Abstract
It is now widely accepted that hippocampal neurogenesis underpins critical cognitive functions, such as learning and memory. To assess the behavioral importance of adult-born neurons, we developed a novel knock-in mouse model that allowed us to specifically and reversibly ablate hippocampal neurons at an immature stage. In these mice, the diphtheria toxin receptor (DTR) is expressed under control of the doublecortin (DCX) promoter, which allows for specific ablation of immature DCX-expressing neurons after administration of diphtheria toxin while leaving the neural precursor pool intact. Using a spatially challenging behavioral test (a modified version of the active place avoidance test), we present direct evidence that immature DCX-expressing neurons are required for successful acquisition of spatial learning, as well as reversal learning, but are not necessary for the retrieval of stored long-term memories. Importantly, the observed learning deficits were rescued as newly generated immature neurons repopulated the granule cell layer upon termination of the toxin treatment. Repeat (or cyclic) depletion of immature neurons reinstated behavioral deficits if the mice were challenged with a novel task. Together, these findings highlight the potential of stimulating neurogenesis as a means to enhance learning.
Introduction
Hippocampal neurogenesis is widely thought to be critical for certain aspects of learning and memory throughout life. Adult-born neurons are generated from a pool of precursor cells within the subgranular zone of the dentate gyrus before maturing and integrating into the hippocampal circuitry (van Praag et al., 2002; Trouche et al., 2009). Adult-born immature neurons, including the doublecortin (DCX)-expressing population, have been hypothesized to underpin ongoing learning and memory formation as these cells are highly excitable (Karl et al., 2005) and have lower thresholds for activation and induction of long-term potentiation than mature granule cells (Wang et al., 2000; Schmidt-Hieber et al., 2004; Ge et al., 2007; Cheng et al., 2011).
To date, most studies aiming to deplete immature neurons have induced sustained interruption of the neurogenic process at the level of the multipotent neural precursors by irradiation, antimitotic agents, or transgenic mouse models (e.g., Saxe et al., 2006; Deng et al., 2009; Garthe et al., 2009; Arruda-Carvalho et al., 2011), thereby ablating the different cell lineages along the neurogenic path. Using these approaches, a number of studies have consequently reported poor performance in different behavioral aspects of hippocampal-dependent tasks (e.g., the Morris water maze and contextual fear conditioning) (Saxe et al., 2006; Deng et al., 2010; Goodman et al., 2010), suggesting that adult-born neurons are involved in extracting critical information, such as spatial location and context from the environment during learning. Consensus is yet to be reached, however, on the precise timing and specificity of behavioral deficits that result from blocking neurogenesis (Deng et al., 2010), and on the contribution of immature neurons to these behavioral outcomes.
In this study, we generated a novel knock-in DCXDTR mouse line to more directly test the premise that immature neurons in the dentate gyrus are important for particular aspects of spatial learning and memory formation. In these mice, the diphtheria toxin receptor (DTR) is expressed under control of the DCX promoter, which allows for specific ablation of DCX-expressing immature neurons after administration of diphtheria toxin (DT) while leaving the neural precursor pool and its non-neuronal progeny intact. Upon cessation of treatment, the immature neuronal population can therefore be regenerated from the unaffected precursor pool.
We used transient ablation in combination with active place avoidance, a hippocampus-dependent task (Cimadevilla et al., 2001; Wesierska et al., 2005) that has been shown to be more sensitive to learning impairments than the water maze test (Cimadevilla et al., 2001; Kubik and Fenton, 2005), to test the significance of immature neurons for behavior. To identify potential differences resulting from ablation of neurogenesis, we designed a challenging training protocol that reduced the number of trials per day to further increase the cognitive demands of the test. Our findings indicate that immature neurons expressing DCX are required for mastering a novel spatial task; however, they are not necessary for recall of a learned spatial task. Importantly, subsequent repopulation of the immature DCX-expressing neurons rescues an animal's ability to learn a new task.
Materials and Methods
Animals
To ablate DCX-positive (DCX+) neurons, we generated a knock-in mouse model in which DTR was expressed under the control of the endogenous DCX promoter sequence via an internal ribosome entry site (IRES) sequence. For this, a targeting vector containing an ires-venus-dtr fusion gene was inserted via homologous recombination into the 3′ untranslated region of the dcx genomic locus, behind the TGA stop codon in exon 7 of the dcx gene (NM_010025 located on the X chromosome; Ensembl gene ID: ENSMUSG00000031285). DCXDTR mice were produced by Ozgene. In brief, the sequences for ires (forward primer: 5′-CGGTCTCTGGTGATGCCATTTC-3′; reverse primer: 5′-TCCTCGCCCTTGCTCACCATGGTTGTGGCCATATTATCATCGTG-3′; 776 bp), the reporter Venus (forward primer: 5′-ATGGTGAGCAAGGGCGAGGA-3′; reverse primer: 5′-CTTGTACAGCTCGTCCATGCCG-3′; 717 bp), and dtr (forward primer: 5′-CGGCATGGACGAGCTGTACAAGATGAAGCTGCTGCCGTCGG-3′; reverse primer: 5′-CATCGATTAATTAATCAGTGGGAATTAGTCATGCCCAA-3′; 663 bp) were amplified from existing constructs with overlap, fused by PCR, and cloned into the ClaI site of the Ozgene plasmid PacL, which was used for construction of the targeting vector. The final fusion product contained an EcoR V restriction site for genomic screening, together with ClaI and NotI sites for cloning. The 5′ homology arm was amplified from genomic C57BL/6 mouse DNA (forward primer: 5′-GTATTATGTCTCTTATACCTTACTAAACCCCGTT-3′; reverse primer: 5′-CGCGGCCGCTAGGCTTTGATTTAGCCTATCATCTTTCAC-3′; 7085 bp) and contained a NotI site for cloning. The 3′ homology arm was similarly amplified from genomic C57BL/6 DNA (forward primer: 5′-CGCGGCCGCATCGATAGTACTTATTGTA GCAGAATACTTTCGCTCAAGTG-3′; reverse primer: 5′-CGCGGCCGCGAAAGCCTTG CTGCAAAGAAACC-3′; 3992 bp) and contained an ScaI site for genomic screening, as well as ClaI and NotI sites for cloning purposes. Cloning of these homology arms upstream and downstream of the ires-venus-dtr fusion fragment completed the targeting vector construct. The correctness of all fragments used for cloning and the final construct was confirmed through sequencing. Next, the targeting vector was excised through digestion with PvuI, and the resulting linearized fragment (16.6 kb) was purified and microinjected into embryonic stem cells. Successful insertion and correct targeting were confirmed through digestion with EcoRV, which yielded an 8.4 kb fragment and an 8.8 kb fragment for the mutant allele (with the latter including the ires-venus-dtr fusion fragment) rather than a single 15.2 kb fragment for the untargeted genomic locus. The identity of these fragments was confirmed through Southern blot hybridization. For this, specific probes were generated through PCR using the forward 5′-CTGTTTCTTTCCTTCCAGTG-3′ and reverse 5′-CTTGTGGGTGTAGAGATAGG-3′ primers for the upstream 8.4 kb fragment (yielding a 629 bp product), or the forward 5′-TCTGCCATTTTGTGAACGGTG-3′ and reverse 5′-GACATTGACATCCCCAAGGTTTC-3′ primers for the downstream 8.8 kb fragment (yielding a 536 bp product). Although the coding sequence for the reporter gene venus was inserted before the dtr sequence, it was found to be nonfunctional as no native fluorescence was observed. DCXDTR knock-in mice were bred on a C57BL/6J background and backcrossed for more than seven generations. Male mice, 7–10 weeks of age, were used for all experiments. Mice were handled for ∼3 min per day for 3 d before the beginning of behavioral experiments. Controls were nonlittermate wild-type (WT) mice on the same genetic background that were housed under similar conditions to the DCXDTR mice. In the active place avoidance task, after DT injection, these nonlittermate WT mice performed similarly to WT mice that were generated directly from heterozygous DCXDTR breeding pairs (F(1,12) = 0.10, p = 0.75). All experiments were conducted in accordance with the Australian Code of Practice for the Care and Use of Animals for Scientific Purposes, with approval from the University of Queensland Animal Ethics Committee. Animals were maintained under standard conditions on a 12-h light/dark cycle (lights on at 7:00 A.M.) with food and water provided ad libitum. All experiments were conducted between 9:00 A.M. and 5 P.M.; animals were group-housed with 2–5 animals per home cage.
Drugs
To deplete DCX+ cells, 10 ng DT (Sigma) per gram of bodyweight was intraperitoneally injected every second day over a period of 11 d (i.e., 6 injections total), except during optimization of the delivery paradigm, when DT was injected daily (10 ng/g) for 10 d. In all experiments, both WT and DCXDTR mice were treated with DT to ensure group effects could not be attributed to nonspecific effects of the toxin. We initially tested an alternative DT delivery regime (i.e., the optimization paradigm), with which we observed a significant reduction in percentage weight change. Daily DT injection resulted in significant weight loss (13.3 ± 3.3%) on day 9 and thereafter compared with the starting weight before DT treatment. The average weight of the mice was not significantly different between genotypes after DT treatment on any of the days, including day 9 (WT: 25.7 ± 1.2 g vs DCXDTR: 22.48 ± 2.2 g; p = 0.22). The negative effect of daily DT treatment was also reflected in reduced total distance covered in the open field behavioral task and a delay in the response to the nociceptive stimulus in the hot-plate test for DCXDTR compared with WT mice (data not shown). For this reason, the daily treatment was not used for any of the behavioral experiments. In contrast, treatment every second day did not produce adverse effects (i.e., no change in weight or explorative behavior). As a result, this protocol was used for all experiments in this study.
