Abstract
Background
Tryptophan hydroxylase (TPH)1 catalyzes the biosynthesis of serotonin (5-hydroxytrptamine; 5-HT) in enterochromaffin (EC) cells, the predominant source of gut 5-HT. Secreted 5-HT regulates various gut functions through diverse 5-HT receptor (5-HTR) families, and 5-HT transporter (5-HTT) sequesters its activity via uptake into surrounding cells. In inflammatory bowel disease (IBD) mucosal 5-HT signaling is altered, including upregulated EC cell numbers and 5-HT levels. We examined key mucosal 5-HT signaling components and blood 5-HT levels and, as part of a pilot study, investigated the association between 5-HTT gene-linked polymorphic region (5HTTLPR) and Crohn’s disease (CD).
Methods
In the context of inflammation, colonic expressions of TPH1, 5-HTT and 5-HTRs were studied in CD patients (n=15) and healthy controls (HC; n=10) using quantitative polymerase chain reaction (qPCR). We also investigated 5HTTLPR in 40 CD patients and HC utilizing PCR and measured platelet-poor plasma (PPP) and plasma 5-HT concentrations.
Results
Compared with HC, inflammation in CD patients was associated with elevated TPH1, 5-HTR3, 5-HTR4, 5-HTR7 and downregulated 5-HTT expressions. In our second cohort of participants, significantly higher PPP and plasma 5-HT levels and higher S-genotype (L/S+S/S) than L/L genotype were observed in CD patients compared with HC.
Conclusion
Our results suggest that augmented mucosal 5-HT signaling and specific 5-HTTLPR genotype–associated decreased efficiency in 5-HT reuptake, the latter through increased 5-HT availability, may contribute to inflammation in CD patients. These findings revealed important information on various components of 5-HT signaling in intestinal inflammation which may ultimately lead to effective strategies targeting this pathway in IBD.
Keywords: Crohn’s disease, IBD, Inflammatory bowel disease, Serotonin, 5-HT, 5-HTTLPR
Crohn’s disease (CD) and ulcerative colitis (UC), the two major forms of inflammatory bowel disease (IBD), are characterized by chronic, relapsing inflammation of the gastrointestinal (GI) tract (1). Inflammatory bowel disease affects approximately two million people in North America, and Canada has one of the highest incidence rates of CD in the world (2–5). Though the exact cause of IBD is yet to be determined, it is believed to result from a continuum of complex interactions among the host genetics, intestinal microbiota, the immune system and environmental factors (6).
The GI tract contains hundreds of thousands of endocrine cells that are dispersed among the intestinal epithelium. Among these cells, enterochromaffin (EC) cells are the best characterized and are well known for synthesizing and releasing serotonin, also known as 5-hydroxytryptamine (5-HT) (6). The GI tract contains about 95% of the body’s 5-HT, and EC cells are the main source of gut-derived 5-HT (6). Enterochromaffin cells via the rate-limiting enzyme tryptophan hydroxylase (TPH)1 produce 5-HT from dietary tryptophan (7). This 5-HT can be released into the gut lumen and surrounding tissue, which can then enter the blood circulation via the dense capillary bed of the lamina propria (7). The 5-HT mediates various GI functions, including motility, sensation and secretion, through an array of 5-HT receptors (5-HTRs); it additionally has immunomodulatory capabilities (6, 8). Five of the seven known receptor families of 5-HT (5-HTR1, 5-HTR2, 5-HTR3, 5-HTR4 and 5-HTR7) are expressed in the gut. Its actions are terminated by uptake into surrounding cells predominantly by the 5-HT transporter (5-HTT) (7). Various cell types express 5-HTT, including enterocytes, immune cells and platelets (7). Platelets do not produce 5-HT; instead, they take up enteric 5-HT using 5-HTT and thus participate in propagating the influence of gut-derived 5-HT beyond the gut (9). The 5-HTT is encoded by the SLC6A4 gene (10). A 44-bp insertion/deletion polymorphism in the promoter region of this gene, known as the 5-HTT gene-linked polymorphic region (5-HTTLPR), results in a short (S) allele and a long (L) allele, where the former is functionally dominant (10–12). Compared with the L/L genotype, which is more common among Caucasians, S/S or S/L genotypes (collectively referred to as the S-genotype) are associated with lower levels of 5-HTT mRNA transcripts and, thereby, associated with lower levels of 5-HTT expression and reduced 5-HT reuptake efficiency (10, 13).
Serotonin, or 5-HT, has been evaluated in IBD and in animal models of colitis. It was shown that TPH1-deficient mice, which have reduced gut 5-HT content, exhibit reduced susceptibility to experimental colitis marked by lower inflammatory cytokine production (14). Moreover, pharmacological blocking of peripheral 5-HT synthesis reduced the severity of both chemical and infection-induced intestinal inflammation (15). In IBD patients, studies have reported increased 5-HT expressing EC cell numbers and reduced 5-HTT expression, the latter particularly observed in association with inflammation in UC patients (16–18). Furthermore, a significant association between 5-HTTLPR and microscopic colitis, a type of colitis which had previously been associated with consumption of selective 5-HT reuptake inhibitors, has been reported (6, 19). Patients with microscopic colitis and UC were found to have significantly higher serum 5-HT levels (19). An elevated level of 5-HT was also observed in a patient with collagenous colitis (20). Taken together, these findings provide compelling evidence for an important role of 5-HT in gut inflammation. Despite the importance of its cognate receptors in 5-HT signaling, only scattered observations about 5-HTRs in IBD have been previously reported by a handful of studies (21, 22).
