Abstract
Background
Conjunctivitis is common in patients with atopic dermatitis (AD) in general and a commonly reported adverse event in AD clinical trials with dupilumab.
Objective
To survey opinions and experience about conjunctivitis occurring in AD, including those during dupilumab treatment in a group of AD experts from the International Eczema Council (IEC).
Methods
Electronic survey and in‐person discussion of management strategies.
Results
Forty‐six (53.5%) IEC members from 19 countries responded to the survey. Consensus was reached for several statements regarding diagnostic workup, referral and treatment. IEC members suggest that patients with AD should (i) routinely be asked about ocular complaints or symptoms, (ii) obtain information about the potential for conjunctivitis before starting dupilumab therapy and (iii) if indicated, be treated with dupilumab despite previous or current conjunctivitis. In cases of new‐onset conjunctivitis, there was consensus that dupilumab treatment should be continued when possible, with appropriate referral to an ophthalmologist.
Limitations
The study relies on expert opinion from dermatologists. Responses from few dermatologists without dupilumab access were not excluded from the survey.
Conclusion
The IEC recommends that dermatologists address conjunctivitis in patients with AD, especially during treatment with dupilumab.
Introduction
Atopic dermatitis (AD) is a chronic inflammatory skin condition.1 Patients with AD have an increased risk of developing keratoconus, cataract, glaucoma and blepharitis, as well as allergic, atopic, vernal and infectious (kerato) conjunctivitis.2, 3
Clinical trials with dupilumab, a monoclonal antibody that inhibits interleukin (IL)‐4 and IL‐13 signalling, have demonstrated efficacy in moderate‐to‐severe AD. However, trials have also shown an increased incidence of conjunctivitis with dupilumab treatment,4, 5, 6 which in some cases leads to cessation of treatment.7 The clinical features of dupilumab‐associated conjunctivitis include bilateral inflammation of the anterior conjunctiva and hyperaemia of the limbus, which may cause nodular swelling.8 In a case series, dupilumab‐associated conjunctivitis appeared to occur more often in patients with severe AD at baseline and with atopic comorbidities. At present, however, predictive factors are unknown.7
There is no literature‐based guidance available about management of dupilumab‐associated conjunctivitis. A group of AD experts, all councillors or associates of the International Eczema Council (IEC), therefore addressed this issue by completing a survey and discussing conjunctivitis and its management in patients with AD.
Materials and methods
The IEC consists of 86 AD experts from 22 countries (http://www.eczemacouncil.org/). In May 2018, a survey developed by three of the authors (J.P.T, M.S.d.B.W. and A.W.) was sent electronically to IEC councillors and associates to examine their opinions regarding management of conjunctivitis in patients with AD focusing on dupilumab‐associated conjunctivitis. The survey consisted of questions and statements followed by possible responses of ‘strongly disagree’, ‘disagree’, ‘neither agree, nor disagree’, ‘agree’ and ‘strongly agree’ as well as closed response categories (for detailed questions, Table 1). All responses were anonymous. No a priori definition of consensus was determined but, post hoc, and it was decided to use rules similar to the Harmonizing Outcome Measures for Eczema (HOME) initiative, which have been used in previous IEC consensus articles.9, 10 Consensus required that less than 30% of the voters disagreed (i.e. no more than 30% had to mark the following responses ‘strongly disagreed’ or ‘disagreed’) for each question with a response rate of at least 90% of respondents in the survey. Descriptive data analysis was performed using Excel (Windows). On 13 September 2018, a round table discussion among IEC members was held on the topic at the European Academy of Dermatology and Venereology meeting in Paris, France. In the remainder of this manuscript, IEC members who completed the survey are respondents; IEC members who participated in the discussion participants.
Table 1.
