Abstract
Background
Infections are a frequent complication of cardiac surgery. The intraoperative use of transesophageal echocardiography (TEE) may be an underrecognized risk factor for post‐operative infections. The aim of this study was to investigate infection rates and outcomes after cardiac surgery in a nationwide cohort, especially in relation to periods where surface damaged TEE probes were used.
Methods
This was a retrospective, observational study at Landspitali University Hospital. All consecutive cardiac surgery patients from 1 January 2013 to 31 December 2017 were included. Patients’ charts were reviewed for evidence of infection, post‐operative complications or death.
Results
During the study period, 973 patients underwent cardiac surgery at Landspitali and 198 (20.3%) developed a post‐operative infection. The most common infections were: Pneumonia (9.1%), superficial surgical site (5.7%), bloodstream (2.8%) and deep sternal wound (1.7%). Risk factors for developing an infection included: The duration of procedure, age, insulin‐dependent diabetes, EuroScore II, reoperation for bleeding and an operation in a period with a surface damaged TEE probe in use. Twenty‐two patients were infected with a multidrug resistant strain of Klebsiella oxytoca, 10 patients with Pseudomonas aeruginosa and two patients developed endocarditis with Enterococcus faecalis. All three pathogens were cultured from the TEE probe in use at respective time, after decontamination. The 30‐day mortality rate in the patient cohort was 3.2%.
Conclusions
The intraoperative use of surface damaged TEE probes caused two serious infection outbreaks in patients after cardiac surgery. TEE probes need careful visual inspection during decontamination and probe sheaths are recommended.
Keywords: cardiac surgery, nosocomial infections, pneumonia, post‐operative complications, transesophageal echocardiography
Editorial comment.
This report describes an analysis of post‐operative infectious complications during a recent period in a single centre cardiac surgical cohort. There is specific focus on risk associated with reused equipment, with tracking to transesophageal echo probes.
1. INTRODUCTION
Infections are among the most frequent complications after cardiac surgery1, 2 and have consistently been linked to longer duration of stay and increased mortality.3, 4, 5 Recent reports have shown rates of surgical site infections from 4.9% to 8.1%,6, 7 urinary tract infections from 0.7% to 6%,6, 8, 9, 10 blood stream infections from 1.1% to 2.3%,3, 8, 10, 11 and deep sternal wound infections from 0.6% to 1.6%.3, 9, 10
Post‐operative pneumonia, in particular, has been the subject of numerous studies, reporting variable incidence rates, from 2.4% to 10.7%.3, 6, 9, 13, 14 Many risk factors for developing pneumonia post‐operatively have been identified and include: age,9, 13, 14, 18 chronic pulmonary disease,3, 9, 13, 14 heart failure,3, 9 female gender,5, 9, 19 duration of surgery3 and cardiopulmonary bypass,14 duration of mechanical ventilation,3, 13, 18, 19 re‐intubation,17, 18, 20 transfusion of blood products14, 17, 18 and emergency surgery.4 Most of these factors are not amenable to modification.
A less well‐known potential causative factor in the development of pneumonia after cardiac surgery is the use of transesophageal echocardiography (TEE) probes, which are used in the majority of all cardiac procedures.21 A few outbreaks have been described linking TEE probes with respiratory infections after cardiac surgery, Enterobacter cloacae 22 (17 patients) and Pseudomonas aeruginosa 23 (eight patients) in Japan and Escherichia coli 24 (eight patients) in the United States. A common factor in all three outbreaks was damage to the probes, presumably preventing adequate decontamination.
1.1. The outbreaks
In the year 2014, multiple patients in the intensive care unit (ICU) at Landspitali University Hospital developed pneumonia with an extensively beta‐lactam resistant Klebsiella oxytoca. After a detailed investigation for possible sources of contamination in the ICU environment, the same strain of K oxytoca was isolated from a TEE probe which had been used in most cardiac operations at the hospital. Careful inspection under magnification revealed a small crack at the junction of the tip and the shaft of the TEE probe. The probe was subsequently replaced with another one that was previously unused but several years old. The use of a protective sheath to cover the probe while in patient use was encouraged, but not enforced. The outbreak strain of K oxytoca was not identified in any cardiac patient after changing the TEE probe. However, in the year 2017, three patients died of septic shock due to P aeruginosa shortly after cardiac surgery and two patients developed endocarditis due to Enterococcus faecalis between 1 and 12 weeks after cardiac surgery. One of those patients died. Inspection of the replacement TEE probe revealed minute damage, see Figure 1, and both pathogens were cultured from the probe despite decontamination.
Figure 1.

