Abstract
Purpose
The purpose of this study was to assess the prevalence of meibomian gland dysfunction (MGD) and its association with tear film and ocular surface parameters in an Austrian clinical population of dry eye patients.
Methods
The records of 1372 consecutive patients from a dry eye unit were analysed retrospectively. Symptoms and objective tear film and ocular surface parameters were evaluated. Patients were classified into pure MGD, pure aqueous tear deficiency (ATD), MGD combined with ATD, pure anterior blepharitis (AB), Sjogren's syndrome (SS) without MGD and SS together with MGD.
Results
Nine‐hundred and sixty‐five patients, that is 70.3% of the investigated population, mean age 55.4 ± 16.6 years, had signs of MGD. Of these, 684 (70.9%) were female. The intensity of symptoms did not differ between subgroups. Four hundred and ninety (50.8%) MGD patients had Schirmer test values ≤10 mm/5 min. The fluorescein break‐up time and Schirmer test values were significantly higher in the pure AB and MGD group. The pure MGD group showed a significantly lower fluorescein staining of the cornea compared to the other groups, except for pure AB. Lissamine green staining of the ocular surface was present in all groups, but was at least pronounced in the pure MGD and AB group.
Conclusion
Meibomian gland dysfunction is a major cause of ocular discomfort and could often be found in combination with a reduced aqueous tear secretion. Although the intensity of subjective complaints was similar to all other subgroups, pure MGD exhibited the lowest severity of signs of ocular surface damage and also affected younger people.
Keywords: Austria, clinical population, dry eye, meibomian gland dysfunction, prevalence, tear film parameters
Introduction
The meibomian glands are large sebaceous glands, located as separate, parallel strands within the tarsal plates of the upper and lower eyelid. Their oily secretion (meibum) is produced by a holocrine secretory process in which, after the formation and storage of lipids, the secretory cells (meibocytes) are completely converted into the meibum (Knop & Knop 2009b).
The meibum forms the superficial lipid layer of the preocular tear film. This lipid layer is of great importance for the stability of the tear film. The meibomian oil is also responsible for the formation of an optically smooth tear film layer (Knop et al. 2009a). A lack of the lipid layer, for example resulting from an obstruction of the ducts of the meibomian glands, leads to an increased evaporation of tears (Mishima & Maurice 1961), consecutively to an increase in tear osmolarity (Gilbard et al. 1989). These changes result in an impaired lubrication, inflammation (2007a) and damage to the ocular surface (Gilbard et al. 1989).
A functional disorder of the meibomian glands is often referred to as posterior blepharitis or meibomian gland dysfunction (MGD). Meibomian gland dysfunction (MGD) is the major cause of evaporative dry eye (EDE; Knop et al. 2009b; Schaumberg et al. 2011).
In 1995, Heiligenhaus and colleagues (Heiligenhaus et al. 1995) found disorders of the lipid layer of the tear film in about 75% of dry eye patients regardless of cause; 65% of patients with symptoms such as eye irritation, burning of the eyes, foreign body sensation and epiphora suffer from MGD (Shimazaki et al. 1995; Knop et al. 2009b). Current literature even suggests that up to 86% of dry eye patients demonstrate signs of MGD (Lemp et al. 2012). However, it has to be noted that MGD can also be present in asymptomatic patients (Korb & Henriquez 1980; Knop et al. 2010).
The primary reason for MGD is thought to be based on an obstructive disorder, caused by increased keratinization of the terminal ducts, the presence of squamous debris and a thickening of the meibum (Knop et al. 2009b).
The prevalence of MGD in the normal population is reported to be highly variable and in various studies ranges between 3.5% and 70%. It seems to be higher in Asian populations (Schein et al. 1997; Schaumberg et al. 2011; Siak et al. 2012).
The purpose of this study was to assess the Austrian prevalence of MGD and the association of MGD with routinely measured tear film and ocular surface parameters, in a large clinical population of dry eye patients, retrospectively.
Materials and Methods
Approval of the local IRB was obtained to analyse the records of patients with dry eye symptoms, such as dryness, foreign body sensation, burning, increased sensitivity to light, sensation of pressure and frequent blinking, who were investigated at the dry eye unit of the ophthalmological department, Medical University of Graz, Austria, between 2004 and 2010. As all data were analysed anonymously, no consent was required. The study and the lack of a written or verbal consent were approved by the IRB with the approval number 26‐270 ex 13/14. The research followed the tenets of the Declaration of Helsinki. Patients who had undergone ocular surgery in the past 6 months were excluded.
