Abstract
Objective
Clinical trial results have shown that, in glucocorticoid‐treated patients, treatment with denosumab 60 mg subcutaneously once every 6 months (Q6M) increased spine and hip bone mineral density (BMD) at month 12 significantly more than treatment with risedronate 5 mg orally once daily (QD). The present analysis was performed to compare efficacy and characterize safety through month 24.
Methods
This phase III study enrolled men and women ≥18 years old who had received ≥7.5 mg daily prednisone or equivalent for <3 months (glucocorticoid‐initiating) or for ≥3 months (glucocorticoid‐continuing) before screening. All patients <50 years old had a history of osteoporotic fracture. Glucocorticoid‐continuing patients ≥50 years old had T scores of −2.0 or less (or −1.0 or less with fracture history). Patients were randomized (1:1) to receive denosumab 60 mg subcutaneously Q6M or risedronate 5 mg orally QD for 24 months, with daily calcium and vitamin D.
Results
Of 795 patients, 590 (74.2%) completed the study (in the glucocorticoid‐initiating group, 109 of 145 patients treated with denosumab and 117 of 145 patients treated with risedronate; in the glucocorticoid‐continuing group, 186 of 253 patients treated with denosumab and 178 of 252 patients treated with risedronate). Denosumab was superior to risedronate in increasing lumbar spine and total hip BMD at all time points assessed, among glucocorticoid‐initiating patients (24‐month lumbar spine: BMD increase of 6.2% versus 1.7%, respectively [P < 0.001]; 24‐month total hip: BMD increase of 3.1% versus 0.0% [P < 0.001]) and among glucocorticoid‐continuing patients (24‐month lumbar spine: BMD increase of 6.4% versus 3.2% [P < 0.001]; 24‐month total hip: BMD increase of 2.9% versus 0.5% [P < 0.001]). Adverse events, serious adverse events (including infections), and fractures were similar between treatment groups.
Conclusion
Denosumab was superior to risedronate in terms of increases in spine and hip BMD through month 24, and the safety profile was similar between treatment groups. Denosumab may offer a new osteoporosis treatment option for glucocorticoid‐treated patients.
Introduction
Long‐term glucocorticoid use is highly prevalent 1 and associated with an increased risk of fracture, even at low daily doses 2. Calcium and vitamin D supplementation is recommended with oral glucocorticoid therapy, and the addition of a bisphosphonate or other osteoporosis treatment is recommended for patients at moderate‐to‐high risk of fracture who are taking oral glucocorticoids 3, 4. These recommendations are supported by several randomized, controlled clinical trials showing that bisphosphonates such as alendronate 5, 6, 7, risedronate 7, 8, 9, 10, or zoledronic acid 11, 12 effectively prevent bone loss in patients receiving oral glucocorticoid therapy. Based on extensions to these studies 13 and meta‐analyses, bisphosphonates may reduce the risk of vertebral fractures associated with glucocorticoid use 5, 7, 9, 10, 13, 14, 15, 16. However, inconvenient dosing regimens and potential side effects of bisphosphonates can lead to low adherence in patients with osteoporosis 17, 18. Furthermore, the increase in bone mineral density (BMD) with bisphosphonate therapy plateaus after 3–4 years 19, 20, 21, 22. Teriparatide may also reduce fracture risk in those taking glucocorticoids 23, but daily injections and restriction to a 2‐year lifetime for treatment limit its use. Therefore, there is great interest in other therapeutic options for patients receiving oral glucocorticoid therapy.
Denosumab is a fully human monoclonal antibody that binds to and neutralizes the activity of human RANKL. In postmenopausal women with osteoporosis, long‐term denosumab treatment for up to 10 years was well tolerated, continued to increase BMD without therapeutic plateau, and was associated with a sustained low incidence of fracture 24. The primary analysis, conducted at month 12 of a 24‐month study of glucocorticoid‐treated patients, has demonstrated that subcutaneous denosumab 60 mg once every 6 months (Q6M) increased BMD at the spine and hip significantly more than oral risedronate 5 mg once daily (QD) 25. However, glucocorticoid treatment often extends beyond 1 year in the clinical setting due to the chronic nature of the inflammatory diseases for which it is taken 26, 27. Therefore, it is important to assess the continued efficacy and safety of denosumab, compared with risedronate, during the second year of treatment. Our report extends the findings of this double‐blind, active‐controlled trial to month 24. The objectives of this final analysis were to compare the effects of denosumab versus risedronate on BMD through month 24 and further characterize the safety profile of continued denosumab treatment in this population.
Patients and methods
Patients
Participants in the study were men and women ≥18 years old who were receiving glucocorticoid therapy (prednisone or its equivalent) at a dose of ≥7.5 mg for <3 months (glucocorticoid‐initiating) or ≥3 months (glucocorticoid‐continuing) before screening. Patients <50 years old in either subpopulation were required to have a history of osteoporosis‐related fracture. Patients ≥50 years old in the glucocorticoid‐continuing subpopulation were required to have a lumbar spine, total hip, or femoral neck BMD T score of −2.0 or less, or a T score of −1.0 or less with a history of osteoporosis‐related fracture. Women of childbearing potential were required to use 2 highly effective forms of contraception through 7 months after the last injection of study medication.
Study design
This was a phase III, international, randomized, double‐blind, double‐dummy, active‐controlled, parallel‐group study (ClinicalTrials.gov identifier: NCT01575873). Patients were randomized 1:1 within each subpopulation to receive subcutaneous denosumab 60 mg Q6M and oral placebo (for risedronate) QD for 24 months, or oral risedronate 5 mg QD (the dosing regimen approved for the treatment and prevention of glucocorticoid‐induced osteoporosis) and subcutaneous placebo (for denosumab) Q6M for 24 months, stratified by sex within each subpopulation using an interactive voice‐response system. The sponsor's Global Randomization and Blinding Group, which was independent of the study team, prepared the randomization before study initiation using a computer‐generated schedule. All patients were to receive daily supplementation with calcium (≥1,000 mg) and vitamin D (≥800 IU). Enrollment of men was restricted to 30–40% in each subpopulation. The patients’ primary or specialist physicians managed their glucocorticoid therapy.
This study was conducted in accordance with the Declaration of Helsinki and followed the International Conference for Harmonisation Guidelines for Good Clinical Practice. An independent review board approved the study design for each center. Written informed consent was obtained from each patient before study participation.
