Abstract
The results of studies on possible effects of radiofrequency electromagnetic fields (RF‐EMFs) on human waking electroencephalography (EEG) have been quite heterogeneous. In the majority of studies, changes in the alpha‐frequency range in subjects who were exposed to different signals of mobile phone‐related EMF sources were observed, whereas other studies did not report any effects. In this review, possible reasons for these inconsistencies are presented and recommendations for future waking EEG studies are made. The physiological basis of underlying brain activity, and the technical requirements and framework conditions for conducting and analyzing the human resting‐state EEG are discussed. Peer‐reviewed articles on possible effects of EMF on waking EEG were evaluated with regard to non‐exposure‐related confounding factors. Recommendations derived from international guidelines on the analysis and reporting of findings are proposed to achieve comparability in future studies. In total, 22 peer‐reviewed studies on possible RF‐EMF effects on human resting‐state EEG were analyzed. EEG power in the alpha frequency range was reported to be increased in 10, decreased in four, and not affected in eight studies. All reviewed studies differ in several ways in terms of the methodologies applied, which might contribute to different results and conclusions about the impact of EMF on human resting‐state EEG. A discussion of various study protocols and different outcome parameters prevents a scientifically sound statement on the impact of RF‐EMF on human brain activity in resting‐state EEG. Further studies which apply comparable, standardized study protocols are recommended. Bioelectromagnetics. 2019;40:291–318. © 2019 The Authors. Bioelectromagnetics Published by Wiley Periodicals, Inc.
Keywords: mobile phone, electroencephalography, brain activity, alpha band power, radiofrequency
INTRODUCTION
Motivation for This Review
Radiofrequency electromagnetic fields (RF‐EMFs) are the basis of mobile communication, which meanwhile is ubiquitous around the world. There is public concern that RF‐EMF, which are associated with wireless technologies, may have a negative impact on health. At the level of the European Union, this concern is documented by a survey conducted in 2010 as face‐to‐face interviews with 26.602 European citizens from 27 member states [European Commission, 2010]. On average 33% of the European citizens believe that mobile phone masts affect their health to a large extent (ranging from 6% in Finland to 79% in Italy). The corresponding figure for mobile phone handsets was 26% (ranging from 7% in the Netherlands and Denmark to 69% in Italy).
Public concerns are one of the reasons why international agencies and organizations from time to time review the scientific evidence for health effects resulting from RF‐EMF. The World Health Organization [WHO] [van Deventer et al., 2011] and the International Commission on Non‐Ionizing Radiation Protection (ICNIRP) are currently in the process of reviewing literature that has been published since their last evaluations [ICNIRP, 1998; Valberg et al., 2007]. The Scientific Committee on Emerging and Newly Identified Health Risks (SCENIHR) updated their 2009 “Opinion on Health Effects of Exposure to EMF.” The updated “Opinion on Potential Health Effects of Exposure to Electromagnetic Fields (EMF)” was published in 2015 [SCENIHR, 2015]. In some countries, e.g. Sweden, literature is reviewed on an almost annual basis by the local authorities [e.g. Swedish Radiation Safety Authority, 2016, 2018].
Research is performed in different domains: in vitro, in vivo, human experimental (or provocation), and epidemiological studies. After reviewing the evidence from all four areas, the International Agency for Research on Cancer (IARC) of the WHO classified RF‐EMF as possibly carcinogenic to humans [IARC, 2011]. One of the main target organs where effects—if present—are expected to occur is the brain, since by using handheld devices the head is usually the most exposed part of the body.
Early human experimental studies seemed to consistently indicate a small physiological effect on brain activity as measured by electroencephalography (EEG). In a strict sense, EEG is a method for registering the rhythmic fluctuations of brain potentials, which are continuously recorded by electrodes fixed to the scalp. Owing to its high temporal resolution, the EEG technique allows for the application of several linear and non‐linear quantitative approaches to analyze the dynamics of brain signals. So far, however, the vast majority of RF‐EMF studies on the waking EEG considered only the results of conventional linear spectral analysis as primary outcome parameters. An effect on the power of sleep EEG in the spindle frequency range and an effect on the alpha power in the waking EEG have been, and are still being, discussed. These observations were taken into account when in 2010 the WHO published the “Research Agenda for Radiofrequency Fields.” Besides further RF‐EMF provocation studies in children of different ages, the WHO defined “Provocation studies to identify neurobiological mechanisms underlying possible effects of RF on brain function, including sleep and resting EEG” [WHO, 2010, p. 16] as high‐priority research needs with regard to human studies. SCENIHR [2015] also recommended further studies on waking EEG with high priority—especially studies that address age and gender‐specific variations in possible effects.
As the literature provides widely differing subject‐ and measurement‐related instructions on how to perform a waking EEG recording [van Diessen et al., 2015], study results are typically hard to compare. The lack of standardization constitutes a major shortcoming since it hampers meaningful comparisons of results from different laboratories and prevents the pooling of data [Jobert et al., 2012]. To achieve comparability between studies, standardization of recording and analysis is an absolute necessity. A comprehensive guideline for EEG recording and evaluation based on power spectra is available for pharmaco‐EEG trials published by the International Pharmaco‐EEG Society (IPEG) in order to ensure a high level of quality that is essential in this field of research [Jobert et al., 2012]. The present paper critically reviews non‐exposure‐related factors that limit the comparability of studies and thus our knowledge on RF‐EMF effects on waking EEG, therefore leading to the question of how consistent are the “consistent” effects on the power in the alpha frequency range of the EEG.
Recording and Analysis of Waking EEG
The non‐resting‐state EEG with eyes open covers a wide frequency spectrum of rhythmic activity; in a normal adult, medium (8–13/s) and fast (14–30/s) oscillations predominate [Chang et al., 2011]. This frequency spectrum is usually broken down into ranges of frequencies, the so‐called frequency bands. Chang et al. [2011] defined frequency bands as follows: delta (<3.5 Hz), theta (4.0–7.5 Hz), alpha (8.0–13.0 Hz), beta (14.0–30.0 Hz), and gamma (>30.0 Hz). Frequency band classifications, however, may vary from study to study (see below) limiting the comparability of results. The alpha rhythm dominates in posterior regions when eyes are closed and is blocked or attenuated by attention processes [Chang et al., 2011]. The occipital dominance of alpha in the resting‐state EEG is shown in Figure 1.
Figure 1.
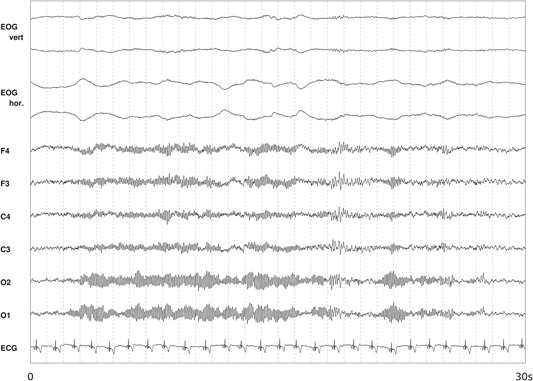
Occipital dominance of alpha in resting‐state electroencephalography (EEG) with eyes closed. Figure shows a 30‐s epoch with six EEG signals (F4, F4, C4, C3, O1, and O2 referenced to the averaged mastoids), a bipolar vertical, horizontal electrooculogram (EOG), and an electrocardiogram (ECG).
In the context of pharmaco‐EEG applications, the traditional parameterization of the EEG is largely based on spectral analysis [Jobert et al., 2012]. Jobert et al. [2012] recommend that absolute spectral EEG values are reported as primary outcome measures. They emphasize that additional spectral parameters such as relative values, dominant frequencies in the alpha and beta frequency bands, should be interpreted in relation to the absolute values. Jobert et al. [2012] recommend frequency bands in units of μV/Hz as follows: delta (1.5 to <6.0 Hz), theta (6.0 to <8.5 Hz), alpha1 (8.5 to <10.5 Hz), alpha2 (10.5 to <12.5 Hz), beta1 (12.5 to <18.5 Hz), beta2 (18.5 to 21.0 Hz), beta3 (21.0 to <30.0 Hz), total power (1.5 to <30.0 Hz), and gamma (30.0 to <40.0 Hz). Note that these frequency bands are defined in the context of identifying drug or substance effects on the EEG while the frequency bands described above originate from the evaluation of the EEG in clinical conditions.
Brain activity recorded from the occipital region in the resting state defines the basic rhythm of an individual's EEG. In adults older than 20 years, the frequency is the most stable feature of the alpha rhythm [Markand, 1990]. The majority has a frequency between 9 and 11 Hz, while the amplitude shows a high inter‐individual variability [Markand, 1990].
To standardize the methodology of EEG recording, Jasper [1958] published a method of electrode placement, the so‐called 10‐20‐system, which is the current basic standard that defines the placement of 21 electrodes (Fig. 2). With currently available technology the number of leads can be increased considerably, e.g. with the 10‐10‐System 80 electrodes that can be positioned. There are also caps available for the recording with even more, e.g. 256 electrodes.
Figure 2.
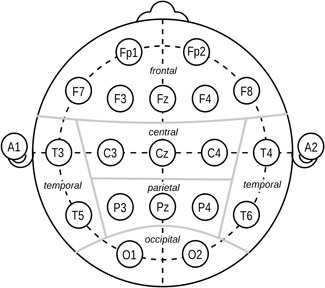
Electrode positions and topographical assignment according to Jasper [1958].
To record the underlying basic rhythm of the EEG, it is necessary to reduce sensory input and to ensure psychological and physical relaxation while simultaneously maintaining a level of vigilance. Jobert et al. [2012, p. 206] state that “the recording should occur in a separate, sound‐attenuated room with constant light (approximately 40 lx), regulated temperature (20–23 °C/68–73 °F) and normal humidity conditions.” For RF‐EMF studies, a further requirement is that the room is shielded. The International Pharmaco‐EEG society also suggests to perform at least one pre‐examination EEG, which allows the subject to become familiar with the apparatus, environment, and recording protocol. This pre‐examination EEG should ideally be performed on a separate day.
METHODS
In the course of our own human experimental studies related to central nervous system (CNS) effects of RF‐EMF, we continuously monitored the publications related to the topic. We covered all publications from 1996 until 2016, which are based on empirical data and were published in the English language, in peer‐reviewed scientific journals. We did not include studies where the waking EEG was derived from an interval of ms prior to an event‐related potential (ERP) or within a cognitive task [e.g. Papageorgiou et al., 2004; Nanou et al., 2005; Hountala et al., 2008; Nanou et al., 2009; Vecchio et al., 2012a; Trunk et al., 2014, 2015]. Following this strategy, we identified 39 publications. We excluded four publications since they were not written in English: Gehlen et al. [1996], Jahre et al. [1996], Spittler et al. [1997], and Krafczyk et al. [2002] (Fig. 3).
Figure 3.
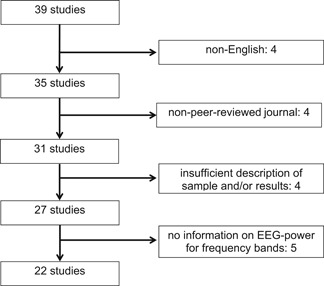
Study selection flow chart. EEG = electroencephalography.
Furthermore, for this review only regular research articles, which were published in peer‐reviewed journals, were considered. This means that four of the 35 publications will not be considered here: Maby et al. [2006], Hinrichs and Heinze [2006], and Smitha and Narayanan [2013, [Link]]. Four other studies were not considered since there is a lack of basic information on the investigated sample and insufficient description of results [Reiser et al., 1995; von Klitzing, 1995; de Seze et al., 1999; Kramarenko and Tan, 2003]. Studies that did not report basic power spectra information and/or amplitudes for the EEG (frequency bands) but used a more complex outcome parameter, e.g. spectral coherence, were also not considered in the present study: Lebedeva et al. [2000], Vecchio et al. [2007, 2010, 2012b], and Lv et al. [2014]. This results in a total of 22 eligible studies for the present review.
Due to public concerns related to possible RF‐EMF effects on human health and a lack of standardization in research related to exploring RF‐EMF effects on the EEG, the EEG studies considered in this review are discussed in the light of the International Pharmaco‐EEG Society's (IPEG) guidelines [Jobert et al., 2012].
RESULTS
The major findings of the 22 studies under consideration are summarized in Figure 4. Overall, around two‐thirds of the studies found an effect of RF‐EMF exposure on the power spectra of waking EEG. The direction of effects, however, is not consistent. One‐third of these studies found a decrease while approximately two‐thirds observed an increase. All studies in some way considered the alpha frequency range. The number of studies, which also looked at other frequency ranges, is much smaller.
Figure 4.
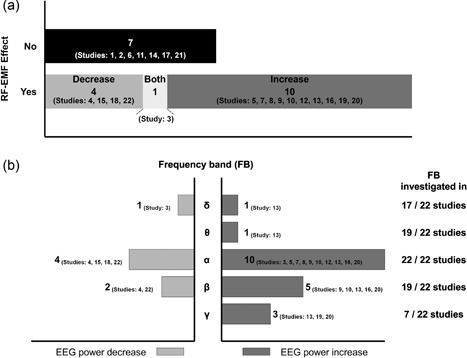
Graphical summary of the major findings observed in the 22 studies under consideration. (a) Number of studies by radiofrequency electromagnetic field (RF‐EMF) effect occurrence (yes/no) and direction of RF‐EMF effect. (b) Number of studies reporting an RF‐EMF effect by frequency band and direction of RF‐EMF effect in relation to the number of studies that investigated the respective frequency band. Studies: 1. Röschke and Mann [1997], 2. Hietanen et al. [2000], 3. Croft et al. [2002], 4. D'Costa et al. [2003], 5. Curcio et al. [2005], 6. Perentos et al. [2007], 7. Regel et al. [2007], 8. Croft et al. [2008], 9. Hinrikus et al. [2008a], 10. Hinrikus et al. [2008b], 11. Kleinlogel et al. [2008], 12. Croft et al. [2010], 13. Hinrikus et al. [2011], 14. Loughran et al. [2013], 15. Perentos et al. [2013], 16. Suhhova et al. [2013], 17. Trunk et al. [2013], 18. Ghosn et al. [2015], 19. Curcio et al. [2015], 20. Roggeveen et al. [2015], 21. Zentai et al. [2015], 22. Yang et al. [2017].
In the following section, the 22 considered studies are discussed with regard to various factors, which limit the comparability of results related to possible RF‐EMF effects on waking EEG.