Objective tear film and ocular surface parameters of both eyes were analysed, including an evaluation of the lid margins and an assessment of subjective symptoms.
The fluorescein tear film break‐up time (FBUT) was determined after the application of dye into the tear film by a fluorescein strip (Haag‐Streit, Bern, CH) moistened with physiological saline. The patient was instructed to blink a few times and then to keep the eyes open. Film break‐up time (FBUT) was always assessed in the right eye first. The precorneal tear film was observed at 10‐fold magnification using a slit lamp with cobalt blue illumination. By a stopwatch, the time until the break‐up of the tear film was measured three times and the mean was documented. Subsequently, the extent of fluorescein staining of the cornea was reported using an area and density (score 0–3; Horwath‐Winter et al. 2003).
The Schirmer test was carried out without prior application of a local anaesthetic. Filter paper strips (Clement Clark International Ltd, Harlow, UK) were bent at the notch and hooked over the lateral lower lid margin for 5 min, with the patient instructed to keep the eyes closed gently. The wetting length of the filter paper strip was read from the calibrated scale, in millimetres and documented.
Further evaluation of the ocular surface was performed by lissamine green staining. The dye was always applied in the same way. The lissamine green strips (HUB Pharmaceuticals, Rancho Cucamonga, CA, USA) were moistened with one drop of physiological saline and dye was introduced into the lower conjunctival sac. Staining of the nasal, central and temporal third of the ocular surface was scored according to van Bijsterveld: all three regions each having a scale of 0–3 points and the results added, resulting in a maximum total score of 9 points per eye (van Bijsterveld 1990).
The intensity of the symptoms was assessed using a visual analogue scale (VAS) from 0 to 100 mm, where ‘0’ represents no complaints and ‘100’ the maximum amount of discomfort. Patients were asked to grade the average intensity of their dry eye symptoms within the last week.
The presence of telangiectasia, erythema and irregularity of the lid margins, a shifting of the openings of the meibomian glands, together with changes in the expressibility and quality of the meibum, for example waxy secretion or no secretion at all, or plugging of the orifices, was counted as signs of MGD (Tomlinson et al. 2011). Expressibility and quality of the meibum were evaluated after applying pressure to the skin of the middle of the lower and upper lid, (the 10 central meibomian glands) with a cotton tip.
For further analysis, the patients were divided into the following subgroups:
pure MGD (reduced expressibility and/or quality of the meibum, as well as morphological changes of the lid margins, such as telangiectasia, irregularity and a shifting of the openings of the meibomian glands) – no signs of anterior blepharitis (AB) or aqueous tear deficiency (ATD)
pure ATD (Schirmer values ≤5 mm/5 min in at least one eye) – no signs of AB or MGD, no Sjogren's syndrome (SS – criteria according to the American‐European Consensus Group (Vitali et al. 2002))
MGD + ATD (both MGD and ATD criteria met) – no signs of AB and SS
pure AB (erythema of the lid margin and the presence of squamous debris and/or crusts at the cilia) – no signs of ATD, MGD and SS
SS without MGD
SS with MGD (both SS and MGD criteria met)
Other ocular surface conditions such as conjunctivochalasis, exposure and blink disorders were included in the group entitled ‘other diagnoses’.
Tear film and ocular surface parameters are described as means, with standard deviations in parentheses and frequencies (with percentages) of categorical parameters are displayed. To assess group differences in continuous parameters, we used the Kruskal–Wallis test with pairwise Wilcoxon signed‐ranks tests as post hoc tests. Differences in categorical parameters are assessed with exact Chi‐square tests. p‐Values <0.05 were considered statistically significant, and for multiple comparisons, p‐values were Bonferroni‐corrected. All computations were carried out using the statistical software IBM spss Statistics (Release 19.0.0. 2010; International Business Machines Corporation, Armonk, NY, USA).
Results
The records of 1372 consecutive patients with dry eye symptoms, who were investigated at the dry eye unit of the ophthalmological department, Medical University of Graz, Austria, between 2004 and 2010, could be analysed. The mean age was 54.3 ± 17.8 years, and 71.3% were female.