Assessments
At the 12‐month and 24‐month screenings, BMD of the lumbar spine, total hip, femoral neck, and 1/3 radius was measured by dual x‐ray absorptiometry (Lunar or Hologic) and analyzed centrally (BioClinica). At months 6 and 18, only BMD of the lumbar spine was measured and analyzed.
In a bone turnover marker substudy, markers of bone resorption (C‐telopeptide of type I collagen [CTX]) and bone formation (N‐propeptide of type I collagen [PINP]) were measured on days 1 and 10, and at months 3, 4, 5, 6, 12, and 24. CTX was assessed centrally (Esoterix Laboratory Services) using IDS‐iSYS based on chemiluminescence technology, and PINP was analyzed centrally (Covance Central Laboratory Services) using UniQ PINP radioimmunoassay.
To identify prevalent and incident vertebral fractures, a central facility (BioClinica) provided Genant semiquantitative grading of lateral thoracic and lumbar spine radiographs obtained on day 1 and at months 12 and 24 or at early termination. Data on clinical and nonvertebral fractures were collected based on adverse event reporting. Clinical fractures were defined as all fractures excluding skull, facial bones, mandible, metacarpus, finger phalanges, toe phalanges, and cervical vertebrae, and were not associated with known high‐trauma severity or pathologic fractures. Participants were queried about adverse events and concomitant medications at each study visit. Potential cases of osteonecrosis of the jaw and atypical femoral fracture, identified based on prespecified search criteria, were reviewed by independent, blinded, external adjudication committees using published case definitions 28, 29.
The final analysis described herein examined secondary end points for the percentage change from baseline through month 24 in lumbar spine and total hip BMD, in both subpopulations separately. Exploratory efficacy end points were the percentage change from baseline through month 24 in femoral neck and 1/3 radius BMD in both subpopulations separately, and in bone turnover markers (CTX and PINP) in the combined subpopulations. Safety objectives compared denosumab with risedronate in the combined subpopulations.
Statistical analysis
Percentage changes from baseline in BMD were assessed using an analysis of covariance (ANCOVA) model with main effects for treatment, sex, baseline BMD, and machine type, and an interaction effect for baseline BMD and machine type. For the glucocorticoid‐continuing subpopulation, duration of prior glucocorticoid use (<12 months versus ≥12 months) was an additional covariate in the model. Because missing BMD values were not imputed, only patients with observed BMD data at baseline and at the time point of interest (e.g., month 12, month 24) were included in the efficacy analysis at each postbaseline visit.
A sensitivity analysis for BMD results was done for the per‐protocol analysis set of patients who did not have important protocol deviations and received all planned denosumab doses (or matching placebo) and at least 80% of the planned risedronate doses (or matching placebo). Additional sensitivity analyses for missing data for BMD results included a repeated‐measures model without imputation, an ANCOVA model with baseline values carried forward, and an ANCOVA model with multiple imputation. Diagnostic plots were generated to examine model assumptions.
Percentage changes from baseline for bone turnover markers were analyzed using nonparametric methodology in the combined subpopulations. Wilcoxon's rank sum test was used to compare treatment groups. Analyses included patients in the bone turnover marker substudy who had values observed at the time points of interest. Undetectable values were imputed using the lower limit of detection value.
Safety analyses included patients who received ≥1 dose of risedronate or denosumab. Patient incidence rates of treatment‐emergent adverse events were summarized in the combined subpopulations, and the Medical Dictionary for Regulatory Activities (MedDRA) version 20.0 was used. Serious infections were assessed in subgroups of patients receiving concomitant biologic and/or nonbiologic immunosuppressants. Statistical comparisons were not conducted for analyses of adverse events.
New and worsening vertebral fractures were assessed in the combined subpopulations for patients who had a baseline spine radiograph and ≥1 postbaseline spine radiograph. Low‐trauma nonvertebral fractures were assessed in the combined subpopulation in patients who received ≥1 dose of risedronate or denosumab. Chi‐square tests were used to compare fracture incidence rates between the treatment groups.
Results
Patient characteristics
This study was conducted at 79 centers in Europe, North America, Latin America, and Korea. Of the 795 patients who were enrolled, 590 (74.2%) completed the 24‐month study, including 226 glucocorticoid‐initiating patients (109 of 145 treated with denosumab and 117 of 145 treated with risedronate) and 364 glucocorticoid‐continuing patients (186 of 253 treated with denosumab and 178 of 252 treated with risedronate) (Figure 1). The leading causes of study discontinuation overall were withdrawal of consent (13.6% denosumab, 12.3% risedronate) and adverse events (4.8% denosumab, 4.0% risedronate). The baseline characteristics of the treatment groups were balanced within each subpopulation (Table 1). Overall, 45.3% of patients were receiving ≥1 immunosuppressant therapy at baseline, including biologic medications in 3.8% of patients and nonbiologic immunosuppressants in 44.2%. Immunosuppressant therapies that at least 5% of patients were using at baseline were methotrexate (33.2%), azathioprine (5.3%), and leflunomide (5.0%).
Figure 1.
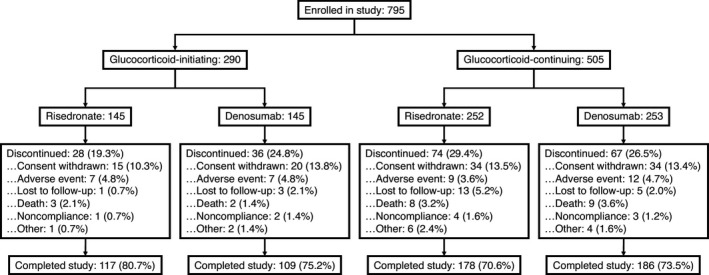
Disposition of the patients enrolled in the study.
Table 1.
