Summary
Background
Skin health declines with age and this is partially attributed to immunosenescence. Mast cells (MCs) are innate immune cells that coordinate tissue immune responses integral to skin homeostasis and disease.
Objectives
To understand how MCs contribute to human skin ageing, we investigated how intrinsic ageing impacts MC phenotype and MC relationships with other immune cells and skin structures.
Methods
In photoprotected skin biopsies from young (≤ 30 years) and aged (≥ 75 years) individuals, immunostaining and spatial morphometry were performed to identify changes in MC phenotype, number, distribution and interaction with the vasculature and nerve fibres. Quantitative polymerase chain reaction was used to measure changes in gene expression related to immune cell activity and neuropeptide signalling.
Results
Skin MCs, macrophages and CD8+ T cells increased in number in intrinsically aged vs. young skin by 40%, 44% and 90%, respectively (P < 0·05), while CD4+ T cells and neutrophils were unchanged. In aged skin, MCs were more numerous in the papillary dermis and showed a reduced incidence of degranulation (50% lower than in young, P < 0·01), a conserved tryptase–chymase phenotype and coexpression of granzyme B. In aged skin, MCs increased their association with macrophages (~ 48% vs. ~27%, P < 0·05) and nerve fibres (~29% vs. 16%, P < 0·001), while reducing their interactions with blood vessels (~34% vs. 45%, P < 0·001). Additionally, we observed modulation of gene expression of vasoactive intestinal peptide (VIP; increased) and substance P (decreased) with age; this was associated with an increased frequency of VIP + nerve fibres (around three times higher in aged skin, P < 0·05), which were strongly associated with MCs (~19% in aged vs. 8% in young, P < 0·05).
Conclusions
In photoprotected skin we observed an accumulation of MCs with increasing age. These MCs have both altered functionality and distribution within the skin, which supports a role for these cells in altered tissue homeostasis during ageing.
Short abstract
What's already known about this topic?
In aged skin, immunity becomes dysregulated leading to greater baseline inflammation and dampened adaptive immunity.
Mast cells (MCs) are regarded as multifunctional regulators of tissue homeostasis and immunity and are known to increase in number in the skin with age.
What does this study add?
This study shows that the increase in MCs in aged skin is localized to the papillary dermis, where these cells are in closer proximity to macrophages but have reduced interaction with the microvasculature and other immune populations.
We show that in aged skin, MCs also exhibit lower amounts of degranulation and form closer interactions with macrophages and vasoactive intestinal peptide‐positive nerve fibres while lessening their association with the dermal vasculature.
What is the translational message?
Alterations in MC frequency, functionality and distribution may contribute to the immunosenescent skin phenotype associated with intrinsic ageing.
The changes in MC behaviour may also play a role in neurogenic inflammation and could provide a therapeutic target for age‐associated pruritus.
Skin health declines with advancing age, resulting in fragility, delayed wound healing and increased susceptibility to infections and skin cancer.1, 2 As the proportion of the global aged population continues to rise, it is becoming increasingly important to invest in skin health research. This importance is recognized by the World Health Organization, which has highlighted cutaneous ageing as an international clinical health challenge.3
In addition to its role as a physical barrier to the environment, the skin also contains a diverse population of innate and adaptive immune cells that contribute to healthy tissue function. In aged patients, global senescence of the immune system combined with the onset of low‐level chronic inflammation, or ‘inflammageing’, is thought to result in dysregulation of tissue homeostasis.4, 5 These changes are thought to result from chronic hyperstimulation of the adaptive and innate immune systems during the course of an individual's lifetime, and, together with the accumulation of molecular lesions and increased proinflammatory cytokine secretion, culminate in a global loss of immunological efficiency.4 In aged skin, the capacity for immunosurveillance is diminished, which is in part attributed to decreased recruitment of CD4+ memory T cells6 and a reduced frequency and migratory capacity of epidermal Langerhans cells.7, 8 However, it has also been reported in human skin that the number of CD45+ cells is gradually increased during ageing, and more specifically mast cells (MCs) are reported to be increased in number in the dermis.9
Mast cells are innate immune cells that contribute to first‐line skin defences, tissue repair and homeostasis.10, 11 They are effective surveillance cells in the skin, responding to a broad range of stimuli via a wide range of surface receptors (including the high‐affinity receptor for IgE, FcεR1). This allows them to provide innate immunity towards microorganisms and helminths, and also to participate in adaptive immune responses through antigen presentation.12 Additionally, they promote local inflammation through recruitment of innate immune cells and secretion of biologically active molecules including proteases (e.g. tryptase, chymase and granzyme B), cytokines, neuropeptides, lipid mediators and monoamines (e.g. histamine and serotonin).
Given the broad‐ranging activities of MCs in maintaining skin health, we sought to explore how they may contribute to changes in the skin's microenvironment during intrinsic ageing.