Overall Sample Selection Criteria
The recruitment of eligible participants is an essential task in research studies. “Eligible” means that volunteers must comply with all predefined selection criteria. An a priori setting of criteria for enrollment is necessary to ensure the safety of potential study candidates and to control for possible confounding factors. According to the Consolidated Standards of Reporting Trials (CONSORT) statement, which is a set of recommendations developed to improve the quality of randomized controlled trial reports, a comprehensive description of the selection criteria is needed [Moher et al., 2001, 2010; Schulz et al., 2010; Moher et al., 2012]. The more stringent the selection criteria are, the more homogeneous is the final sample under investigation, increasing the significance of the results for a specific patient or volunteer group. On the contrary, too restrictive selection criteria affect the external validity of a study, i.e. to which extent the study outcomes can be generalized to the general population [Moher et al., 2010].
The overall criteria for sample selection are summarized in Table 1. It is quite obvious that the eligibility criteria differ considerably between studies. At least the reported information on criteria for selecting eligible subjects varies from just the statement that “healthy subjects or volunteers” were included [e.g. Perentos et al., 2007], to detailed information on inclusion and exclusion criteria [e.g. Regel et al., 2007].
Table 1.
Overall Sample Selection Criteria
| Screening for the history of a | Special consideration given to | Eligibility restrictions on regular (excessive) consumption/intake of | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Authors | Health status | Physical disorder | Neurological disorder | Psychiatric disorder | History of head injury | Handedness | Basic EEG rhythm | Stimulants | Medication | Illicit drugs |
| Röschke and Mann [1997] | HV | + | + | + | − | − | − | + (N) | + | − |
| Hietanen et al. [2000] | HV | NSP a | NSP a | NSP a | − | − | − | − | + b | − |
| Croft et al. [2002] | HV | − | + | − | − | + (R,L) | − | − | − | − |
| D’Costa et al. [2003] | HV | − | − | − | − | − | − | − | + | − |
| Curcio et al. [2005] | HV | − | + | + | − | + (R) | − | − | + | + |
| Perentos et al. [2007] | HV | − | − | − | − | − | − | − | − | − |
| Regel et al. [2007] | HV | + | + | + | − | + (R) | + c | + (A,C,N) d | + | + |
| Croft et al. [2008] | HV | − | + | + | + | + (R,L) | − | − | − | − |
| Hinrikus et al. [2008a] | HV | + | + | + | − | − | − | − | − | − |
| Hinrikus et al. [2008b] | HV | + | + | + | − | − | − | − | − | − |
| Kleinlogel et al. [2008] | HV | + | + | + | + | + (R) | − | − | + b | + |
| Croft et al. [2010] | HV | − | − | + | + | − | − | + (N) | + b | + |
| Hinrikus et al. [2011] | HV | + | + | + | − | − | − | − | − | − |
| Loughran et al. [2013] | HV | − | + | + | − | + (R) | + c | − | + | + |
| Perentos et al. [2013] | HV | − | − | − | − | − | − | − | − | − |
| Suhhova et al. [2013] | HV | + | + | + | − | − | − | − | − | − |
| Trunk et al. [2013] | HV | − | + | − | − | − | − | − | − | − |
| Ghosn et al. [2015] | HV | + | + | + | − | + (R) | − | + (N) | + | − |
| Curcio et al. [2015] | EP | + | + | + | + | − | − | − | + e | − |
| Roggeveen et al. [2015] | HV | + f | + | − | − | − | − | + (N) | − | − |
| Zentai et al. [2015] | HV | − | + | − | − | − | − | − | − | − |
| Yang et al. [2017] | HV | + | + | + | − | + (R) | − | − | − | − |
A = alcohol; C = caffeine; EP = epileptic patients; HV = healthy volunteers; L = left; N = nicotine; NSP = not specified; R = right; + = applies on the basis of the information reported; − = information was not reported.
A health information inquiry was filled out by the partcipants.
Refers only to CNS‐active drugs.
Basic alpha EEG rhythm probably considered as inclusion factor.
Allowed was a moderate alcohol (<5 drinks/week) and caffeine (<3 cups coffee/day) consumption.
Other than anti‐epileptic drugs.
Refers only to cardiac disorders.
Health status and medical history
The vast majority of studies investigated RF‐EMF effects on waking EEG in healthy volunteers. Only one of the 22 studies [Curcio et al., 2015] investigated effects in a sample of epileptic patients (Table 1). Eleven of the 22 studies explicitly stated that subjects were screened for physical disorders, 17 screened for neurological disorders, and 14 for psychiatric disorders. How this screening was performed, i.e. self‐assessment by questionnaire, self‐assessment in an interview performed by a non‐medical staff member, or interview and examination by a medical doctor is not always clearly stated.
Head trauma
A traumatic brain injury (TBI) is caused by a physical force to the head that even in its mildest form may be accompanied by altered brain activities. Rapp et al. [2015] reviewed relevant EEG studies related to this injury and reported that mild TBI is often associated with spectral power changes in the waking EEG. Sandsmark et al. [2017] summarized sleep–wake disturbances and notably excessive daytime sleepiness and spectral changes in sleep EEG after TBI. We therefore looked specifically at whether the studies explicitly mentioned that they considered a history of head trauma in their subjects. Only four studies mention that they controlled for this factor (Table 1).
Handedness
There is general agreement that studies applying EEG should control handedness because of the assumed underlying differences in amplitudes between dominant and non‐dominant hemispheres. For the alpha rhythm, a lateral difference in amplitude can be observed in 60% of healthy adults with an amplitude that is somewhat larger at the right as compared to the left side [Stöhr et al., 1991]. In contrast to earlier assumptions, the observation seems to be physiologic asymmetry with no clear link to handedness or hemispheric‐dominance [Chang et al., 2011]. Studies that conducted repeated recordings of resting‐state EEG showed a stable alpha asymmetry that might represent an individual trait [Tomarken et al., 1992; Papousek and Schulter, 1998]. The number of studies that looked at interhemispheric asymmetry of amplitude in resting‐state EEG are rather scarce, indicating neither a correlation with handedness in a study with children [Petersen and Eeg‐Olofsson, 1971] nor in healthy male adults [Wieneke et al., 1980]. Several studies applying performance tasks during EEG recording show differences in inter‐hemispheric asymmetry, but overall no clear picture can be drawn [French and Beaumont, 1984]. Nevertheless, since it cannot be ruled out that handedness affects the resting‐state EEG, handedness should be considered as a confounding factor. Eight studies provide information on handedness of subjects (Table 1).
Individual basic EEG rhythm
Kubicki and Höller [1980] analyzed the basic EEG rhythm in 1,500 healthy subjects, and observed an alpha‐type rhythm in 86.1% of the subjects, while 7.2% presented with a beta‐rhythm, 5.6% with a theta‐rhythm, and 1.1% presented with a mixed‐type basic EEG rhythm. RF‐EMF effects on the EEG alpha‐power may be hard to find or may be masked when the basic EEG rhythm is different in a sample of subjects with mixed basic rhythms. Table 1 demonstrates that only two studies [Regel et al., 2007; Loughran et al., 2013] probably considered the basic EEG rhythm of subjects included in their studies.
Furthermore, it is known that a low/high natural alpha rhythm may affect EEG responses to drugs [Fink, 2010; Jobert et al., 2012] and that the peak alpha rhythm is largely genetically determined [Markand, 1990; Smit et al., 2005]. If this different responsiveness to drugs is assumed to be observable also for EMF effects, it can be worthwhile to further control this factor, e.g. by genotyping for the catechol‐O‐methyltransferase (COMT) polymorphism. Bodenmann et al. [2009] observed that the Val158MET polymorphism of COMT predicts inter‐individual differences in alpha oscillations in young men, which are in the focus of possible EMF effects on the EEG.
Control of habitual consumption of coffee, alcohol, and nicotine, and intake of medication and illicit drugs
Stimulants such as caffeine, nicotine, and alcohol are the most widely used CNS‐active substances. In addition to acute EEG effects, which are addressed below, they are also associated with long‐lasting alterations in brain activity.
To examine the impact of chronic caffeine intake, Sigmon et al. [2009] compared the effects of daily 400 mg caffeine administration for more than or equal to 14 days on the EEG with those obtained after placebo administration of the same duration. They could show that relative EEG power in the beta2 frequency band (25.0–40.0 Hz) was significantly elevated during chronic caffeine administration. Rass et al. [2015] compared the resting‐state brain activity of daily, non‐daily, and non‐smokers and observed reduced delta (1.5–3.5 Hz) and alpha (8–12.5 Hz) EEG power in non‐deprived chronic smokers. In alcoholics, the resting‐state EEG showed an increased power in beta1 (12.5–16.0 Hz) and beta2 (16.5–20.0 Hz) frequency bands [Rangaswamy et al., 2002] as well as in the theta (3–7 Hz) frequency range [Rangaswamy et al., 2003] compared to age‐ and gender‐matched unaffected controls.
In view of these findings, it is strongly recommended that the habitual consumption of caffeine, nicotine, and alcohol when establishing eligibility criteria for EEG studies is considered. For pharmaco‐EEG trials, IPEG suggests controlling the regular use of these substances by restricting consumption within a certain period of time prior to the start of the study, which may range from a maximum tolerated daily dose to complete abstinence [Jobert et al., 2012].
The application of daily dose restrictions was reported in one study to control for habitual intake of caffeine and alcohol [Regel et al., 2007]. Consumption of nicotine was controlled in five of the 22 studies by defining non‐smoking as inclusion criteria (Table 1). Other studies not reporting the control for habitual caffeine, nicotine, and alcohol consumption does not necessarily imply that they did not do so.
A variety of drugs such as hypnotics and sedatives, anxiolytic drugs including marijuana and cocaine, as well as antibiotics, act on the CNS. Due to the multitude of studies on mostly unspecific acute and chronic effects of CNS‐active drugs on the EEG, we decided not to present any results at this point but refer to an excellent summary of this literature published by Bauer and Bauer [2011]. Medication was controlled at different levels in 10 of the 22 studies, three of them only considered CNS‐active drugs, and the study on epileptic patients controlled for other than epileptic drugs. Five of the 22 studies mention that they controlled for the intake of illicit drugs (Table 1).
Age of the participants
Maturational changes of the brain are reflected by extensive EEG changes [Markand, 1990; Stam, 2011]. In a longitudinal study of school‐age children (6–18 years, 4 years follow‐up), Gmehlin et al. [2011] observed an increase in alpha peak frequency, which was larger in children than in adolescents while the absolute and relative delta and theta power decreased considerably. From age 18 onwards, the EEG remains relatively stable until normal aging becomes visible. With normal aging, a slowing of the alpha frequency, reduced alpha band power (in particular in lower alpha band (8–10.5 Hz), and more uniformly spatial distribution of EEG rhythms have been observed in the elderly [Stam, 2011]. There is a pronounced decrease in the alpha amplitude (8–13 Hz) with increasing age [Rossini et al., 2007]. The amplitude of beta activity is often lower in the elderly, in particular males [Klass and Brenner, 1995]. Furthermore, there is also a global “slowing” of the background EEG with an increase in delta and theta activity in healthy elderly subjects [Rossini et al., 2007], mainly in temporal regions and predominantly on the left side [Klass and Brenner, 1995]. Anderer et al. [2000] did a comprehensive analysis of age‐related changes in absolute and relative EEG power based on 204 healthy volunteers (116 females) in the age range 20–88 years. They observed a significant decrease in the dominant alpha frequency with age starting from 10.3 Hz in 20‐year‐old subjects to 9.3 in 80‐year‐old reflecting a linear decrease of 0.2 Hz per decade. The absolute and relative power of delta, theta, and alpha decreased with increasing age, while the beta power increased in normal aging. Finally, in a large study on 1,498 subjects (735 females) aged 6–86 years, Chiang et al. [2011] observed significant age‐related trends for frequency, position, and amplitude of dominant alpha peaks.
The way of reporting the age of participants varies between the studies under investigation (Fig. 5). In 16 of the 22 studies, an age range is reported. The number of studies reporting a mean age ± a statistic indicating variability is also 16. In some of these studies it is, however, not clear whether the statistic to describe the dispersion is a standard deviation or a standard error of the mean. One study [Croft et al., 2002] reports a mean only. In another study, the weighted mean and standard deviation were computed based on separate information for men and women. One study provides only age information for the total sample and not for the subgroups [Curcio et al., 2005].
Figure 5.
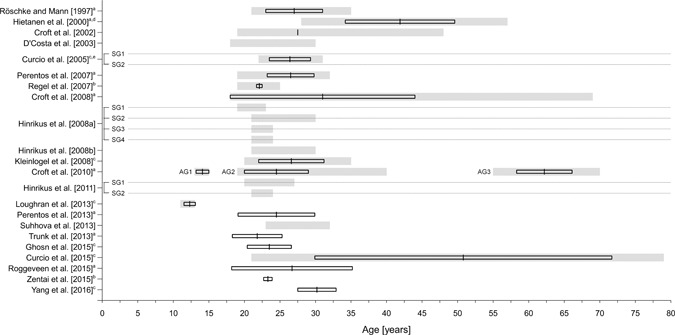
Information on age of the samples in the 22 reviewed studies. Information on age refers either to age ranges (grey‐shaded bars) or to mean values ± a measure of dispersion (black‐framed boxes). Please note that in some cases it was not further specified whether the standard deviation (SD) or standard error (SE) was reported as measure of dispersion. Dotted lines are used for a clear assignment of study subgroups. AG = age group; SG = subgroup; (a) mean ± SD, (b) mean ± SE, (c) not clear whether an SD or SE is reported, (d) weighted mean and standard deviation were computed based on separate information for men and women, (e) no age information was reported for the subgroups.
There are only two studies that investigated RF‐EMF effects on the resting state wake‐EEG in adolescents [Croft et al., 2010; Loughran et al., 2013; Fig. 5]. The large variability with (chronological and additionally maturational) age in children and adolescents has implications for samples sizes, which are necessary to reach statistical significance if an effect is present. In three studies elderly subjects (60+ years) were included. One of them investigated a small sample of (epileptic) patients covering a broad range of ages [21–79 years; Curcio et al., 2015]. In most of the studies, where information on the range is provided, healthy young adults (up to the mid‐thirties) were investigated (Fig. 5). Since the outcome variable spectral power of the resting‐state EEG varies with age, results obtained in a sample of young healthy subjects cannot be generalized to elderly subjects and/or adolescents.