Demographic and disease characteristics of the patients at baselinea
| Glucocorticoid‐initiating | Glucocorticoid‐continuing | |||
|---|---|---|---|---|
| Risedronate (n = 145) | Denosumab (n = 145) | Risedronate (n = 252) | Denosumab (n = 253) | |
| Female sex, no. (%) | 93 (64.1) | 93 (64.1) | 185 (73.4) | 185 (73.1) |
| Premenopause | 7 (7.5) | 10 (10.8) | 25 (13.5) | 24 (13.0) |
| Postmenopause | 83 (89.2) | 82 (88.2) | 157 (84.9) | 159 (85.9) |
| Unknown | 3 (3.2) | 1 (1.1) | 3 (1.6) | 2 (1.1) |
| Race, no. (%) | ||||
| White | 123 (84.8) | 122 (84.1) | 223 (88.5) | 230 (90.9) |
| Asian | 9 (6.2) | 9 (6.2) | 12 (4.8) | 6 (2.4) |
| Black or African American | 2 (1.4) | 2 (1.4) | 4 (1.6) | 4 (1.6) |
| Other | 11 (7.6) | 12 (8.3) | 13 (5.2) | 13 (5.1) |
| Age, mean ± SD years | 64.4 ± 10.0 | 67.5 ± 10.1 | 61.3 ± 11.1 | 61.5 ± 11.6 |
| Condition requiring glucocorticoid, no. (%)b | ||||
| Rheumatologic disorders | 129 (89.0) | 129 (89.0) | 184 (73.0) | 173 (68.4) |
| Rheumatoid arthritis | 46 (31.7) | 49 (33.8) | 119 (47.2) | 96 (37.9) |
| Polymyalgia rheumatica | 52 (35.9) | 51 (35.2) | 18 (7.1) | 21 (8.3) |
| Systemic lupus erythematosus | 4 (2.8) | 2 (1.4) | 16 (6.3) | 15 (5.9) |
| Vasculitis | 10 (6.9) | 7 (4.8) | 9 (3.6) | 15 (5.9) |
| Other | 34 (23.4) | 32 (22.1) | 30 (11.9) | 38 (15.0) |
| Respiratory disorders | 11 (7.6) | 12 (8.3) | 37 (14.7) | 46 (18.2) |
| COPD | 1 (0.7) | 1 (0.7) | 5 (2.0) | 7 (2.8) |
| Asthma | 2 (1.4) | 3 (2.1) | 17 (6.7) | 20 (7.9) |
| Other | 8 (5.5) | 8 (5.5) | 16 (6.3) | 20 (7.9) |
| Inflammatory bowel disease | 0 (0.0) | 1 (0.7) | 5 (2.0) | 3 (1.2) |
| Sarcoidosis | 0 (0.0) | 0 (0.0) | 5 (2.0) | 4 (1.6) |
| Neurologic disorders | 2 (1.4) | 1 (0.7) | 15 (6.0) | 11 (4.3) |
| Dermatologic disorders | 5 (3.4) | 6 (4.1) | 8 (3.2) | 9 (3.6) |
| Other | 11 (7.6) | 12 (8.3) | 37 (14.7) | 46 (18.2) |
| Glucocorticoid dose, mg/dayc | ||||
| Mean ± SD | 15.6 ± 10.25 | 16.6 ± 13.01 | 11.1 ± 7.69 | 12.3 ± 8.09 |
| Median (IQR) | 12.5 (9.0–20.0) | 12.5 (10.0–20.0) | 10.0 (7.5–10.0) | 10.0 (7.5–12.5) |
| Prior glucocorticoid use, no. (%)d | ||||
| 0 to <3 months | 129 (89.0) | 133 (91.7) | 8 (3.2) | 13 (5.1) |
| ≥3 months | 16 (11.0) | 10 (6.9) | 242 (96.0) | 239 (94.5) |
| 3 to <12 months | 8 (5.5) | 7 (4.8) | 75 (29.8) | 81 (32.0) |
| ≥12 months | 8 (5.5) | 3 (2.1) | 167 (66.3) | 158 (62.5) |
| Missing data | 0 (0.0) | 2 (1.4) | 2 (0.8) | 1 (0.4) |
| Immunosuppressant therapy at baseline, no. (%) | 51 (35.2) | 52 (35.9) | 135 (53.6) | 122 (48.2) |
| Biologic medication | 6 (4.1) | 5 (3.4) | 12 (4.8) | 7 (2.8) |
| Nonbiologic immunosuppressant | 48 (33.1) | 50 (34.5) | 133 (52.8) | 120 (47.4) |
| 25(OH)D, median (IQR) ng/ml | 28.6 (24.2–36.4) | 28.8 (23.6–36.0) | 28.0 (23.6–36.3) | 29.2 (24.2–37.6) |
| BMD T score, mean ± SD | ||||
| Lumbar spine | −1.06 ± 1.57 | –0.92 ± 1.86 | –1.96 ± 1.38 | –1.92 ± 1.38 |
| Total hip | –0.98 ± 1.07 | –1.14 ± 1.00 | –1.56 ± 0.96 | –1.66 ± 0.96 |
| Osteoporotic fracture after age ≥18 years, no. (%) | 51 (35.2) | 49 (33.8) | 135 (53.6) | 136 (53.8) |
| Prevalent vertebral fracture, no. (%) | 26 (17.9) | 21 (14.5) | 81 (32.1) | 67 (26.5) |
| Serum CTX, median (IQR) ng/liter | 230 (115–321) | 259 (150–375) | 140 (85–264) | 205 (111–344) |
| Fracture risk, median (IQR)e | ||||
| Major osteoporotic fracture | 11.3 (7.3–17.2) | 11.5 (7.6–17.9) | 14.0 (8.1–23.1) | 14.5 (7.8–24.5) |
| Hip fracture | 2.7 (0.9–5.8) | 3.1 (1.4–6.0) | 4.2 (1.5–8.1) | 4.4 (1.8–8.2) |
COPD = chronic obstructive pulmonary disease; IQR = interquartile range; 25(OH)D = 25‐hydroxyvitamin D; BMD = bone mineral density; CTX = C‐telopeptide of type I collagen.
Patients could have >1 medical condition requiring glucocorticoid therapy.
Dose in prednisone equivalents.
Investigators assigned each patient to a subpopulation (glucocorticoid‐initiating or glucocorticoid‐continuing) and recorded the start date of glucocorticoid use separately. The start date was used to calculate the duration of glucocorticoid use for each patient. Thus, in some instances (<10%), there was a mismatch.
Determined using Fracture Risk Assessment Tool.
