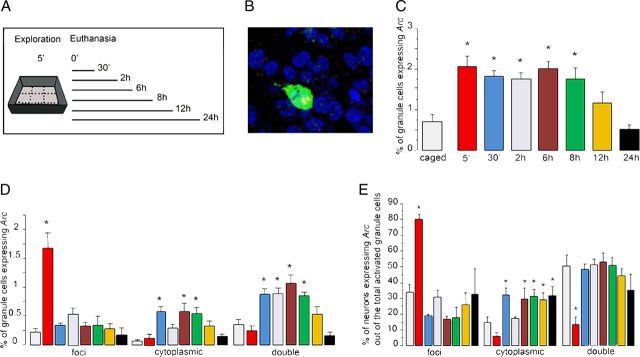
Figure 1.
A, Experimental design used to induce Arc expression with a single spatial exploration experience. All animals explored a novel environment for 5 min and were killed at different times after exploration (5 min, n = 3; 30 min, n = 3; 2 h, n = 3; 6 h, n = 4; 8 h, n = 4; 12 h, n = 4; 24 h, n = 3; caged, n = 4). Caged refers to the animals killed immediately after being taken from their home cages and is a negative control for behaviorally induced Arc. B, Representative confocal image taken with a 40×/1.3 NA objective from the DG granule cell layer stained for the intronic Arc (pre-mRNA) intranuclear foci revealed with Cy3 (red) and the full-length cytoplasmic Arc mRNA probe revealed with FITC (green); the intranuclear staining appears as yellow because the staining is overimposed with green cytoplasmic staining. Note that the cytoplasmic staining is so dense that with only one color it is not reliable for determining whether the cell presents intranuclear foci staining or not; cell body nuclei are counterstained with DAPI (blue). C, The kinetics of exploration-induced Arc mRNA is reported as the percentage of total DG granule cells, which express Arc mRNA; each bar represents the mean proportion of DG cells classified as Arc-positive (foci + cytoplasmic + double) for each treatment group (*p < 0.01 compared with caged, ± SEM). D, Compartment analysis of temporal activity by catFISH of Arc. The Arc FISH staining was found either in the nucleus only (foci), in the cytoplasm only (cytoplasmic), or in both compartments (double). The bars represent the mean proportion of cells, out of the total population of DG neurons, for each classification ± SEM, *p < 0.01 compared with caged controls. E, Proportion of activated cells with foci-only, cytoplasmic-only, or double (those with foci and cytoplasmic) Arc staining, out of the total population of Arc-positive neurons, *p < 0.01 compared with caged controls. The colors in D and E bars refer to the same time point groups indicated in C.
The cell compartment analysis of Arc expression showed that at 5 min after exploration, ∼1.6% of the DG granular cells were classified as Arc foci positive (Fig. 1D), while at later time points <0.5% of the DG granular neurons contained Arc-foci. The ANOVA analysis yielded differences among groups (ANOVA, F(7,20) = 8.36, p < 0.01), and the Bonferroni post hoc analysis indicated differences between the 5 min group and the later time points, with no differences among these later groups (p < 0.05). The proportion of DG neurons with recent transcription only at 5 min after exploration is significantly higher than for caged controls, indicating that spatial exploration-associated activity of DG granule cells driving Arc mRNA expression is observed as recent transcription in the nucleus as early as 5 min after behaviorally induced neural activity. Importantly, these newly activated neurons, those with Arc-foci only, were observed only in the 5 min group and not in the rest of the behaviorally treated animals killed at later time points. This suggests that the same cells that were activated by the original behavioral experience are the ones that express Arc at later time points (see Discussion).
Thirty minutes after exploration Arc mRNA transcription in granule cells, induced during spatial exploration, has already been spliced and translocated into the cytoplasm; interestingly however, only ∼30% of the Arc-positive neurons was classified exclusively as Arc-cyto (Fig. 1E); most were classified as Arc-double (∼50%) and fewer (∼20%) were classified as Arc-foci only. This high proportion of DG neurons classified as Arc-double was observed also at 2 h, 6 h, and 8 h, when the number of Arc-positive neurons remained higher than in the caged control group. This indicates that most of the activated neurons that translocated Arc mRNA into the cytoplasm also exhibited recent Arc transcription (they present both Arc pre-mRNA foci and Arc mRNA in the cytoplasm).
Interestingly, the few activated neurons observed in caged control animals were also classified as double, suggesting that even the small amount of neural activity driving Arc expression in caged control conditions tends to also show this pattern of sustained transcription (Fig. 1E).
Overall, these results suggest that after spatial exploration the persistent presence of Arc (cyto and foci; Fig. 1D,E) in DG granule cells, which lasts for ∼8 h, could result from sustained transcription that maintains a stable level of recent Arc mRNA expression. The results show that recent transcription found in the nucleus is observed in a large subpopulation of neurons whose cytoplasm also contains earlier transcripts. This indicates that ∼50% of the granule cells activated earlier exhibits a new round of transcription at longer times after exploration. Because the proportion of activated neurons does not fall to caged control levels until 12 h after exploration, the majority of the behaviorally activated neurons appear to undergo new rounds of transcription. The proportion of Arc-expressing neurons found in caged control animals is as low as has been reported previously, but interestingly, we found that most of these granule cells show Arc pre-mRNA foci, indicative of recent Arc transcription.