In the present study, we investigated key elements of mucosal 5-HT signaling, including 5-HTRs, in CD patients for a better understanding of the role of 5-HT in IBD. In colonic biopsy specimens from CD patients, we observed significant upregulation of TPH1, 5-HTR3, 5-HTR7 expressions and downregulation of 5-HTT expression in association with intestinal inflammation. Mucosal 5-HTR4 expression was also markedly elevated in these patients. Additionally, in a separate group of CD patients, we found significantly higher platelet-poor plasma (PPP) and plasma 5-HT concentrations in comparison with healthy controls (HC). In the latter group of CD patients, a higher frequency of the S-genotype was also observed compared with HC.
METHODS
This study was approved by the Hamilton Integrated Research Ethics Board (HiREB). All biopsy and blood samples were obtained at the McMaster University Medical Centre (MUMC) from consenting adults (clinicaltrials.gov # NCT01650311).
Sample Collection, Histopathological Assessment and 5-HT ELISA
Fifteen HC and 27 patients with confirmed diagnosis of CD provided informed consent to donate colonic biopsy samples. The HC group was comprised of individuals undergoing routine colorectal screening, and exclusion criteria included any previous history of GI complaints, long-term use of nonsteroidal anti-inflammatory drugs, steroids, antidepressants, or any biologics. Additionally, participants were excluded from the study if they were deemed unfit to partake for any reason by the gastroenterologist, for example, due to serious comorbid disease or difficult colonoscopy. This resulted in the withdrawal of four HC and 12 CD patients. Two mucosal biopsies were obtained from 10 HC patients, and one HC patient donated only one sample and thus was excluded from any analysis. Fifteen patients with CD donated two biopsy samples from both inflamed and noninflamed regions of the colon. Noninflamed regions were defined as those without any endoscopic features of inflammation and at least 10 cm from any area of active inflammation (23). Immediately following sample collection, one of the samples was flash frozen for later analysis of mRNA expression using quantitative polymerase chain reaction (qPCR). The second sample was fixed in 10% buffered formalin and was processed within 24 hours for histopathological assessment. A pathologist unaware of the diagnosis performed the histological evaluations, and specimens were graded on a numerical scale based on standard criteria (24).
In a separate study, we invited 109 Caucasian participants to donate 8 mL of venous blood, 12 of whom refused, and 97 provided informed consent, which consisted of 55 patients with confirmed diagnosis of CD and 42 HC patients. Forty HC patients and 40 patients with CD donated the full 8 mL of blood and completed the required questionnaire. Samples were collected in EDTA tubes, and 4 mL of blood was used to measure PPP and plasma 5-HT levels using a commercially available ELISA kit (Beckman Coulter, Fullerton, CA).
Immunofluorescence of 5-HT Expressing EC Cells
Immunofluorescent analysis of 5-HT expressing EC cells was performed on paraffin wax–embedded colonic sections. Sections were deparaffinized in CitriSolv (Thermo Fisher Scientific, Waltham, MA) and rehydrated through a graded series of ethanol. After washing, heat-mediated antigen retrieval in citrate buffer was performed, following which, 5% normal goat serum in phosphate buffered saline (PBS) was used to block nonspecific binding. Then the sections were incubated at 4°C overnight with rabbit anti-5-HT antibody or isotype control (IgG) antibody (Immunostar, Hudson, WI; concentration: 1:3000). Alexa Fluor 594-goat anti-rabbit IgG (Thermo Fisher Scientific) was used as the secondary antibody. ProLong™ gold antifade mountant with DAPI (Thermo Fisher Scientific) was used for nuclear staining and mounting of the sections, which were studied with a Nikon fluorescence microscope. The 5-HT-positive EC cells were quantified and expressed as number of cells/mm2.
RNA Isolation and qPCR Protocols
RNA was isolated from mucosal samples using RNesay plus a universal mini-kit (Qiagen, Hilden, Germany), and its quality was determined using NANOdrop (Thermo Fisher Scientific). The cDNA was prepared using the quantitect reverse transcription kit (Qiagen). The reference gene selected was 18s rRNA (forward: 5′-TCCACAGGAGGCCTACACGCC-3′; reverse: 5′-TTTCCGCCGCCCATCGATGTT-3′), as it was more stable (M=0.496) compared with glyceraldehyde-3-phosphate gehydrogenase (GAPDH) (M=0.548) and β-Actin (M=0.528). TPH1 mRNA expressions were quantified using previously published methods and primer sequences (25). Prevalidated primers from Bio-Rad (Hercules, CA) were used for 5-HTT (Bio-Rad assay ID qHsaCID0016255; NCBI RefSeq NG_011747.1), 5HTR1A (Bio-Rad assay ID qHsaCED0047309; NCBI RefSeq NT_006713.15), 2A (Bio-Rad assay ID qHsaCED0057396; NCBI RefSeq NG_013011.1), 3A (Bio-Rad assay ID qHsaCID0015498; NCBI RefSeq NG_013058.1), 4 (Bio-Rad assay ID qHsaCIP0028308; NCBI RefSeq NT_029289.11) and 7 (Bio-Rad assay ID qHsaCID0015403; NCBI RefSeq NT_030059.13), as well as for tumor necrosis factor (TNF)-α (Bio-Rad assay ID qHsaCEP0040184; NCBI RefSeq NG_007462.1), interleukin (IL)-13 (Bio-Rad assay ID qHsaCID0020181; NCBI RefSeq NG_012090.1) and S100 calcium-binding protein A9 (S100A9; Bio-Rad assay ID qHsaCED0046059; NCBI RefSeq NT_004487.19), according to manufacturer’s instructions.