Materials and methods
Participants and skin sampling
Ethical approval was granted by the University of Manchester research ethics committee (reference 14415). Volunteers provided written informed consent in accordance with the Declaration of Helsinki principles. In total, 40 white volunteers (n = 22 aged ≤ 30 years, n = 18 aged ≥ 75 years) were recruited to the Dermatopharmacology Unit at Salford Royal NHS Hospital. Volunteers were excluded if they had used a sunbed or sunbathed in the 6 weeks prior to recruitment; if they had pre‐existing skin conditions; or if they were taking significant anti‐inflammatory medication. Skin punch biopsies (6 mm) were taken from photoprotected buttock skin under local anaesthetic (lidocaine 2%; Antigen Pharmaceuticals Ltd, Dublin, Ireland) for immunostaining and RNA extraction as previously described.8 Photoprotected buttock skin was sampled so as to mitigate any other skin pathology induced by the skin's interaction with the environment, for example chronic exposure to sunlight (photoageing).13, 14
Standard peroxidase immunohistochemistry assays
On formalin‐fixed paraffin‐embedded (FFPE) skin sections, reactivity with monoclonal antibodies for CD4, CD8, MC tryptase, neutrophil elastase and CD31 (for vascular endothelium) was visualized using horseradish peroxidase‐coupled secondary antibody kits and substrates according to the manufacturer's instructions (Table S1; see Supporting Information). Images were acquired using either a 20×/0·80 or a 40×/0·80 Plan Apo objective using the Pannoramic 250 Flash II slide scanner (3DHISTECH Ltd, Budapest, Hungary). The number of positively stained cells in three high‐power fields per section was counted for neutrophils, CD8+ T cells, CD68+ macrophages and MCs (final magnification × 200) and in four high‐power fields for CD4+ T cells (final magnification × 400). Vascular endothelium was quantified by calculating the percentage area of expression in the dermis using Image J software (https://imagej.nih.gov/ij).
Immunofluorescence
On FFPE skin sections, antibody reactivity for MC tryptase, CD8, CD68, CD163, PGP 9·5, von Willebrand factor and granzyme B was probed and visualized via fluorochrome‐conjugated secondary antibodies (Table S1; see Supporting Information). To identify PGP 9·5, vasoactive intestinal peptide (VIP) and tryptase targets simultaneously, a method adapted from Hunyady et al.15 and Toth and Mezey16 was used. Briefly, skin sections were dewaxed, rehydrated and antigenic sites revealed by heating in citrate buffer for 20 min. Sections were incubated in tyramide blocking buffer (biotin–tyramide amplification kit; PerkinElmer, Waltham, MA, U.S.A.) for 30 min at room temperature followed by incubation with primary antibody for 1 h.
Following washing, sections were incubated for 30 min with horseradish peroxidase‐conjugated secondary IgG (Vector Laboratories Ltd, Peterborough, U.K.) before incubation in biotin–tyramide amplification reagent according to the manufacturer's instructions. The sections were heated again in citrate buffer for 5 min to elute the first layer of antibodies. Next, sections were incubated with the next primary antibody of interest for 1 h before incubation for 30 min in DyLight 649‐conjugated secondary IgG (Vector Laboratories) and Cy2‐conjugated streptavidin (GE Healthcare, Little Chalfont, U.K.). Sections were blocked with 2·5% normal horse serum (Vector Laboratories) and incubated with the final primary antibody of interest for 1 h. Finally, sections were incubated with Excel Amplification Kit with DyLight 594 (Vector Laboratories) according to the manufacturer's instructions. Sections were mounted using Fluoroshield mounting medium containing 4′,6‐diamidino‐2‐phenylindole (Abcam, Cambridge, U.K.) prior to image analysis.
May–Grünwald–Giemsa staining
May–Grünwald–Giemsa staining was performed to identify MCs and their degranulation status. Using this stain, MCs appear magenta, with nondegranulated MCs more intensely stained than degranulated MCs. In brief, skin sections were dewaxed, rehydrated and stained in May–Grünwald solution followed by Giemsa solution (Sigma‐Aldrich, Poole, U.K.) according to the manufacturer's instructions. Stained sections were examined using a DMRB microscope (Leica Microsystems, Milton Keynes, U.K.) with SPOT digital camera and associated software (RTKE/SE; Diagnostic Instruments Inc., Sterling Heights, MI, U.S.A.). Ten consecutive, nonoverlapping images of the papillary dermis were taken using a 20×/0·80 Plan Apo objective lens (n = 5 for each age group).
Real‐time quantitative polymerase chain reaction
Skin samples were homogenized and RNA extracted as previously described.8 Polymerase chain reaction was performed using Taqman gene expression primer and probe sets for IL2, IFNG, TNF, TAC1 (substance P), VIP, CALCA and CALCB (calcitonin gene‐related peptide; CGRP), F2RL1 (proteinase‐activated receptor 2; PAR2) and RPL27 (internal control gene) on a Step One Plus Real Time PCR machine with Taqman Fast Universal Mastermix No AmpErase® UNG (Thermo Fisher Scientific Inc., Waltham, MA, U.S.A.) for 40 cycles. Samples were assayed in triplicate and gene expression changes were calculated using the comparative CT method, where gene expression changes are then expressed as the fold change of the ∆∆CT (∆CT relative to the average young group baseline ∆CT value for each gene). Where no amplification was detected a CT value of 40 was substituted to allow the data to be included in the analyses.
Spatial association and morphometry and coefficient of association
To assess spatial association we performed morphometry as described by Armstrong.17 In brief, a grid of 325‐μm2 squares was overlaid on each image using ImageJ software, which produced a grid of 130 squares per image. The number of squares containing feature A (e.g. an MC) was counted, then those containing feature B (e.g. a CD8+ T cell), followed by those containing both features and finally those containing neither. This process was repeated for 10 consecutive but not overlapping images for each volunteer and the results were totalled. Using these values a 2 × 2 contingency table was constructed and was used to calculate the coefficient of association.
Statistics
Statistical analysis of data was performed in GraphPad Prism v7·0 software (GraphPad Software, La Jolla, CA, U.S.A.). Normally distributed data were analysed by unpaired, two‐tailed Student's t‐test, with the unpaired, two‐tailed Mann–Whitney U‐test employed if data were nonparametric. All reported P‐values are two‐sided, with P < 0·05 deemed statistically significant.
