Abstract
Uremic syndrome (also known as uraemic syndrome) in patients with advanced chronic kidney disease involves the accumulation in plasma of small-molecule uremic solutes and uremic toxins (also known as uraemic toxins), dysfunction of multiple organs and dysbiosis of the gut microbiota. As such, uremic syndrome can be viewed as a disease of perturbed inter-organ and inter-organism (host–microbiota) communication. Multiple biological pathways are affected, including those controlled by solute carrier (SLC) and ATP-binding cassette (ABC) transporters and drug-metabolizing enzymes, many of which are also involved in drug absorption, distribution, metabolism and elimination (ADME). The remote sensing and signaling hypothesis identifies SLC and ABC transporter-mediated communication between organs and/or between the host and gut microbiota as key to the homeostasis of metabolites, antioxidants, signaling molecules, microbiota-derived products and dietary components in body tissues and fluid compartments. Thus, this hypothesis provides a useful perspective on the pathobiology of uremic syndrome. Pathways considered central to drug ADME might be particularly important for the body’s attempts to restore homeostasis, including the correction of disturbances due to kidney injury and the accumulation of uremic solutes and toxins. This Review discusses how the remote sensing and signaling hypothesis helps to provide a systems-level understanding of aspects of uremia that could lead to novel approaches to its treatment.
The term uremic syndrome refers to various signs and symptoms associated with generalized organ dysfunction occurring in patients with chronic kidney disease (CKD), which results in the accumulation in plasma of many protein-bound and water-soluble metabolites, referred to as uremic solutes. This complex systemic metabolic disorder involves metabolic derangements and aberrant signaling events that occur throughout the body, many of which are mediated by uremic solutes. Accordingly, this disease might best be considered from a systems biology perspective, especially given the growing amount of relevant ‘omics’ data as well as the availability of molecular and cellular functional information comparing diseased and healthy states. These data indicate that a multi-organ network of transporters and drug-metabolizing enzymes (DMEs) plays an important part in sensing, regulating and/or modulating the concentrations of these various small-molecule uremic solutes in tissues and body fluids1.
In patients with advanced CKD, uremic solutes accumulate in the circulation owing to deficient renal clearance. Some of these products are considered uremic toxins and are believed to contribute to the uremic syndrome. Many uremic solutes are produced by the dysbiotic gut flora and/or the action of enzymes in organs such as the liver. These solutes are transported via solute carrier (SLC) and ATP-binding cassette (ABC) transporters into different organs, where they are thought to exert toxic effects or disrupt key signaling and metabolic pathways, before being eliminated via what remains of the injured proximal tubule2–4. Similar to drugs such as diuretics and nonsteroidal anti-inflammatory drugs (NSAIDs), many uremic solutes are small organic molecules that circulate bound to plasma proteins, and both groups of molecules are transported into tissues and body fluid compartments by members of the SLC and ABC transporter superfamilies5,6. These transporters, together with phase 1 and phase 2 DMEs, are prominent in the pharmacological literature owing to their role in drug absorption, distribution, metabolism and excretion (ADME).
A number of these transporters have been identified as particularly important in the transport of uremic solutes, including in the transport of molecules involved in the regulation of key metabolic and signaling pathways, antioxidants and mediators of cellular toxicity7,8 (Table 1). Such transporter-mediated movement of uremic toxins into tissues and body fluids, or from plasma into proximal tubule cells of the kidney where they can be eliminated via the urine, generally occurs via pathways not dissimilar from those involved in the distribution of drugs. Some of these small organic molecules also seem to be toxic to proximal tubule cells9–12 and are thought to be associated with the progression of CKD. Hence, information could be transmitted between cells, organs and tissues via the movement of these small organic molecules. This remote communication involves multi-specific transporters and other ADME-related proteins that are differentially expressed in the cells that line fluid-containing body compartments, such as the intestine, kidney, liver, muscle and central nervous system (CNS)5,13. Accordingly, uremic syndrome could be viewed as a systemic disease resulting in part from perturbed inter-organ and inter-organism (that is, host–microbiota) communication. The changing profile of uremic solutes in progressive CKD is both a result of dysregulated local and systemic homeostasis and a cause of it. In this Review, we frame our discussion of uremic syndrome in the context of the remote sensing and signaling hypothesis, which is more fully elucidated elsewhere1,5,13–15 (Fig. 1). In essence, this hypothesis emphasizes the central role of transporters and DMEs in regulating remote communication between tissues and organs via small organic molecules (Figs 1,2). As we argue, many aspects of uremic syndrome can be viewed as disordered remote sensing and signaling.
Table 1 |.
Transporters in the kidney of importance for uremic toxins
| Transporter name | ligand polarity | Tissue expression | examples of drugs interacting with transporter | examples of uremic toxins interacting with transporter | |
|---|---|---|---|---|---|
| protein | gene | ||||
| Basolateral (influx) transporters | |||||
| OAT1 | SLC22A6 | Anion | Kidney and choroid plexus | Probenecid, adefovir and indometacin | Anthranilic acid, indoxyl sulfate and p-cresol sulfate |
| OAT3 | SLC22A8 | Anion | Kidney, choroid plexus and blood- brain barrier | Probenecid, methotrexate and cimetidine | Indoxyl sulfate, p-cresol sulfate and CMPF |
| OATP1B1 | SLCO1B1 | Anion | Liver | Methotrexate and lopinavir | Kynurenic acid, indole-3-acetic acid and indoxyl sulfate |
| OATP1B3 | SLCO1B3 | Anion | Liver | Methotrexate and telmisartan | Kynurenic acid, indole-3-acetic acid and indoxyl sulfate |
| OATP4C1 | SLCO4C1 | Anion | Kidney | Ritonavir, crizotinib and saquinavir | Symmetric dimethylarginine, guanidine succinate and trans-aconitate |
| OCT1 | SLC22A1 | Cation | Kidney and liver | Metformin, ganciclovir and acyclovir | TMAO and methylguanidine |
| OCT2 | SLC22A2 | Cation | Kidney | Metformin, cimetidine and lamivudine | TMAO, putrescine and methylguanidine |
| Apical (efflux) transporters | |||||
| MRP2 | ABCC2 | Anion | Kidney, intestine and liver | Probenecid, methotrexate and amoxicillin | Anthranilic acid, indoxyl sulfate and p-cresol sulfate |
| MRP4 | ABCC4 | Anion | Kidney, liver and blood–brain barrier | Methotrexate, cefotaxime and furosemide | Anthranilic acid, indoxyl sulfate and p-cresol sulfate |
| MATE1 | SLC47A1 | Cation | Kidney and liver | Metformin, cimetidine and topotecan | TMAO and creatinine |
| MATE2K | SLC47A2 | Cation | Kidney | Metformin, cimetidine and topotecan | TMAO and creatinine |
| BCRP | ABCG2 | Anion | Kidney, intestine, liver and blood- brain barrier | Methotrexate, gefitinib and imatinib | Uric acid, indoxyl sulfate, hippuric acid and kynurenic acid |
CMPF, 3-carboxy-4-methyl-5-propyl-2-furanpropanoic acid; MATE1, multi-drug and toxin extrusion protein 1; MATE2K, 2K splice variant of MATE2; OAT, organic anion transporter; OATP, organic anion-transporting polypeptide; OCT, organic cation transporter; TMAO, trimethylamine-N-oxide.
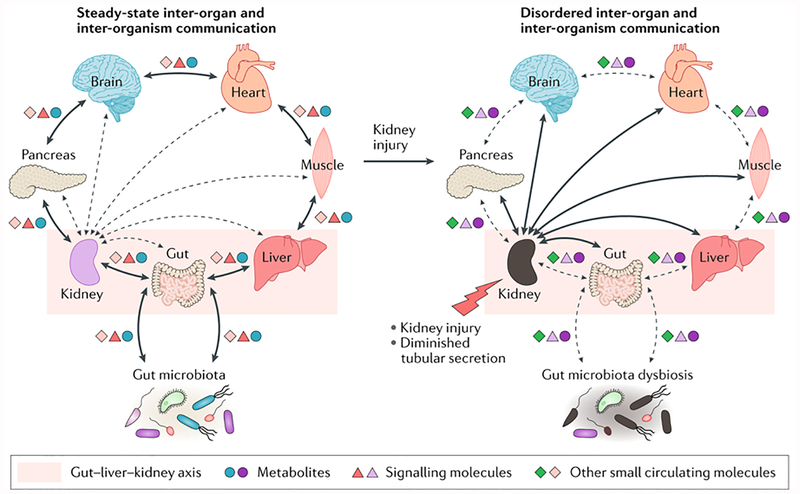
Figure 1. Aberrant inter-organ and inter-organism communication contributes to uremic syndrome.
According to the remote sensing and signaling hypothesis, illustrated here from the perspective of the kidney, steady-state communication between the kidney and other organs and body fluids involves the movement of metabolites, signaling molecules and other small circulating molecules via solute carrier (SLC) and ATP-binding cassette (ABC) transporters. These transporters are expressed in many tissues, including the kidney, liver, pancreas, brain, intestine and muscle. Therefore, injury to the kidney leads to diminished tubular secretion and results in disordered remote communication. Some of these molecules can regulate the expression (via nuclear receptor activation) and/or function of SLC and ABC transporters or phase 1 and phase 2 drug-metabolizing enzymes (DMEs) in distinct cells, tissues and organs, thereby effecting local and global physiological alterations, accompanied by dysbiosis of gut microbiota (including the loss or gain of some bacterial strains). The multi-specificity of some ABC and SLC transporters, together with their different and modifiable tissue expression and/or trafficking, might help to restore homeostasis after organ dysfunction and injury. The differences in color of the symbols representing metabolites, signaling molecules and other small circulating molecules that interact with transporters represent alterations in the concentration and/or identity of these compounds under physiological versus pathological conditions.
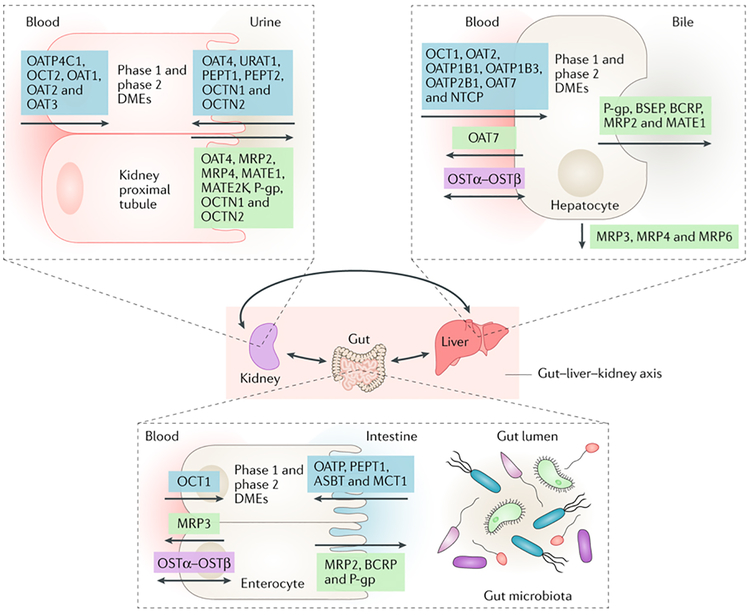
Figure 2. The gut–liver–kidney axis.