Overview of responses given by IEC members in a survey about conjunctivitis. Statements that reached consensus are highlighted with grey. Consensus required that less than 30% of the voters disagreed (i.e. no more than 30% had to mark the following responses ‘strongly disagreed’ or ‘disagreed’) for each question with a response rate of at least 90% of the respondents
| Survey Questions | Strongly agree | Agree | Neither agree nor disagree | Disagree | Strongly disagree | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| % | n | % | n | % | n | % | n | % | n | |
| Atopic dermatitis patients should routinely be asked about ocular complaints or symptoms | 55.81 | 24 | 32.56 | 14 | 9.30 | 4 | 2.33 | 1 | 0.00 | 0 |
| I have observed corneal transplant in one or several of my atopic dermatitis patients where I assume it was due to excessive rubbing (both children and adults) | 15.91 | 7 | 34.09 | 15 | 15.91 | 7 | 22.73 | 10 | 11.36 | 5 |
| Atopic dermatitis patients with conjunctivitis should routinely be referred to an ophthalmologist for diagnostic work‐up and treatment | 23.26 | 10 | 53.49 | 23 | 9.30 | 4 | 13.95 | 6 | 0.00 | 0 |
| Dermatologists should initiate relevant treatment with eye drops/eye ointment or oral antihistamines for conjunctivitis themselves before referring to an ophthalmologist | 6.82 | 3 | 40.91 | 18 | 31.82 | 14 | 18.18 | 8 | 2.27 | 1 |
| Atopic dermatitis patients with conjunctivitis should undergo (or be referred to) skin prick testing (or specific IgE) for common aeroallergens | 25.58 | 11 | 25.58 | 11 | 30.23 | 13 | 16.28 | 7 | 2.33 | 1 |
| Atopic dermatitis patients with conjunctivitis should undergo (or be referred to) skin prick testing to eyedrops if such are used | 9.09 | 4 | 25.00 | 11 | 25.00 | 11 | 38.64 | 17 | 2.27 | 1 |
| Atopic dermatitis patients with conjunctivitis should undergo (or be referred to) patch testing with a standardized ophthalmologic series, and native eye drops/ointments to diagnose possible delayed type hypersensitivity reactions to topical ingredients if these are used | 9.30 | 4 | 41.86 | 18 | 23.26 | 10 | 25.58 | 11 | 0.00 | 0 |
| Dermatologists should leave the indication to use of ciclosporin or tacrolimus eye drops for conjunctivitis to ophthalmologists | 18.18 | 8 | 36.36 | 16 | 25.00 | 11 | 18.18 | 8 | 2.27 | 1 |
| Dermatologists should leave the indication to use of corticosteroid eye drops for conjunctivitis to ophthalmologists | 15.91 | 7 | 38.64 | 17 | 18.18 | 8 | 25.00 | 11 | 2.27 | 1 |
| It is important to routinely inform about possible conjunctivitis in your atopic dermatitis patients before prescribing dupilumab (Dupixent) | 59.09 | 26 | 31.82 | 14 | 6.82 | 3 | 2.27 | 1 | 0.00 | 0 |
| Patients with atopic dermatitis should be referred to an ophthalmologist before initiation of dupilumab therapy | 4.88 | 2 | 14.63 | 6 | 14.63 | 6 | 56.10 | 23 | 9.76 | 4 |
| The risk of conjunctivitis when using dupilumab may prevent me from using the drug in patients with previous (kerato‐) conjunctivitis? | 4.55 | 2 | 13.64 | 6 | 22.73 | 10 | 47.73 | 21 | 11.36 | 5 |
| The risk of conjunctivitis when using dupilumab may prevent me from using the drug in patients with current (kerato‐) conjunctivitis? | 4.65 | 2 | 41.86 | 18 | 25.58 | 11 | 23.26 | 10 | 4.65 | 2 |
| Patients with new onset conjunctivitis during dupilumab treatment should always be referred to an ophthalmologist | 20.45 | 9 | 47.73 | 21 | 20.45 | 9 | 11.36 | 5 | 0.00 | 0 |
| Patients with new onset conjunctivitis during dupilumab treatment should be referred to an ophthalmologist in more severe cases, (inadequate response to artificial tears and/or antihistamine eye drops) | 50.00 | 22 | 34.09 | 15 | 6.82 | 3 | 9.09 | 4 | 0.00 | 0 |
| New onset conjunctivitis during dupilumab should result in referral of the patient to an ophthalmologist but treatment should be continued | 11.36 | 5 | 56.82 | 25 | 25.00 | 11 | 6.82 | 3 | 0.00 | 0 |
| New onset conjunctivitis during dupilumab should result in referral of the patient to an ophthalmologist but treatment should be paused | 4.55 | 2 | 4.55 | 2 | 31.82 | 14 | 52.27 | 23 | 6.82 | 3 |
Results
Survey results
The following sections highlight key findings from the survey. Question and response details are shown in Table 1.
Respondent characteristics
A total of 46 councillors and associates from six continents responded to the survey (participation rate 53.5%). Respondents came from Germany (n = 8), the United States (n = 7), the Netherlands (n = 4), United Kingdom (n = 4), Denmark (n = 3), France (n = 3), Korea (n = 2), Japan (n = 2), Australia (n = 2) and Canada (n = 2), as well as, Brazil, China, India, Ireland, Israel, Italy, Switzerland, Taiwan and Tanzania (each n = 1).