Bone mineral density
Denosumab was superior to risedronate with regard to the percentage change from baseline in lumbar spine BMD and total hip BMD at each assessment through month 24 in each subpopulation (Figure 2). Denosumab was also superior to risedronate with regard to the percentage change from baseline to month 24 in BMD at the femoral neck and 1/3 radius (Figure 2). The difference between denosumab and risedronate treatment groups in mean percentage change in BMD from baseline to month 24 was 4.5% (95% confidence interval [95% CI] 3.2–5.8%) for the lumbar spine, 3.1% (95% CI 2.2–3.9%) for the total hip, 2.5% (95% CI 1.3–3.6%) for the femoral neck, and 1.5% (95% CI 0.5–2.5%) for the 1/3 radius in the glucocorticoid‐initiating subpopulation. In the glucocorticoid‐continuing subpopulation, the difference in the mean percentage change was 3.2% (95% CI 2.0–4.3%) for the lumbar spine, 2.5% (95% CI 1.7–3.2%) for the total hip, 1.8% (95% CI 0.7–2.9%) for the femoral neck, and 1.6% (95% CI 0.7–2.4%) for the 1/3 radius. Results of the sensitivity analyses for BMD were consistent with those of the planned analysis.
Figure 2.
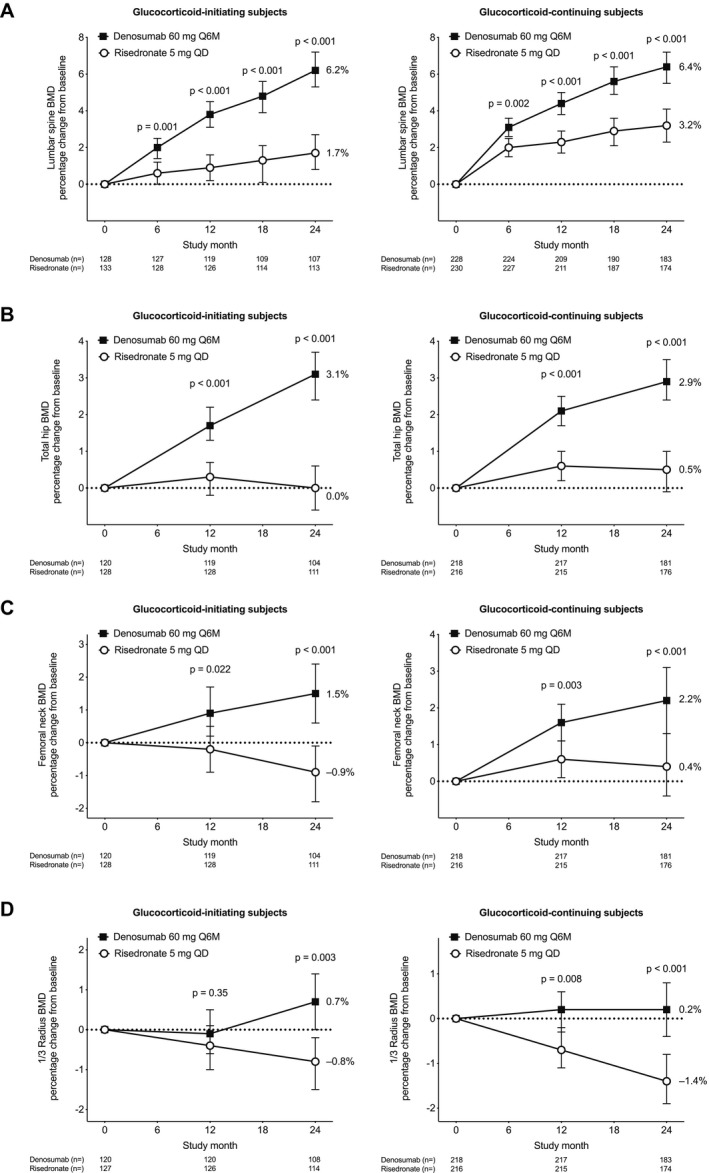
Percentage change from baseline in bone mineral density (BMD) at the lumbar spine (A), total hip (B), femoral neck (C), and 1/3 radius (D) for each subpopulation. Between‐group comparisons are based on analysis of covariance models with adjustment for treatment, baseline BMD, sex, machine type, and baseline BMD × machine type interaction. For the glucocorticoid‐continuing subpopulation, duration of prior glucocorticoid use (<12 months versus ≥12 months) was included as an additional covariate. Values are the least squares means and 95% confidence intervals. Q6M = once every 6 months; QD = once daily.
Bone turnover markers
The bone turnover marker substudy included 269 patients (140 treated with denosumab and 129 treated with risedronate). In both treatment groups, bone turnover markers for resorption (CTX) and formation (PINP) decreased from baseline throughout the study (Figure 3). The reduction in levels from baseline were significantly greater in the denosumab group than in the risedronate group for both CTX (from day 10 to month 12) and PINP (from month 3 to month 12). By month 24, the reductions from baseline were not significantly different between the denosumab and risedronate groups.
Figure 3.
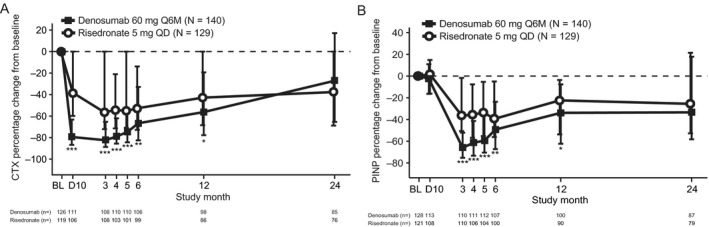
Percentage change from baseline (BL) in serum concentrations of C‐telopeptide of type I collagen (CTX) (A), a marker of bone resorption, and of N‐propeptide of type I collagen (PINP) (B), a marker of bone formation, in the combined subpopulations of the bone turnover marker substudy. Values are the median and interquartile range.* = P ≤ 0.05; ** = P ≤ 0.025; *** = P ≤ 0.001, by Wilcoxon's rank sum test. D10 = day 10; Q6M = once every 6 months; QD = once daily.
Adverse events
Incidence rates of adverse events, serious adverse events, and adverse events leading to discontinuation of study treatment or discontinuation from study were similar between treatment groups (Table 2). The incidence of any infection in the denosumab and risedronate groups was 36.3% (143 of 394) and 36.4% (140 of 385), respectively, and the incidence of any serious infection was 5.8% (23 of 394) and 6.5% (25 of 385). Incidence rates of serious infections in high‐risk subgroups were as follows for those receiving a concomitant biologic: 0% (0 of 23) in the denosumab group and 12.1% (4 of 33) in the risedronate group. In patients receiving a concomitant biologic, or any biologic or nonbiologic immunosuppressant, the rates were as follows: 3.6% (7 of 195) in the denosumab group and 6.8% (14 of 207) in the risedronate group. There was no fatal infection in the denosumab group and 1 fatal infection (septic shock) in the risedronate group. Study treatment was discontinued due to an infection in 1 patient in the denosumab group and 4 patients in the risedronate group.
