Abstract
Numerous studies link decreased serotonin metabolites with increased impulsive and aggressive traits. However, although pharmacological depletion of serotonin is associated with increased aggression, interventions aimed at directly decreasing serotonin neuron activity have supported the opposite association. Furthermore, it is not clear if altered serotonin activity during development may contribute to some of the observed associations. Here, we used two pharmacogenetic approaches in transgenic mice to selectively and reversibly reduce the firing of serotonin neurons in behaving animals. Conditional overexpression of the serotonin 1A receptor (Htr1a) in serotonin neurons showed that a chronic reduction in serotonin neuron firing was associated with heightened aggression. Overexpression of Htr1a in adulthood, but not during development, was sufficient to increase aggression. Rapid suppression of serotonin neuron firing by agonist treatment of mice expressing Htr1a exclusively in serotonin neurons also led to increased aggression. These data confirm a role of serotonin activity in setting thresholds for aggressive behavior and support a direct association between low levels of serotonin homeostasis and increased aggression.
Introduction
Studies across a wide range of metazoan species have identified the brain neurotransmitter serotonin (5-HT) as a major modulator of impulsive and aggressive behavior. Reduced CSF levels of the serotonin metabolite 5-hydroxyindole acetic acid (5-HIAA) have been found in aggressive psychiatric patients, suicide victims and impulsive, violent men (Brown et al., 1982; Linnoila et al., 1983; Virkkunen et al., 1994). A similar negative association between serotonin metabolites and aggressive behavior has been reported in a wide range of species, including macaques (Mehlman et al., 1994), mice (Caramaschi et al., 2007), and fish (Clotfelter et al., 2007), suggesting a causal link between reduced serotonin activity and aggression. Such a causal link is supported by pharmacological studies in which aggression is increased by depletion of tryptophan, an essential precursor of serotonin (Chamberlain et al., 1987; Bjork et al., 1999), or inhibition of serotonin synthesis (Vergnes et al., 1986).
Genetic variants in the rate-limiting enzymes in serotonin synthesis and metabolism, tryptophan hydroxylase 2 (TPH2) and monoamine oxidase A (MAOA), respectively, have been associated with increased aggression in humans, macaques, and mice (Brunner et al., 1993; Manuck et al., 1999; Huang et al., 2004; Newman et al., 2005; Osipova et al., 2009). However, while the pharmacological experiments suggest a negative association between serotonin neurotransmission and aggression, the genetic studies are equivocal with decreased synthesis and decreased degradation of serotonin both being linked to similar behavioral changes. Discrepancies in genetic data may come from the compensatory role of multiple feedback mechanisms that maintain serotonin homeostasis.
The simple association between low serotonin activity and increased aggression has been challenged by data showing that direct injection into the dorsal raphe nucleus of agonists for Htr1a, the receptor which provide direct negative feedback on the firing and release of serotonin, leads to decreased aggressive behavior in rats (Mos et al., 1993; De Almeida and Lucion, 1997; van der Vegt et al., 2003). Furthermore, pharmacological agonists of Htr1a receptors have been shown to decrease aggressive behavior in resident mice (Miczek et al., 1998; de Boer et al., 2000; de Boer and Koolhaas, 2005; Caramaschi et al., 2007). These data raise the possibility that autoregulatory mechanisms obscure a simple linear relationship between aggression and serotonin neuron firing and/or neurotransmission.
Here, we tested the hypothesis that decreased serotonin activity is sufficient to increase aggression and whether decreased serotonin activity during development can modulate aggression in adulthood. We used two different cell-type specific pharmacogenetic tools in transgenic mice to selectively reduce serotonin activity either chronically or acutely and then tested for aggressive behavior in the resident intruder test. First, we used conditional overexpression of Htr1a autoreceptors to chronically decrease serotonin activity either during development or in adulthood. Second, we treated mice engineered to express Htr1a only in serotonin neurons with an Htr1a agonist to acutely reduce serotonin neuron firing. Our data demonstrate that a reduction of serotonin neuron activity during adulthood, but not during development, is sufficient to increase aggression, consistent with the low serotonin/high aggression hypothesis.
Materials and Methods
Transgenic mice.
Slc6a4tTA mice (EM:04891) were produced by replacing portions of exon 2 of the serotonin transporter gene starting at the initiating ATG with the coding sequence of the tetracycline transactivator protein (tTA) followed by bGH polyA sequences and an FRT-flanked neomycin resistance cassette using gene targeting in W9.5 ES cells as previously described (Audero et al., 2008). Htr1atetO mice were produced by removing the loxP-flanked neomycin resistance transcriptional stop cassette from Htr1aSTOP-tetO knock-out mice (Gross et al., 2002) as previously described (Audero et al., 2008). The Htr1atetO allele carries a tetO-CMV promoter targeted to the 5′-UTR of the endogenous Htr1a gene that mediates tTA-dependent overexpression of the receptor. Htr1aRO and control littermates were produced by breeding +/+; Htr1atetO/Htr1atetO and Slc6a4tTA/+; Htr1atetO/Htr1atetO mice. All mice were maintained on a 129S6/SvEvTac;C57BL/6;CBA background and were derived from the same breeding colony as mice used in the study by Audero et al. (2008). The Htr1aRR line was produced by crossing a line carrying a Tph2-Htr1a transgene with Htr1aKO mice (Ramboz et al., 1998). Tph2-Htr1a mice were produced by pronuclear injection in [C57BL/6JxCBA/J]xC57BL/6J embryos of a circular mouse BAC (RP23–112F24; Chori-BACPAC Resources) containing 220 kb of the Tph2 gene in which Htr1a coding sequences followed by a bovine growth hormone polyadenylation sequence and an FRT-flanked kanamycin resistance marker (FLP deleted in bacteria before DNA injection) had been inserted at the start codon of the Tph2 gene. Founders carrying the transgene were identified and genotyped by PCR, crossed to Htr1aKO mice, and maintained on a mixed C57BL/6J;CBA/J;129S6/SvEvTac background. Expression levels in two independent founder lines (Tg10 and Tg24) were similar and a mixture of the two lines was used for all behavioral experiments. Htr1aRR and control littermates were produced by breeding +/+; Htr1aKO/Htr1aKO and Tph2-Htr1a/+; Htr1aKO/Htr1aKO mice.
Animal husbandry.
Mice were group housed (3–5/cage) on a 12/12 h light/dark cycle (lights off at 7:00 P.M.) in a temperature-controlled environment (21 ± 0.5°C). Food and water were provided ad libitum. Doxycycline was given to the animals or pregnant mothers in the form of food pellets (40 mg/kg food; Bio-Serv).
Immunohistochemistry.
Animals were deeply anesthetized by injection of 25% avertin (Sigma-Aldrich) and transcardially perfused with 4% paraformaldehyde (PFA, Sigma-Aldrich). Brains were removed from the skull, postfixed with 4% PFA in PBS and cryoprotected in 30% sucrose. Coronal sections (12 μm) were cut in a cryostat and mounted on glass slides (Superfrost Plus, Thermo Scientific). Sections were allowed to dry at room temperature and incubated in 10 mm citrate buffer pH 6.0 containing 0.05% Tween at 96°C for 10 min to allow unmasking of the epitope. After blocking endogenous peroxidase activity for 30 min with 0.6% H2O2 in TBS (Tris-buffered saline), sections were preincubated for 1 h at room temperature in blocking solution containing 5% fetal bovine serum, 2% BSA (bovine serum albumine) and 0.25% Triton X-100 diluted in TBS. Sections were then incubated in mouse monoclonal anti-Tph antibody (1:400, Sigma-Aldrich) overnight at 4°C. Detection was performed with biotinylated secondary antibodies (horse anti-mouse IgG, 1:300; Vector Laboratories) diluted in blocking solution for 90 min, followed by incubation in streptavidin-biotin-peroxidase complex (Vector Laboratories) for 1 h at room temperature. Peroxidase activity was visualized using diaminobenzidine substrate (Sigma-Aldrich) for 10 min at room temperature. Sections were dehydrated in ethanol and mounted in mounting medium (Eukitt, Sigma-Aldrich). Quantification was performed by counting Tph-positive neurons in dorsal and median raphe in every third section.
Behavioral testing.
Procedures were performed according to Italian guidelines for ethical animal treatment following protocols authorized by the Italian Ministry of Health. Mice were transferred to the phenotyping facility 1 week before testing. Each behavioral test was performed on independent cohorts of 8–10-week-old animals. Illumination of the testing rooms was 150 Lux.
Open field.
Mice were habituated to the testing room for 60 min before being placed into a gray plastic arena (50 × 50 cm) for 60 min. Locomotion data were collected by a video tracking system (TSE Systems). Animals were initially placed along one side of the arena, and the center region was defined as the central 26 × 26 cm area.
Elevated-plus maze.
Mice were habituated to the room for 10 min before been placed into the central platform (5 × 5 cm) of a gray plastic maze facing toward an open arm (open arms, 30 × 5 cm, surrounded by a 0.25 cm high border; closed arms, 30 × 5 cm, surrounded by 15 cm high walls). The entire apparatus was elevated 45 cm above the floor. Locomotion data were collected for 5 min by a video tracking system (TSE Systems).
Resident-intruder assay.