The tip of one of the transesophageal echocardiography probes in use during the study period. With magnification and specific lighting used in this photograph, numerous superficial scratches and a crack (arrow) in the plastic casing can be visualized. The damage was not obvious to the naked eye. The outbreak strains of Pseudomonas aeruginosa and Enterococcus faecalis were isolated from this crack, after the probe had been decontaminated and was ready for use (Photo: Thorkell Thorkelsson) [Colour figure can be viewed at wileyonlinelibrary.com]
Given the two recent outbreaks described above, the aim of our study was to examine the incidence of infections after cardiac surgery in a nationwide population and how it related to periods with a contaminated TEE probe in use. Additionally, we describe the risk factors for infections and the general outcome of cardiac surgery patients in Iceland.
2. METHODS
2.1. Study design and setting
This was a retrospective, nationwide, observational study conducted at Landspitali University Hospital in Reykjavik, Iceland. Landspitali is a tertiary care centre serving a population of 350 000 with around 200 open‐heart surgeries performed each year. It is the sole provider of cardiac surgery in Iceland. During the study period, a single TEE probe was located in the cardiac surgery theatre and used in the majority of procedures. A second probe could be obtained if needed from the ICU (eg, in case of two concurrent operations). Post‐operative cardiac surgery patients were admitted to a 7 bed multidisciplinary ICU for at least one night. Per protocol cefazolin was used for surgical antibiotic prophylaxis (1 g every 8 hours for 48 hours) or clindamycin in patients allergic to beta‐lactam antibiotics. Microbiology cultures were only requested in cardiac surgery patients upon clinical suspicion of infection.
2.2. Patient selection and data collection
All consecutive patients (≥18 years) who underwent open‐heart surgery at Landspitali from 1 January 2013 to 31 December 2017 were included. Data collected from patients´ electronic medical charts, hospital laboratories and the operation planning system were entered into an Excel spreadsheet (Microsoft Office 2016, Version 1810). The European System for Cardiac Operative Risk Evaluation (EuroScore II) scoring system was used to assess preoperative and surgical risk factors.25 The occurrence or absence of infection in each patient was judged by study authors using all available clinical data. Infections were defined and classified based on the definitions of the European Center for Disease Prevention and Control.26
Microbiology cultures were reviewed in all patients for 30 days after surgery, or up to 90 days for deep surgical infections and endocarditis. Additional outcome data collected included the development of a new‐onset atrial fibrillation, stroke, need for pleurocentesis, pericardiocentesis, renal replacement therapy, circulatory mechanical assist devices or reoperation, length of ventilation, need for re‐intubation, length of stay in the ICU and hospital, and 30‐day and in‐hospital mortality. Two periods with a damaged and contaminated TEE probe in use were defined: From the date of surgery of the first (30 October 2013) and the last (12 November 2014) patient with K oxytoca pneumonia and the first (15 September 2016) and last (12 April 2017) patient with P aeruginosa pneumonia.
2.3. End points
The main end point was the diagnosis of a new infection within 30 days after cardiac surgery, or 90 days for deep surgical site infections and endocarditis. Secondary end points included the incidence of complications as described above and mortality after cardiac surgery.
2.4. Statistical analysis
Statistical analysis was performed using SPSS (IBM, Build 1.0.0.1126). Proportions are presented as percentage with confidence intervals. For normally distributed data, means and standard deviation (SD) are reported. The median and interquartile range (IQR) are reported for non‐normally distributed data. Pearson's chi‐squared test was used for categorical variables and odds ratios (OR) are reported with 95% confidence intervals (CI). Mann‐Whitney U test was used for comparing length of stay between groups. For analysis of risk factors for infections and mortality, patient characteristics were entered into a univariate logistic regression with development of an infection or mortality as the dependent variable. Factors associated individually with the development of an infection (P < 0.05) were entered into a forward conditional multivariate logistic model. Missing values were common for preoperative pulmonary artery pressure (67% of subjects), preoperative left ventricular ejection fraction (11% of subjects) and ventilation time (17% of subjects) but otherwise missing data were <1%. When calculating the EuroScore II, missing data were presumed to have been normal.
3. RESULTS
3.1. The patient cohort
Nine hundred seventy‐three patients underwent open‐heart surgery at Landspitali in the 5‐year study period (2013‐2017), with the annual number of operations decreasing from 224 in the first year to 141 in the last year of the study. Patient characteristics are presented in Table 1.
Table 1.
