Key Points
Question
Is edonerpic maleate, an experimental drug that protects against neurotoxic effects, preserves synapses, and improves memory deficits in preclinical models related to Alzheimer disease, efficacious and safe for patients with Alzheimer disease?
Findings
In this randomized phase 2 clinical trial that included 484 patients with mild to moderate Alzheimer disease treated with cholinesterase inhibitors with or without memantine, neither dose of edonerpic improved cognition or function over 52 weeks compared with placebo. Edonerpic also did not have clear effects on amyloid, tau, or brain magnetic resonance imaging biomarkers.
Meaning
In advancing from preclinical testing, edonerpic has not demonstrated clinical proof of concept in Alzheimer disease.
Abstract
Importance
Edonerpic maleate (T-817MA) protects against Aβ40-induced neurotoxic effects and memory deficits, promotes neurite outgrowth, and preserves hippocampal synapses and spatial memory in tau transgenic mice. These effects may be mediated via sigma-1 receptor activation, delivery of synaptic AMPA receptors, or modulation of microglial function and may benefit patients with Alzheimer disease.
Objective
To assess the efficacy, safety, and tolerability of edonerpic for patients with mild to moderate Alzheimer disease.
Design, Setting, and Participants
Randomized, double-blind, placebo-controlled, parallel-group, phase 2 clinical trial conducted over 52 weeks from June 2, 2014, to December 14, 2016, at 52 US clinical and academic centers. Of 822 outpatients screened, 484 met the following criteria and were randomly assigned to treatment: 55 to 85 years of age, probable Alzheimer disease, Mini-Mental State Examination scores from 12 to 22, and taking stable doses of donepezil or rivastigmine with or without memantine.
Interventions
Random assignment (1:1:1 allocation) to placebo or 224 mg or 448 mg of edonerpic maleate, once per day.
Main Outcomes and Measures
Coprimary outcomes were scores on the Alzheimer Disease Assessment Scale–Cognitive Subscale (ADAS-cog) and Alzheimer’s Disease Cooperative Study–Clinical Impression of Change (ADCS-CGIC) at week 52. Biomarkers were brain, lateral ventricular, and hippocampal volumes, as determined on magnetic resonance imaging, and cerebrospinal fluid Aβ40, Aβ42, total tau, and phospho-tau181. The primary efficacy analysis was performed on the coprimary end points for the modified intention-to-treat population.
Results
Of 482 participants in the safety population, 140 of 158 participants (88.6%) assigned to placebo, 117 of 166 participants (70.5%) to 224 mg of edonerpic maleate, and 120 of 158 participants (76.0%) to 448 mg of edonerpic maleate completed the trial. The mean ADAS-cog score change at week 52 was 7.91 for the placebo group, 7.45 for the 224-mg group, and 7.08 for the 448-mg group. Mean differences from placebo were −0.47 (95% CI, −2.36 to 1.43; P = .63) for the 224-mg group and −0.84 (95% CI, −2.75 to 1.08; P = .39) for the 448-mg group. Mean ADCS-CGIC scores were 5.22 for the placebo group, 5.24 for the 224-mg group, and 5.25 for the 448-mg group, with mean differences from placebo of 0.03 (95% CI, −0.20 to 0.25; P = .81) for the 224-mg group and 0.04 (95% CI, −0.19 to 0.26; P = .76) for the 448-mg group. In the safety population, a total of 7 of 158 participants (4.4%) in the placebo group, 23 of 166 participants (13.9%) in the 224-mg group, and 23 of 158 participants (14.6%) in the 448-mg group discontinued because of adverse events. The most frequent adverse events were diarrhea and vomiting.
Conclusions and Relevance
Edonerpic maleate appeared to be safe and tolerable, with expected gastrointestinal symptoms occurring early but without evidence for a clinical effect among patients with mild to moderate Alzheimer disease.
Trial Registration
ClinicalTrials.gov identifier: NCT02079909
This phase 2 randomized clinical trial assesses the efficacy, safety, and tolerability of edonerpic maleate for patients with mild to moderate Alzheimer disease.
Introduction
Edonerpic maleate (T-817MA, 1-{3-[2-(1-benzothiophen-5-yl)ethoxy]propyl}azetidin-3-ol maleate) is a low-molecular-weight compound that protects against Aβ40-induced neurotoxic effects and memory deficits in a rat model,1 increases cortical and neurite outgrowth in rat hippocampal slices,2 preserves hippocampal synapses and spatial memory in tau transgenic P301L mice,3 and enhances neuroplasticity and motor function recovery after injury in nonhuman primates.4 These effects may be mediated through sigma-1 receptor activation,5 modulation of microglia function,6 and interaction with collapsin-response-mediator-protein 2 (CRMP2)4 facilitation of synaptic AMPA (α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid) receptor delivery.
Sixteen phase 1 clinical trials, 1 phase 2a trial (NCT00663936),7 and 1 phase 2 clinical trial undertaken in Japan (JP201, JapicCTI-142499(ja)) have been completed that included 1068 patients who received medication. Edonerpic maleate was well tolerated at a single dose up to 896 mg and at once-daily doses of 672 mg for up to 2 weeks.
An earlier phase 2a trial7 in Alzheimer disease demonstrated safety and tolerability of edonerpic maleate at a 224-mg/d dose during 1 year, a dose expected to achieve exposures sufficient for neuroprotective effects based on the preclinical studies. The trial did not demonstrate efficacy on primary and secondary outcomes, but there was post hoc evidence for a positive cognitive effect in patients with moderate impairment who had Mini-Mental State Examination (MMSE) scores less than 20.7 The main adverse effect was mild diarrhea during the first 10 weeks of treatment.
The main objectives of this phase 2 trial were to assess efficacy, safety, and tolerability of edonerpic maleate in participants with mild to moderate Alzheimer disease using a higher dose of 448 mg/d and a larger sample. Clinical outcomes were supplemented by assessments of brain volumes as measured on magnetic resonance imaging (MRI), cerebrospinal fluid (CSF) amyloid and tau concentrations, and population pharmacokinetics.