Results
The balance of skin‐resident leucocytes is altered with intrinsic ageing: numbers of dermal mast cells and CD8+ T cells are significantly increased in intrinsically aged, photoprotected skin
To identify potential immune hallmarks of cutaneous ageing we investigated dermal cell subsets involved in both innate and adaptive immunity (Fig. 1). Few neutrophils (Fig. 1a) were present in photoprotected skin; of the cell types examined, CD68+ macrophages were the most abundant (Fig. 1b), but increasing volunteer age did not alter their number. Similarly, numbers of CD4+ T cells were also constant (Fig. 1c). In contrast, the mean numbers of tryptase‐positive MCs (Fig. 1d–f) and CD8+ T cells (Fig. 1g–i) were significantly increased in aged skin compared with young skin, by ~40% and 90%, respectively (both P = 0·02; unpaired two‐tailed Student's t‐test).
Figure 1.
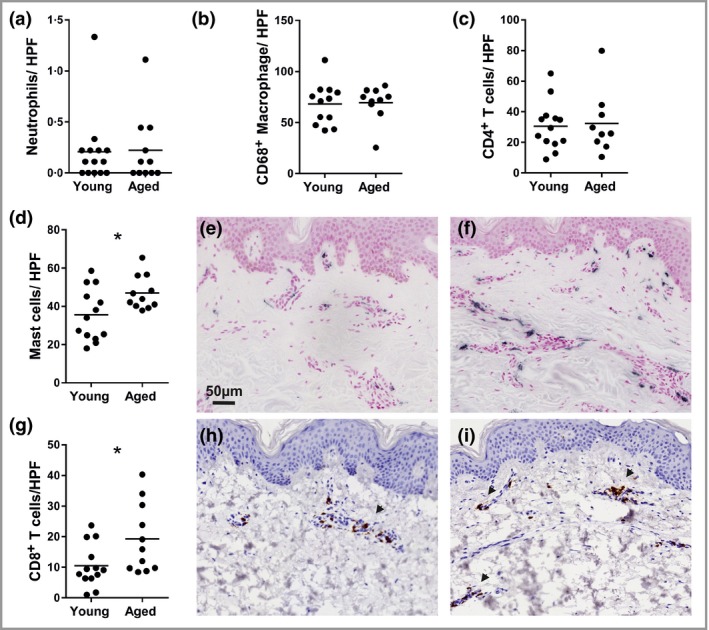
The frequency of some, but not all, innate and adaptive immune cells is altered in photoprotected, intrinsically aged human skin. Immunohistochemistry was used to identify dermal immune cell subsets that were quantified per high‐power field (HPF). The numbers of (a) neutrophils, (b) CD68+ macrophages and (c) CD4+ T cells were similar in both age groups, while (d) mast cells and (g) CD8+ T cells were significantly increased in frequency in intrinsically aged photoprotected skin. Representative photomicrographs of (e) young and (f) aged skin show resident mast cells (black) and (h) young and (i) aged skin stained for the presence of CD8+ T cells (brown; shown by arrows). Data were collected from skin biopsies of 13 young and 11 aged individuals and analysed by unpaired two‐tailed Student's t‐test; *P < 0·05.
In intrinsically aged skin increased numbers of mast cells are confined to the papillary dermis but their protease expression is unaltered
Next we examined the effect of ageing on cutaneous MC protease expression (tryptase and chymase; Fig. 2) by quantifying MCs in the papillary and reticular dermis separately (Fig. 2a, b). Dual labelling identified the most frequent MC phenotype as those cells that coexpressed tryptase and chymase (Fig. 2c); increased MC numbers were observed in aged skin and these were localized to the papillary dermis (Fig. 2d, e). Chymase‐dominated MCs represented around 20% of the papillary dermal population in each age group, while tryptase‐dominated MCs were the least prevalent (only 3–6% of the papillary dermal population). In a smaller cohort of individuals (n = 5), we also examined MC degranulation (Fig. 3) using May–Grünwald–Giemsa staining and found a substantially higher incidence of degranulated MCs (e.g. cytoplasmic granules exhibiting staining alterations, fusion and/or exteriorization) in the younger age group (~40% vs. ~20%; unpaired two‐tailed Mann–Whitney U‐test; P = 0·008).
Figure 2.
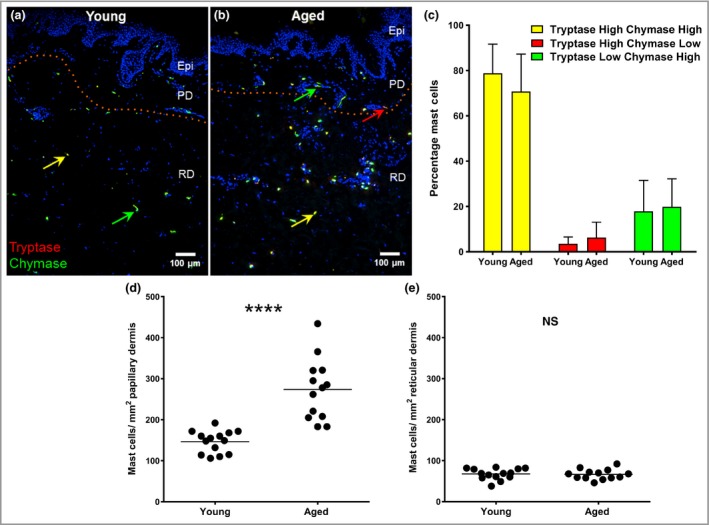
Increased mast cell (MC) numbers in aged skin are localized to the papillary dermis but show no change in protease phenotype. Dual immunohistochemistry for MC tryptase (red) and MC chymase (green) in (a) young and (b) aged skin. Some MCs show strong immunofluorescence for both enzymes (yellow arrows) while others are chymase (green arrows) or tryptase dominant (red arrow). (c) The proportions of MC phenotypes were the same for both age groups (mean ± SEM percentage); however, an age‐related increase in numbers was found in the papillary dermis (d) but not the reticular dermis (e). Epi, epidermis; PD, papillary dermis; RD, reticular dermis. Data from 14 young and 13 aged individuals; ****P < 0·001; NS, not statistically significant using the unpaired two‐tailed Mann–Whitney U‐test.
