Abstract
Neural plasticity is crucial for understanding the experience-dependent reorganization of brain regulatory circuits and the pathophysiology of schizophrenia. An important circuit-level feature derived from functional magnetic resonance imaging (fMRI) is prefrontal-hippocampal seeded connectivity during working memory, the best established intermediate connectivity phenotype of schizophrenia risk to date. The phenotype is a promising marker for the effects of plasticity-enhancing interventions, such as high-frequency repetitive transcranial magnetic stimulation (rTMS), and can be studied in healthy volunteers in the absence of illness-related confounds, but the relationship to brain plasticity is unexplored. We recruited 39 healthy volunteers to investigate the effects of 5 Hz rTMS on prefrontal-hippocampal coupling during working memory and rest. In a randomized and sham-controlled experiment, neuronavigation-guided rTMS was applied to the right dorsolateral prefrontal cortex (DLPFC), and fMRI and functional connectivity analyses [seeded connectivity and psychophysiological interaction (PPI)] were used as readouts. Moreover, the test-retest reliability of working-memory related connectivity markers was evaluated. rTMS provoked a significant decrease in seeded functional connectivity of the right DLPFC and left hippocampus during working memory that proved to be relatively time-invariant and robust. PPI analyses provided evidence for a nominal effect of rTMS and poor test-retest reliability. No effects on n-back-related activation and DLPFC–hippocampus resting-state connectivity were observed. These data provide the first in vivo evidence for the effects of plasticity induction on human prefrontal-hippocampal network dynamics, offer insights into the biological mechanisms of a well established intermediate phenotype linked to schizophrenia, and underscores the importance of the choice of outcome measures in test-retest designs.
Introduction
Neural plasticity is critical for mental health, and is mediated by fine-tuned mechanisms such as long-term potentiation (LTP) which modify the dynamic properties of neurons and facilitate the experience-dependent modification of brain regulatory networks (Bosch and Hayashi, 2012). Particular interest has been directed to disturbed plasticity processes in neurological and psychiatric disorders (Wassermann and Zimmermann, 2012). Specifically, schizophrenia is increasingly recognized as a behavioral outcome of a neural “disconnection syndrome” linked to altered neural development (Friston and Frith, 1995; Stephan et al., 2006; Meyer-Lindenberg, 2010). Modern pathophysiological accounts on schizophrenia propose that genetic and environmental risk factors cause an early developmental insult in the brain that interferes with the normal maturation of neural trajectories, especially in circuits connecting the dorsolateral prefrontal cortex (DLPFC) and hippocampus (Weinberger, 1987). Later in life, the impaired stabilization of these circuits is thought to promote persistent deficits in experience-dependent plasticity, abnormal long-range connectivity, and psychosis (Meyer-Lindenberg, 2011).
Consistent with this, multiple lines of evidence highlight the role of disintegrated prefrontal network dynamics in the neural risk architecture of schizophrenia (Meyer-Lindenberg, 2009). At the brain-systems level, disturbed prefrontal-temporal functional connectivity is one of the best-validated abnormalities observable in chronic schizophrenia, first-episode patients, and prodromal states (Meyer-Lindenberg et al., 2001; Crossley et al., 2009; Rasetti et al., 2011). In working memory tasks engaging the right DLPFC, these abnormal functional interactions manifest as a persistence in the coupling of the left hippocampus (Meyer-Lindenberg et al., 2005; Rasetti et al., 2011). Anomalies in DLPFC–hippocampus connectivity are similarly evident in unaffected relatives of patients (Rasetti et al., 2011), healthy carriers of a genome-wide supported schizophrenia risk variant (Esslinger et al., 2009; Rasetti et al., 2011), and genetic animal models of the disorder linked to altered neuroplasticity (Sigurdsson et al., 2010). Notably, the phenotype itself is a quantitative trait measure whose biological mechanisms can be studied in healthy volunteers in the absence of confounding effects related to illness, chronicity, and medication (Meyer-Lindenberg and Weinberger, 2006). Specifically, whereas reduced coupling is indicative of a relatively “normal” state during n-back working memory (a task challenging spatial but not spatial-relational maintenance), a relative increase in coupling is observed in conditions of schizophrenia risk (Meyer-Lindenberg et al., 2005; Esslinger et al., 2009). These data make prefrontal-hippocampal functional coupling the best established intermediate connectivity phenotype of schizophrenia risk to date, and highlight this circuit-level feature as a potential neuroimaging marker for altered neural plasticity (Meyer-Lindenberg, 2010). However, it is unclear to what extent prefrontal-hippocampal coupling relates to brain plasticity.
Here, we used high-frequency rTMS, a noninvasive method for plasticity induction and promoter of the functional reorganization of neural networks that has gained popularity as a therapeutic tool for brain disorders including schizophrenia (Slotema et al., 2010). We studied a large sample of normal volunteers in a fully randomized and placebo-controlled experiment involving functional neuronavigation, high-frequency rTMS of the right DLPFC, and quantification of effects on DLPFC and hippocampus functional connectivity by means of functional magnetic resonance imaging (fMRI) and a well established n-back working memory paradigm (Callicott et al., 2003). Specifically, we examined the effects of rTMS on working memory-related seeded functional connectivity and psychophysiological interaction, established the test-retest reliability properties of these coupling markers, and compared our findings to the effects of rTMS on DLPFC–hippocampus connectivity during resting state. Based on the evidence reviewed above, we expected to detect a decrease in seeded functional connectivity of the right DLPFC and left hippocampus (i.e., a sign for a more efficient functional decoupling of the circuitry during working memory).
Materials and Methods
Subjects.
