Abstract
Hypothyroidism is a common condition of thyroid hormone deficiency, which is readily diagnosed and managed but potentially fatal in severe cases if untreated. The definition of hypothyroidism is based on statistical reference ranges of the relevant biochemical parameters and is increasingly a matter of debate. Clinical manifestations of hypothyroidism range from life threatening to no signs or symptoms. The most common symptoms in adults are fatigue, lethargy, cold intolerance, weight gain, constipation, change in voice, and dry skin, but clinical presentation can differ with age and sex, among other factors. The standard treatment is thyroid hormone replacement therapy with levothyroxine. However, a substantial proportion of patients who reach biochemical treatment targets have persistent complaints. In this Seminar, we discuss the epidemiology, causes, and symptoms of hypothyroidism; summarise evidence on diagnosis, long-term risk, treatment, and management; and highlight future directions for research.
Introduction
Hypothyroidism refers to the common pathological condition of thyroid hormone deficiency. If untreated, it can lead to serious adverse health effects and ultimately death. Because of the large variation in clinical presentation and general absence of symptom specificity, the definition of hypothyroidism is pre-dominantly biochemical. Overt or clinical primary hypothyroidism is defined as thyroid-stimulating hormone (TSH) concentrations above the reference range and free thyroxine concentrations below the reference range. Mild or subclinical hypothyroidism, which is commonly regarded as a sign of early thyroid failure, is defined by TSH concentrations above the reference range and free thyroxine concentrations within the normal range. Subclinical hypothyroidism has been reviewed in a previous Lancet Seminar1 and is therefore not the focus here.
Whether the existing reference ranges of TSH and free thyroxine should be used to define thyroid dysfunction is a matter of debate. This issue is of clinical importance because the reference ranges are generally used as a threshold for treatment. Thyroid hormone replacement with levothyroxine is the standard treatment for patients with hypothyroidism. However, a substantial proportion of patients treated with levothyroxine have persistent complaints despite reaching the biochemical therapy targets, which has prompted the question of whether levothyroxine treatment is sufficient for all patients or whether alternative therapies (eg, combination with liothyronine preparations) could be adopted. Hypothyroidism in children and pregnant women are considered separate topics and have been discussed elsewhere.2,3
Epidemiology
Prevalence and risk factors
The prevalence of overt hypothyroidism in the general population varies between 0–3% and 3–7% in the USA and between 0–2% and 5–3% in Europe,4–8 depending on the definition used. A meta-analysis7 of studies across nine European countries estimated the prevalence of undiagnosed hypothyroidism, including both overt and mild cases, at around 5%. Differences in iodine status affect the prevalence of hypothyroidism, which occurs more frequently both in populations with a relatively high iodine intake and in severely iodine-deficient populations.9,10 Hypothyroidism occurs more frequently in women, in older people (>65 years), and in white individuals, although data on ethnic differences are scarce.5,11,12 Hypothyroidism is more common in patients with autoimmune diseases, such as type 1 diabetes, autoimmune gastric atrophy, and coeliac disease, and can occur as part of multiple autoimmune endocrinopathies. Individuals with Downs’ syndrome or Turners’ syndrome have an increased risk of hypothyroidism. By contrast, tobacco smoking and moderate alcohol intake are associated with a reduced risk of hypothyroidism.13,14
Genetic epidemiology
The heritability of TSH and free thyroxine concentrations in serum is estimated to be 65% and 23–65%, respectively.15,16 Results from genome-wide association studies have so far explained only a small proportion of thyroid function variability,17 and only three studies18–20 have focused on hypothyroidism specifically. The loci most consistently implicated in hypothyroidism include autoimmunity-related genes and thyroid-specific regulatory genes (panel). Most of these loci are also associated with serum TSH concentrations within the reference range.17 Monogenetic disorders leading to congenital hypothyroidism are rare and include TSH resistance (due to an inactivating mutation in the TSH receptor), thyroid dysgenesis, and thyroid dyshormonogenesis.21
Causes
Hypothyroidism can be classified as primary (due to thyroid hormone deficiency), secondary (due to TSH deficiency), tertiary (due to thyrotropin-releasing hormone deficiency), and peripheral (extra-thyroidal; panel). Central hypothyroidism (including both secondary and tertiary) and peripheral hypothyroidism are rare and account for less than 1% of cases.22
Primary hypothyroidism
In iodine-sufficient areas, the most common cause of hypothyroidism is chronic autoimmune thyroiditis (also known as Hashimoto’s disease). High concentrations of anti-thyroid antibodies (predominantly thyroid peroxidase antibodies and anti-thyroglobulin antibodies) are present in most patients with autoimmune thyroiditis. Raised concentrations of thyroid peroxidase antibodies are also detected in about 11% of the general population.8 In patients with subclinical hypothyroidism, thyroid peroxidase antibody measurements help to predict progression to overt disease.23,24 The exact mechanisms underlying autoimmune thyroiditis are not known, but both genetic and environmental factors are involved. A higher genetic risk score—calculated using five genetic variants for thyroid peroxidase antibodies identified by genome-wide association studies—showed a graded association with higher TSH concentrations and clinical hypothyroidism.25,26 Smokers have lower thyroid peroxidase antibody concentrations than non-smokers, and incidence of autoimmune thyroiditis increases after smoking cessation.27,28 Other environmental factors implicated in autoimmune thyroiditis are vitamin D and selenium deficiency, and moderate alcohol intake.29
Iodine is an essential component of thyroid hormone. Iodine deficiency can result in goitre, thyroid nodules, and hypothyroidism. The most severe consequence of iodine deficiency is cretinism (ie, restricted mental and physical development in utero and during childhood). Iodine fortification programmes are one of the safest and cheapest public health interventions for the prevention of cognitive and physical impairment.30,31 Despite such efforts, suboptimal iodine status still affects large parts of Africa and Asia, as well as specific subpopulations in several high-income countries—most notably, pregnant women in some areas of Italy, USA, and the UK.31–33 In populations that shift from severe to mild iodine deficiency, the prevalence of hypothyroidism decreases; in populations shifting from mild deficiency to optimum or excessive intake of iodine, the prevalence of autoimmune hypothyroidism increases.34,35
Iodine-containing drugs (eg, amiodarone) can restrict thyroid hormone production through iodine overload, immediately blocking thyroid hormone synthesis (ie, Wolff-Chaikoff effect). About 14% of patients treated with amiodarone develop hypothyroidism.36 Lithium also causes hypothyroidism via effects on thyroid hormone synthesis and release.37 In a large population-based cohort study,38 6% of patients needed levothyroxine therapy within 18 months of starting lithium treatment. Tyrosine kinase inhibitors are used as targeted therapy for several cancers. An analysis of clinical reports from the US Food and Drug Administration Adverse Event Reporting System,39 found that patients who received sunitinib developed hypothyroidism more frequently than patients treated with sorafenib. Several other drugs—including interferon-alfa, thalidomide, some monoclonal antibodies, antiepileptic drugs, and drugs for second-line treatment of multidrug-resistant tuberculosis—can also cause primary hypothyroidism (panel).
Hypothyroidism is common after radioiodine treatment, after hemithyroidectomy, and after neck radiation or surgery for cancer therapy.40–44 In the long term, about 80% of patients with Grave’s disease who are treated with radioiodine will develop hypothyroidism, even when low doses are used. Hypothyroidism is reported to occur in 55% of patients treated for toxic nodular goitre40 and about 8% of patients treated for solitary toxic nodules.45 In a meta-analysis43 of 32 studies, 20% of patients developed hypothyroidism after hemithyroidectomy. Other causes of primary hypothyroidism include transient thyroiditis and infiltrative disease (panel).
Central hypothyroidism
Central hypothyroidism is rare and affects both sexes equally. It is more often associated with pituitary than hypothalamic disorders but frequently involves both.22 Biochemically, central hypothyroidism is defined by low or low-to-normal TSH concentrations and a disproportionately low concentration of free thyroxine. Occasionally, TSH concentration is mildly elevated, probably because of decreased bioactivity.46 Over half of central hypothyroidism cases are caused by pituitary adenomas.22 Other causes of central hypothyroidism include pituitary or hypothalamic dysfunction due to head trauma, pituitary apoplexy, Sheehan’s syndrome, surgery, radiotherapy, genetic, and infiltrative disease. Several drugs are known to affect the hypothalamic–pituitary–thyroid axis (panel).47,48
Peripheral hypothyroidism
Consumptive hypothyroidism is caused by aberrant expression of the deiodinase 3 enzyme (which inactivates thyroid hormone) in tumour tissues. Although very rare, such overexpression can induce severe hypothyroidism. Elevated concentration of deiodinase 3 was first described in a newborn baby with infantile hepatic haemangiomatosis, but can also occur in patients with vascular and fibrotic tumours and gastrointestinal stromal tumours.49 Patients with rare genetic syndromes that lead to a reduced sensitivity to thyroid hormone (panel) usually have normal TSH concentrations, but can also present with tissue-specific hypothyroidism.50
Clinical presentation and implications
Myxedema coma and severe hypothyroidism
The clinical manifestations of hypothyroidism range from life threatening—in the case of myxedema coma—to no signs or symptoms. Myxedema coma, which was first described in the late 1900s as an outcome of long-standing untreated and severe hypothyroidism, has become a rare condition. Nevertheless, because the disease course is striking, with mortality of 40% despite treatment, early recognition is vital.51 Myxedema coma leads to an altered mental status, hypothermia, progressive lethargy, and bradycardia and can eventually result in multiple organ dysfunction syndrome and death. Therefore, early initiation of thyroid hormone therapy and other supportive measures is crucial.52
Although very rare, severe primary hypothyroidism can lead to pituitary hyperplasia with concomitant pituitary pathology (eg, secondary adrenal insufficiency) and symptoms (eg, amenorrhoea).53
Signs and symptoms
The most common symptoms of hypothyroidism in adults are fatigue, lethargy, cold intolerance, weight gain, constipation, change in voice, and dry skin, but the clinical presentation can include a wide variety of symptoms that differ with age, sex, and time between onset and diagnosis (table 1).54,55 The symptoms for the diagnosis of hypothyroidism are non-specific, especially in elderly patients who present with fewer and less classic signs and symptoms than younger individuals.55 An increase in the severity of symptoms might predict hypothyroidism, since a change in seven or more symptoms in the past year increases the likelihood of hypothyroidism (likelihood ratio 8–7).56 However, in a case-control study,57 none of 34 hypothyroidism-related symptoms could be used to identify patients with hypothyroidism. Furthermore, 15% of patients with autoimmune hypothyroidism are asymptomatic or report only one hypothyroidism-associated symptom, whereas 70% of euthyroid controls have one or more thyroid- associated complaints.57
Table 1:
Clinical presentation and implications of hypothyroidism
| Presentation | Signs and implications | |
|---|---|---|
| General metabolism | Weight gain, cold intolerance, fatigue | Increase in body-mass index, low metabolic rate, myxedema*, hypothermia* |
| Cardiovascular | Fatigue on exertion, shortness of breath | Dyslipidaemia, bradycardia, hypertension, endothelial dysfunction or increased intima-media thickness*, diastolic dysfunction*, pericardial effusion*, hyperhomocysteinemia*, electrocardiogram changes* |
| Neurosensory | Hoarseness of voice, decreased taste, vision, or hearing | Neuropathy, cochlear dysfunction, decreased olfactory and gustatory sensitivity |
| Neurological and psychiatric | Impaired memory, paresthesia, mood impairment | Impaired cognitive function, delayed relaxation of tendon reflexes, depression*, dementia*, ataxia*, Carpal tunnel syndrome and other nerve entrapment syndromes*, myxedema coma* |
| Gastrointestinal | Constipation | Reduced oesophageal motility, non-alcoholic fatty liver disease*, ascites (very rare) |
| Endocrinological | Infertility and subfertility, menstrual disturbance, galactorrhoea | Goiter, glucose metabolism dysregulation, infertility, sexual dysfunction, increased prolactin, pituitary hyperplasia* |
| Musculoskeletal | Muscle weakness, muscle cramps, arthralgia | Creatine phosphokinase elevation, Hoffman’s syndrome*, osteoporotic fracture* (most probably caused by overtreatment) |
| Haemostasis and haematological | Bleeding, fatigue | Mild anaemia, acquired von Willebrand disease*, decreased protein C and S*, increased red cell distribution width*, increased mean platelet volume* |
| Skin and hair | Dry skin, hair loss | Coarse skin, loss of lateral eyebrows*, yellow palms of the hand*, alopecia areata* |
| Electrolytes and kidney function | Deterioration of kidney function | Decreased estimated glomerular filtration rate, hyponatraemia* |
Uncommon presentation.