Signs of meibomian gland dysfunction were found in 965 (70.3%) of all patients. The mean age of these patients was 55.4 ± 16.6 years, and 684 (70.9%) were female. Four hundred and ninety (50.8%) of the MGD patients showed Schirmer values less than or equal to 10 mm/5 min, and 283 (29.3%) less than or equal to 5 mm/5 min. Anterior blepharitis (AB) was seen in 99 (10.3%) of the MGD patients. Sjogren's syndrome (SS) was seen in 39 (4.0%) of patients with signs of MGD.
According to the classification into the distinct subgroups, pure MGD was present in 598 (43.6%), isolated ATD in 101 (7.4%), MGD combined with ATD in 229 (16.7%) and pure AB in 51 (3.7%) of all patients. Sjogren's syndrome (SS) combined with MGD was diagnosed in 39 (2.8%) and SS without MGD in 40 (2.9%) of all patients, thus resulting in a study population of 1058 patients.
Table 1 shows the results of the individual analysis of the specified parameters for the evaluated subgroups (pure MGD, pure ATD, MGD + ATD, pure AB, SS−MGD and SS + MGD). The following mixed groups were not included in the table for a better visualization (MGD + AB: n = 69 (5.0%), MGD + ATD + AB: n = 30 (2.2%), ATD + AB: n = 18 (1.3%) and other diagnoses: n = 197 (14.4%)).
Table 1.
Tear film and ocular surface parameters of the defined subgroups
| Pure MGD | Pure ATD | MGD + ATD | Pure AB | SS + MGD | SS−MGD | |
|---|---|---|---|---|---|---|
| Patients, n (%) | 598 (43.6) | 101 (7.4) | 229 (16.7) | 51 (3.7) | 39 (2.8) | 40 (2.9) |
| Age, mean (SD), years | 53.1 (16.9)a | 55.9 (17.1) | 58.0 (15.3) | 58.8 (22.9) | 56.1 (13.2) | 52.2 (14.5) |
| Female, n (%) of patients | 422 (70.6) | 77 (76.2) | 167 (72.9) | 24 (47.1)b | 34 (87.2) | 36 (90.0) |
| VAS, mean (SD), score 0–100 | 57.6 (21.5) | 57.4 (26.4) | 57.6 (21.0) | 55.0 (8.7) | 57.4 (25.3) | 56.0 (19.8) |
| FBUT, mean (SD), seconds | 5.9 (3.8)c | 4.1 (2.8) | 4.1 (2.9)f | 6.2 (3.6)e | 3.8 (3.9) | 2.8 (2.2) |
| Schirmer, mean (SD), mm | 20.8 (10.1)c | 4.6 (3.9) | 4.6 (2.9) | 20.6 (11.2)e | 9.8 (11.1) | 6.4 (7.8) |
| Fluorescein staining, mean (SD), score 0–3 | 0.28 (0.53)c | 0.65 (0.72) | 0.52 (0.65) | 0.54 (0.73) | 0.89 (0.84) | 1.17 (0.88)d |
| Lissamine green staining, mean (SD), score 0–3 | 1.33 (1.43)c | 2.77 (2.36) | 2.11 (1.82) | 1.54 (1.47) | 4.48 (2.48)g | 4.50 (2.45)d |
AB = anterior blepharitis; ATD = aqueous tear deficiency; FBUT = film break‐up time; MGD = meibomian gland dysfunction; SD = standard deviation; SS = Sjogren's syndrome; VAS = visual analogue scale.
Value indicates statistically significant difference (p < 0.05) between the pure MGD and MGD + ATD group.
Value indicates statistically significant difference (p < 0.05) between pure AB and all other groups.
Value indicates statistically significant difference (p < 0.05) between pure MGD and pure ATD, MGD + ATD, SS + MGD and SS−MGD groups.
Value indicates statistically significant difference (p < 0.05) between SS−MGD and pure MGD, pure ATD, MGD + ATD and pure AB groups.
Value indicates statistically significant difference (p < 0.05) between pure AB and pure ATD, MGD + ATD, SS + MGD and SS−MGD groups.
Value indicates statistically significant difference (p < 0.05) between MGD + ATD and SS−MGD group.