In CA1 pyramidal neurons Arc transcription after exploration is transient
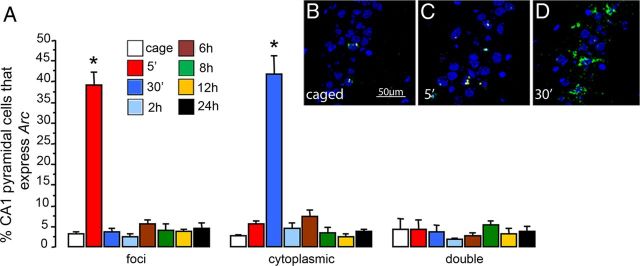
The proportion of CA1 pyramidal neurons that express intranuclear Arc immediately after a 5 min exploration was ∼39%. Intranuclear expression of Arc was elevated only in animals killed immediately after the 5 min exploration (ANOVA, F(7,20) = 72.2, p < 0.01 vs caged controls) (Fig. 2). No differences were found in CA1 when animals were killed 30 min, 2 h, 6 h, 8 h, 12 h, or 24 h after the initial exploration. The proportion of pyramidal neurons with cytoplasmic Arc mRNA in animals killed 30 min after novel environment exploration was significantly different compared with caged controls (ANOVA, F(7,20) = 56.22, p < 0.01; Fig. 2). In contrast to what is observed in granule cells, there were no differences in the percentage of pyramidal neurons classified as double, those with both recent Arc pre-mRNA intranuclear foci and cytoplasmic Arc mRNA, at any of the different time points compared with the caged controls (ANOVA, F(7,20) = 0.462, p = 0.85; Fig. 2). This result clearly indicates that exploration-induced Arc transcription in the CA1 pyramidal neurons is transient, the mRNA lasts ∼5 min in the nucleus, then translocates to the cytoplasm ∼30 min after spatial exploration and is degraded afterward. This result is in striking contrast to what is observed in granule cells.
Figure 2.
A, Mean (± SEM) proportion of CA1 pyramidal neurons that present intronic Arc pre-mRNA intranuclear foci staining (foci), Arc mRNA in the cytoplasm (cytoplasmic), and both (double), in the groups of animals killed at different time points after spatial exploration of a novel environment, *p < 0.01. Representative confocal images taken with a 40×/1.3 NA objective of the CA1 region from a caged control animal (B), an animal killed 5 min after spatial exploration (C), and an animal killed 30 min after spatial exploration (D).
Dynamics of Actinomycin D inhibition of behaviorally induced Arc mRNA transcription
We evaluated the length of time during which an ICV administration of Actinomycin D inhibits exploration-induced Arc transcription in the hippocampus. This analysis was conducted in both the CA1 and the DG regions. As detailed previously (see Materials and Methods), animals were injected with Actinomycin D at time 0 and exposed to spatial exploration 15 min, 1 h, 2 h, or 8 h later, and killed immediately afterward. Vehicle-treated animals were exposed to 5 min of spatial exploration and killed immediately afterward (Fig. 3A).
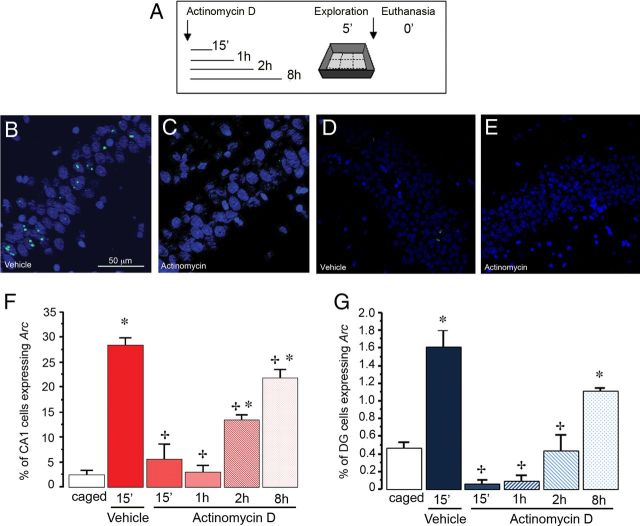
Figure 3.
A, Schematic drawing of the experimental design. Animals were allowed to explore a novel environment at different times after an initial injection of Actinomycin D and were killed immediately after the 5 min exploration. Representative confocal images of Arc mRNA expression in the CA1 (B, C) and DG (D, E) regions of the hippocampus taken with a 40×/1.3 NA objective. B, D, Intranuclear Arc mRNA foci were evident 5 min after exploration in control animals injected with vehicle. C, E, No Arc mRNA was evident in animals injected with Actinomycin D 15 min before exploratory behavior. Proportion of CA1 pyramidal neurons (F) and DG granule cell neurons (G) that express Arc mRNA (intranuclear foci) in animals killed immediately after 5 min of spatial exploration, which received Actinomycin D at different time points before the experience (0.15 min, 1 h, 2 h, 8 h; n = 3 each group). Vehicle injection was administered 15 min before exploration and was used as the positive control for behaviorally induced Arc mRNA expression. At this time point Arc mRNA was observed in the nucleus because the behavioral induction began 5 min earlier. Note that the proportion of Arc-expressing cells was significantly higher as compared with caged controls (*p < 0.01) in the 15 min vehicle group and the animals exposed to exploration 8 h after the drug administration. +p < 0.01 compared with vehicle control.