A total of 38% and 22% of respondents reported seeing 0–20 paediatric and adult patients with AD per week, respectively, whereas 27% and 31% of respondents reported seeing 21–40 paediatric and adults patients with AD per week, respectively. The proportion of respondents that reported seeing 41–100 paediatric and adult patients per week was 31% and 33%, respectively. Remaining respondents reported seeing more than 100 paediatric and adult patients with AD per week.
Dermatologist‐estimated frequency of ocular surface disease and diagnostic workup
Respondents reported relatively frequent occurrence of conjunctivitis and blepharitis in their patients with AD (Fig. 1a and b). As an indication of disease severity, half of the respondents reported having seen a paediatric or adult AD patient who required corneal transplant. Consensus was reached that patients with AD routinely should be asked about ocular complaints or symptoms, and in case of conjunctivitis, routinely referred to an ophthalmologist for diagnostic workup and treatment. Consensus was reached that AD patients with conjunctivitis should undergo (or be referred to) skin prick or specific IgE testing for common aeroallergen and be referred for patch testing with an ophthalmological series and native eye drops and ointments to detect possible delayed‐type hypersensitivity to topical ingredients.
Figure 1.
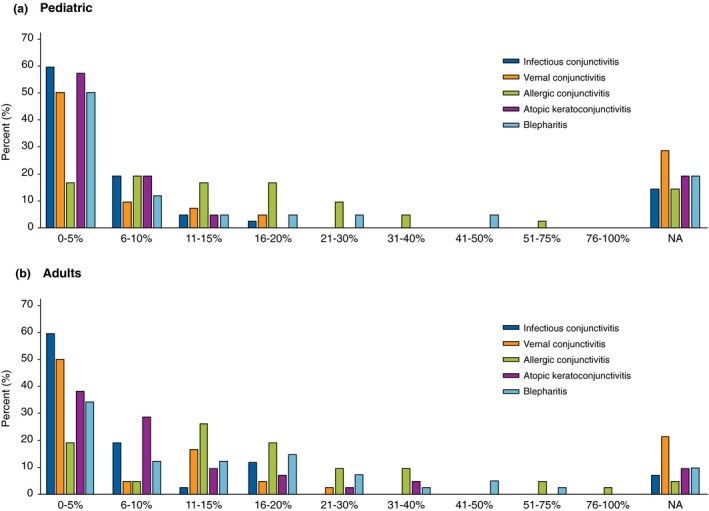
Proportion of IEC member who report having seen pediatric (a) and adult (b) patients with AD and who have been diagnosed with conjunctivitis and blepharitis within the 7 past 12 months.
Treatment
Consensus was reached that dermatologists should initiate relevant treatment with eye drops/ointment, or oral antihistamines, for conjunctivitis before referring to an ophthalmologist, but also dermatologists should leave the indication to use of corticosteroid, ciclosporin or tacrolimus eye drops for conjunctivitis to ophthalmologists. Consensus was reached that patients with AD should be informed about possible conjunctivitis before prescribing dupilumab. There was also consensus that previous, (kerato) conjunctivitis in patients with AD should not prevent the use of dupilumab (Fig. 2; for discussion on dupilumab treatment initiation in patients with current conjunctivitis, see detailed discussion below). There was consensus that patients with new‐onset conjunctivitis during dupilumab treatment should always be referred to an ophthalmologist, but also that dupilumab treatment should be continued while awaiting ophthalmologist consultation.
Figure 2.
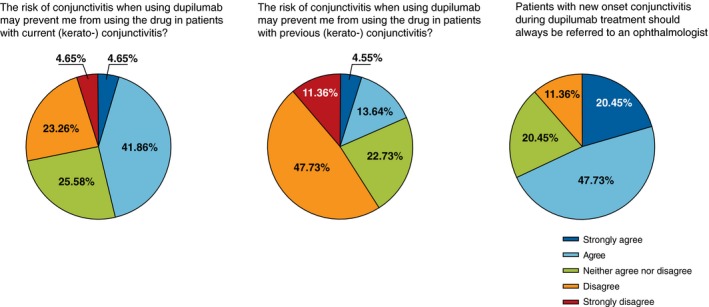
Key findings from the survey regarding management of AD patients with conjunctivitis treated with dupilumab.