Table 2.
Incidence of clinically relevant treatment‐emergent adverse events through month 24a
| Risedronate (n = 385) | Denosumab (n = 394) | |
|---|---|---|
| Total adverse events | 300 (77.9) | 324 (82.2) |
| Leading to discontinuation of study drug | 37 (9.6) | 31 (7.9) |
| Leading to discontinuation from study | 15 (3.9) | 18 (4.6) |
| Fatal | 9 (2.3)b | 13 (3.3) |
| Most frequently reported | ||
| Back pain | 23 (6.0) | 25 (6.3) |
| Arthralgia | 34 (8.8) | 23 (5.8) |
| Hypertension | 15 (3.9) | 21 (5.3) |
| Viral upper respiratory tract infection | 19 (4.9) | 20 (5.1) |
| Upper respiratory tract infection | 12 (3.1) | 20 (5.1) |
| Bronchitis | 17 (4.4) | 19 (4.8) |
| Urinary tract infection | 13 (3.4) | 18 (4.6) |
| Headache | 9 (2.3) | 16 (4.1) |
| Nausea | 17 (4.4) | 15 (3.8) |
| Cataract | 20 (5.2) | 10 (2.5) |
| Osteoarthritis | 16 (4.2) | 10 (2.5) |
| Selected adverse events of interest | ||
| Atypical femoral fracturec | 0 (0.0) | 1 (0.3) |
| Osteonecrosis of the jawc | 0 (0.0) | 0 (0.0) |
| Malignancy | 7 (1.8) | 12 (3.0) |
| Any serious infection | 25 (6.5) | 23 (5.8) |
| Serious infections reported for >1 patient | ||
| Pneumonia | 8 (2.1) | 7 (1.8) |
| Diverticulitis | 1 (0.3) | 2 (0.5) |
| Erysipelas | 1 (0.3) | 2 (0.5) |
| Bronchitis | 2 (0.5) | 0 (0.0) |
| Sepsis | 2 (0.5) | 0 (0.0) |
Patient count indicates number of patients who received ≥1 dose of investigational product; categories include data based on patients reporting ≥1 event. There were no significant differences between treatment groups. Values are the number (%).
Two additional deaths (due to pneumonia bacterial and polymyositis) were reported in patients randomized to receive risedronate. These patients were not included in the safety analysis set, because it was not possible to confirm that they had taken ≥1 dose of oral investigational product. The 2 patients died prior to the 6‐month visit and oral investigational product accountability verification.
Osteonecrosis of the jaw and atypical femoral fracture were positively adjudicated by independent, blinded, external adjudication committees using published case definitions.
As reported previously 25, 1 instance of positively adjudicated atypical femoral fracture in the denosumab group occurred in a 60‐year‐old man with a >30‐year history of glucocorticoid use for asthma, ~2 months after his second dose of denosumab. This event was treated with surgical fixation and resolved within ~7 months. There were no cases of positively adjudicated osteonecrosis of the jaw.
Fractures were a safety end point. Osteoporosis‐related fractures through month 24 were reported in 8.8% of patients in the denosumab group and 9.1% of patients in the risedronate group (Table 3). New and worsening vertebral fractures were reported in 4.4% of patients in the denosumab group and 6.9% of patients in the risedronate group. Low‐trauma nonvertebral fractures occurred in 5.3% of patients in the denosumab group and 3.8% of patients in the risedronate group. None of the above differences in fracture end points between the 2 treatment groups were statistically significant.
Table 3.
Incidence of fractures through month 24a
| Risedronate | Denosumab | |
|---|---|---|
| Any osteoporosis‐related fractureb | 36/397 (9.1) | 35/398 (8.8) |
| New and worsening vertebral fracturec | 24/346 (6.9) | 15/338 (4.4) |
| Men | 5/101 (5.0) | 1/100 (1.0) |
| Women | 19/245 (7.8) | 14/238 (5.9) |
| Premenopause | 2/29 (6.9) | 2/33 (6.1) |
| Postmenopause | 16/211 (7.6) | 12/202 (5.9) |
| Unknown | 1/5 (20.0) | 0/3 (0.0) |
| Nonvertebral fracture (low‐trauma) | 15/397 (3.8) | 21/398 (5.3) |
Values are the number/total (%).
Includes new and worsening vertebral fracture and low‐trauma nonvertebral fracture.
Defined as an increase of ≥1 grade from baseline; evaluated in patients with a baseline assessment and ≥1 postbaseline assessment of vertebral fracture.
Discussion
This final analysis of a 24‐month, prospective, randomized, double‐blind, double‐dummy trial provided additional evidence of the efficacy and safety of denosumab, compared with risedronate, an established therapy, in glucocorticoid‐treated individuals at high risk of fracture. The superior effect of denosumab versus risedronate on spine and hip BMD that was observed in the primary analysis at month 12 25 continued through month 24 in each subpopulation: the glucocorticoid‐initiating subpopulation, in which patients had recently initiated glucocorticoid treatment, and the glucocorticoid‐continuing subpopulation, in which patients had been using a glucocorticoid for ≥3 months before study entry. Increases in BMD at the femoral neck were also significantly greater with denosumab, compared with risedronate, in each subpopulation at month 12 and at month 24. In the glucocorticoid‐initiating subpopulation, BMD at the femoral neck declined ~1% by month 24 with risedronate, compared with an increase of ~1% with denosumab. Changes in BMD at the 1/3 radius have been shown to be limited with many antiresorptives 30, 31; however, a statistically significant increase in BMD of the 1/3 radius was observed with denosumab treatment, compared with risedronate treatment, at month 24. The treatment difference for BMD at each skeletal site was larger at month 24 than at month 12, reflecting the progressive increase for most measures of BMD in the denosumab group versus the plateau in the risedronate group beyond 12 months, which has also been shown in other studies 19, 20, 21, 22.