Within the remote sensing and signaling communication network, the gut–liver–kidney axis drives the absorption, distribution, metabolism and excretion of small molecules, including endogenous metabolites, signaling molecules, products of the gut microbiota, nuclear receptors and antioxidants. Products derived from gut microbiota that cross the intestinal barrier are removed from the blood by solute carrier (SLC) and ATP-binding cassette (ABC) transporters on hepatocytes, where they (and other small molecules) are metabolized by phase 1 and phase 2 drug-metabolizing enzymes (DMEs). Ultimately, many of these products are cleared from the body via the kidney in the urine through the action of SLC and ABC transporters on the proximal tubule cells. Some transporters in different tissues are thought to function in both directions. MATE1, multi-drug and toxin extrusion protein 1; MATE2K, 2K splice variant of MATE2; OAT, organic anion transporter; OATP, organic anion-transporting polypeptide; OCT, organic cation transporter; P-gp, P-glycoprotein.
Remote sensing and signaling
The remote sensing and signaling hypothesis was formulated over a decade ago14,16 to explain the role of SLC and ABC transporters in the physiology of various organ systems, which had been suggested by the results of metabolomics studies and the phenotypes of mice with knockout of Slc22a6 (which encodes SLC family 22 member 6; also known as organic anion transporter 1(OAT1)) and other transporters. Alongside unexpected developmental expression patterns17 and the discovery of unusual transporter family members (such as OAT6, which seems to be an odorant transporter in olfactory mucosa18,19), one of the main findings from studies of mice lacking the ability to renally eliminate organic anions via OAT1 and/or OAT3 was the presence of many metabolic alterations, including elevated plasma levels of numerous uremic solutes, many of which were derived from the gut microbiota7,8.
Many SLC and ABC transporter family members are evolutionarily highly conserved, multi-specific and have a wide range of endogenous substrates, including well-known signaling molecules, such as cAMP, prostaglandins, bile acids and short-chain fatty acids; metabolites such as α-ketoglutarate; antioxidants such as urate and ergothionine; and vitamins or cofactors such as pantothenic acid5. Networks of apical (efflux) and/or basolateral (influx) multi-specific SLC and ABC transporters often work to maintain metabolite homeostasis in concert with closely related (and sometimes also with unrelated) transporters of limited specificity or relative monospecificity. These homeostatic networks can be sited within a single organ or involve several organs20. For example, three multi-specific transporters (OAT1, OAT3 and ABC subfamily G member 2 (ABCG2)) work closely with two limited-specificity transporters (URAT1 (also known as SLC22A12) and GLUT9 (also known as SLC2A9)) to control urate homeostasis21. In the absence of end-stage renal disease (ESRD), the bulk of urate handling in the kidney is carried out by these and several other transporters. Moreover, multi-specific SLC and ABC transporters in the gut, liver and kidney work in concert with monospecific and oligospecific transporters and DMEs to regulate the homeostasis of bile acids, which are involved in fat digestion and signaling via G protein-coupled receptors (GPCRs) and nuclear receptors20 (reviewed elsewhere22–24).
Although DMEs were not emphasized in initial descriptions of the remote sensing and signaling hypothesis, these enzymes modify various molecules, including drugs and gut-derived metabolites, thereby increasing their solubility (Box 1). As these modifications generate additional signaling molecules and putative uremic toxins, DMEs are intimately connected to remote communication pathways involving ADME-related transporters and enzymes. This connection is particularly important in uremia, because some of the most important uremic toxins (including indoxyl sulfate and p-cresol sulfate) are generated as a result of the actions of phase 2 DMEs on their microbiota-derived precursors (indole and p-cresol, respectively), resulting in both cellular toxicity and the potential for diverse types of cell signaling7,8.
Box 1 |. Xenobiotic absorption, distribution, metabolism and elimination.
After intestinal absorption, xenobiotic metabolism modifies the chemical structure of potentially hazardous compounds, including exogenous drugs and toxins and endogenous metabolites and signalling molecules. This biotransformation increases the water solubility of lipophilic compounds, which facilitates their excretion from the body. Hepatocytes are the primary site of xenobiotic metabolism, but it also occurs in cells of the gastrointestinal tract, lungs, kidneys and skin179,180.
Phase 1 xenobiotic metabolism involves the introduction or unmasking of a functional group through the action of various drug-metabolizing enzymes (DMEs), including oxidases, reductases, hydrolases and hydroxylases. The most important enzyme family involved in biotransformation is the cytochrome P450 superfamily179,180. Xenobiotics can also undergo phase 2 metabolism, in which conjugation reactions attach species such as acetyl groups, methyl groups, glutathione, sulfate, glucuronic acid and glycine179,180. The resulting compounds are generally very polar and can be rapidly eliminated from the body.
Some solute carrier (SLC) and ATP-binding cassette (ABC) transporters in tissues such as the gut, liver and kidney are key to the absorption, distribution, metabolism and elimination (ADME) not only of xenobiotics but also of metabolites, signalling molecules, nutrients and antioxidants. Sometimes, transporter-mediated ADME is referred to as phase 3 xenobiotic metabolism179,181–183. For example, in the liver, transporter-mediated uptake clears xenobiotics from blood into hepatocytes182,183. The biotransformed metabolites of these xenobiotics are directly secreted into bile by ABC transporters expressed on the canalicular membrane and excreted via the gut (although some metabolites are resorbed back into the blood from the intestine)180. In addition, some biotransformed metabolites are transported from hepatocytes into the blood by ABC transporters expressed on the basolateral cell membrane. Once in the systemic circulation, these metabolites — some of which are active and/or toxic (for example, indoxyl sulfate) — can access other organs and body fluids via other SLC and ABC transporters. Ultimately, they are cleared from the blood and eliminated in the urine via the SLC transporters organic anion transporter 1 (OAT1), OAT3 and organic cation transporter 2 (OCT2) and the ABC transporters P-glycoprotein, multidrug resistance- associated protein 2 (MRP2; also known as canalicular multispecific organic anion transporter 1) and MRP4 on renal proximal tubule cells.
Thus, a multi-scale, multi-compartment network of transporters and DMEs is involved in sending and receiving information to various tissues and body fluid compartments1,5–8,13–15. This information is transmitted via small organic metabolites (such as α-ketoglutarate, polyamines, tryptophan metabolites and uric acid) and signaling molecules (such as prostaglandins, cyclic nucleotides, odorants and neurotransmitters) that are substrates of SLC and ABC transporters (Figs 1,2). These metabolites and signaling molecules, in turn, feed into well-known metabolic pathways, modulate kinase activity and/or bind to GPCRs (including metabolite GPCRs) and nuclear receptors. This organization also extends to intracellular pathways that affect transcription, signaling, protein trafficking and macromolecular interactions (including transporter–cytoskeleton interactions). Together, this network involves over 100 ADME-related proteins, which are encoded by genes that are differentially and highly expressed in epithelial cells (Fig. 2), such as those in the kidney, liver, gut and pancreas, and in non-epithelial cells, such as the endothelial cells of the blood–brain barrier and circulating cells. This network is considered to act in parallel with the neuroendocrine system, growth factors and cytokines, as well as being analogous to these classic homeostatic systems.
Furthermore, several of the metabolites and signaling molecules transported into and out of various body fluids and tissues have the ability to interact with other organisms. Remote communication consisting of unidirectional or bidirectional transport of metabolites and signaling molecules across an epithelial, endothelial or similar barrier can occur between individuals of the same species, via breast milk, amniotic fluid or urine; between the host and gut microbiota; or between different microbiota species1,5,7. All these remote communication examples could be relevant to ESRD in certain contexts, although (as discussed below) most attention has been paid to bidirectional communication between the host and the gut microbiota in patients with ESRD25,26. Inter-organ communication involves both well-defined physiological pathways, such as transport of bile acids and nutrients via the gut–liver–kidney axis, and those occurring in pathological states. Although DMEs and transporters have been identified and characterized in virtually every tissue of the body, inter-organ communication in the context of uremia involves the brain, muscle, pancreas, gut, liver and heart, among other tissues. The composition of metabolites, signaling molecules, antioxidants, nutrients, vitamins and cofactors in body fluids found in epithelium-lined compartments, including cerebrospinal fluid (CSF), bile, urine, plasma, amniotic fluid, breast milk and ocular fluid, is partly or largely regulated by SLC and ABC transporters. For example, the choroid plexus (which regulates the metabolite composition of CSF) is morphologically and functionally similar to the proximal tubule27, and this similarity extends to the expression of OATs and organic cation transporters (OCTs)28. The reader should keep in mind that, in the setting of ESRD, the composition and volume of these various body fluids can change substantially29,30 and that other compartments (including interstitial, peritoneal and pericardial spaces) can become important27,31.
The remote sensing and signaling hypothesis emphasizes the homeostatic role of SLC and ABC multi-specific, monospecific or oligospecific transporters (particularly in settings such as renal impairment) because this system is thought to be of comparable importance to the neuroendocrine, growth factor and cytokine systems in the resetting of homeostatic mechanisms after perturbation or injury1,5,7. However, the hypothesis also emphasizes the critical interconnections between transporter-based mechanisms and other homeostatic systems. For example, organic anion-transporting polypeptides (OATPs) are transporters of thyroid hormones and knocking out an OATP family member in the mouse brain capillary endothelium leads to an underdeveloped CNS32. Similarly, expression of OAT3 in pancreatic β-cells is necessary for transport of 3-carboxy-4-methyl5-propyl-2-furanpropanoic acid (CMPF), a uremic solute that is involved in the regulation of insulin secretion33. A defect in this pathway has been linked to human gestational diabetes.
This multi-scale systems biology model comprises inter-organism communication, whole-organism homeostasis, organs, tissues, body fluids and transporterexpressing cells. This model incorporates many points of potential regulation and dysregulation, including transporter trafficking, phosphorylation, transcription and transporter–cytoskeletal associations. The modelalso includes multiple points for sensing of metabolites, signaling molecules, antioxidants, nutrients and other molecules, including, but not limited to, nuclear receptors and transcription factors, GPCRs, kinases and sensors involved in redox pathways. Indeed, many of the established and probable in vivo endogenous substrates for SLC and ABC multi-specific transporters are believed to have essential roles in activating and modulating these sensing mechanisms, which in turn can regulate the expression and/or function of DMEs as well as that of the transporters themselves5. As yet, not enough integrated data are available to accurately represent this model in healthy individuals, much less in patients with uremia or CKD. However, networks based on a single transporter (such as OAT1, which is one of the most important uremic toxin transporters) that include all the numerous metabolites and signaling molecules with which the transporter interacts have been developed. Building such networks is complicated, as it involves the integration of transcriptomic and metabolomic data from transporter-knockout mice, in vitro data and computational reconstruction of metabolic networks34–36. Nevertheless, the elucidation of metabolic and signaling networks incorporating multiple transporters in health and disease should be possible in the next few years. We emphasize that this is an iterative process informed by the publication of ever-more comprehensive omics data, new functional data and improved tools for data integration and network reconstruction. Therefore, the publication of accurate networks of this kind is probably years away, particularly for complex diseases such as human CKD. This goal is likely to be more tractable in animal models of CKD.