Value indicates statistically significant difference (p < 0.05) between SS + MGD and pure MGD, pure ATD, MGD + ATD and pure AB groups.
The age distribution within the subgroups is further illustrated in Fig. 1.
Figure 1.
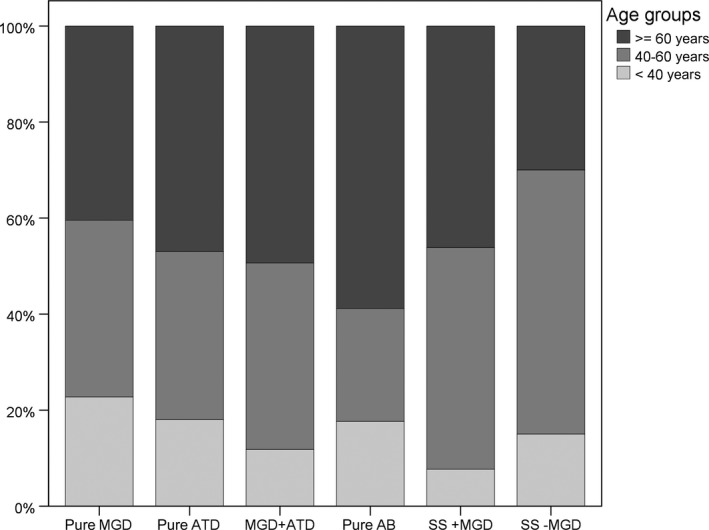
Three‐category age distribution in the six diagnosis groups. The distribution of the three‐category age (younger than 40 years, 40 to 60 years and older or equal than 60 years) shows a higher proportion of over 60‐year‐old patients in the pure anterior blepharitis group and a higher proportion of younger than 40‐year‐old patients in the pure meibomian gland dysfunction group.
The pure MGD group had a significantly lower age than the MGD +ATD group.
In all groups, except for the pure AB group, women were more often affected than men. Relatively more women were affected in the SS group.
The intensity of the subjective symptoms according to the VAS was not different between the groups.
The evaluation of the tear film and ocular surface parameters in pure MGD showed a lower total severity of the ocular surface disease compared to patients suffering from isolated ATD.
The FBUT and Schirmer values were significantly higher in the pure AB and pure MGD group than in all other groups, with no differences between these two groups.
The pure MGD group showed a significantly lower fluorescein staining of the cornea compared to the other subgroups, except for pure AB.
Lissamine green staining of the ocular surface was present in all groups. The pure MGDs lissamine green staining was significantly less than all other groups except for pure AB. The two SS groups showed significantly higher LG staining than all other groups.
Systemic diseases other than SS with known influence on ocular surface disease were present in some patients of the study population, for example allergies (29.2%), arterial hypertension (20.3%), depression (4.3%), diabetes (3.8%), migraine (5.3%), skin disease (7.2%) and thyroid disease (21.2%). There was no statistically significant difference between the subgroups, except for skin diseases, with the highest occurrence in the pure AB group (15.7%).
One‐hundred and ninety‐one (18.1%) patients of the study population had previous ocular surgery, none of them within the last 6 months. The highest occurrence was observed in the pure AB group (39.2%).
Eighty‐four (7.9%) patients of the study population were contact lens wearers. There was no statistically significant difference between the subgroups.
Discussion
Dry eye may be divided into two major pathogenetic categories. Either caused by a lack of tears (aqueous tear‐deficient dry eye, ATD) or by excessive evaporation (evaporative dry eye, EDE) often leading to a vicious inflammatory cycle, including the formation of reactive oxygen species (2007a; Baudouin et al. 2017; Craig et al. 2017; Pflugfelder & de Paiva 2017; Seen & Tong 2017). It is important to note that these groups are not strictly separated from each other and mixed forms occur (Horwath‐Winter et al. 2003; Lemp et al. 2012).
One of the most common causes of EDE is thought to be MGD (Nichols et al. 2011). Very often, a hyposecretory, obstructive condition of the meibomian glands can be observed. This is thought to be due to an increased keratinization of the terminal ducts, the presence of squamous debris and a more viscous meibomian lipid (Knop et al. 2011). The literature also indicates a variant of hypersecretory MGD. There are controversial views regarding the definition, distribution and prevalence (Nelson et al. 2011). In our study population, the hyposecretory, obstructive variant of MGD was present almost exclusively. This may be because the average age of our MGD patients was 55.4 ± 16.6 years. According to Nien and colleagues, the hypersecretory MGD can primarily be found in younger patients (Nien et al. 2011).
