Abstract
Long noncoding RNAs (lncRNAs) CASC11 is an oncogenic lncRNA in gastric cancer and colorectal cancer. Our study aimed to investigate the role of lncRNA CASC11 in bladder cancer. In this study we showed that plasma lncRNA CASC11 was upregulated, while plasma miRNA‐150 was downregulated in patients with early‐stage bladder cancer than in healthy controls. Altered expression of plasma lncRNA CASC11 and miRNA‐150 separated patients with bladder cancer from healthy controls. lncRNA CASC11 expression was inversely correlated with miRNA‐150 expression in patients with bladder cance but not in healthy controls. Overexpression of lncRNA CASC11 mediated the inhibition of miRNA‐150 expression in cancer cells, while miRNA‐150 overexpression did not significantly alter lncRNA CASC11 expression. lncRNA CASC11 overexpression promoted, while miRNA‐150 overexpression inhibited cancer cell proliferation. miRNA‐150 also attenuated the enhancing effects of lncRNA CASC11 overexpression on cancer cell proliferation. However, overexpression of lncRNA CASC11 showed no significant effects on cancer cell migration and invasion. Therefore, lncRNA CASC11 may promote cancer cell proliferation in bladder cancer, and the actions of lncRNA CASC11 are likely through miRNA‐150.
Keywords: bladder cancer, cell proliferation, diagnosis, long noncoding RNA CASC11, miRNA‐150
1. INTRODUCTION
As one of the most frequently diagnosed solid tumor and the second most common malignant tumor of the genitourinary tract, bladder cancer affects more than 400 000 new cases every year.1 At present, more than two million patients were suffering from this disease.2 With efforts made on cancer prevention and treatment, mortality rate of bladder cancer has dropped significantly during past several decades in many countries,3, 4 especially developing countries, such as China.5, 6 However, patients with bladder cancer are prone to develop cancer metastasis, and the prognosis of metastatic bladder cancer is still poor.7 At present, early diagnosis and treatment is still the key of bladder cancer treatment.
The occurrence and development of bladder cancer are affected by multiple internal and external factors.8 Previous studies have shown that the development of bladder cancer is accompanied with changes in expression pattern of large set of noncoding RNAs (ncRNAs), such as long noncoding RNAs (lncRNAs) and microRNAs (miRNAs).9, 10 lncRNA CASC11 is an oncogenic lncRNA in gastric cancer and colorectal cancer,11, 12 while its involvement in bladder cancer is unknown. miRNA‐150 is a well‐characterized tumor suppression miRNA in different types of cancers, such as ovarian cancer.13 In the present study we showed that lncRNA CASC11 promoted cancer cell proliferation in bladder cancer possibly through miRNA‐150.
2. MATERIALS AND METHODS
2.1. Human plasma and cell lines
Plasma samples were obtained from 89 patients with bladder cancer and 62 healthy volunteers who were admitted to Tongji Hospital from June 2014 to June 2018. Inclusion criteria of bladder cancer patients: 1) patients diagnosed by pathological biopsies; 2) patients at stage I or II, which are two early stages; 3) patients with complete medical record; 4) patients and their families who were willing to participate. Exclusion criteria of patients with bladder cancer: 1) patients who were treated within 3 months before admission; 2) patients who were combined with other diseases, such as chronic diseases and metabolic diseases; 3) patients who were not willing to donate plasma samples. The 62 healthy volunteers were randomly selected from the people who received systemic physiological examinations in Tongji Hospital. All those healthy volunteers showed physiological indexes within normal range. The 89 bladder cancer patients included 53 males and 36 females, with an age range of 27 to 67 years and a mean age of 46.4 ± 5.1 years. The 62 healthy volunteers included 40 males and 22 females, with an age range of 26 to 66 years and a mean age of 46.1 ± 4.7 years. Patient and control groups showed similar age and sex distributions. This study passed the review of Ethics Committee of Tongji Hospital before enrollment of patients. All participants signed informed consent.
HT‐1197 and HT‐1376 two urinary bladder cancer cell lines were included in this study. Cells of these two cell lines were bought from American Type Culture Collection (ATCC). ATCC‐formulated Eagle Minimum Essential Medium (Catalog No. 30‐2003) with fetal bovine serum (FBS) added to a final concentration of 10% was used to cultivate cells in an incubator (37°C; 5% CO2).