For isolation-induced aggression, male resident mice were isolated for at least 4 weeks before the first resident-intruder test and maintained isolated throughout testing. Aggressive behavior was monitored during 15 min exposure to unique wild-type C57BL/6J intruder mice that had been group-housed (5/cage). Three test trials were conducted, one trial per week. The latency to first biting attack, total number of biting attacks, and time spent ano-genital sniffing, crawling over, and social grooming were scored from videotape. A latency time of 900 s was assigned to the animals that did not display any attacks during the test. The Htr1a agonist 8-OH-DPAT (8-hydroxy-N-[di-n-propyl]-aminotetralin; Sigma-Aldrich) and Htr1a antagonist WAY100635 (Sigma-Aldrich) were dissolved in 0.9% saline and injected subcutaneously 30 min before the third trial.
Receptor autoradiography.
Brains were removed, frozen in dry ice, and stored at −80°C before sectioning. Coronal sections (16 μm) were cut in a cryostat, preincubated in binding buffer (50 mm Tris-HCl pH 7.4, 2 mm MgCl2) for 30 min before incubation in binding buffer containing 0.14 nm 125I-MPPI (Kung et al., 1995) (kindly provided by Karl Ploessl and Hank Kung, Department of Radiology, University of Pennsylvania, PA) for 60 min. Sections were washed in ice-cold binding buffer (2 × 10 min), rinsed in distilled water, and air-dried. Slides were exposed to single-sided Kodak BioMax film (Kodak) for 2–4 d.
Hypothermia.
Animals were singly housed the day before testing. 8-OH-DPAT was dissolved in 0.9% saline and injected subcutaneously at a concentration of 0.5 mg/kg body weight. Control animals were treated with 0.9% saline in equivalent volumes. Body temperature was recorded in 10 min intervals with a digital rectal thermometer (Bioseb).
Electrophysiology.
Mice (P24–P60) were anesthetized with isofluorane and decapitated. The brain was rapidly removed, dissected in ice-cold gassed (95% O2 and 5% CO2) ACSF (artificial CSF) composed of: 124 mm NaCl, 2.75 mm KCl, 1.25 mm NaH2PO4, 1.3 mm MgCl2, 2 mm CaCl2, 26 mm NaHCO3, 11 mm d-glucose, and the brainstem sliced coronally into 250-μm-thick slices with a vibratome (DSK, T1000; Ted Pella, Inc.). After recovery for at least 2 h at room temperature, the slices were individually transferred into the recording chamber and perfused continuously at a rate of 2 ml min−1. Neurons within dorsal raphe nucleus were visualized by infrared differential interference contrast video microscopy with a Newicon camera (C2400–07; Hamamatsu Photonics) mounted on an upright microscope (Axioskop; Zeiss). Recordings were made using an EPC-10 amplifier (HEKA Elektronic). Signals were filtered at 1–10 kHz and digitized at 5–40 kHz. Data were analyzed using Fitmaster 2 (HEKA Elektronic) and Clampfit 9.2 (Molecular Devices). Patch pipettes (2–5 MΩ resistance) were prepared from thick-walled borosilicate glass on a P-97 Brown-Flaming electrode puller (Sutter Instruments).
Whole-cell recordings.
Whole-cell recordings were done on slices from P24–P36 mice on 28.5 ± 0.5°C. To block synaptic transmission, in all experiments extracellular solution consisted of oxygenated ACSF supplemented with 10 μm NBQX (2,3-dioxo-6-nitro-1,2,3,4-tetrahydrobenzo[f]quinoxaline-7-sulfonamide disodium salt), 20 μm d-APV (d-(-)-2-amino-5-phosphonopentanoic acid), 10 μm strychnine hydrochloride, 10 μm SR-95531 (6-imino-3-(4-methoxyphenyl)-1(6H)-pyridazinebutanoic acid hydrobromide), and 2 μm CGP 55845A (3-N[1-(S)-(3,4-dichlorophenyl)ethyl]amino-2-(S)-hydroxypropyl-P-benzyl-phosphinic acid). Pipette solution consisted of: 120 mm K Gluconate, 15 mm KCl, 2 mm MgCl2, 10 mm HEPES, 0.1 mm EGTA, 10 mm Na2Phosphocreatine, 4 mm MgATP, 0.3 mm Na3GTP (pH 7.35 with KOH). Serotonergic and nonserotonergic neurons were classified according to existing electrophysiological criteria (Vandermaelen and Aghajanian, 1983; Li et al., 2001; de Kock et al., 2006). Serotonergic cells were identified on the basis of electrophysiological properties displayed in current-clamp mode after establishing whole-cell recording configuration: action potential half-height width >1.5 ms; absence of fast afterhyperpolarization; absence of depolarizing sag in response to hyperpolarizing pulse (from −60/−65 to −110/−120 mV); maximal sustained firing rate <12 Hz (in response to long depolarizing current pulses). Cells were considered nonserotonergic when they had action potential half-height width <1.2 ms; fast afterhyperpolarization; depolarizing sag; maximal sustained firing rate >20 Hz. If neurons displayed intermediate electrophysiological properties (<15% of cells) the recording was aborted. All included neurons had action potential height (peak-to-peak) >100 mV. The access resistance ranged from 5–18 MΩ and was not compensated. Recordings were terminated if it changed >15%. Current–voltage relationships were obtained by averaging 5–7 single responses to hyperpolarizing ramps (from −65 to −130 mV, 100 mV s−1).
Loose-seal cell-attached recordings.
Loose cell-attached recordings were done on slices from P30–P60 mice on 34.5 ± 0.5°C. To facilitate firing, extracellular saline, otherwise the same as in whole-cell recordings, was supplemented with the 10 μm phenylephrine (Baraban and Aghajanian, 1980; Vandermaelen and Aghajanian, 1983). Pipette solution contained the following: 125 mm NaCl, 10 mm HEPES, 2.75 mm KCl, 2 mm CaCl2, 1.3 mm MgCl2 (pH 7.4 with NaOH). Pipette potential was set at 0 mV in voltage-clamp mode. Recordings were aborted if firing frequency was sensitive to changes in pipette holding potential or if shape of action current changed. Neurons were identified according to electrophysiological criteria (Vandermaelen and Aghajanian, 1983; Allers and Sharp, 2003). Neurons were considered serotonergic if they displayed slow and steady firing rate (1–4 Hz with COV <15%), and asymmetric action current (ratio of upstroke to downstroke >3.5) with long peak-to-peak interval (>1.2 ms, proportional to action potential half-height width) during at least a 5-min-long control period at the beginning of the recording. Nonserotonergic cells fired either irregularly (COV >30%) or fast and regularly (firing rate >6 Hz) and showed more symmetric action current with shorter peak-to-peak interval (<0.9 ms) than serotonergic neurons. For experiments that depended on endogenous serotonin, i.e., with tryptophan application, recordings were done from neurons located at least 50 μm below the slice surface (Mlinar et al., 2005). In experiments in which single agonist concentration was applied, steady-state values were calculated as average firing rate over the last 3 min of the application, while in concentration-response experiments, values correspond to the last minute of the application.
Statistical analysis.
Statistical analysis was performed using StatView (version 5.0, SAS Institute) and Prism 4 (GraphPad Software). Unless otherwise indicated, the effects of genotype or treatment on dependent variables were analyzed by ANOVA using repeated measures where indicated, followed by unpaired Student's t test in case of significance. Chi-squared testing was used to assess the effect of genotype on the proportion of mice attacking. For the pharmacological challenge tests, data were not normally distributed and nonparametric analysis was performed using paired Wilcoxon matched pairs test.
Results
Heightened aggression in mice with chronic decrease in serotonin activity
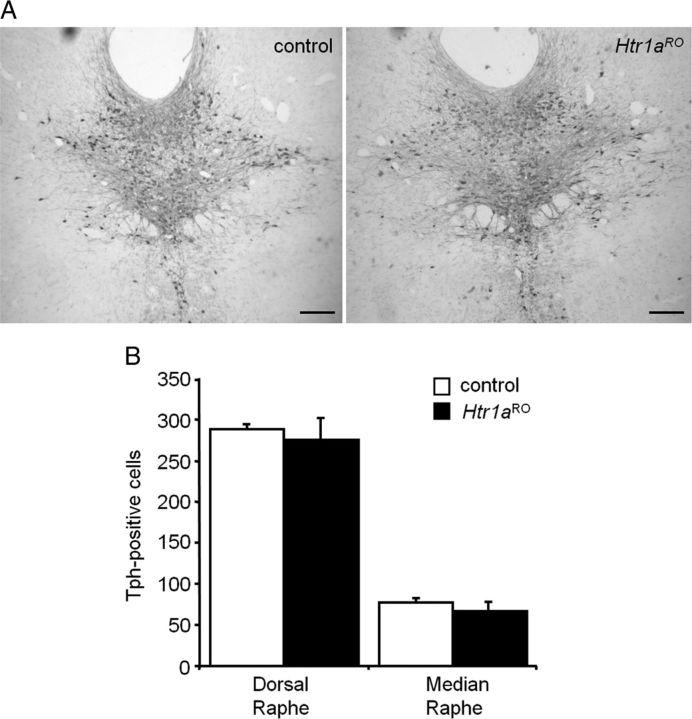
To examine the behavioral consequences of a chronic reduction in serotonin neuron firing we used a double transgenic mouse line in which negative feedback inhibition of serotonin neurons was enhanced by the selective overexpression of the Htr1a autoreceptor (Audero et al., 2008). Tissue-specific conditional overexpression of Htr1a autoreceptor was obtained using the tet-OFF system. Mice carrying the tetracycline transactivator (tTA) gene under control of the endogenous serotonin transporter promoter (called Slc6a4tTA) were crossed to mice carrying a tTA-inducible allele of the endogenous Htr1a gene (called Htr1atetO) (Fig. 1A). Double transgenic mice (called Htr1a raphe overexpressing, or Htr1aRO) showed Htr1a protein overexpression exclusively in raphe nuclei of the mid- and hindbrain and electrophysiological studies in slices taken from these animals revealed decreased serotonin neuron firing in the presence of the serotonin precursor tryptophan (Audero et al., 2008). Neurochemical analysis of whole brain tissue samples confirmed reduced serotonin turnover (ratio of 5-HIAA to 5-HT) consistent with decreased steady-state serotonin neuron activity (Audero et al., 2008). Importantly, Htr1aRO mice showed no change in the number of Tph-immunoreactive cell bodies in dorsal and median raphe nuclei (Fig. 1A,B), demonstrating that increased serotonin auto-inhibition does not interfere with the proliferation or maintenance of serotonin neurons.