Preoperative and surgical characteristics of 973 consecutive patients undergoing cardiac surgery in Iceland
| Patient characteristics (n = 973) | ||
|---|---|---|
| Preoperative | ||
| Age (mean) | 66.7 y | SD 11.50 |
| Males | 76% | |
| Weight (mean) | 86.1 kg | SD 15.67 |
| Chronic lung diseasea | 77 | 7.9% |
| Extracardiac arteriopathyb | 58 | 6.0% |
| Insulin‐dependent diabetes mellitus | 51 | 5.2% |
| Dialysis dependent | 13 | 1.3% |
| Angina pectoris at rest | 134 | 13.8% |
| Recent myocardial infarctionc | 178 | 18.3% |
| Critical pre‐op conditiond | 48 | 4.9% |
| LV Ejection fraction (mean) | 53% | SD 11.0 |
| NYHA classe | ||
| 0 | 94 | 9.7% |
| I | 97 | 10.0% |
| II | 357 | 36.7% |
| III | 238 | 24.5% |
| IV | 187 | 19.2% |
| Surgical | ||
| Proceduref | ||
| CABG | 483 | 49.6% |
| AVR | 156 | 16.0% |
| CABG + valve | 113 | 11.6% |
| Aortic surgery | 61 | 6.3% |
| MVR | 29 | 3.0% |
| OPCAB | 26 | 2.7% |
| Others | 105 | 10.8% |
| Duration of procedure (mean) | 242 min | SD 90.8 |
| Re‐do procedure | 25 | 2.6% |
| Active endocarditisg | 18 | 1.8% |
| Urgencyh | ||
| Elective | 511 | 52.5% |
| Urgent | 386 | 39.7% |
| Emergency | 68 | 7.0% |
| Salvage | 8 | 0.8% |
| EuroScore II (median) | 1.82 | IQR 1.07‐3.77 |
| EuroScore II (mean) | 3.78 | SD 5.8 |
Long‐term use of bronchodilators or steroids for lung disease.
One or more of the following: carotid occlusion or >50% stenosis, amputation for arterial disease, previous or planned intervention on the abdominal aorta, limb arteries or carotids.
Myocardial infarction within 90 d.
Critical preoperative state: Ventricular tachycardia or ventricular fibrillation or aborted sudden death, preoperative cardiac massage, preoperative ventilation before anaesthetic room, preoperative inotropes or IABP, preoperative acute renal failure (anuria or oliguria <10 mL/h).
New York Heart Association class.
AVR, aortic valve replacement; CABG, Coronary artery bypass grafting; MVR, mitral valve replacement; OPCAB, Off‐pump coronary bypass.
Active endocarditis: Patient still on antibiotic treatment for endocarditis at time of surgery.
Elective: Routine admission for operation. Urgent: Patients who have not been electively admitted for operation but who require intervention or surgery on the current admission for medical reasons. Emergency: Operation before the beginning of the next working day after decision to operate. Salvage: Patients requiring cardiopulmonary resuscitation (external cardiac massage) en route to the operating theatre or prior to induction of anaesthesia.
3.2. Post‐operative infections
One hundred ninety‐eight (20.3%) patients developed one or more infection post‐operatively, see Table 2. The total number of registered infections was 273.
Table 2.
Post‐operative infections diagnosed in 973 consecutive cardiac surgery patients in Iceland
| Post‐operative infections (n = 973) | ||
|---|---|---|
| Pneumonia | 89 | 9.1% |
| With positive cultures | 61 | 6.2% |
| Clinical diagnosis only | 28 | 2.9% |
| Surgical site infection | 73 | 7.3% |
| Deep sternal | 17 | 1.7% |
| Superficial (sternum or vein harvest site) | 56 | 5.7% |
| Urinary tract infection | 52 | 5.3% |
| Symptomatic | 14 | 1.4% |
| Asymptomatic bacteriuria | 38 | 3.9% |
| Bloodstream infection | 27 | 2.8% |
| Endocarditisa | 5 | 0.5% |
| Clostridium difficile enterocolitis | 3 | 0.3% |
| Others | 24 | 2.4% |
Patients who developed endocarditis post‐operatively in a valve prosthesis. Patients with endocarditis prior to surgery not included.
Pneumonia was the most common infection and was diagnosed in 89 patients (9.1%). The incidence rate was 13.8% (95% CI 10.5%‐17.8%) in the two periods with a damaged TEE probe in use and 6.6% (95% CI 5.0%‐8.9%) in other periods. A marked drop in pneumonia incidence was observed after TEE probe changes, see Figure 2.
Figure 2.