Genotyping and Polymerase Chain Reaction Protocol
The insertion/deletion polymorphism in the 5-HTT gene was typed by polymerase chain reaction (PCR)-based method using CFX96TM Real-Time System (Bio-RAD). DNA was isolated from blood using a commercially available kit (Qiagen), and a modified version of the methods described by Sikander et al. (19) was utilized. The findings of the genotyping experiment were confirmed by the Centre for Applied Genomics (TCAG) operated by the Hospital for Sick Children (SickKids), Toronto, Canada.
Statistical Analysis
All statistical analyses were performed using GraphPad Prism 5 (La Jolla, CA) by means of Chi-square test, two-sided Fisher exact test, one-way ANOVA with Dunnett test or Newman-Keuls post hoc, as appropriate, where P < 0.05 was considered statistically significant.
RESULTS
Characterization of Study Participants
The demographic information of all study participants is shown in Table 1. At the time of blood donation, in the second group of participants, 21 (52.5%) of the 40 patients with CD reported experiencing symptoms. Additionally, 17 (42.5%) patients tested positive for C-reactive protein (CRP) (CPR+ defined as greater than upper limit of normal where CRP > 5.1 mg/L (26, 27)), 18 (45%) had features of reactive thrombocytosis (RT+ defined as platelet count > 450 × 109/L), and 6 (15%) were on some form of antidepressants (excluded from all analysis of PPP or plasma 5-HT levels).
Table 1.
Demographic data of healthy controls and patients with Crohn’s disease. Cohort 1: the investigation of mucosal 5-HT signaling; and cohort 2: the investigation of blood 5-HT concentrations and 5-HT transporter gene-linked polymorphic region (5-HTTLPR) in inflammatory bowel disease
| Healthy controls (HC) | Crohn’s Disease (CD) | |
|---|---|---|
| Cohort 1 | ||
| Number of participants | 10 | 15 |
| Mean age (range) in years | 61 (50–76) | 36.5 (19–68) |
| % Female | 40 | 47 |
| Mean disease duration (range) in years | n/a | 10.3 (1–28) |
| Cohort 2 | ||
| Number of participants | 40 | 40 |
| Mean age (range) in years | 32.8 (19–62) | 37.8 (19–77) |
| % Female | 50 | 57.5 |
| Mean disease duration (range) in years | - | 11.6 (2–34) |
| Symptomatic (% of group) | - | 21 (52.5) |
Montreal Classification, Microscopic Evaluation and Mucosal Cytokine mRNA Expressions
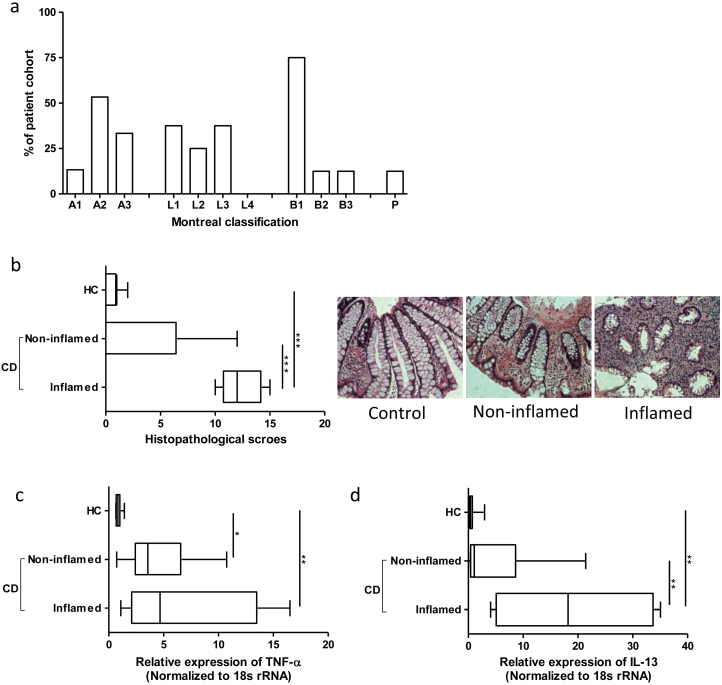
Montreal classification, which takes into account age at diagnosis (A), location (L) and disease behaviour with perianal (P) modifier, of the patients who donated biopsy specimens are presented in Figure 1a. Histological evaluation of biopsies confirmed the macroscopic assessment of the endoscopist during specimen collection (Figure 1b). For further confirmation, we measured the expression of S100A9, which dimerizes with S100A8 to form calprotectin (28). Though low expression of tissue S100A9 was observed, it was significantly higher in inflamed tissue compared with healthy and noninflamed tissue (data not shown). In CD patients, TNF-α expression was significantly higher compared with HC patients (Figure 1c). Moreover, inflammation-associated significant upregulation of IL-13 expression was observed in CD patients (Figure 1d).
Figure 1.