In the CA1 region, the vehicle-treated group exhibited ∼30% of cells with nuclear Arc expression in response to spatial exploration, which is significantly higher compared with caged controls (ANOVA, F(5,13) = 71.04, p < 0.01; Bonferroni post hoc p < 0.01) (Fig. 3F). Given this, the 15 min vehicle group was considered to be the behaviorally activated control. The number of Arc-expressing cells was greatly reduced in the 15 min Actinomycin D-treated group compared with the 15 min vehicle control group, and it remained low at 1 h (ps < 0.01). Two hours after Actinomycin D administration, the number of Arc-expressing cells after spatial exploration was still low as compared with the 15 min vehicle control (p < 0.01), but was already significantly greater than in caged control animals (p < 0.01). At 8 h after Actinomycin D administration, the proportion of Arc-expressing cells was again greater than in caged controls (p < 0.01), but was still significantly lower than in the vehicle control group. Importantly, both the 2 and 8 h Actinomycin D-treated groups showed significant increases in the proportion of Arc-expressing cells as compared with the 1 h Actinomycin D-treated animals.
In the DG (Fig. 3G), the results were similar to those obtained in the CA1 region. The proportion of Arc-expressing cells in the 15 min vehicle-treated animals was significantly higher than in the cage control animals (∼1.6%, ANOVA, F(5,13) = 30.09, p < 0.01; Bonferroni post hoc p < 0.01), and was dramatically reduced in the 15 min and 1 h Actinomycin D-treated groups compared with the 15 min vehicle control group (ps < 0.01). At 15 min and 1 h the proportion of Arc-expressing neurons was lower but not significantly different from the caged control animals. In contrast to what is seen in CA1, in the DG at 2 h after Actinomycin D administration, the proportion of Arc-expressing cells was still lower than the 15 min vehicle control (p < 0.01), but was not significantly different from the caged control animals. At 8 h after Actinomycin D administration, the proportion of Arc-expressing cells was again greater than the caged controls (p < 0.01), but still significantly lower than in the vehicle control group. Only the 8 h Actinomycin D-treated group showed a significant increase in the proportion of Arc-expressing cells as compared with the 1 h Actinomycin D-treated animals.
These results indicate that treatment with Actinomycin D affects behaviorally induced Arc transcription; the strongest effect lasts for ∼1 h; nevertheless, transcription continues to be reduced for at least 8 h. At this later time point, however, the number of cells that express Arc in response to spatial exploration is significantly higher than that is observed in caged control animals, indicating that the effect of Actinomycin D is wearing off and the transcription machinery is working again.
Actinomycin D blocked the sustained presence of Arc in granule cells after spatial exploration
In this experiment, we administered vehicle or Actinomycin D immediately after spatial exploration, and then killed the animals at different time points (Fig. 4A).
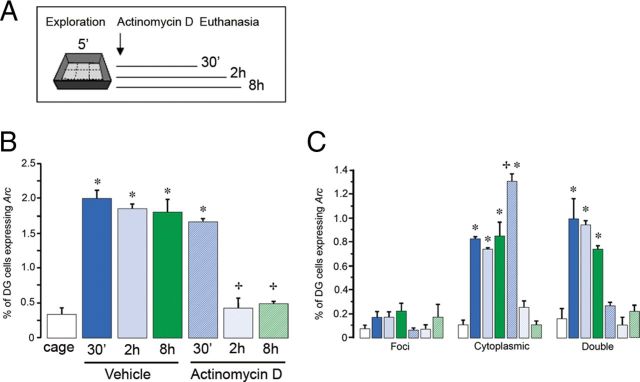
Figure 4.
A, Schematic drawing of the experimental design used. All animals were exposed to a 5 min exploration of a novel environment, then immediately injected with Actinomycin D or vehicle, followed by kill at 30 min, 2 h, or 8 h later. B, Kinetics of Arc mRNA expression in DG neurons of vehicle-treated animals compared with animals injected with Actinomycin D is reported as the percentage of total cells that were classified as Arc-positive; these are cells that express behaviorally induced Arc mRNA (foci, cytoplasmic, and both). Caged refers to the rats killed immediately after being taken from their home cages and is a negative control for behaviorally induced Arc (*p < 0.01 vs caged, +p < 0.01 vs 5 min vehicle). C, Compartment analysis of Arc mRNA-expressing cells either in the nucleus only (foci), in the cytoplasm only (cytoplasmic), or in both compartments (double), *p < 0.01.
In the vehicle-treated animals killed at different time points after exploration (30 min, 2 h, and 8 h), ∼2% of granule cells were classified as Arc-positive (Fig. 4B), and this proportion was significantly higher than that observed in caged controls (ANOVA, F(6,18) = 46.79, p < 0.01; Bonferroni post hoc p < 0.01). No differences were found between all vehicle-treated animals.
In the Actinomycin D-treated animals the greater proportion of Arc-expressing cells was found in the group of animals killed 30 min after spatial exploration (∼2%, Fig. 4B), and this proportion of Arc-positive neurons was significantly higher when compared with caged controls (ANOVA, F(6,18) = 59.83, p < 0.01; Bonferroni post hoc ps < 0.01). Interestingly, animals treated with Actinomycin D and killed at 2 and 8 h after behavioral exploration showed a proportion of Arc-positive neurons in the DG that do not differ from caged controls, but was significantly lower as compared with the Actinomycin D-treated animals killed at 30 min (ps < 0.01). It is of interest that inhibition of Arc expression was also observed 8 h after exploration, when the effect of Actinomycin D is already returning to normal levels (compare with Fig. 3D).