In different studies, the prevalence of MGD in the general population is reported very variable with about 3.5% to 70%. It was found to be higher in the Asian than in the Caucasian population (Schein et al. 1997; Schaumberg et al. 2011; Siak et al. 2012). Frequencies of 20% to 90% were reported in clinical populations. Most of the investigations were performed on selected populations such as contact lens wearers or patients with dry eye (Korb & Henriquez 1980; Ong & Larke 1990; Shimazaki et al. 1995). A possible reason for the divergent results regarding the prevalence of MGD in the previously published literature is the use of different definitions for the existence of MGD (Viso et al. 2012). The MGD report defines the presence of telangiectasia, erythema and irregularity of the lid margins, a shifting of the meibomian glands orifices, as well as changes in the expressibility and quality of the meibum as signs of MGD (Arita et al. 2009; Tomlinson et al. 2011).
In the present study, the medical records of 1372 consecutive patients with dry eye symptoms were analysed retrospectively.
In 70.3% of patients, signs of MGD, as defined by the MGD report, could be observed. Our results show a high prevalence of MGD in this large, clinical, Austrian population. As already reported by Shimazaki and colleagues (Shimazaki et al. 1995), MGD was often combined with ATD. 490 (50.8%) of patients with signs of MGD showed Schirmer values less than or equal to 10 mm/5 min, and 283 (29.3%) less than or equal to 5 mm/5 min, by definition, an ATD. The majority of these patients were female (70.9%). This distribution is consistent with previously published gender distributions in the context of studies on ocular surface disease, especially in relation to ATD (2007b;. However, it is thought that the presence of EDE due solely to MGD is observed in men more often than in women (Viso et al. 2012).
Corroborating Lemp and colleagues (Lemp et al. 2012), we observed that dry eye patients classified with isolated EDE characterized by signs of MGD were approximately six times more common than purely aqueous‐deficient patients.
No significant differences between the subgroups in terms of subjective symptoms could be found. The overlap of symptoms between EDE and ATD‐induced dry eye makes a distinction based on subjective symptoms difficult (Tong et al. 2011; Bartlett et al. 2015). There is still no standardized questionnaire specifically targeting EDE and MGD available. Additionally to the VAS, the Ocular Surface Disease Index questionnaire (OSDI) is used as a standardized survey for ocular surface discomfort (Schiffman et al. 2000). Unfortunately, it could not be evaluated in this study, because it had not been established at our dry eye unit at that time. Nevertheless, visual analogue scales have been used frequently for the assessment of both chronic and acute pain in a variety of conditions and are still seen as a reliable measure in dry eye studies (McCormack et al. 1988; Gaston‐Johansson 1996; Bijur et al. 2001; Schaumberg et al. 2007; Ishida et al. 2008). Currently, we routinely use the VAS, as well as the OSDI for the assessment of subjective symptoms.
The intensity of subjective symptoms in MGD was equal to that in ATD, despite a lesser staining of the ocular surface. Rosenthal and colleagues concluded that the link between MGD and chronic corneal symptoms may be governed by other factors – in particular, the sensitivity of responsive corneal nociceptors (Rosenthal & Borsook 2012). A possible drawback of our study is that we did not measure corneal sensitivity routinely.
Patients with pure MGD had a longer FBUT than those suffering from pure ATD, MGD + ATD, SS + MGD and SS−MGD. Film break‐up time (FBUT) is a well‐known global parameter for dry eye rather than a parameter only related to MGD. Pflugfelder et al. as well as Lemp and colleagues already reported a short FBUT in pure ATD (Pflugfelder et al. 1998; Lemp et al. 2012).
The function of the meibomian glands depends on various endogenous and exogenous factors. The development and progression of MGD can be influenced by ophthalmic, systemic, hormonal and genetic factors, as well as drugs, chemical and mechanical noxious agents (Schaumberg et al. 2011). As MGD is a potentially multifactorial disease, further studies on the extent of the various influences are warranted. Studies targeted on the prevalence of asymptomatic MGD within the normal population are sparse, as it can be assumed that in this early stage, potential long‐term consequences, such as the irreversible loss of meibomian glands, might possibly be prevented (Knop & Knop 2009a, Viso et al. 2012).