Methods
Study Design
This is a multicenter, randomized, double-blind, placebo-controlled, parallel-group clinical trial with a 52-week treatment period for patients with Alzheimer disease who were taking donepezil hydrochloride or rivastigmine transdermal system with or without memantine hydrochloride prior to baseline. The trial was conducted from June 2, 2014, to December 14, 2016, at 52 sites in the United States. The University of California San Diego Institutional Review Board and each site’s institutional review board approved the trial. The sites obtained written informed consent from all study participants, legally authorized representatives, or both and from participants’ study partners according to local institutional review board regulations.
Participants
Participants were required to be between the ages of 55 and 85 years inclusive, have a diagnosis of probable Alzheimer disease by DSM-IV criteria and National Institute of Neurological and Communicative Disorders and Stroke and the Alzheimer Disease and Related Disorders Association criteria,8 brain MRI or computed tomography scan consistent with the clinical diagnosis, MMSE9 score from 12 to 22, modified Hachinski score10 of 4 or less, and a study partner. Participants were required to be taking donepezil or rivastigmine transdermal system, with or without memantine, for at least 4 months and be at stable doses for at least 3 months prior to baseline. Other medications for Alzheimer disease, including other cholinesterase inhibitors and donepezil doses greater than 10 mg/d were not permitted (see the statistical analysis plan for eligibility criteria and trial protocol in Supplement 1).
Randomization and Masking
Study medication was dispensed as identically appearing, film-coated tablets each containing edonerpic maleate, 112 mg (80-mg free base), or placebo. Participants were randomized to 1 of 3 treatment groups: placebo, 224 mg/d of edonerpic maleate, or 448 mg/d of edonerpic maleate in a 1:1:1 allocation using a permuted block randomization, stratified by site and MMSE score. Medication was to be taken once daily after breakfast. The 448-mg group initially received two 112-mg edonerpic maleate tablets and 2 placebo tablets for 4 weeks and then four 112-mg edonerpic maleate tablets for 48 weeks. The 224-mg group received two 112-mg edonerpic maleate tablets and 2 placebo tablets for 52 weeks. The placebo group received 4 placebo tablets for 52 weeks. Medication was manufactured by the Toyama Chemical Co Ltd, packaged in blister packs of 28 tablets, and dispensed at each visit to provide for 1 week beyond the next scheduled visit.
Procedures
The screening prebaseline period consisted of obtaining informed consent, demographic information, medical history, prior medications, height and weight, vital signs, clinical laboratory test results, electrocardiogram results, MMSE scores, brain imaging results, Alzheimer Disease Assessment Scale–cognitive subscales (ADAS-cog) scores11; performing a physical examination; and determining eligibility criteria. A central medical reviewer at the Alzheimer’s Disease Cooperative Study (ADCS) coordinating center determined eligibility and permitted randomization of participants.
After baseline, patients were assessed every 4 weeks for the first 12 weeks, every 6 weeks thereafter until week 36, at week 44, and at week 52 (see eTable 1 in Supplement 2 for trial outline and flow). There was an optional, open-label, 1-year extension period. Safety evaluations were performed at each visit. Efficacy was assessed at weeks 12, 24, 36, 44, and 52. A final safety assessment was conducted 4 weeks after discontinuation of study medications.
Magnetic resonance imaging brain scans were performed during screening and at week 52. A subset of patients agreed to undergo lumbar punctures at baseline and week 52 for the evaluation of CSF biomarkers. Blood was drawn at baseline and weeks 12, 24, 36, 44, and 52 for population pharmacokinetic assessments and APOE (OMIM 107741) genotype at baseline.
Outcomes
Coprimary clinical outcomes were scores on the ADAS-cog and Alzheimer’s Disease Cooperative Study–Clinical Impression of Change (ADCS-CGIC; ie, the Clinician’s Interview-Based Impression of Change with caregiver input)12 at week 52. Secondary outcomes were scores on the Alzheimer’s Disease Cooperative Study–Activities of Daily Living (ADCS-ADL),13 MMSE, Functional Activities Questionnaire,14 and Neuropsychiatric Inventory15 at week 52 and scores on the ADAS-cog and ADCS-CGIC at weeks 12, 24, 36, and 44. Biomarkers were whole brain volumes, lateral ventricular volumes, and hippocampal volumes as measured on MRI and CSF concentrations of Aβ40, Aβ42, total tau, and phospho-tau181 (p-tau). Exploratory responder analyses were conducted as part of the statistical analysis plan (eAppendix 1 and eTables 2 and 3 in Supplement 2).
Quarc,16 a nonlinear MRI registration method, was used to quantify deformation in the whole brain, hippocampus, and lateral ventricle from baseline to 52-week follow-up and to analyze volume changes. Volumes measured using NeuroQuant software (CorTechs Labs Inc) at baseline and week 52 were used post hoc to calculate changes for comparative purposes (eTable 7 in Supplement 2). Magnetic resonance imaging scans were performed using General Electric, Philips, and Siemens 1.5-T and 3.0-T scanners at baseline and week 52 using the same scanner loaded with a defined protocol to ensure that parameters were held constant across visits. Sagittal, nonaccelerated 3-dimensional T1-weighted sequences were acquired. The methods, sequences, across-site standardization, quality checks, and generation of brain volumes have been previously described.17,18 NeuroQuant software was used for image preprocessing and segmentation.
Levels of Aβ40, Aβ42, and total tau in CSF were assayed using MesoScale Discovery platforms, quantified, and quality controlled by the ADCS Biomarker Core.17 Phospho-tau181 was detected using FujireBio Innotest enzyme-linked immunosorbent assay. Baseline and end point CSF specimens from each participant were performed on the same plate. Internal standards were used to adjust for plate-to-plate variation and assess freezer storage effects; the layout of each plate was designed to control for storage time.
Safety assessments included adverse events, vital signs, and electrocardiogram, hematology, chemistry, and urinalysis results at every visit plus physical examinations and the Pediatric Columbia–Suicide Severity Rating Scale19 prior to baseline and at weeks 12, 24, 36, and 52.
Sample Size and Power
We expected the change in the ADAS-cog score in the placebo group to be 3.5 points and differences between each treatment group and placebo to be 2.5 points at 52 weeks, with an SD of 6.5. The required number of participants to show a significant difference was estimated at 110 per group based on 80% statistical power and a 5%, 2-sided α level. We planned to randomize at least 450 patients (150 per group), expecting 26% to discontinue.