Figure 1.
Normal number of serotonin neurons in Htr1aRO mice. A, Representative images of brain sections containing the dorsal raphe nucleus taken from Htr1aRO and control littermates stained with anti-Tph antibodies. B, No significant effect of genotype on total number of Tph-positive cells in dorsal and median raphe was observed (mean ± SEM, N = 4). Scale bars, 200 μm.
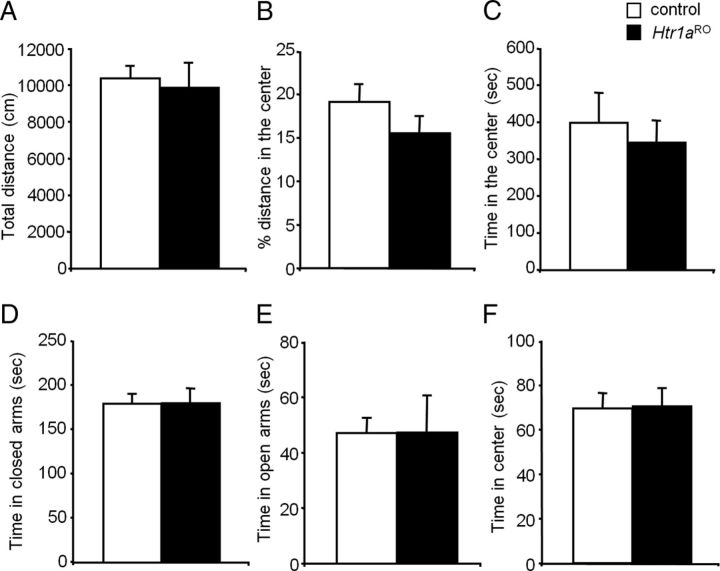
To assess the consequences of chronically reduced serotonin activity on anxiety-like and aggressive behavior, we tested Htr1aRO and control littermates in the open field, elevated plus maze, and resident-intruder tests. Htr1aRO mice showed normal levels of total locomotion (ANOVA: F(1,21) = 0.086, p = 0.772), percentage distance in center (ANOVA: F(1,21) = 1.445, p = 0.242), and time in center (ANOVA: F(1,21) = 0.237, p = 0.631) in the open field (Fig. 2A–C) and time in closed arms (ANOVA: F(1,25) = 3.65E-6, p = 0.998), time in open arms (ANOVA: F(1,25) = 0.002, p = 0.963), and time in center platform (ANOVA: F(1,25) = 0.017, p = 0.898) in the elevated-plus maze tests (Fig. 2D–F), suggesting normal reactivity to a novel aversive environment. No interaction between sex and genotype was observed [OF total locomotion (ANOVA genotype × sex: F(1,21) = 1429, p = 0.2427), OF percentage distance in center (ANOVA genotype × sex: F(1,21) = 1611, p = 0.156), OF time in the center (ANOVA genotype × sex: F(1,21) = 1095, p = 0.3050), EPM time in closed arms (ANOVA genotype × sex: F(1,25) = 0127, p = 0.723), EPM time in open arms (ANOVA genotype × sex: F(1,25) = 0143, p = 0.143), and EPM time in center platform (ANOVA genotype × sex: F(1,25) = 0005, p = 0.941)].
Figure 2.
Normal anxiety behavior in Htr1aRO mice. No differences were detected between Htr1aRO and control littermates in total distance traveled (A), fractional distance in center (B), and time in center in the open field test (C), and time in closed arms (D), time in open arms (E), and time in center platform in the elevated-plus maze (F; open field—control: N = 12, Htr1aRO: N = 11; elevated-plus maze—control: N = 12, Htr1aRO: N = 15).
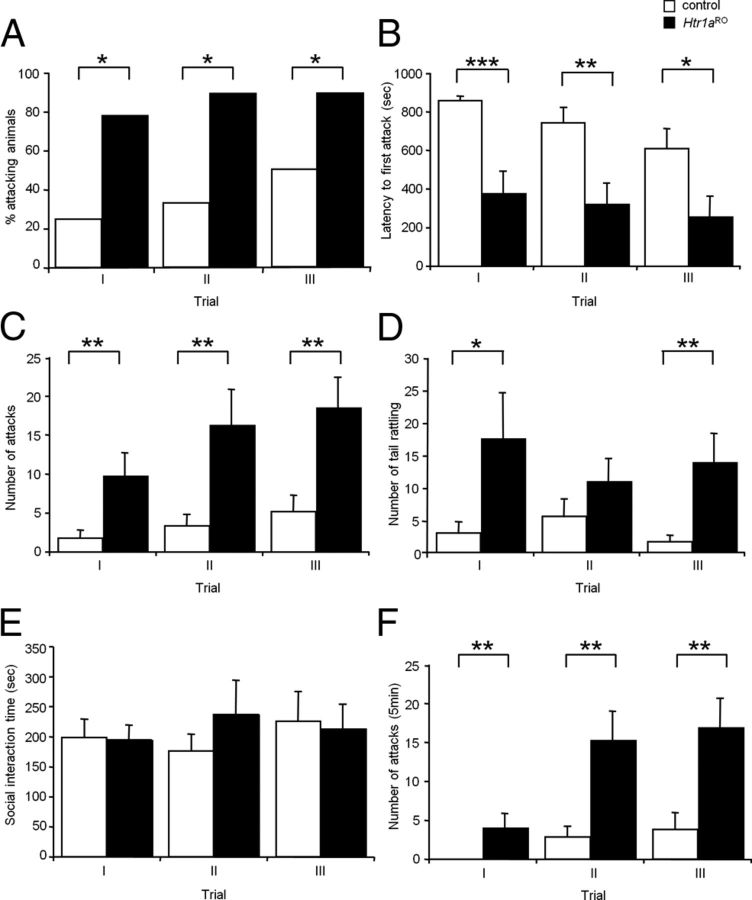
We examined aggressive behavior in Htr1aRO mice in a standard resident-intruder assay of isolation-induced intermale aggression (Schneider et al., 1992; Saudou et al., 1994; Hendricks et al., 2003). In this assay, an unfamiliar male intruder is placed in the home cage of a singly housed resident test mouse. Display of territorial aggression is measured by counting the number of attacks and tail rattling episodes of the resident mouse and the latency to the first attack. Under these conditions, the proportion of control mice attacking a conspecific intruder (Fig. 3A) and the total number of attacks (Fig. 3C) increased and the latency to attack (Fig. 3B) decreased across trials, demonstrating a sensitization of aggressive behavior over time. The proportion of male Htr1aRO mice attacking an intruder was significantly higher compared with control littermates (χ2 test, trial I p = 0.0166; trial II p = 0.0109; trial III p = 0.0121; Fig. 3A). Male Htr1aRO mice showed a significant reduction in the latency to attack (repeated measure ANOVA—main effect of genotype: F(19,38) = 16.11, p = 0.0007; main effect of trial: F(19,38) = 3.48, p = 0.041; trial × genotype interaction: F(19,38) = 0.42, p = 0.662) and increase in the number of attacks (repeated measure ANOVA—main effect of genotype: F(19,38) = 15.80, p = 0.0008; main effect of trial: F(19,38) = 4.32, p = 0.020; trial × genotype interaction: F(19,38) = 1.04, p = 0.362) and tail rattling episodes (repeated measure ANOVA—main effect of genotype: F(19,38) = 6.917, p = 0.0165; main effect of trial: F(19,38) = 0.68, p = 0.770; trial × genotype interaction: F(19,38) = 1.56, p = 0.222) over all three sessions when compared with control littermates (Fig. 3B–D). The time spent in ano-genital sniffing, crawling over, and social grooming with the intruder did not differ between Htr1aRO mice and control littermates (repeated measure ANOVA—main effect of genotype: F(19,38) = 0.138, p = 0.714; main effect of trial: F(19,38) = 0.34, p = 0.713; trial × genotype interaction: F(19,38) = 0.81, p = 0.450; Fig. 3E). An increased number of attacks and tail rattling episodes could be simply a consequence of the shorter attack latency in Htr1aRO animals. However, the number of attacks occurring during the 5 min following the first biting attack were also significantly increased in Htr1aRO animals compared with controls (repeated measure ANOVA—main effect of genotype: F(19,38) = 16.78, p = 0.0006; Fig. 3F). Differences in frequency of attacks and tail rattling between Htr1aRO mice and control littermates were also evident in plots of attacks and tail rattling events across individual mice (Fig. 4A,B). These data demonstrate that long-term reductions in serotonin activity are associated with heightened aggression in the absence of changes in anxiety or motivation for social interaction. In addition, these differences do not appear to be the result of altered learning or escalation of violence as similar differences were seen on the first and subsequent testing days.
Figure 3.