Meibomian gland dysfunction patients tended to be younger, whereas MGD + ATD occurred more frequently in the elderly. It is thought that changes in lifestyle during the last decades, like the increased use of electronic media and video display terminals, might be partly responsible for more and more young people affected (Schaumberg et al. 2011).
The performance of accurate diagnostics and classification is essential for the clinical management and targeted therapy of dry eye patients.
The present study included patients with dry eye symptoms, who were investigated at our dry eye unit and was lacking a healthy control group. By our study design, we may have missed up to 40% of patients with dry eye signs not presenting with symptoms (Nichols et al. 2004; Sullivan et al. 2012a).
A further limitation of our study is the use of traditional dry eye tests without the newer technologies providing a more accurate picture of objective changes in dry eye disease (e.g. noninvasive tear film break‐up time, osmolarity testing, visualization of the lipid layer and meibomian gland morphology; Sullivan et al. 2012b; Wolffsohn et al. 2017). Despite their potential advantages over common tests, these are usually not a part of the routine ocular examination (e.g. for cost reasons) and further studies are needed to determine their precise role in the diagnosis and follow‐up of patients with dry eye (Thulasi & Djalilian 2017).
Our study, showing that MGD is a major cause of ocular discomfort, often combined with a reduced aqueous tear secretion, represents a snapshot in time and location, which might be useful in forming a good baseline for comparison with studies using newer technology in diagnosis and management of dry eye.
Special thanks to Christine Wachswender for her excellent technical assistance.
References
- No authors listed (2007a): The definition and classification of dry eye disease: report of the Definition and Classification Subcommittee of the International Dry Eye WorkShop (2007). Ocul Surf 5: 75–92. [DOI] [PubMed] [Google Scholar]
- No authors listed (2007b): The epidemiology of dry eye disease: report of the Epidemiology Subcommittee of the International Dry Eye WorkShop (2007). Ocul Surf 5: 93–107. [DOI] [PubMed] [Google Scholar]
- Arita R, Itoh K, Maeda S, Maeda K, Furuta A, Fukuoka S, Tomidokoro A & Amano S (2009): Proposed diagnostic criteria for obstructive meibomian gland dysfunction. Ophthalmology 116: 2058–2063. e1. [DOI] [PubMed] [Google Scholar]
- Bartlett J, Keith M, Sudharshan L & Snedecor S (2015): Associations between signs and symptoms of dry eye disease: a systematic review. Clin Ophthalmol 9: 1719–1730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baudouin C, Irkeç M, Messmer EM et al. (2017): Clinical impact of inflammation in dry eye disease: proceedings of the ODISSEY group meeting. Acta Ophthalmol 98: 1001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Bijsterveld OP (1990): Diagnosis and differential diagnosis of keratoconjunctivitis sicca associated with tear gland degeneration. Clin Exp Rheumatol 8(Suppl 5): 3–6. [PubMed] [Google Scholar]
- Bijur PE, Silver W & Gallagher EJ (2001): Reliability of the visual analog scale for measurement of acute pain. Acad Emerg Med 8: 1153–1157. [DOI] [PubMed] [Google Scholar]
- Craig JP, Nichols KK, Akpek EK et al. (2017): TFOS DEWS II definition and classification report. Ocul Surf 15: 276–283. [DOI] [PubMed] [Google Scholar]
- Gaston‐Johansson F (1996): Measurement of pain: the psychometric properties of the Pain‐O‐Meter, a simple, inexpensive pain assessment tool that could change health care practices. J Pain Symptom Manage 12: 172–181. [DOI] [PubMed] [Google Scholar]
- Gilbard JP, Rossi SR & Heyda KG (1989): Tear film and ocular surface changes after closure of the meibomian gland orifices in the rabbit. Ophthalmology 96: 1180–1186. [DOI] [PubMed] [Google Scholar]