Given these variations in the waking EEG, an age‐stratified analysis is recommended when a broad age range is covered in a large sample. This strategy was followed by Croft et al. [2010] (adolescents: 13–15 years; adults: 19–40 years and elderly: 55–70 years) who observed differential RF‐EMF effects by age. Other approaches for large samples could be to control for age effects statistically, e.g. by the use of z‐scores or by introducing age as an additional factor in the statistical model.
Sex distribution
As already pointed out earlier, age‐related changes are more pronounced in men [Klass and Brenner, 1995]. For adolescents, larger age‐related changes in the absolute power of the EEG—except alpha—were observed for boys [Gmehlin et al., 2011]. Clarke et al. [2001] observed less theta and more alpha in 8 to 12‐year‐old boys than in girls. A study that investigated the EEGs of 1416 healthy subjects aged between 6 and 39 years stated that the percentage of alpha time and alpha continuity were greater in males than in females after adolescence, while the percentage of beta time was higher in females than in males at all ages [Matsuura et al., 1985]. Wada et al. [1994] observed sex differences in EEG activity at rest and during photic stimulation in a sample of 40 healthy subjects (20 males and 20 females) in the age range 19–26 years. Females showed higher amplitudes in the resting‐state EEG, which were statistically significant for the frequency bands delta, theta, alpha2, and beta bands. Sex differences were even more pronounced during photic stimulation. Sex differences have also been observed in a sample of 119 healthy elderly subjects (53 males and 66 females) in the age range from 60 to 87 years [Brenner et al., 1995]. In Brenner et al.’s study, females showed a significant higher mean frequency of beta1 and beta2, while the mean frequency of alpha2 and theta‐beta were lower. Finally, sex differences in EEG activity have also been observed in a study on spontaneous alpha rhythms in mild cognitive impairment and control subjects. Garcés et al. [2013] observed a higher alpha peak frequency in females as compared to males in both groups. The trend was observed over the whole brain, whereas statistically significant differences occurred only over some posterior and right frontal areas.
As Figure 6 indicates, 24 of the 28 samples analyzed in the 22 studies that were comprised of men and women, one study included only women [Roggeveen et al., 2015] and four studied only men [Röschke and Mann, 1997; Regel et al., 2007; Kleinlogel et al., 2008, Yang et al., 2017].
Figure 6.
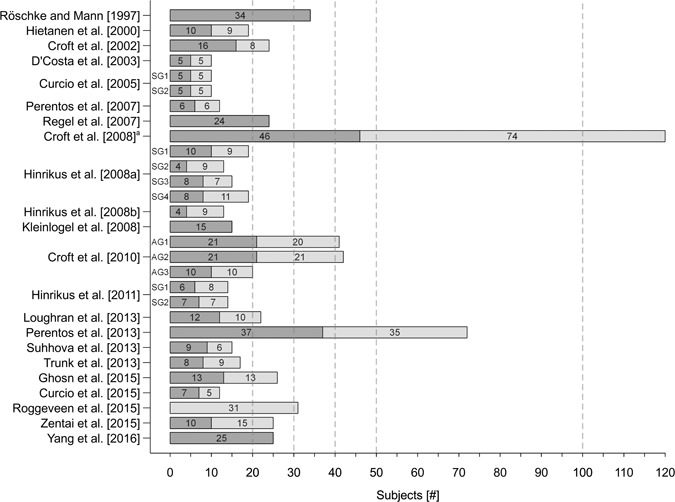
Size and sex distribution of the samples in the 22 reviewed studies. Dark grey bars display the number of males and light grey bars display the number of females. a)Maximum number of subjects considered for analyses was n = 109. AG = age group; SG = subgroup.
Overall Study Characteristics and Procedures
The overall study characteristics and procedures are summarized in Table 2. Except for the study design, which is crossover in all 22 studies, the control of factors known to affect the outcome parameter was treated quite differently between the studies.
Table 2.
Overall Study Characteristics and Procedures
| Controlling for | Instructions for stimulant consumption prior to testing | Risk of carry over effects taken into consideration by | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Authors | Menstrual cycle | Time of day | Regular sleep–wake schedule | Abstinence | Period of abstinence | Study design | ≥24 h wash out periods | Pre‐exposure MCU restrictions | Blindness |
| Röschke and Mann [1997] | NA | + (M) | − | + (Al,C) | 4 days | CO f | − | − | SB |
| Hietanen et al. [2000] | − | − | − | − | − | CO g | + | − | SB |
| Croft et al. [2002] | − | − | − | − | − | CO f | − | − | SB |
| D’Costa et al. [2003] | − | − | − | − | − | CO g | − | − | SB |
| Curcio et al. [2005] | − | + (M) | − | − | − | CO g h | + | + | DB |
| Perentos et al. [2007] | − | − | − | − | − | CO f | − | − | SB, DB |
| Regel et al. [2007] | NA | + (A) | + a | + (Al,C) | 3 days | CO f | + | + | DB |
| Croft et al. [2008] | − | − | − | − | − | CO f | + | − | DB |
| Hinrikus et al. [2008a] | − | + (M) | + b | − | − | CO g , h | + | − | DB |
| Hinrikus et al. [2008b] | − | + (M) | + b | − | − | CO g | + | − | DB |
| Kleinlogel et al. [2008] | NA | + (NSP) | − | + (Al,C,N) | 2 h (C,N), 12 h (A) | CO g | + | + | DB |
| Croft et al. [2010] | − | − | − | + (Al,C) | 24 h | CO f | + | − | DB |
| Hinrikus et al. [2011] | − | + (M) | + b | − | − | COg,h | + | − | DB |
| Loughran et al. [2013] | − | + (NSP) | + a | + (C) | 3 days | CO f | + | + | DB |
| Perentos et al. [2013] | − | − | − | + (Al,C) | 6 h (C), 24 h (A) | CO f | − | + | DB |
| Suhhova et al. [2013] | − | − | + b | − | − | CO g | − | − | SB |
| Trunk et al. [2013] | − | + (M,A) | − | + (C) | NS | CO f | + | − | DB |
| Ghosn et al. [2015] | + (FP) | + (M,A) | + c | + (Al,C) | 24 h | CO f | + | + | DB |
| Curcio et al. [2015] | − | + (M) | + a | + (Al,C) | 2 days | CO f | + | + | DB |
| Roggeveen et al. [2015] | − | + (M,A) | + d | + (Al,C) | 4 h (C), 12 h (A) | CO f | − | − | SB |
| Zentai et al. [2015] | − | + (M,A) | + e | + (Al,C) | 12 h | CO f | + | − | DB |
| Yang et al. [2017] | NA | − | − | + (Al,C,N) | 24 h | CO f | + | + | DB |
A = afternoon; Al = alcohol; C = caffeine; CO = cross over; DB = double blind; FP = folicular phase; M = morning; MCU = mobile communication usage; N = nicotine; NA = not applicabale; NSP = not specified; SB = single blind; + = applies on the basis of the information reported; − = information was not reported.
Controlled by actigraphy and/or sleep logs.
Tired or sleepy participants were excluded.
Applies owing to the eligibility criteria.
Sufficient night rest was ensured.
Duration of sleep prior to the experiments ≥6 h.
Order of exposure conditions was (nearly) counterbalanced.
Order of exposure conditions was randomized.
Conditions to be studied were investigated in subgroups.
Time‐of‐day effects
Time‐of‐day is an important issue in the recording of the EEG. Diurnal variation of EEG spectral power has been reported by Regen et al. [2013] in the course of 40 h of sustained wakefulness. For alpha1 (8.5–10.5 Hz), theta (5.5–8.5 Hz), delta (1.5–5.5 Hz), and beta1 (12.0–18.0 Hz), maximal values have been observed around 12:00–14:00 h. Higuchi et al. [2001] observed an increase in alpha power (8.6–13.3 Hz) recorded at Cz related to a repeated vigilance task. The increase was greatest at 14:00 h and significantly larger than at 8:00 h and 20:00 h. The repetition of a task did not have a similar effect on theta (3.9–7.8 Hz) and beta (14.1–20.3 Hz).
IPEG emphasizes that in studies with repeated assessments recordings should be performed at the same time of the day, preferably between 09:00 and 13:00 h to avoid interference with meal times and postprandial vigilance fluctuations [Jobert et al., 2012].
Out of the seven studies that used a crossover design with assessments on the same day, only two reported that the time of day was kept constant [Röschke and Mann 1997, Roggeveen et al., 2015]. Roggeveen et al. [2015] specify that the time interval in which the experiments were performed ranged from 9 am to 5 pm. The other five studies did not provide any information on the time of day. Nine of the 15 studies that used a crossover design with assessments on different days provided information on the time of day. Five mentioned that the experimental sessions were conducted in the morning, one study reported evening assessments, and in three studies the specified time interval in which the experiments took place ranged again from 9 am to 5 pm. Out of the remaining six crossover studies with assessments on different days, two just mentioned that for all individuals time was kept constant without specifying the exact time of day, and four did not provide any information on this aspect (Table 2). This issue is also relevant for comparability of results from different studies.
Menstrual cycle
Another aspect to consider is that spectral power of the resting‐state EEG in women varies with the menstrual cycle [Creutzfeldt et al., 1976; Becker et al., 1982; Chang et al., 2011]. According to Harding and Thompson [1976], as cited by Chang et al. [2011], the findings of early studies can be summarized as follows: in the preovulatory phase (days 5–14) alpha frequency and the amount of beta increases, in the luteal phase (days 15–23) alpha becomes slower and the amount of alpha increases, while less beta and more theta activity are observed. In the premenstrual phase (days 23–28) alpha frequency increases, the amount of alpha is reduced, and there is more beta activity and less theta activity. In the menstrual phase (days 1–5) alpha frequency is again slower and the amount is increased. In this phase there is less beta and more theta.
Baker and Colrain [2010] analyzed waking EEG spectral power with the menstrual cycle in nine women with severe premenstrual syndrome and in eight female controls. They observed that spectral power of delta/theta frequencies (2–6 Hz) and fast alpha frequency (11–12 Hz) increased in women in the late luteal phase relative to the follicular phase. Brötzner et al. [2014], who investigated the individual alpha frequency (IAF) throughout the menstrual cycle in 57 women with a natural cycle and in 57 women using oral contraceptives, observed that the alpha frequency was related to the menstrual cycle. IAF was highest in the luteal phase and lowest in the late follicular phase. In their study, estradiol levels correlated with alpha frequencies. The absolute power of delta, theta, beta, and prefrontal theta was higher in women than in men [Morgan et al., 2005].
Only one of the 17 studies investigating possible effects of RF‐EMF on the EEG, which included men and women, controlled for variability introduced by cyclic hormonal changes in women (Table 2). Ghosn et al. [2015] investigated women exclusively in the follicular phase of the menstrual cycle. Roggeveen et al. [2015], who studied only women of child‐bearing age, did not provide information on menstrual cycle control. For all other studies that include males and females, there is also no information.
Regular sleep–wake cycle
The resting‐state EEG reflects one aspect of the level of alertness of a subject. Lafrance and Dumont [2000] investigated the diurnal variation of the amplitude of waking EEG assessed at intervals of 2 h from 10 am to midnight in 16 healthy young subjects (12 females), who maintained a regular sleep–wake schedule (with bedtimes around midnight and wake times around 8 am) for four consecutive nights preceding the EEG daytime assessments. The alpha (8.00–11.75 Hz) amplitude was stable from 10 am to noon, increased afterwards steadily in a curvilinear manner until 6 pm, were then stable again until 8 pm, and decreased again until midnight. In a 40‐h sustained wakefulness study [Regen et al., 2013] performed with 24 healthy young subjects (15 females), alpha (8.5–10.5 Hz) EEG activity increased starting at 1 am to a maximum observed at 1 pm. In contrast to Lafrance and Dumont [2000], alpha EEG activity already started to decrease at 1 pm in this sleep‐deprived group. To control for wake time‐dependent variations in EEG alpha power, subjects should be instructed to keep a regular sleep–wake schedule prior to the experimental days. Ten of the 22 studies reported that they considered a regular sleep–wake cycle in one way or the other (Table 2). One study applied a regular sleep–wake schedule just as an inclusion criterion. In four studies, tired or sleepy subjects were excluded from testing, one study reported that a sufficient night sleep was ensured, and another one stated that sleep duration in the night preceding the experiment(s) had to be more than or equal to 6 h. Finally, three studies reported that the regularity of sleep–wake schedules was controlled by actigraphy and/or sleep logs.
Control of caffeine, alcohol, and nicotine consumption prior to testing
As already mentioned above, acute intake of caffeine has an activating effect on the EEG. For example, Dimpfel et al. [1993] observed decreases in the spectral power in the theta and alpha ranges after administration of 200 and 400 mg caffeine compared to placebo administration. In a double‐blind crossover study in which the subjects received either caffeine or placebo, Barry et al. [2005] observed a global reduction of the EEG alpha‐power 30 min after administration of a 250 mg dose of caffeine assessed in a 2‐min eyes‐closed condition, while there was an overall increase in alpha frequency. In 2011, the same group [Barry et al., 2011] could show that intake of caffeine had a larger effect on the EEG alpha amplitude as a measure of arousal than opening of the eyes. Fisher et al. [2012] investigated the electro‐cortical response to nicotine and to a placebo in 20 non‐smoking right‐handed subjects in a study with a double‐blind crossover design. They analyzed five EEG frequency bands and observed significantly greater frontal power in the alpha2 frequency band (10.5–13.0 Hz) after the administration of 6 mg nicotine. Acute effects of alcohol on the EEG in young male social drinkers were studied by Ehlerset al. [1989] in a double‐blind crossover design. Following ingestion of either a low dose of ethanol (0.75 ml/kg) or a placebo drink, the participants completed three 6‐min resting‐state EEG recordings with eyes closed at baseline, 90 min and 150 min post consumption. Alcohol intake resulted in an increased EEG power in the theta (4–7.5 Hz) and slow alpha (7.5–9 Hz) frequency range as well as in decreases in fast alpha (9–12 Hz) peak frequency at anterior and posterior loci that were significant at 90 min, and to some extent also at 150 min post consumption.
To control for these acute effects Jobert et al. [2012, p. 204] stated that: “subjects should refrain from alcohol and caffeine for at least 24 h, and from tobacco or nicotine products for at least 4 h (preferably 8 h) prior to an EEG recording.”