Montreal classification, colonic histopathology and cytokine mRNA expression in patients with CD. A) Montreal classification of CD patient cohort (n=15), where age at diagnosis is indicated as A1 (16 years or younger), A2 (17–40 years), A3 (over 40 years), location is denoted by L1 (terminal ileum), L2 (colon), L3 (ileocolon) or L4 (upper GI), and disease behaviour is characterized by B1 (nonstricturing, nonpenetrating), B2 (stricturing) or B3 (penetrating) with perianal disease (P). B) Histological assessment confirming endoscopists’ assessments during biopsy collection and representative micrographs. mRNA expression of cytokines (C) TNF-α and (D) IL-13 in inflamed and noninflamed regions of the colon. Box-whisker plot representing mean, maximum and minimum, where *P < 0.05 and **P < 0.01. HC, healthy controls; CD, Crohn’s disease; TNF, tumor necrosis factor; IL, interleukin.
TPH1, 5-HTT and 5-HTR mRNA Expressions in CD
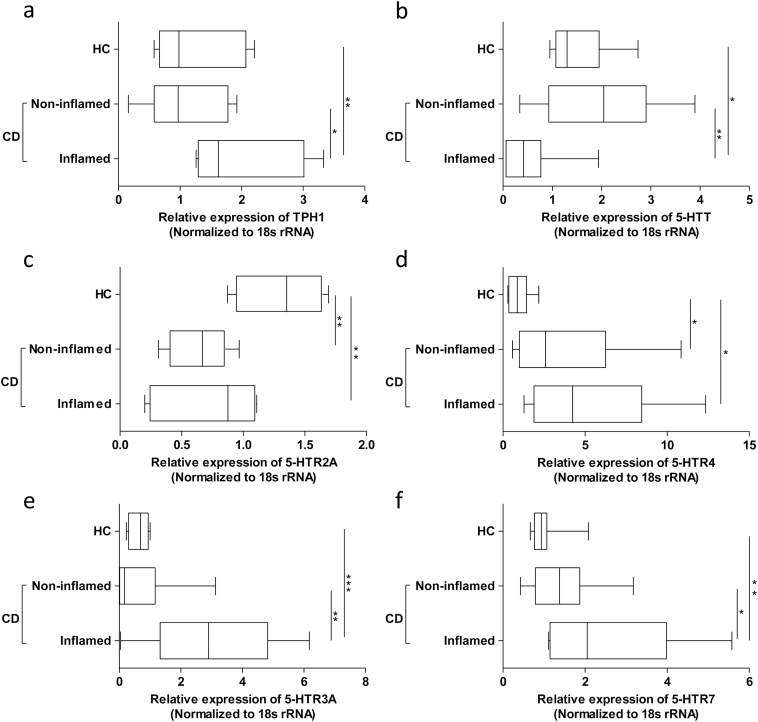
In CD patients, colonic TPH1 expression was significantly higher in inflamed regions compared with noninflamed regions and controls (Figure 2a). However, 5-HT-expressing EC cell numbers were not significantly different among the groups compared (data not shown). Significant reduction in the expression of 5-HTT was observed in inflamed specimens compared with noninflamed regions and controls (Figure 2b). Minimal expression of mucosal 5-HTR1A was detected in all specimens examined (data not shown). An overall low expression of mucosal 5-HTR2A was observed; however, it was significantly lower in CD patients compared with HC (Figure 2c). Conversely, mucosal 5-HTR4 expression was significantly higher in CD patients irrespective of inflammation in comparison with HC patients (Figure 2d). Additionally, inflammation-associated significant upregulation of 5-HTR3A and 7 expressions were observed in CD patients (Figure 2e and f).
Figure 2.
Alterations in key components of mucosal 5-hydroxytraptamine signaling machinery. Differences in mRNA expression of (A) TPH1, (B) 5-HTT and 5-HTR, (C) 2A, (D) 4, (E) 3A and (F) 7 in inflamed and noninflamed regions of the colon in patients with CD and HC. Box-whisker plot representing mean, maximum and minimum, where *P < 0.05, **P < 0.01 and ***P < 0.001. TPH, tryptophan hydroxylase; 5-HTT, 5-HT transporter; 5-HTR, 5-HT receptor: healthy controls (HC); Crohn’s disease (CD).