2.2. Real‐time quantitative polymerase chain reaction (qRT‐PCR)
To detected the expression of miRNA‐150, miRNeasy Kit(Qiagen, Valencia, CA) was used to extract miRNA samples, miScript II RT Kit (Qiagen) was used to synthesize complementary DNA (cDNA) and PCR reaction systems were prepared using miScript SYBR Green PCR Kit (Qiagen) with U6 as the endogenous control. To detect the expression of lncRNA CASC11, GenElute Total RNA Purification Kit (Sigma‐Aldrich, MO) was used to extract total RNA, cDNA was prepared using Applied Biosystems High‐Capacity cDNA Reverse Transcription Kit and SuperScript III Platinum One‐Step qRT‐PCR Kit (Thermo Fisher Scientific, Schwerte, Germany) was used to prepare PCR reaction systems with glyceraldehyde 3‐phosphate dehydrogenase as the endogenous control. All PCR reactions were carried out on ABI 7500 System. Reaction conditions were: 1 minute 10 seconds at 95°C, and then 40 cycles of 12 seconds at 95°C and 30 seconds at 58.5°C. C t values were processed using method.
2.3. Vectors and cell transfections
Vectors expressing CASC11 were constructed by GenePharma (Shanghai, China). Empty vectors were also purchased from GenePharma. MISSION microRNA Mimic hsa‐miR‐150* and Scrambled negative control miRNA 1 were purchased from Sigma‐Aldrich. Cell transfections were performed using Lipofectamine 2000 Reagent (Invitrogen, Carlsbad, CA) with all operations performed in strict accordance with manufacturer's instructions. Dosages of vectors and miRNAs were 15 and 40 nM, respectively. Incubation with Lipofectamine 2000 Reagent only was control. Transfection with Scrambled negative control miRNA1 or empty vectors was negative control.
2.4. Cell proliferation assay
At 24 hours after transfection, overexpression rates of miRNA‐150 and lncRNA CASC11 both reached 200%. Cell proliferation abilities were then tested at 24 hours after transfection. Cell suspensions (3 × 104/mL) were prepared and transferred to a 96‐well plate with 100 µL in each well. Cells were cultivated in an incubator (37°C, 5% CO2) and 10 μL Cell Counting Kit‐8 solution (Dojindo, Kumamoto, Japan) was added 24, 48, 72, and 96 hours later. After cell cultivation for additional 4 hours, Epoch Microplate Spectrophotometer (Bio Tek, Winooski, VT) was used to measure OD values at 450 nm to reflect cell proliferation.
2.5. Transwell cell migration and invasion assay
At 24 hours after transfection, overexpression rates of miRNA‐150, and lncRNA CASC11 both reached 200%. Cell migration and invasion abilities were then tested at 24 hours after transfection. Cell suspensions (3 × 104/mL) were prepared using serum‐free medium and transferred to the upper chamber with 100 µL in each well. The lower chamber was filled with ATCC‐formulated Eagle Minimum Essential Medium with FBS added to a final concentration of 20%. Cells were cultivated in an incubator (37°C; 5% CO2) for 24 hours. After that, the upper chamber was collected and membranes were stained with 0.5% crystal violet (Sigma‐Aldrich) at room temperature for 20 minutes. The upper chamber was precoated with Matrigel (356234; Millipore, Bedford, MA) before the invasion assay. Stained cells were counted under an optical microscope (Olympus, Tokyo, Japan). Cell migration and invasion were normalized to cell proliferation.
2.6. Statistical analysis
Graphpad Prism 6 software (http://www.graphpad.com/support/fagid/1850) was used in this study to perform all statistical analyses. All experiments were repeated three times and mean ± standard deviation was calculated. Pearson's correlation coefficient was used for the correlation analysis between plasma levels of lncRNA CASC11 and miRNA‐150. Diagnostic potentials of plasma levels of lncRNA CASC11 and miRNA‐150 for bladder cancer were evaluated by receiver operating characteristic (ROC) curve analysis in patients with bladder cancer as true positive cases and healthy controls as true negative cases. Data comparisons were performed by either unpaired t test (between two groups) or analysis of variance followed by Tukey's test (among three groups). P < 0.05 was considered to be statistically significant.