We studied 39 right-handed healthy volunteers from the normal population of the city of Mannheim, Germany (mean age = 24.9 ± 2.2 years, mean education = 12.9 ± 0.5 years, 27 males). All participants provided written informed consent for a protocol approved by the Ethics Committee of the University of Heidelberg. Structured clinical screening interviews were acquired by a trained psychologist to ensure the absence of a lifetime history of psychiatric or neurological illness (Mini-DIPS) (Margraf, 1994). Other specific exclusion criteria included an IQ <85, pregnancy, significant general medical problems including liver, cardiac, or renal dysfunctions, a history of head trauma, and current alcohol or drug abuse.
rTMS protocol.
rTMS was performed in accordance with the recommendations of the International Workshop on the Safety of Repetitive Transcranial Magnetic Stimulation (Wassermann, 1998). Stimuli were applied using a MagPro X100 stimulator (MagVenture) and a standard butterfly coil (MFC-B65). Stimulus intensity was set to 90% of the individual active motor threshold of the right primary motor cortex “hot spot” as defined by the lowest stimulator output resulting in a visible twitch of the first dorsal interosseal muscle of the left hand in 5 of 10 stimulations (mean motor threshold = 51.7 ± 7.7% of maximum stimulator output). During these measurements, participants kept the hand muscles in an active state by abducting the left index finger by ∼30° relative to the dorsum of the hand. Before rTMS, and to identify the individual target sites within DLPFC, subjects completed one run of the fMRI working memory task described below. DLPFC target sites (Fig. 1A) were defined as the individual local activation maximum in the right DLPFC during working memory performance and marked for rTMS using functional neuronavigation software (TMS Navigator 1.7.2; Localite). DLPFC stimulations were performed off-line shortly before the actual fMRI experiments (mean delay: 132 ± 5.14 s). Specifically, rTMS was applied at a frequency of 5 Hz and consisted of seven 1 m stimulus trains separated by 1 min intertrain intervals (i.e., a total of 2100 stimuli over a period of 13 min). Sham stimulations proceeded exactly as described for rTMS with the coil being flipped 180° around its main axis so that the integrated cooling system of the coil was sited between the skull and the coil center. Although the sham procedure provoked comparable acoustic and vibration effects, the resulting increase in spatial distance translated in a reduction in stimulation intensity of ∼80% (MagVenture). Each subject completed the full MRI protocol twice, once after rTMS and sham TMS stimulation, respectively. The fMRI protocol included a 4.5 min n-back task, followed by a 5 min resting-state experiment (both tasks described in detail below), and a 10 min Flanker task not further examined here. In all sessions, experimental procedures followed a highly standardized protocol in which only the investigator applying the TMS pulses (but not the participant, MR technician, investigator responsible for the fMRI experiments, or other supportive staff) was informed about the TMS stimulation condition. Poststimulation interviews confirmed the absence of any systematic knowledge of the participants on the TMS session type. The order of treatment conditions was fully randomized over subjects, and an intersession interval of 7 d was observed.
Figure 1.
A, Spatial consistency of rTMS target sites in the right DLPFC. For illustration purposes, individual coordinates of the center of stimulation sites in DLPFC were derived from the functional neuronavigation software, approximated by 5 mm spheres, normalized to standard MNI space, and superimposed to create a heat map of TMS stimulation sites for all subjects. The group map is overlaid on sagittal (left) and transversal (right) sections of a structural template. Color bar represents number of subjects with overlapping stimulation targets at a given voxel in normalized space. Please note that the figure underestimates the topology of locally induced electrical fields. B, Effects of rTMS on DLPFC–hippocampus functional coupling as quantified with a correlative measure (“seeded connectivity”). Left, Significant decrease in functional connectivity of the right DLPFC and left hippocampus under rTMS relative to sham TMS during working memory performance, but no significant effects at rest. Bar plots depict the mean value for the connectivity estimates of the peak voxel stratified by treatment condition. Error bars illustrate the variance of parameter estimates (±SEM). Right, heat map of the n-back group connectivity statistics overlaid on a coronal template section of the brain (p = 0.005 uncorrected, for presentation purposes). Color bar represents t values. SC, Seeded connectivity; HF, hippocampus formation; NS, not significant.
n-back working memory task.
Brain function was studied with a well established spatial working memory task that reliably provokes cortical activations in prefrontal–parietal working memory networks, specifically in DLPFC (n-back task) (Callicott et al., 2003). In this block-designed task, a series of visual stimuli (nos. 1–4) are displayed on a screen in a random order at set locations in a diamond-shaped box (stimulus presentation time: 500 ms, interstimulus interval: 1500 ms). In the working memory condition (2-back), subjects were asked to encode a currently seen number and simultaneously respond with the button that corresponds to the spatial location of the stimulus presented two presentations previously. In the control condition, (0-back) subjects were asked to press the key on the button box that corresponds to the position of the currently seen number presentation. Participants were required to respond to every item, and the number and spatial position of the stimuli were the only criteria relevant for the response. Notably, the spatial position of a given number remained constant over the experiment, i.e., the working memory task did not challenge the maintenance of the spatial relation of the presented stimuli. The task consists of four alternating blocks of each condition, summing up to a total task time of 4.5 min or 128 whole-brain MRI scans. To minimize the likelihood of TMS-related behavioral confounding during fMRI, all subjects were thoroughly trained on a test version of the n-back task before the scan until they reached a stable high performance level (2-back condition: 90.4 ± 13% correct responses).
Resting-state experiment.
In the resting-state experiment subjects were instructed to relax, focus a fixation cross-displayed on the screen, and do not engage in any particular mental activity during the scan (task duration: 5.0 min or 150 whole-brain scans). After each scan, investigators confirmed with the participant that they had not fallen asleep in the scanner.
fMRI data acquisition and preprocessing.
fMRI data were acquired on a 3T MRI scanner (Siemens Trio, Siemens) using a gradient-echo echoplanar imaging (EPI) sequence with the following specifications: 28 slices, 4 mm slice thickness, 1 mm gap, TR = 2000 ms, TE = 30 ms, field of view = 192 × 192 mm, flip angle = 80°. Standard procedures implemented in the Statistical Parametric Mapping software (SPM8; http://www.fil.ion.ucl.ac.uk/spm/software/spm8/) were used for all data preprocessing and analysis routines. All images were realigned to the first image of the scan run, slice time corrected, and smoothed with a 9 mm FWHM Gaussian filter. For activation analysis, images were normalized to standard stereotactic space [as defined by the Montreal Neurological Institute (MNI)] before smoothing. Functional connectivity analyses were performed in native space after extracting time series from individual target seed coordinates derived from the functional neuronavigation software. Here, data were normalized after the calculation of individual DLPFC connectivity maps and contrast images in native space.
Activation analysis.