PPP and Plasma 5-HT Levels in CD Patients
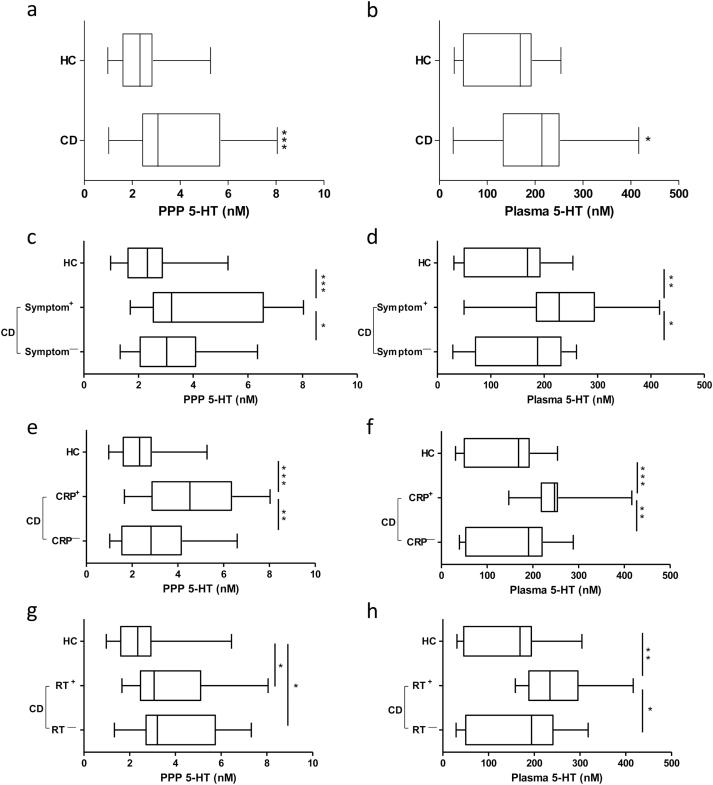
In comparison with HC patients, significantly higher PPP and plasma 5-HT levels were observed in CD patients (Figure 3a and b). Symptomatic CD patients also had higher PPP and plasma 5-HT concentrations in comparison with asymptomatic (Symptom—) patients and HC patients (Figure 3c and d). When CD patients were divided into CRP+ or CRP-negative (CRP—) groups, we found CRP+ patients had significantly higher PPP and plasma 5-HT levels compared with CRP— patients and HC patients (Figure 3e and f). Platelet numbers in CD patients were elevated compared with HC patients, but the difference did not reach statistical significance. However, CD patients who reported experiencing symptoms had significantly higher platelet numbers compared with HC patients (465 x 109/L ± 50 × 109/L versus 367 × 109/L ± 16 × 109/L; mean ± SEM; P < 0.05). In comparison with HC patients, PPP 5-HT levels were significantly higher in CD patients irrespective of platelet count; whereas, only patients with platelet count >450 × 109/L (RT+), but not <450 × 109/L (RTˉ), had significantly higher plasma 5-HT levels (Figure 3g and h).
Figure 3.
Platelet-poor plasma (PPP) and plasma 5-HT levels in inflammatory bowel disease. Comparison of 5-HT concentrations, (A) PPP and (B) plasma between HC and patients with CD (C) PPP and (D) plasma among HC, symptomatic (Symptom+) and asymptomatic (Symptomˉ) patients with CD (E) PPP and (F) plasma among HC, C-reactive protein positive (CRP+; CRP > 5.1 mg/L) and negative (CRPˉ; CRPP < 5.1 mg/L) patients with CD, (G) PPP and (H) plasma among HC and CD patients with platelet counts greater than 450 x 109/L (RT+) and CD patients with platelet count less than 450 x 109/L (RTˉ). Box-whisker plot representing mean, maximum and minimum. *P < 0.05, **P < 0.01, ***P < 0.001. HC, healthy controls; CD, Crohn’s disease; PPP, platelet-poor plasma; 5-HT, 5-hydroxytraptamine. RT, reactive thrombocytosis.
5-HTTLPR Polymorphism and CD
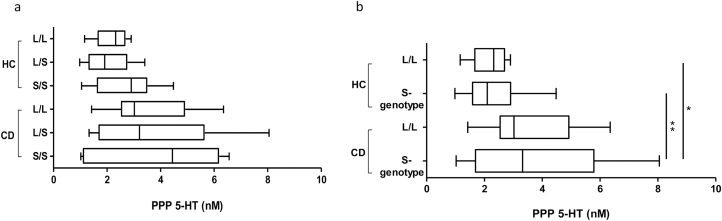
In our second cohort of participants, there were no significant differences in 5-HTTLPR genotype distribution (χ2=5, df=2, p ~0.08) or allele frequency (Fisher exact test, two-sided P=0.05) between CD patients and HC patients (Table 2). Additionally, PPP 5-HT levels were not significantly different among the genotypes of HC and CD patients (Figure 4a). The genotype distribution of our cohort did not deviate from the Hardy–Weinberg equilibrium, and no significant association with gender were observed. However, when grouped together based on the functional dominance of the S allele (11), the S-genotype (L/S and S/S combined) was significantly higher than L/L genotype (Fisher exact test, two-sided P=0.04) between CD and HC patients (Table 2). Furthermore, PPP 5-HT levels in CD patients with S-genotype was significantly higher compared with HC patients with L/L and S-genotypes (Figure 4b).
Table 2.
Distribution of 5-HT transporter gene-linked polymorphism in the healthy controls and in patients with Crohn’s disease
| Genotype distribution | Healthy controls (HC) n=40 (%) | Crohn’s disease (CD) n=40 (%) |
|---|---|---|
| Wild-type (L/L) | 16 (40%) | 7 (17.5%) |
| Heterozygous (L/S) | 16 (40%) | 21 (52.5%) |
|
Homozygous polymorphism (S/S) S-genotype (L/S + S/S) |
8 (20%) 24 (60%) |
12 (30%) 33 (82.3%)* |
| Allele (no. and %) | ||
| Allele (L) | 48 (60%) | 35 (43.8%) |
| Allele (S) | 32 (40%) | 45 (56.2%) |
Healthy controls vs. patients with Crohn’s disease
*Fisher exact test, two-sided P=0.04
Figure 4.
The 5-HT transporter gene-linked polymorphism and associated platelet-poor plasma (PPP) of 5-HT levels in HC and patients with inflammatory bowel disease. Comparison of PPP 5-HT concentrations (A) among different genotypes of HC and patients with CD (B) among L/L and S-genotype of HC and patients with CD. Box-whisker plot representing mean, maximum and minimum, where *P < 0.05 and **P < 0.01. Healthy controls (HC); Crohn’s disease (CD).