Round table discussion
The survey results were presented at the meeting in Paris where 20 councillors and associates were present. Most of the discussion concerned dupilumab‐associated conjunctivitis, which most IEC members had seen and treated in their adult patients with AD. Dupilumab‐associated conjunctivitis had also been seen in paediatric patients treated in clinical trials with dupilumab. It was agreed that secondary analyses of completed clinical trials with dupilumab and ongoing postmarketing registries are urgently needed to identify sub‐groups with increased risk of conjunctivitis after dupilumab administration. Better understanding of the aetiopathogenesis of dupilumab‐associated conjunctivitis to identify susceptible patients and future treatments was emphasized. Although conjunctivitis has been associated with the experimental use of IL‐13 inhibitors for AD, the numbers in trials to date are small and discussants warned about the possibility of similar ocular complications in AD patients treated with IL‐13 inhibitors.
It was the experience of IEC members that many AD patients with dupilumab‐associated conjunctivitis clear their ocular symptoms, some spontaneously and some after a short course of treatment with lubricating eye drops and ointments containing corticosteroids or tacrolimus. Several IEC members had good experiences with the use of fluorometholone 0.1% eye drops or tacrolimus 0.03% ointment.8 Corticosteroid eye drops should be used with caution, since they increase the risk of superinfection and glaucoma. Nevertheless, few patients stop dupilumab treatment due to conjunctivitis and conjunctivitis may improve dramatically during dupilumab use. These varied observations regarding course further stress the need to study these patients and find correlates with outcome. Some IEC members recommended artificial lubricating tears when starting a patient on dupilumab as prevention to reduce ocular complaints and the risk of conjunctivitis but, while there is a scientific rationale for this intervention, more work is needed to develop optimal regimens and assess its efficacy. A strategy pursued by some IEC members is to taper dupilumab injections e.g. to every 3rd week, but potential correlation with extending the dosing interval and increasing the risk of developing anti‐drug antibodies against dupilumab was stressed.
The clinical course and prognosis of already existing conjunctivitis following initiation of dupilumab is difficult to predict. In the experience of IEC councillors, some patients with AD may experience worsening of ocular symptoms due to unrecognized effects of dupilumab treatment, some may experience stable and unchanged conjunctivitis, and some may experience improvement and even complete resolution of ocular symptoms. Patients should be informed about the potential of conjunctivitis with dupilumab and should be referred to an ophthalmological when clinically indicated. It was stressed that at this moment there are also no reliable data on the course of atopic keratoconjunctivitis and vernal keratoconjunctivitis during dupilumab treatment. Patient experiencing these should be carefully monitored by an ophthalmologist before and during treatment with dupilumab. The AD severity, conjunctivitis severity, possible contraindications, possible effect of dupilumab therapy on concomitant asthma or other comorbidities, as well as other treatment options, should be considered on an individual patient basis.
Discussion
This survey of IEC councillors and associates, representing 19 countries from six continents, examined opinions regarding proper management of paediatric and adult patients with AD with conjunctivitis, and in particular, conjunctivitis occurring during dupilumab treatment. Participants in this study care for many patients with AD weekly.
Few studies have examined the exact prevalence and incidence of conjunctivitis in patients with AD. In young children from a U.S. trial, the prevalence of allergic conjunctivitis was 16%,11 whereas chronic conjunctivitis and superficial keratitis were observed in 32% and 7% of 44 adult patients with AD when examined ophthalmologically.12 In Danish adults, 12% and 18% of adults with mild and severe AD, respectively, had used anti‐inflammatory ocular agents, and 13 of 1000 severe patients with AD per year had used an ocular anti‐inflammatory agent compared with 6 of 1000 individuals (sex‐ and age‐adjusted) from the general population.3 These data emphasize that conjunctivitis is more common in patients with AD than controls and, accordingly, the IEC reached consensus and recommended that patients with AD routinely should be asked about signs and symptoms of conjunctivitis. Recommendations about eye examination for conjunctivitis in AD, as well as diagnostic workup, referral and proper management, have not been included in recent European and North American guidelines for AD.1, 13, 14, 15, 16, 17, 18