When we looked at the compartmental expression of Arc (distinguishing between foci, cytoplasmic, and double Arc staining) in the different groups (Fig. 4C), we found that the cytoplasmic Arc mRNA accounted for the majority of the Arc-positive neurons found in the Actinomycin D-treated animals killed at 30 min after exploration. This was due to the fact that during spatial exploration the transcription machinery was intact, allowing the induction of Arc mRNA expression in ∼2% of DG neurons. Thirty minutes later Arc mRNA was already translocated into the cytoplasm, but at this time point transcription was already inhibited by Actinomycin D. For this reason the proportion of Arc-double stained cells and Arc-foci was significantly lower than the vehicle-treated groups (30 min, 2 h, and 8 h) (ANOVA, F(6,18) = 25.60, p < 0.01; Bonferroni post hoc ps < 0.01). This indicated that Actinomycin D applied immediately after exploration inhibited further rounds of Arc transcription responsible for the long-term presence of Arc in DG neurons after spatial exploration. Because the proportion of Arc-expressing cells remains stable for 8 h, we would expect that administration of Actinomycin D at other time points after exploration, others than those used in the present experiment, would also result in cells with only cytoplasmic staining if the animals are killed 30 min later.
Discussion
Here we demonstrate that the long-term presence of Arc in DG granule cells in response to spatial exploration (Ramírez-Amaya et al., 2005; Marrone et al., 2012) results from a sustained transcription mechanism. This conclusion is based on several arguments: first, the neurons recruited during the initial exploration experience are the same ones that express Arc at later time points; second, the detection of Arc pre-mRNA with the intronic probe (intranuclear Arc foci) indicates recent transcription; and finally, this recent transcription is sustained.
Using Arc compartment analysis of temporal activity by fluorescence in situ hybridization (catFISH), Chawla et al. (2005) showed that after consecutive exploration of two environments (with a 20 min interval) there was a small independent population of granule cells that responded to the second environment when it was different from the first one (expressing Arc intranuclear foci only). This suggests that if a population of DG neurons different from those originally recruited was activated a long time after a single exploration, significantly more DG neurons with intranuclear Arc foci only (Arc pre-mRNA) should have been observed in the groups of animals killed at long-time intervals. As can be seen (Fig. 1D,E), no significant changes were observed in the proportion of DG neurons with recent Arc transcription across the different time points (>5 min) after exploration. Moreover, Alme et al. (2010) using catFISH and electrophysiology demonstrated that the same population of granule cells activated by the first exploration tended to be activated during the consecutive exploration of 4 different environments. Together, this evidence strongly suggests that the neurons originally activated by a single exploration continue to be the ones that express Arc at later time points.
To confirm that the presence of recent Arc pre-mRNA (detected with the intronic probe) at long times after exploration resulted from sustained Arc transcription, we performed a pharmacological experiment in which transcription was inhibited by the administration of Actinomycin D immediately after exploration of a novel environment. When the animals were killed 30 min later, it was observed that ∼2% of DG granule neurons had already translocated Arc into the cytoplasm but, importantly, no intranuclear Arc pre-mRNA was observed (Fig. 4C). This demonstrates that Arc pre-mRNA staining observed in the nuclei is indeed a result of recent transcription.
By simultaneously detecting Arc pre-mRNA in the nucleus using the intronic probe and mature Arc mRNA using the full-length probe, we were able to reliably distinguish between recent (∼5 min) and earlier (∼30 min) Arc transcription (Rosi et al., 2009). We observed that ∼50% of DG granule neurons that were activated by the behavioral exploration experience (at least 30 min earlier, characterized by cytoplasmic Arc mRNA) also contained intranuclear Arc pre-mRNA staining across all time points (Fig. 1E). These data suggest that from 30 min to 8 h after spatial exploration, the transcription of Arc is initiated again in a subpopulation of the originally activated neurons. Because the average total proportion of activated neurons does not vary across the different time points (Fig. 1C), it is either possible that Arc mRNA transcription is being turned on and off, or that splicing is active or inactive in an Arc pre-mRNA concentration-dependent manner. Importantly, our histological detection of Arc pre-mRNA suggests that the Arc splicing mechanism lasts ∼5 min. In either case, a continuous transcription or in pulses, we considered this to constitute “sustained transcription of Arc after spatial exploration.”
The mechanism by which Arc transcription is sustained for ∼8 h currently is unknown. There are at least two possibilities, network or intrinsic cellular mechanisms (Ramírez-Amaya et al., 2005; Trifilieff et al., 2006). While the current data cannot conclusively distinguish between these, it is interesting to note that 8 h after Actinomycin D administration, even though the number of Arc mRNA-positive neurons after exploration does not completely return to the levels in vehicle-treated animals, its inhibitory effect is clearly diminished (Fig. 3G). If the mechanism responsible for the sustained transcription is reverberatory network activity, it would have been expected that when the transcriptional machinery begins to recover, Arc transcription should also begin to return. It is important to note that electrophysiological studies do not observe continuous spiking after spatial exploration, although it has been shown that off-line reactivation activity does occur intermittently during periods of sleep or rest (Shen et al., 1998). Together, these observations tend to favor an intrinsic cellular mechanism underlying the sustained transcription of Arc, although this remains to be demonstrated.