3. RESULTS
3.1. lncRNA CASC11 and miRNA‐150 showed opposite expression patterns in patients with bladder cancer
Plasma levels of CASC11 and miRNA‐150 in both patients with bladder cancer and healthy volunteers were measured by RT‐qPCR. Compared with healthy controls, plasma levels of CASC11 significantly increased (Figure 1A; P < 0.05), while plasma levels of miRNA‐150 significantly decreased (Figure 1B; P < 0.05) in patients with bladder cancer.
Figure 1.
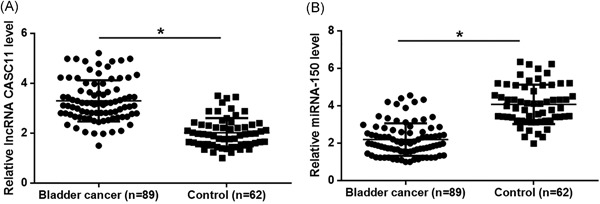
lncRNA CASC11 and miRNA‐150 showed opposite expression patterns in bladder cancer patients plasma levels of CASC11 significantly increased (A), while plasma levels of miRNA‐150 significantly decreased (B) in patients with bladder cancer (*P < 0.05). lncRNA, long noncoding RNA
3.2. Plasma lncRNA CASC11 and miRNA‐150 were with diagnostic potentials for patients with bladder cancer
Diagnostic potentials of plasma levels of lncRNA CASC11 and miRNA‐150 for bladder cancer were evaluated by ROC curve analysis with patients with bladder cancer as true positive cases and healthy controls as true negative cases. For plasma lncRNA CASC11, area under the curve was 0.8990, with standard error of 0.02478% and 95% confidence interval of 0.8505‐0.9476 (P < 0.0001; Figure 2A). For plasma miRNA‐150, area under the curve was 0.9147, with standard error of 0.02243% and 95% confidence interval of 0.8707‐0.9586 (P < 0.0001; Figure 2B).
Figure 2.
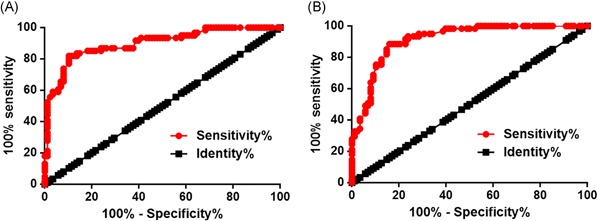
Plasma lncRNA CASC11 and miRNA‐150 were with diagnostic potentials for patients with bladder cancer ROC curve analysis showed that altered expression of plasma lncRNA CASC11 (A) and miRNA‐150 (B) separated patients with bladder cancer from healthy controls. lncRNA, long noncoding RNA; ROC, receiver operating characteristic
3.3. A significantly inverse correlation between plasma levels of lncRNA CASC11 and miRNA‐150 was observed in bladder cancer patients
Pearson's correlation coefficient was used for the correlation analysis between plasma levels of lncRNA CASC11 and miRNA‐150. Results showed that lncRNA CASC11 expression was inversely and significantly correlated with miRNA‐150 in patients with bladder cancer (Figure 3A). However, lncRNA CASC11 expression was not significantly correlated with miRNA‐150 in healthy controls (Figure 3B).
Figure 3.