Reductions in serum concentrations of bone turnover markers were significantly greater with denosumab versus risedronate through month 12 but were not significantly different between the treatment groups at month 24 in this analysis. In contrast to the FREEDOM study, in which >7,000 postmenopausal women with osteoporosis were enrolled and followed up for >3 years, and in which a placebo group and not a bisphosphonate group was used as comparator 32, the present study was not designed with adequate statistical power to detect fracture differences between treatment groups: ~700 patients were followed up for >2 years. Moreover, only 1 clinical trial in glucocorticoid‐induced osteoporosis demonstrated a significant risk reduction for fractures in the primary analysis, with fewer vertebral fractures in the teriparatide group than in the alendronate group 23. In that study, there was a greater proportion of postmenopausal women compared with other similarly designed studies. Significant fracture risk reduction with bisphosphonates, compared with placebo, was seen in extensions to the original alendronate clinical trials 5, 13 or when 2 risedronate trials were combined 9. In the present study, consistent with the results of the primary analysis at month 12, the cumulative incidence of fracture at month 24 was low in both groups and similar between treatment groups.
It is notable that the relationship between BMD and fracture risk may differ between patients with glucocorticoid‐induced osteoporosis and postmenopausal women with osteoporosis, and patients receiving glucocorticoids may have a different BMD threshold for fracture from those not receiving such medications 33. Thus, it is important to examine both BMD and fracture in studies of patients with glucocorticoid‐induced osteoporosis. Denosumab treatment in this active‐controlled study was associated with greater BMD gains at all skeletal sites measured compared with a treatment (risedronate) that has shown trends toward fracture risk reduction relative to placebo in previous trials in glucocorticoid‐induced osteoporosis 9. Furthermore, denosumab has demonstrated antifracture effects in both primary (postmenopausal) and secondary (androgen deprivation therapy or aromatase inhibitor therapy) osteoporosis settings 32, 34, 35. A meta‐analysis of bisphosphonate studies showed that the effects on the incidence of vertebral and, to a lesser degree, nonvertebral fractures in glucocorticoid‐induced osteoporosis were comparable to those observed in postmenopausal osteoporosis 16.
The overall incidences of adverse events, serious adverse events, and adverse events leading to discontinuation of study drug were similar between the denosumab and risedronate groups in the combined subpopulations. Consistent with the results of the primary analysis at month 12, rates of infection, including serious infections, were similar between treatment groups through month 24 in this study of patients with an inflammatory disease who were taking glucocorticoids, with or without biologics and other immunomodulatory agents. However, the study was not powered for this exploratory safety end point in a small subset of patients at higher risk of infection. Atypical femoral fracture and osteonecrosis of the jaw have been identified as risks with antiresorptive treatment, including denosumab 24, 36. As previously reported in the analysis at month 12 of this study, there was 1 case of positively adjudicated atypical femoral fracture in the denosumab group. No additional cases of atypical femoral fracture, or any cases of osteonecrosis of the jaw, were reported in either group with continued treatment beyond month 12.
Strengths of this study include the large sample size, long‐term follow‐up with study completion by 74% of patients in each treatment group, and use of an active treatment control group instead of a placebo control group. A potential limitation of the study is that it was neither designed nor powered to evaluate fracture as an efficacy end point; fracture data were collected as a safety end point. However, it would be difficult to show efficacy for fracture prevention in this setting due to the very large number of patients that would be needed in order to show superiority with respect to a fracture end point, especially compared with patients receiving active treatment. This study compared denosumab with only one drug—risedronate. Thus, how denosumab compares with other bisphosphonates or other bone‐specific agents is unknown. We did not stratify randomization by the underlying glucocorticoid‐requiring illness because of the difficulty of recruiting for such a study. This could introduce heterogeneity, but this issue should be adequately addressed by randomizations. Another potential limitation was a lack of data on treatment patterns or outcomes after completion of denosumab dosing. It is established that the effects of denosumab are reversible when discontinued without follow‐on therapy. In the setting of denosumab discontinuation in postmenopausal osteoporosis, a transient rise in bone turnover above baseline and a decline in BMD toward baseline have been shown 37, 38, 39. This is associated with a return of fracture risk to that of an untreated patient and an increased risk of multiple vertebral fracture 40. Similar consideration of the need to either continue denosumab or transition to another antiresorptive agent is warranted in patients with glucocorticoid‐induced osteoporosis.
In conclusion, denosumab continued to be superior to risedronate with regard to increases in spine and hip BMD through month 24. The overall safety profile was similar between treatment groups. Denosumab may offer a new option for treatment of at least 24 months for patients with glucocorticoid‐induced osteoporosis.
Author contributions
All authors were involved in drafting the article or revising it critically for important intellectual content, and all authors approved the final version to be published. Dr. Saag had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Study conception and design
Saag.
Acquisition of data
Saag, Geusens, Adachi, Messina, Morales‐Torres, Emkey, Lems.
Analysis and interpretation of data
Saag, Pannacciulli, Geusens, Adachi, Messina, Morales‐Torres, Emkey, Butler, Yin, Lems.
Role of the study sponsor
Amgen Inc. funded the study and participated in the study design. The authors collected the data, interpreted the results, and had the final decision to submit the manuscript for publication. Medical writing assistance was provided by Lisa Humphries (Amgen Inc.) and Jonathan Latham (PharmaScribe, LLC, on behalf of Amgen Inc.).
Acknowledgments
The authors thank the study centers and study patients.
ClinicalTrials.gov identifier: NCT01575873.
Supported by Amgen Inc.
Dr. Saag has received consulting fees, speaking fees, and/or honoraria from AbbVie, Ironwood/AstraZeneca, Bayer, Gilead, Horizon, Kowa, Radius, Sobi, and Teijin (less than $10,000 each) and from Amgen, Roche/Genentech, and Takeda (more than $10,000 each). Drs. Pannacciulli, Butler, and Yin own stock or stock options in Amgen. Dr. Geusens has received honoraria from Amgen and Eli Lilly (less than $10,000 each) and research support from Abbott, Amgen, Bristol‐Myers Squibb, Celgene, Janssen, Eli Lilly, MSD, Novartis, Pfizer, Roche, UCB, and Will‐Pharma. Dr. Adachi has received consulting fees, speaking fees, and/or honoraria from Amgen and Eli Lilly (less than $10,000 each) and research support from Amgen. Dr. Messina has received consulting fees, speaking fees, and/or honoraria from Amgen and the American Health Foundation (less than $10,000 each). Dr. Morales‐Torres has received consulting fees, speaking fees, and/or honoraria from Amgen (less than $10,000). Dr. Emkey has received consulting fees, speaking fees, and/or honoraria from Amgen (more than $10,000). Dr. Lems has received consulting fees, speaking fees, and/or honoraria from Amgen, MSD, Pfizer, and Eli Lilly (less than $10,000 each).