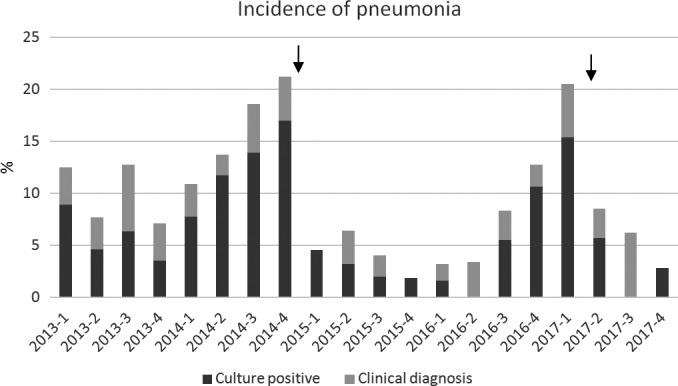
Incidence of pneumonia after cardiac surgery over a 5‐year period. Each column represents one quarter year. Arrows indicate the time of TEE probe changes (November 2014 and April 2017)
Of all 89 patients with pneumonia, 67 (74.1%) were diagnosed while still in the ICU. The median time of diagnosis was on post‐operative day 3 (IQR 2‐4). The majority of patients with pneumonia (61 [68.5%]) had positive microbiological cultures, these came from tracheal aspirates (42), bronchoalveolar lavage (11) and sputum (8). The most commonly isolated pathogens were: K oxytoca (23), P aeruginosa (10), Serratia marcescens (8), E cloacae (8), Klebsiella pneumoniae (7) and E coli (5). Incidence rates for E cloacae, K pneumoniae and E coli were low throughout the study period but K oxytoca and P aeruginosa exhibited peaks in incidence, see Figure 3.
Figure 3.
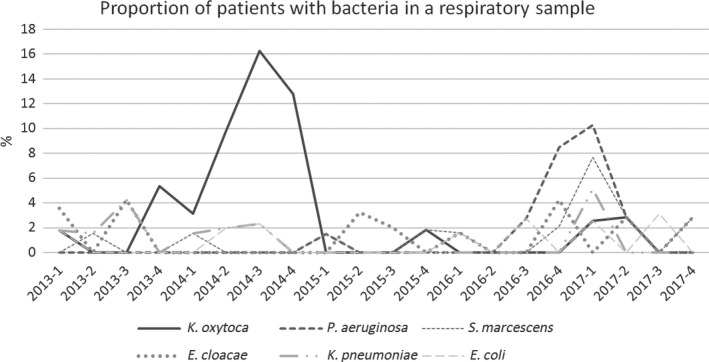
The figure shows the proportion of all cardiac surgery patients with specific bacteria isolated from a post‐operative respiratory sample
3.3. Two outbreaks traced to damaged transesophageal echocardiography probes
An extensively beta‐lactam resistant strain of K oxytoca was found in respiratory samples of 22 patients after intraoperative use of the first contaminated TEE probe. One patient died during the hospital stay but the death was not considered to be related to the infection. With the second probe, a total of 10 patients had P aeruginosa in respiratory samples, of which three also had a bloodstream infection. All three presented with septic shock within 3 days of their surgery and died in hospital. One additional patient with P aeruginosa pneumonia died before hospital discharge. Also with the second probe, two patients developed endocarditis with E faecalis in a biologic valve prosthesis within a 3‐month period, one of whom died. All three bacteria (K oxytoca, P aeruginosa and E faecalis) could be cultured from the TEE probe in use at the respective time (after decontamination).
3.4. Risk factors for and the impact of infections
Several factors were associated with the development of a post‐operative infection, see Table 3.
Table 3.
Presented in the table are factors associated with the development of post‐operative infection in a cohort of 973 cardiac surgery patients
| Risk factors for developing a post‐operative infection—a univariate analysis | ||||
|---|---|---|---|---|
| Variable | Infected (n = 198) | No infection (n = 775) | Unadj. OR (95% CI) | P‐value |
| Duration of procedure | 279 min (SD 121.1) | 233 min (SD 78.7) | 1.005 (1.003‐1.006) | <0.001 |
| Age (y) | 69.8 (SD 10.48) | 65.9 (SD 11.62) | 1.034 (1.018‐1.050) | <0.001 |
| Extracardiac arteriopathya | 18 (9.1%) | 40 (5.2%) | 1.837 (1.029‐3.281) | 0.040 |
| Critical pre‐op conditiona | 20 (10.1%) | 28 (3.6%) | 2.998 (1.651‐5.444) | <0.001 |
| Insulin‐dep. diabetes mellitus | 20 (10.1%) | 31 (4.0%) | 2.697 (1.502‐4.843) | 0.001 |
| NYHA class | ||||
| 0 | 17 (8.6%) | 77 (9.9%) | Reference | |
| I | 13 (6.6%) | 84 (10.8%) | 0.701 (0.320‐1.538) | 0.375 |
| II | 50 (25.3%) | 307 (39.6%) | 0.738 (0.403‐1.350) | 0.324 |
| III | 57 (28.8%) | 181 (23.5%) | 1.426 (0.780‐2.609) | 0.249 |
| IV | 61 (30.8%) | 126 (16.3%) | 2.193 (1.194‐4.027) | 0.011 |
| Angina at rest | 37 (18.7%) | 97 (12.5%) | 1.606 (1.060‐2.435) | 0.026 |
| Ejection fraction | 50.1% (SD 12.20) | 54.1% (SD 10.49) | 0.970 (0.956‐0.984) | <0.001 |
| Pulm. art. pressureb | 42 mm Hg (SD 12.7) | 38 mm Hg (SD 13.4) | 1.024 (1.005‐1.043) | 0.015 |
| Recent MIa | 48 (24.2%) | 130 (16.8%) | 1.588 (1.090‐2.312) | 0.016 |
| Urgency of procedurea | ||||
| Elective | 94 (47.4%) | 417 (53.8%) | Reference | |
| Urgent | 76 (38.4%) | 310 (40.0%) | 1.088 (0.777‐1.522) | 0.625 |
| Emergency | 26 (13.1%) | 42 (5.4%) | 2.746 (1.604‐4.702) | <0.001 |
| Salvage | 2 (1.0%) | 6 (0.8%) | 1.479 (0.294‐7.441) | 0.635 |
| Euroscore II | 2.9 (IQR 1.62‐8.70) | 1.6 (IQR 1.00‐3.10) | 1.096 (1.066‐1.127) | <0.001 |
| Reoperation for bleeding | 21 (10.6%) | 43 (5.5%) | 2.020 (1.169‐3.490) | 0.012 |
| Contaminated TEE periodc | 79 (40.1%) | 249 (32.1%) | 1.402 (1.016‐1.935) | 0.040 |
See definitions under Table 1.