Despite the importance of this aspect, the participants were instructed to abstain from stimulant consumption for a certain period of time prior to testing in only 12 of the 22 studies. Moreover, the specified abstinence periods varied widely between these studies; seven studies completely complied with the IPEG recommendations and one study partially complied (Table 2). Again, that other studies did not report the control for caffeine, nicotine, and alcohol consumption prior to testing does not necessarily imply that they did not do so.
Study design
The fact that EEG power spectra are highly heritable [Stassen et al., 1987], with highest heritability around the alpha peak frequency and lower heritability in the theta and delta bands [Smit et al., 2005], has implications for the design of studies investigating RF‐EMF effects on EEG activity. Inter‐individual differences are much more marked than intra‐individual variations. Since effects—if present—are expected to be small, a crossover design is more appropriate than a parallel‐group design. In statistical terms, a crossover design should result in smaller standard errors for comparisons between “treatments” than a design where treatments are assigned to different subjects [parallel group design, Johnson, 2010]. All 22 studies used a crossover design (Table 2). A disadvantage of crossover trials is the “order” effect, which can be overcome when subjects are randomly assigned to a sequence of treatments in a counterbalanced design, e.g. the same treatments are presented to each individual in a different balanced order.
Carry‐over effect
Another disadvantage might be a “carry‐over” effect, i.e. the persistence of an effect from one period to the period of a subsequent treatment. Given that in sleep studies, effects on the spectral power of the non‐rapid eye movement (NREM) sleep EEG have been observed after 30‐min exposure prior to sleep [e.g. Huber et al., 2000; Loughran et al., 2005], carry‐over effects might be expected in those studies, in which the exposure conditions are applied on the same day. The same applies to prior mobile communication system usage. In seven studies with a crossover design, different exposures were delivered on the same day. This carry‐over effect can be overcome by scheduling a sufficiently long time interval between applications of different treatments (Table 2).
With the same reasoning, i.e. that exposure effects can be observed even after exposure prior to assessment, it is necessary to control mobile phone use prior to any testing. This factor was considered in eight of the 22 studies. In seven studies, subjects were asked not to use their mobile phone within a certain period preceding the experimental sessions. The instructions varied from 2–3 h to more than 12 h. One study did not prohibit mobile phone use during the day prior to the experiments but reported that usage was not more than 10 min [Yang et al., 2017; Table 2].
Effects of single versus double‐blind designs
Another important aspect of the study design is the “blinding” of subjects to the exposure condition. In a single‐blind study the participants are not aware of the exposure condition while the investigator, who is carrying out the experiments with the subject, knows the exposure condition. In a double‐blind study, neither the participant nor the researcher knows the current exposure condition. This ensures the avoidance of conscious and unconscious biases, which is also a requirement of CONSORT. Instead of using the terms single‐ or double‐blind, CONSORT recommends specifying the blinding status of all people involved in the research project (participants, data collectors, data analysts, healthcare providers, etc. [Moher et al., 2012]. Early human experimental studies on RF‐EMF effects on the EEG, i.e. those published up to 2003 [Röschke and Mann, 1997; Hietanen et al., 2000; Croft et al., 2002; D'Costa et al., 2003], used a single‐blind design. With two exceptions [Suhhova et al., 2013; Roggeveen et al., 2015], studies published since 2005 were performed in a double‐blinded manner. Perentos et al. [2007] mentioned that the study was performed double‐blind, but analysis was single‐blind since extremely low frequency (ELF) artifacts were present during verum exposure, which according to the authors, most likely originated from the pulse‐modulated RF signal demodulated in the EEG amplifiers, which were sometimes visible in the recordings (Table 2).
EEG Recordings
Eyes open versus eyes closed
EEG spectral power differs between recording situations with eyes closed and eyes open. As already mentioned, the alpha rhythm is attenuated when eyes are opened (Fig. 7).
Figure 7.
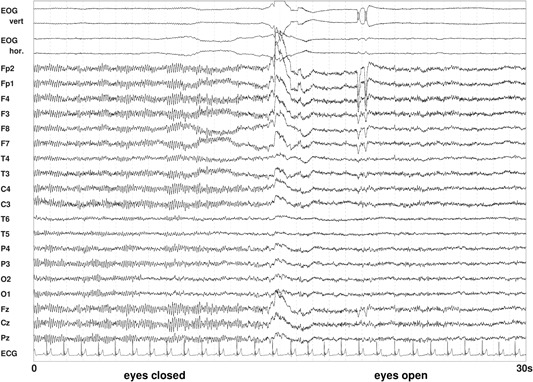
Resting‐state electroencephalography (EEG) with eyes closed (left) and eyes open (right). Figure shows a 30‐s epoch with 19 EEG signals referenced to the averaged mastoids, a bipolar vertical and horizontal electrooculogram (EOG), and an electrocardiogram (ECG). The blockade of alpha in the EEG by opening of the eyes is obvious.
The International Pharmaco‐EEG Society [Jobert et al., 2012] suggests using one of the following three summarized options to ensure standardization and comparability of results:
-
(1)
Vigilance‐controlled EEG (duration 5 min): recorded with eyes open, the vigilance level is controlled by a simple continuous performance task, a fixation point is used to minimize eye movement artifacts, and short external interventions are permitted for vigilance stimulation.
-
(2)
Resting‐state EEG with eyes closed (duration 5–15 min): fluctuations of vigilance are permitted, no task to complete, instruction to sit quietly and keep eyes closed.
-
(3)
Resting EEG with open and closed eyes (duration of each state is 5 min): alternating periods of eyes open and closed of 1‐min duration, standardize visual environment to minimize eye movement artifacts in the eyes‐open condition (no fixation demanded), no vigilance control procedures, and separate analysis for both states is recommended.
Jobert et al. [2012] furthermore recommend that an EEG recording is always done prior to any additional testing (e.g. cognitive paradigms).
In 12 studies the EEG was solely recorded in an eyes‐closed condition and four studies recorded the EEG under an eyes‐open and eyes‐closed condition. One of these four studies, however, only reported results for the eyes‐open condition. Thus, the majority (15 out of 22 studies) provided results for an eyes‐closed condition, in which the basic rhythm can be determined and EEG is not influenced by visual input. The remaining studies—most of them were from the same group [Croft et al., 2002, 2008, 2010; Perentos et al., 2013]—reported results solely for an eyes‐open condition, which limits the comparability of results with those from other studies. Croft et al. [2002, p. 1624] argues for the eyes‐open condition as follows: “We required subjects to keep their eyes open in the resting EEG, to keep them relatively awake, as drowsiness can affect the “alpha” range, and such variance would act as noise and reduce the chances of detecting MP‐related changes to alpha.” In three of the studies that recorded the EEG with eyes open, the participants watched a documentary clip during recording [Trunk et al., 2015; Roggeveen et al., 2015; Zentai et al., 2015; Table 3]. In one study [Kleinlogel et al., 2008], subjects had to react to a randomly presented tone (every 20–40 s) over a headphone during an eyes‐closed EEG condition.
Table 3.
Waking Electroencephalography (EEG) Specifications
| EEG segment length analyzed per exposure condition | |||||
|---|---|---|---|---|---|
| Authors | Eyes open/closed | Prior to exposure | During exposure | After exposure | Vigilance control during EEG |
| Röschke and Mann [1997] | Closed | NA | 3.4 min | NA | − |
| Hietanen et al. [2000] | Closed | NA | 1 min | NA | + |
| Croft et al. [2002] | Open | NA | 4 × 30–90 s | NA | − |
| D’Costa et al. [2003] | Closed | NA | 25 min | NA | − |
| Curcio et al. [2005] | Closed | NA | 7 min b | 7 min b | − |
| Perentos et al. [2007] | Closed | 7.5 min | NA | 7.5 min | − |
| Regel et al. [2007] | Both | 6 min c | NA | 3 × 6 min c | + |
| Croft et al. [2008] | Open | NA | 10 min | 10 min | − |
| Hinrikus et al. [2008a] | Closed | 10 × 1 min | 10 × 1 min | NA | − |
| Hinrikus et al. [2008b] | Closed | 5 × 1 min | 5 × 1 min | NA | − |
| Kleinlogel et al. [2008] | Closed d | 4 min | 4 min + 2 min | 6 min | + |
| Croft et al. [2010] | Both e | 5 min c | 10 min + 2 × 5 min c | 5 min c | − |
| Hinrikus et al. [2011] | Closed | (5 or 10 b ) × 1 min | (5 or 10 b ) × 1 min | NA | − |
| Loughran et al. [2013] | Both g | 6 min c | NA | 3 × 6 min c | + |
| Perentos et al. [2013] | Open | 5 min | 4 × 5 min | 5 min | − |
| Suhhova et al. [2013] | Closed | 5 × 1 min | 5 × 1 min | NA | − |
| Trunk et al. [2013] | Open g | 10 min | NA | 5 × 2 min | − |
| Ghosn et al. [2015] | Both f | 12 min c | 18 min c | 12 min c | − |
| Curcio et al. [2015] | Closed | 45 min | 45 min | 45 min | + |
| Roggeveen et al. [2015] | Open g | NA | (2 + 4 a ) × 15 min | NA | − |
| Zentai et al. [2015] | Open g | 10 min | 10 min | 5 × 2 min | − |
| Yang et al. [2017] | Closed | 10 min | 3 × 10 min | 10 min | + |
EEG = electroencephalography; NA = not applicable; + = applies on the basis of the information reported; − = Information was not reported.
Time indicated refers to verum and sham exposure applied within the same experimental session.
Time indicated refers to different sub groups.
Time indicated includes equally lasting periods of eyes‐open and eyes‐closed.
Repeated reactions to a tone were demanded during EEG.
Only open reported.
Only closed reported.
Participants watched a documentary clip during EEG.
Vigilance‐control versus no vigilance‐control during EEG recording
Jobert et al. [2012] suggested standardizing recording conditions or at least describing the methods in detail to facilitate comparisons between studies. Different kinds of interventions, such as conducting simple tasks, instructions to open or close eyes or to relax, or fixating on a point, lead to changes in vigilance. If no intervention is applied, drowsiness may occur or even sleep instead of wakefulness. From Table 3 it becomes clear that there is a wide variety of recording methods between studies with regard to the combination of eyes closed and/or open and different methods of vigilance control or no vigilance control at all.
None of the studies conducted a vigilance‐controlled EEG as in the first of the three recommended EEG recording conditions from Jobert et al. [2012; see 1) in the prior section]. Two studies monitored vigilance of subjects during eyes‐open and eyes‐closed sessions, but no performance task was conducted during eyes‐open sessions [Regel et al., 2007; Loughran et al., 2013]. In four studies, vigilance was controlled online during eyes‐closed conditions [Hietanen et al., 2000; Kleinlogel et al., 2008; Curcio et al., 2015; Yang et al., 2017; Table 3], and in two of them subjects were alerted whenever they showed signs of drowsiness [Hietanen et al., 2000; Curcio et al., 2015]. In one study, EEG recordings under eyes‐closed conditions were inspected retrospectively and recordings were repeated on another day if subjects were drowsy [Suhhova et al., 2013]. From the remaining seven studies that applied or referred to only eyes‐open conditions, none reported online monitoring and/or interventions during EEG recording [Croft et al., 2002, 2008, 2010; Perentos et al., 2013; Trunk et al., 2013; Roggeveen et al., 2015; Zentai et al., 2015].
Four studies [Röschke and Mann, 1997; D'Costa et al., 2003; Curcio et al., 2005; Perentos et al., 2007] recorded the resting EEG in a sitting position with closed eyes without vigilance control under conditions similar to those described by Jobert et al. [2012; see 2) in the above section]. In the studies of Hinrikus et al. [2008a,2008b, 2011], the EEG was recorded under eyes‐closed conditions without any intervention, but subjects were lying in a relaxed position with their ears blocked.
The third kind of recording condition, with both eyes open and eyes closed without any vigilance control [Jobert et al., 2012], was only partially conducted in the study of Ghosn et al. [2015], since they instructed their subjects to look at a screen 1 m ahead of them. They reported results of the eyes‐closed condition only.
EEG segment lengths
The EEG is subject to fluctuations over time. Especially in the resting‐state EEG there are systematic trends. For example, EEG spectra in the beginning of a 5 min eyes‐closed resting EEG differ from those in the end [Jobert and Wilson, 2015]. Therefore, both the duration of analyzed EEG blocks and their temporal arrangement within the session may affect results. Systematic trends will introduce differences if two conditions to be compared are applied consecutively and only once in one session. The same is true for a pre–post comparison. Crossover and counterbalancing of conditions will reduce this influence on results, but systematic trends in the EEG still introduce additional variance. Effects of EEG fluctuations over time can also be reduced by applying a design with interleaving the different conditions in short segments. However, if carry‐over or other than short‐time effects are assumed, such an interleaved design will not find them. In general, variance of EEG variables will rise with shorter total analyzed segment length per condition.
In the publications reviewed here, rather different arrangements of conditions and analyzed blocks of EEG were applied. The time elapsed from the start of one condition to the start of the next one ranges between 1 and 45 min. The total duration of analyzed EEG segments per condition also ranges from 1 to 45 min. Of the studies reviewed here, four [Perentos et al., 2007; Regel et al., 2007; Loughran et al., 2013; Trunk et al., 2013] only analyzed EEG pre‐ and post‐exposure (Table 3).
If RF signals enter the EEG recording device, they may cause technical artifacts. Analyzing EEG recorded during RF‐EMF exposure without generating false positive results can therefore be a challenge. It is planned to address this in a second part of the review focusing on technical aspects. Of the publications reviewed here, 18 include analyses of EEG segments recorded during exposure (Table 3).
Number of leads, topographical distribution, reference
The number of considered electrodes has implications and challenges for the statistical analyses (e.g. control for multiple testing). The number of electrodes used to analyze possible RF‐EMF effects on the EEG varies from one [Regel et al., 2007] to 61 [Croft et al., 2010] (Fig. 8). Six out of the 22 studies considered five or fewer electrodes (Fig. 8). The four Estonian studies [Hinrikus et al., 2008a,2008b, 2011; Suhhova et al., 2013] used eight electrodes, and Roggeveen et al. [2015] used 12 electrodes (omitting the temporal, some frontal, and frontopolar derivations from the 10–20‐system). Six studies recorded the EEG basically at those 19 electrodes with positions defined according to the international 10‐20 system. The recording from 21 electrode positions placed according to the 10‐20 system [Jasper, 1958] is recommended as the minimal electrode configuration by IPEG for pharmaco‐EEG studies.