Increased aggression in Htr1aRO mice. Male mice were isolated for 4 weeks before testing during three trials of the resident-intruder assay at one-week intervals starting at P60. A, The proportion of Htr1aRO mice attacking an intruder was significantly higher compared with controls. B–D, Htr1aRO mice showed a significant decrease in latency to the first attack (B), increase in total number of attacks (C), increase in tail rattling episodes (D), but no difference in total social interaction time (E; ano-genital sniffing, crawling over, and social grooming) during each of the three trials when compared with control littermates. Htr1aRO mice also showed a significantly increased number of attacks during the 5 min following the first biting attack (F; controls: N = 9, Htr1aRO: N = 12; *p < 0.05, **p < 0.01, ***p < 0.001).
Figure 4.
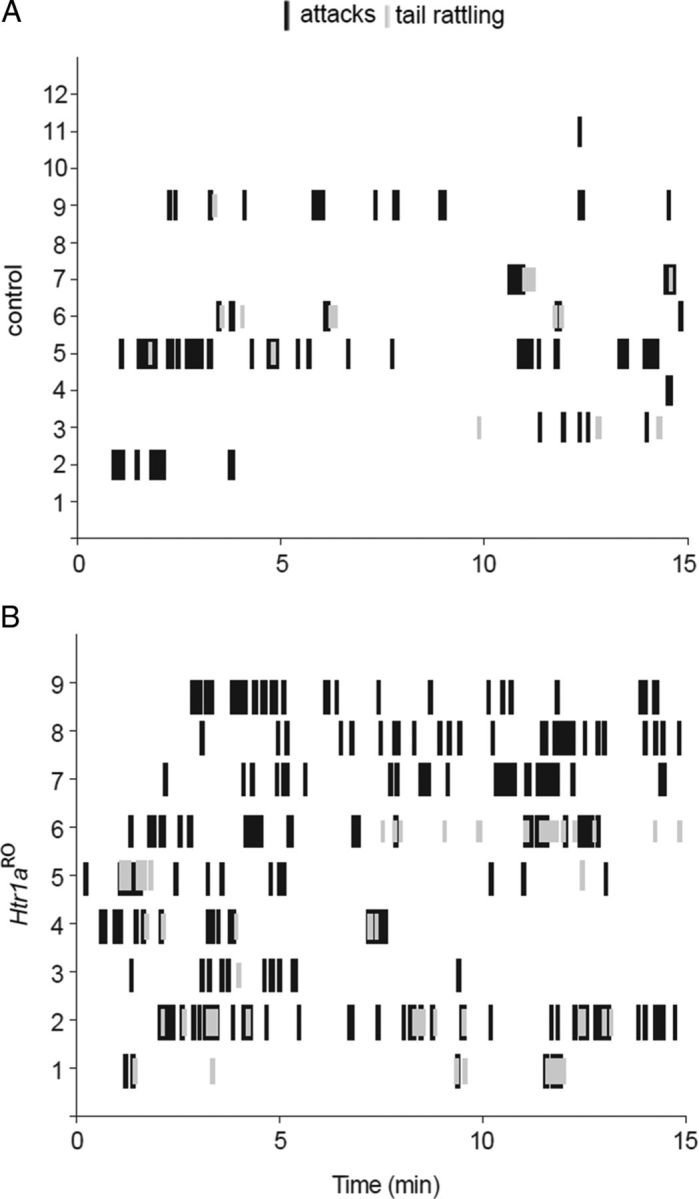
Pattern of aggressive behavior in Htr1aRO and control mice. A, B, Timeline (15 min) showing initiation and duration of attacks and tail rattling events for individual mice in control (A) and Hr1aRO animals (B) during the third trial of the resident-intruder assay.
Heightened aggression is not developmentally programmed
To test whether the increased aggression seen in Htr1aRO mice was due to reduced serotonin activity during development, adulthood, or both, we turned on and off the receptor by treating Htr1aRO mice during different periods of life with doxycycline. Doxycycline blocks the binding of tTA protein to its target tetO sequence and can be used to prevent or reverse overexpression of Htr1a in Htr1aRO mice (Audero et al., 2008). Suppression of Htr1a overexpression during development was achieved by doxycycline treatment of mothers and their Htr1aRO and control littermate offspring until postnatal day 40 (P40; Fig. 5A). When tested in the resident-intruder test at P60, Htr1aRO mice treated during development with doxycycline showed a significant decrease in latency to attack (repeated measure ANOVA—main effect of genotype: F(17,34) = 11.633, p = 0.0033) and increase in number of attacks (repeated measure ANOVA—main effect of genotype: F(17,34) = 10.627, p = 0.0046) toward the intruder when compared with control animals. Similar heightened aggression was seen on the first and subsequent encounters (Fig. 5B,C). Again, no effect of genotype was seen on time spent in social interaction (repeated measure ANOVA—main effect of genotype: F(17,34) = 0.21, p = 0.6523, Fig. 5D), suggesting that adult overexpression of Htr1a was sufficient to explain the heightened aggression seen in untreated Htr1aRO mice (Fig. 3). On the other hand, when Htr1aRO and control littermates were treated with doxycycline beginning at P21 and tested at P60 (Fig. 5E), no significant effect of genotype on latency to attack (repeated measure ANOVA—main effect of genotype: F(13,26) = 1.406, p = 0.2569), number of attacks (repeated measure ANOVA—main effect of genotype: F(13,26) = 0.025, p = 0.8763), or time spent in social interaction (repeated measure ANOVA—main effect of genotype: F(13,26) = 3.286, p = 0.0903) was seen (Fig. 5F–H). Together, these data demonstrate that chronic suppression of serotonin activity during adulthood, but not development, was sufficient to cause increased aggression.
Figure 5.
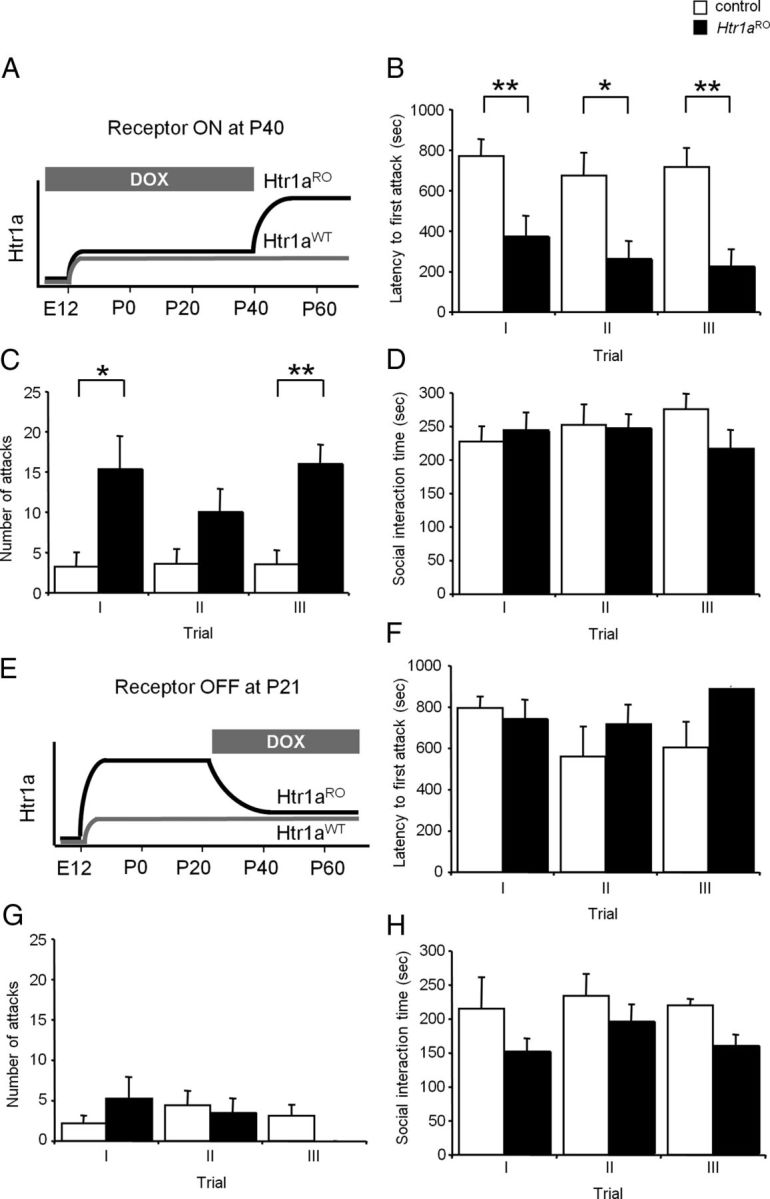
Increased aggression in Htr1aRO mice overexpressing Htr1a in adulthood. A, Htr1aRO and control littermates were treated until P40 with doxycycline to block overexpression of Htr1a during development and tested during three trials of the resident intruder assay at one-week intervals starting at P60. B–D, Htr1aRO mice showed a significant decrease in latency to the first attack (B), increase in total number of attacks (C), but no difference in total social interaction time (D; ano-genital sniffing, crawling over, and social grooming) when compared with control littermates (controls: N = 10, Htr1aRO: N = 9). E, Htr1aRO and control littermates were treated from P21 with doxycycline to block overexpression of Htr1a during adulthood and tested during three trials of the resident intruder assay at one-week intervals starting at P60. F–H, Htr1aRO mice showed no significant differences in latency to the first attack (F), total number of attacks (G), or total social interaction time (H; ano-genital sniffing, crawling over, and social grooming) when compared with control littermates with the exception of the last trial where Htr1aRO animals showed a significant decrease in social interaction behavior (controls: N = 7, Htr1aRO: N = 8; *p < 0.05, **p < 0.01).