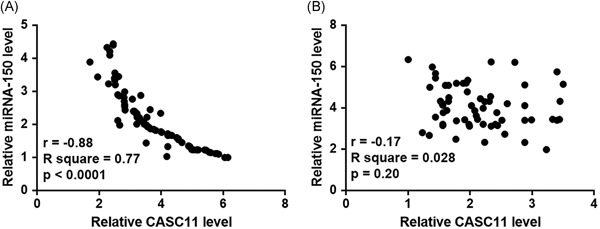
A significantly inverse correlation between plasma levels of lncRNA CASC11 and miRNA‐150 was observed in patients with bladder cancer Pearson's correlation coefficient revealed a significantly inverse correlation between plasma levels of lncRNA CASC11 and miRNA‐150 was observed in patients with bladder cancer (A) but not in healthy controls (B). lncRNA, long noncoding RNA
3.4. Overexpression of lncRNA CASC11 led to inhibited miRNA‐150 expression in cells of bladder cancer cell lines
At 24 hours after transfection, overexpression of lncRNA CASC11 and miRNA‐150 both reach 200% in cells of both HT‐1197 and HT‐1376 urinary bladder cancer cell lines (Figure 4A; P < 0.05). Compared with control (C) and negative control (NC) groups, overexpression of lncRNA CASC11 led to inhibited miRNA‐150 expression in cells of both cell lines (Figure 4B; P < 0.05). In contrast, overexpression of miRNA‐150 failed to significantly affect the expression of lncRNA CASC11 (Figure 4C; P < 0.05).
Figure 4.
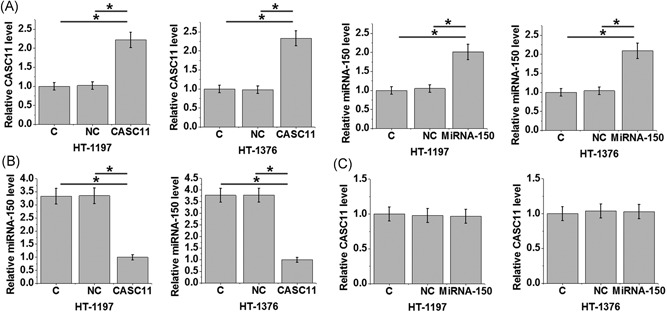
Overexpression of lncRNA CASC11 led to inhibited miRNA‐150 expression in cells of bladder cancer cell lines At 24 hours after transfection, overexpression of lncRNA CASC11 and miRNA‐150 both reach 200% in cells of both HT‐1197 and HT‐1376 urinary bladder cancer cell lines (A). Overexpression of lncRNA CASC11 led to inhibited miRNA‐150 expression in cells of both cell lines (B). In contrast, overexpression of miRNA‐150 failed to significantly affect the expression of lncRNA CASC11 (C) (*P < 0.05). lncRNA, long noncoding RNA
3.5. Overexpression of lncRNA CASC11 promoted cell proliferation possibly by inhibiting miRNA‐150
Results of cell proliferation assay showed that, compared with control (C) and negative control (NC) groups, overexpression of lncRNA CASC11 led to promoted, while overexpression of miRNA‐150 led to inhibited proliferation of cells of both HT‐1197 and HT‐1376 urinary bladder cancer cell lines (Figure 5; P < 0.05). In addition, overexpression of miRNA‐150 totally reversed the enhancing effect of lncRNA CASC11 overexpression on cancer cell proliferation. However, overexpression of lncRNA CASC11 failed to significantly affect cancer cell migration and invasion (data not shown).
Figure 5.
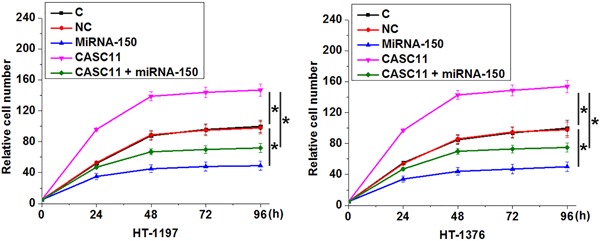
Overexpression of lncRNA CASC11 promoted cell proliferation possibly by inhibiting miRNA‐150 Overexpression of lncRNA CASC11 led to promoted, while overexpression of miRNA‐150 led to inhibited proliferation of cells of both HT‐1197 and HT‐1376 urinary bladder cancer cell lines. In addition, overexpression of miRNA‐150 totally reversed the enhancing effect of lncRNA CASC11 overexpression on cancer cell proliferation (*P < 0.05). lncRNA, long noncoding RNA
4. DISCUSSION
lncRNA CASC11 is a potential oncogenic lncRNA with functionality characterized only in gastric cancer and colorectal cancer.11, 12 Our study first reported that lncRNA CASC11 was also likely an oncogenic lncRNA in bladder cancer. We also showed that the oncogenic functions of lncRNA CASC11 are likely mediated by the inhibition of miRNA‐150, which is a well‐characterized tumor suppressor.