Statistical Analysis
The primary efficacy analysis was performed on the coprimary end points for the modified intention-to-treat population, which included all randomized participants who took at least 1 dose of study medication and had at least 1 efficacy evaluation after baseline. We used separate longitudinal mixed models with repeated measures (MMRM) for each efficacy end point and used SAS PROC MIXED (SAS Institute Inc) to fit an MMRM. Terms in the model were age (covariate), baseline efficacy score (covariate), baseline MMSE score (covariate), clinical site, treatment, apolipoprotein ε4 (APOE ε4) status, time defined categorically, time by treatment interaction, and baseline by time interaction. The covariance structure for the repeated measures was first-order heterogeneous autoregressive to account for decreasing correlation with time between visits. (The MMRM approach assumes that data are missing at random within groups. Although the assumption is not possible to confirm, withdrawals seemed to differ mainly by treatment group and baseline score, which were both included as terms in the model).
A gatekeeper strategy was used to preserve the familywise α error at the 2-sided level of P = .05 between coprimary end points and the multiple doses. The first stage compared the high-dose edonerpic group with the placebo group for ADAS-cog score at P = .05. If statistically significant, then the high-dose group for the ADCS-CGIC score would be formally compared with the placebo group at P = .05. If both comparisons were statistically significant, then the low-dose edonerpic group would similarly be compared with the placebo group.
For MRI volumes, an MMRM model was used, and for CSF concentrations, analysis of covariance was used. A protocol-specified, exploratory responder analysis examined the relationship between clinical outcomes and treatment with response defined as a better score or no change at week 52 compared with baseline on individual and several composite outcomes (eAppendix 1 and eTables 2 and 3 in Supplement 2).
Adverse events were categorized for the safety population, which included all participants who took at least 1 dose of study medication, by seriousness, severity, and relationship to study drug. Adverse events that occurred in 5% or more of any treatment or placebo group were tabulated and analyzed descriptively.
Results
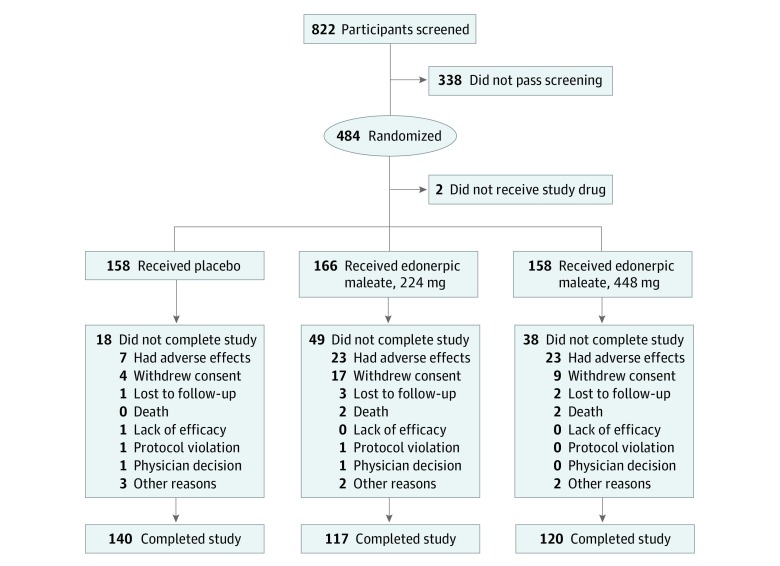
Of the safety population, 140 of 158 participants (88.6%) assigned to placebo, 117 of 166 participants (70.5%) assigned to 224 mg/d of edonerpic maleate, and 120 of 158 participants (76.0%) assigned to 448 mg/d of edonerpic maleate completed the trial. The most common reasons for discontinuation were adverse events and withdrawal of consent (Figure 1; eAppendix 2 in Supplement 2). In the modified intention-to-treat population, women comprised 53.7% of participants (252 of 469), 91.3% (428 of 469) were white, mean age was 71.9 years (range, 55-85 years), 59.7% of those genotyped (280 of 469) were APOE ε4 carriers, 82.3% (386 of 469) were taking donepezil, 17.3% (81 of 469) were using a rivastigmine patch, and 63.1% (296 of 469) were taking memantine (Table 1). Five participants took prohibited donepezil doses of 15 or 23 mg/d.
Figure 1. Participant Disposition.
Table 1. Demographic and Clinical Characteristics at Baseline, mITT Population.
| Characteristic | Participants, No (%) | ||
|---|---|---|---|
| Placebo (n = 156) | Edonerpic Maleate, 224 mg (n = 159) | Edonerpic Maleate, 448 mg (n = 154) | |
| Sex | |||
| Male | 67 (42.9) | 78 (49.1) | 72 (46.8) |
| Female | 89 (57.1) | 81 (50.9) | 82 (53.2) |
| Age, mean (SD), y | 71.8 (7.5) | 71.9 (8.2) | 71.8 (7.8) |
| Race/ethnicity | |||
| Asian | 2 (1.3) | 3 (1.9) | 3 (1.9) |
| Black or African American | 11 (7.1) | 7 (4.4) | 8 (5.2) |
| Other or not reported | 1 (0.6) | 3 (1.9) | 3 (1.9) |
| White | 142 (91.0) | 146 (91.8) | 140 (90.9) |
| ApoE4 genotype | |||
| Noncarriers | 50 (32.1) | 57 (35.8) | 54 (35.1) |
| Heterozygotes | 71 (45.5) | 74 (46.5) | 55 (35.7) |
| Homozygotes | 27 (17.3) | 21 (13.2) | 32 (20.8) |
| Unknown | 8 (5.1) | 7 (4.4) | 13 (8.4) |
| Concomitant medications | |||
| Donepezil hydrochloride | 131 (84.0) | 134 (84.3) | 121 (78.6) |
| Rivastigmine transdermal system | 24 (15.4) | 25 (15.7) | 32 (20.8) |
| Memantine hydrochloride | 102 (65.4) | 98 (61.6) | 96 (62.3) |
| Clinical scales | |||
| MMSE score, mean (SD) | 18.2 (3.8) | 18.2 (3.8) | 18.4 (3.9) |
Abbreviation: MMSE, Mini-Mental State Examination.
Of 91 baseline CSF samples, 89 (97.8%) had a positive Alzheimer disease index (>0.81).20 A total of 86 CSF samples (94.5%) had Aβ42 levels below the 592-pg/mL cutoff and 70 (76.9%) had p-tau above the 76.9-pg/mL cutoff.