Three signal transduction pathways are known to regulate Arc transcription: (1) glutamate, (2) brain-derived neurotrophic factor (BDNF), and (3) acetylcholine. Strong synaptic activity results in glutamate NMDA receptor-dependent Arc transcription (Lyford et al., 1995; Martin, 2001; Steward and Worley, 2001; Czerniawski et al., 2011; El-Sayed et al., 2011), but electrophysiological recordings of granule cells in behaving rats suggest that it is unlikely that strong synaptic activity is maintained continuously (Shen et al., 1998). It is possible, however, that NMDA receptors in granule cells may become hypersensitive by anchoring the protein kinase CaMKIIβ for a prolonged period of time (Shen et al., 2000), resulting in the trigger of Arc mRNA transcription with more subtle synaptic activity in NMDA-sensitized granule cells. Because Arc expression is also induced by BDNF in DG granule cells (Bramham and Messaoudi, 2005) and sustained release of BDNF has been suggested to occur after certain LTP induction protocols (Kang et al., 1997; Aicardi et al., 2004; Santi et al., 2006), it is possible that sustained BDNF release could play a role in the observed sustained Arc transcription after spatial exploration. Finally, the activation of metabotropic acetylcholine receptors is known to play an important role in Arc mRNA transcription (Teber et al., 2004; Fletcher et al., 2007) and cholinergic activity is induced primarily during information acquisition (Miranda and Bermúdez-Rattoni, 1999; Sarter et al., 2003). Thus, it is possible that the sustained transcription of Arc in DG occurs only after exploration of a novel and not after a familiar environment, a possibility that we are currently investigating.
Arc protein is important for synaptic plasticity (Guzowski et al., 2000). The idea is that Arc may work as a synaptic tag, since it is known that its mRNA travels into the activated region of the dendrites (Steward and Worley, 2001). Recently a conceptually similar idea was proposed, indicating that Arc actually mediates what is known as “inverse synaptic tagging” by promoting synaptic scaling in the nonactivated synapses (Okuno et al., 2012), eliminating nonspecific changes that occur during the establishment of synaptic plasticity. It has also been demonstrated that Arc mRNA is rapidly degraded by a nonsense-mediated mRNA decay mechanism (Giorgi et al., 2007; Bramham et al., 2010), indicating that Arc mRNA may go through only a single round of translation (Maquat, 2004), and also Arc protein is rapidly degraded (Ramírez-Amaya et al., 2005; Greer et al., 2010; Shepherd and Bear, 2011). The question that arises in the face of these observations is how such transitory presence of Arc protein can engage the synaptic plasticity machinery necessary for synaptic consolidation (Guzowski et al., 2000; Messaoudi et al., 2002; Plath et al., 2006). For these reasons the prolonged availability of Arc in granule cells has been suggested to provide a mechanism that could maintain sufficient protein levels to achieve synaptic consolidation (Bramham and Messaoudi, 2005; Messaoudi et al., 2007). Notably, the data reported here show that behavior can induce sustained transcription of Arc in DG neurons, possibly providing the conditions under which Arc can mediate synaptic plasticity, in DG neurons.
The sustained transcription of Arc mRNA in the hippocampus occurs only in DG granule cells and not CA1 pyramidal cells; this is noteworthy considering that in the DG only ∼2% of neurons become activated in response to experience (a sparse coding scheme; McNaughton and Morris, 1987). This sparse code may be due to particular features of DG neurons such as the high levels of depolarization before action potential threshold is reached (Barnes and McNaughton, 1980), a different transcriptional repression profile (Penner et al., 2010), and perhaps the number of spikes elicited to a given behavior (Leutgeb et al., 2007). This sparse code theoretically allows the DG to store a large amount of nonoverlapping information, but the code in the DG tends to use the same neurons for several different representations (Alme et al., 2010). Therefore, it appears that the DG uses different activity sequences with the same neurons for each representation, in contrast to other regions of hippocampus and cortex, where different spatial experiences tend to recruit independent and large groups (∼40%) of pyramidal neurons. Given the role of Arc in synaptic plasticity (Guzowski et al., 2000; Messaoudi et al., 2002; Plath et al., 2006; Okuno et al., 2012), it is conceivable that the sustained transcription of Arc in a population of neurons that is sparse in their original activation might ensure an efficient modification of the synaptic strengths of the behaviorally recruited neural network.
Footnotes
This work was supported by funding provided by CONACyT P51028325 and 130802 (V.R.-A.) and DGAPA UNAM PAPIIT IN216510-23 (V.R.-A.), McKnight Brain Research Foundation (C.A.B.), the state of Arizona and ADHS (C.A.B.), and R01CA133216 (S.R.). We thank Dr. Hiroyuki Okuno for his comments, which helped in the discussion of this manuscript; B.S. Damaris Ketino Rangel Guerrero for manuscript reviewing; Dr. Dorothy Pless and Michelle Carroll for proofreading this manuscript; M.C. Carlos Lozano Flores for technical assistance; Nidia Hernandez Rios from the INB microscope core unit for technical assistance; M.V.Z. Martín García Servín for animal care; and Cutberto Dorado Mendieta for his laboratory assistance.
The authors declare no competing financial interests.