The functionality of miRNA‐150 has been characterized in several types of human malignancies. Downregulation of microRNA‐150 not only promote cancer cell invasion in ovarian cancer,13 but also contributes to the development of drug resistance.14 In the development of pancreatic cancer, miRNA‐150 directly targets MUC4 to inhibit cancer cell growth and malignant behaviors.15 miRNA‐150 also modulates cisplatin chemosensitivity of bladder cancer cells.16 Consistent with previous studies, our study also reported that miRNA‐150 was downregulated in bladder cancer and overexpression of miRNA‐150 led to inhibited bladder cancer cell proliferation. Therefore, overexpression of miRNA‐150 may serve as a potential therapeutic target for bladder cancer.
lncRNA CASC11 is upregulated in gastric cancer and colorectal cancer.11, 12 Our study first reported that plasma lncRNA CASC11 was also upregulated in patients with bladder cancer than in healthy controls. It has been reported that CASC11 can promote gastric cancer cell proliferation, migration and invasion through its interactions with cell cycle pathway.11 However, our study showed that lncRNA CASC11 overexpression only affected the proliferation but not the migration and invasion of bladder cancer cells. Therefore, lncRNA CASC11 may play different roles in different types of human malignancies.
In spite of the efforts made on the treatment of metastatic bladder cancer,17, 18 prognosis is still poor. Therefore, early diagnosis and treatment are still critical for the survival of patients with metastatic bladder cancer. Our study only included patients with bladder cancer at stage I or II to investigate the diagnostic values of plasma lncRNA CASC11 and miRNA‐150 for early‐stage bladder. The ROC curve analysis showed that altered plasma levels of lncRNA CASC11 and miRNA‐150 effectively distinguished patients with early‐stage bladder cancer from healthy controls. Therefore, plasma lncRNA CASC11 and miRNA‐150 may serve as potential biomarkers for the early diagnosis of bladder cancer.
The crosstalk between lncRNAs and miRNAs is common in cancer biology.19 In the present study we showed that lncRNA CASC11 overexpression mediated the inhibition of miRNA‐150. The crosstalk between lncRNA CASC11 and miRNA‐150 participates in the proliferation of bladder cancer cells. These data enriched our knowledge of the functionality of lncRNA CASC11. It is also worth noting that plasma levels of lncRNA CASC11 and miRNA‐150 were not closely correlated in healthy controls. Therefore, the interaction between lncRNA CASC11 and miRNA‐150 is likely mediated by pathological factors.
In conclusion, lncRNA CASC11 was upregulated, while miRNA‐150 was downregulated in patients with early‐stage bladder cancer. lncRNA CASC11 may promote bladder cancer cell proliferation through the inhibition of miRNA‐150.
CONFLICT OF INTERESTS
The authors declare that there are no conflict of interests.
AUTHOR CONTRIBUTIONS
HL, CX, and TW designed the study. HL, CX, TW, WL, and BG participated in the experiment and analysis. HL, CX, TW, and BG prepared the manuscript draft. WL made the manuscript editing. TW revised the manuscript.