Pharmacogenetic tool for the rapid suppression of serotonin activity
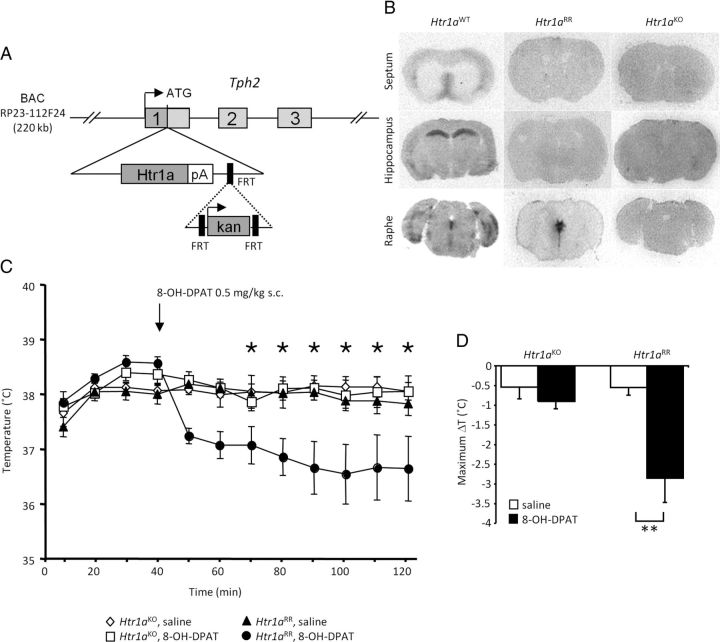
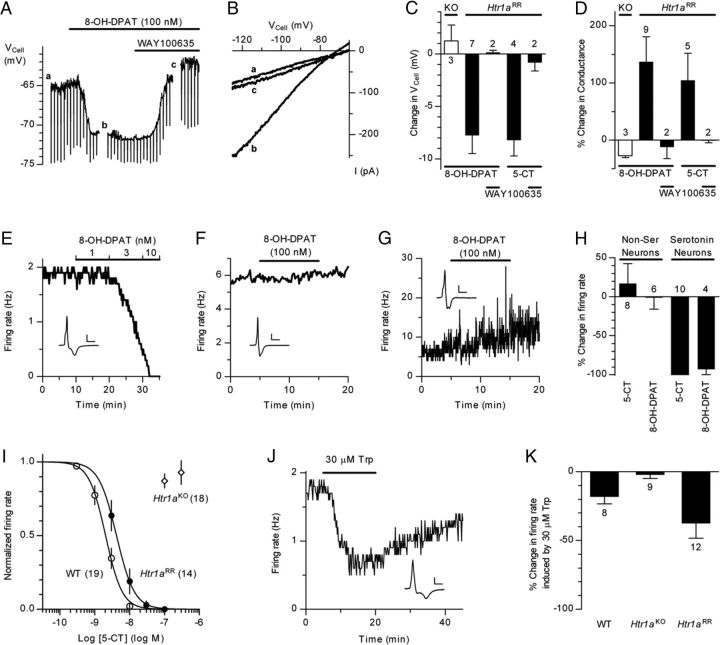
Next, we investigated whether the increased aggression associated with the chronic suppression of serotonin activity would also be seen following rapid, acute inhibition of serotonin neuron firing. Because of its relatively slow kinetics (Kistner et al., 1996), the tet-OFF system does not lend itself to rapid manipulations of gene expression. To facilitate the rapid suppression of serotonin neuron firing, we treated mice in which Htr1a was exclusively expressed in serotonin neurons with the Htr1a selective agonist 8-OH-DPAT (Tsetsenis et al., 2007). Exclusive expression of Htr1a in serotonin neurons was achieved by crossing Htr1a knock-out mice (Ramboz et al., 1998) to a transgenic line in which Htr1a expression was driven by the tryptophan hydroxylase 2 (Tph2) promoter (called Htr1a raphe rescue, or Htr1aRR mice; Fig. 6A). Autoradiography with the Htr1a-selective ligand, 125I-MPPI, confirmed exclusive localization of Htr1a in the raphe nuclei of Htr1aRR mice (Fig. 6B). To confirm functional rescue of Htr1a autoreceptor function in vivo, we measured hypothermic responses to systemic administration of 8-OH-DPAT (Martin et al., 1992). Htr1aRR mice showed a significant hypothermic response to the agonist that was absent in Htr1aKO littermates (ANOVA: F(42,462) = 5.163, p = 0.0283; Fig. 6C,D).
Figure 6.
Functional rescue of Htr1a autoreceptor. A, Schematic representation of Tph2-Htr1a transgene showing insertion of the Htr1a coding sequence at the start codon of the Tph2 gene in a mouse BAC. B, Crossing of the Tph2-Htr1a transgene to Htr1aKO mice produced Htr1aRR mice in which Htr1a expression as assessed by 125I-MPPI autoradiography was restricted to raphe nuclei. C, Htr1aRR mice showed significant hypothermia in response to the Htr1a agonist 8-OH-DPAT (0.5 mg/kg, s.c.). Time course and maximum hypothermic response (D) following systemic administration of the Htr1a agonist or saline in Htr1aKO and Htr1aRR littermates (N = 8; **p < 0.01).
Electrophysiological recordings made in brain slices containing the dorsal raphe nucleus taken from Htr1aRR and Htr1aKO littermates confirmed functional restoration of Htr1a activity selectively in serotonin neurons. Bath application of the selective (8-OH-DPAT, 100 nm) or semiselective, but high efficacy (5-CT, 300 nm) Htr1a agonist during intracellular whole-cell recording caused significant membrane hyperpolarization (two-tailed paired t test: p = 0.004 and p = 0.012, respectively) and an increase in conductance slope (two-tailed paired t test: p = 0.002 and p = 0.0183, respectively) in dorsal raphe cells from Htr1aRR, but not Htr1aKO littermates (Fig. 7A–D). Agonist effects were reversed by the selective Htr1a antagonist, WAY100635 (50 nm). Similarly, application of high concentrations of 8-OH-DPAT (100 nm) and 5-CT (100 nm) suppressed spontaneous firing of serotonin, but not nonserotonin neurons (Fig. 7E–H). Full dose–response curves revealed an EC50 for agonist-induced suppression of firing in Htr1aRR mice (4.18 nm, 95% C.I. 3.18–5.51 nm) that was indistinguishable from wild-type mice (2.03 nm, 95% C.I. 1.69–2.45 nm), suggesting complete rescue of autoreceptor function (Fig. 7I). Finally, treatment of dorsal raphe slices with the serotonin precursor tryptophan caused significant autoreceptor-mediated suppression of neuronal firing in wild-type and Htr1aRR (two-tailed paired t test: p = 0.026 and p = 0.007, respectively), but not Htr1aKO mice (Fig. 7J,K). Together, these data demonstrate functional rescue of Htr1a selectively in serotonin neurons of Htr1aRR mice.
Figure 7.
Electrophysiological characterization of Htr1aRR mice. A–D, Whole-cell recordings. A, Effect of the Htr1a agonist 8-OH-DPAT on the membrane potential of dorsal raphe nucleus serotonin neurons in Htr1aRR mice and its reversal by treatment with the Htr1a antagonist WAY100635 (50 nm). Downward deflections are responses to current pulses (−10 pA) used to monitor cell membrane resistance. B, Current–voltage relationships at different time points (a–c) of cell shown in A obtained with hyperpolarizing ramps. C, Effect of 8-OH-DPAT (100 nm) and 5-CT (300 nm) on membrane potential (VCell) of cells from Htr1aKO (KO) and Htr1aRR mice and their reversal by WAY100635 (50 nm). Numbers indicate the number of cells in each group. D, Effect of 8-OH-DPAT (100 nm) and 5-CT (300 nm) on the slope conductance (between −120 and −100 mV) determined from current–voltage ramps in cells from Htr1aKO and Htr1aRR mice and their reversal by WAY100635 (50 nm). E–K, Loose-seal cell-attached recordings. Representative data of the suppression of firing of serotonin (E), but not nonserotonin, constant frequency (F) and nonserotonin, irregular frequency (G) firing cells from Htr1aRR mice by treatment with increasing doses of 8-OH-DPAT. Action current waveforms used to distinguish serotonin from nonserotonin cells are shown in the insets (calibration: E, 1 ms, 100 pA; F, 1 ms, 50 pA; G: 1 ms, 20 pA). H, Effect of single supramaximal concentration of 8-OH-DPAT (100 nm) and 5-CT (100 nm) on firing rate of serotonin and nonserotonin (Non-Ser) neurons. I, EC50 curves for suppression of firing in dorsal raphe serotonin neurons in response to 5-CT showing wild-type control (WT) and functional recovery of Htr1a in Htr1aRR. No response to 5-CT was seen in Htr1aKO mice. Numbers indicate the total number of cells used for each genotype. J, Representative and summary (K) of data for the suppression of dorsal raphe serotonin neuron firing in wild-type control (WT), Htr1aRR (shown in J), but not Htr1aKO mice following treatment with the serotonin precursor tryptophan (Trp). Inset shows action current waveform used to identify serotonin neurons (calibration: 1 ms, 25 pA).