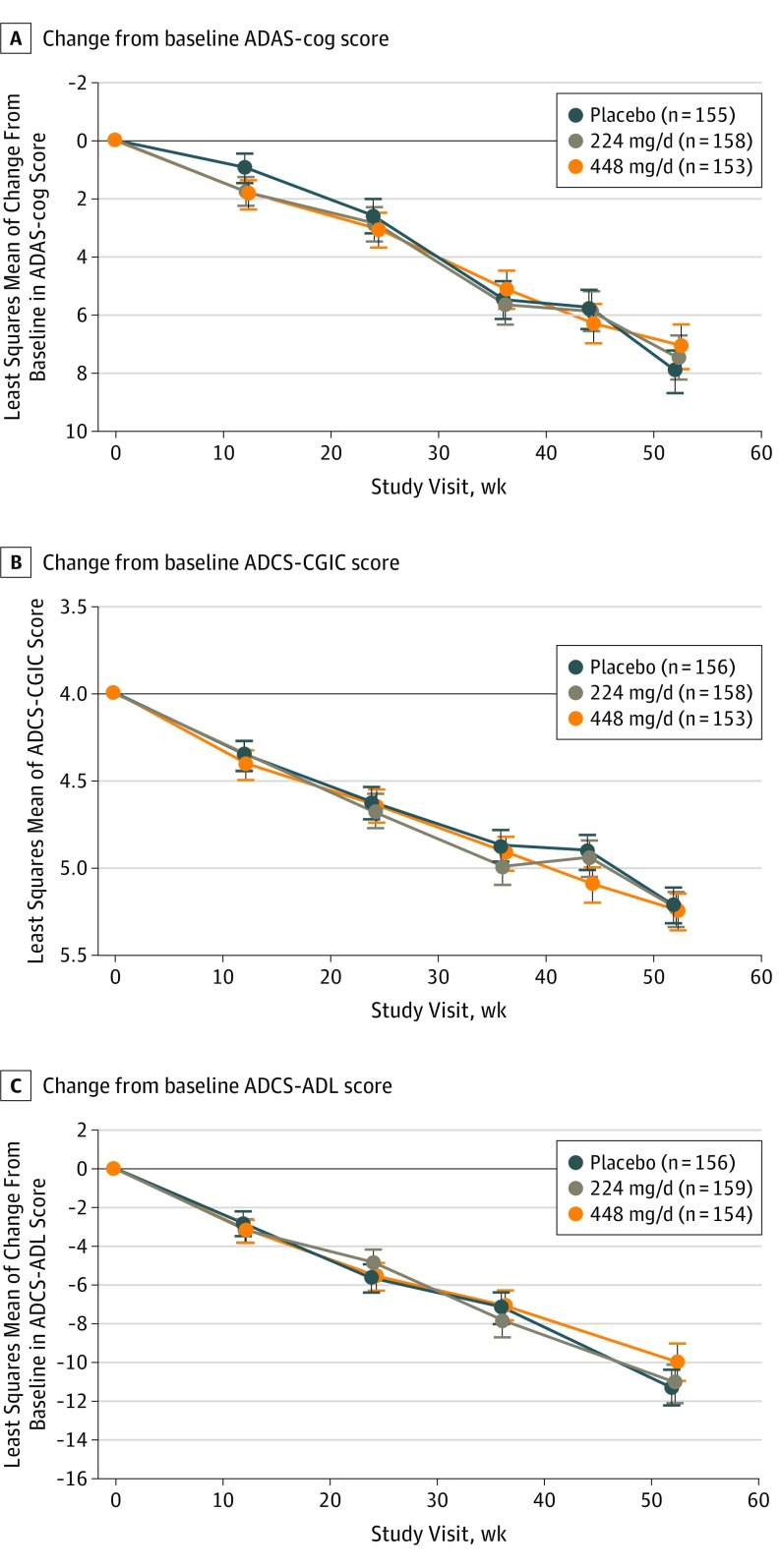
The mean ADAS-cog score adjusted change at week 52 was 7.91 for the placebo group, 7.45 for the 224-mg edonerpic maleate group, and 7.08 for the 448-mg edonerpic maleate group. The mean differences from placebo were −0.47 (95% CI, −2.36 to 1.43; P = .63) for the 224-mg group and −0.84 (95% CI, −2.75 to 1.08; P = .39) for the 448-mg group. Mean ADCS-CGIC scores were 5.22 for the placebo group, 5.24 for the 224-mg edonerpic maleate group, and 5.25 for the 448-mg edonerpic maleate group. Mean differences from placebo were 0.03 (95% CI, −0.20 to 0.25; P = .81) for the 224-mg group and 0.04 (95% CI, −0.19 to 0.26; P = .76) for the 448-mg group (Table 2; Figure 2A and B).
Table 2. Primary and Secondary Clinical Outcomes and MRI Volumes at Week 52.