Figure 8.
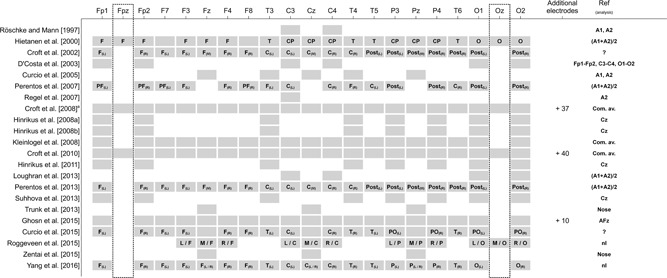
Number and topographical distribution of EEG electrodes (including EEG analysis references) used in the 22 studies under consideration. Figure shows all 19 EEG electrode positions according to the 10–20‐system [Jasper, 1958] plus electrode positions Fpz and Oz (black dotted‐framed boxes) that do not belong to the standard electrode locations of the 10–20‐system. More than these 21 electrodes were considered in three of the 22 studies. Some studies did not clearly provide information on the reference used for analysis. This lack of information is either indicated by a “?” (not clear whether the specification refers to the recording or to the analysis reference) or by an “nI” (no information is provided about the analysis reference). C = Central; Com. av. = common average; CP = Centroparietal; EEG = electroencephalography; F = Frontal; P = Parietal; PF = Prefrontal; PO = Parieto‐Occipital; Post = Posterior; O = Occipital; L = Left; M = Midline; R = Right; T = Temporal. 1)Not further specified electrodes were grouped into front ipsilateral, front contralateral, posterior ipsilateral, and posterior contralateral scalp regions.
Three studies used a much larger number of electrodes [Croft et al., 2002, p. 58; Croft et al., 2010, p. 61; Ghosn et al., 2015, p. 29; Fig. 8]. For the recording of a higher number of electrodes, usually specific electrode caps are used, which are available for different head sizes. Out of the nine studies that recorded the EEG from 19 or more electrodes, seven mention that they used a cap. For two there is no information about the use of a cap. In those studies that recorded a lower number of electrodes, these were also positioned according to the 10‐20 system. Croft et al. [2008], who recorded the EEG from 58 electrodes, used a standard procedure comparable to the 10‐10 international system [Nuwer, 1987].
Considering a high number of electrodes enables us to distinguish topographical variation (e.g. left–central–right; frontal–parietal–occipital) in possible effects. Eight studies grouped electrodes according to scalp regions. Out of these, Hietanen et al. [2000] were the only ones who did not differentiate between hemispheres but only between frontal, temporal, centroparietal, and occipital regions. The others grouped electrodes according to frontal–occipital orientation and hemisphere. One study did further specify which electrodes were used for grouping [Croft et al., 2008]. Two studies omitted the central electrodes Cz, Fz, and Pz [Perentos et al., 2007; Curcio et al., 2015], and one included these three electrodes both for a grouping of right hemisphere electrodes and left hemisphere electrodes [Yang et al., 2017]. The other three studies used the three central electrodes as distinct entity [Croft et al., 2002; Perentos et al., 2013, Roggeveen et al., 2015]. Besides the differences in dealing with central electrodes (Fz, Cz, and Pz), there is variability in regard to the grouping of electrodes—even for research from the same group (Fig. 8).
The EEG reference used in the analysis may affect the outcome (Fig. 9). EEG signals are always differences in voltages between two electrodes varying with time. While one of the electrodes is the location mentioned, the other one is the reference location. In favor of more robustness against disturbances, there are also recording techniques using several reference locations combined for real measurement within the electronics of the EEG acquisition device. Nowadays, in most cases recordings are done with a common reference for all locations. The resulting data can then be easily converted to another montage, i.e. to signals based on another (common) reference or several references.
Figure 9.
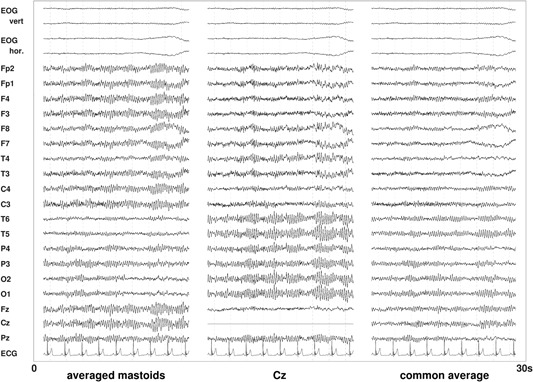
Examples of the impact of different references on the resting‐state electroencephalography (EEG). In the averaged mastoids montage, for most locations comparably high alpha amplitudes occur, with the lowest amplitudes for the temporal and frontal regions. With the Cz (single electrode) reference, the central and frontal regions show lower alpha amplitudes (the Cz location itself is zero in this diagram, of course.) A common average reference (mean of all scalp locations) leads to a generally weaker alpha rhythm.
While the reference (or reference combination) actually used in the recording does not influence the analysis results (provided that there are no technical problems with the recording quality), the reference chosen for analysis of changes in the EEG at given locations does (see example for resting‐state EEG with eyes closed in Fig. 9). Depending on the reference chosen, the same segment of the EEG recording shows different amplitudes for most locations. This is especially true for signals that are generated over broader regions of the brain, like the alpha rhythm.
Based on dense (129‐channel) eyes‐closed EEG, Qin et al. [2010] observed significant differences in the absolute power for seven EEG frequency bands between different references (reconstructed infinity (REST), average of electrodes, linked mastoids, and left mastoid) and mentioned that: “All comparisons demonstrated frequency dependent reference effects” [Qin et al., 2010, p. 1981].
Jobert et al. [2012] recommend a recording against a single reference electrode (e.g. Cz, A1, or A2) or the arithmetic mean of A1 and A2 (“linked ears” or “linked mastoids”). However, to be able to correct problems affecting the quality of the EEG (e.g. different impedances of A1 and A2) the data should be stored in a format that allows for using another reference after recording (e.g. common average reference).
In 18 of the 22 studies, the analysis reference is explicitly reported, and two other studies mention a reference without specifying whether it is a recording or an analysis reference [Croft et al., 2002; Curcio et al., 2015]. Another two studies only mention the recording reference without specifying the analysis reference [Roggeveen et al., 2015; Yang et al., 2017]. Figure 8 demonstrates that there is a large variation in the analysis reference for EEG recordings between studies. Ten studies used either single or “linked” (averaged) mastoids as a reference. Using averaged mastoids is preferable according to Qin et al. [2010]. Four studies used a Cz reference. Another three studies did their analyses on bipolar derivations or on EEG referenced to the nose. The nose as a reference introduces a higher level of eye movement or eye blink artifacts.
Overall, the use of different references, different numbers of electrodes, and different topographical clustering may contribute to heterogeneity of the observed results.
Frequency bands
The definition of frequency bands varies from study to study (Fig. 10). All 22 studies considered the alpha frequency range in their analyses. While 16 studies looked at alpha frequencies that covered most or all of the alpha frequency band(s) defined by Chang et al. [2011] and Jobert et al. [2012], three studies looked at narrower frequency bins within the alpha frequency range [Hinrikus et al., 2011; Trunk et al., 2013; Zentai et al., 2015], and another three did not report on specific frequency bands at all [Curcio et al., 2005; Regel et al., 2007; Loughran et al., 2013].
Figure 10.
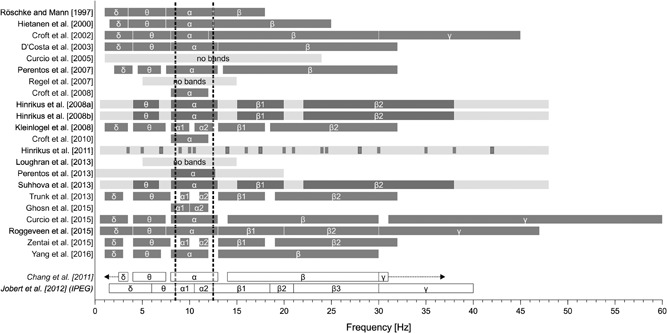
Frequency bands used in the 22 studies under consideration. Dark grey‐shaded bars indicate the frequency bands, while the light grey‐shaded bars indicate the whole frequency range if no clear frequency band breakdown was reported. For comparison, the reference frequency band classifications according to Chang et al. [2011] and Jobert et al. [2012] are displayed at the bottom of the figure. The dotted lines mark the alpha frequency range used in pharmaco‐electroencephalography (EEG) studies.
Data preprocessing (signal filtering, artifact detection, and elimination)
The acquired EEG signals depend on the conditions for recording and those for the subject recorded. Settings of the recording device or parameters chosen within the preprocessing may affect the outcome of studies. If, e.g., no notch filter for suppression of artifacts from the utility grid (50 or 60 Hz) is applied, these disturbances may interfere with the artifact rejection (peak amplitudes of EEG, 50/60 Hz disturbance, and other artifacts add linearly). When amplitude thresholds are used as an artifact criterion, the sensitivity of the artifact rejection will rise depending on the amplitude of the 50/60 Hz disturbance. In general, artifact rejection algorithms determine the segments included in the analysis, and this selection may be correlated with properties of the EEG.
There are some other settings affecting spectra. High‐pass filters are used to damp slow (drift) signals and therefore reduce spectral power contribution resulting from unwanted edge effects in the spectral analysis. The segment length and the applied tapering function also have an effect. High‐pass filters also reduce signal amplitudes beyond their cutoff frequency [Dworetzky et al., 2011] and directly change the spectrum. Additionally, different properties of the recording devices and electrodes may lead to varying artifact levels and different relations between movements and artifacts in experiments.
Artifact identification and elimination is crucial for a meaningful quantitative analysis of the EEG [Jobert et al., 2012]. Artifacts can originate from various sources, e.g. ocular, cardiac, muscular, behavioral, sweating, and respiratory. To be able to detect ocular artifacts in the EEG and correct for the artifacts offline, the recording of a bipolar vertical and horizontal electrooculogram (EOG) is required [Jobert et al., 2012]. Even when automated EOG artifact rejection is applied, an additional visual inspection is recommended [Jobert et al., 2012]. Another approach to reduce EEG artifacts caused by electrical signals from eye movements and/or eye blinks, including small, subliminal disturbances, is usually termed as “EOG correction.” Various methods can be applied for EOG correction [Croft and Barry, 2000]. Often a requirement for these methods is a recording of eye movements (which is especially dedicated to this purpose) for calibration of the artifact removal (compensation) calculation. This accounts for anatomical variability and for variance in the geometry of electrode placement.
Whenever bands of higher frequencies (beta and gamma) are the focus, special interest should be paid to muscle artifacts. Therefore, the recording of an electromyogram (EMG) is also recommended. Furthermore, the recording of an electrocardiogram is recommended by IPEG [Jobert et al., 2012] to assess the activity of the autonomous nervous system.
In the 22 EMF‐related studies discussed here, artifact detection and elimination is reported to be done at different levels. Ten studies explicitly mention that an EOG was recorded [Croft et al., 2002; Curcio et al., 2005; Croft et al., 2008, 2010; Loughran et al., 2013; Perentos et al., 2013; Trunk et al., 2013; Curcio et al., 2015; Roggeveen et al., 2015; Zentai et al., 2015]. Some of them mention that they applied an amplitude criterion [Croft et al., 2008: rejection of voltages greater ± 200 µV; Trunk et al., 2013: artifact at ±100 µV]. Most of the studies (additionally) used a visual inspection, and some even had the data double‐checked by independent experts [e.g. Curcio et al. 2015].
However, even in most of those studies where a recording of an EOG is not explicitly mentioned, some artifact detection and rejection was considered. Röschke and Mann [1997] visually removed epochs with eye or movement artifacts, Hietanen et al. [2000] used artifact‐free epochs without specifying how they identified them, Perentos et al. [2007] identified artifacts based on an amplitude criterion (rejection automatically ≥60 µV), Kleinlogel et al. [2008] identified artifacts if the EEG amplitude was greater than ±60 µV or less than 0.5 µV, Yang et al. [2017] used the EEGLAB Toolbox for artifact rejection and an amplitude more than ±100 µV, Hinrikus et al. [2008a] mentioned an off‐line filtering without further specification, and Hinrikus et al. [2008b] removed modulation frequencies of the EMF signal as artifacts. Regel et al. [2007], Loughran et al. [2013], and Suhhova et al. [2013] finally reported that they did a visual inspection for artifacts without further specification. There are only very few studies where no information on artifact handling is given [D'Costa et al., 2003; Hinrikus et al., 2011; Ghosn et al., 2015].
Statistics
Sample size
An accurate a priori calculation of sample size increases the probability that a study is capable of detecting a statistically significant difference of a given magnitude if such a difference exists. While undersized studies may miss “true” treatment effects, even tiny effects of meaningless clinical and/or biological relevance can reach statistical significance in oversized studies. Both scenarios should be avoided for economic (waste of resources) and ethical (unjustified exposure to potentially harmful interventions) reasons [Lenth, 2001].
According to CONSORT, detailed information about how the sample size was determined should be indicated and conclusively justified in publications [Moher et al., 2001; Schulz et al., 2010]. The CONSORT guidelines state that all parameters necessary for the calculation of sample size must be specified: the significance level α, the statistical power 1‐β, the estimated outcomes in each group and, for continuous outcomes, the assumed standard deviation of the measurements [Moher et al., 2010]. Effect size measures can be used instead of the last two elements. Estimates for these values can usually be derived from previous studies or from pilot projects. Sample size determination for within‐person randomized trials further requires an estimate of the expected within‐person correlation of outcomes [Pandis et al., 2017].
Given the assumption that expected RF‐EMF effects on the EEG are small, it usually needs large samples to detect significant effects. In most of the reviewed studies, however, sample size is comparatively small (Fig. 6). Exceptions are the studies by Croft et al. [2008, 2010] and Perentos et al. [2013] with 120, 63, and 72 subjects, respectively. For all other studies, sample size is less than 35. In 14 studies, the samples size is less than 20. Consequently, statistical power, i.e. the probability that non‐significant results really reflect non‐existing effects, is rather low in most studies. Notably, none of the 22 studies under review provide information about a priori sample size calculation.
EEG parameters
IPEG prefers absolute spectral power values over relative spectral power values as primary outcome measure in EEG studies [Jobert et al., 2012].