DISCUSSION
Inflammatory bowel disease remains incurable and is on the rise, with Canada having one of the highest rates of prevalence and incidence in the world (4). The present study evaluated the expression of key 5-HT signaling components in CD patients and found a significant upregulation of mucosal 5-HT signaling. Inflammation in CD was associated with a significantly higher expression of colonic TPH1, along with IL-13. Interleukin-13 is a pleiotropic cytokine that has emerged as an important mediator in UC and fistulizing CD (29). Additionally, previous work from our laboratory has identified the role IL-13 in increasing TPH1 expression in BON cells and 5-HT production in vitro and in animal models of intestinal inflammation (21, 30), thus implicating IL-13 in the augmented production of 5-HT via increased availability of the rate-limiting enzyme. Interleukin-13 has also been shown to increase intestinal epithelial cell turnover and EC cell numbers (21, 30). However, in our patient group, we did not observe a significant upregulation in the number of EC cells. This could potentially be due to inflammation-associated destruction of epithelial architecture observed in CD; additionally, it could be due to the varying locations of inflamed and noninflamed tissue collection within our cohort, as EC cell density varies along the length of the colon. Other important factors in IBD, such as the gut microbiota, may also influence host 5-HT production. The role of gut microbiota in 5-HT production, via the regulation of TPH1, has already been established (31). Though the findings of Yano et al. (31) are not in the context of inflammation and the precise role of the gut microbiome in IBD pathogenesis is not fully understood, it is possible that the increase in TPH1 expression may be associated with dysbiosis observed in IBD. Notably, a recent study has also shown that increased availability of 5-HT, due to 5-HTT deficiency, is associated with dysbiosis (32). We also found inflammation-associated significant downregulation of 5-HTT expression in CD patients. We are the first to report this reduction 5-HTT expression in patients with active CD, which was previously reported in severe UC patients and in the inflamed mucosa of UC patients compared with the healing mucosa (17, 18). This may be a contributing factor in the attenuated clearance of 5-HT from the GI tract, which may ultimately lead to the increased PPP and plasma 5-HT levels that we observed in our second cohort of CD patients. We also found that PPP and plasma 5-HT concentrations in symptomatic patients were increased compared with HC and asymptomatic patients. In this cohort, we were unable to directly associate these finding with disease activity in the intestine; it does, however, indicate that increased 5-HT production may play a role in patients’ experiences of IBD-associated symptoms. Reactive thrombocytosis is a nonspecific response to inflammation, which is known to occur in chronic inflammatory conditions (33). It is now well established that thrombocytosis is related to IBD activity and severity (34), and we found that plasma 5-HT levels were upregulated in patients with reactive thrombocytosis compared with patients without and HC patients. Elevated levels of gut-derived 5-HT in circulation may potentially underlie the increased number of platelets we observed in our patients. Platelets do not produce 5-HT because they lack TPH1 (9); they uptake gut-derived 5-HT entering circulation by 5-HTT and have a finite capacity for its storage. Therefore, if more 5-HT is available to enter the circulation, then more 5-HT will be taken into platelets and will be stored there. As part of a pilot investigation, we examined the association of 5-HTTLPR polymorphism with CD. We observed that the S-genotype, associated with decreased clearance of 5-HT (12), was significantly more common than L/L genotype between CD and HC.
A vast number of 5-HTRs mediate the diverse effects of 5-HT in the gut. Five families of 5-HTRs, with the exceptions of 5-HTR5 and 5-HTR6, are expressed throughout the human GI tract (7). However, to date, very few studies have investigated alterations in 5-HTR expressions in IBD. In our study, we found that CD is associated with upregulation of 5-HTR4, 5-HTR7 and 5-HTR3A (essential for the formation of a functional 5-HTR3 channel (7)), with the latter two upregulated only in the inflamed regions of the colon. Our observation of increased 5-HTR7 expression associated with inflammation in CD is in agreement with previous findings by Guseva et al. (21). The changes observed in 5-HTR expression may contribute to various symptoms experienced by IBD patients, including abnormal gut motility and sensation of pain. These 5-HTRs, in addition to being expressed on various GI cell types, are found on immune cells, and alterations observed in the expression of these receptors may potentiate the pro-inflammatory influence of 5-HT (7). The role of 5-HT as a pro-inflammatory mediator in animal models of intestinal inflammation is well established. It has been shown that antagonism of 5-HTR3 by tropisetron and granisetron can reduce the severity of acetic, acid-induced colitis in rats. Tropisetron also reduces the severity of trintrobezenesulfonic acid (TBNS)–induced colitis in rats (35–37). We have made similar observations by antagonizing 5-HTR7 in the dextran sulfate sodium (DSS) and dintrobezenesulfonic acid (DNBS)–induced colitis models (38). In addition to targeting receptors of 5-HT, blocking the synthesis of peripheral 5-HT using telotristat etiprate, a TPH inhibitor, has been shown to attenuate intestinal inflammation (15).
The findings of our study are based on two small cohorts of participants, which is one of the limitations that must be considered, in addition to the incomplete phenotypic profile of inflammation in the second cohort of participants, where we investigated prevalence of 5-HTTLPR-associated genotype among CD patients, PPP and plasma 5-HT levels. However, our studies revealed important findings and do highlight the need for future studies with larger populations and functional studies to demonstrate cause-effect relationships.