| Characteristic | Placebo | Edonerpic Maleate, 224 mg | P Value | Edonerpic Maleate, 448 mg | P Value | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Baseline, Mean (SD) | Change From Baseline, Mean (SE) | Baseline, Mean (SD) | Change From Baseline, Mean (SE) | Difference vs Placebo, Mean (95% CI) | Baseline, Mean (SD) | Change From Baseline, Mean (SE) | Difference vs Placebo, Mean (95% CI) | |||
| Primary outcomesa | ||||||||||
| ADAS-cog score | 28.1 (10.0) | 7.91 (0.727) | 27.7 (8.6) | 7.45 (0.756) | −0.47 (−2.36 to 1.43) | .63 | 27.8 (10.1) | 7.08 (0.756) | −0.84 (−2.75 to 1.08) | .39 |
| ADCS-CGIC score | NA | 5.22 (0.097) | NA | 5.24 (0.101) | 0.03 (−0.20 to 0.25) | .81 | NA | 5.25 (0.101) | 0.04 (−0.19 to 0.26) | .76 |
| Secondary outcomes | ||||||||||
| ADCS-ADL score | 60.3 (11.11) | −11.29 (0.957) | 60.4 (10.59) | −11.07 (0.992) | 0.23 (−2.29 to 2.74) | .86 | 61.0 (10.92) | −10.01 (0.991) | 1.29 (−1.26 to 3.83) | .32 |
| MMSE score | 18.2 (3.8) | −2.94 (0.416) | 18.2 (3.8) | −2.89 (0.428) | 0.05 (−0.96 to 1.06) | .92 | 18.4 (3.9) | −3.00 (0.426) | −0.06 (−1.08 to 0.96) | .91 |
| Functional Activities Questionnaire scoreb | 17.7 (7.46) | 4.35 (0.433) | 17.8 (7.05) | 5.05 (0.448) | 0.70 (−0.33 to 1.73) | .18 | 16.9 (7.74) | 4.25 (0.448) | −0.10 (−1.14 to 0.93) | .85 |
| Neuropsychiatric Inventory score | 9.3 (10.54) | 4.36 (1.312) | 9.3 (10.31) | 6.77 (1.370) | 2.41 (−1.01 to 5.83) | .17 | 9.5 (10.83) | 3.01 (1.368) | −1.35 (−4.79 to 2.09) | .44 |
| MRI outcomesc,d | ||||||||||
| Brain volume, mL (baseline) | 822.6 (89.8) | NA | 820.7 (102.5) | NA | NA | NA | 840.2 (99.0) | NA | NA | NA |
| Brain volume, pdu | NA | −1.84 (0.29) | NA | −1.48 (0.29) | 0.36 (−0.18 to 0.91) | .19 | NA | −1.48 (0.30) | 0.36 (−0.18 to 0.91) | .19 |
| Lateral ventricle volume, right and left, mL (baseline) | 63.84 (24.3) | NA | 62.18 (23.7) | NA | NA | NA | 65.49 (30.1) | NA | NA | NA |
| Lateral ventricle volume, right and left, pdu | NA | 23.17 (2.00) | NA | 24.66 (2.02) | 1.49 (−2.92 to 5.90) | .51 | NA | 29.16 (2.12) | 5.99 (1.52 to 10.45) | .009 |
| Hippocampal volume, right and left, mL (baseline) | 4.86 (1.02) | NA | 4.88 (0.98) | NA | NA | NA | 4.79 (1.12) | NA | NA | NA |
| Hippocampal volume, right and left, pdu | NA | −4.06 (0.62) | NA | −3.70 (0.63) | 0.36 (−0.93 to 1.65) | .58 | NA | −4.07 (0.66) | −0.01 (−1.31 to 1.29) | .99 |
Abbreviations: ADAS-cog, Alzheimer Disease Assessment Scale–Cognitive Subscale; ADCS-ADL, Alzheimer’s Disease Cooperative Study–Activities of Daily Living; ADCS-CGIC, Alzheimer’s Disease Cooperative Study–Clinical Impression of Change; MMSE, Mini-Mental State Examination; MRI, magnetic resonance imaging; NA, not applicable; pdu, procedure-defined units.
Placebo, n = 156; edonerpic maleate, 224 mg, n = 159; and edonerpic maleate, 448 mg, n = 154.
For 158 participants at baseline for 224-mg dose on Functional Activities Questionnaire.
Placebo, n = 107; edonerpic maleate, 224 mg, n = 89; and edonerpic maleate, 448 mg, n = 85.
MRI outcomes at week 52 estimated by mixed models with repeated measures with covariates.
Figure 2. Clinical Outcomes.
A, Change from baseline Alzheimer Disease Assessment Scale–Cognitive Subscale (ADAS-cog) score. B, Change from baseline Alzheimer’s Disease Cooperative Study–Clinical Impression of Change (ADCS-CGIC) score. C, Change from baseline Alzheimer’s Disease Cooperative Study–Activities of Daily Living (ADCS-ADL) score.
There were no significant differences between the edonerpic groups and placebo for any of the secondary outcomes, ADCS-ADL score (Figure 2C), MMSE score, Functional Activities Questionnaire score, Neuropsychiatric Inventory score (Table 2), and ADAS-cog or ADCS-CGIC scores at weeks 12, 24, 36, and 44 (eFigure in Supplement 2). None of the individual or composite exploratory responder analyses favored edonerpic treatment; responses using the MMSE and Neuropsychiatric Inventory showed nominally significant effects for placebo (eAppendix 1 and eTables 2 and 3 in Supplement 2).
Magnetic resonance imaging segmentation results were obtained for 412 patients at baseline (55 had computed tomography scans, 3 had nonvolumetric clinical MRI scans, and 14 had MRI scans that were not of sufficient quality or failed quality control processing). Follow-up MRI scans were performed on 325 participants, and 281 pairs of scans (86.5%) were of sufficient quality for registration and analysis with Quarc (Table 2). The main reason for rejection was that 1 or both of the scan pairs had poor image quality for registration and alignment across time points. More participants in the placebo group had baseline and follow-up pairs of analyzable MRI scans than those in the 224-mg and 448-mg groups (107 of 158 [67.7%] vs 89 of 166 [53.6%] vs 85 of 158 [53.8%]). There were no significant differences in deformation of brain and hippocampal volumes between placebo and either dose of edonerpic maleate. There was, however, an increase in ventricular volume in the 448-mg edonerpic maleate group compared with placebo (29.16 procedure-defined units vs 23.17 procedure-defined units) (Table 2). Procedure-defined units reflect percentage of QUARC deformation within a given brain structure.
Ninety-one CSF samples were obtained at baseline, 60 were obtained at week 52, and 59 pairs were available for analysis. There were no differences between groups in the proportion of sample pairs available for analysis (placebo, 18 of 156 [11.5%]; 224 mg, 17 of 159 [10.7%]; and 448 mg, 24 of 154 [15.6%]). Phospho-tau181 and total tau appeared to decrease in the 448-mg group compared with placebo; Aβ42 and Aβ40 comparisons were not significant (eTable 4 in Supplement 2).
Pharmacokinetics
The mean (SD) maximum plasma concentration of edonerpic was 271.30 (99.67) ng/mL for the 224-mg group and 618.68 (222.28) ng/mL for the 448-mg group. Mean (SD) area under the curve was 3409.90 (1521.07) ng · h/mL for the 224-mg group and 7345.74 (3390.85) ng · h/mL for the 448-mg group (eTable 5 in Supplement 2).
The mean (SD) plasma level of edonerpic was 437.4 (279.2) ng/mL and mean (SD) CSF level was 41.07 (31.22) ng/mL; the mean (SD) plasma level of the main M5 metabolite was 390.5 (305.9) ng/mL and mean CSF concentration of the main M5 metabolite was 9.57 (10.71) ng/mL (eTable 6 in Supplement 2). Cerebrospinal fluid penetration of edonerpic was about 11%. The ratio of CSF to plasma M5 metabolite was 0.03, suggesting that very little edonerpic is metabolized in the CSF or crosses the blood-brain barrier.