Remote sensing and signaling in CKD
In animal models of CKD, declining renal function is associated with altered renal expression of many SLC and ABC transporters. Furthermore, changes occur in the gene expression and/or function of ADME-related proteins (namely, multi-specific transporters and DMEs) in the liver and intestine as well as other tissues37. Some of these changes could be viewed as compensatory efforts to preserve homeostasis. Indeed, the changes in expression and/or function of the efflux transporter, ABCG2, in the intestine in the setting of ESRD might be an example of such a compensatory response. Abcg2knockout mice subjected to adenine-induced CKD not only displayed impaired survival compared with wild-type controls but also had substantially increased plasma concentrations of uremic toxins, including indoxyl sulfate (a substrate of ABCG2)38. Patients with ESRD who have dysfunctional variants of ABCG2 have substantially elevated plasma levels of uric acid39, also considered a uremic toxin. These data support the notion that increased expression or activity of ABCG2 (largely in the intestine) is a compensatory mechanism that decreases plasma levels of indoxyl sulfate and urate via their excretion into the gut lumen in the setting of renal insufficiency38–43. Importantly, the gut microbiota also undergoes changes in response to the increased uric acid load, including the growth of bacteria that produce uricase44, an enzyme involved in the reduction of uric acid to allantoin. Together with the findings in mice described above, these data suggest that intestinal ABCG2 (in addition to its role in the increased excretion of uric acid and indoxyl sulfate in CKD) plays an important part in inter-organism remote sensing and signaling through its transport of uric acid and other uremic toxins.
Other multi-specific transporters also show increased expression in the gut and liver in response to impaired renal function37. By contrast, many DMEs show opposing changes in expression in the gut and liver45–48 as well as decreased functional activity45 in this setting. Interestingly, some of these DME changes can be reversed by renal transplantation49. As DMEs are involved in the generation of many uremic toxins (including indoxyl sulfate), the decrease in expression and/or activity of some DMEs might be a mechanism for decreasing the organism’s uremic toxin burden.
The expression of genes encoding nuclear receptors such as nuclear receptor subfamily 1 group I member 2 (PXR) and hepatocyte nuclear factor 4α (HNF4α) is also decreased in animal models of CKD45. These proteins are sometimes considered master regulators of the expression of many ADME-related genes and as such are potential sensors in a remote sensing and signaling network5,50–54. Decreased expression of these nuclear receptors could explain the decreases in the expression of certain DMEs but probably does not explain the observed increases in transporter expression. Other transcription factors, such as the aryl hydrocarbon receptor (AHR), probably regulate the expression of multi-specific transporters, such as P-glycoprotein (P-gp), apparently involved in resetting the system in the presence of renal impairment55,56.
Analyses of omics data indicate considerable alteration in the gene expression and/or activity of transporters and other ADME-related proteins involved in remote sensing and signaling in the setting of renal insufficiency. Some of these alterations might be interpreted as an attempt to reset homeostasis by decreasing levels of uremic toxins such as indoxyl sulfate and urate40. This interpretation, together with the many changes in expression and/or function of multi-specific transporters and DMEs across tissues, suggests that resetting of the inter-organ and inter-organism communication network occurs to preserve homeostasis in the setting of ESRD.
The example of indoxyl sulfate
A growing body of research relates to the toxic, metabolic and signaling effects of individual uremic toxins, such as albumin-bound indoxyl sulfate, which is one of the main uremic toxins implicated in uremic syndrome9–12,57. Indoxyl sulfate is not optimally cleared during dialysis, and considerable translational and clinical research has focused on strategies to reduce serum levels of this molecule in patients with CKD57–64.
Indoxyl sulfate ispartofan inter-organism (microbiota- host) and inter-organ (gut, liver, kidney and brain) communication network regulated by transporters and DMEs (Fig. 3). Indole is produced by the gut microbiota as a result of the metabolism of tryptophan. Once absorbed across the intestinal wall into the blood, indole is taken up by hepatocytes, where it is metabolized first to indoxyl by hepatic DMEs (namely, CYP2E1) and then to indoxyl sulfate by sulfotransferases65. Indoxyl sulfate is then secreted back into the blood by hepatocyte transporters, whereupon it interacts with various tissues, organs and body fluids, largely via SLC and ABC transporters. In vitro data indicate that indoxyl sulfate can participate in or at least affect numerous intracellular signaling pathways, including those involving transcription factors (such as AHR) and various kinases66–74. Indoxyl sulfate is ultimately cleared from the blood via OAT1 and OAT3 transporters expressed on the basolateral membrane of kidney proximal tubule cells7,8. This uremic solute is then secreted into the tubular lumen via apical efflux transporters (although precisely which transporters are involved remains unclear at present) and eliminated from the body in urine. However, high levels of indoxyl sulfate seem to exert toxicity in proximal tubule cells as well as cells of the vasculature and CNS57. Movement of indoxyl sulfate into these cells is probably mediated by OAT3 and other transporters75. Moreover, AHR (to which indoxyl sulfate binds67,76) regulates the expression of members of the cytochrome P450 family, other DMEs and transporters77,78. At concentrations observed in the serum of patients with uremia, indoxyl sulfate activates expression of CYP1A1, CYP1A2 and CYP1B1 genes, as well as expression of UGT1A1 and UGT1A6 (encoding the phase 2 DMEs UDP-glucuronosyltransferase 1–1 and UDP-glucuronosyltransferase 1–6, respectively) in primary human hepatocytes69,76. This uremic toxin also regulates the expression of hepatic P-gp via binding to AHR in rodent and cell culture models of CKD55. The observation that patients with CKD who have high plasma levels of indoxyl sulfate also show increased hepatic metabolism of cyclosporin, a P-gp substrate, suggests that this increased expression is likely to be clinically relevant55.
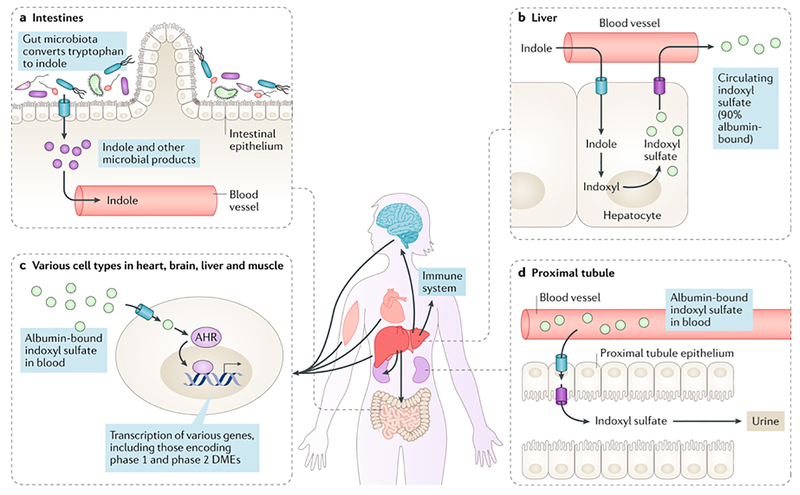
Figure 3. The role of indoxyl sulfate in inter-organism and inter-organ remote communication.
a) Indole is created in the lumen of the gut, via the metabolism of tryptophan by the gut microbiota, and absorbed across the gut wall into the blood. b) Circulating indole is taken up by hepatocytes, where it is metabolized first to indoxyl and then to indoxyl sulfate. Indoxyl sulfate is transported back into the circulation where the majority of it circulates bound to albumin and where it is distributed to and interacts with other organs, such as the brain, immune system and muscle, and with the gut microbiota. c) Indoxyl sulfate gains access to tissues and cells, where it signals through the aryl hydrocarbon receptor (AHR), leading to alterations in the expression of a number of genes in these tissues. d) Indoxyl sulfate is ultimately excreted by the kidney via solute carrier (SLC) and ATP-binding cassette (ABC) transporters located in the basolateral (such as organic anion transporter 1 (OAT1) and OAT3) and apical (such as multidrug resistance-associated protein 4 (MRP4)) membranes of proximal tubule cells (influx and efflux transporters are indicated by blue and purple barrels, respectively).
These data are consistent with the notion that remote communication between the failing kidney and the liver occurs via one or more uremic toxins, which act through nuclear receptor signaling. In addition to activation of AHR, indoxyl sulfate can also activate nuclear factor-κB (NF-κB) and mitogen-activated protein kinase (MAPK) signaling pathways in macrophages, as demonstrated by increased phosphorylation of p38 MAPKs, c-Jun N-terminal kinases (JNKs) and the NF-κB p65 subunit70,79. However, the relevance of many of these intriguing findings remains to be determined in human CKD. Indoxyl sulfate is but one example of a well-studied uremic solute or toxin involved in inter-organ and/or inter-organism communication. Later in this article, we discuss other examples.
ADME networks in resetting homeostasis
Under physiological circumstances, numerous multi-specific, oligospecific and monospecific transporters, DMEs and their substrates function as a regulated local and systemic small-molecule remote communication network that is closely linked to the neuroendocrine, autonomic, growth factor and cytokine systems traditionally associated with homeostasis (Figs 1,2,4). As with other homeostatic systems, the ADME-related remote sensing and signaling network adapts to help the organism recover from or compensate for disturbances1,5,13–15 (Fig. 4). For example, in a rodent model of obesity-associated renal disease, diminished function of OAT3 was causedbyits reduced expression at theplasma membrane, possibly owing to increased internalization of OAT380. Feeding a probiotic to these rodents prevented renal damage and resulted in increased activity of OAT3 at the plasma membrane. This observation was interpreted by the authors as an instance of remote sensing and signaling between the intestine, gut microflora and kidney80.