The estimated prevalence of ocular surface disease provided by the IEC members should be interpreted with caution because of the risk of recall bias and the skewed population towards more severe disease seen by IEC members. Moreover, there was no information about the severity or accuracy of diagnosis of the conjunctivitis (e.g. if ophthalmologist‐confirmed). Nonetheless, IEC members experienced that adults with AD frequently suffer from conjunctivitis and blepharitis. It is currently unclear whether blepharitis should be regarded as part of AD or, instead, occurs as a result of co‐pathogenic factors and is a complication or comorbidity. Importantly, other aetiologies of blepharitis, e.g. allergic contact dermatitis, or infestation with demodex mites, should also be considered in patients with AD. Sometimes, aetiological classification of conjunctivitis is straightforward, e.g. onset of allergic rhinoconjunctivitis during pollen season in AD patients with known allergic disease and positive response to antihistamines, or purulent infectious conjunctivitis in conjunction with impetiginized AD, a positive culture, and a favourable response to antibiotics. At other times, it can be clinically difficult to categorize conjunctivitis and, in this case, more extensive diagnostic workup is required. There was consensus to recommend pursuit of a diagnostic workup for type 1 and 4 allergy in AD patients with conjunctivitis. Notably, in severe conjunctivitis cases, it is important that an ophthalmologist rule out atopic keratoconjunctivitis, which may lead to keratitis and blindness.2, 19
There was consensus that dermatologists should prescribe lubricating eye drops, ointments or oral antihistamines in AD patients with conjunctivitis, but also that dermatologists should leave the use of corticosteroid, ciclosporin or tacrolimus eye drops to ophthalmologists. A general concern with corticosteroid eye drop use, besides the risk of superinfection, is induction of increased ocular pressure, which may result in glaucoma. The price of commercially available ciclosporin eyedrop treatment is relatively high and may limit its use, but ciclosporin eye drops are generally well tolerated and are effective for vernal and atopic keratoconjunctivitis.20 Compounding of ciclosporin eyedrops in a pharmacy may be an alternative, if available. The use of tacrolimus eye drops (and tacrolimus as ointment) has also proven favourable with no systemic side‐effects in a large trial; however, incident herpetic and bacterial infections can occur, warranting ophthalmological monitoring.21 Despite the more limited experience with eye drops by dermatologists, rapid access to ophthalmological service may be difficult, sometimes warranting a short course of corticosteroid eye drop without ophthalmological consultations. However, persistent or recurrent conjunctivitis requiring repeated or prolonged use of corticosteroid, tacrolimus and ciclosporin containing eye drops, must be managed by an ophthalmologist, given the risk of glaucoma, cataract and infections.
Conjunctivitis occurring during dupilumab treatment is observed in 9–28% of every 2‐week dupilumab users in clinical trials4, 5, 6 and in real‐world evidence from US, Dutch and French registries (oral presentations, EADV, Paris, September, 2018).7 Observations in other clinical trials may aid in understanding the pathogenesis of conjunctivitis occurring during dupilumab treatment. For example, the risk of conjunctivitis in patients with asthma receiving treatment with dupilumab is similar to that of controls,22 raising the question of ocular or immune differences between patients with AD and asthma. Furthermore, larger trials of IL‐13 inhibitors may indicate the role of IL‐13 inhibition directly vs. IL‐4 inhibition or the combination through IL4Ra targeting; in small phase II trials, an increased incidence of conjunctivitis was observed with lebrikizumab, but not tralokinumab.23, 24 One possible mechanism of conjunctivitis occurring during dupilumab treatment involves the observed early increases in blood total eosinophil counts, since eosinophil chemotactic and activating peptides, eotaxin 1 and 2, are increased in the mucus and tears of patients with vernal and atopic keratoconjunctivitis.25 Other hypotheses include a pathogenic role of increased OX40 ligand activity,26 or IL‐17 driven inflammation following colonization with demodex mites.27 Notably, a recent histopathology case study showed marked depletion of goblet cells in conjunctivae of patients with dupilumab‐associated conjunctivitis.28
The limitations of our study include the following: 1) reliance on expert dermatologist opinion; 2) while discussion participants were all dermatologists experienced with dupilumab use (and managing dupilumab‐associated conjunctivitis) from clinical trials and dermatology office use; number of respondents was relatively small; and survey respondents included few councillors from countries lacking access to dupilumab.
Conclusion
This small IEC member survey and round table discussion provided guidance from a group of AD experts regarding general diagnostic workup and management of conjunctivitis occurring during dupilumab treatment. We achieved consensus for several statements regarding management of conjunctivitis in patients with AD, also before and during dupilumab therapy, but emphasize that correct classification and treatment of conjunctivitis is important and that the threshold of contacting ophthalmologists should be low. Topical ophthalmological therapies recommended by IEC members have proved successful and have allowed dupilumab treatment continuation, but consideration of alternative diagnoses that require further assessment and/or initiation of treatment other than topical anti‐inflammatory medication is critical before initiation of therapy29; these management decisions may be beyond the ability and comfort of a dermatologist or other dupilumab prescriber and may require joint management with an ophthalmologist. Future studies on the aetiopathogenesis, predictive factors and approach to conjunctivitis occurring during dupilumab treatment are warranted.