Adverse Events
In the safety population, 7 of 158 participants (4.4%) in the placebo group, 23 of 166 participants (13.9%) in the 224-mg group, and 23 of 158 participants (14.6%) in the 448-mg group discontinued treatment because of adverse events. The most frequent adverse events were diarrhea (placebo, 20 of 158 [12.7%]; edonerpic maleate, 224 mg, 34 of 166 [20.5%]; and edonerpic maleate, 448 mg, 49 of 158 [31.0%]) and nausea (placebo, 6 of 158 [3.8%]; edonerpic maleate, 244 mg, 13 of 166 [7.8%]; and edonerpic maleate, 448 mg, 9 of 158 [5.7%]) and tended to occur within the first 24 weeks. Infections, injuries, falls, agitation, and anxiety were more common with placebo (Table 3). As the exposure in the edonerpic maleate–treated groups was 89% of that in the placebo group (mean, 341.6 treatment-days per participant for the placebo-treated group, 303.4 treatment days for the 224-mg group, and 304.2 treatment-days for the 448-mg group), rates of adverse events would be expected at 89% of placebo if there were no treatment differences. Other than mild diarrhea, nausea, weight loss, and dizziness at the higher dose, there were no adverse events reported at a greater rate than placebo.
Table 3. Treatment-Emergent Adverse Events Occurring in 5% or More of Participants and SAEs (Safety Population).
| Characteristic | Participants, No. (%) | ||
|---|---|---|---|
| Placebo (n = 158) | Edonerpic Maleate, 224 mg (n = 166) | Edonerpic Maleate, 448 mg (n = 158) | |
| Patient-days exposure, mean (SD) | 341.6 (76.9) | 303.4 (111.2) | 304.2 (116.1) |
| Patients with at least 1 adverse event | 122 (77.2) | 134 (80.7) | 136 (86.1) |
| Total adverse events, No. | 446 | 470 | 472 |
| Patients with at least 1 SAE | 20 (12.7) | 25 (15.1) | 26 (16.5) |
| Total SAEs, No. | 25 | 35 | 34 |
| Deaths, No. | 0 | 2 (1.2) | 2 (1.3) |
| Patients with an SAE considered related | 3 (1.9) | 3 (1.8) | 2 (1.3) |
| Total SAEs considered related, No. | 4 | 3 | 3 |
| Adverse events (≥5%, MEDRA terms) | |||
| Nasopharyngitis | 9 (5.7) | 4 (2.4) | 4 (2.5) |
| Urinary tract infection | 22 (13.9) | 17 (10.2) | 21 (13.3) |
| Agitation | 14 (8.9) | 5 (3.0) | 4 (2.5) |
| Anxiety | 12 (7.6) | 9 (5.4) | 7 (4.4) |
| Depression | 7 (4.4) | 6 (3.6) | 8 (5.1) |
| Dizziness | 3 (1.9) | 7 (4.2) | 11 (7.0) |
| Headache | 10 (6.3) | 16 (9.6) | 9 (5.7) |
| Diarrhea | 20 (12.7) | 34 (20.5) | 49 (31.0) |
| Nausea | 6 (3.8) | 13 (7.8) | 9 (5.7) |
| Vomiting | 7 (4.4) | 8 (4.8) | 9 (5.7) |
| Weight decreased | 3 (1.9) | 4 (2.4) | 8 (5.1) |
| Falls | 17 (10.8) | 14 (8.4) | 12 (7.6) |
| SAEs (>1 in any group) | |||
| Myocardial infarction or cardiac arrest | 2 (1.3) | 1 (0.6) | 2 (1.3) |
| Chest pain | 2 (1.3) | 2 (1.3) | 0 |
| Urinary tract infection | 1 (0.6) | 1 (0.6) | 2 (1.3) |
| Cellulitis | 0 | 0 | 2 (1.3) |
| Sepsis | 0 | 2 (1.3) | 0 |
| Fractures | 2 (1.3) | 3 (1.8) | 3 (1.9) |
| Dehydration | 0 | 2 (1.3) | 2 (1.3) |
| Breast cancer | 0 | 2 (1.3) | 0 |
| Cerebrovascular accident or hemorrhage | 0 | 3 (1.8) | 0 |
| Transient ischemic attack | 0 | 0 | 2 (1.3) |
| Syncope | 3 (1.9) | 2 (1.3) | 0 |
| Agitation | 2 (1.3) | 0 | 0 |
| Confusional state and delirium | 1 (0.6) | 2 (1.3) | 4 (2.6) |
Abbreviations: MEDRA, Medical Dictionary for Regulatory Activities; SAE, serious adverse event.
A total of 94 serious adverse events were observed in 71 participants (Table 3). Four serious adverse events judged possibly related to treatment were in the placebo group, 3 in the 224-mg group, and 3 in the 448-mg group (1 of the latter was considered probably related). Two deaths occurred in the 224-mg group due to sepsis and cerebral hemorrhage and 2 in the 448-mg group due to cardiac arrest and suicide; 3 were judged unrelated and 1 unlikely related to study medications.
Discussion
There were no significant differences favoring edonerpic maleate for the primary or secondary outcomes. Edonerpic maleate appeared to be tolerable and safe, with expected gastrointestinal symptoms early in treatment. Other than mild diarrhea, nausea, weight loss, and dizziness at the higher dose, there were no adverse events reported at a greater rate than placebo, despite more treatment discontinuations.
The trial was well conducted and valid, with no deficiencies in conduct that we could identify. Withdrawals were only 11% in the placebo group, which was less than the 26% that was expected. The clinical diagnosis of Alzheimer disease was strongly supported by CSF Aβ42 and p-tau biomarkers. A total of 97.8% of participants in the CSF substudy had a positive Alzheimer disease CSF index,20 and 94% had Aβ42 levels below the standard cutoff for the assay (592 pg/mL).