- Heiligenhaus A, Koch JM, Kruse FE, Schwarz C & Waubke TN (1995): [Diagnosis and and differentiation of dry eye disorders]. Ophthalmologe 92: 6–11. [PubMed] [Google Scholar]
- Horwath‐Winter J, Berghold A, Schmut O, Floegel I, Solhdju V, Bodner E, Schwantzer G & Haller‐Schober E‐M (2003): Evaluation of the clinical course of dry eye syndrome. Arch Ophthalmol 121: 1364–1368. [DOI] [PubMed] [Google Scholar]
- Ishida R, Matsumoto Y, Onguchi T et al. (2008): Tear film with ‘Orgahexa EyeMasks’ in patients with meibomian gland dysfunction. Optom Vis Sci 85: 684–691. [DOI] [PubMed] [Google Scholar]
- Knop E & Knop N (2009a): [Meibomian glands: part IV. Functional interactions in the pathogenesis of meibomian gland dysfunction (MGD)]. Ophthalmologe 106: 980–987. [DOI] [PubMed] [Google Scholar]
- Knop N & Knop E (2009b): [Meibomian glands. Part I: anatomy, embryology and histology of the Meibomian glands]. Ophthalmologe 106: 872–883. [DOI] [PubMed] [Google Scholar]
- Knop E, Knop N & Schirra F (2009a): [Meibomian glands. Part II: physiology, characteristics, distribution and function of meibomian oil]. Ophthalmologe 106: 884–892. [DOI] [PubMed] [Google Scholar]
- Knop E, Knop N, Brewitt H, Pleyer U, Rieck P, Seitz B & Schirra F (2009b): [Meibomian glands: part III. Dysfunction – argument for a discrete disease entity and as an important cause of dry eye]. Ophthalmologe 106: 966–979. [DOI] [PubMed] [Google Scholar]
- Knop E, Korb DR, Blackie CA & Knop N (2010): The lid margin is an underestimated structure for preservation of ocular surface health and development of dry eye disease. Dev Ophthalmol 45: 108–122. [DOI] [PubMed] [Google Scholar]
- Knop E, Knop N, Millar T, Obata H & Sullivan DA (2011): The International Workshop on Meibomian Gland Dysfunction: Report of the Subcommittee on Anatomy, Physiology, and Pathophysiology of the Meibomian Gland. Invest Ophthalmol Vis Sci 52: 1938–1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korb DR & Henriquez AS (1980): Meibomian gland dysfunction and contact lens intolerance. J Am Optom Assoc 51: 243–251. [PubMed] [Google Scholar]
- Lemp MA, Crews LA, Bron AJ, Foulks GN & Sullivan BD (2012): Distribution of aqueous‐deficient and evaporative dry eye in a clinic‐based patient cohort: a retrospective study. Cornea 31: 472–478. [DOI] [PubMed] [Google Scholar]
- McCormack HM, Horne DJ & Sheather S (1988): Clinical applications of visual analogue scales: a critical review. Psychol Med 18: 1007–1019. [DOI] [PubMed] [Google Scholar]
- Mishima S & Maurice DM (1961): The oily layer of the tear film and evaporation from the corneal surface. Exp Eye Res 1: 39–45. [DOI] [PubMed] [Google Scholar]
- Nelson JD, Shimazaki J, Benitez‐del‐Castillo JM, Craig JP, McCulley JP, Den S & Foulks GN (2011): The International Workshop on Meibomian Gland Dysfunction: Report of the Definition and Classification Subcommittee. Invest Ophthalmol Vis Sci 52: 1930–1937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nichols KK, Nichols JJ & Mitchell GL (2004): The lack of association between signs and symptoms in patients with dry eye disease. Cornea 23: 762–770. [DOI] [PubMed] [Google Scholar]
- Nichols KK, Foulks GN, Bron AJ, Glasgow BJ, Dogru M, Tsubota K, Lemp MA & Sullivan DA (2011): The International Workshop on Meibomian Gland Dysfunction: Executive Summary. Invest Ophthalmol Vis Sci 52: 1922–1929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nien CJ, Massei S, Lin G, Nabavi C, Tao J, Brown DJ, Paugh JR & Jester JV (2011): Effects of age and dysfunction on human meibomian glands. Arch Ophthalmol 129: 462–469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ong BL & Larke JR (1990): Meibomian gland dysfunction: some clinical, biochemical and physical observations. Ophthalmic Physiol Opt 10: 144–148. [DOI] [PubMed] [Google Scholar]