Funding sources
No funding was obtained for this study.
Conflict of interest
Dr. Thyssen has attended advisory boards for Roche, Eli Lilly and Sanofi Genzyme and received speaker honorarium from LEO Pharma and Sanofi Genzyme. Dr. de Bruin‐Weller M has been a principal investigator, advisory board member and consultant for Regeneron Pharmaceuticals, Inc. and Sanofi Genzyme and a principal investigator, advisory board member for AbbVie. Dr. Paller has been a principal investigator for AbbVie, Anaptysbio, Eli Lilly, Galderma, Incyte, Leo, Janssen, Novartis and Regeneron, and a consultant for AbbVie, Amgen, Asana, Dermavant, Dermira, Galderma, Eli Lilly, Forte, Leo, MatriSys, Menlo, MorphoSys/Galapagos, Novartis, Pfizer, Regeneron and Sanofi. Dr. Leshem has been a principal investigator, consultant and/or advisory board member for and/or received honoraria from AbbVie Inc., Sanofi and Regeneron Pharmaceuticals Inc., Pfizer, Dexcel Pharma, Genentech and Eli Lilly. Dr Vestergaard has been a investigator, speaker or consultant for Novartis, AbbVie, Sanofi, Leo Pharma and Eli Lilly. Dr. Deleuran has been a principal investigator, speaker, advisory board member and/or consultant for LEO Pharma, Lilly, Novartis, Pfizer, Regeneron Pharmaceuticals, Inc., Sanofi Genzyme, MorphoSys‐Galapagos, Pierre Fabre and MEDA. Dr. Drucker has served as a investigator and has received research funding from Sanofi and Regeneron and has been a consultant for Sanofi, RTI Health Solutions, Eczema Society of Canada and Canadian Agency for Drugs and Technology in Health. He has received honoraria from Prime Inc, Spire Learning, CME Outfitters and Eczema Society of Canada. His institution has received educational grants from Sanofi. Dr. Fölster‐Holst has been a investigator, speaker or consultant for Astellas, Almirall Hermal, Beiersdorf, Johnson & Johnson, La Roche‐Posay, LEO Pharma, Neubourg GmbH, Novartis, Pierre Fabre, Procter & Gamble and Regeneron Pharmaceuticals. Dr Traidl‐Hoffmann has been a investigator, speaker or consultant for Novartis, Lilly, Regeneron Pharmaceuticals, Sanofi Genzyme and Sebapharma. Dr. Kilian Eyerich has been a principal investigator, advisory board member or consultant for AbbVie, Almirall, Berlin‐Chemie, Hexal, Janssen, Leo, Lilly, Novartis and Sanofi. Dr Alain Taïeb has been a consultant or investigator for Pierre Fabre, Galderma, Novartis, Johnson and Johnson and Sanofi. Dr Su has been a principal investigator, consultant or advisory board member for Amgen, AbbVie, Eli Lilly, Janssen, Meda, Novartis, Pfizer, Pierre Fabre and Sanofi. Dr. Bieber has been a principal investigator, advisory board member or consultant for Regeneron, Sanofi, GSK, Celgene, AbbVie, AnaptysBio, MedImmune, Chugai, Pierre Fabre, Novartis, Asana Biosciences, LEO, Galapagos/MorphoSys, BioVersys, Galderma, Kymab, Glenmark, Astellas, Daiichi‐Sankyo, Lilly, Pfizer, MenloTx, Dermavant and Almirall. Dr Michael J. Cork is a investigator and consultant for Regeneron, Sanofi Genzyme, Pfizer, Leo, Galapagos, Novartis, Boots, L'Oreal, Dermavant, Menlo, Reckitt Benckiser, Oxagen, Johnson & Johnson, Hyphens, Astellas, AbbVie, Galderma and Procter & Gamble. Dr. Eichenfield has been a principal investigator, consultant or advisory board member for Amgen, Dermavant, Dermira, Eli Lilly, Forte, Galderma, Leo, MatriSys, Menlo, MorphoSys, Novartis, Ortho Dermatologics, Pfizer, Pierre Fabre and Regeneron/Sanofi. Dr Guttman‐Yassky is an employee of Mount Sinai and has received research funds (grants paid to the institution) from AbbVie, Celgene, Eli Lilly, Janssen, MedImmune/Astra Zeneca, Novartis, Pfizer, Regeneron, Vitae, Glenmark, Galderma, Asana, Innovaderm, Dermira, UCB, Kiniksa and Novan. EGY is also a consultant for Sanofi Aventis, Regeneron, Stiefel/GlaxoSmithKline, MedImmune, Celgene, AnaptysBio, Dermira, Galderma, Glenmark, Novartis, Pfizer, Vitae, Leo Pharma, AbbVie, Eli Lilly, Kyowa Kirin, Mitsubishi Tanabe, Asana Biosciences, Promius, Kiniksa, Novan and Evelo biosciences. Dr. Wollenberg has been a principal investigator, advisory board member or consultant for Galderma, LEO Pharma, Lilly, MedImmune, Novartis, Pfizer, Regeneron Pharmaceuticals, Inc. and Sanofi Genzyme, and received speaker honoraria from Chugai, Galderma, LEO Pharma, MedImmune, Pfizer, Regeneron Pharmaceuticals, Inc. and Sanofi Genzyme. Corporate sponsorship was provided to the International Eczema Council by AbbVie, Asana, Celgene, Chugai, Dermavant, Dermira, Eli Lilly, Galderma, Leo Pharma, Novartis, Pfizer, Pierre Fabre, Sanofi, Genzyme and Regeneron Pharmaceuticals, Sienna and Valeant. This research was performed independently through the authors’ academic university and hospital affiliations.