Processed images were entered into random-effects statistical analyses in a two-level procedure. For n-back functional activation analysis, a separate general linear model (GLM) was specified for each subject modeling the alternating fMRI task conditions by convolving a boxcar reference vector with the canonical hemodynamic response function as implemented in SPM8. At the model estimation stage, the data were high-pass filtered with a cutoff of 128 s to remove low-frequency drifts, and an autoregressive model of the first order was applied to account for serial correlations. Contrast images were calculated for each subject to identify brain regions with greater activity during working memory performance relative to the control task and rTMS relative to sham TMS treatment [rTMS (2-back > 0-back) > sham TMS (2-back > 0-back)].
Functional connectivity analyses.
Seeded functional connectivity analyses were conducted as previously described (Esslinger et al., 2009, 2011). Briefly, seed time series were extracted from 6 mm spheres centered on the spatial location of individual TMS target sites within DLPFC. For the n-back task, individual first-level multiple regression models were defined with the subject-specific DLPFC time series as regressor of interest, and the following regressors of no interest: (1) the movement parameters from the realignment step, (2) first eigenvariates derived from CSF and white matter masks, and (3) regressors encoding the experimental conditions (for removal of task-related variance). During model estimation, a high-pass filter of 128 s (SPMs built-in DCT filter) and an autoregressive model of the first order were applied. With exception of the lack of task regressors, and the use of a 0.01–0.08 Hz bandpass filter, the seeded connectivity analysis of the resting-state experiment followed the procedures described for the n-back task. Individual first-level psychophysiological interaction (PPI) models for the n-back task consisted of the following parts: (1) a regressor that captures the task conditions, (2) the DLPFC seed time series described above, (3) the PPI regressor of interest that captures the interaction between the task condition-series and the seed time-series, and (4) the same movement parameters from the realignment step, and CSF and white matter eigenvariates also used for the n-back seeded functional connectivity analysis. The resulting statistical parametric maps of these analyses constituted t-statistics for DLPFC functional connectivity during working memory (seeded connectivity, PPI: 2-back > 0-back) and during resting state (seeded connectivity) for each voxel and every subject, and were subsequently contrasted by the TMS conditions (rTMS > sham TMS). The relationship between DLPFC–hippocampus seeded functional connectivity and n-back task performance was examined for the sham TMS measurements using a multiple regression model that included the n-back seeded functional connectivity maps as dependent variable, the mean individual reaction times as independent variable, and the mean individual accuracy indices of the 2-back condition as covariate of no interest.
Statistical inference.
To examine the effects of rTMS versus sham rTMS on brain activation and DLPFC connectivity, individual first-level contrast images (n-back), seeded connectivity maps (n-back, resting state), and PPI connectivity maps (n-back) were subjected to second-level statistical inference using one-sample t tests. Based on our a priori defined hypothesis, significance was measured at p < 0.05 familywise error (FWE) corrected for multiple-comparisons in predefined anatomical masks of the bilateral hippocampus and DLPFC derived from the Wake Forest University PickAtlas (www.fmri.wfubmc.edu/downloads). Outside these prehypothesized regions of interest (ROIs), activation, and connectivity findings were considered significant if they passed a significance threshold of p < 0.05 FWE corrected for multiple-comparisons across the whole brain. For the n-back paradigm, behavioral measures of interest were computed as mean percentage of correct responses and mean reaction time during task performance, respectively. Behavioral data were subjected individually to repeated measurement ANOVAs with task (2-back vs 0-back) and treatment (rTMS vs sham TMS) as factors. Analyses were performed using predictive analysis software (PASW Statistics 18, SPSS).
Test-retest reliability of DLPFC–hippocampus functional connectivity measures.
The within-subject robustness of DLPFC–hippocampus functional connectivity measures derived from the seeded connectivity and PPI analyses was examined by reanalysis of the test-retest reliability data reported in detail in (Plichta et al., 2012). Briefly, in this study, 25 healthy subjects (mean age: 24.4 years, 10 males) were scanned twice with an fMRI test battery that included the n-back task described above (mean retest interval: 14.6 d). To keep the methods consistent with our TMS study, individual “pseudo-targets” (i.e., 6 mm spheres centered on the spatial location of the maximum peak activations in the 2-back > 0-back activation contrast) were defined in the right DLPFC based on the activation data of the first session. The extraction of time series and the seeded connectivity and PPI analyses followed the procedures of the TMS study. In keeping with Shrout and Fleiss (1979) and Plichta et al. (2012), we calculated intraclass correlation coefficients (ICCs; 2,1) for the following n-back outcome measures between sessions: DLPFC–hippocampus seeded functional connectivity (averaged over the left hippocampus), DLPFC–hippocampus PPI (averaged over the left hippocampus), and the 2-back > 0-back activation estimates of the DLPFC pseudo-targets. Further methods details on the calculation of ICCs are given by Plichta et al. (2012).
Results
Task performance
Analysis of behavioral data showed a significant main effect of task manifesting as lower percentage of correct responses (mean0-back 99.7%, SD = 0.7; mean2-back = 90.4%, SD = 12.8; F(1,38) = 24.7, p < 0.001) and faster reaction times (mean0-back 461.5 ms, SD = 146.3; mean2-back = 378.1 ms, SD = 302.6; F(1,38) = 4.7, p < 0.05) in the 2-back condition. No significant effects of treatment (rTMS vs sham TMS) or task by treatment interaction effects on behavior were observed (all p values > 0.69). Post hoc correlation analysis of individual task condition-specific differences in correct responses [%correct (2-back − 0-back)] and reaction times [RT (2-back − 0-back)] did not suggest a systematic speed-accuracy tradeoff in either the sham TMS (r < 0.1, p > 0.55) or the rTMS measurements (r < 0.01, p > 0.98).
n-back and resting-state functional connectivity analyses
The regression analysis of the n-back seeded functional connectivity maps and 2-back reaction times of the sham condition showed a significant positive relationship in the left (MNI −27 −13 −24, t = 3.52, p = 0.05 FWE-corrected within ROI) (Fig. 2D) and right hippocampus (MNI 27 −10 −27, t = 3.63, p = 0.038 FWE-corrected within ROI), a finding consistent with the idea that decreased DLPFC–hippocampus coupling may reflect an increase in the efficiency of working memory processes. Analogous interpretations of reaction time indices have been offered in prior working memory studies with within-subject designs and ceiling accuracy measures (Furey et al., 1997; Luber et al., 2008; Finn et al., 2010; Esslinger et al., 2012).