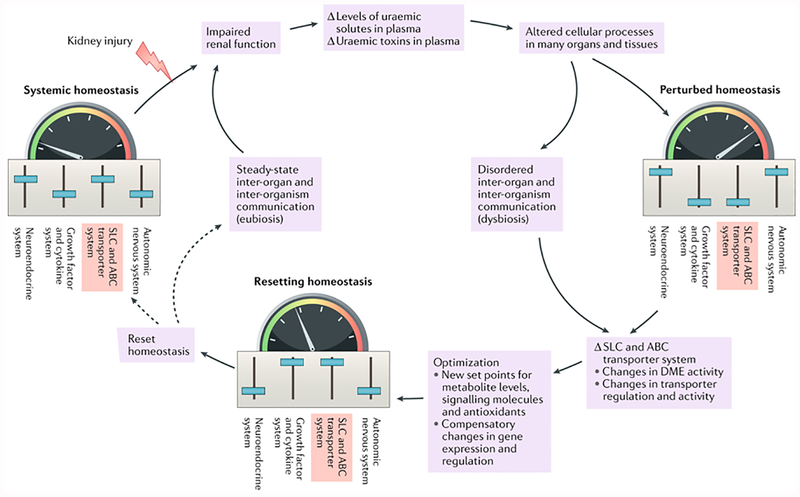
Figure 4. A remote sensing and signalling system maintains homeostasis in the steady state and resets homeostasis following perturbations due to kidney dysfunction and microbiota dysbiosis.
Small molecules with informational content are important in transmitting signals to remote tissues and/or organs in the maintenance of homeostasis. In essence, the small-molecule substrates of multi-specific solute carrier (SLC) and ATP-binding cassette (ABC) transporters act in concert with other transporters of limited specificity, as well as with phase 1 and phase 2 drug-metabolizing enzymes (DMEs), as part of a highly flexible inter-organ and inter-organism communication network. Under physiological conditions, this network works in concert with other systems to maintain steady-state homeostasis. Injury to the kidney resulting in diminished kidney function and reduced tubular secretion can lead to the accumulation of uremic solutes and uremic toxins in plasma. The increased levels of these small molecules can lead to alterations in cell functions and processes in multiple tissues and organs, resulting in perturbed homeostasis. The components of this network optimize metabolic pathways, signaling pathways, the control of redox status and other mechanisms necessary for homeostasis in different tissues and body fluid compartments, as well as in different organisms. This system is intertwined with and works in parallel with other homeostatic mediators, such as growth factors, cytokines and the neuroendocrine and vasoregulatory systems. The flexibility of the system enables homeostasis to be restored or reset at a new (presumably compensatory) set point despite the presence or progression of renal disease. Sliders in each picture represent the set points of each of the homeostatic systems, which are altered following organ injury and adjusted during the resetting of homeostasis. The colored scale and arrow represent the homeostatic setting in the various stages.
Therefore, regulation of the expression of SLC and ABC transporters (as well as DMEs) results in a highly adaptable system5,15,81–83 capable of responding to acute or chronic perturbations of homeostasis, such as occur in CKD and acute AKI (Fig. 4). For example, the internalization, degradation, membrane trafficking and cytoskeletal association of transporters are affected by post-translational modifications, any of which could modify their function and cell surface expression in response to changing levels of substrate, such as are seen in CKD81,83. In addition, covalent modifications might also affect protein–protein interactions involving the transporters. For example, OAT1 seems to exist as a functional homo-oligomer, and the association of OAT1 into homo-oligomers might be dynamic83,84. The expression of OAT4 and other transporters also seems to be regulated by PDZ domain-containing proteins85–91.
As discussed above, the functioning of this remote communication network is likely to be altered in patients with CKD and early uremia. Indeed, many of the components of this network (including multi-specific transporters, transporters of limited specificity and DMEs) show altered expression in the kidney, gut, liver and other tissues in the presence of renal dysfunction.
Gut dysbiosis and remote communication
Many uremic toxins are derived from the gut microflora66,92–94, and numerous reviews have highlighted the role of the gut microbiota as a source of uremic toxins in patients with CKD25,65,94,95. Here, however, we focus on the gut dysbiosis associated with CKD as an indicator of disordered host–microbiota communication. It is becoming increasingly clear that the relationship between the gut microbiota and the host is very complicated, particularly in the setting of CKD. For example, gut dysbiosis is a typical feature of CKD and is characterized by increases in bacterial species involved in the production of several gut-derived uremic toxins65, many of which can alter the function and expression of transporters and DMEs and potentially contribute to the progression of CKD65. These bacteria are believed to communicate not only with the host but also among themselves96, in part through transporters that are evolutionarily related to the ABC and SLC transporters involved in inter-organ communication within the host. Therefore, bidirectional communication seems to operate between the gut microbiota and the host, mediated by small-molecule metabolites derived from the gut microbiota. These metabolites alter the expression and function of transporters and DMEs in the kidney, liver and other tissues, leading to the accumulation of uremic toxins in the blood. In addition, changes in the host ADME-protein-related signaling and communication network in the progressively failing kidney will, in turn, probably lead to further alterations in the gut microbiota. The mechanisms of gut dysbiosis are not well understood, and it is possible that the types and amounts of uremic toxins arising from the gut microbiota could differ depending on the cause and stage of CKD.
Intriguingly, many microbiota-derived uremic solutes have well-characterized roles in normal and aberrant signaling pathways (kynurenine)97, carbohydrate metabolism (CMPF)98, redox state (urate)99 and cell proliferation (polyamines)100 and are also considered to be uremic toxins, suggesting that they have a dual toxic and regulatory or metabolic function. This duality might hold important clues for understanding the pathophysiology of CKD at a systems level and for understanding the complex metabolic aberrations that characterize the uremic syndrome in patients with different types and stages of kidney disease. The efficacy of interventions aimed at influencing the gut microbiota might differ according to the type and stage of renal disease.
OAT1 and OAT3 knockout metabolomics
The prototypic OAT, OAT1 (originally identified by us in mice as NKT101), is the main probenecid-sensitive transporter of para-aminohippurate in the kidney102,103. The OAT1 pathway has been extremely well studied from the viewpoint of renal physiology, toxicology and pharmacokinetics13,15 because it is the route through which many toxins, protein-bound antibiotics, NSAIDs, diuretics and antiviral agents are eliminated102,104–108. For example, mercury, a highly toxic environmental pollutant, is conjugated to glutathione or cystathione and then eliminated from the circulation via OAT1 (reFs109–111). OAT1 also seems to be a major transporter of indoxyl sulfate7,8,112.
Much of our understanding of the role of OAT1 and OAT3 is based on data from Slc22a6-knockout and Slc22a8-knockout mouse models5,13,15. Metabolomic data and other studies in these mice indicate that OAT1 directly and indirectly influences a wide range of metabolic pathways via transport of many metabolites (such as α-ketoglutarate, urate and pantothenic acid) and signaling molecules, including prostaglandins, cyclic nucleotides, fatty acids and odorants7,34,35.
Although plasma levels of indoxyl sulfate display a major increase in Slc22a6-knockout mice7,8, OAT1 also transports other uremic solutes, including p-cresol sulfate, kynurenine and hippurate7,8. These molecules (and many others, including kynurenate, indolelactate and xanthurenate) are also transported by the closely related transporter OAT3, which is also found on the basolateral surface of the proximal tubule8,20. OAT1 and OAT3 have partly overlapping specificities for metabolites, signaling molecules and gut microbiota products, including uremic toxins8,15,108,113. Many other uremic solutes thought to be of relevance to CKD or uremia are transported from blood to urine by OAT1 and OAT3, including CMPF, 1-methylguanosine and urate7,8. Indeed, a substantial fraction of all uremic solutes identified thus far interact with OAT1 and/or OAT3 (Table 2), as well as with other SLC family 22 members92,93,114. Single-nucleotide polymorphisms (SNPs) in SLC22A6 are associated with human CKD and have been suggested to affect the ability of the kidney to handle uremic toxins115
Table 2 |.
Some uremic toxins that interact with OAT1 and/or OAT3
| Metabolite | In vivo metabolomicsa | In vitro Interaction | ||
|---|---|---|---|---|
| OAT1 | OAT3 | OAT1 | OAT3 | |
| CMPF | NS | ✓ | ✓ | ✓ |
| Creatinine | ✓ | ✓ | ✓ | ✓ |
| Cysteine | ✓ | NS | ✓ | ✓ |
| Hypoxanthine | NS | NS | ✓ | ✓ |
| Indoleacetate | NS | ✓ | ✓ | ✓ |
| Indoxyl sulfate | ✓ | ✓ | ✓ | ✓ |
| Kynurenate | ✓ | NS | ✓ | ✓ |
| Kynurenine | ✓ | ✓ | ✓ | ND |
| N2,N2-dimethyl-guanosine | ✓ | NS | ND | ND |
| N6-methyladenosine | ✓ | NS | ND | ND |
| Orotate | ✓ | NS | ND | ND |
| p-Cresol sulfate | NS | ✓ | ✓ | ✓ |
| Putrescine | NS | ✓ | ND | ND |
| S-adenosylhomocysteine | ✓ | NS | ND | ND |
| Trimethylamine-N-oxide | NS | ✓ | ND | ND |
| Uric acid | ✓ | ✓ | ✓ | ✓ |
CMPF; 3-carboxy-4-methyl-5-propyl-2-furanpropanoic acid; ND, not done; NS, no significant change; OAT, organic anion transporter.
Other uremic solute transporters
OCT2 (encoded by SLC22A2) transports cationic uremic solutes, including polyamines (putrescine, spermine and spermidine) and creatinine116, although some of these cationic or zwitterionic molecules can also, to a limited degree, be transported by OATs34,117.
Another member of SLC family 22, OAT2 (encoded by SLC22A7), is expressed on erythrocytes118,119. OAT2 seems to be responsible for transporting indoxyl sulfate into erythrocytes, where it induces cell death owing to the production of reactive oxygen species (ROS) via the NADPH oxidase-dependent pathway113,119.
OATP4C1, a member of the OATP family (encoded by SLCO4C1), transports a somewhat different set of uremic solutes120. Transgenic mice that overexpress human SLCO4C1 display decreased plasma levels of some uremic toxins, including asymmetric dimethylarginine, guanidine succinate and trans-aconitate, compared with wild-type controls120.
Transport of the cationic uremic toxin trimethylamine-N-oxide (TMAO), which is strongly associated with cardiovascular morbidity121,122, is likely to be mediated by OCT2 and several ABC transporters123. TMAO accumulates in the plasma of Slc22a8knockout mice, although whether TMAO is actually transported by OAT3 is not clear8. The apical (luminal) efflux of cationic uremic solutes probably involves the SLC47 family of transporters, including multi-drug and toxin extrusion protein 1 (MATE1) and the 2K splice variant of MATE2 (MATE2K)124–126. In general, however, the apical efflux transporters for uremic solutes are not well studied.
Pharmacokinetic implications
Patients with CKD show diminished transport of drugs that are normally excreted in bile as well as increased levels of uremic toxins. These observations suggest that these toxins compete with the drugs for transport via OATP1B1 and OATP1B3, which are expressed in the sinusoidal membrane of hepatocytes as well as other non-renal tissues127. In support of this notion, the uremic toxins kynurenate and indoxyl sulfate inhibit OATP1B1-mediated and OATP1B3-mediated transport of methotrexate in a dose-dependent manner127.