Acute suppression of serotonin neuron firing increases aggression
To test whether acute reductions in serotonin neuron activity directly control aggressive behavior we examined the effect of treating Htr1aRR mice with the Htr1a agonist 8-OH-DPAT in the resident-intruder assay. Animals were subjected to two encounters with an intruder to measure baseline aggression and then, 30 min before the third trial, treated with either the Htr1a agonist 8-OH-DPAT (0.2 mg/kg, s.c.) or antagonist WAY100635 (0.2 mg/kg, s.c.). A group of control animals was left untreated. Similar levels of aggression and social interaction were seen in all three groups in the first and second trials (Fig. 8A–C). During the third trial, however, agonist, but not control or antagonist treated resident mice showed a significant increase in the number of attacks toward the intruder when compared with the second trial (Wilconox matched pairs, trial II and III, p = 0.0076, trial I and III, p = 0.0229, trial I and II, p = 0.8927) (Fig. 8B). However, only a trend for a decrease in latency to the first attack was seen in agonist treated mice when compared with control or antagonist treated mice on trial three (Fig. 8A), suggesting that acute suppression of serotonin firing primarily affects the persistence and/or recurrence of attacks rather than the timing of their initial occurrence. Again, changes in social interaction time did not explain the effect of agonist or antagonist treatment on aggression measures (ANOVA: F(2,31) = 1.281, p = 0.292) (Fig. 8C). These data demonstrate that acute suppression of serotonin neuron firing is sufficient to promote heightened aggression.
Figure 8.
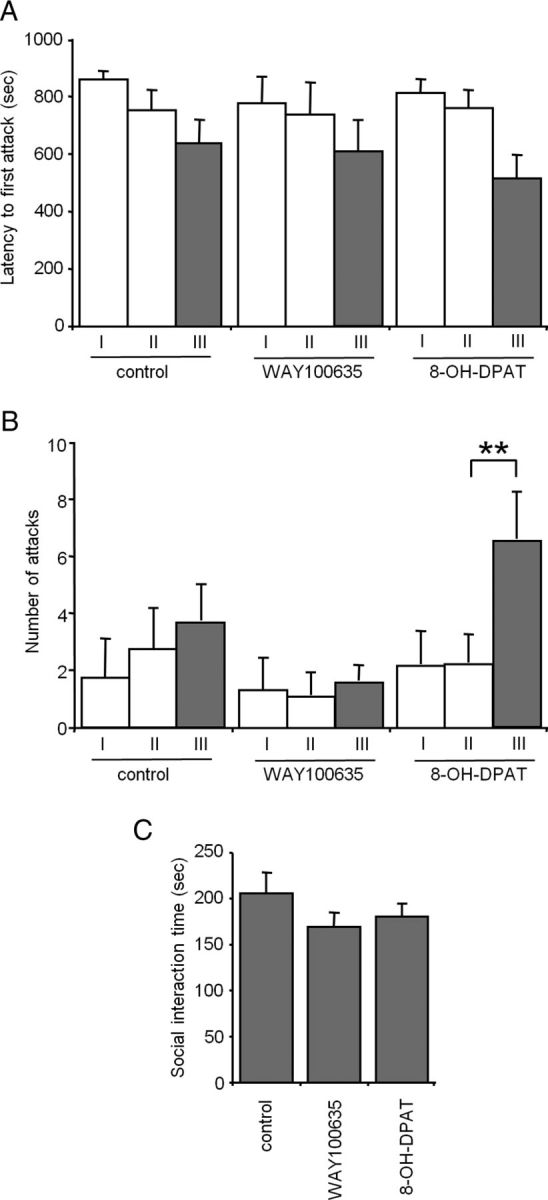
Increased aggression in Htr1aRR mice treated with 8-OH-DPAT. Three groups of male Htr1aRR mice were isolated for 4 weeks before testing during three trials of the resident-intruder test starting at P60. Before testing on trial three, resident mice were injected with either the Htr1a agonist 8-OH-DPAT (0.2 mg/kg, s.c.) or antagonist WAY100635 (0.2 mg/kg, s.c.). A third group of mice received no injection (control). A, B, Treatment with 8-OH-DPAT caused a nonsignificant decrease in latency to first attack (A) and a significant increase in total attacks (B) toward the intruder when compared with behavior on the second trial or to behavior of WAY100635-treated mice. C, No significant difference in total social interaction time (ano-genital sniffing, crawling over, and social grooming) was observed between the three groups. Notably, no significant effect of WAY100635 was seen for any measure. No differences in behavior were detected between the three groups on the first two trials (control: N = 16, 8-OH-DPAT: N = 20, WAY100635: N = 9; **p < 0.01 Wilcoxon matched pairs test).
Discussion
Our data demonstrate that chronic reductions in serotonin activity induced by overexpression of the Htr1a autoreceptor were associated with a reduced latency to initiate and an increased number of attacks against an unfamiliar male intruder mouse. Overexpression of the Htr1a autoreceptor during development was neither necessary nor sufficient to reproduce this heightened aggression phenotype arguing against a long-term effect of reduced serotonin activity on adult aggressive behavior. On the other hand, an acute reduction of serotonin activity in adult mice was sufficient to increase the number of attacks against an unfamiliar intruder, although it had only a moderate effect on reducing the latency to the initial attack. This finding is consistent with studies in the male tree shrew showing a transient reduction in serotonin neuron firing immediately before and during offensive, but not defensive antagonistic encounters (Walletschek and Raab, 1982). These findings argue that the firing of serotonin neurons directly modulates the timing and intensity of aggressive behavior toward threatening conspecifics and provide direct support for the low serotonin/high aggression hypothesis.
Several implications arise from our findings. First, our data support a direct causal relationship between low levels of the serotonin metabolite, 5-HIAA, and aggression-related behavior as has been postulated by associations between these measures across several species (Brown et al., 1982; Linnoila et al., 1983; Mehlman et al., 1994; Caramaschi et al., 2007; Clotfelter et al., 2007). Specifically, our findings suggest that it is at least partly the lack of sufficient tonic serotonin neurotransmission in these subjects that reduces their threshold to aggressive acts. This interpretation is consistent with recordings from serotonin neurons made in male tree shrews during antagonistic encounters. In this study serotonin neuron firing rates were reduced in dominant males and increased in subordinate males immediately before the encounter (Walletschek and Raab, 1982). We hypothesize that lower levels of extracellular serotonin in critical target tissues is insufficient to suppress the initiation of aggressive behavioral programs under social conditions in which they tend to be elicited. Thus, an important question for further study will be the precise neural circuits targeted by serotonin that mediate this altered threshold.
Notably, our findings appear to be at odds with pharmacological experiments in which Htr1a agonists were delivered directly into the dorsal raphe nucleus of rats with the aim of suppressing serotonin neuron activity (Mos et al., 1993; De Almeida and Lucion, 1997; van der Vegt et al., 2003). Those studies reported significant reductions in aggression in the resident intruder test and were interpreted to support a role for serotonin in promoting aggression under nonescalated, baseline conditions. However, Htr1a is expressed on serotonin and a subset of nonserotonin, GABA neurons in the raphe (Kirby et al., 2003; Marinelli et al., 2004; Bonnavion et al., 2010), and simultaneous inhibition of these two potentially antagonistic populations might underlie the behavioral findings reported. In contrast, our electrophysiological studies showed that agonist treatment of Htr1aRR mice exclusively suppressed firing of serotonin neurons, leaving nonserotonin neurons unaffected (Fig. 7H). We hypothesize, then, that local GABA neurons are likely to play a critical role in modulating serotonin homeostasis and function.
Our findings do not support a role for serotonin activity in influencing the escalation of aggression as suggested by some studies (Fish et al., 1999; de Almeida and Miczek, 2002; Caramaschi et al., 2008). Htr1aRO mice showed a similar decrease in latency to attack and increase in number of attacks across all three sessions (Fig. 3B,C). However, it remains to be determined whether acute reductions in serotonin at different stages of escalation might have differential effects on thresholds to aggression or whether more extended escalation of aggression could be differentially affected. It should be noted that the level of aggression elicited in the control strain we used was relatively low compared with that seen in some outbred strains (Parmigiani et al., 1999).
Our data also do not support a major role for the programming of adult aggression by developmental reduction in serotonin homeostasis. These findings seem to contradict pharmacological data showing that transient postnatal treatment with inhibitors of MAOA (Mejia et al., 2002) as well as hypomorphic alleles of MAOA (Brunner et al., 1993; Caspi et al., 2002; Mejia et al., 2002; Kim-Cohen et al., 2006; Frazzetto et al., 2007) are associated with increased aggression and anti-social behavior. One possibility is that reduced degradation of serotonin by MAOA in these cases acts via a mechanism other than that modeled by the increased serotonin autoregulation seen in Htr1aRO mice. Alternatively, an action of MAOA on nonserotonergic monoamine metabolism may play a critical role in the developmental programming of aggression by MAOA.
Strikingly, no effect of reducing serotonin neuron firing during either development or adulthood was seen on anxiety behavior in the open field test (Fig. 2A–C), consistent with the effects of other global reductions in serotonin (Savelieva et al., 2008), but seemingly at odds with the long-term effects of serotonin 1A receptor signaling during development (Gross et al., 2002; Tsetsenis et al., 2007; Lo Iacono and Gross, 2008) and changes in anxiety reported following other genetic manipulations of the serotonin system (Hendricks et al., 2003). Thus, balanced regulation between multiple receptors must play a role in buffering the effects of global reductions in serotonin, at least for anxiety circuits.