Figure 3.
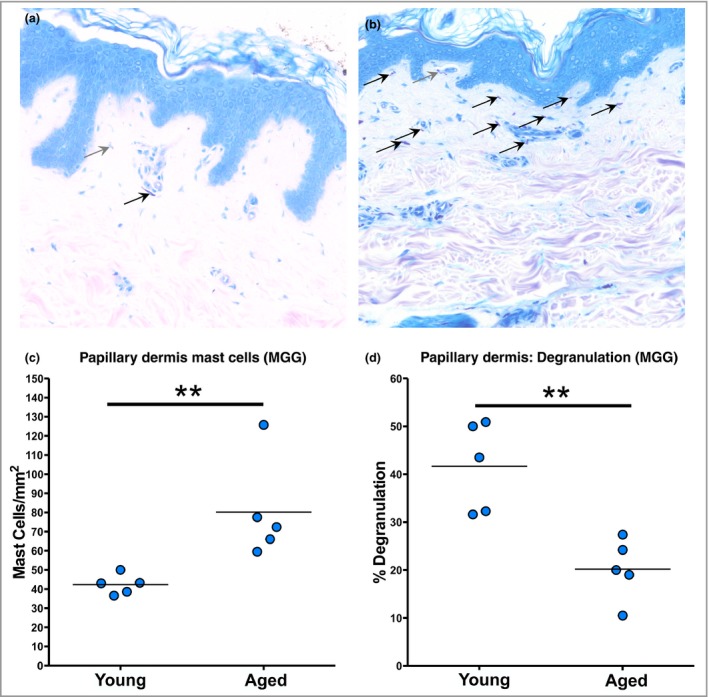
Degranulation of mast cells (MCs) is significantly reduced in aged human skin. May–Grünwald–Giemsa (MGG) staining was performed to assess degranulation of MCs in vivo. Representative photomicrographs of (a) young and (b) aged skin. Grey arrows identify degranulating MCs; black arrows identify quiescent MCs. (c) When quantified, there were significantly more MCs in the papillary dermis of the aged group but with (d) significantly less degranulation (n = 5 per group). **P < 0·01 using the unpaired two‐tailed Mann–Whitney U‐test.
Mast cells, and not CD8+ T cells, remain the key source of granzyme B in aged skin
Activated CD8+ T cells are reported to express granzyme B,18 a cytotoxic molecule that can mediate cleavage of the extracellular matrix (ECM).19, 20 However, in both young and aged skin, granzyme B reactivity did not colocalize with CD8+ T cells (data not shown), but did with MCs (Fig. S1; see Supporting Information). In the papillary dermis of skin from both age groups, the majority of MCs strongly expressed granzyme B (70–90%) and these double‐positive cells were more numerous in aged, as compared with young, papillary dermis (data analysed using the unpaired two‐tailed Student's t‐test, P < 0·001).
IL2 and IFNG gene expression was also examined as an indicator of CD8+ T‐cell activation (Fig. S2; see Supporting Information). No difference in IL2 was found, while IFNG gene expression was increased in aged skin compared with young skin (unpaired two‐tailed Student's t‐test, P = 0·008).
Ageing does not affect mast cell‐CD8+ T‐cell interaction but is associated with increased proximity of mast cells to macrophages
We next examined the interaction of MCs with CD8+ T cells and macrophages in the papillary dermis by morphometry (an example of which is displayed in Figure 4). In aged skin the mean distance between MCs and CD8+ T cells was 11 μm, compared with 14 μm in young skin (data analysed using the unpaired two‐tailed Mann–Whitney U‐test, P < 0·001; Fig. S3; see Supporting Information). Around 45% of MCs in aged skin were within 9 μm of a CD8+ T cell compared with around 31% in young skin. In order to identify whether this change in proximity was due to a dynamic interaction between the cells or whether it was a function of the increased numbers of MCs and CD8+ T cells in aged skin, a test for spatial association was performed; this found no significant difference between the age groups.
Figure 4.
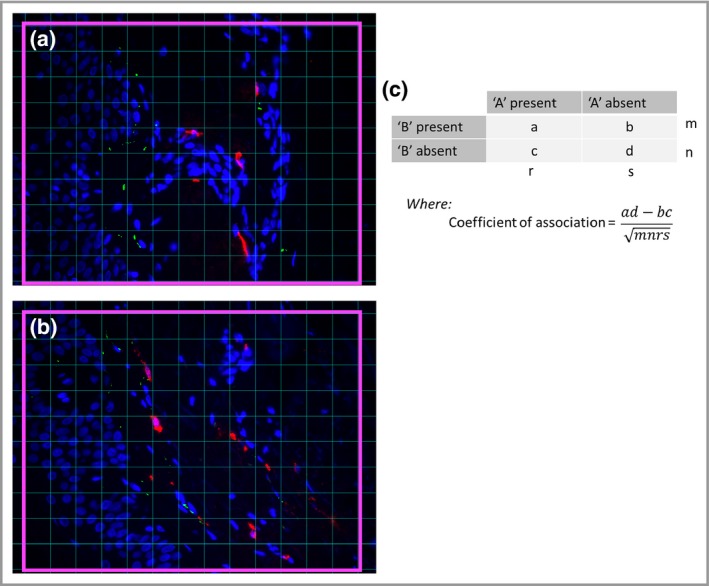
Calculation of the coefficient of association. (a) Using ImageJ software a grid containing 130 squares (pink boxes) was overlaid onto an image. The numbers of squares containing feature ‘A’, feature ‘B’, both features or neither feature were counted. This was repeated for 10 images and the total numbers of squares for each condition were entered into a 2 × 2 contingency table (b). The values m, n, r and s are the sums of a + b, c + d, a + c and b + d, respectively. The coefficient of association is then calculated from these values, where 0 represents an absence of association and +1 or −1 represents complete association. Positive values represent increased association of the two features with each other, while negative values represent increased association of each feature with itself. Values obtained from young and aged individuals were compared using the unpaired two‐tailed Mann–Whitney U‐test.