Hypothyroidism has clinical implications related to nearly all major organs (table 1), but the cardiovascular system is the most robustly studied. Hypothyroidism results in increased vascular resistance, decreased cardiac output, decreased left ventricular function, and changes in several other markers of cardiovascular contractility. Myocardial injuries and pericardial effusions are more common in patients with hypothyroidism than in matched euthyroid controls.58 Furthermore, patients with hypothyroidism have a higher prevalence of cardiovascular risk factors and often have features of metabolic syndrome, including hypertension, increased waist circumference, and dyslipidaemia.59 Hypothyroidism also increases total cholesterol, low-density lipoprotein, and homocysteine concentrations.
Patients with acute hypothyroidism, in the context of thyroid cancer treatment, show a decline in mood and quality of life.60 Hypothyroidism is considered a cause of reversible dementia; however, how often this occurs and in what proportion of patients dementia is truly reversible is unclear.61 Other manifestations include neurosensory, musculoskeletal, and gastrointestinal signs and symptoms (table 1). Because of the pleiotropic effects of thyroid hormone, hypothyroidism can also affect the course of other disorders. For example, statin intolerance is more prevalent in individuals with hypothyroidism than in controls without hypothyroidism.62
Long-term outcomes
Most long-term consequences of hypothyroidism have been studied in the context of subclinical hypothyroidism, because overt hypothyroidism is generally treated. Few studies63–65 have investigated the association of hypothyroidism with all-cause mortality and results are mainly available for subclinical hypothyroidism. Results from a population-based follow-up study65 of 599 participants aged 85 years suggested that, in elderly populations, subclinical hypothyroidism might be associated with better survival than euthyroidism or suppressed TSH. However, this finding was not confirmed in a large individual participant-based meta-analysis63 that included more than 2500 participants (aged >80 years). This meta-analysis63 showed an increased risk of coronary heart disease events and mortality in those with higher TSH concentrations, particularly those with TSH concentrations above 10 mIU/L. However, the association between hypothyroidism and coronary artery disease has been recognised for a long time.66 Subclinical hypothyroidism with TSH concentrations above 10 mIU/L has also been associated with an increased risk of heart failure.63,67 Patients with hypothyroidism undergoing percutaneous coronary intervention have more major adverse cardiovascular and cerebral events than those with normal thyroid function and those with adequately treated hypothyroidism.68 By contrast, the association with stroke is less evident and might be apparent only in younger individuals (<65 years).69 Patients with hypothyroidism have fewer neurological deficits post-stroke than controls without elevated TSH concentrations;70 normally after a stroke localised hypothyroidism is observed as a result of deiodinase 3 induction in the ischaemic brain area.71,72 The risk of coronary heart disease in patients with subclinical hypothyroidism does not differ by thyroid peroxidase antibody concentrations, suggesting that autoimmunity per se is not a contributing factor to the association.73 Hypothyroidism can present with cognitive impairments and dementia but the association is controversial, because results from several population-based cohort studies74–77 showed a protective effect of elevated TSH concentrations on the risk of dementia.
Hypothyroidism has also been associated with nonalcoholic fatty liver disease, cancer mortality, arthritis, kidney dysfunction, and diabetes; however, in most cases causality is suggested but not proven.78–82
Diagnosis
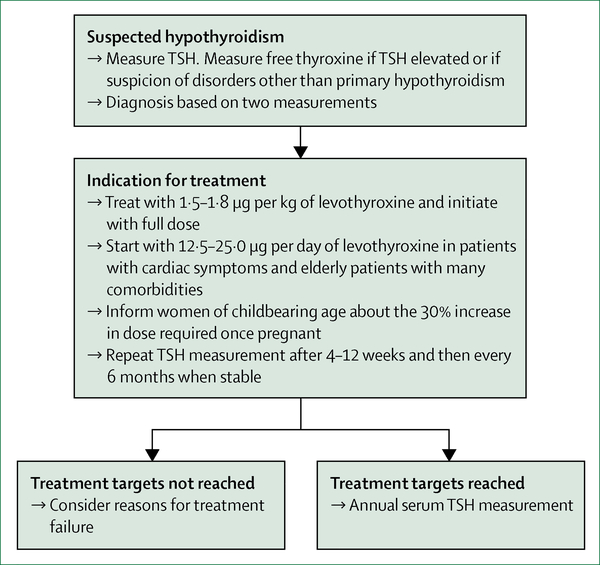
Primary hypothyroidism is defined by TSH concentrations above the reference range (most commonly used 0–4-4–0 mIU/L) and free thyroxine concentrations below the reference range, which is dependent on the type of assay used and the population studied (figure 1). The US Preventive Service Task Force83 has suggested reserving the term overt hypothyroidism for cases in which patients present with symptoms. However, such a definition is challenging in practice because of the large variability in presentation of even severe hypothyroidism. Additionally, patients might recognise previous symptoms only after the initiation of levothyroxine treatment.
Figure 1: Diagnosis and treatment of primary hypothyroidism.
TSH=thryoid-stimulating hormone.
TSH has circadian fluctuations, with higher concentrations towards the evening. Patients with severe hypothyroidism show irregularity of TSH secretion.84 Seasonal variations have also been described, with higher TSH concentrations in winter and spring than in autumn and summer.85 No indications exist for routine measurement of total tri-iodothyronine, total thyroxine, or free tri-iodothyronine. Measurement of thyroid peroxidase antibody is not strictly necessary to diagnose hypothyroidism but is useful to affirm the diagnosis of autoimmune primary hypothyroidism. Hypothyroidism is often characterised by a hypoechogenic pattern on thyroid sonography, even in the absence of raised thyroid peroxidase antibody concentrations. However, in the absence of additional clinical indications, such as abnormal thyroid palpation, an ultrasound is not required.
Reference ranges of thyroid function tests
Most commercially available TSH and free thyroxine assays are immunoassays, and their reference ranges are statistically defined as between the 2 · 5th and 97 · 5th percentile in an apparently healthy population. Therefore, the reference ranges do not consider symptoms or the risk of adverse events or disease, which is demonstrated by studies showing an increased risk of adverse events with variations in thyroid function even within these reference ranges.76,86–89 Furthermore, the reference ranges differ with age, sex, and ethnic origin.90 The applied reference ranges for thyroid function have been a matter of debate in recent years.91,92 The upper limit of TSH reference ranges typically increases with age in adults, and age-specific reference ranges gave conflicting results in younger individuals in studies from the UK and Australia.93,94 Nevertheless, in both studies,93,94 the use of age-specific reference ranges led to a reclassification from abnormal to normal thyroid function predominantly in older individuals. Little information exists about the consequences of treatment and thus no convincing arguments have been put forward to change the applied reference ranges.
Conditions that interfere with diagnosis
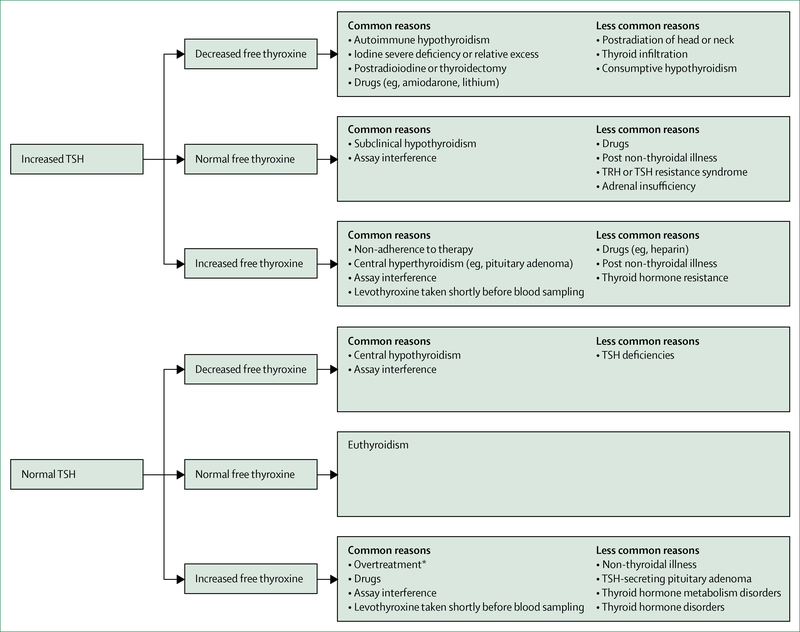
Several conditions can interfere with the laboratory measurements of thyroid analytes. Interference should be suspected when thyroid function tests do not match the clinical presentation (figure 2). Human anti-animal antibodies in patient’s serum can cause falsely high TSH concentrations and can interfere with free thyroxine equilibrium dialysis platform assays. Heparin, including low-molecular-weight heparin, can lead to falsely elevated concentrations of free thyroxine.95 High intake of biotin, a popular over-the-counter supplement, can interfere with biotin-based hormone assays, leading to false results of thyroid function tests.