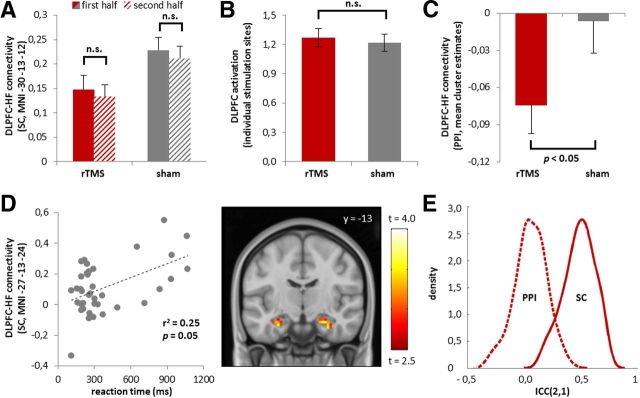
Figure 2.
A, No significant main effect of time or rTMS condition by time interaction effect on DLPFC–hippocampus seeded connectivity in a split-half analysis of the n-back data (p > 0.05, see results section for details). B, No significant effects of rTMS on working-memory related brain activations in DLPFC in the area under the coil (p > 0.05, see Results for details). The bar plot depicts parameter estimates derived from individual stimulation sites in native space. C, Nominal significant effect of rTMS on DLPFC–hippocampus functional connectivity as quantified with PPI. Bar plot depicts the mean contrast estimates derived from nominally significant clusters (p < 0.05, uncorrected, see Results for details). All error bars illustrate the variance of parameter estimates (±SEM). D, Significant association of DLPFC–hippocampus seeded connectivity estimates with n-back-related reaction times in the sham condition (p = 0.05, corrected, see Results for details). The heat map depicts the corresponding group statistic on a coronal template section of the brain (p = 0.005 uncorrected, for presentation purposes). Color bar represents t values. E, Test-retest reliability of n-back-related DLPFC–hippocampus connectivity estimates. The graph depicts ICC (2,1) density plots across hippocampus voxels for PPI (dashed line) and seeded connectivity (solid line) estimates.
Seeded functional connectivity analyses demonstrated significant effects of rTMS on prefrontal network dynamics in the n-back task that were not evident during rest (Fig. 1B). Specifically, compared with sham TMS, the application of rTMS resulted in a significant reduction in functional connectivity between right DLPFC and left hippocampus during working memory performance (MNI −30 −13 −12, t = 3.75, p = 0.039 FWE-corrected within ROI). A trend-level finding in the same direction was observed for the functional connectivity between right DLPFC and right hippocampus (MNI 18 −4 −12, t = 3.59, p = 0.059 FWE-corrected within ROI). No significant effects of rTMS on DLPFC seeded functional connectivity during working memory performance were observed outside the prehypothesized ROIs. In a post hoc analysis we tested whether the observed effects of rTMS on DLPFC–hippocampus seeded connectivity during working memory performance were time-variant, i.e., faded over the course of the experiment. For this, we split the task-related time series in two time segments (first half, second half), recalculated our seeded connectivity measure for these segments separately, and examined the hippocampal region with the observed rTMS effect (MNI −30, −13, −12) for potential rTMS condition by time interaction effects. The analysis confirmed a significant main effect of rTMS on DLPFC–hippocampus seeded connectivity in both hemispheres (left hippocampus: F = 11.5, p = 0.002; right hippocampus: F = 7.4, p = 0.01), but provided no evidence for either a significant main effect of time (left hippocampus: F < 0.64, p > 0.42; right hippocampus: F < 0.54, p > 0.46) or rTMS condition by time interaction effect (left hippocampus: F < 0.007, p > 0.93; right hippocampus: F < 0.092, p > 0.76) (Fig. 2A).
Notably, no corrected significant effects of rTMS on DLPFC–hippocampus functional connectivity during n-back performance were observed when PPI connectivity estimates were used as outcome measures (p > 0.05, FWE-corrected within ROI). Exploratory analysis at a nominal significance threshold revealed a total of 5 clusters in the hippocampus with a stronger load-dependent decoupling from DLPFC under rTMS relative to sham TMS [Tmax = 2.75, MNImax −15 −34 9, Pmin = 0.004 uncorrected, contrast: rTMS (PPI: 2-back < 0-back) < sham TMS (PPI: 2-back < 0-back)] (Fig. 2C). The reverse contrast did not reveal any effects at a nominal level of significance (Tmax < 0.58, Pmin > 0.25, uncorrected). In the resting-state experiment, no significant effects of rTMS on the coupling of right DLPFC and left hippocampus were observed when the same seeded functional connectivity estimates were examined as outcome measures (p > 0.65 FWE-corrected within ROI). Supplementary focal analyses in the location of the hot spot of rTMS effects for the n-back task in the left hippocampus (MNI −30 −13 −12) confirmed the absence of significant effects of rTMS in the resting brain even at a nominal significant threshold (t < 0.58; p > 0.56, uncorrected) (Fig. 1B), and the presence of a significant task by TMS interaction effect (F > 4.50, p < 0.042) in a 2 × 2 repeated-measures ANOVA model that included task (n-back, resting state) and treatment condition (rTMS, sham) as within-subject factors.
n-back activation analysis
In both TMS conditions, 2-back performance relative to 0-back performance resulted in a significant signal increase in a well known prefrontal–parietal network linked to spatial working memory (Callicott et al., 2003). No significant effects of rTMS stimulation on brain activation were detected inside or outside the prehypothesized ROIs in DLPFC and hippocampus. Supplementary focal analyses confirmed the absence of significant effects of rTMS on working memory-related brain activations in the area under the coil both at the group level in normalized space (p > 0.05, uncorrected) as well as in a paired t test of contrast estimates extracted from individual DLPFC activation peaks (t < 0.72; p > 0.47, uncorrected) (Fig. 2B).
Test-retest reliability of n-back functional connectivity estimates
The robustness of n-back activation measures in the DLPFC was previously established (Plichta et al., 2012). With an ICC (2,1) of 0.54, the analysis of the activation estimates from the DLPFC pseudo-targets revealed a comparably good outcome. The reliability study of the DLPFC–hippocampus functional connectivity measures provided a more diverse result, namely, an ICC (2,1) of 0.50 for seeded functional connectivity and an ICC (2,1) of 0.05 for DLPFC–hippocampus PPI connectivity (Fig. 2E). In keeping with Fleiss (1986) and Plichta et al. (2012), these findings indicate a fair-to-good robustness of the individual DLPFC activation peaks and DLPFC–hippocampus seeded connectivity measures, but a poor within-subject reliability of the examined DLPFC–hippocampus PPI connectivity measures.