As MCs are reported to recruit macrophages21 we employed a more specific macrophage marker (CD163) in combination with MC tryptase and found that higher macrophage numbers were present in the papillary dermis of intrinsically aged, as compared with young, skin (unpaired two‐tailed Mann–Whitney U‐test, P < 0·001). Additionally, the mean distance between MCs and their nearest macrophage was lower in aged skin (9 μm, compared with 14 μm in young skin; unpaired two‐tailed Mann–Whitney U‐test, P < 0·001). While around 48% of MCs were within 9 μm of a macrophage in aged skin, this decreased to around 27% in young skin. Furthermore, a statistically significant increase in spatial association of MCs and macrophages was found in the aged compared with young skin (unpaired two‐tailed Mann–Whitney U‐test, P = 0·031), suggesting a functional interaction.
The close relationship of mast cells and blood vessels is lessened in aged papillary dermis
Next we examined the interaction between MCs and the papillary dermal microvasculature and found that in aged skin, the distance of MCs from vascular elements was increased (14 μm compared with about 9 μm in young skin; unpaired two‐tailed Mann–Whitney U‐test, P < 0·001; Fig. S4; see Supporting Information). While around 45% of MCs in young skin were within 9 μm of a blood vessel this was reduced to around 34% in aged skin when the data were displayed as a frequency distribution. No difference in the number or density of vascular elements was found between the age groups, suggesting an active relocation of MCs away from the microvasculature in the aged skin.
Mast cells increase their contacts with nerve fibres in the papillary dermis of aged skin and strongly associate with vasoactive intestinal peptide‐positive nerve fibres
MCs are increased in number in the papillary dermis of aged skin, and an increase in their frequency is reported to contribute to pruritus, a common symptom described by elderly patients.22 Therefore we assessed the spatial relationship of MCs to papillary dermis PGP 9·5‐ and VIP+ nerve fibres (Fig. S5; see Supporting Information). In aged skin (Fig. S5b), MCs were located closer to PGP 9·5‐positive nerve fibres than in young skin (Fig. S5a, 13 μm vs. 18 μm; Fig. S5c, unpaired two‐tailed Mann–Whitney U‐test, P < 0·001) and around 29% of the MCs in aged skin were within 9 μm of such a nerve fibre compared with 16% in young skin (Fig. S5d). Spatial analysis showed a statistically significant increase in association of MCs and nerve fibres in aged skin compared with young skin (P < 0·001; Fig. S5e), despite a reduction in nerve fibre abundance in the aged skin (unpaired two‐tailed Mann–Whitney U‐test, P < 0·001; Fig. S5f).
Immunofluorescence for tryptase, PGP 9·5 and VIP identified no VIP+ MCs, but the frequency of VIP+ nerve fibres was increased in aged (62 fibres mm−2) compared with young papillary dermis (23 fibres mm−2; unpaired two‐tailed Mann–Whitney U‐test, P = 0·04; Fig. S5l). MCs in aged papillary dermis were located closer to VIP+ nerve fibres than those in young papillary dermis (16·5 μm vs. 25·1 μm, P = 0·0076; Fig. S5i). The proportion of MCs within 9 μm of a VIP+ nerve fibre increased from 8% in young skin to 19% in aged skin (Fig. S5j). Furthermore, the spatial association of MCs to VIP+ nerve fibres was increased significantly in aged compared with young skin (P = 0·026; Fig. S5k), indicating a functional interaction.
CGRP genes were either not detected (CALCA) or showed no difference (CALCB) in expression between the age groups, and no change in PAR2 (F2RL1) gene expression was found (data not shown). However, expression of TAC, the gene encoding substance P, was lower (P = 0·01) and VIP expression higher in aged compared with young skin (unpaired two‐tailed Mann–Whitney U‐test, P = 0·04).
Discussion
Intrinsic ageing is associated with a decline in immune robustness and an increase in systemic inflammation. However, the impact of ageing on skin immunity is not well understood. We aimed to characterize key changes occurring in the complex system of resident immune cells in aged human skin. We found that the composition of dermal leucocytes becomes skewed with age, favouring MCs, CD8+ T cells and macrophages, and that these changes are confined to the papillary dermis. As MCs are local coordinators of immunity in epithelial tissues we performed detailed analysis of MC distribution and activity in ageing skin. We found that MCs were more closely associated with macrophages and VIP+ nerves fibres but showed a relaxed association with the papillary dermal vasculature. No effect of ageing was found on MC protease expression, but the incidence of degranulation was lower in aged skin. Therefore, increased numbers of MCs in combination with an altered distribution in aged skin could impact on activation of other dermal immune cells and papillary dermal structures, with important consequences for healthy tissue function.
MCs express a broad range of receptors, facilitating their active involvement in skin homeostasis and inflammation. The imbalance of MCs, macrophages and CD8+ T cells is localized to the papillary dermis of aged skin, which likely increases the chances of interaction and cross‐talk of these cells with one another. Human MCs can activate T cells,23 and in this study we found that MCs and CD8+ T cells were very closely associated in the papillary dermis; however, no significant effect of age was found. This suggests that communication between MCs and CD8 T cells has an important role for skin health in general but may not be a key factor involved in declining immune health in aged skin.