Several uremic toxins (including CMPF, hippuric acid, indole-3-acetate and indoxyl sulfate) inhibit OATP1B1-mediated uptake of an active metabolite of irinotecan in cells engineered to stably express this transporter128. These findings might partially explain why the half-life of this drug metabolite, which is normally excreted in bile, is increased in patients with ESRD128. Together, these data provide evidence for potential drug–metabolite interactions at the level of the transporters in non-renal tissues, a phenomenon that seems to be exacerbated in uremia. Indeed, the data also support the notion that non-OAT transporters (such as OATPs) are important for the movement of uremic toxins into non-renal tissue.
Under physiological circumstances, the expression of phase 1 and phase 2 DMEs and transporters is regulated, at least in part, by the gut microbiota129–133. Therefore, not surprisingly, many gut-derived uremic toxins have adverse effects on the expression and function of phase 1 and phase 2 DMEs and transporters65. Patients with CKD are routinely treated with many drugs134,135 (an average of 12 different medications135) that require DMEs and transporters for ADME. Uremic-toxinmediated alterations in the function or expression of DMEs and transporters have the potential to drastically affect biotransformation, leading to increased drug half-lives and plasma levels.
Moreover, to the extent that uremia is partly a remote sensing and signaling disorder involving multi-specific SLC and ABC drug transporters, it follows that drug–metabolite interactions resulting from competition for transporters, such as those described above, can exert widespread and unpredictable effects on metabolism and signaling in patients with kidney disease6,15. As CKD progresses and uremic toxins continue to accumulate, drug–metabolite interactions involving transporters and/or DMEs can become more prevalent and more deleterious, potentially contributing to new and/or increased drug toxicities65. For example, competition between paracetamol and p-cresol for liver sulfotransferases not only leads to increased glucuronidation of paracetamol, instead of its sulfation, but also leads to the production of toxic metabolites of this analgesic owing to shunting of paracetamol to a different metabolic enzyme65,136. In addition, sulfated conjugates of morinidazole are dramatically increased in the plasma of patients with CKD, leading to increased drug exposure, perhaps because this drug competes with uremic toxins for OAT3mediated uptake in the proximal tubule137. Similarly, administration of NSAIDs that inhibit OAT1-mediated and OAT3-mediated transport (namely, ketoprofen and diclofenac)138,139 markedly decreases the renal clearance of indoxyl sulfate. The resulting increase in systemic exposure to this uremic toxin140 could have important clinical ramifications.
Furthermore, the OAT1 and OAT3 metabolic networks extrapolated from omics data obtained in healthy individuals suggest that OAT-transported drugs affect a broad range of metabolic pathways beyond those involved in transporter-level drug–metabolite competition34–36. Although similar metabolic networks have not yet been constructed to reflect the changes resulting from uremia, a similarly wide range of pathways is likely to be affected by OAT-transported drugs5. A detailed catalogue of all potential drug–metabolite interactions occurring as a result of competition between drugs and uremic solutes at the transporter level is greatly needed to provide a sound basis for drug dosing in the setting of renal disease6.
The reader should also keep in mind that transporters are important therapeutic targets and that almost one-third of the top 200 most frequently prescribed drugs depend upon active secretion in the proximal tubule of the kidney for elimination141. Thus, although drug–drug interactions occur even in patients without renal impairment, they are likely to be even more important in patients with CKD owing to the presence of high levels of uremic solutes. Many uremic solutes also compete with administered drugs and with each other for transporters and DMEs65. Hence, drug–drug interactions, drug–uremic solute interactions and uremic solute–uremic solute interactions are likely to be very complicated6.
Remote signaling by uremic solutes
Several uremic solutes that are toxic to cells and tissues at high concentrations also directly affect or participate in signaling pathways at low concentrations, some of which are discussed below. However, we emphasize that much of the data come from experiments performed using solute concentrations that differ from those occurring at the relevant tissue site in uremia. Much more data need to be obtained on local levels of uremic toxins, as well as the cellular expression and function of their putative targets, to assess which particular pathways are likely to be affected in patients with renal disease. Nevertheless, the available reports give an indication of potential mechanisms by which uremic toxins can modulate a wide range of metabolic pathways and signaling events.
Kynurenic acid is a ligand of both AHR and GPCR 35 (GPR35)142–144 and is important in CNS signaling both under physiological conditions and in disorders such as depression, schizophrenia and Alzheimer disease144–146. Kynurenic acid is synthesized via the kynurenine pathway (through which >95% of dietary tryptophan is metabolized147) from kynurenine, an intermediate of tryptophan metabolism, by the gut microflora. In the CNS, kynurenic acid is thought to act as an antagonist of N-methyl-d-aspartate, kainite and α-amino-3-hydroxy5-methyl-4-isoxazolepropionic acid receptors148, as well as the α7-nicotinic receptor146.
Outside the CNS, kynurenic acid affects a number of metabolic and immune system pathways. For example, this uremic toxin modulates immune cell function149,150 and attenuates the increases in expression and production of tumour necrosis factor induced by treatment with lipopolysaccharide145. Kynurenic acid-mediated GPR35 signaling also regulates energy metabolism by stimulating lipid metabolism, thermogenesis and the expression of anti-inflammatory genes in adipose tissue151. Kynurenic acid also suppresses weight gain and improves glucose tolerance in animals fed a high-fat diet151. Some of these findings might be relevant to the uremic syndrome.
Another uremic toxin, CMPF (which is transported into pancreatic islet cells by OAT3) is involved in insulin secretion, and this mechanism seems to become aberrant in the setting of gestational diabetes33. Administration of CMPF to mice fed a high-fat diet not only prevented insulin resistance but also inhibited the activity of acetyl-CoA carboxylase and induced long-term reductions in the expression of several genes (namely, Acaca, Acacb, Srebp1 and Cyp7a1) involved in the regulation of lipogenesis and glucose metabolism152. These gene expression changes were correlated with increased levels of fibroblast growth factor 21 and alterations in the expression of components of the mechanistic target of rapamycin (mTOR) pathway152.
Similar to indoxyl sulfate, p-cresol sulfate can affect many intracellular kinase pathways, including those involving cAMP and MAPKs, after being transported into cells153,154. For example, osteoblasts exposed to p-cresol sulfate showed evidence of intracellular oxidative stress, including an increase in ROS that could be inhibited by probenecid (presumably reflecting the involvement of an OAT) and activation of the JNK or p38 MAPK pathways leading to apoptotic cell death153. Likewise, treatment of human renal proximal tubular epithelial cells with indoxyl sulfate not only induced ROS production but also activated the MAPK, NF-κB p65 and RACα serine/threonine-protein kinase (AKT) signaling pathways155.
Polyamines that accumulate in patients with CKD, such as spermidine, spermine and putrescine, are implicated in many cellular pathways including apoptosis; cell division, differentiation and proliferation; and signal transduction156,157. For example, activation of eukaryotic translation initiation factor 5A1 (eIF5A1) requires the unusual post-translational modification of a lysine residue at position 50 to hypusine, an essential mechanism for control of cell proliferation that is dependent upon spermidine158. Binding of polyamines such as spermine to inwardly rectifying K+ (Kir) channels also modulates cell proliferation159.
Uremic solutes potentially affect a wide array of other signaling pathways. For example, redox potential is altered by urate, as are signaling events160–162. In vascular smooth muscle cells, urate activates p38 MAPKs, ERK1 and ERK2 (also known as MAPK3 and MAPK1, respectively), as well as the transcription factors NF-κB and activator protein 1 (AP-1), leading to increased cellular proliferation and a pro-inflammatory phenotype163. Pathways involving nitric oxide are affected by asymmetric and symmetric dimethylarginines as well as some guanidine compounds164–166. For example, asymmetric dimethylarginine is an inhibitor of nitric oxide synthase and directly competes with arginine for the binding site of this enzyme, leading to a reduction in nitric oxide formation. Symmetric dimethylarginine perturbs nitric oxide concentration by inhibiting arginine entry into cells via amino acid transporters167.
Another uremic toxin, 4-ethylphenyl sulfate (a benzoate-derived compound), is one of several gutderived uremic toxins that show substantially increased levels in plasma, liver, heart and kidneys in a rat model of CKD168. Interestingly, this compound accumulates in plasma in a mouse model of autism (which could be reversed by inoculation with the gut bacterium Bacteroides fragilis)169; moreover, administration of 4-ethylphenyl sulfate induces anxiety-like behaviour in wild-type mice169. This uremic toxin is a component of the urine of male rats, and both 4-ethylphenyl sulfate and its unconjugated form, 4-ethylphenol, are thought to participate in pheromonal communication170. Taken together, these data suggest that this uremic toxin is involved in inter-organism and inter-organ remote sensing and signaling.
Clinical implications
The remote sensing and signaling hypothesis addresses the roles of drug transporters and DMEs in inter-organ and inter-organism communication via small organic molecules in health and disease. These small organic molecules include metabolites, signaling molecules, gut microbiota products, antioxidants, vitamins and dietary components1,5,13–15. Within the conceptual framework of the remote sensing and signaling hypothesis, uremia can be considered a disorder of inter-organism and inter-organ communication mediated by multi-specific SLC and ABC transporters that are involved in normal metabolism and signaling as well as in the restoration of homeostasis.
Ultimately, this conceptual framework might lead to the development of a formal biological understanding of uremia at a systems biology level and new approaches to the treatment of uremia. For example, inter-organism communication might be modulated by treatment with prebiotics and probiotics, which affect the gut microbiota171 and would presumably reduce levels of gut-microbiota-derived uremic toxins. Another approach is to perturb inter-organism communication by oral administration of agents that absorb uremic toxins, such as AST-120172. Moreover, given that uremia can be considered a disorder of specific metabolic and signaling pathways, these pathways (as is the case for other complex metabolic diseases) might be amenable not only to nutritional therapy but also to specific targeting by drugs that are already approved for use in other settings. Alone or in combination, these strategies could, perhaps quite rapidly, be translated into novel approaches in the clinic.
Another potential approach involves modulating the expression or function of transporters located in the proximal tubule, perhaps through drugs that affect key transcriptional regulators of these transporters, with the goal of enhancing the renal excretion of uremic toxins. In this regard, it is worth noting that OATs, OCTs and other transporters seem to be regulated by nuclear receptors and transcription factors, including the hepatocyte nuclear factors HNF1α and HNF4α51,52. Strategies that lower the levels of uremic toxins in various tissues and body fluid compartments by targeting influx (SLC) or efflux (mostly ABC) transporters are expected to alter local or systemic toxin levels and might help to ameliorate uremic syndrome. Some anionic uremic solutes (such as hippurate and benzoate) contribute to chronic metabolic acidosis in patients with CKD, and diminishing blood levels of these compounds could attenuate this symptom.