Discussion
In this work, we aimed to identify a neuroimaging signature of short-term plasticity induction on one of the best established intermediate phenotypes in neuropsychiatry to date, DLPFC–hippocampus connectivity during working memory performance. Consistent with our expectations, we observed significant effects of rTMS on prefrontal network dynamics manifesting as a decrease in the functional interaction of DLPFC and hippocampus as quantified with a correlative functional connectivity measure. In contrast, no effects of rTMS were seen when the same correlational outcome measure was examined during rest, and only a nominally significant (i.e., uncorrected) effect on DLPFC–hippocampus coupling was detected when the same working memory data were examined with a PPI approach. We will discuss our data in light of these findings and their neurobiological implications.
The first and most obvious question to address is whether plasticity was provoked in prefrontal neural networks and, if so, how. Consistent with earlier experiments (Esslinger et al., 2012; Gromann et al., 2012), we demonstrate effects of rTMS on n-back seeded interregional connectivity that outlasted the stimulation period by minutes and were relatively time-invariant over the course of the experiment. These observations support the induction of adaptive changes in neural network organization (Wassermann and Zimmermann, 2012), an interpretation that has been repeatedly offered for other single-session rTMS experiments (Siebner and Rothwell, 2003), although the precise time-frame of effects remains to be clarified [e.g., the study by Rizzo et al., (2004) suggests a duration of effects up to 1 h poststimulation]. Interestingly, the evidenced TMS-induced changes in seeded connectivity were only partially corroborated by our PPI analysis. The reliability study of the examined n-back functional connectivity measures provides a plausible explanation for this observation: although our seeded connectivity index showed fair-to-good (Fleiss, 1986) reliability equivalent to that previously reported for BOLD activation measures (Bennett and Miller, 2010; Plichta et al., 2012), the PPI indices derived from the same fMRI data proved to be unstable, at least in a test-retest design comparable to that of our rTMS study.
As for the “how” part of the question, valuable clues on rTMS-induced plasticity arise from prior systems-level research in humans, although knowledge on the underlying mechanisms is sparse due to the lack of suitable animal models (Wassermann and Zimmermann, 2012). At the neural systems level, a convincing body of evidence points to a prolonged facilitatory impact of high-frequency rTMS on neural circuit function (Wassermann and Zimmermann, 2012). These effects are mediated, at least in parts, by LTP-dependent changes in glutamate signaling, as evidenced, for example, by the modulatory impact of NMDA antagonists (Huang et al., 2007). Further insights into the chemical intermediates come from positron emission tomography (PET) studies demonstrating a lasting rTMS-induced increase in endogenous dopamine release in downstream functional nodes of the lateral prefrontal cortex, in particular in striatum and medial prefrontal lobe (Strafella et al., 2001; Cho and Strafella, 2009). At the molecular level, rTMS has been linked to the function of plasticity genes, as suggested by the upregulation of brain-derived neurotrophic factor (BDNF) gene expression (Gersner et al., 2011) and increased tyrosine receptor kinase B (TrkB) and NMDA receptor signaling (Wang et al., 2011) after repeated stimulations, and the detrimental effects of a common functional variant in BDNF on rTMS-induced changes in cortical excitability (Jayasekeran et al., 2011). Together, these observations suggest that our experiment was successful in provoking neural plasticity in prefrontal regulatory circuits, likely through facilitating effects of rTMS on glutamate and dopamine neurotransmission and related modifications in the efficacy of intermediate excitatory synapses. Because the effects on seeded connectivity were relatively sustained but of an undefined absolute duration, we believe an early LTP-dependent plasticity process is the most parsimonious explanation (Siebner and Rothwell, 2003). If prior experience with longitudinal rTMS designs is a guide, repeated rTMS applications may be suitable to prolong these effects, possibly to a duration of months (Slotema et al., 2010).
Even though our experiment allowed for a precise and homogenous definition of target sites within DLPFC, no significant changes in working memory-related BOLD response were detected inside or outside the prefrontal cortex following off-line conditioning with rTMS. Although a negative result in local BOLD response does not exclude the presence of changes in cortical excitability (Norup Nielsen and Lauritzen, 2001), the absence of activation effects in the area under the coil is consistent with previous studies, in particular those who have adopted similar subthreshold high-frequency rTMS protocols (Bestmann et al., 2003; Rounis et al., 2006; Esslinger et al., 2012). In contradistinction, activity changes in interconnected neural areas have been repeatedly described by researchers challenging different target sites and/or functional domains (Bestmann et al., 2003; Rounis et al., 2006). Our data confirm the efficacy of rTMS in working memory networks as such, as we detected sustained effects on DLPFC functional coupling and our experimental methods were either optimized (e.g., large sample size, fully randomized within-subject design, well established phenotypes, robust task, spatially consistent targets, short time interval between stimulation and probe) or followed established procedures (Wassermann and Zimmermann, 2012). Moreover, absent regional activation effects of 5 Hz subthreshold rTMS at the stimulation site in conjunction with clear downstream functional effects are consistent with prior evidence suggesting that fMRI activation measures are largely driven by the energetic demands of the synaptic input side (Logothetis et al., 2001), whereas 5 Hz rTMS facilitates (directly or indirectly) postsynaptic activity in remote but anatomically connected areas (Rounis et al., 2006).
Interestingly, the evidenced dissociation of working memory-related activation and connectivity findings may also point to a particularly close biological link (or “marker quality”) of fMRI coupling indices to prefrontal-hippocampal plasticity mechanisms. Notably, a recent fMRI study (Rasetti et al., 2011) demonstrated that although deficits in DLPFC activation and DLPFC–hippocampus connectivity in the n-back task are both intimately linked to the genetic risk for schizophrenia, they seem to be two independent neurobiological phenomena. The authors concluded that their observations likely reflect two unrelated intermediate physiological phenotypes that may be modulated by different risk-associated genes affecting DLPFC local neural activity and interregional functional connectivity, respectively. This notion receives indirect support by the specific effects of plasticity induction on n-back-related DLPFC functional connectivity, but not activation, observed here. Although tentative, our data thus draw attention to the possibility that DLPFC functional dynamics during working memory may be particularly sensitive to experimental manipulations and genetic variants linked to experience-dependent plasticity, and highlight a potential avenue for future clinical applications targeting this phenotype.