Figure 2: Interpretation of thyroid function tests associated with hypothyroidism.
TSH=thryoid-stimulating hormone. TRH=thyrotropin-releasing hormone. *TSH can also be suppressed.
Free thyroxine measurement is important to the diagnosis of hypothyroidism (eg, central hypothyroidism) and during follow-up and treatment. The accuracy of free thyroxine immunoassays has been questioned in conditions that affect binding protein concentrations (ie, albumin or thyroxine-binding globulin), such as pregnancy or acute illness. However, free thyroxine assays generally perform well in daily clinical practice. Free thyroxine measured by liquid chromatography-tandem mass spectrometry seems to perform better than immunoassays in certain clinical conditions, such as acute illness and pregnancy,96 but is not available in most health-care facilities.
Independent of measurement artifacts, severe illness is characterised by low thyroid hormone status, but TSH concentration is generally within the normal range, although it can be transiently increased during recovery from a non-thyroidal illness.97 Changes in thyroid hormone metabolism, thyroid hormone transporters, and thyroid hormone receptors all have a role in the pathophysiology of non-thyroidal illness. Non-thyroidal illness occurs in different disease states, but is present in almost all critically ill patients.97 Thyroid function testing should therefore not be done in these patients, unless thyroid disease or central hypothyroidism is suspected. No evidence exists that thyroid hormone replacement therapy is beneficial in critically ill patients.
Screening
Despite the high prevalence of hypothyroidism, easy diagnostics, and cheap treatment, no consensus has been reached about TSH screening in specific subgroups of the general population. Several organisations—including the American Thyroid Association, American Association of Clinical Endocrinologists, and the Latin American Thyroid Society—recommend screening above a particular age (ranging from every 5 years for individuals aged >35 years to periodically for those aged ≥60 years), especially in women.98,99 The US Preventive Services Task Force83 found no evidence for or against screening, whereas the UK Royal College of Physicians100 suggests that screening of the general population is unjustified. However, evidence98 does support case finding in patients with dementia, infertility, autoimmune diseases, hypercholesterolaemia, dysmenorrhoea, or a family history of autoimmune hypothyroidism, in patients taking amiodarone or lithium, or in those at risk of iatrogenic hypothyroidism (eg, after neck radiation).
Treatment
Levothyroxine monotherapy in solid formulation, taken on an empty stomach, is the treatment of choice. The presence of clinical features of hypothyroidism, with biochemical confirmation of overt hypothyroidism, is the indication for treatment initiation. No rationale exists for avoiding the prescription of generic preparations, but switches between levothyroxine products in patients who are stable are not recommended.101 The optimal daily dose in overt hypothyroidism is 1·5—1·8 μg per kg of bodyweight.101–103 In patients with coronary artery disease, the starting dose is generally 12·5—25·0 μg per day and should be gradually increased on the basis of symptoms and TSH concentrations.101 This regimen is often preferred in the elderly, especially in patients with many co-morbidities.101,102 In younger patients without comorbidities, the full dose can usually be given from the start with adequate monitoring to avoid overtreatment. After the initiation of therapy, TSH measurement is repeated after 4–12 weeks and then every 6 months and, once stabilised, annually. Adjustments should be made according to laboratory findings, keeping in mind that in some patients (ie, those with low bodyweight or older patients) small changes in dose can have substantial effects on serum TSH concentrations. The clinical significance of low tri-iodothyronine concentrations in some patients despite reaching normal TSH concentrations is unknown. Routine measurement of tri-iodothyronine should not be used to assess treatment effectiveness.104
Women of childbearing age
Because of physiological changes during pregnancy, an increase in levothyroxine dose is required to maintain euthyroidism.105 Therefore, women of childbearing age with levothyroxine-treated hypothyroidism should be informed to increase their dose by 30% once pregnant and directly contact their physician for further guidance.106 Screening, the definition of subclinical hypothyroidism, and potential treatment during pregnancy are beyond the scope of this Seminar.107,108
Treatment targets
Treatment targets include normalisation of TSH concentrations and resolution of physical and mental complaints, while avoiding undertreatment or overtreatment.101 Nevertheless, an estimated 35—60% of patients treated with levothyroxine do not reach the target range of TSH (either overtreated or undertreated).109,110 Results from a retrospective cohort study in the UK109 showed that, after 5 years of levothyroxine therapy, almost 6% of patients have TSH concentrations below 0–1 mIU/L and more than 10% have TSH concentrations above 10–0 mIU/L. Overtreatment (ie, iatrogenic subclinical or overt hyperthyroidism) can have deleterious health effects, such as atrial fibrillation and osteoporosis, and should always be avoided, especially in the elderly and postmenopausal women. Undertreatment (ie, persistent thyroid hormone deficiency) can result in an increased risk of cardiovascular disease and persistent signs and symptoms. Treatment targets for central hypothyroidism are different from primary hypothyroidism because clinicians cannot rely on the so- called reflex TSH strategy. Further information on the treatment of central hypothyroidism can be found elsewhere.22
Failure to reach treatment targets
Factors that prevent patients from reaching therapy targets include prescription or intake of inadequate doses, interaction with supplements or medication, concurrent medical conditions, and non-adherence to therapy (table 2). Lower levothyroxine doses are needed to suppress TSH secretion in the elderly and higher doses are needed after thyroidectomy. Levothyroxine treatment and therapy targets in the context of thyroid malignancy are beyond the scope of this Seminar.
Table 2:
Reasons for failure to reach levothyroxine treatment goals and recommendations
| Recommendations | |
|---|---|
| Elevated TSH with or without (persistent) symptoms | |
| Inadequate dose | Consider higher doses, especially in patients with no remaining functional thyroid capacity (eg, after total thyroidectomy or radioablation therapy for Graves’ disease) |
| Simultaneous intake of levothyroxine with food can impair absorption | Intake 30–60 min before breakfast or at bedtime (2–3 h after evening meal); discuss patient preference |
| Some drugs can affect levothyroxine absorption—eg, calcium carbonate*, ferrous sulfate*, proton pump inhibitors, aluminium containing antacid, sucralfate, and orlistat | Separate intake of levothyroxine from interfering medications and supplements (eg,4 h) |
| Some drugs can affect levothyroxine availability and requirement— eg, oestrogens, androgens, sertraline, phenobartbital†, carbamazepine, phenytoin, and rifampicin | Monitor TSH at initiation and adjust levothyroxine dose if required |
| Malabsorption due to gastrointestinal disease and conditions | Helicobacter pylori gastritis, coeliac disease, autoimmune atrophic gastritis, and diabetic gastropathy should be considered and treated if possible |
| Non-adherence | Common cause, but should be suspected after other causes have been excluded; consider thyroxine absorption test |
| Normal TSH and (persistent) symptoms | |
| Concurrent (autoimmune) disease or causes | Autoimmune atrophic gastritis with pernicious anaemia, Addison’s disease, diabetes, and rheumatoid arthritis could be considered |
| Inadequate thyroid hormone concentrations at the tissue level | Acknowledgment of the patient’s symptoms; check if the patient feels better at a different TSH concentration in the normal range (ie, an individual set-point); a trial of levothyroxine–liothyronine combination therapy can be considered in adherent patients with long-lasting steady state of TSH in serum |
| Low TSH with or without (persistent) symptoms | |
| Overtreatment due to high doses | Consider lower doses in elderly individuals and patients with subclinical hypothyroidism; ask if the patient takes any over-the-counter preparations that might contain thyroid hormone |
| Medical conditions—certain drugs (eg, metformin) and weight loss can decrease TSH concentrations | Consider lower doses |
TSH= thyroid-stimulating hormone.
Evidence from prospective trials.
Antiepileptic drugs accelerate thyroxine and tri-iodothyronine conjugation but serum concentrations of TSH do not necessarily increase.
Levothyroxine is absorbed in the small intestine and intake is advised in the morning 30–60 min before breakfast. Intake before bedtime (2–3 h after last meal) might improve absorption and can be considered to increase compliance.111 Several drugs can interfere with the absorption, availability, or metabolism of levothyroxine, although evidence for some of these preparations comes from n-of-1 trials. Gastrointestinal conditions that reduce levothyroxine absorption include Helicobacter pylori gastritis, coeliac disease, and autoimmune atrophic gastritis. Results from some studies112,113 suggest that liquid and soft gel formulations of levothyroxine do not depend on gastric pH for absorption, and could provide a solution for patients with difficulties ingesting levothyroxine 30–60 min before breakfast. In a double-blind randomised crossover trial114of liquid thyroxine in 77 treatment-naive patients with hypothyroidism, no significant differences in thyroid function tests were seen when the liquid preparation was ingested at breakfast or 30 min before breakfast. However, no studies have compared liquid gel formulations of levothyroxine with solid formulations in relation to clinical outcomes.