The selection of patients with mild to moderate Alzheimer disease was based on post hoc analyses from a previous phase 2a trial7 that suggested that benefits for edonerpic were seen in individuals with an MMSE score less than 20. Furthermore, its preclinical actions, including effects through sigma-1 receptors, interactions with CRMP2 on AMPA receptor delivery, behavioral effects including spatial memory benefits, and increased neurite length in rat hippocampal slices, supported its testing for symptomatic effects in Alzheimer disease. The trial was undertaken without the use of pharmacodynamic or companion biomarker measures of edonerpic’s potential effects on its targets, which is an intrinsic limitation to its development strategy. Also, it is possible that a behavioral or cognitive intervention given along with edonerpic could have effected clinical change as observed in preclinical models.4
The higher 448-mg daily dose of edonerpic maleate was selected based on the limited outcomes of the 224-mg dose used in previous studies. Plasma drug levels were as expected and the 11% penetration of edonerpic into CSF was reasonable considering that approximately 80% of edonerpic is bound to plasma proteins. A higher dose than 448 mg in any future trial, however, is likely not feasible based on gastrointestinal adverse effects.
The trial design, duration, outcomes, and sample size were similar to other trials in patients with mild to moderate Alzheimer disease.21 The ADAS-cog and ADCS-CGIC coprimary outcomes were appropriate and sufficiently sensitive to have detected moderately small drug effects as planned for in this trial. Secondary and exploratory outcomes did not suggest any potential for efficacy, and, in several instances, exploratory responder analyses favored placebo (eAppendix 1 and eTables 2 and 3 in Supplement 2). We also performed a range of post hoc subgroup analyses, including median splits on age, cognition, MMSE score, APOE4 ε4 carriers or not, and taking or not taking memantine, but these analyses did not show significant interactions or suggest efficacy.
Limitations
Biomarker outcomes in trials require consideration within the context of the clinical outcomes. Lumbar punctures were voluntary, and there was no planned sample size for either the CSF or MRI analyses. The CSF analysis set of only 59 pairs was small (13% of the planned trials sample size). Although there may be a suggestion that the 448-mg/d edonerpic maleate dose was associated with decreased p-tau and total tau, baseline values were not balanced and the effect sizes were implausibly large. Any inference that edonerpic showed neuroprotection in this study would be speculative and would lack supporting clinical evidence. The possibility exists, however, that edonerpic eventually may be shown to have neuroprotective effects in other conditions, other tauopathy, earlier-stage Alzheimer disease, and potentially in cerebrovascular injury models.4
The higher rate of follow-up MRI scans in the placebo group is likely because of greater withdrawals in the treatment groups due to adverse events. The increase in lateral ventricular volumes with edonerpic was unexpected. Although increased ventricular volumes generally have been taken to suggest brain volume loss, its meaning in this trial is uncertain, as it was observed only in the high-dose group. Where such findings have been described with amyloid-lowering treatment, the effect has been explained as related to amyloid removal, fluid shifts,22 or neuroinflammatory effects with fluid shifts; when described in the context of other drugs, the effect has been explained as atrophy and neurodegeneration as well as fluid shifts.23 The Quarc volume analyses of coregistered images used here have better measurement properties than does subtraction of estimated volumes through segmentation of each scan.16 We also performed the latter volume analyses to facilitate comparisons with other studies (eTable 7 in Supplement 2) and found only reduced hippocampal volume loss with the lower edonerpic maleate dose compared with placebo, which is also of unclear significance in the absence of clinical effect.
Conclusions
Beyond the preclinical data, the clinical outcomes of 3 phase 2 trials and the use of maximal doses do not provide evidence of clinical proof of concept for edonerpic maleate for patients with mild to moderate Alzheimer disease.
Trial Protocol
eTable 1. Clinical Trial Outline and Flow
eTable 2. Individual Clinical Response Counting Non-completers as Non-responders, mITT Population
eTable 3. Multiple Clinical Response Definitions Counting Non-completers as Non-responders, mITT Population
eTable 4. Cerebrospinal Fluid Amyloid and Tau Analyses
eTable 5. Pharmacokinetic Parameters of Edonerpic
eTable 6. Penetration of Edonerpic and the M5 Metabolite Into CSF
eTable 7. Exploratory MRI Outcomes Performed Using NeuroQuant Volumes
eAppendix 1. Protocol-Specified Responder Analyses
eAppendix 2. CONSORT 2010 Checklist of Information to Include When Reporting a Randomized Trial
eFigure. Protocol-Specified Secondary Analyses
Data Sharing Statement
References
- 1.Nguyen PTH, Kimura T, Ho SA, Tran AH, Ono T, Nishijo H. Ameliorative effects of a neuroprotective agent, T-817MA, on place learning deficits induced by continuous infusion of amyloid-β peptide (1-40) in rats. Hippocampus. 2007;17(6):443-455. doi: 10.1002/hipo.20281 [DOI] [PubMed] [Google Scholar]
- 2.Hirata K, Yamaguchi H, Takamura Y, et al. . A novel neurotrophic agent, T-817MA [1-{3-[2-(1-benzothiophen-5-yl) ethoxy] propyl}-3-azetidinol maleate], attenuates amyloid-beta–induced neurotoxicity and promotes neurite outgrowth in rat cultured central nervous system neurons. J Pharmacol Exp Ther. 2005;314(1):252-259. doi: 10.1124/jpet.105.083543 [DOI] [PubMed] [Google Scholar]
- 3.Fukushima T, Nakamura A, Iwakami N, et al. . T-817MA, a neuroprotective agent, attenuates the motor and cognitive impairments associated with neuronal degeneration in P301L tau transgenic mice. Biochem Biophys Res Commun. 2011;407(4):730-734. doi: 10.1016/j.bbrc.2011.03.091 [DOI] [PubMed] [Google Scholar]