Although prior experiments underscore the favorable impact of high-frequency rTMS on prefrontal cortical functions (Guse et al., 2010), the cognitive implications of the observed systems-level effects of plasticity induction are, at first glance, unclear. It should be noted that the application of rTMS did not alter cognitive performance in our thoroughly trained sample, a result that is optimal for our neuroimaging purposes as the experimental confounding of treatment conditions and overt behavior would have rendered the detected BOLD signal changes uninterpretable. However, circuit-specific neurocognitive accounts have been offered for schizophrenia, namely, that the deficient decoupling of DLPFC and hippocampus in the n-back task contributes to the characteristic working memory deficits seen in these patients (Meyer-Lindenberg, 2010; Rasetti et al., 2011), possibly by a lack of inhibition of interfering cognitive processes, such as the parallel encoding of stimuli in episodic memory (Meyer-Lindenberg et al., 2005). The formation of episodic memories and the maintenance of spatial-relational stimulus properties are indeed critically dependent on precise neural timing, in particular hippocampal rhythms that trigger phase synchronous activations in higher order prefrontal association cortices (Jones and Wilson, 2005; Cashdollar et al., 2009; Cohen, 2011; Rattenborg and Martinez-Gonzalez, 2011). This network dynamic may be detrimental during the n-back paradigm, which primarily recruits prefrontal–parietal circuits and does not challenge these functions (Callicott et al., 2003). Prefrontal-hippocampal rhythms are modulated, among others, by direct glutamatergic projections connecting the hippocampus with the medial prefrontal cortex (Thierry et al., 2000), an area directly linked to DLPFC and a likely functional intermediate in this study given the preferential propagation of rTMS-induced currents via larger tracts that run tangentially to the plane of the coil (Wassermann and Zimmermann, 2012). Our data further point to a specific modulatory impact of rTMS on the task-related interaction of these areas, because no alterations in DLPFC–hippocampus connectivity were seen at rest, a finding that is highly consistent with prior data demonstrating the state dependency of this risk phenotype (Esslinger et al., 2011). The alternative scenario of an already faded rTMS effect, in contrast, is rather unlikely, because the connectivity decrease in the preceding n-back task was relatively sustained and effects of longer duration were seen in a prior study with similar rTMS and fMRI protocols (Esslinger et al., 2012). Together, the decrease in n-back-related functional interaction of DLPFC and hippocampus is indeed consistent with a regulatory impact of rTMS on episodic memory circuits, an effect that may plausibly translate into a cognitive advantage in the context of concurrent working memory processes. Our PPI analysis supports, if anything, the idea of an rTMS-induced increase the decoupling of the hippocampus from higher-order DLPFC under working memory load. However, this specific conclusion has to be taken with caution given the evidenced reliability constraints of the PPI measure per se and the uncorrected significance level of the corresponding results.
In conclusion, to our knowledge, this is the first in vivo demonstration of the effects of high-frequency rTMS on prefrontal-hippocampal network dynamics in humans. As predicted, plasticity induction with rTMS provoked a sustained decrease in the functional coupling of DLPFC and hippocampus during n-back performance. Although tentative, these observations are in line with the dynamic reorganization of temporally coordinated firing patterns in prefrontal regulatory circuits through the induction of LTP-related processes, and of potential significance for the capacity of the DLPFC to constrain interfering cognitive processes during n-back working memory performance. Given the high clinical relevance of the examined phenotype, and the potential of rTMS as a therapeutic modality with low costs and relatively mild side effects, future studies are warranted that examine its efficacy for the modulation of compromised prefrontal network dynamics in schizophrenia. However, substantial efforts will be needed to establish the clinical utility of rTMS for the examined risk phenotype, in particular the accumulation of large control databases supporting data interpretation, and the validation of the potential prolongation of effects through repeated stimulations.
Footnotes
E.B. is a Ph.D. grant awardee of the SFB 636 International Graduate Program in Translational Neuroscience funded by Deutsche Forschungsgemeinschaft (DFG). A.M.-L., M.R., and T.G.S. received grant support by DFG (Collaborative Research Center SFB 636, subproject B7). H.T. received grant support by the German Federal Ministry of Education and Research (Grant BMBF 01GQ1102). We thank Claudia Stief and Dagmar Gass for research assistance.
The authors declare no competing financial interests.