The pulmonary arterial pressure was excluded from the multivariate analysis due to a large number of missing values.
Operation in a time period where a damaged and contaminated TEE probe was in use.
Statistically significant values in bold (P < 0.05)
In a multivariate model, the duration of procedure (adjusted OR 1.006, 95% CI 1.004‐1.008, P < 0.001), age (adj. OR 1.035, 95% CI 1.015‐1.054, P < 0.001), insulin‐dependent diabetes (adj. OR 3.174, 95% CI 1.665‐6.052, P < 0.001), the EuroScore II (adj. OR 1.049, 95% CI 1.016‐1.083, P = 0.03), reoperation for bleeding (adj. OR 1.939, 95% CI 1.053‐3.572, P = 0.034) and operation within a contaminated TEE period (adj OR 1.558, 95% CI 1.078‐2.253, P = 0.018) remained independently associated with the development of an infection.
3.5. Complications and outcome
Thirty‐one patients died within 30 days of the index surgery (3.2%) and further three after that while still in hospital (in‐hospital mortality 3.5%). Of those who died within 30 days, the most common causes of death were: Cardiovascular (13 patients), infectious (12 patients), stroke (two patients) and unknown (four patients). The most frequent complications were new‐onset atrial fibrillation (31.6%), infections (20.3%) and the need for pleurocentesis (10%). Apart from atrial fibrillation and pericardiocentesis, all complications were associated with an increased risk of death on a univariate analysis, see Table 4.
Table 4.
Rates of complications in 973 consecutive cardiac surgical patients in Iceland and their impact on survival in a univariate analysis with 30 d mortality as the dependent factor
| Rates of complications and risk of death after cardiac surgery | ||||
|---|---|---|---|---|
| Complications | n | % | Unadj. OR for 30 d mortality (95% CI) | P‐value |
| New‐onset atrial fibrillation | 307 | 31.6 | 0.888 (0.404‐1.953) | 0.768 |
| Infection (any) | 198 | 20.3 | 4.454 (2.162‐9.176) | <0.0001 |
| Pleurocentesis | 97 | 10.0 | 2.761 (1.157‐6.587) | 0.022 |
| Mech. ventilation >48 h | 83 | 8.5 | 13.930 (6.600‐29.400) | <0.0001 |
| Reoperation for bleeding | 64 | 6.6 | 2.878 (1.067‐7.767) | 0.037 |
| Mechanical assist device[Link] | 49 | 5.0 | 18.176 (8.271‐39.941) | <0.0001 |
| Re‐intubation | 37 | 3.8 | 13.354 (5.640‐31.617) | <0.0001 |
| Pericardiocentesis | 27 | 2.8 | 1.174 (0.154‐8.943) | 0.877 |
| Cerebrovascular accident | 22 | 2.3 | 7.605 (2.410‐23.994) | <0.0001 |
| Renal replacement therapya | 17 | 1.7 | 20.313 (6.960‐59.284) | <0.0001 |
| Reoperation for graft failure | 6 | 0.6 | na | na |
Aorta balloon pump or venoarterial extracorporeal membrane oxygenation (VA‐ECMO) post‐operatively.
For post‐operative acute kidney injury.
Statistically significant values in bold (P < 0.05)
The median length of stay in the ICU was 1 day (IQR 1‐2) and in hospital was 8 days (IQR 7‐12). In patients extubated within 24 hours, extubation time was at a median of 6 hours and 32 minutes post‐surgery (IQR 4:30‐8:52). Re‐admissions to the ICU were 42 (4.3%).