Cell culture
Cortical neuronal cultures.
A small piece of dorsolateral cerebral cortex was dissected from postnatal day 1 (P1) WT and DCXDTR mice. The tissue was then minced with a scalpel and dissociated in 1 ml of 0.05% trypsin (Invitrogen) for 6 min at 37°C. Next, 1 ml of trypsin inhibitor was added and the tissue was triturated into a single-cell suspension. The sample was washed with 15 ml of Eagle's minimum essential medium and passed through a 40 μm filter, then spun at 100 g for 7 min, and the pellet suspended in culture medium (DMEM containing 10% fetal calf serum). The cells were plated in an 8-well chamber slide coated with poly-l-lysine (0.1 mg/ml; Sigma) at 20,000 cells/well, with each sample being plated across two duplicate wells. At the time of plating, DT was added at 50 ng/ml and the cells were cultured for 4 d at 37°C under 5% CO2. Next, the cells were fixed with 4% paraformaldehyde (Sigma) in 0.1 m PBS. After washing with PBS, the cells were incubated for 1 h at room temperature (RT) with blocking solution: 5% fetal calf serum plus 5% normal goat serum (Sigma-Aldrich) in 0.1 m PBS containing 0.1% Triton X-100 (Sigma). The blocking solution was replaced with fresh solution containing monoclonal mouse anti-DCX antibody (1:500; Abcam), and the cells were incubated in this fresh solution for 1 h at RT. Cells were washed with PBS and incubated for 30 min at RT in blocking solution containing AlexaFluor-488 anti-rabbit antibody (1:1000; Invitrogen). After washing with PBS, slides were coverslipped with fluorescence mounting medium (Dako Cytomation) containing DAPI (1:5000; Sigma). The cells were viewed on a Zeiss inverted fluorescence microscope and the number of DCX-expressing cells quantified across five random fields per well.
Differentiation of subventricular zone neurospheres.
To assess depletion of DCX+ cells in culture, DT was added to differentiated subventricular zone (SVZ) neurospheres. In brief, adult mice (n = 4) were killed by cervical dislocation, their brains immediately removed, and the SVZ dissected. The SVZ tissue was digested by incubation in a mixture containing 0.1% papain (Worthington Biochemical) and 0.1% DNaseI (Roche) in Hank's buffered salt solution (Thermo Scientific) for 16 min at 37°C. After brief trituration, 2 ml of neurosphere growth medium was added and the cell suspension centrifuged at 250 g for 5 min. The neurosphere growth medium consisted of mouse NeuroCult NSC basal medium containing mouse NeuroCult NSC proliferation supplements (Stem Cell Technologies), 2% BSA (Invitrogen), 2 μg/ml heparin (Sigma), 20 ng/ml purified mouse receptor-grade epidermal-like growth factor (EGF; BD Biosciences), and 10 ng/ml recombinant bovine fibroblast growth factor-2 (FGF-2; Roche). The pellet was resuspended in another 2 ml of neurosphere growth medium and mechanically triturated until smooth, to obtain a single-cell suspension. The cell preparation was then plated in neurosphere growth medium in a 24 well plate and cultured for a period of 7 d at 37°C in 5% CO2.
To allow for differentiation, neurospheres were transferred to a 4 well slide coated with poly-d-lysine and l-orthonine and cultured in medium without the growth factors EGF and FGF-2 for a period of 5 d at 37°C in humidified 5% CO2. On day 4 of differentiation, DT was added to half of the wells at 50 ng/ml. Twenty-four hours later, the neurospheres were fixed and stained for DCX, as described above. The neurospheres were viewed on a Zeiss inverted fluorescence microscope, and the number of DCX-expressing cells in each neurosphere was quantified to obtain the percentage of neurospheres containing <10, 10–25, or >25 DCX-expressing cells.
Tissue processing and analysis
Staining procedures.
DCX staining was used to evaluate the number of immature neurons in the hippocampus, Arc and c-Fos staining was used to identify immediate early gene-expressing cells involved in memory consolidation (Shepherd and Bear, 2011), Ki-67 staining was used to examine the number of proliferating cells in the subgranular layer, and Iba1 and GFAP staining was used to identify microglia and astrocytes, respectively. For this, mice were deeply anesthetized with sodium pentobarbitone (150 mg/kg; Virbac) and transcardially perfused with 10 ml of PBS, pH 7.5, followed by 30 ml of 10% neutral buffered formalin, pH 7.5. The brains were postfixed in formalin, cryoprotected in 30% sucrose, and sectioned at 40 μm thickness using a sledge microtome. All staining was done on free-floating sections (1 in 6 series) through the entire hippocampus. Sections were washed three times for 10 min at RT and then incubated for 1 h in a blocking solution (1% BSA, 0.2% Triton X-100 in PBS). Sections were then left overnight at 4°C immersed in diluent containing monoclonal rabbit anti-DCX (1:500; Abcam), guinea pig anti-Arc (1:500; Sysy), goat anti-c-Fos (1:500; Santa Cruz Biotechnology), rabbit anti-Ki-67 (1:1000; Abcam), rabbit anti-Iba1 (1:500; Wako), or mouse anti-GFAP (1:500; Millipore) antibody. The following day, sections were washed and incubated for 1 h at RT with goat anti-rabbit AlexaFluor-488 (1:1000) or biotinylated anti-guinea pig IgG (1:1000; Vector Laboratories) followed by Cy3-streptavidin (1:1000). Sections were washed three times in PBS at RT (5 min per wash), then immediately mounted in Dako fluorescence mounting medium containing the nuclear stain DAPI (1:1000).
Double immunofluorescence staining was performed to determine the distribution of DTR in DCXDTR mice. For this immunolabeling, DCXDTR and WT mice were deeply anesthetized with sodium pentobarbitone, then transcardially perfused with 1% sodium nitrite in 0.1 m PBS, pH 7.4, followed by 4% paraformaldehyde, 14% v/v picric acid in 0.1 m phosphate buffer. Brains were removed from the skull, hemisected, and postfixed via immersion in the same fixative for 2 h at RT. Tissues were washed three times in 0.1 m PBS and processed into paraffin wax. Next, 10 μm coronal sections containing the hippocampus were cut and collected onto SuperFrost Plus slides (Menzel-Gläser). The paraffin wax was removed through immersion in 100% xylene, after which the sections were rehydrated through a graded series of ethanol and H2O. Sections were incubated in an antigen recovery solution (Revealit-ag, ImmunoSolution) for 10 min at 85°C and washed three times in 0.1 m PBS. Wells were created around sections using a Pap pen (Pro SciTech) before tissues were incubated in blocking solution for 30 min. The sections were then incubated overnight in blocking solution containing the primary antibodies goat anti-HB-EGF (1:50; R&D Systems) and rabbit anti-DCX (1:500; Abcam). Sections were washed and incubated for 5 h with biotinylated anti-goat IgG (1:500; Jackson Biosciences), then washed and incubated overnight in streptavidin-AlexaFluor-488 (1:1000, Invitrogen) and anti-rabbit IgG-AlexaFluor-647 (1:1000; Invitrogen). Finally, sections were washed three times in PBS at RT before being mounted in Dako fluorescence mounting medium. All incubations were performed at 4°C in a humidified chamber unless otherwise indicated. The specificity of DTR staining was confirmed through omission of the primary antibody incubation step as well as the use of WT tissue.
Image analysis and quantification.
Images were taken at 20× magnification using a Zeiss Axio Imager microscope and AxiocamMRm/3 camera together with AxioVision Software (Zeiss, Version 4.8.2). To determine the number of cells stained for DCX, Ki-67, Arc or c-Fos within each section, Z-stacks were taken at 1.75 μm intervals throughout the entire 40 μm section; quantitative counts were expressed as cells per unit length of dentate gyrus (Vukovic et al., 2012). Counts for Iba1- and GFAP-immunoreactive cells were performed using Stereo Investigator stereologic software. Using every sixth section throughout the rostrocaudal extent of the hippocampus, the total number of Iba1- and GFAP-immunoreactive cells was estimated by the optical fractionator method using a 40× plan-apo objective with a 1.4 numerical aperture. For every section analyzed, upper and lower “guard zones” of 5 μm were established. The hippocampus was outlined under a objective and ∼20% of the outlined area was analyzed using a systematic random sampling design. The section thickness was measured every 5 counting frames, with each contour having 8–22 evenly spaced counting frames, depending on the size of the contour. The counting frame was set at 500 × 500 pixels during analysis as this yielded a sufficiently low coefficient of error (<0.1).