References
- Bennett CM, Miller MB. How reliable are the results from functional magnetic resonance imaging? Ann N Y Acad Sci. 2010;1191:133–155. doi: 10.1111/j.1749-6632.2010.05446.x. [DOI] [PubMed] [Google Scholar]
- Bestmann S, Baudewig J, Siebner HR, Rothwell JC, Frahm J. Subthreshold high-frequency TMS of human primary motor cortex modulates interconnected frontal motor areas as detected by interleaved fMRI-TMS. Neuroimage. 2003;20:1685–1696. doi: 10.1016/j.neuroimage.2003.07.028. [DOI] [PubMed] [Google Scholar]
- Bosch M, Hayashi Y. Structural plasticity of dendritic spines. Curr Opin Neurobiol. 2012;22:383–388. doi: 10.1016/j.conb.2011.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Callicott JH, Egan MF, Mattay VS, Bertolino A, Bone AD, Verchinksi B, Weinberger DR. Abnormal fMRI response of the dorsolateral prefrontal cortex in cognitively intact siblings of patients with schizophrenia. Am J Psychiatry. 2003;160:709–719. doi: 10.1176/appi.ajp.160.4.709. [DOI] [PubMed] [Google Scholar]
- Cashdollar N, Malecki U, Rugg-Gunn FJ, Duncan JS, Lavie N, Duzel E. Hippocampus-dependent and -independent theta-networks of active maintenance. Proc Natl Acad Sci U S A. 2009;106:20493–20498. doi: 10.1073/pnas.0904823106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho SS, Strafella AP. rTMS of the left dorsolateral prefrontal cortex modulates dopamine release in the ipsilateral anterior cingulate cortex and orbitofrontal cortex. PLoS One. 2009;4:e6725. doi: 10.1371/journal.pone.0006725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen MX. Hippocampal-prefrontal connectivity predicts midfrontal oscillations and long-term memory performance. Curr Biol. 2011;21:1900–1905. doi: 10.1016/j.cub.2011.09.036. [DOI] [PubMed] [Google Scholar]
- Crossley NA, Mechelli A, Fusar-Poli P, Broome MR, Matthiasson P, Johns LC, Bramon E, Valmaggia L, Williams SC, McGuire PK. Superior temporal lobe dysfunction and frontotemporal dysconnectivity in subjects at risk of psychosis and in first-episode psychosis. Hum Brain Mapp. 2009;30:4129–4137. doi: 10.1002/hbm.20834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esslinger C, Walter H, Kirsch P, Erk S, Schnell K, Arnold C, Haddad L, Mier D, Opitz von Boberfeld C, Raab K, Witt SH, Rietschel M, Cichon S, Meyer-Lindenberg A. Neural mechanisms of a genome-wide supported psychosis variant. Science. 2009;324:605. doi: 10.1126/science.1167768. [DOI] [PubMed] [Google Scholar]
- Esslinger C, Kirsch P, Haddad L, Mier D, Sauer C, Erk S, Schnell K, Arnold C, Witt SH, Rietschel M, Cichon S, Walter H, Meyer-Lindenberg A. Cognitive state and connectivity effects of the genome-wide significant psychosis variant in ZNF804A. Neuroimage. 2011;54:2514–2523. doi: 10.1016/j.neuroimage.2010.10.012. [DOI] [PubMed] [Google Scholar]
- Esslinger C, Schuler N, Sauer C, Gass D, Mier D, Braun U, Ochs E, Schulze TG, Rietschel M, Kirsch P, Meyer-Lindenberg A. Induction and quantification of prefrontal cortical network plasticity using 5 Hz rTMS and fMRI. Hum Brain Mapp. 2012 doi: 10.1002/hbm.22165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finn AS, Sheridan MA, Kam CL, Hinshaw S, D'Esposito M. Longitudinal evidence for functional specialization of the neural circuit supporting working memory in the human brain. J Neurosci. 2010;30:11062–11067. doi: 10.1523/JNEUROSCI.6266-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fleiss JL. The design and analysis of clinical experiments. New York: Wiley; 1986. [Google Scholar]
- Friston KJ, Frith CD. Schizophrenia: a disconnection syndrome? Clin Neurosci. 1995;3:89–97. [PubMed] [Google Scholar]
- Furey ML, Pietrini P, Haxby JV, Alexander GE, Lee HC, VanMeter J, Grady CL, Shetty U, Rapoport SI, Schapiro MB, Freo U. Cholinergic stimulation alters performance and task-specific regional cerebral blood flow during working memory. Proc Natl Acad Sci U S A. 1997;94:6512–6516. doi: 10.1073/pnas.94.12.6512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gersner R, Kravetz E, Feil J, Pell G, Zangen A. Long-term effects of repetitive transcranial magnetic stimulation on markers for neuroplasticity: differential outcomes in anesthetized and awake animals. J Neurosci. 2011;31:7521–7526. doi: 10.1523/JNEUROSCI.6751-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gromann PM, Tracy DK, Giampietro V, Brammer MJ, Krabbendam L, Shergill SS. Examining frontotemporal connectivity and rTMS in healthy controls: implications for auditory hallucinations in schizophrenia. Neuropsychology. 2012;26:127–132. doi: 10.1037/a0026603. [DOI] [PubMed] [Google Scholar]
- Guse B, Falkai P, Wobrock T. Cognitive effects of high-frequency repetitive transcranial magnetic stimulation: a systematic review. J Neural Transm. 2010;117:105–122. doi: 10.1007/s00702-009-0333-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang YZ, Chen RS, Rothwell JC, Wen HY. The after-effect of human theta burst stimulation is NMDA receptor dependent. Clin Neurophysiol. 2007;118:1028–1032. doi: 10.1016/j.clinph.2007.01.021. [DOI] [PubMed] [Google Scholar]
- Jayasekeran V, Pendleton N, Holland G, Payton A, Jefferson S, Michou E, Vasant D, Ollier B, Horan M, Rothwell J, Hamdy S. Val66Met in brain-derived neurotrophic factor affects stimulus-induced plasticity in the human pharyngeal motor cortex. Gastroenterology. 2011;141:827–836.e1–e3. doi: 10.1053/j.gastro.2011.05.047. [DOI] [PubMed] [Google Scholar]
- Jones MW, Wilson MA. Theta rhythms coordinate hippocampal-prefrontal interactions in a spatial memory task. PLoS Biol. 2005;3:e402. doi: 10.1371/journal.pbio.0030402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Logothetis NK, Pauls J, Augath M, Trinath T, Oeltermann A. Neurophysiological investigation of the basis of the fMRI signal. Nature. 2001;412:150–157. doi: 10.1038/35084005. [DOI] [PubMed] [Google Scholar]
- Luber B, Stanford AD, Bulow P, Nguyen T, Rakitin BC, Habeck C, Basner R, Stern Y, Lisanby SH. Remediation of sleep-deprivation-induced working memory impairment with fMRI-guided transcranial magnetic stimulation. Cereb Cortex. 2008;18:2077–2085. doi: 10.1093/cercor/bhm231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Margraf J. Diagnostisches kurz-interview bei psychischen störungen. Berlin: Springer; 1994. [Google Scholar]