References
- 1. Wollenberg A, Oranje A, Deleuran M et al ETFAD/EADV Eczema task force 2015 position paper on diagnosis and treatment of atopic dermatitis in adult and paediatric patients. J Eur Acad Dermatol Venereol 2016; 30: 729–747. [DOI] [PubMed] [Google Scholar]
- 2. Chen JJ, Applebaum DS, Sun GS, Pflugfelder SC. Atopic keratoconjunctivitis: a review. J Am Acad Dermatol 2014; 70: 569–575. [DOI] [PubMed] [Google Scholar]
- 3. Thyssen JP, Toft PB, Halling‐Overgaard AS, Gislason GH, Skov L, Egeberg A. Incidence, prevalence, and risk of selected ocular disease in adults with atopic dermatitis. J Am Acad Dermatol 2017; 77: 280–286. [DOI] [PubMed] [Google Scholar]
- 4. Blauvelt A, de Bruin‐Weller M, Gooderham M et al Long‐term management of moderate‐to‐severe atopic dermatitis with dupilumab and concomitant topical corticosteroids (LIBERTY AD CHRONOS): a 1‐year, randomised, double‐blinded, placebo‐controlled, phase 3 trial. Lancet 2017; 389: 2287–2303. [DOI] [PubMed] [Google Scholar]
- 5. de Bruin‐Weller M, Thaci D, Smith CH et al Dupilumab with concomitant topical corticosteroid treatment in adults with atopic dermatitis with an inadequate response or intolerance to ciclosporin A or when this treatment is medically inadvisable: a placebo‐controlled, randomized phase III clinical trial (LIBERTY AD CAFE). Br J Dermatol 2018; 178: 1083–1101. [DOI] [PubMed] [Google Scholar]
- 6. Simpson EL, Bieber T, Guttman‐Yassky E et al Two phase 3 trials of dupilumab versus placebo in atopic dermatitis. N Engl J Med 2016; 375: 2335–2348. [DOI] [PubMed] [Google Scholar]
- 7. Treister AD, Kraff‐Cooper C, Lio PA. Risk factors for dupilumab‐associated conjunctivitis in patients with atopic dermatitis. JAMA Dermatol 2019. 10.1001/jamadermatol.2019.0209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Wollenberg A, Ariens L, Thurau S, van LC, Seegraber M, de Bruin‐Weller M. Conjunctivitis occurring in atopic dermatitis patients treated with dupilumab‐clinical characteristics and treatment. J Allergy Clin Immunol Pract 2018; 6: 1778–1780. [DOI] [PubMed] [Google Scholar]
- 9. Drucker AM, Eyerich K, de Bruin‐Weller MS et al Use of systemic corticosteroids for atopic dermatitis: International Eczema Council consensus statement. Br J Dermatol 2018; 178: 768–775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Silverberg JI, Thyssen JP, Paller AS et al What's in a name? Atopic dermatitis or atopic eczema, but not eczema alone. Allergy 2017; 72: 2026–2030. [DOI] [PubMed] [Google Scholar]
- 11. Schneider L, Hanifin J, Boguniewicz M et al Study of the atopic march: development of atopic comorbidities. Pediatr Dermatol 2016; 33: 388–398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Amemiya T, Matsuda H, Uehara M. Ocular findings in atopic dermatitis with special reference to the clinical features of atopic cataract. Ophthalmologica 1980; 180: 129–132. [DOI] [PubMed] [Google Scholar]
- 13. Eichenfield LF, Tom WL, Berger TG et al Guidelines of care for the management of atopic dermatitis: section 2. Management and treatment of atopic dermatitis with topical therapies. J Am Acad Dermatol 2014; 71: 116–132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Eichenfield LF, Tom WL, Chamlin SL et al Guidelines of care for the management of atopic dermatitis: section 1. Diagnosis and assessment of atopic dermatitis. J Am Acad Dermatol 2014; 70: 338–351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Sidbury R, Davis DM, Cohen