Behavioral apparatus and procedures
The active place avoidance task is a hippocampus-dependent spatial learning task (Cimadevilla et al., 2001; Wesierska et al., 2005). The apparatus (Bio-Signal Group) consisted of an elevated arena with a grid floor fenced with a 32-cm-high transparent circular boundary (enclosed arena diameter 77 cm), located in a room with visual cues on the walls (see Fig. 2A). The arena rotated counterclockwise (0.75 rpm) and an electric shock could be delivered through the grid floor, which also rotated. The position of the animal in the arena was tracked using an overhead camera linked to Tracker software (Bio-Signal Group). During trials, a mouse was placed in the arena and trained to avoid a 60° shock zone, the positioning of which was kept constant (i.e., did not rotate) in relation to the room coordinates; the mouse's “start” position was always near the wall on the side of the arena opposite the shock zone. Entrance into the shock zone led to the delivery of a brief foot shock (500 ms, 60 Hz, 0.6 mA). If, after the initial shock, the animal remained in the shock zone, further shocks were delivered at 1.5 s intervals until the animal moved out of the zone. Each training session lasted 10 min (habituation sessions were 5 min long), and recorded tracks were analyzed offline using Track Analysis software (Bio-Signal Group). Mice were habituated to the training environment 24 h before the initial experiment (i.e., a mouse was placed in the rotating arena with the shock turned off and allowed to explore the arena for 5 min).
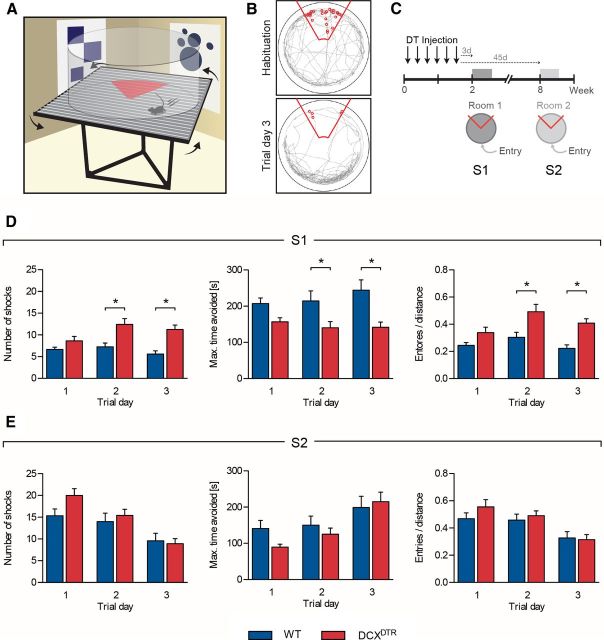
Figure 2.
Ablation of DCX+ cells impairs performance of the active place avoidance task; repopulation of DCX+ cells rescues acquisition of the task. A, Schematic representation of the active place avoidance task. Experimental mice were placed on top of a turntable, confined in a clear circular barrier. Using distal visual cues as a reference, the animals were trained to avoid a stationary shock zone (red triangle). B, Depiction of the path tracked by the experimental mice (gray line) within the arena during habituation (top) and after trial day 3 of training (bottom). Red line indicates shock zone of 60°; small red circles represent “shocks” delivered. C, Experimental design used to assess the importance of DCX+ cells for learning and memory in the active place avoidance task. D, Acquisition of the active place avoidance task was significantly impaired in DCXDTR mice 3 d after DT treatment. DCXDTR mice received more shocks than control mice (left: F(1,22) = 17.37, p < 0.001), had shorter maximum shock zone avoidance time (middle: F(1,22) = 12.81, p < 0.01), and had a higher number of entries into the shock zone per distance covered (right: F(1,22) = 15.06, p < 0.001). E, At 45 d after ablation (i.e., after recovery of the DCX+ cell population), the DCXDTR mice performed as well as control animals in the new active place avoidance task. There was no difference observed in the number of shocks received (F(1,22) = 1.23, p = 0.28), maximum avoidance time (F(1,22) = 0.76, p = 0.39), or entries per distance (F(1,22) = 0.56, p = 0.46) between genotypes. S indicates experimental session (i.e., S1, Session 1). **p < 0.05, Significance between genotypes for individual trial days (Bonferroni post hoc test). n = 12 per experimental group.
To test the role of adult neurogenesis at different stages of spatial learning and memory, we conducted three experiments using different cohorts of animals. Each experiment started with a habituation session, followed by a series of experimental blocks. Each block consisted of three 10 min training sessions per day. The position of the shock zone was kept constant with respect to the spatial frame of the room. To assess the extent of learning, the following parameters were measured: number of foot shocks delivered during a 10 min trial, maximal avoidance time, latency to first entry into the shock zone, and number of entries per distance covered (in meters).
The open field test (Harms et al., 2008) was used to assay the animals' locomotor activity. The open field tests were conducted using a square arena (27.3 × 27.3 × 20.3 cm) equipped with infrared beams (Med Associates). In each individual experiment, the mouse was placed in the center of the arena and the total distance traveled over 10 min was recorded, along with the number of entries into the central quadrant of the arena, rearings, and defecations.
The rotarod task was used to assay motor coordination, balance, and motor learning by measuring latency to the first fall from an accelerating rotating rod, with latencies averaged over multiple trials (Lalonde et al., 1995). The animal was placed on an accelerating rotating rod (acceleration 4–40 revolutions/min over 5 min) and its motor function was measured as latency to the first fall. Each mouse was tested in two trials per day (10 min intertrial interval) for 2 d.
The hot-plate test was used to assess mouse pain sensitivity, as any differences in pain threshold between genotypes might affect avoidance learning (Bannon and Malmberg, 2007). An automated hot-plate (Harvard Instruments) was heated to 53°C, each mouse was placed individually on the plate, and the latency of the mouse to lick its hindpaw was used as the measure of nociception (cutoff time 20 s).
The elevated plus maze was used to obtain a measure of anxiety-related behavior (Carobrez and Bertoglio, 2005). The elevated plus maze was made from opaque gray acrylic and consisted of four opposing pairs of arms, two open (5 × 30 cm) and two closed (5 × 30 × 30 cm), extending from a central platform (5 × 5 cm) positioned 50 cm above the ground. Mice were placed on the central platform facing one of the open arms and allowed to freely explore the maze for 10 min while their behavior was videorecorded. The Ethovision 3.1 program (Noldus) was used for tracking and analysis. The distance mice moved in different arms of the maze was measured, as was the frequency of head-dipping, stretching, and rearing. The time that animals spent in the open arms of the maze, relative to the closed arms, was used as the primary measure of anxiety-related behavior.
In all behavioral experiments, the floor and walls of the corresponding experimental apparatus were wiped with 70% ethanol after each trial. The experiments were conducted in such a way as to minimize bias in that WT and knock-in animals were tested in an alternating fashion. Three cohorts of mice were examined for general behavior between 1 and 3 d after the conclusion of DT treatment. One cohort was used for open-field and hot-plate testing, which took place one day after the last DT injection. The remaining two cohorts were used for testing rotarod and elevated plus maze performance at 1 and 3 d after the last DT injection, respectively.
Data analysis
Statistical analysis was performed using GraphPad Prism (GraphPad Software, Version 5.0c). Data were analyzed using an unpaired two-tailed Student's t test or a repeated-measures two-way ANOVA with Bonferroni post hoc test, as appropriate. Values are expressed as mean ± SEM with significance determined at p < 0.05.
Results
DCXDTR mice: a novel knock-in mouse model for the ablation of immature hippocampal neurons
To examine the role of immature neurons in the hippocampus, we used genetic targeting to specifically ablate DCX-expressing (DCX+) cells. To do so, we generated a knock-in model (DCXDTR mice), in which the human version of the receptor for DTR was constitutively expressed under the control of the DCX promoter. As such, all DCX+ cells are primed to undergo apoptotic cell death once human DT has bound to the DTR. In the mouse, cells lacking DTR expression are insensitive to DT-induced cell death (Middlebrook and Dorland, 1977; Buch et al., 2005).
The efficacy of DT-induced DCX+ cell depletion was first tested in vitro. Cortical cultures were established from P1 pups, as DCX protein is highly expressed by neurons during postnatal development, after which DT was added to the culture medium. The total number of DCX+ cells was significantly reduced in cultures derived from heterozygous and homozygous DCXDTR mice in the presence of DT compared with WT mice (Fig. 1A). The loss of immature neurons was particularly pronounced in homozygous DCXDTR mice where no DCX+ cells remained after DT treatment. Cultures derived from heterozygous DCXDTR mice showed an intermediate phenotype, whereas the number of DCX+ cells in cultures derived from WT mice did not change in the presence of DT.
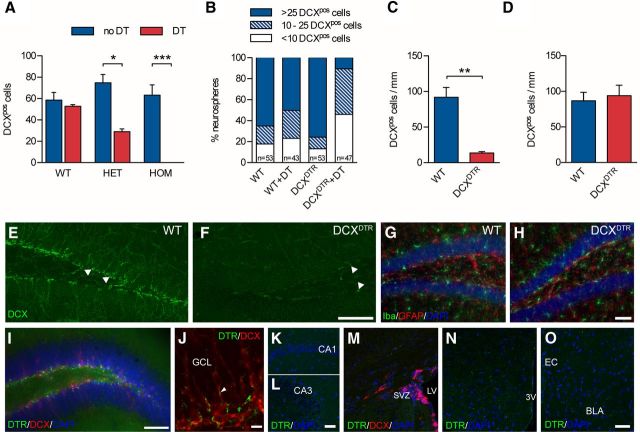
Figure 1.