Infected patients had a longer duration of stay in the ICU (median 2 days, IQR 1‐6) than patients without an infection (median 1 day, IQR 1‐1, P < 0.001) and they also had a longer duration of stay in the hospital, median 14 days (IQR 9‐27.8) vs 8 days (IQR 7‐10, P < 0.001). Patients who developed a post‐operative infection had a higher 30‐day mortality rate (8.1%) compared with 1.9% for non‐infected patients (OR 4.454, 95% CI 2.162‐9.176, P < 0.001). The highest 30‐day mortality rates were encountered in patients with bloodstream infections (19%, 95% CI 8.2‐36.7) and pneumonia (17%, 95% CI 10.5‐26.0).
4. DISCUSSION
We report epidemiological data on post‐operative infections and outcomes from a nationwide cohort of cardiac surgery patients. During the study period, there were two large outbreaks of infections associated with damaged and contaminated TEE probes, which had a major effect on our infection rates.
The total infection rate (20.3%) at our institution is higher than has been reported in recent large cardiac surgery cohorts (13.3%7‐14%9). We report a particularly high pneumonia incidence (13.8%) from periods with a damaged TEE probe in use but the incidence in other periods (6.6%) is within the range others have reported (2.4%‐10.7%).3, 6, 9, 13, 14
The rates of other infections, such as surgical site infections (5.7%), bloodstream infections (2.8%), deep sternal infection (1.7%) and symptomatic urinary tract infections (1.4%) are similar to reports from other centres.3, 8, 9 Analysis of our data confirms previously known risk factors for the development of an infection, such as age, duration of procedure and insulin‐dependent diabetes mellitus, but we are the first to report increased infection rates related to which TEE probe was in use at the time of surgery. This is (to the authors knowledge) the largest reported outbreak related to TEE use and the first with several fatalities.
At the height of the K oxytoca outbreak, 16.2% of all cardiac surgery patients had the pathogen in a respiratory tract sample (Figure 3) and 61% of all respiratory tract samples from cardiac patients were positive for K oxytoca (microbiology cultures were only taken on clinical suspicion of an infection). The bacteria therefore seems to have been very efficient at colonising the respiratory tract of exposed patients. Several factors may have aided this. The TEE probe slides past the laryngeal inlet on insertion and is routinely left in place for several hours during surgery. Host defence mechanisms are also compromised due to numerous factors, such as general anesthesia, endotracheal tube, atelectasis, sedative drugs and the stress of a major operation. This efficiency of respiratory tract colonisation and subsequent development of pneumonia suggests that aspiration of secretions from the pharynx or oesophagus into the respiratory tract during surgery and intubation may be more common than is generally appreciated. This risk may be increased when a TEE probe is used concurrently.
The clinical presentation of infections related to TEE use was in most cases an early‐onset pneumonia within 3 days of surgery. Interestingly, no patient with K oxytoca had a bloodstream infection and there were no fatalities directly attributed to the K oxytoca infections. The P aeruginosa seemed to be more virulent, with 3 of 10 patients developing bloodstream infection with septic shock. P aeruginosa has been associated with a higher mortality than other pathogens in previous studies on post‐operative pneumonia.27
The K oxytoca outbreak went unnoticed for some time in our institution. The Department of infection control has an active surveillance system for several pathogens, such as extended spectrum beta‐lactamase (ESBL) producing strains of Enterobacteriaceae, but the K oxytoca did not produce ESBL even though it was multidrug resistant. The patient cohort was cared for post‐operatively in a general mixed ICU where the majority of physicians have short rotations and this may have hindered detection of the outbreak. The discovery of the P aeruginosa outbreak was quicker. Although P aeruginosa is a relatively common pathogen in the ICU, the unusual clinical presentation of septic shock in two post‐operative cardiac patients within a short period led to an alert to infection control.
Our experience with two damaged probes over a relatively short time period suggests that TEE probe damage causing decontamination failure may be more common than is appreciated and that this damage can be difficult to detect. The use of protective sheaths on the TEE probe reduces the risk of cross‐infection in case there is a decontamination failure. However, sheaths can be impractical as some users find they impair ultrasound views of the heart and not all sheaths are robust enough to maintain integrity during a lengthy surgery. We made informal enquiries to centres in both Europe and the United States of America and in most cases protective sheaths were not used on TEE probes during cardiac surgery. In collaboration with the Department of Infection Control, we have enhanced cleaning routines for TEE probes, which now include ultraviolet light decontamination and regular visual examination under magnification and enhanced lighting for signs of damage. The use of probe sheaths is now mandatory at our clinic.