In conclusion, inflammation in CD is associated with enhancement of mucosal 5-HT signaling, which is marked by upregulation of TPH1 expression and downregulation of 5-HTT expression. Additionally, this coincides with elevated expression of IL-13, a cytokine associated with increased 5-HT production. Increased 5-HT availability due to enhanced production and impaired clearance may play an important role in perpetuating intestinal inflammation and in associated symptom manifestation, where the enhancement of various 5-HTR expression observed may be vital. Initial data indicate that the role of 5-HT in IBD-associated inflammation may also have a genetic component, where 5-HTTLPR-associated genotypes may potentially be involved in CD. Our findings revealed new and important information on the key elements of 5-HT signaling in CD and support the need for more studies with larger populations investigating 5-HT signaling in IBD patients with both CD and UC, which may ultimately lead to the development of new therapeutic strategies in the management of IBD.
Acknowledgements
Authors’ contributions
WIK, JKM and MSS conceived the idea for this project. MSS and UC recruited all study participants. MSS performed all experiments with help from SA and YC. MSS analyzed and interpreted the data and wrote and prepared the manuscript for publication. DA and SLSH critically appraised the manuscript. JKM and WIK edited and revised the manuscript. Thanks to Drs. Marcus M. Manocha, Walter Reinisch, Tariq Aziz and Terence Agbor for valuable discussion in study design. This work is supported by a grant awarded to Dr Waliul I. Khan by the Canadian Institutes of Health Research (CIHR).
References
- 1. Matricon J, Barnich N, Ardid D. Immunopathogenesis of inflammatory bowel disease. Self Nonself 2010;1:299–309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Loftus EV. Clinical epidemiology of inflammatory bowel disease: Incidence, prevalence, and environmental influences. Gastroenterology 2004;126:1504–17. [DOI] [PubMed] [Google Scholar]
- 3. Bernstein CN, Wajda A, Svenson LW, et al. The epidemiology of inflammatory bowel disease in Canada: A population-based study. Am J Gastroenterol 2006;101:1559–68. [DOI] [PubMed] [Google Scholar]
- 4. Qin X. What made Canada become a country with the highest incidence of inflammatory bowel disease: Could sucralose be the culprit?Can J Gastroenterol 2011;25:511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Fedorak RN, Wong K, Bridges R. Canadian digestive health foundation public impact series. inflammatory bowel disease in Canada: Incidence, prevalence, and direct and indirect economic impact. Can J Gastroenterol 2010;24:651–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Manocha M, Khan WI. Serotonin and GI Disorders: An Update on Clinical and Experimental Studies. Clin Transl Gastroenterol 2012;3:e13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Shajib MS, Khan WI. The role of serotonin and its receptors in activation of immune responses and inflammation. Acta Physiol 2015;213:561–74. [DOI] [PubMed] [Google Scholar]
- 8. Mawe GM, Hoffman JM. Serotonin signaling in the gut-functions, dysfunctions and therapeutic targets. Nat Rev Gastroenterol Hepatol 2013;10:473–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Gershon MD. 5-Hydroxytryptamine (serotonin) in the gastrointestinal tract. Curr Opin Endocrinol Diabetes Obes 2013;20:14–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Kenna GA, Roder-Hanna N, Leggio L, et al. Association of the 5-HTT gene-linked promoter region (5-HTTLPR) polymorphism with psychiatric disorders: Review of psychopathology and pharmacotherapy. Pharmgenomics Pers Med 2012;5:19–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Willeit M, Praschak-Rieder N, Neumeister A, et al. A polymorphism (5-HTTLPR) in the serotonin transporter promoter gene is associated with DSM-IV depression subtypes in seasonal affective disorder. Mol Psychiatry 2003;8:942–46. [DOI] [PubMed] [Google Scholar]
- 12. Lesch KP, Bengel D, Heils A, et al. Association of anxiety-related traits with a polymorphism in the serotonin transporter gene regulatory region. Science 1996;274:1527–31. [DOI] [PubMed] [Google Scholar]
- 13. Odgerel Z, Talati A, Hamilton SP, et al. Genotyping serotonin transporter polymorphisms 5-HTTLPR and rs25531 in European- and African-American subjects from the National Institute of Mental Health’s Collaborative Center for Genomic Studies. Transl Psychiatry 2013;3:e307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Ghia J, Li N, Wang H, et al. Serotonin Has a Key Role in Pathogenesis of Experimental Colitis. Gastroenterology 2009;137: 1649–60. [DOI] [PubMed] [Google Scholar]
- 15. Kim JJ, Wang H, Terc JD, et al. Blocking peripheral serotonin synthesis by telotristat etiprate (LX1032/LX1606) reduces severity of both chemical- and infection-induced intestinal inflammation. Am J Physiol Gastrointest Liver Physiol 2015;309:G455–65. [DOI] [PubMed] [Google Scholar]