Depletion of immature neurons in DCXDTR knock-in mice. A, DT ablated DCX+ cells in cultures of embryonic cortical neurons derived from heterozygous (HET; n = 3) and homozygous (HOM; n = 4) DCXDTR mice but not from WT controls (n = 2). B, Addition of DT to differentiated neurospheres ablated DCX+ cells. Note the dramatic reduction in the number of DCX+ cells encountered within neurospheres from DCXDTR mice after the addition of DT. C, In vivo DT administration resulted in depletion of DCX+ cells in the dentate gyrus of DCXDTR mice (n = 3) but not WT controls (n = 3). D, Recovery of DCX+ cell numbers in the dentate gyrus in DCXDTR mice was observed 28 d after the last DT injection (n = 4 per condition). E, F, Representative images showing depletion of DCX+ cells in the dentate gyrus of DCXDTR but not WT mice after DT treatment. White arrowheads indicate DCX+ cells. G, H, Representative images of the distribution of Iba1+ microglia and GFAP+ astrocytes in WT and DCXDTR mice after DT treatment. I, Photomicrograph showing staining for DTR (green) and DCX (red) in the dentate gyrus of DCXDTR mice (cell nuclei are counterstained blue with DAPI). J, Higher-power image showing punctate DTR staining (green) in close association with DCX+ cells (red) and their processes (white arrowhead). K–O, No detectable DTR staining was observed in other regions of the hippocampus (i.e., the CA1 and CA3) (K,L), the SVZ (M), hypothalamus (N), or amygdala (O). Scale bars: E, F, 100 μm; I, 70 μm; J, 10 μm; K–O, 50 μm. *p < 0.05. **p < 0.01. ***p < 0.001. GCL, Granule cell layer; LV, lateral ventricle; 3V, third ventricle; BLA, basolateral amygdala; EC, external capsule.
To further corroborate our findings, we performed additional in vitro experiments to test whether DT could also ablate DCX+ cells within differentiated neurospheres cultured from the adult SVZ. Differentiation of neurospheres generated from this brain region results in the production of a large number of DCX+ cells. Differentiated neurospheres from DCXDTR mice produced a similar number of DCX+ cells to WT neurospheres in the absence of DT. However, exposure of these neurospheres to DT led to a marked decline in DCX+ cells in preparations derived from homozygous DCXDTR mice (hereafter referred to as DCXDTR mice; Fig. 1B), with an overall shift toward neurospheres containing only a few immature neurons. In DCXDTR cultures, ∼10% of neurospheres contained >25 DCX+ cells/neurosphere compared with 50% of WT neurospheres.
Having established that DT exposure was an effective means by which to induce loss of DCX+ cells in vitro, we next assessed the efficacy of depletion in vivo. Both WT and DCXDTR mice were injected with DT every second day for 11 d (six injections in total). They were then killed on the day after the final injection, after which we assessed the number of DCX+ cells present in the dentate gyrus. After DT injection, there was an 85% reduction in the number of DCX+ cells in the DCXDTR dentate gyrus compared with the control region (p < 0.01; Fig. 1C,E,F). The number of proliferating Ki-67+ cells was, however, similar between the genotypes (WT: 14 ± 0.4 cells/mm vs DCXDTR: 15 ± 0.4 cells/mm), as was the number of hippocampal neurospheres (data not shown). We also found no evidence of gliosis or microglial activation in DCXDTR mice as a result of DT treatment and the subsequent ablation of immature neurons (Fig. 1G,H). Specifically, there was no difference between genotypes in the number of Iba+ microglia (WT: 74,443 ± 13,840 cells, n = 4, vs DCXDTR: 71,749 ± 5758 cells, n = 5) or GFAP+ astrocytes (WT: 120,108 ± 13,133, n = 3, vs DCXDTR 110,317 ± 5635, n = 5) in the hippocampus after DT injections. There was also no significant difference in the proportion of proliferating (i.e., BrdU+) microglia and astrocytes between genotypes (data not shown).
Staining for DTR was observed within the basal aspect of the granule cell layer of the DCXDTR dentate gyrus, where DCX+ cells are located (Fig. 1I). Inspection at higher magnification showed a punctate, surface receptor-like staining pattern for DTR in close association with DCX+ cells (Fig. 1J). No detectable levels of DTR staining were observed in other areas for which DCX expression has been reported, including the SVZ, rostral migratory stream, olfactory bulb, piriform cortex, hypothalamus, or amygdala (Fig. 1K–O). A similarly restricted pattern of reporter gene expression was noted in the Rosa26-EYFP line when crossed with a transgenic mouse that expressed CreERT2 under control of the full-length dcx promoter (Cheng et al., 2011), perhaps as a result of variations in DCX expression between different brain regions (Zhang et al., 2010).
Next, in a separate cohort of experimental mice, we quantified the number of DCX+ cells present at 28 and 49 d after the last DT injection and observed that the population of DCX+ cells in the DCXDTR mice had completely recovered at these time points (28 d: Fig. 1D; 49 d: data not shown). Thus, the DCXDTR transgenic mouse represents a model in which DCX+ immature hippocampal neurons can be specifically targeted and ablated.
Ablation of immature hippocampal neurons results in learning deficits, which can be rescued after restoration of this population
Having successfully established a transgenic mouse model that allows for in vivo ablation of immature neurons, we next aimed to evaluate the importance of these cells for hippocampal/dentate gyrus-dependent behavior by examining changes in learning and memory after DCX+ cell ablation. Previous studies have drawn links between impairments in hippocampal neurogenic processes and deficits in spatial learning using the Morris water maze (Dupret et al., 2008; Farioli-Vecchioli et al., 2008; Zhang et al., 2008), although the reported outcomes have been variable (Deng et al., 2010). Therefore, in this study, we aimed to test spatial learning in a more controlled fashion with a challenging version of the active place avoidance task. The apparatus used for this behavioral task, together with the visual cues displayed on the walls of the testing room, are schematically represented in Figure 2A. Experimental mice were placed on a turntable and tested for their ability to learn to avoid an unmarked shock zone using the distal visual cues present in the testing room as their reference. The readout from the active place avoidance task is depicted in Figure 2B. During the habituation phase of the test, experimental animals explored the arena randomly as indicated by the gray tracking path throughout the entire testing arena (Fig. 2B, top). After activation of the shock zone, animals normally learned to avoid this region, typically preferring to stay on the opposite side of the arena (Fig. 2B, bottom).
To test hippocampal-dependent learning in the absence of immature neurons, both DCXDTR and WT mice were treated with DT and subsequently tested in the active place avoidance task in “Room 1,” 3 d after the last DT injection. The experimental design is shown in Figure 2C. In the 5 min habituation phase, during which the shock zone was not activated, both DCXDTR and WT mice explored the arena to a similar extent. The total distance covered (WT: 30.0 ± 1.4 m vs DCXDTR: 26.7 ± 1.5 m), the number of “virtual shocks” (WT: 27.9 ± 2.0 vs DCXDTR: 24.33 ± 1.6), and the number of entries into the shock zone (WT: 13.3 ± 0.8 vs DCXDTR: 12.1 ± 1.0) were similar between genotypes (n = 12 per experimental group). During habituation, the number of “virtual shocks” was typically higher than the number of entries into the shock zone because test animals were not presented with an aversive stimulus to encourage their exit from this area. However, after activation of the shock zone, the number of shocks received tended to equal the number of entries, as animals quickly escaped the aversive area. During test trials, DCXDTR mice had greater difficulty in avoiding the shock zone after DT treatment compared with their WT counterparts (Fig. 2D). DCXDTR mice not only received significantly more shocks (Fig. 2D, left), they also avoided the shock zone for a reduced period of time (Fig. 2D, middle). The number of entries into the shock zone per distance covered (in meters) was also significantly greater for the DCXDTR cohort (Fig. 2D, right). These deficits in performance were particularly apparent on days 2 and 3 of the testing period. Several other parameters also reflected impaired performance of the DCXDTR mice in the active place avoidance task, with significant increases observed in the total number of entries into the shock zone (WT: 5.1 ± 0.6 vs DCXDTR: 9.3 ± 0.7; p < 0.01) and time spent in the quadrant containing the shock zone (WT: 6.7 ± 1.1 s vs DCXDTR 13.4 ± 1.5 s; p < 0.001) on trial day 3 (n = 12 per genotype). In a separate cohort of mice, behavior was tested 17 d after DT treatment, revealing ongoing learning deficits. The number of shocks received here by DCXDTR mice was still significantly higher than that experienced by WT animals on both trial day 2 (WT: 5.5 ± 1.3 vs DCXDTR: 10.75 ± 2.1; p < 0.05) and trial day 3 (WT: 2.3 ± 0.7 vs DCXDTR: 8.0 ± 0.9; p < 0.05; n = 4 per genotype).