Several published studies indicate that disproportionately high tubular secretion, including of OAT-transported uremic toxins, probably occurs in patients with impaired renal function3,173–176. The disproportionately active residual function of the proximal tubule indicates its probable importance in regulating uremic toxin levels and thereby uremia-induced metabolism. Therefore, residual function (and particularly OAT activity) in the proximal tubule might be especially important in patients with declining kidney function in CKD — not only for eliminating uremic toxins and drugs but also for regulating systemic metabolism, possibly via remote communication between the proximal tubule and other organs and/or the gut microbiota. We speculate that there could be a period early in the development of uremic syndrome when local and systemic aberrations are subtle and affect a limited number of disparate metabolic and signaling pathways. This situation might be analogous to metabolic syndrome, which is a potentially reversible precursor to the development of type 2 diabetes mellitus. Perhaps early uremic syndrome might be similarly amenable to drug-based or dietary interventions that selectively target specific metabolic and signaling pathways. However, as uremia progresses, similar or different but overlapping sets of molecules might cause overt cellular toxicity and further progression of CKD and pleiotropically affect many aspects of metabolism and signaling. In this situation, approaches that target multiple pathways might be necessary.
Among patients with similar levels of renal dysfunction, who will get uremic syndrome and which tissues will primarily be affected is difficult to predict. This difficulty might, in substantial part, be a function of the specific SLC influx and ABC efflux transporters expressed in susceptible tissue, their levels of expression at the cell surface and the functional capacity of these transporters. Thus, we might expect that SNPs or combinations of SNPs that determine either the level of expression (non-coding SNPs) or the function (coding SNPs) of a transporter might adversely affect the handling of uremic toxins177,178. Patients with ESRD on dialysis bearing SNPs in ABCG2 (Q126X, rs72552713; and Q141K, rs22331142) that impair the function of the corresponding protein, a renal and intestinal transporter, not only had significantly higher serum levels of urate than patients with normal versions of the protein but also required earlier initiation of dialysis39. A single SNP in OAT1 has also been associated with CKD115. As net levels of uremic toxins depend on influx as well as efflux, the function of both influx and efflux transporters needs to be considered178.
The links between these drug transporters and phase 1 and phase 2 DMEs provide another opportunity to alter the levels of toxic uremic solutes, many of which are substrates for DMEs. The function and/or expression of phase 1 or phase 2 DMEs could potentially be modulated by enzyme activators and/or inhibitors or nuclear receptor agonists that regulate DME expression and thereby affect local or systemic levels of toxic uremic solutes. Given the plethora of DMEs, which are likely to act on dozens of clinically important uremic solutes, many potential targets can be explored.
Finally, it is obvious from the complexity of the metabolic networks constructed around OAT1 and other transporters34,35 that uremic toxins might not simply compete directly for transport with drugs and physiologically beneficial metabolites. Rather, the competition between drugs, key endogenous metabolites and uremic toxins could lead to unexpected and complex cascade effects. These will be important to define in future studies on the role of OATs and other uremic toxin transporters in regulating metabolic networks in both healthy states and in CKD.
Conclusions
A characteristic feature of kidney failure and the uremic syndrome is the accumulation of protein-bound and free small molecules in plasma. Many are endogenous compounds derived from cellular metabolism but others are derived from the gut microbiota, which also undergoes dramatic changes in response to the host’s loss of kidney function. Dysbiosis of the gut microbiota leads to increased production of existing as well as new uremic solutes and toxins. ADME of many of these compounds is mediated by a complex network of SLC and ABC transporters and DMEs. Consequently, dynamic changes occur in transporter-mediated and DME-mediated small-molecule remote communication between the gut microbiota and the failing kidney (and possibly other organs). In other words, the reduced elimination and plasma accumulation of these small molecules, driven by the progressively failing kidney, as well as their continued generation by the gut microbiota, reset the ‘normal’ levels of these substrates for the network of SLC and ABC transporters and DMEs involved in their ADME. This resetting of the system (which probably involves organs other than the failing kidney) occurs in addition to the resetting of homeostasis associated with the loss of other renal functions. These other renal functions might or might not directly involve transporters or DMEs but could still be indirectly related to ADME via growth factor, cytokine and neuroendocrine pathways.
Moreover, many gut-microbiota-derived uremic solutes also function as signaling molecules. These signaling molecules regulate or modulate the expression of genes encoding ADME-related proteins via their effects on nuclear receptors, GPCRs, kinases and redox status. Accordingly, these small molecules transmit critical situational (homeostatic) information back and forth between cells (including between organelles), tissues and organs (that is, the intestine, liver, kidney and brain) and between organisms (namely, the host and gut microbiota). Thus, from the perspective of the remote sensing and signaling hypothesis, the failing kidney and worsening uremic syndrome profoundly influence both inter-organism and inter-organ small-molecule remote communication. The many changes in expression and/or function of multi-specific transporters and DMEs across tissues in the setting of CKD suggest that alterations in this inter-organ and inter-organism communication network represent an attempt to preserve homeostasis in this pathophysiological setting. As this network of genes and proteins is also involved in drug ADME, perturbations of remote sensing and signaling are likely to be further exacerbated by the many pharmacological agents administered to patients with CKD owing to increased competition between these drugs and uremic solutes for transporters that are part of the ADME network. Understanding the uremic syndrome from the systems biology perspective of the remote sensing and signaling hypothesis could have important clinical ramifications. For example, implicit in this framework is the idea that early in the development of kidney failure and uremic syndrome, the number of ADME pathways affected by alterations in remote communication and sensing might be limited and therefore could be specifically targeted to forestall full-blown uremia.
Key points.
The uremic syndrome (also known as uraemic syndrome) associated with chronic kidney disease (CKD) is characterized by complex local and systemic derangements in metabolism and signaling.
CKD involves aberrant inter-organ (gut–liver–kidney–brain) and inter-organism (host–gut microbiota) remote communication via small molecules, including uremic solutes, metabolites and signaling molecules.
Aspects of uremic syndrome can be considered disordered remote sensing and signaling mediated by a multi-organ network of solute carrier (SLC) and ATP-binding cassette (ABC) transporters and drug-metabolizing enzymes (DMEs).
The remote sensing and signaling hypothesis provides a systems biology framework for understanding the role of these transporters and DMEs in small-molecule-mediated inter-organ and inter-organism communication.
Transported uremic solutes (including gut-microbiota-derived indoxyl sulfate) can affect multiple signaling pathways.
Viewing CKD and uremic syndrome through the lens of the remote sensing and signaling hypothesis provides fresh perspectives on the metabolic derangements of CKD that might lead to novel therapies.
Acknowledgements
The authors’ work referred to in this Review was partly supported by US National Institutes of Health grants DK109392 and HD090259 (U54) to S.K.N.
Footnotes
Competing interests
The authors declare no competing interests.
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Reviewer information
Nature Reviews Nephrology thanks T. D. Nolin and the other anonymous reviewer(s) for their contribution to the peer review of this work.
REFERENCS
- 1.Wu W, Dnyanmote AV & Nigam SK Remote communication through solute carriers and ATP binding cassette drug transporter pathways: an update on the remote sensing and signaling hypothesis. Mol. Pharmacol 79, 795–805 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Liu X & Dai C Advances in understanding and management of residual renal function in patients with chronic kidney disease. Kidney Dis 2, 187–196 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lowenstein J & Grantham JJ Residual renal function: a paradigm shift. Kidney Int 91, 561–565 (2017). [DOI] [PubMed] [Google Scholar]
- 4.Shafi T, Mullangi S, Toth-Manikowski SM, Hwang S & Michels WM Residual kidney function: implications in the era of personalized medicine. Semin. Dial 30, 241–245 (2017). [DOI] [PubMed] [Google Scholar]
- 5.Nigam SK What do drug transporters really do? Nat. Rev. Drug Discov 14, 29–44 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nigam SK et al. Handling of drugs, metabolites, and uremic toxins by kidney proximal tubule drug transporters. Clin. J. Am. Soc. Nephrol 10, 2039–2049 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wikoff WR, Nagle MA, Kouznetsova VL, Tsigelny IF & Nigam SK Untargeted metabolomics identifies enterobiome metabolites and putative uremic toxins as substrates of organic anion transporter 1 (Oat1). J. Proteome Res 10, 2842–2851 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wu W, Bush KT & Nigam SK Key role for the organic anion transporters, OAT1 and OAT3, in the in vivo handling of uremic toxins and solutes. Sci. Rep 7, 4939 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kim SH, Yu MA, Ryu ES, Jang YH & Kang DH Indoxyl sulfate-induced epithelial-to-mesenchymal transition and apoptosis of renal tubular cells as novel mechanisms of progression of renal disease. Lab. Invest 92, 488–498 (2012). [DOI] [PubMed] [Google Scholar]
- 10.Sun CY, Chang SC & Wu MS Uremic toxins induce kidney fibrosis by activating intrarenal renin-angiotensin-aldosterone system associated epithelial-to-mesenchymal transition. PLOS ONE 7, e34026 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bolati D, Shimizu H, Higashiyama Y, Nishijima F & Niwa T Indoxyl sulfate induces epithelial-tomesenchymal transition in rat kidneys and human proximal tubular cells. Am. J. Nephrol 34, 318–323 (2011). [DOI] [PubMed] [Google Scholar]