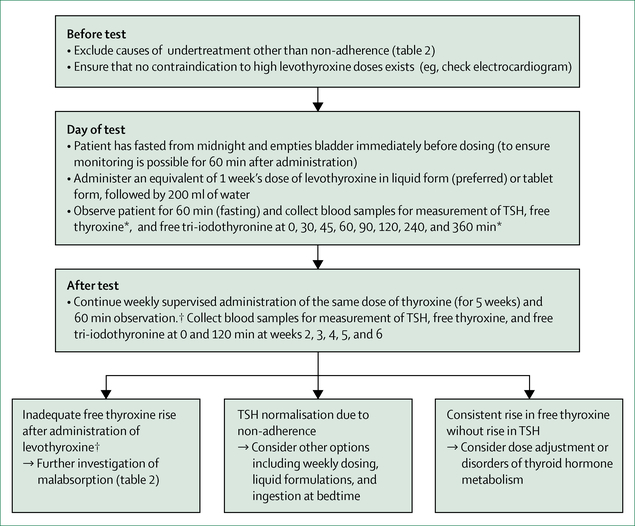
For individuals in whom high TSH concentrations persist and other causes have been excluded, the possibility of non-adherence, a common cause of therapy failure, should be considered and discussed with the patient. High TSH concentrations with normal or highnormal free thyroxine concentrations can be a result of levothyroxine tablets taken shortly before blood sampling. A supervised thyroxine absorption test to distinguish non-adherence from other reasons for undertreatment should be considered. Figure 3 shows a suggested protocol that combines both acute and long-term supervised administration.115,116
Figure 3: Thyroxine absorption test.
Protocol for supervised thyroxine absorption test, followed by weekly supervised thyroxine administration. Adapted from reference 115, by permission of Elsevier. TSH=thryoid-stimulating hormone. *Laboratory test values, especially an increase in free thyroxine, can be interpreted already at this stage. †Adequate free thyroxine rise is estimated to be roughly 50% from baseline at 120 min.116
Persistent complaints despite biochemical normalisation of TSH
About 5–10% of biochemically well controlled patients with levothyroxine-treated hypothyroidism have persistent symptoms, such as depression and impaired mental wellbeing.117 Concurrent diseases or perimenopausal status might cause these complaints and should be excluded. Patients with hypothyroidism have a higher prevalence of autoimmune diseases than the general population, which could give rise to similar symptoms. Autoimmunity per se has been suggested to lead to persistent symptoms.118
Circulating thyroid hormone concentrations are regulated by the hypothalamic–pituitary–thyroid axis with an individually determined set-point, reflected by a smaller intraindividual variability than interindividual variability.119 Therefore, the TSH concentration needed to achieve similar concentrations of circulating thyroid hormones differs between individuals. Differences in individual set-point could explain why patients with similar TSH concentrations under treatment respond differently. However, the individual set-point before hypothyroidism is rarely known. Also, studies120,121 that target different TSH goals generally do not show alterations in wellbeing or other clinical parameters.
An alternative explanation for persistent complaints in specific patients might be the imperfections oflevothyroxine monotherapy itself. Generally, levothyroxine can ensure adequate concentrations of circulating thyroxine that can then be converted into tri-iodothyronine by deiodination via deiodinase 1 and deiodinase 2. However, in euthyroid patients, about 20% of circulating tri-iodothyronine is derived from direct thyroidal secretion, whereas in patients on levothyroxine monotherapy all tri-iodothyronine is derived from the conversion of peripheral thyroxine to triiodothyronine. Consequently, patients on levothyroxine monotherapy have higher free thyroxine to triiodothyronine ratios than euthyroid individuals. Some patients with normalised TSH have serum tri-iodothyronine concentrations below the reference range, while free thyroxine serum concentrations are high.122–124 The clinical significance of this occurrence is unknown. However, levothyroxine monotherapy cannot restore physiological tri-iodothyronine concentrations or thyroid hormone-dependent biological effects in all tissues of hypothyroid rats.125,126 Although a normal TSH concentration reflects euthyroidism at the level of the pituitary, this might not necessarily reflect euthyroidism in all tissues. Tissue-specific differences in the inactivation of deiodinase 2 could play a part, resulting in the normalisation of triiodothyronine concentrations in the hypothalamus before tri-iodothyronine concentrations have fully normalised in the rest of the body.125
Levothyroxine–liothyronine combination therapy
Several trials using combined levothyroxine–liothyronine therapy have been done in the past 15 years.127 Although some studies128–131 show a beneficial effect, such as patient preference for combination therapy or an improved metabolic profile, patients on combination therapy generally do not have improved outcomes compared with those on levothyroxine monotherapy.132 Possible explanations include inadequate levothyroxine and liothyronine doses or frequency of administration.133 Liothyronine has a short half-life and no previous studies used a slow-release tri-iodothyronine. Additionally, most trials were of short duration (<4 months) and used questionnaires to record patients’ symptoms, which might not have been targeted or sensitive enough.
Alternatively, trials might have failed to identify the appropriate subgroups that would benefit from combination therapy. Most trials did not specifically recruit patients who feel unwell on levothyroxine or those with particularly low serum tri-iodothyronine concentrations. Individuals with genetic variations in thyroid hormone metabolism have not been specifically targeted.133 A subgroup that could be targeted is individuals with common genetic variations in DIO2, which encodes the deiodinase 2 enzyme that converts thyroxine to tri-iodothyronine locally in several tissues, including the brain.134 The Thr92Ala polymorphism in DIO2 gives rise to deiodinase 2 with a longer half-life than the wild-type enzyme and ectopic localisation in the Golgi apparatus. It was shown to alter expression profiles in the cerebral cortex in a similar pattern as seen in neurodegenerative disease, without evidence of altered thyroid hormone signalling.135 In a study136 of 552 people, the Thr92Ala polymorphism in DIO2 was associated with lower baseline psychological wellbeing in patients on levothyroxine replacement therapy and with better response to combination therapy, compared with patients without the polymorphism on levothyroxine replacement therapy. However, after appropriate multiple testing correction, the results were not significant. Results from a population-based cohort study137 showed no effect of the Thr92Ala polymorphism on quality of life or cognitive function measures. Sufficiently powered prospective randomised controlled trials are therefore needed before conclusions can be drawn.
Although the American Thyroid Association101 and European Thyroid Association138 guidelines generally recommend against the routine use of combination therapy in patients with hypothyroidism, the recommendations concerning trials in patients with persistent symptoms differ slightly. The European Thyroid Association states that a 3 month trial of levothyroxine–liothyronine combination might be considered experimentally in adherent, biochemically well controlled patients who have persistent complaints despite levothyroxine treatment138 and provides methods for calculating levothyroxine and liothyronine doses.138 However, treatment should be initiated only by accredited doctors of internal medicine or endocrinologists, closely monitored, and discontinued if no improvement is seen. By contrast, the American Thyroid Association recommends against any routine use of such trials outside of formal research and clinical trials, mainly because of uncertainty regarding benefit and long-term safety.101 Both the European Thyroid Association and American Thyroid Association agree on the need for long-term randomised controlled trials to assess risk-benefit ratios. Such trials would need to incorporate investigation of the ideal thyroid parameters to monitor during combination therapy, and whether tri-iodothyronine concentrations would be an important parameter. The timing of phlebotomy is also important, particularly if liothyronine is being administered more than once daily.
Little evidence exists to support other therapies for hypothyroidism. The use of thyroid extracts or liothyronine monotherapy is generally not recommended because of potential safety concerns associated with the presence of supraphysiological serum tri-iodothyronine concentrations and a paucity of long-term safety outcome data. The use of compounded thyroid hormones, dietary supplements, and any over-the-counter drug for the treatment of hypothyroidism is discouraged.
Directions for future research
Although great advances have been made in the identification of causes, knowledge of clinical implications, diagnosis, and treatment of hypothyroidism, several unanswered questions remain, especially regarding diagnosis and treatment.
Many risk factors have been identified for abnormal TSH concentrations, free thyroxine concentrations, and thyroid disease, but only a small proportion of the variability is explained.139 Therefore, identification of risk factors is important. Increasing evidence shows that endocrine-disrupting chemicals might be casual factors for endocrine diseases. Thyroid-disrupting chemical exposure can come from sources ranging from environmental (eg, flame retardants) to dietary (eg, food packaging material).140 A transatlantic call for action has been made to answer these questions in a collaborative effort.141
The association of hypothyroidism with cardiovascular disease has been established and replicated in several studies.63,67 However, the mechanisms behind this association remain unclear. The link between hypothyroidism and several cardiovascular diseases seems independent of traditional cardiovascular risk factors.63,67,69 Further research focused on novel cardiovascular risk factors or other pathways could shed light on the exact mechanisms, which would be crucial to support treatment decisions and monitor strategies in patients with asymptomatic hypothyroidism.
The diagnosis of hypothyroidism is currently based on statistically defined reference ranges for TSH and free thyroxine, which do not consider whether patients are at risk to develop disease. Because of the arbitrary nature of the cutoffs that define mild and overt hypothyroidism, an alternative grading system based on thyroid function tests has been proposed. The arbitrary nature of these cutoffs was also highlighted by the US Preventive Service Task Force83 as one of the important factors hampering decision making for screening of thyroid dysfunction in asymptomatic patients. These cutoffs also affect treatment decisions in asymptomatic patients with hypothyroidism. More research is needed to identify which adverse health events occur after long-term thyroid dysfunction. Furthermore, which concentrations of TSH and free thyroxine are accompanied by an increased risk of disease needs to be established. This information can be obtained from collaborative observational cohort studies with sufficiently long follow-up. Once this information is available, randomised controlled trials can assess whether treatment of thyroid function beyond these cutoffs for thyroid function test concentrations reduces excess risk and also the risk–benefit ratio of treatment.
Levothyroxine monotherapy is the standard treatment for hypothyroidism. However, several unresolved issues exist concerning patients who are biochemically well controlled but unsatisfied with their treatment outcome. Future studies should address whether alternative regimens could provide a solution for at least a proportion of patients with residual symptoms. Research areas most urgently in need of progress include: identification of the causes of persistent symptoms in biochemically well controlled patients, assessment of whether a more adequate dose (eg, tailored to patient serum triiodothyronine or to a patient’s own particular TSH set-point) produces more satisfactory outcomes, investigation of whether new formulations (eg, slow-release liothyronine) or increased administration frequency of levothyroxine–liothyronine combination therapy (eg, liothyronine three times daily) can ameliorate patient symptoms, and identification of patient subgroups that could benefit from therapies other than levothyroxine monotherapy (eg, by identifying additional genetic polymorphisms that could provide information on the individual thyroid set-point). Genome-wide association studies that include a large number of individuals with detailed genotyping could provide such information.