Having demonstrated the importance of DCX+ immature neurons for learning and mastering a spatial task (Fig. 2D), we hypothesized that the behavioral deficits observed in the DCXDTR mice should be alleviated upon withdrawal of the DT treatment, which would allow for replenishment of the DCX+ cell population. To investigate this, the same cohort of mice that was tested at 3 d after DT treatment (see above) and retested at 45 d, but in a different room (Room 2) (Fig. 2E). This provided the animals with a novel environment and a new set of distal visual cues, thereby presenting them with a new active place avoidance learning task (Fig. 2C). This late time point, 8 weeks after the first DT injection, was selected based on a previous study, which reported that 6- to 8-week-old new neurons are preferentially recruited during new learning tasks (Kee et al., 2007). No significant differences were observed during Room 2 testing; both genotypes received a similar number of shocks on each trial day, avoided the shock zone for a similar period of time, and made the same number of entrances per distance into the shock area (Fig. 2E). Thus, the effects observed after DT-induced ablation of DCX+ cells could be reversed once treatment ceased.
It should be noted that the relatively flat learning curve of the WT group in our initial experiments, resulting from very quick learning to avoid the shock sector during the first training trial (relative to the number of entries into this sector during habituation session; Fig. 2D), appears to be characteristic of the active place avoidance test and has also been reported by other research groups (Cimadevilla et al., 2001; Burghardt et al., 2012). In our experiment, this quick acquisition might have been further facilitated by running habituation on the rotating arena in the presence of the distal cues, thus allowing animals to preform a “spatial map” in advance of the first training trial session. In support of this, a clear trend toward a learning curve was observed during acquisition of the active avoidance task after a room change when habituation was omitted during the retesting (Fig. 2E). Two-way ANOVA confirmed improved performance during consecutive trials in this scenario, with mice receiving fewer shocks over time regardless of genotype (F(2,22) = 20.64, p < 0.0001). Similar results were obtained in later experiments during shock zone reversal (see below and Fig. 5, S3; Fig. 6, S4). These findings lend strong support to the notion that habituation allows experimental mice to partially construct a spatial memory, making it easier/faster for them to map the location of the shock sector during actual test trials. The overall greater number of shocks received by the control group during the second round of active place avoidance learning (Fig. 2E, S2) is likely to have resulted from a combination of experimental factors: (1) the lack of a habituation session before the start of training in Room 2 may have contributed to a slower pace of learning (and consequently more foot shocks being received); and (2) it must also be taken into consideration that these mice were already familiar with the active place avoidance task and therefore may have learned to associate the experimental rig with foot shocks. This may have made them more anxious upon reentering the arena, resulting in a greater number of entries into the shock zone. These possibilities are not mutually exclusive. As a very similar result was obtained during later experiments when test rooms were changed between training sessions 1 and 2 (see below and Fig. 6, S2), this profile appears to be a feature of the experimental paradigm rather than the result of irregular poor performance.
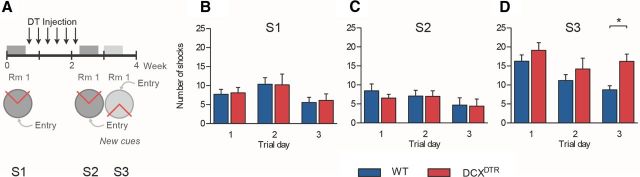
Figure 5.
Immature DCX+ neurons are not important for recall of a previously learned task but are important for learning new hippocampal-dependent tasks. A, Experimental design used to assess recall of the already learned task after DT treatment, as well as learning of a new task where the cues and entry point have been altered. B, There was no difference in the number of shocks delivered during the initial learning period in the active place avoidance task (F(1,18) = 0.02, p = 0.88). C, When retested after DCX+ cell ablation, DCXDTR mice performed equally as well as controls (F(1,18) = 0.16, p = 0.70). D, Changing the distal visual cues and shock/entry zone positions precipitated a deficit in DCX-ablated animals, which showed impaired acquisition of this new task as evidenced by a significant increase in the number of shocks received by this group on day 3 of the new training protocol (F(1,18) = 4.51, p < 0.05). S indicates experimental session (i.e., S1, Session 1). *p < 0.05, significance between genotypes for individual trial days (Bonferroni post hoc test). WT: n = 11; DCXDTR: n = 9.
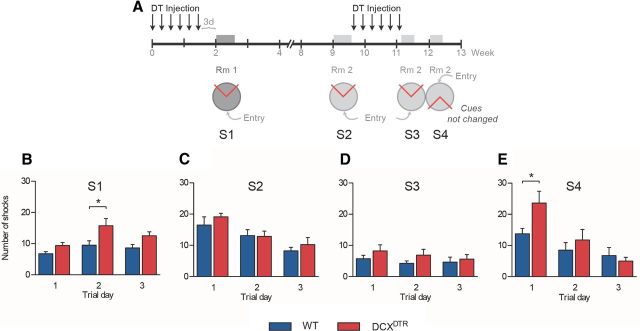
Figure 6.
Repeated depletion of immature neurons in the DCXDTR transgenic mouse model impairs reversal learning in the active place avoidance task. A, Experimental design used to test acquisition deficits (S1), recovery (S2), recall (S3), and reversal learning (S4) after multiple rounds of DT-induced DCX+ cell ablation. B, DCXDTR mice received a greater number of shocks when tested in the active place avoidance task after initial depletion of the immature neurons (F(1,14) = 11.28, p < 0.01). C, This deficit was rescued after a rest period during which time the immature neurons repopulated the dentate gyrus in either genotype (F(1,14) = 0.68, p = 0.42). D, The second depletion did not produce a deficit when animals were retested in the already familiar task (F(1,14) = 1.70, p = 0.21). E, When presented with a reversal of location of the shock and entry zones, DCXDTR mice were slower than WT mice in adjusting their behavior to avoid the shock zone, with two-way ANOVA showing an interaction between genotype and time (F(2,14) = 3.28, p = 0.05). S indicates experimental session (i.e., S1, Session 1). *p < 0.05, Significance between genotypes for individual trial days (Bonferroni post hoc test). n = 8 per experimental group.
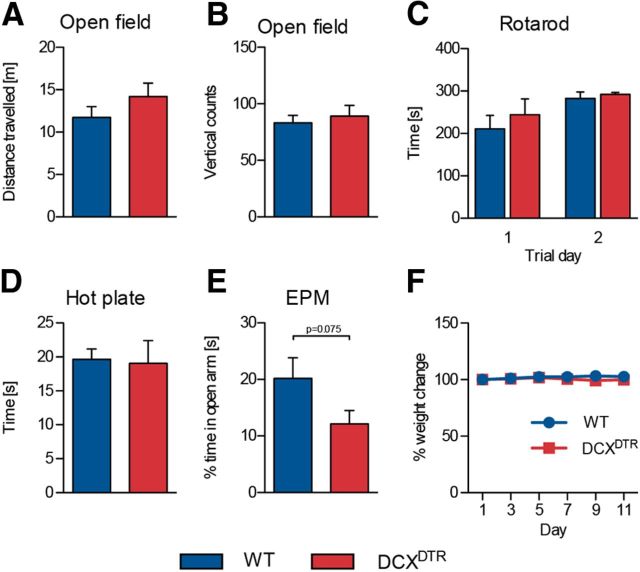
As changes in some types of behavior, such as altered motor activity or pain sensitivity, could affect performance in the active place avoidance test, we also conducted several additional tests using two separate cohorts of mice. One day after the last DT injection, we found behavior in the open field test to be unchanged between the WT and DCXDTR animals, with no significant differences recorded in distance traveled, times the mouse reared onto its hind legs (Fig. 3A,B), or the number of entries into the center of the arena (WT: 129 ± 8 vs DCXDTR: 143 ± 15; p > 0.05; n = 4–6). Similarly, motor coordination, balance, and motor learning in the repeated rotarod test were not altered by DT-induced depletion of immature neurons (Fig. 3C). In addition, DT administration did not affect pain sensitivity as measured in the hot-plate test (Fig. 3D). DCXDTR mice did have a tendency to spend less time in the open arms in the elevated plus maze; however, this difference was not significant (Fig. 3E). Finally, as a measure of general health status, we closely monitored the animals' body weight during the DT treatment. Our treatment protocol of DT administration on alternating days (one injection every other day over 11 d) did not induce any significant weight loss, and no difference in the percentage weight change was observed between DCXDTR and WT mice (Fig. 3F).
Figure 3.
Depletion of DCX+ cells does not alter animals' performance in a range of general nonhippocampal-related tasks. A, B, DT treatment did not affect behavior of experimental mice in the open field test. Similarly, motor performance on the accelerating rotarod (C) and pain sensitivity, as assessed by the hot-plate test (D) (WT: n = 6; DCXDTR: n = 5), were unaffected. DCXDTR mice appeared to spend less time in the open arms of an elevated plus maze (EPM) (E), although this was not statistically significant (WT: n = 9; DCXDTR: n = 11). F, DT administration did not negatively affect the body weight of experimental mice (WT: n = 16; DCXDTR: n = 16).