Luo H, Xu C, Le W, Ge B, Wang T. lncRNA CASC11 promotes cancer cell proliferation in bladder cancer through miRNA‐150. J Cell Biochem. 2019;120:13487‐13493. 10.1002/jcb.28622
References
REFERENCES
- 1. Antoni S, Ferlay J, Soerjomataram I, Znaor A, Jemal A, Bray F. Bladder cancer incidence and mortality: a global overview and recent trends. Eur Urol. 2017;71(1):96‐108. [DOI] [PubMed] [Google Scholar]
- 2. Ploeg M, Aben KKH, Kiemeney LA. The present and future burden of urinary bladder cancer in the world. World J Urol. 2009;27(3):289‐293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Smith ND, Prasad SM, Patel AR, et al. Bladder cancer mortality in the united states: a geographic and temporal analysis of socioeconomic and environmental factors. J Urol. 2016;195(2):290‐296. [DOI] [PubMed] [Google Scholar]
- 4. Chavan S, Bray F, Lortet‐Tieulent J, Goodman M, Jemal A. International variations in bladder cancer incidence and mortality. Eur Urol. 2014;66(1):59‐73. [DOI] [PubMed] [Google Scholar]
- 5. Chen W, Zheng R, Zhang S, et al. Cancer incidence and mortality in China, 2013. Cancer Lett. 2017;401:63‐71. [DOI] [PubMed] [Google Scholar]
- 6. Mahdavifar N, Ghoncheh M, Pakzad R, Momenimovahed Z, Salehiniya H. Epidemiology, incidence and mortality of bladder cancer and their relationship with the development index in the world. Asian Pac J Cancer Prev. 2016;17(1):381‐386. [DOI] [PubMed] [Google Scholar]
- 7. Stenzl A, Cowan NC, De Santis M, et al. Treatment of muscle‐invasive and metastatic bladder cancer: update of the EAU guidelines. Eur Urol. 2011;59(6):1009‐1018. [DOI] [PubMed] [Google Scholar]
- 8. Czerniak B, Dinney C, McConkey D. Origins of bladder cancer. Annu Rev Pathol. 2016;11:149‐174. [DOI] [PubMed] [Google Scholar]
- 9. Dyrskjot L, Ostenfeld MS, Bramsen JB, et al. Genomic profiling of microRNAs in bladder cancer: miR‐129 is associated with poor outcome and promotes cell death in vitro. Cancer Res. 2009;69(11):4851‐4860. [DOI] [PubMed] [Google Scholar]
- 10. Peter S, Borkowska E, Drayton RM, et al. Identification of differentially expressed long non‐coding RNAs in bladder cancer. Clin Cancer Res, clincanres. 2014;0706:2014. [DOI] [PubMed] [Google Scholar]
- 11. Zhang L, Kang W, Lu X, Ma S, Dong L, Zou B. LncRNA CASC11 promoted gastric cancer cell proliferation, migration and invasion in vitro by regulating cell cycle pathway. Cell Cycle. 2018;17(15):1886‐1900. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 12. Zhang Z, Zhou C, Chang Y, et al. Long non‐coding RNA CASC11 interacts with hnRNP‐K and activates the WNT/β‐catenin pathway to promote growth and metastasis in colorectal cancer. Cancer Lett. 2016;376(1):62‐73. [DOI] [PubMed] [Google Scholar]
- 13. Jin M, Yang Z, Ye W, Xu H, Hua X. MicroRNA‐150 predicts a favorable prognosis in patients with epithelial ovarian cancer, and inhibits cell invasion and metastasis by suppressing transcriptional repressor ZEB1. PloS 0ne. 2014;9(8):e103965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Wuerkenbieke D, Wang J, Li Y, Ma C. miRNA‐150 downregulation promotes pertuzumab resistance in ovarian cancer cells via AKT activation. Arch Gynecol Obstet. 2015;292(5):1109‐1116. [DOI] [PubMed] [Google Scholar]
- 15. Srivastava SK, Bhardwaj A, Singh S, et al. MicroRNA‐150 directly targets MUC4 and suppresses growth and malignant behavior of pancreatic cancer cells. Carcinogenesis. 2011;32(12):1832‐1839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Lei Y, Hu X, Li B, et al. miR‐150 modulates cisplatin chemosensitivity and invasiveness of muscle‐invasive bladder cancer cells via targeting PDCD4 in vitro. Med Sci Monit. 2014;20:1850‐1857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Powles T, Eder JP, Fine GD, et al. MPDL3280A (anti‐PD‐L1) treatment leads to clinical activity in metastatic bladder cancer. Nature. 2014;515(7528):558‐562. [DOI] [PubMed] [Google Scholar]
- 18. Witjes JA, Compérat E, Cowan NC, et al. EAU guidelines on muscle‐invasive and metastatic bladder cancer: summary of the 2013 guidelines. Eur Urol. 2014;65(4):778‐792. [DOI] [PubMed] [Google Scholar]
- 19. Yu Y, Nangia‐Makker P, Farhana L, Majumdar APN. A novel mechanism of lncRNA and miRNA interaction: CCAT2 regulates miR‐145 expression by suppressing its maturation process in colon cancer cells. Mol Cancer. 2017;16(1):155. [DOI] [PMC free article] [PubMed] [Google Scholar]