We cannot exclude that the high pneumonia rates we observed may have influenced the rates of other complications, such as the need for pleurocentesis (10%) and mechanical ventilation >48 hours (8.5%). Both were higher than the corresponding rates published in the Swedeheart 2017 Annual report (6.3% and 5.1% respectively).10 The Swedeheart Annual report publishes outcome data annually from around 6000 cardiac operations in Sweden, with patient characteristics and EuroScore II (mean 3.1%‐5.1%) very similar to the cohort described in our study (3.78%). The rates of other complications such as cerebrovascular accidents (2.3%) and renal replacement therapy (1.7%) were similar to those reported in Swedeheart (2.0% and 3.0% respectively), as was the 30‐day mortality, 3.2% vs 1.6%‐3.5% in the Swedeheart cohort.
The strength of this study is the nationwide cohort from a single institution over a 5‐year period. Rather than relying on diagnostic codes, we reviewed the charts of every cardiac patient during the study period. A limitation is the retrospective design and the inherent difficulties in diagnosing infections retrospectively. Especially pneumonia can be difficult to ascertain, given that critically ill cardiac surgery patients invariably have infiltrates on their chest X‐rays and frequently exhibit a systemic inflammatory response syndrome post cardiopulmonary bypass.
5. CONCLUSIONS
Minor surface damage and subsequent contamination of TEE probes were responsible for a surge in pneumonia rates and bloodstream infections with fatal outcomes in cardiac surgery patients in Iceland. This study highlights the importance of TEE probes as a potential risk factor for pneumonia following cardiac surgery and the necessity of adequate procedures for handling, inspection and decontamination of TEE probes.
ETHICS APPROVAL
The study was approved by the National Bioethics Committee of Iceland, the Icelandic Data Protection Authority and the chief medical executive of Landspitali University Hospital. The need for informed consent was waived given the observational nature of the study.
CONSENT FOR PUBLICATION
Not applicable.
AVAILABILITY OF DATA AND MATERIALS
The datasets analysed in the current study are available from the corresponding author on reasonable request.
CONFLICT OF INTEREST
The authors declare no conflicts of interest.
AUTHORS’ CONTRIBUTIONS
E. V. and S. K. designed the study. E. V. and K. O. H. collected patient data. E. V. did statistical analysis. All authors co‐wrote the manuscript.
ACKNOWLEDGEMENT
Gratitude to Gudmundur K. Klemenzson memorial fund for supporting Open Access publication of this article.
Vesteinsdottir E, Helgason KO, Sverrisson KO, Gudlaugsson O, Karason S. Infections and outcomes after cardiac surgery—The impact of outbreaks traced to transesophageal echocardiography probes. Acta Anaesthesiol Scand. 2019;63:871–878. 10.1111/aas.13360
Funding information
Departmental funding only.
REFERENCES
- 1. Rahmanian PB, Kröner A, Langebartels G, Özel O, Wipperman J, Wahlers T. Impact of major non‐cardiac complications on outcome following cardiac surgery procedures: logistic regression analysis in a very recent patient cohort. Interact Cardiovasc Thorac Surg. 2013;17:319‐327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Welsby IJ, Bennet‐Guerrero E, Atwell D, et al. The association of complication type with mortality and prolonged stay after cardiac surgery with cardiopulmonary bypass. Anesth Analg. 2002;94:1072‐1078. [DOI] [PubMed] [Google Scholar]
- 3. Gelijns AC, Moskowitz AJ, Acker MA, et al. Management practices and major infections after cardiac surgery. J Am Coll Cardiol. 2014;64:372‐381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Chen LF, Arduino JM, Sheng S, et al. Epidemiology and outcome of major postoperative infections following cardiac surgery: risk factors and impact of pathogen type. Am J Infect Control. 2012;40:963‐968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Fowler VG, O’Brien SM, Muhlbaier LH, Corey GR, Ferguson TB, Peterson ED. Clinical predictors of major infections after cardiac surgery. Circulation. 2005;112(Suppl I):I358‐I365. [DOI] [PubMed] [Google Scholar]
- 6. Cotogni P, Barbero C, Passera R, Fossati L, Olivero G, Rinaldi M. Violation of prophylactic vancomycin administration timing is a potential risk factor for rate of surgical site infections in cardiac surgery patients: a prospective cohort study. BMC Cardiovasc Disord. 2017;17:73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. McClure GR, Belley EP, Harlock J, et al. Steroids in cardiac surgery trial: a substudy of surgical site infections. Can J Anesth. 66:182‐192. Ahead of print. [DOI] [PubMed] [Google Scholar]
- 8. Hortal J, Muňos P, Cuerpo G, Litvan H, Rosseel PM, Bouza E. Ventilator‐associated pneumonia in patients undergoing major heart surgery: an incidence study in Europe. Crit Care. 2009;13:R80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Mocanu V, Buth KJ, Johnston LB, Davis I, Hirsch GM, Légaré J‐F. The importance of continued quality improvement efforts in monitoring hospital‐aquired infection rates: a cardiac surgery experience. Ann Thorac Surg. 2015;99:2061‐2069. [DOI] [PubMed] [Google Scholar]
- 10. Swedeheart Annual Report. 2017. http://www.ucr.uu.se/swedeheart/arsrapport-2017/swedeheart-annual-report-2017. Accessed November 27, 2018.