References
- Aicardi G, Argilli E, Cappello S, Santi S, Riccio M, Thoenen H, Canossa M. Induction for long-term potentiation and depression is reflected by corresponding changes in secretion of endogenous brain-derived neurotrophic factor. Proc Natl Acad Sci U S A. 2004;101:15788–15792. doi: 10.1073/pnas.0406960101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alme CB, Buzzetti RA, Marrone DF, Leutgeb JK, Chawla MK, Schaner MJ, Bohanick JD, Khoboko T, Leutgeb S, Moser EI, Moser MB, McNaughton BL, Barnes CA. Hippocampal granule cells opt for early retirement. Hippocampus. 2010;20:1109–1123. doi: 10.1002/hipo.20810. [DOI] [PubMed] [Google Scholar]
- Barnes CA, McNaughton BL. Physiological compensation for loss of afferent synapses in rat hippocampal granule cells during senescence. J Physiol. 1980;309:473–485. doi: 10.1113/jphysiol.1980.sp013521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bekinschtein P, Cammarota M, Katche C, Slipczuk L, Rossato JI, Goldin A, Izquierdo I, Medina JH. BDNF is essential to promote persistence of long-term memory storage. Proc Natl Acad Sci U S A. 2008;105:2711–2716. doi: 10.1073/pnas.0711863105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bingol B, Sheng M. Deconstruction for reconstruction: the role of proteolysis in neural plasticity and disease. Neuron. 2011;69:22–32. doi: 10.1016/j.neuron.2010.11.006. [DOI] [PubMed] [Google Scholar]
- Bramham CR, Messaoudi E. BDNF function in adult synaptic plasticity: the synaptic consolidation hypothesis. Prog Neurobiol. 2005;76:99–125. doi: 10.1016/j.pneurobio.2005.06.003. [DOI] [PubMed] [Google Scholar]
- Bramham CR, Alme MN, Bittins M, Kuipers SD, Nair RR, Pai B, Panja D, Schubert M, Soule J, Tiron A, Wibrand K. The Arc of synaptic memory. Exp Brain Res. 2010;200:125–140. doi: 10.1007/s00221-009-1959-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chawla MK, Guzowski JF, Ramirez-Amaya V, Lipa P, Hoffman KL, Marriott LK, Worley PF, McNaughton BL, Barnes CA. Sparse, environmentally selective expression of Arc RNA in the upper blade of the rodent fascia dentata by brief spatial experience. Hippocampus. 2005;15:579–586. doi: 10.1002/hipo.20091. [DOI] [PubMed] [Google Scholar]
- Cole AJ, Saffen DW, Baraban JM, Worley PF. Rapid increase of an immediate early gene messenger RNA in hippocampal neurons by synaptic NMDA receptor activation. Nature. 1989;340:474–476. doi: 10.1038/340474a0. [DOI] [PubMed] [Google Scholar]
- Czerniawski J, Ree F, Chia C, Ramamoorthi K, Kumata Y, Otto TA. The importance of having Arc: expression of the immediate-early gene Arc is required for hippocampus-dependent fear conditioning and blocked by NMDA receptor antagonism. J Neurosci. 2011;31:11200–11207. doi: 10.1523/JNEUROSCI.2211-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El-Sayed M, Hofman-Bang J, Mikkelsen JD. Effect of brain-derived neurotrophic factor on activity-regulated cytoskeleton-associated protein gene expression in primary frontal cortical neurons. Comparison with NMDA and AMPA. Eur J Pharmacol. 2011;660:351–357. doi: 10.1016/j.ejphar.2011.03.055. [DOI] [PubMed] [Google Scholar]
- Fletcher BR, Baxter MG, Guzowski JF, Shapiro ML, Rapp PR. Selective cholinergic depletion of the hippocampus spares both behaviorally induced Arc transcription and spatial learning and memory. Hippocampus. 2007;17:227–234. doi: 10.1002/hipo.20261. [DOI] [PubMed] [Google Scholar]
- Giorgi C, Yeo GW, Stone ME, Katz DB, Burge C, Turrigiano G, Moore MJ. The EJC factor eIF4AIII modulates synaptic strength and neuronal protein expression. Cell. 2007;130:179–191. doi: 10.1016/j.cell.2007.05.028. [DOI] [PubMed] [Google Scholar]
- Greer PL, Hanayama R, Bloodgood BL, Mardinly AR, Lipton DM, Flavell SW, Kim TK, Griffith EC, Waldon Z, Maehr R, Ploegh HL, Chowdhury S, Worley PF, Steen J, Greenberg ME. The Angelman Syndrome protein Ube3A regulates synapse development by ubiquitinating Arc. Cell. 2010;140:704–716. doi: 10.1016/j.cell.2010.01.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guzowski JF, McNaughton BL, Barnes CA, Worley PF. Environment-specific expression of the immediate-early gene Arc in hippocampal neuronal ensembles. Nat Neurosci. 1999;2:1120–1124. doi: 10.1038/16046. [DOI] [PubMed] [Google Scholar]
- Guzowski JF, Lyford GL, Stevenson GD, Houston FP, McGaugh JL, Worley PF, Barnes CA. Inhibition of activity-dependent arc protein expression in the rat hippocampus impairs the maintenance of long-term potentiation and the consolidation of long-term memory. J Neurosci. 2000;20:3993–4001. doi: 10.1523/JNEUROSCI.20-11-03993.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guzowski JF, Miyashita T, Chawla MK, Sanderson J, Maes LI, Houston FP, Lipa P, McNaughton BL, Worley PF, Barnes CA. Recent behavioral history modifies coupling between cell activity and Arc gene transcription in hippocampal CA1 neurons. Proc Natl Acad Sci U S A. 2006;103:1077–1082. doi: 10.1073/pnas.0505519103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holahan MR, Routtenberg A. Post-translational synaptic protein modification as substrate for long-lasting, remote memory: an initial test. Hippocampus. 2007;17:93–97. doi: 10.1002/hipo.20245. [DOI] [PubMed] [Google Scholar]