Ablation of immature hippocampal neurons is associated with reduced expression of immediate early genes
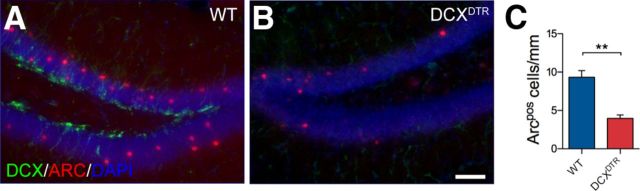
It was noted during the initial characterization of the DCXDTR line that DT treatment reduced the number of Arc-positive cells in the dentate gyrus (WT: 16.6 ± 1.3 cells/mm vs DCXDTR: 10.3 ± 0.4 cells/mm, n = 3 per genotype; p < 0.01). The vast majority of these Arc+ cells were observed within the granule cell layer, which typically contains more mature neurons. Consistent with this finding, only a very small percentage (<1.5%) of immature DCX+ neurons cells were found to express Arc. Nonetheless, the number of Arc-expressing DCX+ cells was also significantly reduced in the DCXDTR mice (WT: 0.5 ± 0.05 cells/mm, n = 4, vs DCXDTR: 0.2 ± 0.03 cells/mm, n = 5; p < 0.01).
As Arc is an immediate early gene thought to be involved in memory consolidation (Shepherd and Bear, 2011), we next examined its expression in relation to learning and subsequent recovery of the DCX+ cell population. To assess Arc expression during learning, additional cohorts of WT and DCXDTR mice were treated with DT and trained in the active place task for 2 d, as per our previous experiments. On day 3, the shock zone was deactivated to avoid the possibility that the induction of Arc expression could have arisen, at least in part, from the delivery of electric shocks rather than learning. Animals were then allowed to navigate the rig for 3 min before being perfused within 60 min. Consistent with our initial observations, the overall number of Arc-expressing cells under this experimental paradigm was significantly reduced in DCXDTR mice (WT: 9.3 ± 0.9 cells/mm; vs DCXDTR: 4.0 ± 0.5 cells/mm, n = 4 per genotype; p < 0.01; Fig. 4A–C). The number of cells expressing c-Fos, another immediate early gene activated by a variety of stimuli, was not different between genotypes (WT: 224 ± 21 vs DCXDTR 199 ± 26 cells per dentate gyrus, n = 4 per genotype; p = 0.5). When specifically examining Arc expression in immature neurons, we found a significant increase in the number of DCX+Arc+ cells in WT mice after active avoidance learning compared with their naive counterparts (i.e., WT mice that had been treated with DT but were not exposed to the experimental rig) (WT: 0.2 ± 0.04 cells/mm vs WT naive: 0.1 ± 0.01 cells/mm, n = 4 per genotype; p < 0.05). Virtually no Arc-expressing DCX+ cells were observed in the DCXDTR mice because of the DT-induced ablation of immature neurons (WT: 0.2 ± 0.04 cells/mm vs DCXDTR: 0.01 ± 0.01 cells/mm, n = 4 per genotype; p < 0.01). If, however, DCXDTR mice were allowed to recover from DT treatment for 8 weeks, thereby allowing immature neurons to repopulate the dentate gyrus before being exposed to the active place avoidance task and subsequently killed 24 h later, the number of Arc-expressing DCX+ cells was not different between genotypes (WT: 0.1 ± 0.02 cells, n = 5, vs DCXDTR: 0.1 ± 0.02 cells, n = 4). Overall, these data suggest a tight relationship between expression of the immediate early gene Arc in immature neurons and behavioral performance.
Figure 4.
Arc expression is reduced in DCXDTR mice during learning. A, B, Representative images of Arc (red) and DCX (green) expression in DCXDTR mice, compared with WT mice, during learning (cell nuclei are counterstained blue with DAPI). C, Quantification of Arc+ cells after active place avoidance learning revealed that the overall number of cells expressing Arc was significantly reduced in DCXDTR mice. Asterisks indicate significance between genotypes as determined by Student's t test. **p < 0.01 (WT: n = 4; DCXDTR: n = 4). Scale bars: A, B, 50 μm.
Immature neurons are not required for recall of a familiar spatial task
Having illustrated the importance of DCX+ immature neurons for mastering a novel and challenging spatial task, we next tested the premise that retrieval of a learned memory in relation to the now familiar task may also require the presence of such cells (experimental design, Fig. 5A). For this, new cohorts of naive mice were tested in the active place avoidance task. In the absence of DT treatment, DCXDTR and WT mice exhibited no significant differences in any of the parameters measured, confirming that genomic insertion of the DCXDTR construct (in the absence of DT treatment) had no effect on the animals' performance. Without DT treatment, DCXDTR and WT animals received a similar number of shocks (Fig. 5B). No significant differences were observed between genotypes in, for example, the “maximal time of avoidance” (WT: 246 ± 31 s, n = 11, vs DCXDTR: 307 ± 46 s, n = 9) or the “number of entries per distance” (WT: 0.19 ± 0.04, n = 11, vs DCXDTR: 0.20 ± 0.04, n = 9) on trial day 3 (when differences would have otherwise been present with DT treatment). Next, the animals were treated with DT and retested under the same conditions (i.e., unaltered visual cues or positioning of the entry and shock zones). Again, no significant differences in behavior were observed between genotypes (Fig. 5C). These results suggest that the lack of an immature neuron population in the hippocampus did not impact on performance in the familiar spatial task, as both DCXDTR and WT mice learned to avoid the shock zone with similar success. To further corroborate our finding that immature neurons are particularly important for mastering a novel spatial task, the animals were retested, but with altered visual cues and position of the shock zone. Under these circumstances, DCXDTR mice were unable to master the novel spatial task as well as WT mice, receiving significantly more shocks on trial day 3 (Fig. 5D). Together, these findings suggest that an intact population of DCX+ immature neurons is critical for mastering a novel spatial task, but that these cells are redundant for memory retrieval in a familiar spatial task.
Repeated ablation of immature neurons induces spatial learning deficits
Finally, we tested whether impairments in learning a spatial task could be repeatedly induced in the same cohort of mice by exposing the animals to another round of DT treatment 8 weeks after administration of the first round of DT. A schematic overview of the experimental design is shown in Figure 6A. In brief, mice were tested in the active place avoidance task after the initial ablation of DCX+ cells, after recovery of the DCX+ cell population upon cessation of round 1 of DT treatment and directly after the second round of DT-induced DCX+ cell ablation. Mice were retested in both the familiar task (session 3) and a novel configuration (session 4) in which the position of the shock and entry zones was reversed but the spatial cues remained the same. In line with our previous findings, DCXDTR mice received a greater number of shocks when tested in the active place avoidance task after initial depletion of the immature neurons (Fig. 6B). This deficit in the ability to rapidly master a challenging spatial learning task was again rescued after a rest period (to allow replenishment of DCX+ population), as no difference was observed in the number of shocks received by mice of either genotype at 8 weeks after DT treatment (Fig. 6C). In line with our previous observations, the second depletion did not induce a significant performance deficit when animals were retested in the already familiar task (Fig. 6D). However, when presented with reversal of the location of the shock and entry zones, DCXDTR mice received significantly more shocks during trial day 1, although on trial days 2 and 3 a similar number of shocks was delivered to DCXDTR and WT mice (Fig. 6E). Thus, repeated depletion of immature neurons in the DCXDTR transgenic mouse model significantly slowed reversal learning in the active place avoidance task, suggesting a negative effect on cognitive flexibility.
Discussion
To define the significance of adult-born immature hippocampal neurons, we generated a knock-in mouse model that allowed for effective and targeted depletion of DCX+ cells. It has been proposed that the unique properties of immature neurons (e.g., increased excitability) underpin some types of learning (Ge et al., 2007). To date, however, this hypothesis has not been tested genetically through their specific depletion. Previous studies have mostly disrupted neurogenesis at an early stage by preventing proliferation of neural precursor cells through irradiation, antimitotic agents, or, more recently, transgenic approaches targeting Nestin-expressing and/or GFAP-expressing cells (Arruda-Carvalho et al., 2011; Snyder et al., 2011). These approaches result in sustained impairment in production of both immature neurons (including DCX+ cells) and other cell types arising from the neural cell precursor pool, exerting a depletion effect for prolonged periods of time. Using DTR expression under endogenous DCX promoter control, we were able to selectively target immature neurons, as DCX has been shown to colocalize with early neuronal markers, such as βIII-tubulin and Map2 but not neural precursor/stem cell markers, including Nestin and GFAP (Karl et al., 2005). This approach thereby allowed us to transiently deplete immature (DCX+) neurons after only a relatively short treatment with DT (6 injections over 11 d), and to also repeatedly examine the contribution of immature neurons to different stages of learning and memory through multiple rounds of depletion.