MCs were significantly more closely associated with macrophages in aged skin, which could indicate a change in macrophage and/or MC function, and altered expression of chemoattractive substances. Macrophages have multiple important functions in maintaining skin health such as antigen presentation and phagocytosis, and are among the first line of defence following breach of the epidermal barrier. While macrophages produce proinflammatory molecules, this function is reported to be impaired with increasing age, and secretion of immunosuppressive interleukin‐10 and prostaglandin E2 is increased.24
Macrophages are activated by interferon (IFN)‐γ, and we observed an increase in IFN‐γ gene expression in aged skin, potentially as a result of increased CD8+ T‐cell numbers. However, while this could mean increased availability of IFN‐γ protein in aged skin, macrophages in aged tissues are reported to be less receptive to IFN‐γ activation.25, 26 Therefore, increased numbers of macrophages in aged skin may indicate not an increased inflammatory state, but instead a reaction towards reduced integrity of the epithelial barrier, or a compensatory measure in response to an overall reduced efficacy of function at the single‐cell level. Macrophages are also targets for therapeutic intervention in tissue regeneration due to their plasticity and diverse roles in inflammation, resolution and healing.27 Their further characterization in aged skin is required to determine their role in intrinsic ageing.
In humans, two common MC phenotypes are recognized in epithelial tissues according to protease expression: tryptase+/chymase− and tryptase+/chymase+,28 with the different phenotypes associated with different biological functions.29 In human skin, tryptase+/chymase+ MCs are the most common, but we found no age‐related differences in MC tryptase–chymase phenotypes. We also found that MCs, and not CD8+ T cells, were the predominant source of granzyme B in healthy skin and that the majority of MCs in both age groups expressed this protease. Granzyme B is a serine protease that cleaves extracellular proteins such a fibrinogen, laminins, fibronectin and decorin, and clotting proteins such as von Willebrand factor and fibrinogen.30
Mouse models of premature ageing have demonstrated that MC‐derived granzyme B is increased in aged skin and promotes skin atrophy due to decreased dermal collagen density and reduced levels of decorin.19 In line with this action, granzyme B may promote ‘inflammageing’ through generation of ECM fragments, such as biglycan and decorin, which can activate macrophages via Toll‐like receptors (2 and 4) to release proinflammatory cytokines.31, 32, 33 MCs also express Toll‐like receptors 2 and 4,34 and therefore may also respond to ECM fragments by producing proinflammatory cytokines. While little evidence of inflammation was apparent in intrinsically aged skin, increased numbers of MCs and macrophages may contribute to dysregulated inflammation during bacterial or viral infection, and could contribute to impaired wound healing via aberrant cleavage of ECM proteins and clotting factors with increasing age.
Despite increased MC numbers in aged skin, a higher incidence of MC degranulation was observed in young skin, yet it was not associated with a detectable inflammatory response. Activation of different MC receptors can affect the type and time course of granule release. Stimulation via the FcεR1 receptor initiates a slow and sustained release of large granules rich in inflammatory mediators,35 while activation via MRGPRX2 can result in a rapid and transient release of smaller less inflammatory granules.35 Thus, the degranulation seen in young skin may be beneficial in maintenance of the dermal microenvironment, promoting turnover and remodelling of ECM by proteases, and local deactivation of potent signalling molecules such as VIP, substance P and endothelin 1 to limit inflammatory responses.36 In aged skin, reduced MC degranulation could reflect altered functionality and a senescent phenotype. This is supported by lower expression of the substance P gene, TAC1, as substance P is a trigger for human MC degranulation.37 Furthermore, as MCs become less closely associated with the vasculature in aged skin, this could imply that they are less active in recruitment of immune cells from the circulation.
We also found that MCs strongly associate with VIP+ nerve fibres, suggesting that this neuropeptide may influence MC distribution and function in aged skin. It has been reported that stimulation with VIP activates MCs but suppresses degranulation,38, 39 potentially explaining the decreased incidence of degranulation we observed in aged skin. While the overall density of nerve fibres was decreased in the papillary dermis of aged skin, VIP+ nerve fibres were increased and this correlated with increased VIP gene expression. In Parkinsonian rat brains VIP treatment reportedly increases expression of nerve growth factor in cerebral MCs, increasing neuronal survival.40 Therefore, recruitment of MCs to VIP+ nerve fibres in aged skin may represent an attempt to avert the process of nerve fibre attrition, a feature of aged skin.
MCs in close proximity to nerve fibres may limit neurogenic inflammation through tryptase detoxification of neuronal CGRP.36 However, tryptase can also activate PAR2,41 which is implicated in itch in atopic dermatitis.42 PAR2 is reported to be expressed by keratinocytes in human skin,43 and while we found no age‐associated differences in the genes for CGRP and PAR2 in this study, the presence of a greater number of MCs in aged skin adjacent to the epidermis, and in close association with nerves, may make these neuropeptides more susceptible to tryptase cleavage. While this may prove beneficial with regard to inactivation of CGRP, activation of PAR2 could contribute to the pruritus that is commonly described in elderly patients.
Thus, in aged skin a shift in the balance of resident papillary dermal immune cells towards those efficient at producing inflammatory mediators could indicate the presence of a heightened inflammatory microenvironment. While this could have repercussions for the integrity of adjacent skin structures and the dermal ECM, closer investigation of the expanded MC population in aged skin revealed a phenotype that is more suggestive of a senescent, or compensatory–protective role. However, it cannot be excluded that the intensified interaction of MCs with nerve fibres in aged skin may contribute to neurogenic inflammation. These findings, in concert with the versatility of MCs in regulating tissue processes, highlight these cells as potential targets for the improvement of skin health in the ageing population.