- Hopkins ME, Bucci DJ. BDNF expression in perirhinal cortex is associated with exercise-induced improvement in object recognition memory. Neurobiol Learn Mem. 2010;94:278–284. doi: 10.1016/j.nlm.2010.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung MW, McNaughton BL. Spatial selectivity of unit activity in the hippocampal granular layer. Hippocampus. 1993;3:165–182. doi: 10.1002/hipo.450030209. [DOI] [PubMed] [Google Scholar]
- Kang H, Welcher AA, Shelton D, Schuman EM. Neurotrophins and time: different roles for TrkB signaling in hippocampal long-term potentiation. Neuron. 1997;19:653–664. doi: 10.1016/s0896-6273(00)80378-5. [DOI] [PubMed] [Google Scholar]
- Lanahan A, Worley P. Immediate-early genes and synaptic function. Neurobiol Learn Mem. 1998;70:37–43. doi: 10.1006/nlme.1998.3836. [DOI] [PubMed] [Google Scholar]
- Leutgeb JK, Leutgeb S, Moser MB, Moser EI. Pattern separation in the dentate gyrus and CA3 of the hippocampus. Science. 2007;315:961–966. doi: 10.1126/science.1135801. [DOI] [PubMed] [Google Scholar]
- Link W, Konietzko U, Kauselmann G, Krug M, Schwanke B, Frey U, Kuhl D. Somatodendritic expression of an immediate early gene is regulated by synaptic activity. Proc Natl Acad Sci U S A. 1995;92:5734–5738. doi: 10.1073/pnas.92.12.5734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyford GL, Yamagata K, Kaufmann WE, Barnes CA, Sanders LK, Copeland NG, Gilbert DJ, Jenkins NA, Lanahan AA, Worley PF. Arc, a growth factor and activity-regulated gene, encodes a novel cytoskeleton-associated protein that is enriched in neuronal dendrites. Neuron. 1995;14:433–445. doi: 10.1016/0896-6273(95)90299-6. [DOI] [PubMed] [Google Scholar]
- Maquat LE. Nonsense-mediated mRNA decay: splicing, translation and mRNP dynamics. Nat Rev Mol Cell Biol. 2004;5:89–99. doi: 10.1038/nrm1310. [DOI] [PubMed] [Google Scholar]
- Marrone DF, Satvat E, Shaner MJ, Worley PF, Barnes CA. Attenuated long-term Arc expression in the aged fascia dentata. Neurobiol Aging. 2012;33:979–990. doi: 10.1016/j.neurobiolaging.2010.07.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin KC. Arc mRNA dynamics: return to sender–the NMDA receptor provides the targeting address for Arc mRNA. Trends Neurosci. 2001;24:621–623. doi: 10.1016/s0166-2236(00)01926-3. [DOI] [PubMed] [Google Scholar]
- Martínez-Moreno A, Rodríguez-Durán LF, Escobar ML. Late protein synthesis-dependent phases in CTA long-term memory: BDNF requirement. Front Behav Neurosci. 2011;5:61. doi: 10.3389/fnbeh.2011.00061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNaughton BL, Morris RGM. Hippocampal synaptic enhancement and information storage within a distributed memory system. Trends Neurosci. 1987;10:408–415. [Google Scholar]
- Messaoudi E, Ying SW, Kanhema T, Croll SD, Bramham CR. Brain-derived neurotrophic factor triggers transcription-dependent, late phase long-term potentiation in vivo. J Neurosci. 2002;22:7453–7461. doi: 10.1523/JNEUROSCI.22-17-07453.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Messaoudi E, Kanhema T, Soulé J, Tiron A, Dagyte G, da Silva B, Bramham CR. Sustained Arc/Arg3.1 synthesis controls long-term potentiation consolidation through regulation of local actin polymerization in the dentate gyrus in vivo. J Neurosci. 2007;27:10445–10455. doi: 10.1523/JNEUROSCI.2883-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miranda MI, Bermúdez-Rattoni F. Reversible inactivation of the nucleus basalis magnocellularis induces disruption of cortical acetylcholine release and acquisition, but not retrieval, of aversive memories. Proc Natl Acad Sci U S A. 1999;96:6478–6482. doi: 10.1073/pnas.96.11.6478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyashita T, Kubik S, Lewandowski G, Guzowski JF. Networks of neurons, networks of genes: an integrated view of memory consolidation. Neurobiol Learn Mem. 2008;89:269–284. doi: 10.1016/j.nlm.2007.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan JI, Curran T. Stimulus-transcription coupling in neurons: role of cellular immediate-early genes. Trends Neurosci. 1989;12:459–462. doi: 10.1016/0166-2236(89)90096-9. [DOI] [PubMed] [Google Scholar]