Immature neurons are important for acquisition of spatial learning
Ablation of immature neurons caused deficits in the acquisition of the active place avoidance task for up to at least 17 d after cessation of DT treatment (i.e., 28 d after the first injection). Typically, no significant difference in acquisition was observed between DCXDTR and WT animals on trial day 1. However, difficulty in learning consolidation became readily apparent in animals deficient in immature neurons on trial days 2 and 3. The majority of previous studies using a classical spatial task, the Morris water maze, observed no such deficits in acquisition, with impaired performance only becoming apparent during probe trials (Saxe et al., 2006; Zhang et al., 2008; Deng et al., 2009; Arruda-Carvalho et al., 2011). In comparison with the water maze, the active place avoidance task adds extra complexity by requiring the animal to be constantly aware of its changing location in relation to the shock zone because of the continuous rotation of the apparatus. As such, the animal has to detect subtle changes in movement and the location of spatial cues to navigate optimally. One could therefore hypothesize that the task requires a similar level of hippocampal processing to the one present during pattern separation tasks in which the ability of animals to discriminate between minimal changes in the environment is tested. Our findings therefore accord with those of several recent studies reporting deficits in pattern separation after ablation of neurogenesis (Clelland et al., 2009; Sahay et al., 2011; Tronel et al., 2011; Kheirbek et al., 2012; Nakashiba et al., 2012). Nonetheless, it must be noted that, in their recent study, Burghardt et al. (2012) did not observe a deficit in acquisition of the active place avoidance task when neurogenesis was impaired through ablation of neural precursors. However, the active place avoidance task in this study was conducted by administering multiple trials per day, with intertrial intervals allowing for robust acquisition of the task. In our study, animals were only tested once per day, but over 3 d, aiming for more gradual learning with an increased requirement for efficient long-term consolidation of the memory trace between training days. We think that this is a critical difference that allowed us to expose differences in learning after depletion of immature neurons. Similarly, different outcomes have been observed for contextual fear conditioning, where the behavioral significance of neurogenesis was illustrated when a single shock was paired with a context, but not with multiple shock pairings (Drew et al., 2010). Considering that neurogenesis appears to be involved in the consolidation of subtle but critical information, the level of difficulty of the task should therefore be a major factor for consideration when seeking to detect behavioral deficits under conditions where neurogenesis is impaired.
Our depletion strategy also provided us with a unique opportunity to examine whether the observed behavioral deficits could be reversed after withdrawal of DT treatment, thereby allowing the rapid replenishment of immature neurons from the unaffected neural precursor pool. The acquisition phenotype in the active place avoidance task indeed disappeared after recovery of the DCX+ cell population, thereby conclusively demonstrating its importance for learning. These findings also illustrate the robustness of the adult neurogenic niche, particularly its ability to successfully reconstitute a fully functional population of immature neurons. The only other study to show recovery of normal behavior after transient blockade of neurogenesis was performed by Deng et al. (2009), whose findings suggest that the presence of immature neurons (∼4 weeks of age) is important for reducing extinction of learned spatial preference in the Morris water maze task. To our knowledge, the present study is the first to report recovery from acquisition deficits.
In our model, learning deficits remained present for up to at least 17 d after DT treatment, indicating that DCX+ cells must be at least ∼3 weeks of age before they can make a significant contribution to spatial learning in the active place avoidance task. Such a finding is in line with previous studies showing that the initial synaptic connections of adult-born neurons with afferent connections from the entorhinal cortex are only established at day 16 (Zhao et al., 2006; Toni et al., 2008). Expression of immediate early genes, which are important for synaptic plasticity and thus learning (Lanahan and Worley, 1998; Shepherd and Bear, 2011), also appears to peak 3 weeks after the birth of immature neurons (Jessberger and Kempermann, 2003; Kee et al., 2007; Snyder et al., 2009), and these cells are thought to become preferentially recruited during learning from 4 weeks of age (Kee et al., 2007). In the present study, we observed that a reduction in the number of DCX+ cells also led to a profound decrease in Arc expression, suggesting an important role for immature neurons in regulating overall synaptic plasticity within the dentate gyrus during learning.
Immature DCX+ neurons are not important for memory recall in a spatial task
We also examined whether the presence of DCX+ cells was required for remembering an already familiar (i.e., learned) task. In our study, we observed no behavioral recall deficits if DCX+ cells were ablated after acquisition of the active place avoidance task, suggesting that this pool of immature neurons was not important for recalling a familiar task. These findings appear somewhat at odds with two recent reports stating that adult-born neurons are required for memory recall (Arruda-Carvalho et al., 2011; Gu et al., 2012). Although the initial study by Arruda-Carvalho et al. (2011) used a DT-based depletion strategy that ablated adult-born neurons up to ∼16 weeks of age, the more recent paper by Gu et al. (2012) used elegant optogenetic approaches to demonstrate that integration of a cohort of ∼4 week-old cells appears particularly important for hippocampal memory retrieval. As the DCX protein is only highly expressed up to 3 weeks after the birth of neurons (Snyder et al., 2009), a likely explanation for the differences between our studies is therefore that the DCXDTR mouse model depletes adult-born neurons before their integration, with the majority of these cells simply being too young to commit to a memory trace and play a role in recall. It is therefore important to note that our findings do not preclude a role for adult-born neurons in the recall of a spatial memory once these cells have sufficiently matured and integrated into the existing neural circuitry at ∼4 weeks of age. Rather, they show that young immature neurons in the DCX+ cell population do not yet have such a role, at least not in the active place avoidance task. Future experiments should reveal whether a more similar spatial learning task and timing of the recall can further reconcile the differences between previous studies and the present findings.
In our final experimental protocol, we induced a second round of depletion of DCX+ cells in a cohort of animals that had recovered from the first episode, again confirming that immature neurons were not required to remember the already familiar active place avoidance task. However, behavioral deficits again became apparent for DCXDTR mice when we tested reversal learning (i.e., changing the shock zone location). The reversal task tests cognitive flexibility, as an animal needs to learn to suppress some aspects of the previously learned response, transfer some of the other aspects, and simultaneously learn new spatial locations to adapt to the changed circumstances and complete the task successfully. This type of task highlights deficits in mice with reduced neurogenesis (Garthe et al., 2009; Burghardt et al., 2012).
In conclusion, although adult-born immature neurons are important for learning a spatial task, they appear redundant in remembering a learned task. These findings have importance for our understanding of conditions that affect memory formation and demonstrate the potential of stimulating neurogenesis as a means of alleviating learning deficits.
Footnotes
This work was supported by a National Health and Medical Research Council Program Grant and ARC Special Research Initiative in Stem Cells Science (SR110001002), to P.F.B. and the Estate of Dr. Clem Jones AO. J.V. was supported by the Queensland Government Smart Futures Fellowship. M.J.R. was supported by a SpinalCure Australia Career Development Fellowship. We thank the staff of the University of Queensland Biological Resources facility for breeding and maintaining the animals used in this study; Estella Newcombe and Deaglan Barnes for technical assistance; Ashley Cooper and Rowan Tweedale for editorial assistance; and Dee McGrath for assistance with illustrations.
The authors declare no competing financial interests.
References
- Arruda-Carvalho M, Sakaguchi M, Akers KG, Josselyn SA, Frankland PW. Posttraining ablation of adult-generated neurons degrades previously acquired memories. J Neurosci. 2011;31:15113–15127. doi: 10.1523/JNEUROSCI.3432-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bannon AW, Malmberg AB. Models of nociception: hot-plate, tail-flick, and formalin tests in rodents. Curr Protoc Neurosci. 2007;8 doi: 10.1002/0471142301.ns0809s41. Unit 8.9. [DOI] [PubMed] [Google Scholar]
- Buch T, Heppner FL, Tertilt C, Heinen TJ, Kremer M, Wunderlich FT, Jung S, Waisman A. A Cre-inducible diphtheria toxin receptor mediates cell lineage ablation after toxin administration. Nat Methods. 2005;2:419–426. doi: 10.1038/nmeth762. [DOI] [PubMed] [Google Scholar]
- Burghardt NS, Park EH, Hen R, Fenton AA. Adult-born hippocampal neurons promote cognitive flexibility in mice. Hippocampus. 2012;22:1795–1808. doi: 10.1002/hipo.22013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carobrez AP, Bertoglio LJ. Ethological and temporal analyses of anxiety-like behavior: the elevated plus-maze model 20 years on. Neurosci Biobehav Rev. 2005;29:1193–1205. doi: 10.1016/j.neubiorev.2005.04.017. [DOI] [PubMed] [Google Scholar]