DE et al Guidelines of care for the management of atopic dermatitis: section 3. Management and treatment with phototherapy and systemic agents. J Am Acad Dermatol 2014; 71: 327–349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Sidbury R, Tom WL, Bergman JN et al Guidelines of care for the management of atopic dermatitis: section 4. Prevention of disease flares and use of adjunctive therapies and approaches. J Am Acad Dermatol 2014; 71: 1218–1233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Wollenberg A, Barbarot S, Bieber T et al Consensus‐based European guidelines for treatment of atopic eczema (atopic dermatitis) in adults and children: part II. J Eur Acad Dermatol Venereol 2018; 32: 850–878. [DOI] [PubMed] [Google Scholar]
- 18. Wollenberg A, Barbarot S, Bieber T et al Consensus‐based European guidelines for treatment of atopic eczema (atopic dermatitis) in adults and children: part I. J Eur Acad Dermatol Venereol 2018; 32: 657–682. [DOI] [PubMed] [Google Scholar]
- 19. Guglielmetti S, Dart JK, Calder V. Atopic keratoconjunctivitis and atopic dermatitis. Curr Opin Allergy Clin Immunol 2010; 10: 478–485. [DOI] [PubMed] [Google Scholar]
- 20. Ebihara N, Ohashi Y, Uchio E et al A large prospective observational study of novel cyclosporine 0.1% aqueous ophthalmic solution in the treatment of severe allergic conjunctivitis. J Ocul Pharmacol Ther 2009; 25: 365–372. [DOI] [PubMed] [Google Scholar]
- 21. Fukushima A, Ohashi Y, Ebihara N et al Therapeutic effects of 0.1% tacrolimus eye drops for refractory allergic ocular diseases with proliferative lesion or corneal involvement. Br J Ophthalmol 2014; 98: 1023–1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Castro M, Corren J, Pavord ID et al Dupilumab efficacy and safety in moderate‐to‐severe uncontrolled asthma. N Engl J Med 2018; 378: 2486–2496. [DOI] [PubMed] [Google Scholar]
- 23. Simpson EL, Flohr C, Eichenfield LF et al Efficacy and safety of lebrikizumab (an anti‐IL‐13 monoclonal antibody) in adults with moderate‐to‐severe atopic dermatitis inadequately controlled by topical corticosteroids: a randomized, placebo‐controlled phase II trial (TREBLE). J Am Acad Dermatol 2018; 78: 863–871. [DOI] [PubMed] [Google Scholar]
- 24. Wollenberg A, Howell MD, Guttman‐Yassky E et al Treatment of atopic dermatitis with tralokinumab, an anti‐IL‐13 mAb. J Allergy Clin Immunol 2019; 143: 135–141. 10.1016/j.jaci.2018.05.029 [DOI] [PubMed] [Google Scholar]
- 25. Leonardi A, Jose PJ, Zhan H, Calder VL. Tear and mucus eotaxin‐1 and eotaxin‐2 in allergic keratoconjunctivitis. Ophthalmology 2003; 110: 487–492. [DOI] [PubMed] [Google Scholar]
- 26. Mennini M, Dahdah L, Fiocchi A. Two phase 3 trials of dupilumab versus placebo in atopic dermatitis. N Engl J Med 2017; 376: 1090. [DOI] [PubMed] [Google Scholar]
- 27. Thyssen JP. Could conjunctivitis in patients with atopic dermatitis treated with dupilumab be caused by colonization with Demodex and increased interleukin‐17 levels? Br J Dermatol 2018; 178: 1220. [DOI] [PubMed] [Google Scholar]
- 28. Bakker DS, Ariens LFM, van LC et al Goblet cell scarcity and conjunctival inflammation during treatment with dupilumab in patients with atopic dermatitis. Br J Dermatol 2019; 180: 1248–1249. 10.1111/bjd.17538 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Cronau H, Kankanala RR, Mauger T. Diagnosis and management of red eye in primary care. Am Fam Physician 2010; 81: 137–144. [PubMed] [Google Scholar]