Finally, subtle differences between the behavioral effects of chronic and acute reductions of serotonin neuron firing (e.g., only a trend for a reduction in latency to attack in the latter case, Fig. 5B,C vs Fig. 8A,B) suggest that the aggressive phenotype of Htr1aRO mice might be sustained in part by homeostatic mechanisms. For example, gradual sensitization and/or desensitization of Htr1b or Htr1a autoreceptors or serotonin transporter function might amplify serotonergic deficits in Htr1aRO mice (Chaput et al., 1986). It also may be that acute and chronic reductions in serotonin neuron firing moderate different aspects of aggression, with the latter having a stronger effect on impulsivity as reflected in shorter latencies to attack. Alternatively, the difference in attack latency could be explained by a difference in the amount of Htr1a autoreceptor feedback available in the two animals, with higher feedback in Htr1aRO mice conferring unique kinetics of firing during the social encounter that might reduce attack thresholds. Testing these hypotheses will require the use of neural silencing systems not dependent on serotonin signaling.
In summary, our data confirm a role of serotonin activity in setting thresholds for aggressive behavior and help clarify a long-standing controversy about the direction and causality of the association between serotonin and aggression. Future studies aimed at measuring the activity of serotonin neurons under pharmacogenetic or optogenetic control in awake behaving mice will shed light on the precise relationship between the firing dynamics of serotonin neurons and thresholds to aggression.
Footnotes
This work was supported by funds from European Molecular Biology Laboratory (EMBL) (E.A., Z.K.S., C.G.) and grants from the European Commission (FP7 DEVANX; C.G.), Compagnia San Paolo (Programma Neuroscienze-2008.2265; R.C.), and Regione Toscana (Ricerca Regionale in Materia di Salute 2009-Project 82; R.C.). We thank Jeanette Rientjes and Theodoros Tsetsenis for cloning the Tph2-Htr1a construct; Francesca Zonfrillo and Stefania Rizzo for animal husbandry; Olga Schubert for help in behavioral testing; and Jose Gonzalez and the EMBL Transgenic Facility for animal production.
References
- Allers KA, Sharp T. Neurochemical and anatomical identification of fast- and slow-firing neurones in the rat dorsal raphe nucleus using juxtacellular labelling methods in vivo. Neuroscience. 2003;122:193–204. doi: 10.1016/S0306-4522(03)00518-9. [DOI] [PubMed] [Google Scholar]
- Audero E, Coppi E, Mlinar B, Rossetti T, Caprioli A, Banchaabouchi MA, Corradetti R, Gross C. Sporadic autonomic dysregulation and death associated with excessive serotonin autoinhibition. Science. 2008;321:130–133. doi: 10.1126/science.1157871. [DOI] [PubMed] [Google Scholar]
- Baraban JM, Aghajanian GK. Suppression of firing activity of 5-HT neurons in the dorsal raphe by alpha-adrenoceptor antagonists. Neuropharmacology. 1980;19:355–363. doi: 10.1016/0028-3908(80)90187-2. [DOI] [PubMed] [Google Scholar]
- Bjork JM, Dougherty DM, Moeller FG, Cherek DR, Swann AC. The effects of tryptophan depletion and loading on laboratory aggression in men: time course and a food-restricted control. Psychopharmacology. 1999;142:24–30. doi: 10.1007/s002130050858. [DOI] [PubMed] [Google Scholar]
- Bonnavion P, Bernard JF, Hamon M, Adrien J, Fabre V. Heterogeneous distribution of the serotonin 5-HT(1A) receptor mRNA in chemically identified neurons of the mouse rostral brainstem: Implications for the role of serotonin in the regulation of wakefulness and REM sleep. J Comp Neurol. 2010;518:2744–2770. doi: 10.1002/cne.22331. [DOI] [PubMed] [Google Scholar]
- Brown GL, Ebert MH, Goyer PF, Jimerson DC, Klein WJ, Bunney WE, Goodwin FK. Aggression, suicide, and serotonin: relationships to CSF amine metabolites. Am J Psychiatry. 1982;139:741–746. doi: 10.1176/ajp.139.6.741. [DOI] [PubMed] [Google Scholar]
- Brunner HG, Nelen M, Breakefield XO, Ropers HH, van Oost BA. Abnormal behavior associated with a point mutation in the structural gene for monoamine oxidase A. Science. 1993;262:578–580. doi: 10.1126/science.8211186. [DOI] [PubMed] [Google Scholar]
- Caramaschi D, de Boer SF, Koolhaas JM. Differential role of the 5-HT1A receptor in aggressive and nonaggressive mice: an across-strain comparison. Physiol Behav. 2007;90:590–601. doi: 10.1016/j.physbeh.2006.11.010. [DOI] [PubMed] [Google Scholar]
- Caramaschi D, de Boer SF, de Vries H, Koolhaas JM. Development of violence in mice through repeated victory along with changes in prefrontal cortex neurochemistry. Behav Brain Res. 2008;189:263–272. doi: 10.1016/j.bbr.2008.01.003. [DOI] [PubMed] [Google Scholar]
- Caspi A, McClay J, Moffitt TE, Mill J, Martin J, Craig IW, Taylor A, Poulton R. Role of genotype in the cycle of violence in maltreated children. Science. 2002;297:851–854. doi: 10.1126/science.1072290. [DOI] [PubMed] [Google Scholar]
- Chamberlain B, Ervin FR, Pihl RO, Young SN. The effect of raising or lowering tryptophan levels on aggression in vervet monkeys. Pharmacol Biochem Behav. 1987;28:503–510. doi: 10.1016/0091-3057(87)90513-2. [DOI] [PubMed] [Google Scholar]
- Chaput Y, de Montigny C, Blier P. Effects of a selective 5-HT reuptake blocker, citalopram, on the sensitivity of 5-HT autoreceptors: electrophysiological studies in the rat brain. Naunyn Schmiedebergs Arch Pharmacol. 1986;333:342–348. doi: 10.1007/BF00500007. [DOI] [PubMed] [Google Scholar]
- Clotfelter ED, O'Hare EP, McNitt MM, Carpenter RE, Summers CH. Serotonin decreases aggression via 5-HT1A receptors in the fighting fish Betta splendens. Pharmacol Biochem Behav. 2007;87:222–231. doi: 10.1016/j.pbb.2007.04.018. [DOI] [PubMed] [Google Scholar]
- De Almeida RM, Lucion AB. 8-OH-DPAT in the median raphe, dorsal periaqueductal gray and corticomedial amygdala nucleus decreases, but in the medial septal area it can increase maternal aggressive behavior in rats. Psychopharmacology. 1997;134:392–400. doi: 10.1007/s002130050476. [DOI] [PubMed] [Google Scholar]
- de Almeida RM, Miczek KA. Aggression escalated by social instigation or by discontinuation of reinforcement (“frustration”) in mice: inhibition by anpirtoline: a 5-HT1B receptor agonist. Neuropsychopharmacology. 2002;27:171–181. doi: 10.1016/S0893-133X(02)00291-9. [DOI] [PubMed] [Google Scholar]
- de Boer SF, Koolhaas JM. 5-HT1A and 5-HT1B receptor agonists and aggression: a pharmacological challenge of the serotonin deficiency hypothesis. Eur J Pharmacol. 2005;526:125–139. doi: 10.1016/j.ejphar.2005.09.065. [DOI] [PubMed] [Google Scholar]
- de Boer SF, Lesourd M, Mocaër E, Koolhaas JM. Somatodendritic 5-HT(1A) autoreceptors mediate the anti-aggressive actions of 5-HT(1A) receptor agonists in rats: an ethopharmacological study with S-15535, alnespirone, and WAY-100635. Neuropsychopharmacology. 2000;23:20–33. doi: 10.1016/S0893-133X(00)00092-0. [DOI] [PubMed] [Google Scholar]
- de Kock CP, Cornelisse LN, Burnashev N, Lodder JC, Timmerman AJ, Couey JJ, Mansvelder HD, Brussaard AB. NMDA receptors trigger neurosecretion of 5-HT within dorsal raphe nucleus of the rat in the absence of action potential firing. J Physiol. 2006;577:891–905. doi: 10.1113/jphysiol.2006.115311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fish EW, Faccidomo S, Miczek KA. Aggression heightened by alcohol or social instigation in mice: reduction by the 5-HT(1B) receptor agonist CP-94,253. Psychopharmacology. 1999;146:391–399. doi: 10.1007/PL00005484. [DOI] [PubMed] [Google Scholar]
- Frazzetto G, Di Lorenzo G, Carola V, Proietti L, Sokolowska E, Siracusano A, Gross C, Troisi A. Early trauma and increased risk for physical aggression during adulthood: the moderating role of MAOA genotype. PLoS One. 2007;2:e486. doi: 10.1371/journal.pone.0000486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gross C, Zhuang X, Stark K, Ramboz S, Oosting R, Kirby L, Santarelli L, Beck S, Hen R. Serotonin1A receptor acts during development to establish normal anxiety-like behaviour in the adult. Nature. 2002;416:396–400. doi: 10.1038/416396a. [DOI] [PubMed] [Google Scholar]
- Hendricks TJ, Fyodorov DV, Wegman LJ, Lelutiu NB, Pehek EA, Yamamoto B, Silver J, Weeber EJ, Sweatt JD, Deneris ES. Pet-1 ETS gene plays a critical role in 5-HT neuron development and is required for normal anxiety-like and aggressive behavior. Neuron. 2003;37:233–247. doi: 10.1016/S0896-6273(02)01167-4. [DOI] [PubMed] [Google Scholar]