In some studies, ratios of power values are included in the analyses. However, arithmetic means calculated from ratios of values measured under different conditions tend to be greater than 1.0 for random data. Arithmetic means of ratios (or of percent values) should therefore be avoided. Geometric means of ratios can be used. Alternatively, all individual ratios could be log‐transformed before being further processed. Another solution would be the use of differences instead of ratios. In eight of the 22 studies under review, certain kinds of ratios had been analyzed (Table 4).
Table 4.
Statistical Specifications
| EEG parameter | Testing for normality | |||||||
|---|---|---|---|---|---|---|---|---|
| Authors | Unity | Change expressed as | Normality check of untransformed data | Log transformation | Normality check of transformed data | Statistical method | Method to control over MCP | Indication of effect size measure |
| Röschke and Mann [1997] | P | D | − | − | − | NP | − | − |
| Hietanen et al. [2000] | P | D | − | − | − | PA | − | |
| Croft et al. [2002] | A | D | − | + | − | PA | + | + |
| D’Costa et al. [2003] | P | D | − | − | − | PA | − | − |
| Curcio et al. [2005] | P | D | + | P | + | − | ||
| Perentos et al. [2007] | P | D | − | − | − | PA | + | − |
| Regel et al. [2007] | P | R a | − | + | P | − | − | |
| Croft et al. [2008] | P | D | + (↓) | − | − | NP | + | |
| Hinrikus et al. [2008a] | P | R b | − | − | − | PA | + | − |
| Hinrikus et al. [2008b] | P | R b | − | − | − | PA | + | − |
| Kleinlogel et al. [2008] | P | D | + (↓) | + | + (↑) | PA | + | − |
| Croft et al. [2010] | P | R c , D | + (↓) | − | − | NP | + | + d |
| Hinrikus et al. [2011] | P | R b | − | − | − | PA | + | − |
| Loughran et al. [2013] | P | R a | − | − | − | PA | + | − |
| Perentos et al. [2013] | A | D | + (↓) | − | − | NP | + | − |
| Suhhova et al. [2013] | P | R b | − | − | − | PA | + | |
| Trunk et al. [2013] | P | D | NSP | + | + (↑) | PA | + | |
| Ghosn et al. [2015] | P | D | − | + | − | PA | + | − |
| Curcio et al. [2015] | P | R c , D | − | + | − | PA | − | |
| Roggeveen et al. [2015] | P | D | + (↓) | + | NSP | PA | + | − |
| Zentai et al. [2015] | P | D | NSP | + | + (↑) | PA | − | + |
| Yang et al. [2017] | P | D | − | − | − | PA | + | − |
A = amplitude; D = difference; NSP = not specified; NP = nonparametric; PA = parametric; P = power; R = ratio; + = applies on the basis of the information reported; − = information was not reported; ↓ = not normally distributed data; ↑ = =normally distributed data.
First post exposure intervals relative to baseline and second verum relative to sham in %.
Dring exposure intervals relative to baseline for each exposure condition.
During and post exposure intervals relative to baseline for each exposure condition.
Only for one subsample.
Statistical analyses
Choosing an appropriate statistical method for hypothesis testing depends on several aspects: the type of study design (paired vs. unpaired samples), the number of dependent variables (univariate vs. multivariate), the number of experimental conditions (two vs. multiple), the scale of the outcome measure (nominal, ordinal, continuous), and certain assumptions about the data (normality, homogeneity of variances) [Glantz, 2012]. The last two aspects determine whether parametric or nonparametric statistics should be used. Parametric tests are preferred to nonparametric methods due to their larger statistical power when the normality assumption is satisfied [Glantz, 2012]. However, violations of the parametric assumptions may have considerable implications on the outcome and can lead to erroneous interpretation of the results. Deviations from normal distribution may be accompanied by a power loss of parametric tests as compared to nonparametric tests, in particular when the deviations from normality are large and the sample size is small with, e.g. fewer than 25 individuals per group [Kitchen, 2009]. If violations of the variance assumptions are present, a correction factor to the degrees of freedom can be applied to overcome this problem. For example, correcting for violations of sphericity in a repeated measures analysis of variance can be accomplished by the Greenhouse and Geisser [1959] or Huynh and Feldt [1976] adjustment method.
The statistical analysis of EEG data constitutes a particularly challenging task for at least two reasons. First, empirical distribution of EEG spectral parameters usually shows quite large deviations from normality [Jobert and Wilson, 2015]. To cope with this problem, it is common practice to apply transformation methods (log transformation, square root transformation, etc.) in order to achieve normally distributed data. However, such transformation attempts often fail to have the desired effect [Gasser et al., 1982; Jobert and Wilson, 2015].
Another difficulty in EEG studies is the multivariate nature of electrophysiological data. EEG data structures may contain either a temporal (different time points), spectral (different frequencies), or spatial (different scalp locations) dimension, or, in more complex analyses, any combination of these dimensions. Since multivariate methods are not easily applicable to every data set, signal averaging over time, localizations, or frequencies is required to reduce the multivariate problem to a univariate one [Maris, 2012]. However, many univariate comparisons risk multiplicity. Multiplicity refers to a type 1 error rate inflation because of multiple significance tests, potentially resulting in an increased probability of a false‐positive finding. To account for multiplicity, the overall significance level may need to be adjusted [Li et al., 2017]. Value adjustment approaches, such as the Bonferroni correction or one of its derivatives [e.g. Holm adjustment, PHolm, 1979; Hochberg adjustment, Hochberg, 1988], are usually advised, but due to very small significance cut‐offs when many statistical tests are performed, they tend to be too conservative [Ferber et al., 1999]. Instead, permutation test procedures either in combination with the maximum‐statistic approach [Groppe et al., 2011] or with cluster statistics [Maris and Oostenveld, 2007; Groppe et al., 2011; Mensen and Khatami, 2013] have been proven to be valid alternatives to control for false‐positive findings in EEG studies.
Merely using p values to interpret results supports only the conclusion whether an effect exists or not, but it does not provide information on the magnitude of the effect. As already mentioned earlier, observed effects can be statistically significant but meaningless with regard to clinical or biological relevance. Hence, reporting effect size estimates along with p values facilitates full understanding of the outcome [Sullivan and Feinn, 2012]. In addition, effect size estimates help other investigators to compute the required number of participants in related research efforts and replication studies, respectively [Lakens, 2013].
Table 4 summarizes some of the statistical specifications of the 22 considered studies related to possible RF‐EMF effects on waking EEG. It shows that the statistical approaches used in these studies differed from each other in several aspects. Parametric statistical procedures were applied in the majority of the studies (18 of 22), although the assumption of normally distributed data was explicitly reported to have been tested only in three of them. In all three studies, normal distribution was achieved by a log‐transformation of the data. A log‐transformation of the data was also performed in six other studies, but whether these attempts yielded the expected result was not further specified. In four studies, the original untransformed data was analyzed by means of nonparametric statistical approaches. The multiple comparison problem was addressed in 15 of the 22 studies. Effect size estimates were only sparsely reported in the 22 EMF‐related studies under review. Only three studies used standardized metrics to indicate the magnitude of an effect [Croft et al., 2002; Trunk et al., 2013; Zentai et al., 2015], while in one study this information was only reported for one subgroup [Croft et al., 2010].
CONCLUSION
The preceding discussion stresses that the currently available studies on RF‐EMF effects on waking EEG differ in many non‐exposure related aspects, which strongly limit comparability and thus prevent reliable conclusions. SCENIHR [2015, p. 222] acknowledges these problems and among others emphasizes that “there is a high priority research need for (preferably multicentre) neurophysiological studies in volunteers with pre‐defined effect sizes, based on a priori considerations of power and sample size (type I and type II errors and adequate sample size for the statistical test(s) to be used) for data analysis according to a predefined analysis protocol.” Thus, it can be concluded that there is a strong need for a standardized assessment under many aspects to ensure comparability of results and reliable conclusions.
Assuming that modulated RF signals exert a modulating effect on the EEG, a spectral analysis based on frequency bands used in clinical EEG or as suggested by IPEG might be inadequate. Additionally, more sophisticated, e.g. nonlinear methods, might be indicated for analysis. Such methods have been successfully applied in other fields, e.g. in anesthesiology or neurology. Introducing such methods in RF‐EMF research might provide further insights.
Conflicts of interest: None.
References
REFERENCES
- Anderer P, Saletu B, Pascual‐Marqui RD, Semlitsch HV. 2000. EEG and ERP topography and tomography in normal aging In: Saletu B, Krijzer F, Ferber G, Anderer P, editors, Electrophysiological Brain Research in Preclinical and Clinical Pharmacology and Related Fields ‐ An Update Wien, Austria: Facultas Universitätsverlag; pp 122–138. [Google Scholar]
- Baker FC, Colrain IM. 2010. Daytime sleepiness, psychomotor performance, waking EEG spectra and evoked potentials in women with severe premenstrual syndrome. J Sleep Res 19:214–227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barry RJ, Rushby JA, Wallace MJ, Clarke AR, Johnstone SJ, Zlojutro I. 2005. Caffeine effects on resting‐state arousal. Clin Neurophysiol 116:2693–2700. [DOI] [PubMed] [Google Scholar]
- Barry RJ, Clarke AR, Johnstone SJ. 2011. Caffeine and opening the eyes have additive effects on resting arousal measures. Clin Neurophysiol 122:2010–2015. [DOI] [PubMed] [Google Scholar]
- Bauer G, Bauer R. 2011. EEG, drug effects, and central nervous system poisoning In: Schomer DL, Lopez da Silva FH, editors, Niedermeyer's Electroencephalography: Basic Principles, Clinical Applications, and Related Fields, Sixth Edition Phliadelphia, PA: Lippincott Williams and Wilkins; pp 901–922. [Google Scholar]
- Becker D, Creutzfeldt OD, Schwibbe M, Wuttke W. 1982. Changes in physiological, EEG and psychological parameters in women during the spontaneous menstrual cycle and following oral contraceptives. Psychoneuroendocrinology 7:75–90. [DOI] [PubMed] [Google Scholar]
- Bodenmann S, Rusterholz T, Durr R, Stoll C, Bachmann V, Geissler E, Jaggi‐Schwarz K, Landolt HP. 2009. The functional Val158Met polymorphism of COMT predicts interindividual differences in brain alpha oscillations in young men. J Neurosci 29:10855–10862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brenner RP, Ulrich RF, Reynolds CF, 3rd . 1995. EEG spectral findings in healthy, elderly men and women‐‐sex differences. Electroencephalogr Clin Neurophysiol 94:1–5. [DOI] [PubMed] [Google Scholar]
- Brötzner CP, Klimesch W, Doppelmayr M, Zauner A, Kerschbaum HH. 2014. Resting state alpha frequency is associated with menstrual cycle phase, estradiol and use of oral contraceptives. Brain Res 1577:36–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang BS, Schomer DL, Niedermayer E. 2011. Normal EEG and sleep: Adults and elderly In: Schomer DL, Lopez da Silva FH, editors, Niedermeyer's Electroencephalography: Basic Principles, Clinical Applications, and Related Fields, Sixth Edition Phliadelphia, PA: Lippincott Williams and Wilkins; pp 183–214. [Google Scholar]
- Chiang AK, Rennie CJ, Robinson PA, van Albada SJ, Kerr CC. 2011. Age trends and sex differences of alpha rhythms including split alpha peaks. Clin Neurophysiol 122:1505–1517. [DOI] [PubMed] [Google Scholar]
- Clarke AR, Barry RJ, McCarthy R, Selikowitz M. 2001. Age and sex effects in the EEG: Development of the normal child. Clin Neurophysiol 112:806–814. [DOI] [PubMed] [Google Scholar]
- Creutzfeldt OD, Arnold PM, Becker D, Langenstein S, Tirsch W, Wilhelm H, Wuttke W. 1976. EEG changes during spontaneous and controlled menstrual cycles and their correlation with psychological performance. Electroencephalogr Clin Neurophysiol 40:113–131. [DOI] [PubMed] [Google Scholar]
- Croft RJ, Barry RJ. 2000. Removal of ocular artifact from the EEG: A review. Neurophysiol Clin 30:5–19. [DOI] [PubMed] [Google Scholar]
- Croft RJ, Chandler JS, Burgess AP, Barry RJ, Williams JD, Clarke AR. 2002. Acute mobile phone operation affects neural function in humans. Clin Neurophysiol 113:1623–1632. [DOI] [PubMed] [Google Scholar]
- Croft RJ, Hamblin DL, Spong J, Wood AW, McKenzie RJ, Stough C. 2008. The effect of mobile phone electromagnetic fields on the alpha rhythm of human electroencephalogram. Bioelectromagnetics 29:1–10. [DOI] [PubMed] [Google Scholar]
- Croft RJ, Leung S, McKenzie RJ, Loughran SP, Iskra S, Hamblin DL, Cooper NR. 2010. Effects of 2G and 3G mobile phones on human alpha rhythms: Resting EEG in adolescents, young adults, and the elderly. Bioelectromagnetics 31:434–444. [DOI] [PubMed] [Google Scholar]
- Curcio G, Ferrara M, Moroni F, D'Inzeo G, Bertini M, De Gennaro L. 2005. Is the brain influenced by a phone call? An EEG study of resting wakefulness. Neurosci Res 53:265–270. [DOI] [PubMed] [Google Scholar]
- Curcio G, Mazzucchi E, Della Marca G, Vollono C, Rossini PM. 2015. Electromagnetic fields and EEG spiking rate in patients with focal epilepsy. Clin Neurophysiol 126:659–666. [DOI] [PubMed] [Google Scholar]
- D'Costa H, Trueman G, Tang L, Abdel‐rahman U, Abdel‐rahman W, Ong K, Cosic I. 2003. Human brain wave activity during exposure to radiofrequency field emissions from mobile phones. Australas Phys Eng Sci Med 26:162–167. [DOI] [PubMed] [Google Scholar]
- de Seze R, Ayoub J, Peray P, Miro L, Touitou Y. 1999. Evaluation in humans of the effects of radiocellular telephones on the circadian patterns of melatonin secretion, a chronobiological rhythm marker. J Pineal Res 27:237–242. [DOI] [PubMed] [Google Scholar]
- Dimpfel W, Schober F, Spüler M. 1993. The influence of caffeine on human EEG under resting condition and during mental loads. Clin Investig 71:197. [DOI] [PubMed] [Google Scholar]
- Dworetzky B, Herman S, Tatum WO. 2011. Artifacts of recording In: Schomer DL, Lopez da Silva FH, editors, Niedermeyer's Electroencephalography: Basic Principles, Clinical Applications, and Related Fields, Sixth Edition Phliadelphia, PA: Lippincott Williams and Wilkins; pp 239–266. [Google Scholar]
- Ehlers CL, Wall TL, Schuckit MA. 1989. EEG spectral characteristics following ethanol administration in young men. Electroencephalogr Clin Neurophysiol 73:179–187. [DOI] [PubMed] [Google Scholar]
- European Commission . 2010. Eurobarometer 347 / Wave 73.3 ‐ TNS Opinion & Social, June 2010 [Internet]. Burxelles (BE): European Commission. Available from http://ec.europa.eu/commfrontoffice/publicopinion/archives/ebs/ebs_347_en.pdf [Last accessed 20 September 2018].