Supplementary Material
Search strategy and selection criteria.
We searched Embase, MEDLINE, and the Cochrane database between Jan 1, 2000 and Sept 22, 2016, for articles published in or translated into English. The full search and search terms are provided in the appendix. We mainly selected publications from the past 3 years, but did not exclude commonly referenced and highly regarded older publications. We also searched the reference lists of articles identified by this search and selected those we judged relevant. We supplemented the search with mainly older records from personal files. Review articles are cited to provide additional details and references.
Panel: Causes of hypothyroidism.
Primary hypothyroidism
Chronic autoimmune thyroiditis (also known as Hashimoto’s thyroiditis)
Iodine—severe iodine deficiency, mild and severe iodine excess
Drugs—eg, amiodarone, lithium, tyrosine kinase inhibitors, interferon-alfa, thalidomide, monoclonal antibodies (eg, ipilimumab and nivolumab), antiepileptic drugs (eg, valproate), drugs for second-line treatment of multidrug-resistant tuberculosis
Iatrogenic—radioiodine treatment (eg, for Graves’ disease or toxic nodular disease), hemithyroidectomy, radiotherapy or surgery in the neck or head region
Transient thyroiditis—viral (De Quervain’s syndrome), post-partum, silent thyroiditis, destructive thyroiditis
Thyroid gland infiltration*—infectious (eg, mycoplasma), malignant (eg, thyroid malignancy, lymphoma, metastasis of malignancy elsewhere), autoimmune (eg, sarcoidosis), inflammatory (eg, Riedels’s thyroiditis)
Genetic*—autoimmunity-related genes (eg, HLA class I region, PTPN22, SH2B3, and VAV3), general and thyroid-specific genes (eg, FOXE1, ATXN2, and PDE8B)
Central hypothyroidism
Pituitary tumours (secreting or non-secreting)
Pituitary dysfunction (eg, Sheehan’s syndrome)
Hypothalamic dysfunction (eg, post-traumatic)
Resistance to thyroid-stimulating hormone (TSH) or thyrotropin-releasing hormone
Drugs (eg, dopamine, somatostatins, glucocorticosteroids, and retinoid X receptor selective ligands)
Increased TSH concentration due to leptin stimulation†
Peripheral (extra-thyroidal) hypothyroidism
Consumptive hypothyroidism
Tissue-specific hypothyroidism due to decreased sensitivity to thyroid hormone (eg, mutations in MCT8 [also known as SLC16A2], SECISBP2, THRA, THRB)
*Rare causes of primary hypothyroidism. †Evidence mainly from animal models.
Acknowledgments
We thank Wichor Bramer (Erasmus Medical Center, Rotterdam, Netherlands) for the important contribution to the literature search.
Footnotes
Declaration of interests
RPP received lecture fees from GoodLife Fertility BV and Institut Biochemique SA. All other authors declare no competing interests.
Contributor Information
Layal Chaker, Academic Centre for Thyroid Disease, Erasmus University Medical Centre, Rotterdam, Netherlands.
Antonio C Bianco, Division of Endocrinology and Metabolism, Rush University Medical Center, Chicago, IL, USA.
Jacqueline Jonklaas, Division of Endocrinology, Georgetown University, Washington, DC, USA.
Robin P Peeters, Academic Centre for Thyroid Disease, Erasmus University Medical Centre, Rotterdam, Netherlands.
References
- 1.Cooper DS, Biondi B. Subclinical thyroid disease. Lancet 2012; 379: 1142–54. [DOI] [PubMed] [Google Scholar]
- 2.Salerno M, Capalbo D, Cerbone M, De Luca F. Subclinical hypothyroidism in childhood—current knowledge and open issues. Nat Rev Endocrinol 2016; 12: 734–46. [DOI] [PubMed] [Google Scholar]
- 3.Teng W, Shan Z, Patil-Sisodia K, Cooper DS. Hypothyroidism in pregnancy. Lancet Diabetes Endocrinol 2013; 1: 228–37 [DOI] [PubMed] [Google Scholar]
- 4.Åsvold BO, Vatten LJ, Bjøro T. Changes in the prevalence of hypothyroidism: the HUNT Study in Norway. Eur J Endocrinol 2013; 169:613–20. [DOI] [PubMed] [Google Scholar]
- 5.Aoki Y, Belin RM, Clickner R, Jeffries R, Phillips L, Mahaffey KR. Serum TSH and total T4 in the United States population and their association with participant characteristics: National Health and Nutrition Examination Survey (NHANES 1999–2002). Thyroid 2007; 17: 1211–23. [DOI] [PubMed] [Google Scholar]
- 6.Canaris GJ, Manowitz NR, Mayor G, Ridgway EC. The Colorado thyroid disease prevalence study. Arch Intern Med 2000; 160: 526–34. [DOI] [PubMed] [Google Scholar]
- 7.Garmendia Madariaga A, Santos Palacios S, Guillén-Grima F, Galofré JC. The incidence and prevalence of thyroid dysfunction in Europe: a meta-analysis. J Clin Endocrinol Metab 2014; 99: 923–31. [DOI] [PubMed] [Google Scholar]
- 8.Hollowell JG, Staehling NW, Flanders WD, et al. Serum TSH, T(4), and thyroid antibodies in the United States population (1988 to 1994): National Health and Nutrition Examination Survey (NHANES III). J Clin Endocrinol Metab 2002; 87: 489–99. [DOI] [PubMed] [Google Scholar]
- 9.Laurberg P, Cerqueira C, Ovesen L, et al. Iodine intake as a determinant of thyroid disorders in populations. Best Pract Res Clin Endocrinol Metab 2010; 24: 13–27 [DOI] [PubMed] [Google Scholar]
- 10.Teng W, Shan Z, Teng X, et al. Effect of iodine intake on thyroid diseases in China. N Engl J Med 2006; 354: 2783–93. [DOI] [PubMed] [Google Scholar]
- 11.Sichieri R, Baima J, Marante T, de Vasconcellos MTL, Moura AS, Vaisman M. Low prevalence of hypothyroidism among black and Mulatto people in a population-based study of Brazilian women. Clin Endocrinol 2007; 66: 803–07 [DOI] [PubMed] [Google Scholar]
- 12.McLeod DS, Caturegli P, Cooper DS, Matos PG, Hutfless S. Variation in rates of autoimmune thyroid disease by race/ethnicity in US military personnel. JAMA 2014; 311: 1563–65. [DOI] [PubMed] [Google Scholar]
- 13.Carlé A, Pedersen IB, Knudsen N, et al. Moderate alcohol consumption may protect against overt autoimmune hypothyroidism: a population-based case-control study. Eur J Endocrinol 2012; 167: 483–90. [DOI] [PubMed] [Google Scholar]
- 14.Asvold BO, Bjøro T, Nilsen TI, Vatten LJ. Tobacco smoking and thyroid function: a population-based study. Arch Intern Med 2007; 167: 1428–32. [DOI] [PubMed] [Google Scholar]
- 15.Hansen PS, Brix TH, Sorensen TI, Kyvik KO, Hegedus L. Major genetic influence on the regulation of the pituitary-thyroid axis: a study of healthy Danish twins. J Clin Endocrinol Metab 2004; 89: 1181–87 [DOI] [PubMed] [Google Scholar]
- 16.Panicker V, Wilson SG, Spector TD, et al. Heritability of serum TSH, free T4 and free T3 concentrations: a study of a large UK twin cohort. Clin Endocrinol 2008; 68: 652–59. [DOI] [PubMed] [Google Scholar]
- 17.Porcu E, Medici M, Pistis G, et al. A meta-analysis of thyroid-related traits reveals novel loci and gender-specific differences in the regulation of thyroid function. PLoS Genet 2013; 9: e1003266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Eriksson N, Tung JY, Kiefer AK, et al. Novel associations for hypothyroidism include known autoimmune risk loci. PLoS One 2012; 7: e34442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Denny JC, Crawford DC, Ritchie MD, et al. Variants near FOXE1 are associated with hypothyroidism and other thyroid conditions: using electronic medical records for genome- and phenome-wide studies. Am J Hum Genet 2011; 89: 529–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pickrell JK, Berisa T, Liu JZ, Segurel L, Tung JY, Hinds DA. Detection and interpretation of shared genetic influences on 42 human traits. Nat Genet 2016; 48: 709–17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Medici M, Visser WE, Visser TJ, Peeters RP. Genetic determination of the hypothalamic-pituitary-thyroid axis: where do we stand? Endocr Rev 2015; 36: 214–44. [DOI] [PubMed] [Google Scholar]
- 22.Persani L Clinical review: Central hypothyroidism: pathogenic, diagnostic, and therapeutic challenges. J Clin Endocrinol Metab 2012; 97: 3068–78. [DOI] [PubMed] [Google Scholar]
- 23.Effraimidis G, Strieder TGA, Tijssen JGP, Wiersinga WM. Natural history of the transition from euthyroidism to overt autoimmune hypo- or hyperthyroidism: a prospective study. Eur J Endocrinol 2011; 164: 107–13. [DOI] [PubMed] [Google Scholar]
- 24.Walsh JP, Bremner AP, Feddema P, Leedman PJ, Brown SJ, O’Leary P. Thyrotropin and thyroid antibodies as predictors of hypothyroidism: a 13-year, longitudinal study of a community-based cohort using current immunoassay techniques. J Clin Endocrinol Metab 2010; 95: 1095–104. [DOI] [PubMed] [Google Scholar]
- 25.Medici M, Porcu E, Pistis G, et al. Identification of novel genetic Loci associated with thyroid peroxidase antibodies and clinical thyroid disease. PLoS Genet 2014; 10: e1004123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Schultheiss UT, Teumer A, Medici M, et al. A genetic risk score for thyroid peroxidase antibodies associates with clinical thyroid disease in community-based populations. J Clin Endocrinol Metab 2015; 100: e799–807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Effraimidis G, Tijssen JG, Wiersinga WM. Discontinuation of smoking increases the risk for developing thyroid peroxidase antibodies and/or thyroglobulin antibodies: a prospective study. J Clin Endocrinol Metab 2009; 94: 1324–28. [DOI] [PubMed] [Google Scholar]