- Okuno H, Akashi K, Ishii Y, Yagishita-Kyo N, Suzuki K, Nonaka M, Kawashima T, Fujii H, Takemoto-Kimura S, Abe M, Natsume R, Chowdhury S, Sakimura K, Worley PF, Bito H. Inverse synaptic tagging of inactive synapses via dynamic interaction of Arc/Arg3.1 with CaMKIIβ. Cell. 2012;149:886–898. doi: 10.1016/j.cell.2012.02.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The rat brain in stereotaxic coordinates. Ed 4. San Diego, CA: Academic; 1998. [DOI] [PubMed] [Google Scholar]
- Penner MR, Roth TL, Barnes CA, Sweatt JD. An epigenetic hypothesis of aging-related cognitive dysfunction. Front Aging Neurosci. 2010;2:9. doi: 10.3389/fnagi.2010.00009. 2010 Mar 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plath N, Ohana O, Dammermann B, Errington ML, Schmitz D, Gross C, Mao X, Engelsberg A, Mahlke C, Welzl H, Kobalz U, Stawrakakis A, Fernandez E, Waltereit R, Bick-Sander A, Therstappen E, Cooke SF, Blanquet V, Wurst W, Salmen B, et al. Arc/Arg3.1 is essential for the consolidation of synaptic plasticity and memories. Neuron. 2006;52:437–444. doi: 10.1016/j.neuron.2006.08.024. [DOI] [PubMed] [Google Scholar]
- Ramírez-Amaya V, Vazdarjanova A, Mikhael D, Rosi S, Worley PF, Barnes CA. Spatial exploration-induced Arc mRNA and protein expression: evidence for selective, network-specific reactivation. J Neurosci. 2005;25:1761–1768. doi: 10.1523/JNEUROSCI.4342-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosi S, Ramirez-Amaya V, Vazdarjanova A, Worley PF, Barnes CA, Wenk GL. Neuroinflammation alters the hippocampal pattern of behaviorally induced Arc expression. J Neurosci. 2005;25:723–7231. doi: 10.1523/JNEUROSCI.4469-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosi S, Ramirez-Amaya V, Vazdarjanova A, Esparza EE, Larkin PB, Fike JR, Wenk GL, Barnes CA. Accuracy of hippocampal network activity is disrupted by neuroinflammation: rescue by memantine. Brain. 2009;132:2464–2477. doi: 10.1093/brain/awp148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandoval CJ, Martínez-Claros M, Bello-Medina PC, Pérez O, Ramírez-Amaya V. When are new hippocampal neurons, born in the adult brain, integrated into the network that processes spatial information? PLoS One. 2011;6:e17689. doi: 10.1371/journal.pone.0017689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santi S, Cappello S, Riccio M, Bergami M, Aicardi G, Schenk U, Matteoli M, Canossa M. Hippocampal neurons recycle BDNF for activity-dependent secretion and LTP maintenance. EMBO J. 2006;25:4372–4380. doi: 10.1038/sj.emboj.7601303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarter M, Bruno JP, Givens B. Attentional functions of cortical cholinergic inputs: what does it mean for learning and memory? Neurobiol Learn Mem. 2003;80:245–256. doi: 10.1016/s1074-7427(03)00070-4. [DOI] [PubMed] [Google Scholar]
- Shen J, Kudrimoti HS, McNaughton BL, Barnes CA. Reactivation of neuronal ensembles in hippocampal dentate gyrus during sleep after spatial experience. J Sleep Res. 1998;7:6–16. doi: 10.1046/j.1365-2869.7.s1.2.x. [DOI] [PubMed] [Google Scholar]
- Shen K, Teruel MN, Connor JH, Shenolikar S, Meyer T. Molecular memory by reversible translocation of calcium/calmodulin-dependent protein kinase II. Nat Neurosci. 2000;3:881–886. doi: 10.1038/78783. [DOI] [PubMed] [Google Scholar]
- Shepherd JD, Bear MF. New views of Arc, a master regulator of synaptic plasticity. Nat Neurosci. 2011;14:279–284. doi: 10.1038/nn.2708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steward O, Worley PF. Selective targeting of newly synthesized Arc mRNA to active synapses requires NMDA receptor activation. Neuron. 2001;30:227–240. doi: 10.1016/s0896-6273(01)00275-6. [DOI] [PubMed] [Google Scholar]
- Teber I, Köhling R, Speckmann EJ, Barnekow A, Kremerskothen J. Muscarinic acetylcholine receptor stimulation induces expression of the activity-regulated cytoskeleton-associatedgene (ARC) Brain Res Mol Brain Res. 2004;121:131–136. doi: 10.1016/j.molbrainres.2003.11.017. [DOI] [PubMed] [Google Scholar]
- Trifilieff P, Herry C, Vanhoutte P, Caboche J, Desmedt A, Riedel G, Mons N, Micheau J. Foreground contextual fear memory consolidation requires two independent phases of hippocampal ERK/CREB activation. Learn Mem. 2006;13:349–358. doi: 10.1101/lm.80206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vazdarjanova A, Guzowski JF. Differences in hippocampal neuronal population responses to modifications of an environmental context: evidence for distinct, yet complementary, functions of CA3 and CA1 ensembles. J Neurosci. 2004;24:6489–6496. doi: 10.1523/JNEUROSCI.0350-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vazdarjanova A, McNaughton BL, Barnes CA, Worley PF, Guzowski JF. Experience-dependent coincident expression of the effector immediate-early genes arc and Homer 1a in hippocampal and neocortical neuronal networks. J Neurosci. 2002;22:10067–10071. doi: 10.1523/JNEUROSCI.22-23-10067.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]