- Cheng X, Li Y, Huang Y, Feng X, Feng G, Xiong ZQ. Pulse labeling and long-term tracing of newborn neurons in the adult subgranular zone. Cell Res. 2011;21:338–349. doi: 10.1038/cr.2010.141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cimadevilla JM, Wesierska M, Fenton AA, Bures J. Inactivating one hippocampus impairs avoidance of a stable room-defined place during dissociation of arena cues from room cues by rotation of the arena. Proc Natl Acad Sci U S A. 2001;98:3531–3536. doi: 10.1073/pnas.051628398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clelland CD, Choi M, Romberg C, Clemenson GD, Jr, Fragniere A, Tyers P, Jessberger S, Saksida LM, Barker RA, Gage FH, Bussey TJ. A functional role for adult hippocampal neurogenesis in spatial pattern separation. Science. 2009;325:210–213. doi: 10.1126/science.1173215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deng W, Saxe MD, Gallina IS, Gage FH. Adult-born hippocampal dentate granule cells undergoing maturation modulate learning and memory in the brain. J Neurosci. 2009;29:13532–13542. doi: 10.1523/JNEUROSCI.3362-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deng W, Aimone JB, Gage FH. New neurons and new memories: how does adult hippocampal neurogenesis affect learning and memory? Nat Rev Neurosci. 2010;11:339–350. doi: 10.1038/nrn2822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drew MR, Denny CA, Hen R. Arrest of adult hippocampal neurogenesis in mice impairs single- but not multiple-trial contextual fear conditioning. Behav Neurosci. 2010;124:446–454. doi: 10.1037/a0020081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dupret D, Revest JM, Koehl M, Ichas F, De Giorgi F, Costet P, Abrous DN, Piazza PV. Spatial relational memory requires hippocampal adult neurogenesis. PLoS One. 2008;3:e1959. doi: 10.1371/journal.pone.0001959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farioli-Vecchioli S, Saraulli D, Costanzi M, Pacioni S, Cinà I, Aceti M, Micheli L, Bacci A, Cestari V, Tirone F. The timing of differentiation of adult hippocampal neurons is crucial for spatial memory. PLoS Biol. 2008;6:e246. doi: 10.1371/journal.pbio.0060246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garthe A, Behr J, Kempermann G. Adult-generated hippocampal neurons allow the flexible use of spatially precise learning strategies. PLoS One. 2009;4:e5464. doi: 10.1371/journal.pone.0005464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ge S, Yang CH, Hsu KS, Ming GL, Song H. A critical period for enhanced synaptic plasticity in newly generated neurons of the adult brain. Neuron. 2007;54:559–566. doi: 10.1016/j.neuron.2007.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodman T, Trouche S, Massou I, Verret L, Zerwas M, Roullet P, Rampon C. Young hippocampal neurons are critical for recent and remote spatial memory in adult mice. Neuroscience. 2010;171:769–778. doi: 10.1016/j.neuroscience.2010.09.047. [DOI] [PubMed] [Google Scholar]
- Gu Y, Arruda-Carvalho M, Wang J, Janoschka SR, Josselyn SA, Frankland PW, Ge Shaoyu. Optical controlling reveals time-dependent roles for adult-born dentate granule cells. Nat Neuorsci. 2012;15:1700–1706. doi: 10.1038/nn.3260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harms LR, Eyles DW, McGrath JJ, Mackay-Sim A, Burne TH. Developmental vitamin D deficiency alters adult behaviour in 129/SvJ and C57BL/6J mice. Behav Brain Res. 2008;187:343–350. doi: 10.1016/j.bbr.2007.09.032. [DOI] [PubMed] [Google Scholar]
- Jessberger S, Kempermann G. Adult-born hippocampal neurons mature into activity-dependent responsiveness. Eur J Neurosci. 2003;18:2707–2712. doi: 10.1111/j.1460-9568.2003.02986.x. [DOI] [PubMed] [Google Scholar]
- Karl C, Couillard-Despres S, Prang P, Munding M, Kilb W, Brigadski T, Plötz S, Mages W, Luhmann H, Winkler J, Bogdahn U, Aigner L. Neuronal precursor-specific activity of a human doublecortin regulatory sequence. J Neurochem. 2005;92:264–282. doi: 10.1111/j.1471-4159.2004.02879.x. [DOI] [PubMed] [Google Scholar]
- Kee N, Teixeira CM, Wang AH, Frankland PW. Preferential incorporation of adult-generated granule cells into spatial memory networks in the dentate gyrus. Nat Neurosci. 2007;10:355–362. doi: 10.1038/nn1847. [DOI] [PubMed] [Google Scholar]
- Kheirbek MA, Tannenholz L, Hen R. NR2B-dependent plasticity of adult-born granule cells is necessary for context discrimination. J Neurosci. 2012;32:8696–8702. doi: 10.1523/JNEUROSCI.1692-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kubík S, Fenton AA. Behavioral evidence that segregation and representation are dissociable hippocampal functions. J Neurosci. 2005;25:9205–9212. doi: 10.1523/JNEUROSCI.1707-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lalonde R, Bensoula AN, Filali M. Rotorod sensorimotor learning in cerebellar mutant mice. Neurosci Res. 1995;22:423–426. doi: 10.1016/0168-0102(95)00916-H. [DOI] [PubMed] [Google Scholar]
- Lanahan A, Worley P. Immediate-early genes and synaptic function. Neurobiol Learn Mem. 1998;70:37–43. doi: 10.1006/nlme.1998.3836. [DOI] [PubMed] [Google Scholar]
- Middlebrook JL, Dorland RB. Response of cultured mammalian cells to the exotoxins of Pseudomonas aeruginosa and Corynebacterium diphtheriae: differential cytotoxicity. Can J Microbiol. 1977;23:183–189. doi: 10.1139/m77-026. [DOI] [PubMed] [Google Scholar]
- Nakashiba T, Cushman JD, Pelkey KA, Renaudineau S, Buhl DL, McHugh TJ, Rodriguez Barrera V, Chittajallu R, Iwamoto KS, McBain CJ, Fanselow MS, Tonegawa S. Young dentate granule cells mediate pattern separation, whereas old granule cells facilitate pattern completion. Cell. 2012;149:188–201. doi: 10.1016/j.cell.2012.01.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sahay A, Scobie KN, Hill AS, O'Carroll CM, Kheirbek MA, Burghardt NS, Fenton AA, Dranovsky A, Hen R. Increasing adult hippocampal neurogenesis is sufficient to improve pattern separation. Nature. 2011;472:466–470. doi: 10.1038/nature09817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saxe MD, Battaglia F, Wang JW, Malleret G, David DJ, Monckton JE, Garcia AD, Sofroniew MV, Kandel ER, Santarelli L, Hen R, Drew MR. Ablation of hippocampal neurogenesis impairs contextual fear conditioning and synaptic plasticity in the dentate gyrus. Proc Natl Acad Sci U S A. 2006;103:17501–17506. doi: 10.1073/pnas.0607207103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt-Hieber C, Jonas P, Bischofberger J. Enhanced synaptic plasticity in newly generated granule cells of the adult hippocampus. Nature. 2004;429:184–187. doi: 10.1038/nature02553. [DOI] [PubMed] [Google Scholar]
- Shepherd JD, Bear MF. New views of Arc, a master regulator of synaptic plasticity. Nat Neurosci. 2011;14:279–284. doi: 10.1038/nn.2708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snyder JS, Choe JS, Clifford MA, Jeurling SI, Hurley P, Brown A, Kamhi JF, Cameron HA. Adult-born hippocampal neurons are more numerous, faster maturing, and more involved in behavior in rats than in mice. J Neurosci. 2009;29:14484–14495. doi: 10.1523/JNEUROSCI.1768-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snyder JS, Soumier A, Brewer M, Pickel J, Cameron HA. Adult hippocampal neurogenesis buffers stress responses and depressive behaviour. Nature. 2011;476:458–461. doi: 10.1038/nature10287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toni N, Laplagne DA, Zhao C, Lombardi G, Ribak CE, Gage FH, Schinder AF. Neurons born in the adult dentate gyrus form functional synapses with target cells. Nat Neurosci. 2008;11:901–907. doi: 10.1038/nn.2156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tronel S, Belnoue L, Grosjean N, Revest JM, Piazza PV, Koehl M, Abrous DN. Adult-born neurons are necessary for extended contextual discrimination. Hippocampus. 2012;22:292–298. doi: 10.1002/hipo.20895. [DOI] [PubMed] [Google Scholar]
- Trouche S, Bontempi B, Roullet P, Rampon C. Recruitment of adult-generated neurons into functional hippocampal networks contributes to updating and strengthening of spatial memory. Proc Natl Acad Sci U S A. 2009;106:5919–5924. doi: 10.1073/pnas.0811054106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Praag H, Schinder AF, Christie BR, Toni N, Palmer TD, Gage FH. Functional neurogenesis in the adult hippocampus. Nature. 2002;415:1030–1034. doi: 10.1038/4151030a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vukovic J, Colditz MJ, Blackmore DG, Ruitenberg MJ, Bartlett PF. Microglia modulate hippocampal neural precursor activity in response to exercise and aging. J Neurosci. 2012;32:6435–6443. doi: 10.1523/JNEUROSCI.5925-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang S, Scott BW, Wojtowicz JM. Heterogenous properties of dentate granule neurons in the adult rat. J Neurobiol. 2000;42:248–257. doi: 10.1002/(SICI)1097-4695(20000205)42:2<248::AID-NEU8>3.0.CO;2-J. [DOI] [PubMed] [Google Scholar]
- Wesierska M, Dockery C, Fenton AA. Beyond memory, navigation, and inhibition: behavioral evidence for hippocampus-dependent cognitive coordination in the rat. J Neurosci. 2005;25:2413–2419. doi: 10.1523/JNEUROSCI.3962-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang CL, Zou Y, He W, Gage FH, Evans RM. A role for adult TLX-positive neural stem cells in learning and behaviour. Nature. 2008;451:1004–1007. doi: 10.1038/nature06562. [DOI] [PubMed] [Google Scholar]
- Zhang J, Giesert F, Kloos K, Vogt Weisenhorn DM, Aigner L, Wurst W, Couillard-Despres S. A powerful transgenic tool for fate mapping and functional analysis of newly generated neurons. BMC Neurosci. 2010;11:158. doi: 10.1186/1471-2202-11-158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao C, Teng EM, Summers RG, Jr, Ming GL, Gage FH. Distinct morphological stages of dentate granule neuron maturation in the adult mouse hippocampus. J Neurosci. 2006;26:3–11. doi: 10.1523/JNEUROSCI.3648-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]