- 28.Belin RM, Astor BC, Powe NR, Ladenson PW. Smoke exposure is associated with a lower prevalence of serum thyroid autoantibodies and thyrotropin concentration elevation and a higher prevalence of mild thyrotropin concentration suppression in the third National Health and Nutrition Examination Survey (NHANES III). J Clin Endocrinol Metab 2004; 89: 6077–86. [DOI] [PubMed] [Google Scholar]
- 29.Wu Q, Rayman MP, Lv H, et al. Low population selenium status is associated with increased prevalence of thyroid disease. J Clin Endocrinol Metab 2015; 100: 4037–47 [DOI] [PubMed] [Google Scholar]
- 30.Bougma K, Aboud FE, Harding KB, Marquis GS. Iodine and mental development of children 5 years old and under: a systematic review and meta-analysis. Nutrients 2013; 5: 1384–416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.WHO. Assessment of iodine deficiency disorders and monitoring their elimination. A guide for programme managers, 3rd edn. Geneva: World Health Organization, 2007. http://apps.who.int/iris/bitstream/10665/43781/1/9789241595827_eng.pdf (accessed Jan 30, 2017). [Google Scholar]
- 32.Bath SC, Steer CD, Golding J, Emmett P, Rayman MP. Effect of inadequate iodine status in UK pregnant women on cognitive outcomes in their children: results from the Avon Longitudinal Study of Parents and Children (ALSPAC). Lancet 2013; 382: 331–37 [DOI] [PubMed] [Google Scholar]
- 33.Caldwell KL, Pan Y, Mortensen ME, Makhmudov A, Merrill L, Moye J. Iodine status in pregnant women in the National Children’s study and in U.S. women (15—44- years), National Health and Nutrition Examination Survey 2005–2010. Thyroid 2013; 23: 927–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Teng X, Shan Z, Chen Y, et al. More than adequate iodine intake may increase subclinical hypothyroidism and autoimmune thyroiditis: a cross-sectional study based on two Chinese communities with different iodine intake levels. Eur J Endocrinol 2011; 164: 943–50. [DOI] [PubMed] [Google Scholar]
- 35.Zimmermann MB, Boelaert K. Iodine deficiency and thyroid disorders. Lancet Diabetes Endocrinol 2015; 3: 286–95. [DOI] [PubMed] [Google Scholar]
- 36.Zhong B, Wang Y, Zhang G, Wang Z. Environmental iodine content, female sex and age are associated with new-onset amiodarone-induced hypothyroidism: a systematic review and meta-analysis of adverse reactions of amiodarone on the thyroid. Cardiology 2016; 134: 366–71. [DOI] [PubMed] [Google Scholar]
- 37.Shine B, McKnight RF, Leaver L, Geddes JR. Long-term effects of lithium on renal, thyroid, and parathyroid function: a retrospective analysis of laboratory data. Lancet 2015; 386: 461–68. [DOI] [PubMed] [Google Scholar]
- 38.Shulman KI, Sykora K, Gill SS, et al. New thyroxine treatment in older adults beginning lithium therapy: implications for clinical practice. Am J Geriatr Psychiatry 2005; 13: 299–304. [DOI] [PubMed] [Google Scholar]
- 39.Shu M, Zai X, Zhang B, Wang R, Lin Z. Hypothyroidism side effect in patients treated with sunitinib or sorafenib: clinical and structural analyses. PLoS One 2016; 11: e0147048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kahraman D, Keller C, Schneider C, et al. Development of hypothyroidism during long-term follow-up of patients with toxic nodular goitre after radioiodine therapy. Clin Endocrinol 2012; 76: 297–303. [DOI] [PubMed] [Google Scholar]
- 41.Krohn T, Hänscheid H, Müller B, et al. Maximum dose rate is a determinant of hypothyroidism after 131I therapy of Graves’ disease but the total thyroid absorbed dose is not. J Clin Endocrinol Metab 2014; 99: 4109–15. [DOI] [PubMed] [Google Scholar]
- 42.Lee V, Chan SY, Choi CW, et al. Dosimetric predictors of hypothyroidism after radical intensity-modulated radiation therapy for non-metastatic nasopharyngeal carcinoma. Clin Oncol (R Coll Radiol) 2016; 28: e52–60. [DOI] [PubMed] [Google Scholar]
- 43.Verloop H, Louwerens M, Schoones JW, Kievit J, Smit JWA, Dekkers OM. Risk of hypothyroidism following hemithyroidectomy: systematic review and meta-analysis of prognostic studies. J Clin Endocrinol Metab 2012; 97: 2243–55. [DOI] [PubMed] [Google Scholar]
- 44.Vogelius IR, Bentzen SM, Maraldo MV, Petersen PM, Specht L. Risk factors for radiation-induced hypothyroidism: a literature-based meta-analysis. Cancer 2011; 117: 5250–60. [DOI] [PubMed] [Google Scholar]
- 45.Nygaard B, Hegedüs L, Nielsen KG, Ulriksen P, Hansen JM. Long-term effect of radioactive iodine on thyroid function and size in patients with solitary autonomously functioning toxic thyroid nodules. Clin Endocrinol 1999; 50: 197–202. [DOI] [PubMed] [Google Scholar]
- 46.Persani L, Ferretti E, Borgato S, Faglia G, Beck-Peccoz P. Circulating thyrotropin bioactivity in sporadic central hypothyroidism. J Clin Endocrinol Metab 2000; 85: 3631–35. [DOI] [PubMed] [Google Scholar]
- 47.Graeppi-Dulac J, Vlaeminck-Guillem V, Perier-Muzet M, Dalle S, Orgiazzi J. Endocrine side-effects of anti-cancer drugs: the impact of retinoids on the thyroid axis. Eur J Endocrinol 2014; 170: R253–62. [DOI] [PubMed] [Google Scholar]
- 48.Grunenwald S, Caron P. Central hypothyroidism in adults: better understanding for better care. Pituitary 2015; 18: 169–75. [DOI] [PubMed] [Google Scholar]
- 49.Huang SA, Tu HM, Harney JW, et al. Severe hypothyroidism caused by type 3 iodothyronine deiodinase in infantile hemangiomas. N Engl J Med 2000; 343: 185–89. [DOI] [PubMed] [Google Scholar]
- 50.Dumitrescu AM, Refetoff S. The syndromes of reduced sensitivity to thyroid hormone. Biochim Biophys Acta 2013; 1830: 3987–4003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Beynon J, Akhtar S, Kearney T. Predictors of outcome in myxoedema coma. Crit Care 2008; 12: 111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wiersinga WM. Myxedema and coma (severe hypothyroidism) In: De Groot LJ, Chrousos G, Dungan K, et al. , eds. Endotext. South Dartmouth, MA: MD Text.com, 2000. [Google Scholar]
- 53.Khawaja NM, Taher BM, Barham ME, et al. Pituitary enlargement in patients with primary hypothyroidism. Endocr Pract 2006; 12: 29–34. [DOI] [PubMed] [Google Scholar]
- 54.Carlé A, Pedersen IB, Knudsen N, Perrild H, Ovesen L, Laurberg P. Gender differences in symptoms of hypothyroidism: a population-based DanThyr study. Clin Endocrinol 2015; 83: 717–25. [DOI] [PubMed] [Google Scholar]
- 55.Carlé A, Pedersen IB, Knudsen N, et al. Hypothyroid symptoms fail to predict thyroid insufficiency in old people: a population-based case-control study. Am J Med 2016; 129: 1082–92. [DOI] [PubMed] [Google Scholar]
- 56.Canaris GJ, Steiner JF, Ridgway EC. Do traditional symptoms of hypothyroidism correlate with biochemical disease? J Gen Intern Med 1997; 12: 544–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Carlé A, Pedersen IB, Knudsen N, Perrild H, Ovesen L, Laurberg P. Hypothyroid symptoms and the likelihood of overt thyroid failure: a population-based case-control study. Eur J Endocrinol 2014; 171: 593–602. [DOI] [PubMed] [Google Scholar]
- 58.Gao X, Liu M, Qu A, et al. Native magnetic resonance Tl-mapping identifies diffuse myocardial injury in hypothyroidism. PLoS One 2016; 11: e0151266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Tiller D, Ittermann T, Greiser KH, et al. Association of serum thyrotropin with anthropometric markers of obesity in the general population. Thyroid 20l6; 26: 1205–14. [DOI] [PubMed] [Google Scholar]
- 60.Shin YW, Choi YM, Kim HS, et al. Diminished quality of life and increased brain functional connectivity in patients with hypothyroidism after total thyroidectomy. Thyroid 2016; 26: 641–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Muangpaisan W, Petcharat C, Srinonprasert V. Prevalence of potentially reversible conditions in dementia and mild cognitive impairment in a geriatric clinic. Geriatr Gerontol Int 2012; 12: 59–64. [DOI] [PubMed] [Google Scholar]
- 62.Robison CD, Bair TL, Horne BD, et al. Hypothyroidism as a risk factor for statin intolerance. J Clin Lipidol 2014; 8: 401–07 [DOI] [PubMed] [Google Scholar]
- 63.Rodondi N, den Elzen WPJ, Bauer DC, et al. , for the Thyroid Studies Collaboration Study Group. Subclinical hypothyroidism and the risk of coronary heart disease and mortality. JAMA 2010; 304: 1365–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Grossman A, Weiss A, Koren-Morag N, Shimon I, Beloosesky Y, Meyerovitch J. Subclinical thyroid disease and mortality in the elderly: a retrospective cohort study. Am J Med 2016; 129: 423–30. [DOI] [PubMed] [Google Scholar]
- 65.Gussekloo J, van Exel E, de Craen AJ, Meinders AE, Frolich M, Westendorp RG. Thyroid status, disability and cognitive function, and survival in old age. JAMA 2004; 292: 2591–99. [DOI] [PubMed] [Google Scholar]
- 66.Vanhaelst L, Neve P, Chailly P, Bastenie PA. Coronary-artery disease in hypothyroidism. Observations in clinical myxoedema. Lancet 1967; 2: 800–02. [DOI] [PubMed] [Google Scholar]