- Meyer-Lindenberg A. Neural connectivity as an intermediate phenotype: brain networks under genetic control. Hum Brain Mapp. 2009;30:1938–1946. doi: 10.1002/hbm.20639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer-Lindenberg A. From maps to mechanisms through neuroimaging of schizophrenia. Nature. 2010;468:194–202. doi: 10.1038/nature09569. [DOI] [PubMed] [Google Scholar]
- Meyer-Lindenberg A. Neuroimaging and the question of neurodegeneration in schizophrenia. Prog Neurobiol. 2011;95:514–516. doi: 10.1016/j.pneurobio.2011.07.007. [DOI] [PubMed] [Google Scholar]
- Meyer-Lindenberg A, Weinberger DR. Intermediate phenotypes and genetic mechanisms of psychiatric disorders. Nat Rev Neurosci. 2006;7:818–827. doi: 10.1038/nrn1993. [DOI] [PubMed] [Google Scholar]
- Meyer-Lindenberg AS, Olsen RK, Kohn PD, Brown T, Egan MF, Weinberger DR, Berman KF. Regionally specific disturbance of dorsolateral prefrontal-hippocampal functional connectivity in schizophrenia. Arch Gen Psychiatry. 2005;62:379–386. doi: 10.1001/archpsyc.62.4.379. [DOI] [PubMed] [Google Scholar]
- Meyer-Lindenberg A, Poline JB, Kohn PD, Holt JL, Egan MF, Weinberger DR, Berman KF. Evidence for abnormal cortical functional connectivity during working memory in schizophrenia. Am J Psychiatry. 2001;158:1809–1817. doi: 10.1176/appi.ajp.158.11.1809. [DOI] [PubMed] [Google Scholar]
- Norup Nielsen A, Lauritzen M. Coupling and uncoupling of activity-dependent increases of neuronal activity and blood flow in rat somatosensory cortex. J Physiol. 2001;533:773–785. doi: 10.1111/j.1469-7793.2001.00773.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plichta MM, Schwarz AJ, Grimm O, Morgen K, Mier D, Haddad L, Gerdes AB, Sauer C, Tost H, Esslinger C, Colman P, Wilson F, Kirsch P, Meyer-Lindenberg A. Test-retest reliability of evoked BOLD signals from a cognitive-emotive fMRI test battery. Neuroimage. 2012;60:1746–1758. doi: 10.1016/j.neuroimage.2012.01.129. [DOI] [PubMed] [Google Scholar]
- Rasetti R, Sambataro F, Chen Q, Callicott JH, Mattay VS, Weinberger DR. Altered cortical network dynamics: a potential intermediate phenotype for schizophrenia and association with ZNF804A. Arch Gen Psychiatry. 2011;68:1207–1217. doi: 10.1001/archgenpsychiatry.2011.103. [DOI] [PubMed] [Google Scholar]
- Rattenborg NC, Martinez-Gonzalez D. A bird-brain view of episodic memory. Behav Brain Res. 2011;222:236–245. doi: 10.1016/j.bbr.2011.03.030. [DOI] [PubMed] [Google Scholar]
- Rizzo V, Siebner HR, Modugno N, Pesenti A, Münchau A, Gerschlager W, Webb RM, Rothwell JC. Shaping the excitability of human motor cortex with premotor rTMS. J Physiol. 2004;554:483–495. doi: 10.1113/jphysiol.2003.048777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rounis E, Stephan KE, Lee L, Siebner HR, Pesenti A, Friston KJ, Rothwell JC, Frackowiak RS. Acute changes in frontoparietal activity after repetitive transcranial magnetic stimulation over the dorsolateral prefrontal cortex in a cued reaction time task. J Neurosci. 2006;26:9629–9638. doi: 10.1523/JNEUROSCI.2657-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shrout PE, Fleiss JL. Intraclass correlations: uses in assessing rater reliability. Psychol Bull. 1979;86:420–428. doi: 10.1037/0033-2909.86.2.420. [DOI] [PubMed] [Google Scholar]
- Siebner HR, Rothwell J. Transcranial magnetic stimulation: new insights into representational cortical plasticity. Exp Brain Res. 2003;148:1–16. doi: 10.1007/s00221-002-1234-2. [DOI] [PubMed] [Google Scholar]
- Sigurdsson T, Stark KL, Karayiorgou M, Gogos JA, Gordon JA. Impaired hippocampal-prefrontal synchrony in a genetic mouse model of schizophrenia. Nature. 2010;464:763–767. doi: 10.1038/nature08855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slotema CW, Blom JD, Hoek HW, Sommer IE. Should we expand the toolbox of psychiatric treatment methods to include repetitive transcranial magnetic stimulation (rTMS)? A meta-analysis of the efficacy of rTMS in psychiatric disorders. J Clin Psychiatry. 2010;71:873–884. doi: 10.4088/JCP.08m04872gre. [DOI] [PubMed] [Google Scholar]
- Stephan KE, Baldeweg T, Friston KJ. Synaptic plasticity and dysconnection in schizophrenia. Biol Psychiatry. 2006;59:929–939. doi: 10.1016/j.biopsych.2005.10.005. [DOI] [PubMed] [Google Scholar]
- Strafella AP, Paus T, Barrett J, Dagher A. Repetitive transcranial magnetic stimulation of the human prefrontal cortex induces dopamine release in the caudate nucleus. J Neurosci. 2001;21:RC157. doi: 10.1523/JNEUROSCI.21-15-j0003.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thierry AM, Gioanni Y, Dégénétais E, Glowinski J. Hippocampo-prefrontal cortex pathway: anatomical and electrophysiological characteristics. Hippocampus. 2000;10:411–419. doi: 10.1002/1098-1063(2000)10:4<411::AID-HIPO7>3.0.CO;2-A. [DOI] [PubMed] [Google Scholar]
- Wang HY, Crupi D, Liu J, Stucky A, Cruciata G, Di Rocco A, Friedman E, Quartarone A, Ghilardi MF. Repetitive transcranial magnetic stimulation enhances BDNF-TrkB signaling in both brain and lymphocyte. J Neurosci. 2011;31:11044–11054. doi: 10.1523/JNEUROSCI.2125-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wassermann EM. Risk and safety of repetitive transcranial magnetic stimulation: report and suggested guidelines from the international workshop on the safety of repetitive transcranial magnetic stimulation, June 5–7, 1996. Electroencephalogr Clin Neurophysiol. 1998;108:1–16. doi: 10.1016/S0168-5597(97)00096-8. [DOI] [PubMed] [Google Scholar]
- Wassermann EM, Zimmermann T. Transcranial magnetic brain stimulation: therapeutic promises and scientific gaps. Pharmacol Ther. 2012;133:98–107. doi: 10.1016/j.pharmthera.2011.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinberger DR. Implications of normal brain development for the pathogenesis of schizophrenia. Arch Gen Psychiatry. 1987;44:660–669. doi: 10.1001/archpsyc.1987.01800190080012. [DOI] [PubMed] [Google Scholar]