- 4.Abe H, Jitsuki S, Nakajima W, et al. . CRMP2-binding compound, edonerpic maleate, accelerates motor function recovery from brain damage. Science. 2018;360(6384):50-57. doi: 10.1126/science.aao2300 [DOI] [PubMed] [Google Scholar]
- 5.Yano T, Tanabe H, Kobayashi K, et al. . Sigma-1 receptor is a molecular target for novel neuroprotectant T-817MA. Alzheimers Dement. 2015;11(7):861. doi: 10.1016/j.jalz.2015.08.038 [DOI] [Google Scholar]
- 6.Quinti L, Forte AM, Kim DY, Griciuc A, Tanzi RE. A novel drug-screening platform in microglial cells identifies potential AD drugs. Alzheimers Dement. 2017;13(7):1485. doi: 10.1016/j.jalz.2017.07.564 [DOI] [Google Scholar]
- 7.Schneider L, Porsteinsson A, Farlow M, Shimakura A, Nakagawa M, Iwakami N. The neuroprotective and neurotrophic agent T-817MA for Alzheimer’s disease: randomized, double-blind, placebo-controlled proof-of-concept trial outcomes. Alzheimers Dement. 2013;9(4):P530-P531. doi: 10.1016/j.jalz.2013.04.272 [DOI] [Google Scholar]
- 8.McKhann G, Drachman D, Folstein M, Katzman R, Price D, Stadlan EM. Clinical diagnosis of Alzheimer’s disease: report of the NINCDS-ADRDA Work Group under the auspices of Department of Health and Human Services Task Force on Alzheimer’s Disease. Neurology. 1984;34(7):939-944. doi: 10.1212/WNL.34.7.939 [DOI] [PubMed] [Google Scholar]
- 9.Folstein MF, Folstein SE, McHugh PR. ‘Mini-mental state’: a practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12(3):189-198. doi: 10.1016/0022-3956(75)90026-6 [DOI] [PubMed] [Google Scholar]
- 10.Rosen WG, Terry RD, Fuld PA, Katzman R, Peck A. Pathological verification of ischemic score in differentiation of dementias. Ann Neurol. 1980;7(5):486-488. doi: 10.1002/ana.410070516 [DOI] [PubMed] [Google Scholar]
- 11.Rosen WG, Mohs RC, Davis KL. A new rating scale for Alzheimer’s disease. Am J Psychiatry. 1984;141(11):1356-1364. doi: 10.1176/ajp.141.11.1356 [DOI] [PubMed] [Google Scholar]
- 12.Schneider LS, Olin JT, Doody RS, et al. . Validity and reliability of the Alzheimer’s Disease Cooperative Study–Clinical Global Impression of Change: the Alzheimer’s Disease Cooperative Study. Alzheimer Dis Assoc Disord. 1997;11(suppl 2):S22-S32. doi: 10.1097/00002093-199700112-00004 [DOI] [PubMed] [Google Scholar]
- 13.Galasko D, Bennett D, Sano M, et al. . An inventory to assess activities of daily living for clinical trials in Alzheimer’s disease: the Alzheimer’s Disease Cooperative Study. Alzheimer Dis Assoc Disord. 1997;11(suppl 2):S33-S39. doi: 10.1097/00002093-199700112-00005 [DOI] [PubMed] [Google Scholar]
- 14.Pfeffer RI, Kurosaki TT, Harrah CH Jr, Chance JM, Filos S. Measurement of functional activities in older adults in the community. J Gerontol. 1982;37(3):323-329. doi: 10.1093/geronj/37.3.323 [DOI] [PubMed] [Google Scholar]
- 15.Cummings JL, Mega M, Gray K, Rosenberg-Thompson S, Carusi DA, Gornbein J. The Neuropsychiatric Inventory: comprehensive assessment of psychopathology in dementia. Neurology. 1994;44(12):2308-2314. doi: 10.1212/WNL.44.12.2308 [DOI] [PubMed] [Google Scholar]
- 16.Holland D, Dale AM; Alzheimer’s Disease Neuroimaging Initiative . Nonlinear registration of longitudinal images and measurement of change in regions of interest. Med Image Anal. 2011;15(4):489-497. doi: 10.1016/j.media.2011.02.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Turner RS, Thomas RG, Craft S, et al. ; Alzheimer’s Disease Cooperative Study . A randomized, double-blind, placebo-controlled trial of resveratrol for Alzheimer disease. Neurology. 2015;85(16):1383-1391. doi: 10.1212/WNL.0000000000002035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Relkin NR, Thomas RG, Rissman RA, et al. ; Alzheimer’s Disease Cooperative Study . A phase 3 trial of IV immunoglobulin for Alzheimer disease. Neurology. 2017;88(18):1768-1775. doi: 10.1212/WNL.0000000000003904 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Posner K, Brown GK, Stanley B, et al. . The Columbia-Suicide Severity Rating Scale: initial validity and internal consistency findings from three multisite studies with adolescents and adults. Am J Psychiatry. 2011;168(12):1266-1277. doi: 10.1176/appi.ajp.2011.10111704 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Molinuevo JL, Gispert JD, Dubois B, et al. . The AD-CSF-index discriminates Alzheimer’s disease patients from healthy controls: a validation study. J Alzheimers Dis. 2013;36(1):67-77. doi: 10.3233/JAD-130203 [DOI] [PubMed] [Google Scholar]
- 21.Schneider LS, Sano M. Current Alzheimer’s disease clinical trials: methods and placebo outcomes. Alzheimers Dement. 2009;5(5):388-397. doi: 10.1016/j.jalz.2009.07.038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fox NC, Black RS, Gilman S, et al. ; AN1792(QS-21)-201 Study . Effects of Aβ immunization (AN1792) on MRI measures of cerebral volume in Alzheimer disease. Neurology. 2005;64(9):1563-1572. doi: 10.1212/01.WNL.0000159743.08996.99 [DOI] [PubMed] [Google Scholar]
- 23.Fleisher AS, Truran D, Mai JT, et al. ; Alzheimer’s Disease Cooperative Study . Chronic divalproex sodium use and brain atrophy in Alzheimer disease. Neurology. 2011;77(13):1263-1271. doi: 10.1212/WNL.0b013e318230a16c [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Trial Protocol
eTable 1. Clinical Trial Outline and Flow
eTable 2. Individual Clinical Response Counting Non-completers as Non-responders, mITT Population
eTable 3. Multiple Clinical Response Definitions Counting Non-completers as Non-responders, mITT Population
eTable 4. Cerebrospinal Fluid Amyloid and Tau Analyses
eTable 5. Pharmacokinetic Parameters of Edonerpic
eTable 6. Penetration of Edonerpic and the M5 Metabolite Into CSF
eTable 7. Exploratory MRI Outcomes Performed Using NeuroQuant Volumes
eAppendix 1. Protocol-Specified Responder Analyses
eAppendix 2. CONSORT 2010 Checklist of Information to Include When Reporting a Randomized Trial
eFigure. Protocol-Specified Secondary Analyses
Data Sharing Statement