- 67.Gencer B, Collet TH, Virgini V, et al. , for the Thyroid Studies Collaboration Study Group. Subclinical thyroid dysfunction and the risk of heart failure events: an individual participant data analysis from 6 prospective cohorts. Circulation 2012; 126: 1040–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Zhang M, Sara JD, Matsuzawa Y, et al. Clinical outcomes of patients with hypothyroidism undergoing percutaneous coronary intervention. Eur Heart J 2016; 37: 2055–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Chaker L, Baumgartner C, den Elzen WPJ, et al. , for the Thyroid Studies Collaboration Study Group. Subclinical hypothyroidism and the risk of stroke events and fatal stroke: An individual participant data analysis. J Clin Endocrinol Metab 2015; 100: 2181–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Alevizaki M, Synetou M, Xynos K, Alevizaki CC, Vemmos KN. Hypothyroidism as a protective factor in acute stroke patients. Clin Endocrinol 2006; 65: 369–72. [DOI] [PubMed] [Google Scholar]
- 71.Freitas BC, Gereben B, Castillo M, et al. Paracrine signaling by glial cell-derived triiodothyronine activates neuronal gene expression in the rodent brain and human cells. J Clin Invest 2010; 120: 2206–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Jo S, Kalló I, Bardoczi Z, et al. Neuronal hypoxia induces Hsp40-mediated nuclear import of type 3 deiodinase as an adaptive mechanism to reduce cellular metabolism. J Neurosci 2012; 32: 8491–500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Collet TH, Bauer DC, Cappola AR, et al. , for the Thyroid Studies Collaboration Study Group. Thyroid antibody status, subclinical hypothyroidism, and the risk of coronary heart disease: an individual participant data analysis. J Clin Endocrinol Metab 2014; 99: 3353–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Pasqualetti G, Pagano G, Rengo G, Ferrara N, Monzani F. Subclinical hypothyroidism and cognitive impairment: systematic review and meta-analysis. J Clin Endocrinol Metab 2015; 100: 4240–48. [DOI] [PubMed] [Google Scholar]
- 75.Tan ZS, Beiser A, Vasan RS, et al. Thyroid function and the risk of Alzheimer disease: the Framingham Study. Arch Intern Med 2008; 168: 1514–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Chaker L, Wolters FJ, Bos D, et al. Thyroid function and the risk of dementia: the Rotterdam study. Neurology 2016; 87: 1688–95. [DOI] [PubMed] [Google Scholar]
- 77.Rieben C, Segna D, da Costa BR, et al. Subclinical thyroid dysfunction and the risk of cognitive decline: a meta-analysis of prospective cohort studies. J Clin Endocrinol Metab 2016; 101: 4945–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Tseng FY, Lin WY, Li CI, Li TC, Lin CC, Huang KC. Subclinical hypothyroidism is associated with increased risk for cancer mortality in adult Taiwanese—a 10 years population-based cohort. PLoS One 2015; 10: e0122955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Bano A, Chaker L, Plompen EP, et al. Thyroid function and the risk of nonalcoholic fatty liver disease: the Rotterdam study. J Clin Endocrinol Metab 2016; 101: 3204–11. [DOI] [PubMed] [Google Scholar]
- 80.Zhang Y, Chang Y, Ryu S, et al. Thyroid hormone levels and incident chronic kidney disease in euthyroid individuals: the Kangbuk Samsung health study. Int J Epidemiol 2014; 43: 1624–32. [DOI] [PubMed] [Google Scholar]
- 81.Gronich N, Deftereos SN, Lavi I, Persidis AS, Abernethy DR, Rennert G. Hypothyroidism is a risk factor for new-onset diabetes: a cohort study. Diabetes Care 2015; 38: 1657–64. [DOI] [PubMed] [Google Scholar]
- 82.Abrahamsen B, Jorgensen HL, Laulund AS, et al. The excess risk of major osteoporotic fractures in hypothyroidism is driven by cumulative hyperthyroid as opposed to hypothyroid time: an observational register-based time-resolved cohort analysis. J Bone Miner Res 2015; 30: 898–905. [DOI] [PubMed] [Google Scholar]
- 83.LeFevre ML, the U.S. Preventive Services Task Force. Screening for thyroid dysfunction: U.S. Preventive Services Task Force recommendation statement. Ann Intern Med 2015; 162: 641–50. [DOI] [PubMed] [Google Scholar]
- 84.Roelfsema F, Pereira AM, Adriaanse R, et al. Thyrotropin secretion in mild and severe primary hypothyroidism is distinguished by amplified burst mass and Basal secretion with increased spikiness and approximate entropy. J Clin Endocrinol Metab 2010; 95: 928–34. [DOI] [PubMed] [Google Scholar]
- 85.Kim TH, Kim KW, Ahn HY, et al. Effect of seasonal changes on the transition between subclinical hypothyroid and euthyroid status. J Clin Endocrinol Metab 2013; 98: 3420–29. [DOI] [PubMed] [Google Scholar]
- 86.Chaker L, Baumgartner C, den Elzen WP, et al. , for the Thyroid Studies Collaboration Study Group. Thyroid function within the reference range and the risk of stroke: an individual participant data analysis. J Clin Endocrinol Metab 2016; 101: 4270–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Chaker L, van den Berg ME, Niemeijer MN, et al. Thyroid function and sudden cardiac death: a prospective population-based cohort study. Circulation 2016; 134: 713–22. [DOI] [PubMed] [Google Scholar]
- 88.Cappola AR, Arnold AM, Wulczyn K, Carlson M, Robbins J, Psaty BM. Thyroid function in the euthyroid range and adverse outcomes in older adults. J Clin Endocrinol Metab 2015; 100: 1088–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Inoue K, Tsujimoto T, Saito J, Sugiyama T. Association between serum thyrotropin levels and mortality among euthyroid adults in the United States. Thyroid 2016; 26: 1457–65. [DOI] [PubMed] [Google Scholar]
- 90.Surks MI, Boucai L. Age- and race-based serum thyrotropin reference limits. J Clin Endocrinol Metab 2010; 95: 496–502. [DOI] [PubMed] [Google Scholar]
- 91.Surks MI. TSH reference limits: new concepts and implications for diagnosis of subclinical hypothyroidism. Endocr Pract 2013; 19: 1066–69. [DOI] [PubMed] [Google Scholar]
- 92.Wartofsky L, Dickey RA. The evidence for a narrower thyrotropin reference range is compelling. J Clin Endocrinol Metab 2005; 90: 5483–88. [DOI] [PubMed] [Google Scholar]
- 93.Kahapola-Arachchige KM, Hadlow N, Wardrop R, Lim EM, Walsh JP. Age-specific TSH reference ranges have minimal impact on the diagnosis of thyroid dysfunction. Clin Endocrinol 2012; 77: 773–79. [DOI] [PubMed] [Google Scholar]
- 94.Vadiveloo T, Donnan PT, Murphy MJ, Leese GP. Age- and gender-specific TSH reference intervals in people with no obvious thyroid disease in Tayside, Scotland: the Thyroid Epidemiology, Audit, and Research Study (TEARS). J Clin Endocrinol Metab 2013; 98: 1147–53. [DOI] [PubMed] [Google Scholar]
- 95.Jaume JC, Mendel CM, Frost PH, Greenspan FS, Laughton CW. Extremely low doses of heparin release lipase activity into the plasma and can thereby cause artifactual elevations in the serum-free thyroxine concentration as measured by equilibrium dialysis. Thyroid 1996; 6: 79–83. [DOI] [PubMed] [Google Scholar]
- 96.van Deventer HE, Mendu DR, Remaley AT, Soldin SJ. Inverse log-linear relationship between thyroid-stimulating hormone and free thyroxine measured by direct analog immunoassay and tandem mass spectrometry. Clin Chem 2011; 57: 122–27. [DOI] [PubMed] [Google Scholar]
- 97.Fliers E, Bianco AC, Langouche L, Boelen A. Thyroid function in critically ill patients. Lancet Diabetes Endocrinol 2015; 3: 816–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Garber JR, Cobin RH, Gharib H, et al. , for the American Association of Clinical Endocrinologists and American Thyroid Association Taskforce on Hypothyroidism in Adults Study Groups. Clinical practice guidelines for hypothyroidism in adults: cosponsored by the American Association of Clinical Endocrinologists and the American Thyroid Association. Endocr Pract 2012; 18: 988–1028. [DOI] [PubMed] [Google Scholar]
- 99.Brenta G, Vaisman M, Sgarbi JA, et al. , for the Task Force on Hypothyroidism of the Latin American Thyroid Society (LATS) Study Group. Clinical practice guidelines for the management of hypothyroidism. Arq Bras Endocrinol Metabol 2013; 57: 265–91. [DOI] [PubMed] [Google Scholar]
- 100.Vanderpump MP, Ahlquist JA, Franklyn JA, Clayton RN. Consensus statement for good practice and audit measures in the management of hypothyroidism and hyperthyroidism. The Research Unit of the Royal College of Physicians of London, the Endocrinology and Diabetes Committee of the Royal College of Physicians of London, and the Society for Endocrinology. BMJ 1996; 313: 539–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Jonklaas J, Bianco AC, Bauer AJ, et al. , for the American Thyroid Association Task Force on Thyroid Hormone Replacement Study Group. Guidelines for the treatment of hypothyroidism: prepared by the American Thyroid Association Task Force on thyroid hormone replacement. Thyroid 2014; 24: 1670–751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Pearce SHS, Brabant G, Duntas LH, et al. 2013 ETA Guideline: management of subclinical hypothyroidism. Eur Thyroid J 2013; 2: 215–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Roos A, Linn-Rasker SP, van Domburg RT, Tijssen JP, Berghout A. The starting dose of levothyroxine in primary hypothyroidism treatment: a prospective, randomized, double-blind trial. Arch Intern Med 2005; 165: 1714–20. [DOI] [PubMed] [Google Scholar]
- 104.Abdalla SM, Bianco AC. Defending plasma T3 is a biological priority. Clin Endocrinol 2014; 81: 633–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Alexander EK, Marqusee E, Lawrence J, Jarolim P, Fischer GA, Larsen PR. Timing and magnitude of increases in levothyroxine requirements during pregnancy in women with hypothyroidism. N Engl J Med 2004; 351: 241–9. [DOI] [PubMed] [Google Scholar]