- 11. Alasmari FA, Tleyjeh IM, Riaz M, et al. Temporal trends in the incidence of surgical site infections in patients undergoing coronary artery bypass graft surgery: a population‐based cohort study, 1993 to 2008. Mayo Clin Proc. 2012;87:1054‐1061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Perrault LP, Kirkwood KA, Chang HL, et al. A prospective multi‐institutional cohort study of mediastinal infections after cardiac operations. Ann Thorac Surg. 2018;105:461‐468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Ailawadi G, Chang HL, O'Gara PT, et al. Pneumonia after cardiac surgery: experience of the National Institutes of Health/Canadian Institutes of Health Research Cardiothoracic Surgical Trials Network. J Thorac Cardiovasc Surg. 2017;153:1384‐1391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Allou N, Bronchard R, Guglielminotti J, et al. Risk factors for postoperative pneumonia after cardiac surgery and development of a preoperative risk score. Crit Care Med. 2014;42:1150‐1156. [DOI] [PubMed] [Google Scholar]
- 15. Leal‐Noval R, Marquez‐Vcaro JA, Garca‐Curiel A, et al. Nosocomial pneumonia in patients undergoing heart surgery. Crit Care Med. 2000;4:935‐940. [DOI] [PubMed] [Google Scholar]
- 16. Likosky DS, Wallace AS, Prager RL, et al. Sources of variation en hospital‐level infection rates after coronary artery bypass grafting: an analysis of the Society of Thoracic Surgeons Adult Heart Surgery Database. Ann Thorac Surg. 2015;100:1570‐1576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. El Solh AA, Bhora M, Pineda L, Dhillon R. Nosocomial pneumonia in elderly patients following cardiac surgery. Respir Med. 2006;100:729‐736. [DOI] [PubMed] [Google Scholar]
- 18. Hortal J, Giannella M, Pérez MJ, et al. Incidence and risk factors for ventilator associated pneumonia after major heart surgery. Intensive Care Med. 2009;35:1518‐1525. [DOI] [PubMed] [Google Scholar]
- 19. Kollef MH, Sharpless L, Vlasnik J, Pasque C, Murphy D, Fraser VJ. The impact of nosocomial infections on patient outcomes following cardiac surgery. Chest. 1997;112:666‐675. [DOI] [PubMed] [Google Scholar]
- 20. Bouza E, Pérez A, Muňos P, et al. Ventilator‐associated pneumonia after heart surgery: a prospective analysis and the value of surveillance. Crit Care Med. 2003;31:1964‐1970. [DOI] [PubMed] [Google Scholar]
- 21. Judge O, Fuhai Ji, Fleming N, Liu H. Current use of the pulmonary artery catheter in cardiac surgery: a survey study. J Cardiothorac Vasc Anesth. 2015;29:69‐75. [DOI] [PubMed] [Google Scholar]
- 22. Kanemitsu K, Endo S, Oda K, et al. An increased incidence of Enterobacter cloacae in a cardiovascular ward. J Hosp Infect. 2007;66:130‐134. [DOI] [PubMed] [Google Scholar]
- 23. Seki M, Machida N, Yamagishi Y, Yoshida H, Tomono K. Nosocomial outbreak of multidrug‐resistant Pseudomonas aeruginosa caused by a damaged transesophageal echocardiogram probe used in cardiovascular surgical operations. J Infect Chemother. 2013;19:677‐681. [DOI] [PubMed] [Google Scholar]
- 24. Bancroft EA, English LT, Terashita D, Yasuda L. Outbreak of Escherichia coli infections associated with a contaminated transesophageal echocardiography probe. Infect Control Hosp Epidemiol. 2013;34:1121‐1122. [DOI] [PubMed] [Google Scholar]
- 25. Nashef S, Roques F, Sharples LD, et al. EuroSCORE II. Eur J Cardiothorac Surg. 2012;41:734‐745. [DOI] [PubMed] [Google Scholar]
- 26. European Center for Disease Prevention and Control . Point prevalence survey of healthcare‐associated infections and antimicrobial use in European acute care hospitals. Protocol version 5.3, ECPC PPS 2016–2017. https://ecdc.europa.eu/sites/portal/files/media/en/publications/Publications/PPS-HAI-antimicrobial-use-EU-acute-care-hospitals-V5-3.pdf. Accessed November 19, 2018.
- 27. Allou N, Kermarrec N, Muller C, et al. Risk factors and prognosis of post‐operative pneumonia due to Pseudomonas aeruginosa following cardiac surgery. J Antimicrob Chemother. 2010;65:806‐807. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets analysed in the current study are available from the corresponding author on reasonable request.