- Ferber G, Abt K, Fichte K, Luthringer R. 1999. IPEG guideline on statistical design and analysis for pharmacodynamic trials. International Pharmaco‐EEG Group. Neuropsychobiology 39:92–100. [DOI] [PubMed] [Google Scholar]
- Fink M. 2010. Remembering the lost neuroscience of pharmaco‐EEG. Acta Psychiatr Scand 121:161–173. [DOI] [PubMed] [Google Scholar]
- Fisher DJ, Daniels R, Jaworska N, Knobelsdorf A, Knott VJ. 2012. Effects of acute nicotine administration on resting EEG in nonsmokers. Exp Clin Psychopharmacol 20:71–75. [DOI] [PubMed] [Google Scholar]
- French CC, Beaumont JG. 1984. A critical review of EEG coherence studies of hemisphere function. Int J Psychophysiol 1:241–254. [DOI] [PubMed] [Google Scholar]
- Garcés P, Vicente R, Wibral M, Pineda‐Pardo JA, Lopez ME, Aurtenetxe S, Marcos A, de Andres ME, Yus M, Sancho M, Maestú F, Fernandez A. 2013. Brain‐wide slowing of spontaneous alpha rhythms in mild cognitive impairment. Front Aging Neurosci 5:1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gasser T, Bächer P, Möcks J. 1982. Transformations towards the normal distribution of broad band spectral parameters of the EEG. Electroencephalogr Clin Neurophysiol 53:119–124. [DOI] [PubMed] [Google Scholar]
- Gehlen W, Spittler JF, Calabrese P. 1996. Biologisch‐zerebrale Effekte in niederfrequent gepulsten Hochfrequenzfeldern.Cerebro‐Biological Effects in Low‐Frequency Pulsed RF Fields. Edition Wissenschaft Forschungsgemeinschaft Funk 12:3–28. [Google Scholar]
- Ghosn R, Yahia‐Cherif L, Hugueville L, Ducorps A, Lemarechal JD, Thuroczy G, de Seze R, Selmaoui B. 2015. Radiofrequency signal affects alpha band in resting electroencephalogram. J Neurophysiol 113:2753–2759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glantz SA. 2012. Primer of Biostatistics, Seventh Edition New York, NY: McGraw‐Hill Medical. [Google Scholar]
- Gmehlin D, Thomas C, Weisbrod M, Walther S, Pfuller U, Resch F, Oelkers‐Ax R. 2011. Individual analysis of EEG background‐activity within school age: Impact of age and sex within a longitudinal data set. Int J Dev Neurosci 29:163–170. [DOI] [PubMed] [Google Scholar]
- Greenhouse SW, Geisser S. 1959. On methods in the analysis of profile data. Psychometrika 24:95–112. [Google Scholar]
- Groppe DM, Urbach TP, Kutas M. 2011. Mass univariate analysis of event‐related brain potentials/fields I: A critical tutorial review. Psychophysiology 48:1711–1725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harding GFA, Thompson CRS. 1976. EEG rhythms and the internal milieu In: Remond A, editor, Handbook of Electroencephalography and Clinical Neurophysiology, 6A:Amsterdam, Netherlands: Elsevier; pp 176–194. [Google Scholar]
- Hietanen M, Kovala T, Hamalainen AM. 2000. Human brain activity during exposure to radiofrequency fields emitted by cellular phones. Scand J Work Environ Health 26:87–92. [DOI] [PubMed] [Google Scholar]
- Higuchi S, Liu Y, Yuasa T, Maeda A, Motohashi Y. 2001. Diurnal variations in alpha power density and subjective sleepiness while performing repeated vigilance tasks. Clin Neurophysiol 112:997–1000. [DOI] [PubMed] [Google Scholar]
- Hinrichs H, Heinze HJ. 2006. High frequency GSM‐1800 fields with various modulations and field strengths: No short term effect on human awake EEG. Edition Wissenschaft Forschungsgemeinschaft Funk 23:4–11. [Google Scholar]
- Hinrikus H, Bachmann M, Lass J. 2011. Parametric mechanism of excitation of the electroencephalographic rhythms by modulated microwave radiation. Int J Radiat Biol 87:1077–1085. [DOI] [PubMed] [Google Scholar]
- Hinrikus H, Bachmann M, Lass J, Karai D, Tuulik V. 2008a. Effect of low frequency modulated microwave exposure on human EEG: Individual sensitivity. Bioelectromagnetics 29:527–538. [DOI] [PubMed] [Google Scholar]
- Hinrikus H, Bachmann M, Lass J, Tomson R, Tuulik V. 2008b. Effect of 7, 14 and 21 Hz modulated 450 MHz microwave radiation on human electroencephalographic rhythms. Int J Radiat Biol 84:69–79. [DOI] [PubMed] [Google Scholar]
- Hochberg Y. 1988. A sharper Bonferroni procedure for multiple tests of significance. Biometrika 75:800–802. [Google Scholar]
- Holm S. 1979. A Simple Sequentially Rejective Multiple Test Procedure. Scand J Statist 6:65–70. [Google Scholar]
- Hountala CD, Maganioti AE, Papageorgiou CC, Nanou ED, Kyprianou MA, Tsiafakis VG, Rabavilas AD, Capsalis CN. 2008. The spectral power coherence of the EEG under different EMF conditions. Neurosci Lett 441:188–192. [DOI] [PubMed] [Google Scholar]
- Huber R, Graf T, Cote KA, Wittmann L, Gallmann E, Matter D, Schuderer J, Kuster N, Borbely AA, Achermann P. 2000. Exposure to pulsed high‐frequency electromagnetic field during waking affects human sleep EEG. Neuroreport 11:3321–3325. [DOI] [PubMed] [Google Scholar]
- Huynh H, Feldt LS. 1976. Estimation of the Box Correction for Degrees of Freedom from Sample Data in Randomized Block and Split‐Plot Designs. J Educ Stat 1:69–82. [Google Scholar]
- IARC . (2011). IARC classifies radiofrequency electromagnetic fields as possibly carcinogenic to humans. Press Relaese No 208 [Internet]. Lyon (FR): International Agency for Reasearch on Cancer. Available from: http://www.iarc.fr/en/media‐centre/pr/2011/pdfs/pr208_E.pdf [Last updated 31 May 2011] [Last accessed 20 February 2019].
- ICNIRP . 1998. Guidelines for limiting exposure to time‐varying electric, magnetic, and electromagnetic fields (up to 300 GHz). Health Phys 74:494–522. [PubMed] [Google Scholar]
- Jahre K, Matkey K, Meckelburg H‐J. 1996. Der Einfluß von gepulsten elektromagnetischen Feldern auf das Elektroenzephalogramm von Menschen. [The effect of pulsed electro‐magnetic fields on the electro‐encephalogram of humans]. Newsletter Edition Wissenschaft Forschungsgemeinschaft Funk 9:3–47. [Google Scholar]
- Jasper HH. 1958. Report of the committee on methods of clinical examination in electroencephalography. Electroencephalogr Clin Neurophysiol 10:370–375. [Google Scholar]
- Jobert M, Wilson FJ. 2015. Advanced Analysis of Pharmaco‐EEG Data in Humans. Neuropsychobiology 72:165–177. [DOI] [PubMed] [Google Scholar]
- Jobert M, Wilson FJ, Ruigt GS, Brunovsky M, Prichep LS, Drinkenburg WH, The IPEG Pharmaco‐EEG Guideline Committee . 2012. Guidelines for the recording and evaluation of pharmaco‐EEG data in man: The International Pharmaco‐EEG Society (IPEG). Neuropsychobiology 66:201–220. [DOI] [PubMed] [Google Scholar]
- Johnson DE. 2010. Crossover experiments. WIREs Comp Stat 2:620–625. [Google Scholar]
- Kitchen CM. 2009. Nonparametric vs parametric tests of location in biomedical research. Am J Ophthalmol 147:571–572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klass DW, Brenner RP. 1995. Electroencephalography of the elderly. J Clin Neurophysiol 12:116–131. [DOI] [PubMed] [Google Scholar]
- Kleinlogel H, Dierks T, Koenig T, Lehmann H, Minder A, Berz R. 2008. Effects of weak mobile phone ‐ electromagnetic fields (GSM, UMTS) on well‐being and resting EEG. Bioelectromagnetics 29:479–487. [DOI] [PubMed] [Google Scholar]
- Krafczyk S, Haberhauer PE, Bötzel K, Brix J 2002. Sind EMF ‐ Effekte auf das Ruhe‐EEG und evozierte Hirnpotentiale nachweisbar? [Are EMF effects on resting EEG and evoked brain potentials detectable?] In Bundesanstalt für Arbeitsschutz und Arbeitsmedizin (ed): Beeinflussen elektromagnetische Felder von Mobiltelefonen zentralnervöse Informationsverarbeitungsleistungen des Menschen? [Do electromagnetic fields of mobile phones affect central nervous information processing in humans?]Schriftenreihe der Bundesanstalt für Arbeitsschutz und Arbeitsmedizin. Dortmund, Berlin, Germany: Bundesanstalt für Arbeitsschutz und Arbeitsmedizin pp 147‐162 (In German)
- Kramarenko AV, Tan U. 2003. Effects of high‐frequency electromagnetic fields on human EEG: A brain mapping study. Int J Neurosci 113:1007–1019. [DOI] [PubMed] [Google Scholar]
- Kubicki ST, Höller L. 1980. Systematische Einteilung der EEG‐Grundrhythmen und ‐Normvarianten. EEG‐Labor 2:32–53. [Google Scholar]
- Lakens D. 2013. Calculating and reporting effect sizes to facilitate cumulative science: A practical primer for t‐tests and ANOVAs. Front Psychol 4:1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lafrance C, Dumont M. 2000. Diurnal variations in the waking EEG: Comparisons with sleep latencies and subjective alertness. J Sleep Res 9:243–248. [DOI] [PubMed] [Google Scholar]
- Lebedeva NN, Sulimov AV, Sulimova OP, Kotrovskaya TI, Gailus T. 2000. Cellular phone electromagnetic field effects on bioelectric activity of human brain. Crit Rev Biomed Eng 28:323–337. [DOI] [PubMed] [Google Scholar]
- Lenth RV. 2001. Some Practical Guidelines for Effective Sample Size Determination. Am Stat 55:187–193. [Google Scholar]
- Li G, Taljaard M, Van den Heuvel ER, Levine MA, Cook DJ, Wells GA, Devereaux PJ, Thabane L. 2017. An introduction to multiplicity issues in clinical trials: The what, why, when and how. Int J Epidemiol 46:746–755. [DOI] [PubMed] [Google Scholar]
- Loughran SP, Benz DC, Schmid MR, Murbach M, Kuster N, Achermann P. 2013. No increased sensitivity in brain activity of adolescents exposed to mobile phone‐like emissions. Clin Neurophysiol 124:1303–1308. [DOI] [PubMed] [Google Scholar]
- Loughran SP, Wood AW, Barton JM, Croft RJ, Thompson B, Stough C. 2005. The effect of electromagnetic fields emitted by mobile phones on human sleep. Neuroreport 16:1973–1976. [DOI] [PubMed] [Google Scholar]
- Lv B, Chen Z, Wu T, Shao Q, Yan D, Ma L, Lu K, Xie Y. 2014. The alteration of spontaneous low frequency oscillations caused by acute electromagnetic fields exposure. Clin Neurophysiol 125:277–286. [DOI] [PubMed] [Google Scholar]
- Maby E, Le Bouquin Jeannes R, Faucon G. 2006. Short‐term effects of GSM mobiles phones on spectral components of the human electroencephalogram. Conf Proc IEEE Eng Med Biol Soc 1:3751–3754. [DOI] [PubMed] [Google Scholar]
- Maris E. 2012. Statistical testing in electrophysiological studies. Psychophysiology 49:549–565. [DOI] [PubMed] [Google Scholar]
- Maris E, Oostenveld R. 2007. Nonparametric statistical testing of EEG‐ and MEG‐data. J Neurosci Methods 164:177–190. [DOI] [PubMed] [Google Scholar]
- Markand ON. 1990. Alpha rhythms. J Clin Neurophysiol 7:163–189. [DOI] [PubMed] [Google Scholar]
- Matsuura M, Yamamoto K, Fukuzawa H, Okubo Y, Uesugi H, Moriiwa M, Kojima T, Shimazono Y. 1985. Age development and sex differences of various EEG elements in healthy children and adults‐‐quantification by a computerized wave form recognition method. Electroencephalogr Clin Neurophysiol 60:394–406. [DOI] [PubMed] [Google Scholar]
- Mensen A, Khatami R. 2013. Advanced EEG analysis using threshold‐free cluster‐enhancement and non‐parametric statistics. NeuroImage 67:111–118. [DOI] [PubMed] [Google Scholar]
- Moher D, Hopewell S, Schulz KF, Montori V, Gotzsche PC, Devereaux PJ, Elbourne D, Egger M, Altman DG. 2010. CONSORT 2010 explanation and elaboration: Updated guidelines for reporting parallel group randomised trials. BMJ 340:c869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moher D, Hopewell S, Schulz KF, Montori V, Gotzsche PC, Devereaux PJ, Elbourne D, Egger M, Altman DG, Consort . 2012. CONSORT 2010 explanation and elaboration: Updated guidelines for reporting parallel group randomised trials. Int J Surg 10:28–55. [DOI] [PubMed] [Google Scholar]
- Moher D, Schulz KF, Altman DG. 2001. The CONSORT statement: Revised recommendations for improving the quality of reports of parallel‐group randomised trials. Lancet 357:1191–1194. [PubMed] [Google Scholar]
- Morgan ML, Witte EA, Cook IA, Leuchter AF, Abrams M, Siegman B. 2005. Influence of age, gender, health status, and depression on quantitative EEG. Neuropsychobiology 52:71–76. [DOI] [PubMed] [Google Scholar]
- Nanou E, Hountala C, Maganioti A, Papageorgiou C, Tsiafakis V, Rabavilas A, Capsalis C. 2009. Influence of a 1,800 MHz electromagnetic field on the EEG energy. Environmentalist 29:205–209. [Google Scholar]
- Nanou E, Tsiafakis V, Kapareliotis E, Papageorgiou C, Rabavilas A, Capsalis C. 2005. Influence of the Interaction of a 900 MHz Signal with Gender on EEG Energy: Experimental Study on the Influence of 900 MHz Radiation on EEG. Environmentalist 25:173–179. [Google Scholar]