- Pflugfelder SC & de Paiva CS (2017): The pathophysiology of dry eye disease: what we know and future directions for research. Ophthalmology 124: S4–S13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pflugfelder SC, Tseng SC, Sanabria O, Kell H, Garcia CG, Felix C, Feuer W & Reis BL (1998): Evaluation of subjective assessments and objective diagnostic tests for diagnosing tear‐film disorders known to cause ocular irritation. Cornea 17: 38–56. [DOI] [PubMed] [Google Scholar]
- Rosenthal P & Borsook D (2012): The corneal pain system. Part I: the missing piece of the dry eye puzzle. Ocul Surf 10: 2–14. [DOI] [PubMed] [Google Scholar]
- Schaumberg DA, Gulati A, Mathers WD, Clinch T, Lemp MA, Nelson JD, Foulks GN & Dana R (2007): Development and validation of a short global dry eye symptom index. Ocul Surf 5: 50–57. [DOI] [PubMed] [Google Scholar]
- Schaumberg DA, Nichols JJ, Papas EB, Tong L, Uchino M & Nichols KK (2011): The International Workshop on Meibomian Gland Dysfunction: Report of the Subcommittee on the Epidemiology of, and Associated Risk Factors for, MGD. Invest Ophthalmol Vis Sci 52: 1994–2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schein OD, Muñoz B, Tielsch JM, Bandeen‐Roche K & West S (1997): Prevalence of dry eye among the elderly. Am J Ophthalmol 124: 723–728. [DOI] [PubMed] [Google Scholar]
- Schiffman RM, Christianson MD, Jacobsen G, Hirsch JD & Reis BL (2000): Reliability and validity of the Ocular Surface Disease Index. Arch Ophthalmol 118: 615–621. [DOI] [PubMed] [Google Scholar]
- Seen S & Tong L (2017): Dry eye disease and oxidative stress. Acta Ophthalmol 77: 193. [DOI] [PubMed] [Google Scholar]
- Shimazaki J, Sakata M & Tsubota K (1995): Ocular surface changes and discomfort in patients with meibomian gland dysfunction. Arch Ophthalmol 113: 1266–1270. [DOI] [PubMed] [Google Scholar]
- Siak JJK, Tong L, Wong WL, Cajucom‐Uy H, Rosman M, Saw SM & Wong TY (2012): Prevalence and risk factors of meibomian gland dysfunction: the Singapore Malay eye study. Cornea 31: 1223–1228. [DOI] [PubMed] [Google Scholar]
- Sullivan BD, Crews LA, Messmer EM et al. (2012a): Correlations between commonly used objective signs and symptoms for the diagnosis of dry eye disease: clinical implications. Acta Ophthalmol 92: 161–166. [DOI] [PubMed] [Google Scholar]
- Sullivan BD, Crews LA, Sönmez B et al. (2012b): Clinical utility of objective tests for dry eye disease: variability over time and implications for clinical trials and disease management. Cornea 31: 1000–1008. [DOI] [PubMed] [Google Scholar]
- Thulasi P & Djalilian AR (2017): update in current diagnostics and therapeutics of dry eye disease. Ophthalmology 124: S27–S33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomlinson A, Bron AJ, Korb DR et al. (2011): The International Workshop on Meibomian Gland Dysfunction: Report of the Diagnosis Subcommittee. Invest Ophthalmol Vis Sci 52: 2006–2049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tong L, Zhou L, Beuerman RW, Zhao SZ & Li XR (2011): Association of tear proteins with Meibomian gland disease and dry eye symptoms. Br J Ophthalmol 95: 848–852. [DOI] [PubMed] [Google Scholar]
- Viso E, Rodríguez‐Ares MT, Abelenda D, Oubiña B & Gude F (2012): Prevalence of asymptomatic and symptomatic meibomian gland dysfunction in the general population of Spain. Invest Ophthalmol Vis Sci 53: 2601–2606. [DOI] [PubMed] [Google Scholar]
- Vitali C, Bombardieri S, Jonsson R et al. (2002): Classification criteria for Sjögren's syndrome: a revised version of the European criteria proposed by the American‐European Consensus Group. Ann Rheum Dis 61: 554–558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolffsohn JS, Arita R, Chalmers R et al. (2017): TFOS DEWS II diagnostic methodology report. Ocul Surf 15: 539–574. [DOI] [PubMed] [Google Scholar]