Supporting information
Fig S1. Mast cells strongly express granzyme B in young and aged skin.
Fig S2. Gene expression of interferon‐γ is significantly elevated in the skin of aged volunteers.
Fig S3. The proximity of mast cells (MCs) to CD8+ T cells in the papillary dermis is not affected by age, but MCs become more closely associated with macrophages in aged skin.
Fig S4. Mast cells are less closely associated with the dermal vasculature in aged skin.
Fig S5. Mast cells become more closely associated with papillary dermal PGP 9·5+ and vasoactive intestinal peptide‐positive nerve fibres in aged skin.
Table S1 Antibody details and methodology for immunohistochemical and dual immunofluorescent staining.
Acknowledgments
We acknowledge the Histology and Bioimaging core facilities at the University of Manchester and Ian Kimber and Rebecca Dearman for scientific discussion.
Funding sources We thank Walgreens Boots Alliance for funding this research. C.E.M.G. is a National Institute for Health Research Senior Investigator. C.E.M.G., R.E.B.W. and S.B.P. are supported in part by the Manchester Biomedical Research Centre (Dermatology Theme).
Conflicts of interest None to declare.
S.M.P. and M.J.B. are joint first authors
References
- 1. Fulop T, Larbi A, Witkowski JM et al Immunosenescence and cancer. Crit Rev Oncog 2013; 18:489–513. [DOI] [PubMed] [Google Scholar]
- 2. Pera A, Campos C, López N et al Immunosenescence: implications for response to infection and vaccination in older people. Maturitas 2015; 82:50–5. [DOI] [PubMed] [Google Scholar]
- 3. World Health Organization . World report on ageing and health 2015. Available at: https://www.who.int/ageing/events/world-report-2015-launch/en (last accessed 18 October 2018).
- 4. Cevenini E, Monti D, Franceschi C. Inflamm‐ageing. Curr Opin Clin Nutr Metab Care 2013; 16:14–20. [DOI] [PubMed] [Google Scholar]
- 5. Freund A, Orjalo AV, Desprez PY, Campisi J. Inflammatory networks during cellular senescence: causes and consequences. Trends Mol Med 2010; 16:238–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Agius E, Lacy KE, Vukmanovic‐Stejic M et al Decreased TNF‐α synthesis by macrophages restricts cutaneous immunosurveillance by memory CD4+ T cells during aging. J Exp Med 2009; 206:1929–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Bhushan M, Cumberbatch M, Dearman RJ et al Tumour necrosis factor‐α‐induced migration of human Langerhans cells: the influence of ageing. Br J Dermatol 2002; 146:32–40. [DOI] [PubMed] [Google Scholar]
- 8. Pilkington SM, Ogden S, Eaton L et al Lower levels of IL‐1β gene expression are associated with impaired Langerhans cell migration in aged human skin. Immunology 2018; 153:60–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Gunin AG, Kornilova NK, Vasilieva OV, Petrov VV. Age‐related changes in proliferation, the numbers of mast cells, eosinophils, and CD45‐positive cells in human dermis. J Gerontol A Biol Sci Med Sci 2011; 66:385–92. [DOI] [PubMed] [Google Scholar]
- 10. Xu Y, Chen G. Mast cell and autoimmune diseases. Mediators Inflamm 2015; 2015:246126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Douaiher J, Succar J, Lancerotto L et al Development of mast cells and importance of their tryptase and chymase serine proteases in inflammation and wound healing. Adv Immunol 2014; 122:211–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Cardamone C, Parente R, Feo GD, Triggiani M. Mast cells as effector cells of innate immunity and regulators of adaptive immunity. Immunol Lett 2016; 178:10–14. [DOI] [PubMed] [Google Scholar]
- 13. Blume‐Peytavi U, Kottner J, Sterry W et al Age‐associated skin conditions and diseases: current perspectives and future options. Gerontologist 2016; 56 (Suppl. 2):S230–42. [DOI] [PubMed] [Google Scholar]
- 14. Naylor EC, Watson REB, Sherratt MJ. Molecular aspects of skin ageing. Maturitas 2011; 69:249–56. [DOI] [PubMed] [Google Scholar]
- 15. Hunyady B, Krempels K, Harta G, Mezey E. Immunohistochemical signal amplification by catalyzed reporter deposition and its application in double immunostaining. J Histochem Cytochem 1996; 44:1353–62. [DOI] [PubMed] [Google Scholar]
- 16. Toth ZE, Mezey E. Simultaneous visualization of multiple antigens with tyramide signal amplification using antibodies from the same species. J Histochem Cytochem 2007; 55:545–54. [DOI] [PubMed] [Google Scholar]
- 17. Armstrong RA. Measuring the degree of spatial correlation between histological features in thin sections of brain tissue. Neuropathology 2003; 23:245–53. [DOI] [PubMed] [Google Scholar]
- 18. Strik MC, de Koning PJ, Kleijmeer MJ et al Human mast cells produce and release the cytotoxic lymphocyte associated protease granzyme B upon activation. Mol Immunol 2007; 44:3462–72. [DOI] [PubMed] [Google Scholar]
- 19. Hiebert PR, Boivin WA, Abraham T et al Granzyme B contributes to extracellular matrix remodeling and skin aging in apolipoprotein E knockout mice. Exp Gerontol 2011; 46:489–99. [DOI] [PubMed] [Google Scholar]
- 20. Parkinson LG, Toro A, Zhao H et al Granzyme B mediates both direct and indirect cleavage of extracellular matrix in skin after chronic low‐dose ultraviolet light irradiation. Aging Cell 2015; 14:67–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Amin K. The role of mast cells in allergic inflammation. Respir Med 2012; 106:9–14. [DOI] [PubMed] [Google Scholar]