- Huang YY, Cate SP, Battistuzzi C, Oquendo MA, Brent D, Mann JJ. An association between a functional polymorphism in the monoamine oxidase a gene promoter, impulsive traits and early abuse experiences. Neuropsychopharmacology. 2004;29:1498–1505. doi: 10.1038/sj.npp.1300455. [DOI] [PubMed] [Google Scholar]
- Kim-Cohen J, Caspi A, Taylor A, Williams B, Newcombe R, Craig IW, Moffitt TE. MAOA, maltreatment, and gene-environment interaction predicting children's mental health: new evidence and a meta-analysis. Mol Psychiatry. 2006;11:903–913. doi: 10.1038/sj.mp.4001851. [DOI] [PubMed] [Google Scholar]
- Kirby LG, Pernar L, Valentino RJ, Beck SG. Distinguishing characteristics of serotonin and nonserotonin-containing cells in the dorsal raphe nucleus: electrophysiological and immunohistochemical studies. Neuroscience. 2003;116:669–683. doi: 10.1016/S0306-4522(02)00584-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kistner A, Gossen M, Zimmermann F, Jerecic J, Ullmer C, Lübbert H, Bujard H. Doxycycline-mediated quantitative and tissue-specific control of gene expression in transgenic mice. Proc Natl Acad Sci U S A. 1996;93:10933–10938. doi: 10.1073/pnas.93.20.10933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kung MP, Frederick D, Mu M, Zhuang ZP, Kung HF. 4-(2′-Methoxy-phenyl)-1-[2′-(n-2“-pyridinyl)-p-iodobenzamido]-ethyl-piperazine ([125I]p-MPPI) as a new selective radioligand of serotonin-1A sites in rat brain: in vitro binding and autoradiographic studies. J Pharmacol Exp Ther. 1995;272:429–437. [PubMed] [Google Scholar]
- Li YQ, Li H, Kaneko T, Mizuno N. Morphological features and electrophysiological properties of serotonergic and nonserotonergic projection neurons in the dorsal raphe nucleus. An intracellular recording and labeling study in rat brain slices. Brain Res. 2001;900:110–118. doi: 10.1016/S0006-8993(01)02272-7. [DOI] [PubMed] [Google Scholar]
- Linnoila M, Virkkunen M, Scheinin M, Nuutila A, Rimon R, Goodwin FK. Low cerebrospinal fluid 5-hydroxyindoleacetic acid concentration differentiates impulsive from nonimpulsive violent behavior. Life Sci. 1983;33:2609–2614. doi: 10.1016/0024-3205(83)90344-2. [DOI] [PubMed] [Google Scholar]
- Lo Iacono L, Gross C. Alpha-Ca2+/calmodulin-dependent protein kinase II contributes to the developmental programming of anxiety in serotonin receptor 1A knock-out mice. J Neurosci. 2008;28:6250–6257. doi: 10.1523/JNEUROSCI.5219-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manuck SB, Flory JD, Ferrell RE, Dent KM, Mann JJ, Muldoon MF. Aggression and anger-related traits associated with a polymorphism of the tryptophan hydroxylase gene. Biol Psychiatry. 1999;45:603–614. doi: 10.1016/S0006-3223(98)00375-8. [DOI] [PubMed] [Google Scholar]
- Marinelli S, Schnell SA, Hack SP, Christie MJ, Wessendorf MW, Vaughan CW. Serotonergic and nonserotonergic dorsal raphe neurons are pharmacologically and electrophysiologically heterogeneous. J Neurophysiol. 2004;92:3532–3537. doi: 10.1152/jn.00437.2004. [DOI] [PubMed] [Google Scholar]
- Martin KF, Phillips I, Hearson M, Prow MR, Heal DJ. Characterization of 8-OH-DPAT-induced hypothermia in mice as a 5-HT1A autoreceptor response and its evaluation as a model to selectively identify antidepressants. Br J Pharmacol. 1992;107:15–21. doi: 10.1111/j.1476-5381.1992.tb14457.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehlman PT, Higley JD, Faucher I, Lilly AA, Taub DM, Vickers J, Suomi SJ, Linnoila M. Low CSF 5-HIAA concentrations and severe aggression and impaired impulse control in nonhuman primates. Am J Psychiatry. 1994;151:1485–1491. doi: 10.1176/ajp.151.10.1485. [DOI] [PubMed] [Google Scholar]
- Mejia JM, Ervin FR, Baker GB, Palmour RM. Monoamine oxidase inhibition during brain development induces pathological aggressive behavior in mice. Biol Psychiatry. 2002;52:811–821. doi: 10.1016/S0006-3223(02)01418-X. [DOI] [PubMed] [Google Scholar]
- Miczek KA, Hussain S, Faccidomo S. Alcohol-heightened aggression in mice: attenuation by 5-HT1A receptor agonists. Psychopharmacology. 1998;139:160–168. doi: 10.1007/s002130050701. [DOI] [PubMed] [Google Scholar]
- Mlinar B, Tatini F, Ballini C, Nencioni S, Della Corte L, Corradetti R. Differential autoinhibition of 5-hydroxytryptamine neurons by 5-hydroxytryptamine in the dorsal raphe nucleus. Neuroreport. 2005;16:1351–1355. doi: 10.1097/01.wnr.0000175249.25535.bf. [DOI] [PubMed] [Google Scholar]
- Mos J, Olivier B, Poth M, Van Oorschot R, Van Aken H. The effects of dorsal raphe administration of eltoprazine, TFMPP and 8-OH-DPAT on resident intruder aggression in the rat. Eur J Pharmacol. 1993;238:411–415. doi: 10.1016/0014-2999(93)90877-K. [DOI] [PubMed] [Google Scholar]
- Newman TK, Syagailo YV, Barr CS, Wendland JR, Champoux M, Graessle M, Suomi SJ, Higley JD, Lesch KP. Monoamine oxidase A gene promoter variation and rearing experience influences aggressive behavior in rhesus monkeys. Biol Psychiatry. 2005;57:167–172. doi: 10.1016/j.biopsych.2004.10.012. [DOI] [PubMed] [Google Scholar]
- Osipova DV, Kulikov AV, Popova NK. C1473G polymorphism in mouse tph2 gene is linked to tryptophan hydroxylase-2 activity in the brain, intermale aggression, and depressive-like behavior in the forced swim test. J Neurosci Res. 2009;87:1168–1174. doi: 10.1002/jnr.21928. [DOI] [PubMed] [Google Scholar]
- Parmigiani S, Palanza P, Rogers J, Ferrari PF. Selection, evolution of behavior and animal models in behavioral neuroscience. Neurosci Biobehav Rev. 1999;23:957–969. doi: 10.1016/S0149-7634(99)00029-9. [DOI] [PubMed] [Google Scholar]
- Ramboz S, Oosting R, Amara DA, Kung HF, Blier P, Mendelsohn M, Mann JJ, Brunner D, Hen R. Serotonin receptor 1A knockout: an animal model of anxiety-related disorder. Proc Natl Acad Sci U S A. 1998;95:14476–14481. doi: 10.1073/pnas.95.24.14476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saudou F, Amara DA, Dierich A, LeMeur M, Ramboz S, Segu L, Buhot MC, Hen R. Enhanced aggressive behavior in mice lacking 5-HT1B receptor. Science. 1994;265:1875–1878. doi: 10.1126/science.8091214. [DOI] [PubMed] [Google Scholar]
- Savelieva KV, Zhao S, Pogorelov VM, Rajan I, Yang Q, Cullinan E, Lanthorn TH. Genetic disruption of both tryptophan hydroxylase genes dramatically reduces serotonin and affects behavior in models sensitive to antidepressants. PLoS One. 2008;3:e3301. doi: 10.1371/journal.pone.0003301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider R, Hoffmann HJ, Schicknick H, Moutier R. Genetic analysis of isolation-induced aggression. I. Comparison between closely related inbred mouse strains. Behav Neural Biol. 1992;57:198–204. doi: 10.1016/0163-1047(92)90150-3. [DOI] [PubMed] [Google Scholar]
- Tsetsenis T, Ma XH, Lo Iacono L, Beck SG, Gross C. Suppression of conditioning to ambiguous cues by pharmacogenetic inhibition of the dentate gyrus. Nat Neurosci. 2007;10:896–902. doi: 10.1038/nn1919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Vegt BJ, Lieuwes N, van de Wall EH, Kato K, Moya-Albiol L, Martínez-Sanchis S, de Boer SF, Koolhaas JM. Activation of serotonergic neurotransmission during the performance of aggressive behavior in rats. Behav Neurosci. 2003;117:667–674. doi: 10.1037/0735-7044.117.4.667. [DOI] [PubMed] [Google Scholar]
- Vandermaelen CP, Aghajanian GK. Electrophysiological and pharmacological characterization of serotonergic dorsal raphe neurons recorded extracellularly and intracellularly in rat brain slices. Brain Res. 1983;289:109–119. doi: 10.1016/0006-8993(83)90011-2. [DOI] [PubMed] [Google Scholar]
- Vergnes M, Depaulis A, Boehrer A. Parachlorophenylalanine-induced serotonin depletion increases offensive but not defensive aggression in male rats. Physiol Behav. 1986;36:653–658. doi: 10.1016/0031-9384(86)90349-5. [DOI] [PubMed] [Google Scholar]
- Virkkunen M, Rawlings R, Tokola R, Poland RE, Guidotti A, Nemeroff C, Bissette G, Kalogeras K, Karonen SL, Linnoila M. CSF biochemistries, glucose metabolism, and diurnal activity rhythms in alcoholic, violent offenders, fire setters, and healthy volunteers. Arch Gen Psychiatry. 1994;51:20–27. doi: 10.1001/archpsyc.1994.03950010020003. [DOI] [PubMed] [Google Scholar]
- Walletschek H, Raab A. Spontaneous activity of dorsal raphe neurons during defensive and offensive encounters in the tree-shrew. Physiol Behav. 1982;28:697–705. doi: 10.1016/0031-9384(82)90054-3. [DOI] [PubMed] [Google Scholar]