- 12.van den Brand JA et al. Uremic solutes in chronic kidney disease and their role in progression. PLOS ONE 11, e0168117 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nigam SK The SLC22 transporter family: a paradigm for the impact of drug transporters on metabolic pathways, signaling, and disease. Annu. Rev. Pharmacol. Toxicol 58, 663–687 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ahn SY & Nigam SK Toward a systems level understanding of organic anion and other multispecific drug transporters: a remote sensing and signaling hypothesis. Mol. Pharmacol 76, 481–490 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nigam SK et al. The organic anion transporter (OAT) family: a systems biology perspective. Physiol. Rev 95, 83–123 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kaler G et al. Structural variation governs substrate specificity for organic anion transporter (OAT) homologs. Potential remote sensing by OAT family members. J. Biol. Chem 282, 23841–23853 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pavlova A et al. Developmentally regulated expression of organic ion transporters NKT (OAT1), OCT1, NLT (OAT2), and ROCT. Am. J. Physiol. Renal Physiol 278, F635–F643 (2000). [DOI] [PubMed] [Google Scholar]
- 18.Kaler G et al. Olfactory mucosa-expressed organic anion transporter, Oat6, manifests high affinity interactions with odorant organic anions. Biochem. Biophys. Res. Commun 351, 872–876 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Monte JC, Nagle MA, Eraly SA & Nigam SK Identification of a novel murine organic anion transporter family member, OAT6, expressed in olfactory mucosa. Biochem. Biophys. Res. Commun 323, 429–436 (2004). [DOI] [PubMed] [Google Scholar]
- 20.Bush KT, Wu W, Lun C & Nigam SK The drug transporter OAT3 (SLC22A8) and endogenous metabolite communication via the gut-liver-kidney axis. J. Biol. Chem 292, 15789–15803 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nigam SK & Bhatnagar V The systems biology of uric acid transporters: the role of remote sensing and signaling. Curr. Opin. Nephrol. Hypertens 27, 305–313 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Li T & Apte U Bile acid metabolism and signaling in cholestasis, inflammation, and cancer. Adv. Pharmacol 74, 263–302 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chiang JY Recent advances in understanding bile acid homeostasis. F1000Res 6, 2029 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Molinaro A, Wahlstrom A & Marschall HU Role of bile acids in metabolic control. Trends Endocrinol. Metab 29, 31–41 (2018). [DOI] [PubMed] [Google Scholar]
- 25.Evenepoel P, Poesen R & Meijers B The gut–kidney axis. Pediatr. Nephrol 32, 2005–2014 (2017). [DOI] [PubMed] [Google Scholar]
- 26.Wing MR, Patel SS, Ramezani A & Raj DS Gut microbiome in chronic kidney disease. Exp. Physiol 101, 471–477 (2016). [DOI] [PubMed] [Google Scholar]
- 27.Spector R, Robert Snodgrass S & Johanson CE A balanced view of the cerebrospinal fluid composition and functions: focus on adult humans. Exp. Neurol 273, 57–68 (2015). [DOI] [PubMed] [Google Scholar]
- 28.Stieger B & Gao B Drug transporters in the central nervous system. Clin. Pharmacokinet 54, 225–242 (2015). [DOI] [PubMed] [Google Scholar]
- 29.Jabbari B & Vaziri ND The nature, consequences, and management of neurological disorders in chronic kidney disease. Hemodial. Int 22, 150–160 (2018). [DOI] [PubMed] [Google Scholar]
- 30.Seifter JL & Samuels MA Uremic encephalopathy and other brain disorders associated with renal failure. Semin. Neurol 31, 139–143 (2011). [DOI] [PubMed] [Google Scholar]
- 31.Ebah LM et al. Subcutaneous interstitial pressure and volume characteristics in renal impairment associated with edema. Kidney Int 84, 980–988 (2013). [DOI] [PubMed] [Google Scholar]
- 32.Mayerl S et al. Transporters MCT8 and OATP1C1 maintain murine brain thyroid hormone homeostasis. J. Clin. Invest 124, 1987–1999 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Prentice KJ et al. The furan fatty acid metabolite CMPF is elevated in diabetes and induces beta cell dysfunction. Cell Metab 19, 653–666 (2014). [DOI] [PubMed] [Google Scholar]
- 34.Ahn SY et al. Linkage of organic anion transporter-1 to metabolic pathways through integrated “omics”driven network and functional analysis. J. Biol. Chem 286, 31522–31531 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Liu HC et al. An organic anion transporter 1 (OAT1)centered metabolic network. J. Biol. Chem 291, 19474–19486 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wu W et al. Multispecific drug transporter Slc22a8 (Oat3) regulates multiple metabolic and signaling pathways. Drug Metab. Dispos 41, 1825–1834 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Liu B et al. Metabolic enzyme system and transport pathways in chronic kidney diseases. Curr. Drug Metab 19, 568–576 (2018). [DOI] [PubMed] [Google Scholar]
- 38.Takada T et al. Identification of ABCG2 as an exporter of uremic toxin indoxyl sulfate in mice and as a crucial factor influencing CKD progression. Sci. Rep 8, 11147 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Matsuo H et al. Hyperuricemia in acute gastroenteritis is caused by decreased urate excretion via ABCG2. Sci. Rep 6, 31003 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bhatnagar V et al. Analysis of ABCG2 and other urate transporters in uric acid homeostasis in chronic kidney disease: potential role of remote sensing and signaling. Clin. Kidney J 9, 444–453 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Jing J et al. Genetics of serum urate concentrations and gout in a high-risk population, patients with chronic kidney disease. Sci. Rep 8, 13184 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Takada T et al. ABCG2 dysfunction increases serum uric acid by decreased intestinal urate excretion. Nucleosides Nucleotides Nucleic Acids 33, 275–281 (2014). [DOI] [PubMed] [Google Scholar]
- 43.Yano H, Tamura Y, Kobayashi K, Tanemoto M & Uchida S Uric acid transporter ABCG2 is increased in the intestine of the 5/6 nephrectomy rat model of chronic kidney disease. Clin. Exp. Nephrol 18, 50–55 (2014). [DOI] [PubMed] [Google Scholar]
- 44.Pahl MV & Vaziri ND The chronic kidney disease–colonic axis. Semin. Dial 28, 459–463 (2015). [DOI] [PubMed] [Google Scholar]
- 45.Velenosi TJ, Fu AY, Luo S, Wang H & Urquhart BL Down-regulation of hepatic CYP3A and CYP2C mediated metabolism in rats with moderate chronic kidney disease. Drug Metab. Dispos 40, 1508–1514 (2012). [DOI] [PubMed] [Google Scholar]
- 46.Naud J, Nolin TD, Leblond FA & Pichette V Current understanding of drug disposition in kidney disease. J. Clin. Pharmacol 52, 10S–22S (2012). [DOI] [PubMed] [Google Scholar]
- 47.Philips BJ, Lane K, Dixon J & Macphee I The effects of acute renal failure on drug metabolism. Expert Opin. Drug Metab. Toxicol 10, 11–23 (2014). [DOI] [PubMed] [Google Scholar]
- 48.Ladda MA & Goralski KB The effects of CKD on cytochrome P450-mediated drug metabolism. Adv. Chronic Kidney Dis 23, 67–75 (2016). [DOI] [PubMed] [Google Scholar]
- 49.Michaud J et al. Effect of hemodialysis on hepatic cytochrome P450 functional expression. J. Pharmacol. Sci 108, 157–163 (2008). [DOI] [PubMed] [Google Scholar]
- 50.Ma X, Idle JR & Gonzalez FJ The pregnane X receptor: from bench to bedside. Expert Opin. Drug Metab. Toxicol 4, 895–908 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Martovetsky G, Bush KT & Nigam SK Kidney versus liver specification of SLC and ABC drug transporters, tight junction molecules, and biomarkers. Drug Metab. Dispos 44, 1050–1060 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Martovetsky G, Tee JB & Nigam SK Hepatocyte nuclear factors 4α and 1α regulate kidney developmental expression of drug-metabolizing enzymes and drug transporters. Mol. Pharmacol 84, 808–823 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Staudinger JL et al. The nuclear receptor PXR is a lithocholic acid sensor that protects against liver toxicity. Proc. Natl Acad. Sci. USA 98, 3369–3374 (2001). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Xie W et al. Humanized xenobiotic response in mice expressing nuclear receptor SXR. Nature 406, 435–439 (2000). [DOI] [PubMed] [Google Scholar]
- 55.Santana Machado T et al. Indoxyl sulfate upregulates liver P-glycoprotein expression and activity through aryl hydrocarbon receptor signaling. J. Am. Soc. Nephrol 29, 906–918 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Xu C, Li CY & Kong AN Induction of phase I, II and III drug metabolism/transport by xenobiotics. Arch. Pharm. Res 28, 249–268 (2005). [DOI] [PubMed] [Google Scholar]
- 57.Leong SC & Sirich TL Indoxyl sulfate — review of toxicity and therapeutic strategies. Toxins 8, E358 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Camacho O et al. Effect of a sustained difference in hemodialytic clearance on the plasma levels of p-cresol sulfate and indoxyl sulfate. Nephrol. Dial. Transplant 31, 1335–1341 (2016). [DOI] [PubMed] [Google Scholar]
- 59.Poesen R et al. The influence of prebiotic arabinoxylan oligosaccharides on microbiota derived uremic retention solutes in patients with chronic kidney disease: a randomized controlled trial. PLOS ONE 11, e0153893 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Chiavaroli L, Mirrahimi A, Sievenpiper JL, Jenkins DJ & Darling PB Dietary fiber effects in chronic kidney disease: a systematic review and meta-analysis of controlled feeding trials. Eur. J. Clin. Nutr 69, 761–768 (2015). [DOI] [PubMed] [Google Scholar]
- 61.Cha RH et al. A randomized, controlled trial of oral intestinal sorbent AST-120 on renal function deterioration in patients with advanced renal dysfunction. Clin. J. Am. Soc. Nephrol 11, 559–567 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Rossi M et al. Synbiotics easing renal failure by improving gut microbiology (SYNERGY): a randomized trial. Clin. J. Am. Soc. Nephrol 11, 223–231 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Schulman G et al. Randomized placebo-controlled EPPIC trials of AST-120 in CKD. J. Am. Soc. Nephrol 26, 1732–1746 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Sirich TL, Plummer NS, Gardner CD, Hostetter TH & Meyer TW Effect of increasing dietary fiber on plasma levels of colon-derived solutes in hemodialysis patients. Clin. J. Am. Soc. Nephrol 9, 1603–1610 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Prokopienko AJ & Nolin TD Microbiota-derived uremic retention solutes: perpetrators of altered nonrenal drug clearance in kidney disease. Expert Rev. Clin. Pharmacol 11, 71–82 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Vanholder R, Schepers E, Pletinck A, Nagler EV & Glorieux G The uremic toxicity of indoxyl sulfate and p-cresyl sulfate: a systematic review. J. Am. Soc. Nephrol 25, 1897–1907 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Kaminski T, Michalowska M & Pawlak D Aryl hydrocarbon receptor (AhR) and its endogenous agonist indoxyl sulfate in chronic kidney disease. Postepy Hig. Med. Dosw 71, 624–632 (2017). [DOI] [PubMed] [Google Scholar]
- 68.Gao H & Liu S Role of uremic toxin indoxyl sulfate in the progression of cardiovascular disease. Life Sci 185, 23–29 (2017). [DOI] [PubMed] [Google Scholar]
- 69.Liu H, Narayanan R, Hoffmann M & Surapaneni S The uremic toxin indoxyl-3-sulfate induces CYP1A2 in primary human hepatocytes. Drug Metab. Lett 10, 195–199 (2016). [DOI] [PubMed] [Google Scholar]