- 16. El-Salhy M, Danielsson A, Stenling R, et al. Colonic endocrine cells in inflammatory bowel disease. J Intern Med 1997;242:413–19. [DOI] [PubMed] [Google Scholar]
- 17. Tada Y, Ishihara S, Kawashima K, et al. Downregulation of serotonin reuptake transporter gene expression in healing colonic mucosa in presence of remaining low-grade inflammation in ulcerative colitis. J Gastroenterol Hepatol 2016;31:1443–52. [DOI] [PubMed] [Google Scholar]
- 18. Coates MD, Mahoney CR, Linden DR, et al. Molecular defects in mucosal serotonin content and decreased serotonin reuptake transporter in ulcerative colitis and irritable bowel syndrome. Gastroenterology 2004;126:1657–64. [DOI] [PubMed] [Google Scholar]
- 19. Sikander A, Sinha SK, Prasad KK, et al. Association of serotonin transporter promoter polymorphism (5-HTTLPR) with microscopic colitis and ulcerative colitis. Dig Dis Sci 2015;60:887–94. [DOI] [PubMed] [Google Scholar]
- 20. Docherty M. Elevated serotonin associated with collagenous colitis. Am J Gastroenterol 2010;105:1449. [DOI] [PubMed] [Google Scholar]
- 21. Guseva D, Holst K, Kaune B, et al. Serotonin 5-HT7 receptor is critically involved in acute and chronic inflammation of the gastrointestinal tract. Inflamm Bowel Dis 2014;20:1516–29. [DOI] [PubMed] [Google Scholar]
- 22. Taquet N, Philippe C, Reimund JM, et al. G-Protein Coupled Receptors (GPCRs) expression profiling with microfluidic cards. In: Karoui S. (ed). Crohn’s Disease. Intech: Rijeka, Croatia, 2012, pp 59–86. [Google Scholar]
- 23. Magro F, Vieira-Coelho MA, Fraga S, et al. Impaired synthesis or cellular storage of norepinephrine, dopamine, and 5-hydroxytryptamine in human inflammatory bowel disease. Dig Dis Sci 2002;47:216–24. [DOI] [PubMed] [Google Scholar]
- 24. Magro F, Langner C, Driessen A, et al. European consensus on the histopathology of inflammatory bowel disease. J Crohns Colitis 2013;7:827–51 [DOI] [PubMed] [Google Scholar]
- 25. Manocha M, Shajib MS, Rahman MM, et al. IL-13-mediated immunological control of enterochromaffin cell hyperplasia and serotonin production in the gut. Mucosal Immunol 2013;6:146–55. [DOI] [PubMed] [Google Scholar]
- 26. Chamouard P, Richert Z, Meyer N, et al. Diagnostic value of C-reactive protein for predicting activity level of Crohn’s disease. Clin Gastroenterol Hepatol 2006;4(7):882–87. [DOI] [PubMed] [Google Scholar]
- 27. Jürgens M, Mahachie John JM, et al. Levels of C-reactive protein are associated with response to infliximab therapy in patients with Crohn’s disease. Clin Gastroenterol Hepatol 2011;9(5):421–27. [DOI] [PubMed] [Google Scholar]
- 28. Ikhtaire S, Shajib MS, Reinisch W, et al. Fecal calprotectin: Its scope and utility in the management of inflammatory bowel disease. J Gastroenterol 2016;51:434–46. [DOI] [PubMed] [Google Scholar]
- 29. Jovani M, Fiorino G, Danese S. Anti-IL-13 in inflammatory bowel disease: From the bench to the bedside. Curr Drug Targets 2013;14:1444–52. [DOI] [PubMed] [Google Scholar]
- 30. Shajib MS, Wang H, Kim JJ, et al. Interleukin 13 and serotonin: Linking the immune and endocrine systems in murine models of intestinal inflammation. PLoS One 2013;8:e72774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Yano JM, Yu K, Donaldson GP, et al. Indigenous bacteria from the gut microbiota regulate host serotonin biosynthesis. Cell 2015;161:264–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. El Aidy S, Ramsteijn AS, Dini-Andreote F, et al. Serotonin transporter genotype modulates the gut microbiota composition in young rats, an effect augmented by early life stress. Front Cell Neurosci 2017;11:222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Danese S, Papa A, Saibeni S, et al. Inflammation and coagulation in inflammatory bowel disease: The clot thickens. Am J Gastroenterol 2007;102:174–86. [DOI] [PubMed] [Google Scholar]
- 34. Talstad I, Rootwelt K, Gjone E. Thrombocytosis in ulcerative colitis and Crohn’s disease. Scand J Gastroenterol 1973;8:135–8. [PubMed] [Google Scholar]
- 35. Motavallian A, Minaiyan M, Rabbani M, et al. Anti-inflammatory effect of ondansetron through 5-HT3 receptors on TNBS-induced colitis in rat. EXCLI J 2012;11:30–44. [PMC free article] [PubMed] [Google Scholar]
- 36. Motavallian A, Minaiyan M, Rabbani M, et al. Involvement of 5HT3 receptors in anti-inflammatory effects of tropisetron on experimental TNBS-induced colitis in rat. Bioimpacts 2013;3:169–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Mousavizadeh K, Rahimian R, Fakhfouri G et al. Anti-inflammatory effects of 5-HT3 receptor antagonist, tropisetron on experimental colitis in rats. Eur J Clin Invest 2009;39: 375–83. [DOI] [PubMed] [Google Scholar]
- 38. Kim JJ, Bridle BW, Ghia JE, et al. Targeted inhibition of serotonin type 7 (5-HT7) receptor function modulates immune responses and reduces the severity of intestinal inflammation. J Immunol 2013;190:4795–804. [DOI] [PubMed] [Google Scholar]