- Nuwer MR. 1987. Recording electrode site nomenclature. J Clin Neurophysiol 4:121–133. [DOI] [PubMed] [Google Scholar]
- Pandis N, Chung B, Scherer RW, Elbourne D, Altman DG. 2017. CONSORT 2010 statement: Extension checklist for reporting within person randomised trials. BMJ 357:j2835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papageorgiou CC, Nanou ED, Tsiafakis VG, Capsalis CN, Rabavilas AD. 2004. Gender related differences on the EEG during a simulated mobile phone signal. Neuroreport 15:2557–2560. [DOI] [PubMed] [Google Scholar]
- Papousek I, Schulter G. 1998. Different temporal stability and partial independence of EEG asymmetries from different locations: Implications for laterality research. Int J Neurosci 93:87–100. [DOI] [PubMed] [Google Scholar]
- Perentos N, Croft RJ, McKenzie RJ, Cosic I. 2013. The alpha band of the resting electroencephalogram under pulsed and continuous radio frequency exposures. IEEE Trans Biomed Eng 60:1702–1710. [DOI] [PubMed] [Google Scholar]
- Perentos N, Croft RJ, McKenzie RJ, Cvetkovic D, Cosic I. 2007. Comparison of the effects of continuous and pulsed mobile phone like RF exposure on the human EEG. Australas Phys Eng Sci Med 30:274–280. [DOI] [PubMed] [Google Scholar]
- Petersen I, Eeg‐Olofsson O. 1971. The development of the electroencephalogram in normal children from the age of 1 through 15 years. Non‐paroxysmal activity. Neuropadiatrie 2:247–304. [DOI] [PubMed] [Google Scholar]
- Qin Y, Xu P, Yao D. 2010. A comparative study of different references for EEG default mode network: The use of the infinity reference. Clin Neurophysiol 121:1981–1991. [DOI] [PubMed] [Google Scholar]
- Rangaswamy M, Porjesz B, Chorlian DB, Wang K, Jones KA, Bauer LO, Rohrbaugh J, O'Connor S, Kuperman S, Reich T, Begleiter H. 2002. Beta power in the EEG of alcoholics. Biol Psychiatry 52:831–842. [DOI] [PubMed] [Google Scholar]
- Rangaswamy M, Porjesz B, Chorlian DB, Choi K, Jones KA, Wang K, Rohrbaugh J, O'Connor S, Kuperman S, Reich T, Begleiter H. 2003. Theta power in the EEG of alcoholics. Alcohol Clin Exp Res 27:607–615. [DOI] [PubMed] [Google Scholar]
- Rapp PE, Keyser DO, Albano A, Hernandez R, Gibson DB, Zambon RA, Hairston WD, Hughes JD, Krystal A, Nichols AS. 2015. Traumatic brain injury detection using electrophysiological methods. Front Hum Neurosci 9:1–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rass O, Ahn WY, O'Donnell BF. 2015. Resting‐state EEG, impulsiveness, and personality in daily and nondaily smokers. Clin Neurophysiol 127:409–418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Regel SJ, Gottselig JM, Schuderer J, Tinguely G, Retey JV, Kuster N, Landolt HP, Achermann P. 2007. Pulsed radio frequency radiation affects cognitive performance and the waking electroencephalogram. Neuroreport 18:803–807. [DOI] [PubMed] [Google Scholar]
- Regen F, Dorn H, Danker‐Hopfe H. 2013. Association between pupillary unrest index and waking electroencephalogram activity in sleep‐deprived healthy adults. Sleep Med 14:902–912. [DOI] [PubMed] [Google Scholar]
- Reiser H, Dimpfel W, Schober F. 1995. The influence of electromagnetic fields on human brain activity. Eur J Med Res 1:27–32. [PubMed] [Google Scholar]
- Roggeveen S, van Os J, Viechtbauer W, Lousberg R. 2015. EEG Changes Due to Experimentally Induced 3G Mobile Phone Radiation. PLoS One 10:e0129496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Röschke J, Mann K. 1997. No short‐term effects of digital mobile radio telephone on the awake human electroencephalogram. Bioelectromagnetics 18:172–176. [PubMed] [Google Scholar]
- Rossini PM, Rossi S, Babiloni C, Polich J. 2007. Clinical neurophysiology of aging brain: From normal aging to neurodegeneration. Prog Neurobiol 83:375–400. [DOI] [PubMed] [Google Scholar]
- Sandsmark DK, Elliott JE, Lim MM. 2017. Sleep‐wake disturbances after traumatic brain injury: Synthesis of human and animal studies. Sleep 40:zsx044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SCENIHR . (2015). Opinion on Potential Health Effects of Exposure to Electromagnetic Fields (EMF) [Internet]. Luxembourg (L): Scientific Committee on Emerging and Newly Identified Health Risks SCENIHR. Available from: https://ec.europa.eu/health/scientific_committees/emerging/docs/scenihr_o_041.pdf [Last updated 20 January 2015].
- Schulz KF, Altman DG, Moher D, CONSORT Group . 2010. CONSORT 2010 Statement: Updated guidelines for reporting parallel group randomised trials. BMC Med 8:1–9. [DOI] [PubMed] [Google Scholar]
- Sigmon SC, Herning RI, Better W, Cadet JL, Griffiths RR. 2009. Caffeine withdrawal, acute effects, tolerance, and absence of net beneficial effects of chronic administration: Cerebral blood flow velocity, quantitative EEG, and subjective effects. Psychopharmacology (Berl) 204:573–585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smit DJ, Posthuma D, Boomsma DI, Geus EJ. 2005. Heritability of background EEG across the power spectrum. Psychophysiology 42:691–697. [DOI] [PubMed] [Google Scholar]
- Smitha CK, Narayanan NK 2013. Effect of mobile phone radiation on EEG. Proceedings –Third International Conference on Advances in Computing and Communications (ICACC), 29‐31 Aug 2013. Cochin, India: pp 111‐114.
- Smitha CK, Narayanan NK Analysis of fractal dimension of EEG signals under mobile phone radiation. IEEE International Conference on Signal Processing, Informatics, Communication and Energy Systems (SPICES), 19‐21 Feb 2015. Kozhikode; India: pp 1‐5.
- Spittler JF, Calabrese P, Gehlen W. 1997. Einfluß elektromagnetischer Felder auf die menschliche Gehirntätigkeit. Medizintechnik 117:176–178. [Google Scholar]
- Stam CJ. 2011. Dementia and EEG In: Schomer DL, Lopes da Silva FH, editors, Niedermeyer's Electroencephalography: Basic Priciples, Clinical Applications, and Related Fields, Sixth Edition Philadelphia, PA: Lippincott Williams and Wilkins; pp 375–393. [Google Scholar]
- Stassen HH, Bomben G, Propping P. 1987. Genetic aspects of the EEG: An investigation into the within‐pair similarity of monozygotic and dizygotic twins with a new method of analysis. Electroencephalogr Clin Neurophysiol 66:489–501. [DOI] [PubMed] [Google Scholar]
- Stöhr M, Riffel B, Pfadenhauer K. 1991. Neurophysiologische Untersuchungsmethoden in der Intensivmedizin Neurophysiological assessment methods in intensive‐care medicine Berlin, Heidelberg, Germany: Springer. [Google Scholar]
- Suhhova A, Bachmann M, Karai D, Lass J, Hinrikus H. 2013. Effect of microwave radiation on human EEG at two different levels of exposure. Bioelectromagnetics 34:264–274. [DOI] [PubMed] [Google Scholar]
- Sullivan GM, Feinn R. 2012. Using effect size—or why the p value is not enough. J Grad Med Educ 4:279–282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swedish Radiation Safety Authority . (2016). Recent Research on EMF and Health Risk ‐ Eleventh report from SSM's Scientific Council on Electromagnetic Fields, 2016, including thirteen yaers of electromagnetic field research monitored by SSM'S Scientific Council on EMF and health: How has the evidence changed over time? [Internet]. Stockholm (SE): SSM's Scientific Council on Electromagetic Fields. Available from: https://www.stralsakerhetsmyndigheten.se/contentassets/98d67d9e3301450da4b8d2e0f6107313/201615‐recent‐research‐on‐emf‐and‐health‐risk‐eleventh‐report‐from‐ssms‐scientific‐council‐on‐electromagnetic‐fields‐2016 [Last updated April 2016].
- Swedish Radiation Safety Authority . (2018). Recent Research on EMF and Health Risk, Twelfth report from SSM's Scientific Council on Electromagnetic Fields, 2017. [Internet]. Stockholm (SE): SSM's Scientific Council on Electromagetic Fields. Available from: https://www.stralsakerhetsmyndigheten.se/contentassets/f34de8333acd4ac2b22a9b072d9b33f9/201809‐recent‐research‐on‐emf‐and‐health‐risk [Last updated April 2018].
- Tomarken AJ, Davidson RJ, Wheeler RE, Kinney L. 1992. Psychometric properties of resting anterior EEG asymmetry: Temporal stability and internal consistency. Psychophysiology 29:576–592. [DOI] [PubMed] [Google Scholar]
- Trunk A, Stefanics G, Zentai N, Bacskay I, Felinger A, Thuroczy G, Hernadi I. 2014. Lack of interaction between concurrent caffeine and mobile phone exposure on visual target detection: An ERP study. Pharmacol Biochem Behav 124:412–420. [DOI] [PubMed] [Google Scholar]
- Trunk A, Stefanics G, Zentai N, Bacskay I, Felinger A, Thuroczy G, Hernadi I. 2015. Effects of concurrent caffeine and mobile phone exposure on local target probability processing in the human brain. Sci Rep 5:1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trunk A, Stefanics G, Zentai N, Kovacs‐Balint Z, Thuroczy G, Hernadi I. 2013. No effects of a single 3G UMTS mobile phone exposure on spontaneous EEG activity, ERP correlates, and automatic deviance detection. Bioelectromagnetics 34:31–42. [DOI] [PubMed] [Google Scholar]
- Valberg PA, van Deventer TE, Repacholi MH. 2007. Workgroup report: Base stations and wireless networks‐radiofrequency (RF) exposures and health consequences. Environ Health Perspect 115:416–424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Deventer E, van Rongen E, Saunders R. 2011. WHO research agenda for radiofrequency fields. Bioelectromagnetics 32:417–421. [DOI] [PubMed] [Google Scholar]
- van Diessen E, Numan T, van Dellen E, van der Kooi AW, Boersma M, Hofman D, van Lutterveld R, van Dijk BW, van Straaten EC, Hillebrand A, Stam CJ. 2015. Opportunities and methodological challenges in EEG and MEG resting state functional brain network research. Clin Neurophysiol 126:1468–1481. [DOI] [PubMed] [Google Scholar]
- Vecchio F, Babiloni C, Ferreri F, Buffo P, Cibelli G, Curcio G, van Dijkman S, Melgari JM, Giambattistelli F, Rossini PM. 2010. Mobile phone emission modulates inter‐hemispheric functional coupling of EEG alpha rhythms in elderly compared to young subjects. Clin Neurophysiol 121:163–171. [DOI] [PubMed] [Google Scholar]
- Vecchio F, Babiloni C, Ferreri F, Curcio G, Fini R, Del Percio C, Rossini PM. 2007. Mobile phone emission modulates interhemispheric functional coupling of EEG alpha rhythms. Eur J Neurosci 25:1908–1913. [DOI] [PubMed] [Google Scholar]
- Vecchio F, Buffo P, Sergio S, Iacoviello D, Rossini PM, Babiloni C. 2012a. Mobile phone emission modulates event‐related desynchronization of alpha rhythms and cognitive‐motor performance in healthy humans. Clin Neurophysiol 123:121–128. [DOI] [PubMed] [Google Scholar]
- Vecchio F, Tombini M, Buffo P, Assenza G, Pellegrino G, Benvenga A, Babiloni C, Rossini PM. 2012b. Mobile phone emission increases inter‐hemispheric functional coupling of electroencephalographic alpha rhythms in epileptic patients. Int J Psychophysiol 84:164–171. [DOI] [PubMed] [Google Scholar]
- von Klitzing L. 1995. Low‐frequency pulsed electromagnetic fields influence EEG of man. Physica Medica 11:77–80. [Google Scholar]
- Wada Y, Takizawa Y, Jiang ZY, Yamaguchi N. 1994. Gender differences in quantitative EEG at rest and during photic stimulation in normal young adults. Clin Electroencephalogr 25:81–85. [DOI] [PubMed] [Google Scholar]
- WHO . (2010). WHO Research Agenda for Radiofrequency Fields. [Internet]. Geneva (CH): Word Health Organization (2010) Availbale from: http://apps.who.int/iris/bitstream/handle/10665/44396/9789241599948_eng.pdf;jsessionid=6880B7747A89B4EE034714851F0D35BB?sequence=1 [Last accessed 20 September 2018]. [Google Scholar]
- Wieneke GH, Deinema CH, Spoelstra P, Storm van Leeuwen W, Versteeg H. 1980. Normative spectral data on alpha rhythm in male adults. Electroencephalogr Clin Neurophysiol 49:636–645. [DOI] [PubMed] [Google Scholar]
- Yang L, Chen Q, Lv B, Wu T. 2017. Long‐Term Evolution Electromagnetic Fields Exposure Modulates the Resting State EEG on Alpha and Beta Bands. Clin EEG Neurosci 48:168–175. [DOI] [PubMed] [Google Scholar]
- Zentai N, Csatho A, Trunk A, Fiocchi S, Parazzini M, Ravazzani P, Thuroczy G, Hernadi I. 2015. No Effects of Acute Exposure to Wi‐Fi Electromagnetic Fields on Spontaneous EEG Activity and Psychomotor Vigilance in Healthy Human Volunteers. Radiat Res 184:568–577. [DOI] [PubMed] [Google Scholar]