Qualified researchers may request data from Amgen clinical studies. Complete details are available at http://www.amgen.com/datasharing.
References
- 1. Overman RA, Yeh JY, Deal CL. Prevalence of oral glucocorticoid usage in the United States: a general population perspective. Arthritis Care Res (Hoboken) 2013;65:294–8. [DOI] [PubMed] [Google Scholar]
- 2. Van Staa TP, Leufkens HG, Cooper C. The epidemiology of corticosteroid‐induced osteoporosis: a meta‐analysis. Osteoporos Int 2002;13:777–87. [DOI] [PubMed] [Google Scholar]
- 3. Buckley L, Guyatt G, Fink HA, Cannon M, Grossman J, Hansen KE, et al. 2017 American College of Rheumatology guideline for the prevention and treatment of glucocorticoid‐induced osteoporosis. Arthritis Rheumatol 2017;69:1521–37. [DOI] [PubMed] [Google Scholar]
- 4. Compston J, Cooper A, Cooper C, Gittoes N, Gregson C, Harvey N, et al. UK clinical guideline for the prevention and treatment of osteoporosis. Arch Osteoporos 2017;12:43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Saag KG, Emkey R, Schnitzer TJ, Brown JP, Hawkins F, Goemaere S, et al, for the Glucocorticoid‐Induced Osteoporosis Intervention Study Group . Alendronate for the prevention and treatment of glucocorticoid‐induced osteoporosis. N Engl J Med 1998;339:292–9. [DOI] [PubMed] [Google Scholar]
- 6. Stoch SA, Saag KG, Greenwald M, Sebba AI, Cohen S, Verbruggen N, et al. Once‐weekly oral alendronate 70 mg in patients with glucocorticoid‐induced bone loss: a 12‐month randomized, placebo‐controlled clinical trial. J Rheumatol 2009;36:1705–14. [DOI] [PubMed] [Google Scholar]
- 7. Reid DM, Hughes RA, Laan RF, Sacco‐Gibson NA, Wenderoth DH, Adami S, et al. Efficacy and safety of daily risedronate in the treatment of corticosteroid‐induced osteoporosis in men and women: a randomized trial. J Bone Miner Res 2000;15:1006–13. [DOI] [PubMed] [Google Scholar]
- 8. Eastell R, Devogelaer JP, Peel NF, Chines AA, Bax DE, Sacco‐Gibson N, et al. Prevention of bone loss with risedronate in glucocorticoid‐treated rheumatoid arthritis patients. Osteoporos Int 2000;11:331–7. [DOI] [PubMed] [Google Scholar]
- 9. Wallach S, Cohen S, Reid DM, Hughes RA, Hosking DJ, Laan RF, et al. Effects of risedronate treatment on bone density and vertebral fracture in patients on corticosteroid therapy. Calcif Tissue Int 2000;67:277–85. [DOI] [PubMed] [Google Scholar]
- 10. Mok CC, Tong KH, To CH, Siu YP, Ma KM. Risedronate for prevention of bone mineral density loss in patients receiving high‐dose glucocorticoids: a randomized double‐blind placebo‐controlled trial. Osteoporos Int 2008;19:357–64. [DOI] [PubMed] [Google Scholar]
- 11. Reid DM, Devogelaer JP, Saag K, Roux C, Lau CS, Reginster JY, et al. Zoledronic acid and risedronate in the prevention and treatment of glucocorticoid‐induced osteoporosis (HORIZON): a multicentre, double‐blind, double‐dummy, randomised controlled trial. Lancet 2009;373:1253–63. [DOI] [PubMed] [Google Scholar]
- 12. Sambrook PN, Roux C, Devogelaer JP, Saag K, Lau CS, Reginster JY, et al. Bisphosphonates and glucocorticoid osteoporosis in men: results of a randomized controlled trial comparing zoledronic acid with risedronate. Bone 2012;50:289–95. [DOI] [PubMed] [Google Scholar]
- 13. Adachi JD, Saag KG, Delmas PD, Liberman UA, Emkey RD, Seeman E, et al. Two‐year effects of alendronate on bone mineral density and vertebral fracture in patients receiving glucocorticoids: a randomized, double‐blind, placebo‐controlled extension trial. Arthritis Rheum 2001;44:202–11. [DOI] [PubMed] [Google Scholar]
- 14. Cohen S, Levy RM, Keller M, Boling E, Emkey RD, Greenwald M, et al. Risedronate therapy prevents corticosteroid‐induced bone loss: a twelve‐month, multicenter, randomized, double‐blind, placebo‐controlled, parallel‐group study. Arthritis Rheum 1999;42:2309–18. [DOI] [PubMed] [Google Scholar]
- 15. Gonnelli S, Rottoli P, Cepollaro C, Pondrelli C, Cappiello V, Vagliasindi M, et al. Prevention of corticosteroid‐induced osteoporosis with alendronate in sarcoid patients. Calcif Tissue Int 1997;61:382–5. [DOI] [PubMed] [Google Scholar]
- 16. Kanis JA, Stevenson M, McCloskey EV, Davis S, Lloyd‐Jones M. Glucocorticoid‐induced osteoporosis: a systematic review and cost‐utility analysis. Health Technol Assess 2007;11:1–231. [DOI] [PubMed] [Google Scholar]
- 17. Recker RR, Gallagher R, MacCosbe PE. Effect of dosing frequency on bisphosphonate medication adherence in a large longitudinal cohort of women. Mayo Clin Proc 2005;80:856–61. [DOI] [PubMed] [Google Scholar]
- 18. Silverman SL, Schousboe JT, Gold DT. Oral bisphosphonate compliance and persistence: a matter of choice? Osteoporos Int 2011;22:21–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Black DM, Schwartz AV, Ensrud KE, Cauley JA, Levis S, Quandt SA, et al. Effects of continuing or stopping alendronate after 5 years of treatment: the Fracture Intervention Trial Long‐term Extension (FLEX): a randomized trial. JAMA 2006;296:2927–38. [DOI] [PubMed] [Google Scholar]
- 20. Bianchi G, Czerwinski E, Kenwright A, Burdeska A, Recker RR, Felsenberg D. Long‐term administration of quarterly IV ibandronate is effective and well tolerated in postmenopausal osteoporosis: 5‐year data from the DIVA study long‐term extension. Osteoporos Int 2012;23:1769–78. [DOI] [PubMed] [Google Scholar]