- 22. Jin X, Zhao W, Kirabo A et al Elevated levels of mast cells are involved in pruritus associated with polycythemia vera in JAK2V617F transgenic mice. J Immunol 2014; 193:477–84. [DOI] [PubMed] [Google Scholar]
- 23. Suurmond J, Dorjee AL, Huizinga TW, Toes RE. Human mast cells costimulate T cells through a CD28‐independent interaction. Eur J Immunol 2016; 46:1132–41. [DOI] [PubMed] [Google Scholar]
- 24. Linehan E, Fitzgerald DC. Ageing and the immune system: focus on macrophages. Eur J Microbiol Immunol 2015; 5:14–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Yoon P, Keylock KT, Hartman ME et al Macrophage hypo‐responsiveness to interferon‐γ in aged mice is associated with impaired signaling through Jak‐STAT. Mech Ageing Dev 2004; 125:137–43. [DOI] [PubMed] [Google Scholar]
- 26. Davila DR, Edwards CK 3rd, Arkins S et al Interferon‐γ‐induced priming for secretion of superoxide anion and tumor necrosis factor‐α declines in macrophages from aged rats. FASEB J 1990; 4:2906–11. [DOI] [PubMed] [Google Scholar]
- 27. Smith TD, Nagalla RR, Chen EY, Liu WF. Harnessing macrophage plasticity for tissue regeneration. Adv Drug Deliv Rev 2017; 114:193–205. [DOI] [PubMed] [Google Scholar]
- 28. Irani AA, Schechter NM, Craig SS et al Two types of human mast cells that have distinct neutral protease compositions. Proc Natl Acad Sci U S A 1986; 83:4464–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Coussens LM, Raymond WW, Bergers G et al Inflammatory mast cells up‐regulate angiogenesis during squamous epithelial carcinogenesis. Genes Dev 1999; 13:1382–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Hiebert PR, Granville DJ. Granzyme B in injury, inflammation, and repair. Trends Mol Med 2012; 18:732–41. [DOI] [PubMed] [Google Scholar]
- 31. Okamura Y, Watari M, Jerud ES et al The extra domain A of fibronectin activates Toll‐like receptor 4. J Biol Chem 2001; 276:10229–33. [DOI] [PubMed] [Google Scholar]
- 32. Schaefer L, Babelova A, Kiss E et al The matrix component biglycan is proinflammatory and signals through Toll‐like receptors 4 and 2 in macrophages. J Clin Invest 2005; 115:2223–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Merline R, Moreth K, Beckmann J et al Signaling by the matrix proteoglycan decorin controls inflammation and cancer through PDCD4 and MicroRNA‐21. Sci Signal 2011; 4:ra75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Sandig H, Bulfone‐Paus S. TLR signaling in mast cells: common and unique features. Front Immunol 2012; 3:185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Gaudenzio N, Sibilano R, Marichal T et al Different activation signals induce distinct mast cell degranulation strategies. J Clin Invest 2016; 126:3981–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Caughey GH. Mast cell proteases as protective and inflammatory mediators In: Mast Cell Biology: Contemporary and Emerging Topics (Gilfillan AM, Metcalfe DD, eds). Austin, TX: Landes Bioscience, 2011; 212–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Kulka M, Sheen CH, Tancowny BP et al Neuropeptides activate human mast cell degranulation and chemokine production. Immunology 2008; 123:398–410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Tunçel N, Töre F, Şahintürk V et al Vasoactive intestinal peptide inhibits degranulation and changes granular content of mast cells: a potential therapeutic strategy in controlling septic shock. Peptides 2000; 21:81–9. [DOI] [PubMed] [Google Scholar]
- 39. Tunçel N, Şener E, Cerit C et al Brain mast cells and therapeutic potential of vasoactive intestinal peptide in a Parkinson's disease model in rats: brain microdialysis, behavior, and microscopy. Peptides 2005; 26:827–36. [DOI] [PubMed] [Google Scholar]
- 40. Korkmaz OT, Tunçel N, Tunçel M et al Vasoactive intestinal peptide (VIP) treatment of Parkinsonian rats increases thalamic gamma‐aminobutyric acid (GABA) levels and alters the release of nerve growth factor (NGF) by mast cells. J Mol Neurosci 2010; 41:278–87. [DOI] [PubMed] [Google Scholar]
- 41. Molino M, Barnathan ES, Numerof R et al Interactions of mast cell tryptase with thrombin receptors and PAR‐2. J Biol Chem 1997; 272:4043–9. [DOI] [PubMed] [Google Scholar]
- 42. Steinhoff M, Neisius U, Ikoma A et al Proteinase‐activated receptor‐2 mediates itch: a novel pathway for pruritus in human skin. J Neurosci 2003; 23:6176–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Steinhoff M, Corvera CU, Thoma MS et al Proteinase‐activated receptor‐2 in human skin: tissue distribution and activation of keratinocytes by mast cell tryptase. Exp Dermatol 1999; 8:282–94. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fig S1. Mast cells strongly express granzyme B in young and aged skin.
Fig S2. Gene expression of interferon‐γ is significantly elevated in the skin of aged volunteers.
Fig S3. The proximity of mast cells (MCs) to CD8+ T cells in the papillary dermis is not affected by age, but MCs become more closely associated with macrophages in aged skin.
Fig S4. Mast cells are less closely associated with the dermal vasculature in aged skin.
Fig S5. Mast cells become more closely associated with papillary dermal PGP 9·5+ and vasoactive intestinal peptide‐positive nerve fibres in aged skin.
Table S1 Antibody details and methodology for immunohistochemical and dual immunofluorescent staining.