- 106.Stagnaro-Green A, Abalovich M, Alexander E, et al. , for the American Thyroid Association Taskforce on Thyroid Disease During Pregnancy and Postpartum Study Group. Guidelines of the American Thyroid Association for the diagnosis and management of thyroid disease during pregnancy and postpartum. Thyroid 2011; 21: 1081–125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Chan S, Boelaert K. Optimal management of hypothyroidism, hypothyroxinaemia and euthyroid TPO antibody positivity preconception and in pregnancy. Clin Endocrinol 2015; 82: 313–26. [DOI] [PubMed] [Google Scholar]
- 108.Medici M, Korevaar TI, Visser WE, Visser TJ, Peeters RP. Thyroid function in pregnancy: what is normal? Clin Chem 2015; 61: 704–13. [DOI] [PubMed] [Google Scholar]
- 109.Taylor PN, Iqbal A, Minassian C, et al. Falling threshold for treatment of borderline elevated thyrotropin levels-balancing benefits and risks: evidence from a large community-based study. JAMA Intern Med 2014; 174: 32–39. [DOI] [PubMed] [Google Scholar]
- 110.Somwaru LL, Arnold AM, Joshi N, Fried LP, Cappola AR. High frequency of and factors associated with thyroid hormone over-replacement and under-replacement in men and women aged 65 and over. J Clin Endocrinol Metab 2009; 94: 1342–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Bolk N, Visser TJ, Nijman J, Jongste IJ, Tijssen JGP, Berghout A. Effects of evening vs morning levothyroxine intake: a randomized double-blind crossover trial. Arch Intern Med 2010; 170:1996–2003. [DOI] [PubMed] [Google Scholar]
- 112.Vita R, Saraceno G, Trimarchi F, Benvenga S. Switching levothyroxine from the tablet to the oral solution formulation corrects the impaired absorption of levothyroxine induced by proton-pump inhibitors. J Clin Endocrinol Metab 2014; 99: 4481–86. [DOI] [PubMed] [Google Scholar]
- 113.Seng Yue C, Benvenga S, Scarsi C, Loprete L, Ducharme MP. When bioequivalence in healthy volunteers may not translate to bioequivalence in patients: differential effects of increased gastric pH on the pharmacokinetics of levothyroxine capsules and tablets. J Pharm Pharm Sci 2015; 18 : 844–55. [DOI] [PubMed] [Google Scholar]
- 114.Cappelli C, Pirola I, Daffini L, et al. A double-blind placebo-controlled trial of liquid thyroxine ingested at breakfast: results of the TICO Study. Thyroid 2016; 26: 197–202. [DOI] [PubMed] [Google Scholar]
- 115.Koulouri O, Moran C, Halsall D, Chatterjee K, Gurnell M. Pitfalls in the measurement and interpretation of thyroid function tests. Best Pract Res Clin Endocrinol Metab 2013; 27: 745–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Walker JN, Shillo P, Ibbotson V, et al. A thyroxine absorption test followed by weekly thyroxine administration: a method to assess non-adherence to treatment. Eur J Endocrinol 2013; 168: 913–17 [DOI] [PubMed] [Google Scholar]
- 117.Saravanan P, Chau WF, Roberts N, Vedhara K, Greenwood R, Dayan CM. Psychological well-being in patients on ‘adequate’ doses of l-thyroxine: results of a large, controlled community-based questionnaire study. Clin Endocrinol 2002; 57: 577–85. [DOI] [PubMed] [Google Scholar]
- 118.Ott J, Promberger R, Kober F, et al. Hashimoto’s thyroiditis affects symptom load and quality of life unrelated to hypothyroidism: a prospective case-control study in women undergoing thyroidectomy for benign goiter. Thyroid 2011; 21: 161–67. [DOI] [PubMed] [Google Scholar]
- 119.Andersen S, Pedersen KM, Bruun NH, Laurberg P. Narrow individual variations in serum T(4) and T(3) in normal subjects: a clue to the understanding of subclinical thyroid disease. J Clin Endocrinol Metab 2002; 87: 1068–72. [DOI] [PubMed] [Google Scholar]
- 120.Walsh JP, Ward LC, Burke V, et al. Small changes in thyroxine dosage do not produce measurable changes in hypothyroid symptoms, well-being, or quality of life: results of a double-blind, randomized clinical trial. J Clin Endocrinol Metab 2006; 91: 2624–30. [DOI] [PubMed] [Google Scholar]
- 121.Boeving A, Paz-Filho G, Radominski RB, Graf H, Amaral de Carvalho G. Low-normal or high-normal thyrotropin target levels during treatment of hypothyroidism: a prospective, comparative study. Thyroid 2011; 21: 355–60. [DOI] [PubMed] [Google Scholar]
- 122.Gullo D, Latina A, Frasca F, Le Moli R, Pellegriti G, Vigneri R. Levothyroxine monotherapy cannot guarantee euthyroidism in all athyreotic patients. PLoS One 2011; 6: e22552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Ito M, Miyauchi A, Morita S, et al. TSH-suppressive doses of levothyroxine are required to achieve preoperative native serum triiodothyronine levels in patients who have undergone total thyroidectomy. Eur J Endocrinol 2012; 167: 373–78. [DOI] [PubMed] [Google Scholar]
- 124.Jonklaas J, Davidson B, Bhagat S, Soldin SJ. Triiodothyronine levels in athyreotic individuals during levothyroxine therapy. JAMA 2008; 299: 769–77 [DOI] [PubMed] [Google Scholar]
- 125.Werneck de Castro JP, Fonseca TL, Ueta CB, et al. Differences in hypothalamic type 2 deiodinase ubiquitination explain localized sensitivity to thyroxine. J Clin Invest 2015; 125: 769–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Escobar-Morreale HF, del Rey FE, Obregón MJ, de Escobar GM. Only the combined treatment with thyroxine and triiodothyronine ensures euthyroidism in all tissues of the thyroidectomized rat. Endocrinology 1996; 137: 2490–502. [DOI] [PubMed] [Google Scholar]
- 127.Bunevicius R, Kazanavicius G, Zalinkevicius R, Prange AJ Jr. Effects of thyroxine as compared with thyroxine plus triiodothyronine in patients with hypothyroidism. N Engl J Med 1999; 340: 424–29. [DOI] [PubMed] [Google Scholar]
- 128.Nygaard B, Jensen EW, Kvetny J, Jarlov A, Faber J. Effect of combination therapy with thyroxine (T4) and 3,5,3 “ -triiodothyronine versus T4 monotherapy in patients with hypothyroidism, a double-blind, randomised cross-over study. Eur J Endocrinol 2009; 161: 895–902. [DOI] [PubMed] [Google Scholar]
- 129.Walsh JP, Shiels L, Lim EM, et al. Combined thyroxine/liothyronine treatment does not improve well-being, quality of life, or cognitive function compared to thyroxine alone: a randomized controlled trial in patients with primary hypothyroidism. J Clin Endocrinol Metab 2003; 88: 4543–50. [DOI] [PubMed] [Google Scholar]
- 130.Escobar-Morreale HF, Botella-Carretero JI, Gómez-Bueno M, Galán JM, Barrios V, Sancho J. Thyroid hormone replacement therapy in primary hypothyroidism: a randomized trial comparing L-thyroxine plus liothyronine with L-thyroxine alone. Ann Intern Med 2005; 142: 412–24. [DOI] [PubMed] [Google Scholar]
- 131.Appelhof BC, Fliers E, Wekking EM, et al. Combined therapy with levothyroxine and liothyronine in two ratios, compared with levothyroxine monotherapy in primary hypothyroidism: a double-blind, randomized, controlled clinical trial. J Clin Endocrinol Metab 2005; 90: 2666–74. [DOI] [PubMed] [Google Scholar]
- 132.Grozinsky-Glasberg S, Fraser A, Nahshoni E, Weizman A, Leibovici L. Thyroxine-triiodothyronine combination therapy versus thyroxine monotherapy for clinical hypothyroidism: meta-analysis of randomized controlled trials. J Clin Endocrinol Metab 2006; 91: 2592–99. [DOI] [PubMed] [Google Scholar]
- 133.Wiersinga WM. Paradigm shifts in thyroid hormone replacement therapies for hypothyroidism. Nat Rev Endocrinol 2014; 10: 164–74. [DOI] [PubMed] [Google Scholar]
- 134.McAninch EA, Bianco AC. New insights into the variable effectiveness of levothyroxine monotherapy for hypothyroidism. Lancet Diabetes Endocrinol 2015; 3: 756–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.McAninch EA, Jo S, Preite NZ, et al. Prevalent polymorphism in thyroid hormone-activating enzyme leaves a genetic fingerprint that underlies associated clinical syndromes. J Clin Endocrinol Metab 2015; 100: 920–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Panicker V, Saravanan P, Vaidya B, et al. Common variation in the DIO2 gene predicts baseline psychological well-being and response to combination thyroxine plus triiodothyronine therapy in hypothyroid patient. J Clin Endocrinol Metab 2009; 94: 1623–29. [DOI] [PubMed] [Google Scholar]
- 137.Wouters HJ, van Loon HC, van der Klauw MM, et al. No effect of the Thr92Ala polymorphism of deiodinase-2 on thyroid hormone parameters, health-related quality of life, and cognitive functioning in a large population-based cohort study. Thyroid 2016; published online Oct 27 DOI: 10.1089/thy.2016.0199. [DOI] [PubMed] [Google Scholar]
- 138.Wiersinga WM, Duntas L, Fadeyev V, Nygaard B, Vanderpump MP. 2012 ETA guidelines: the use of LT-4 + LT-3 in the treatment of hypothyroidism. Eur Thyroid J 2012; 1: 55–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Chaker L, Korevaar TI, Medici M, et al. Thyroid function characteristics and determinants: the Rotterdam study. Thyroid 2016; 26: 1195–204. [DOI] [PubMed] [Google Scholar]
- 140.Boas M, Feldt-Rasmussen U, Main KM. Thyroid effects of endocrine disrupting chemicals. Mol Cell Endocrinol 2012; 355: 240–48. [DOI] [PubMed] [Google Scholar]
- 141.Leung AM, Korevaar TI, Peeters RP, et al. Exposure to thyroid-disrupting chemicals: a transatlantic call for action. Thyroid 2016; 26: 479–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.