- 21. Black DM, Reid IR, Boonen S, Bucci‐Rechtweg C, Cauley JA, Cosman F, et al. The effect of 3 versus 6 years of zoledronic acid treatment of osteoporosis: a randomized extension to the HORIZON‐Pivotal Fracture Trial (PFT). J Bone Miner Res 2012;27:243–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Miller PD, Recker RR, Reginster JY, Riis BJ, Czerwinski E, Masanauskaite D, et al. Efficacy of monthly oral ibandronate is sustained over 5 years: the MOBILE long‐term extension study. Osteoporos Int 2012;23:1747–56. [DOI] [PubMed] [Google Scholar]
- 23. Saag KG, Shane E, Boonen S, Marín F, Donley DW, Taylor KA, et al. Teriparatide or alendronate in glucocorticoid‐induced osteoporosis. N Engl J Med 2007;357:2028–39. [DOI] [PubMed] [Google Scholar]
- 24. Bone HG, Wagman RB, Brandi ML, Brown JP, Chapurlat R, Cummings SR, et al. 10 years of denosumab treatment in postmenopausal women with osteoporosis: results from the phase 3 randomised FREEDOM trial and open‐label extension. Lancet Diabetes Endocrinol 2017;5:513–23. [DOI] [PubMed] [Google Scholar]
- 25. Saag KG, Wagman RB, Geusens P, Adachi JD, Messina OD, Emkey R, et al. Denosumab versus risedronate in glucocorticoid‐induced osteoporosis: a multicentre, randomised, double‐blind, active‐controlled, double‐dummy, non‐inferiority study. Lancet Diabetes Endocrinol 2018;6:445–54. [DOI] [PubMed] [Google Scholar]
- 26. Lems WF, Baak MM, van Tuyl LH, Lodder MC, Dijkmans BA, Boers M. One‐year effects of glucocorticoids on bone density: a meta‐analysis in cohorts on high and low‐dose therapy. RMD Open 2016;2:e000313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Mudano A, Allison J, Hill J, Rothermel T, Saag K. Variations in glucocorticoid induced osteoporosis prevention in a managed care cohort. J Rheumatol 2001;28:1298–305. [PubMed] [Google Scholar]
- 28. Ruggiero SL, Dodson TB, Fantasia J, Goodday R, Aghaloo T, Mehrotra B, et al. American Association of Oral and Maxillofacial Surgeons position paper on medication‐related osteonecrosis of the jaw: 2014 update. J Oral Maxillofac Surg 2014;72:1938–56. [DOI] [PubMed] [Google Scholar]
- 29. Shane E, Burr D, Abrahamsen B, Adler RA, Brown TD, Cheung AM, et al. Atypical subtrochanteric and diaphyseal femoral fractures: second report of a task force of the American Society for Bone and Mineral Research. J Bone Miner Res 2014;29:1–23. [DOI] [PubMed] [Google Scholar]
- 30. Harris ST, Watts NB, Genant HK, McKeever CD, Hangartner T, Keller M, et al, for the Vertebral Efficacy With Risedronate Therapy (VERT) Study Group . Effects of risedronate treatment on vertebral and nonvertebral fractures in women with postmenopausal osteoporosis: a randomized controlled trial. JAMA 1999;282:1344–52. [DOI] [PubMed] [Google Scholar]
- 31. McClung MR, Lewiecki EM, Cohen SB, Bolognese MA, Woodson GC, Moffett AH, et al. Denosumab in postmenopausal women with low bone mineral density. N Engl J Med 2006;354:821–31. [DOI] [PubMed] [Google Scholar]
- 32. Cummings SR, San Martin J, McClung MR, Siris ES, Eastell R, Reid IR, et al. Denosumab for prevention of fractures in postmenopausal women with osteoporosis. N Engl J Med 2009;361:756–65. [DOI] [PubMed] [Google Scholar]
- 33. Van Staa TP, Laan RF, Barton IP, Cohen S, Reid DM, Cooper C. Bone density threshold and other predictors of vertebral fracture in patients receiving oral glucocorticoid therapy. Arthritis Rheum 2003;48:3224–9. [DOI] [PubMed] [Google Scholar]
- 34. Gnant M, Pfeiler G, Dubsky PC, Hubalek M, Greil R, Jakesz R, et al. Adjuvant denosumab in breast cancer (ABCSG‐18): a multicentre, randomised, double‐blind, placebo‐controlled trial. Lancet 2015;386:433–43. [DOI] [PubMed] [Google Scholar]
- 35. Smith MR, Egerdie B, Hernández Toriz N, Feldman R, Tammela TL, Saad F, et al. Denosumab in men receiving androgen‐deprivation therapy for prostate cancer. N Engl J Med 2009;361:745–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Miller PD, Pannacciulli N, Brown JP, Czerwinski E, Nedergaard BS, Bolognese MA, et al. Denosumab or zoledronic acid in postmenopausal women with osteoporosis previously treated with oral bisphosphonates. J Clin Endocrinol Metab 2016;101:3163–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Miller PD, Bolognese MA, Lewiecki EM, McClung MR, Ding B, Austin M, et al. Effect of denosumab on bone density and turnover in postmenopausal women with low bone mass after long‐term continued, discontinued, and restarting of therapy: a randomized blinded phase 2 clinical trial. Bone 2008;43:222–9. [DOI] [PubMed] [Google Scholar]
- 38. Bone HG, Bolognese MA, Yuen CK, Kendler DL, Miller PD, Yang YC, et al. Effects of denosumab treatment and discontinuation on bone mineral density and bone turnover markers in postmenopausal women with low bone mass. J Clin Endocrinol Metab 2011;96:972–80. [DOI] [PubMed] [Google Scholar]
- 39. Brown JP, Dempster DW, Ding B, Dent‐Acosta R, San Martin J, Grauer A, et al. Bone remodeling in postmenopausal women who discontinued denosumab treatment: off‐treatment biopsy study. J Bone Miner Res 2011;26:2737–44. [DOI] [PubMed] [Google Scholar]
- 40. Cummings SR, Ferrari S, Eastell R, Gilchrist N, Jensen JB, McClung M, et al. Vertebral fractures after discontinuation of denosumab: a post hoc analysis of the randomized placebo‐controlled FREEDOM trial and its extension. J Bone Miner Res 2018;33:190–8. [DOI] [PubMed] [Google Scholar]