- 70.Wu Y, Han X, Wang L, Diao Z & Liu W Indoxyl sulfate promotes vascular smooth muscle cell calcification via the JNK/Pit-1 pathway. Ren. Fail 38, 1702–1710 (2016). [DOI] [PubMed] [Google Scholar]
- 71.Muteliefu G et al. Indoxyl sulfate promotes vascular smooth muscle cell senescence with upregulation ofp53, p21, and prelamin A through oxidative stress. Am. J. Physiol. Cell Physiol 303, C126–C134 (2012). [DOI] [PubMed] [Google Scholar]
- 72.Mozar A et al. Uremic toxin indoxyl sulfate inhibits human vascular smooth muscle cell proliferation. Ther. Apher. Dial 15, 135–139 (2011). [DOI] [PubMed] [Google Scholar]
- 73.Shimizu H, Hirose Y, Nishijima F, Tsubakihara Y & Miyazaki H ROS and PDGF-β [corrected] receptors are critically involved in indoxyl sulfate actions that promote vascular smooth muscle cell proliferation and migration. Am. J. Physiol. Cell Physiol 297, C389–C396 (2009). [DOI] [PubMed] [Google Scholar]
- 74.Yamamoto H et al. Indoxyl sulfate stimulates proliferation of rat vascular smooth muscle cells. Kidney Int 69, 1780–1785 (2006). [DOI] [PubMed] [Google Scholar]
- 75.Mair RD, Sirich TL & Meyer TW Uremic toxin clearance and cardiovascular toxicities. Toxins 10, E226 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Schroeder JC et al. The uremic toxin 3-indoxyl sulfate is a potent endogenous agonist for the human aryl hydrocarbon receptor. Biochemistry 49, 393–400 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Esser C & Rannug A The aryl hydrocarbon receptor in barrier organ physiology, immunology, and toxicology. Pharmacol. Rev 67, 259–279 (2015). [DOI] [PubMed] [Google Scholar]
- 78.Roman AC, Carvajal-Gonzalez JM, Merino JM, Mulero-Navarro S & Fernandez-Salguero PM The aryl hydrocarbon receptor in the crossroad of signaling networks with therapeutic value. Pharmacol. Ther 185, 50–63 (2017). [DOI] [PubMed] [Google Scholar]
- 79.Wakamatsu T et al. Indoxyl sulfate promotes macrophage IL-1β production by activating aryl hydrocarbon receptor/NF-κ/MAPK cascades, but the NLRP3 inflammasome was not activated. Toxins 10, E124 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Wanchai K et al. Probiotic Lactobacillus paracasei HII01 protects rats against obese-insulin resistance-induced kidney injury and impaired renal organic anion transporter 3 function. Clin. Sci 132, 1545–1563 (2018). [DOI] [PubMed] [Google Scholar]
- 81.Czuba LC, Hillgren KM & Swaan PW Post-translational modifications of transporters. Pharmacol. Ther 192, 88–99 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Murray M & Zhou F Trafficking and other regulatory mechanisms for organic anion transporting polypeptides and organic anion transporters that modulate cellular drug and xenobiotic influx and that are dysregulated in disease. Br. J. Pharmacol 174, 1908–1924 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Xu D & You G Loops and layers of post-translational modifications of drug transporters. Adv. Drug Deliv. Rev 116, 37–44 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Hong M et al. Human organic anion transporter hOAT1 forms homooligomers. J. Biol. Chem 280, 32285–32290 (2005). [DOI] [PubMed] [Google Scholar]
- 85.Miyazaki H et al. Modulation of renal apical organic anion transporter 4 function by two PDZ domain-containing proteins. J. Am. Soc. Nephrol 16, 3498–3506 (2005). [DOI] [PubMed] [Google Scholar]
- 86.Endou H & Anzai N Urate transport across the apical membrane of renal proximal tubules. Nucleosides Nucleotides Nucleic Acids 27, 578–584 (2008). [DOI] [PubMed] [Google Scholar]
- 87.Zhang Q, Pan Z & You G Regulation of human organic anion transporter 4 by protein kinase C and NHERF-1: altering the endocytosis of the transporter. Pharm. Res 27, 589–596 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Xue P, Crum CM & Thibodeau PH Regulation of ABCC6 trafficking and stability by a conserved C-terminal PDZ-like sequence. PLOS ONE 9, e97360 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Schaletzki Y et al. Several adaptor proteins promote intracellular localisation of the transporter MRP4/ ABCC4 in platelets and haematopoietic cells. Thromb. Haemost 117, 105–115 (2017). [DOI] [PubMed] [Google Scholar]
- 90.Ferreira C et al. The scaffold protein PDZK1 modulates expression and function of the organic anion transporting polypeptide 2B1. Eur. J. Pharm. Sci 120, 181–190 (2018). [DOI] [PubMed] [Google Scholar]
- 91.Venot Q et al. A PDZ-like motif in the biliary transporter ABCB4 interacts with the scaffold protein EBP50 and regulates ABCB4 cell surface expression. PLOS ONE 11, e0146962 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Vanholder R et al. The role of EUTox in uremic toxin research. Semin. Dial 22, 323–328 (2009). [DOI] [PubMed] [Google Scholar]
- 93.Zhao YY Metabolomics in chronic kidney disease. Clin. Chim. Acta 422, 59–69 (2013). [DOI] [PubMed] [Google Scholar]
- 94.Lau WL, Savoj J, Nakata MB & Vaziri ND Altered microbiome in chronic kidney disease: systemic effects of gut-derived uremic toxins. Clin. Sci 132, 509–522 (2018). [DOI] [PubMed] [Google Scholar]
- 95.Mahmoodpoor F, Rahbar Saadat Y, Barzegari A, Ardalan M & Zununi Vahed S The impact of gut microbiota on kidney function and pathogenesis. Biomed. Pharmacother 93, 412–419 (2017). [DOI] [PubMed] [Google Scholar]
- 96.Vogt SL, Pena-Diaz J & Finlay BB Chemical communication in the gut: effects of microbiotagenerated metabolites on gastrointestinal bacterial pathogens. Anaerobe 34, 106–115 (2015). [DOI] [PubMed] [Google Scholar]
- 97.Kennedy PJ, Cryan JF, Dinan TG & Clarke G Kynurenine pathway metabolism and the microbiota-gut-brain axis. Neuropharmacology 112, 399–412 (2017). [DOI] [PubMed] [Google Scholar]
- 98.Xu L et al. Furan fatty acids — beneficial or harmful to health? Prog. Lipid Res 68, 119–137 (2017). [DOI] [PubMed] [Google Scholar]
- 99.El Ridi R & Tallima H Physiological functions and pathogenic potential of uric acid: a review. J. Adv. Res 8, 487–493 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Bae DH, Lane DJR, Jansson PJ & Richardson DR The old and new biochemistry of polyamines. Biochim. Biophys. Acta Gen. Subj 1862, 2053–2068 (2018). [DOI] [PubMed] [Google Scholar]
- 101.Lopez-Nieto CE et al. Molecular cloning and characterization of NKT, a gene product related to the organic cation transporter family that is almost exclusively expressed in the kidney. J. Biol. Chem 272, 6471–6478 (1997). [DOI] [PubMed] [Google Scholar]
- 102.Eraly SA et al. Decreased renal organic anion secretion and plasma accumulation of endogenous organic anions in OAT1 knock-out mice. J. Biol. Chem 281, 5072–5083 (2006). [DOI] [PubMed] [Google Scholar]
- 103.Sweeney DE et al. Functional maturation of drug transporters in the developing, neonatal, and postnatal kidney. Mol. Pharmacol 80, 147–154 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Nagle MA et al. Analysis of three-dimensional systems for developing and mature kidneys clarifies the role of OAT1 and OAT3 in antiviral handling. J. Biol. Chem 286, 243–251 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Nagle MA, Wu W, Eraly SA & Nigam SK Organic anion transport pathways in antiviral handling in choroid plexus in Oat1 (Slc22a6) and Oat3 (Slc22a8) deficient tissue. Neurosci. Lett 534, 133–138 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Truong DM, Kaler G, Khandelwal A, Swaan PW & Nigam SK Multi-level analysis of organic anion transporters 1, 3, and 6 reveals major differences in structural determinants of antiviral discrimination. J. Biol. Chem 283, 8654–8663 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Vallon V et al. Overlapping in vitro and in vivo specificities of the organic anion transporters OAT1 and OAT3 for loop and thiazide diuretics. Am. J. Physiol. Renal Physiol 294, F867–F873 (2008). [DOI] [PubMed] [Google Scholar]
- 108.VanWert AL, Gionfriddo MR & Sweet DH Organic anion transporters: discovery, pharmacology, regulation and roles in pathophysiology. Biopharm. Drug Dispos 31, 1–71 (2010). [DOI] [PubMed] [Google Scholar]
- 109.Rubino FM Toxicity of glutathione-binding metals: a review of targets and mechanisms. Toxics 3, 20–62 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Torres AM, Dnyanmote AV, Bush KT, Wu W & Nigam SK Deletion of multispecific organic anion transporter Oat1/Slc22a6 protects against mercury-induced kidney injury. J. Biol. Chem 286, 26391–26395 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Zalups RK Molecular interactions with mercury in the kidney. Pharmacol. Rev 52, 113–143 (2000). [PubMed] [Google Scholar]
- 112.Wikoff WR et al. Metabolomics analysis reveals large effects of gut microflora on mammalian blood metabolites. Proc. Natl Acad. Sci. USA 106, 3698–3703 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Liu HC et al. Molecular properties of drugs interacting with SLC22 transporters OAT1, OAT3, OCT1, and OCT2: a machine-learning approach. J. Pharmacol. Exp. Ther 359, 215–229 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Rhee EP et al. Metabolite profiling identifies markers of uremia. J. Am. Soc. Nephrol 21, 1041–1051 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Sun CY et al. A novel SNP in the 5’ regulatory region of organic anion transporter 1 is associated with chronic kidney disease. Sci. Rep 8, 8085 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Schophuizen CM et al. Cationic uremic toxins affect human renal proximal tubule cell functioning through interaction with the organic cation transporter. Pflugers Arch 465, 1701–1714 (2013). [DOI] [PubMed] [Google Scholar]
- 117.Ahn SY, Eraly SA, Tsigelny I & Nigam SK Interaction of organic cations with organic anion transporters. J. Biol. Chem 284, 31422–31430 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Sager G, Smaglyukova N & Fuskevaag OM The role of OAT2 (SLC22A7) in the cyclic nucleotide biokinetics of human erythrocytes. J. Cell. Physiol 233, 5972–5980 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Dias GF et al. Indoxyl sulfate, a uremic toxin, stimulates reactive oxygen species production and erythrocyte cell death supposedly by an organic anion transporter 2 (OAT2) and NADPH oxidase activity-dependent pathways. Toxins 10, E280 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Toyohara T et al. SLCO4C1 transporter eliminates uremic toxins and attenuates hypertension and renal inflammation. J. Am. Soc. Nephrol 20, 2546–2555 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Subramaniam S & Fletcher C Trimethylamine N-oxide: breathe new life. Br. J. Pharmacol 175, 1344–1353 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Tang WH et al. Gut microbiota-dependent trimethylamine N-oxide (TMAO) pathway contributes to both development of renal insufficiency and mortality risk in chronic kidney disease. Circ. Res 116, 448–455 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Teft WA et al. Identification and characterization of trimethylamine-N-oxide uptake and efflux transporters. Mol. Pharm 14, 310–318 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Masuda S et al. Identification and functional characterization of a new human kidney-specific H+/ organic cation antiporter, kidney-specific multidrug and toxin extrusion 2. J. Am. Soc. Nephrol 17, 2127–2135 (2006). [DOI] [PubMed] [Google Scholar]
- 125.Motohashi H & Inui K Organic cation transporter OCTs (SLC22) and MATEs (SLC47) in the human kidney. AAPS J 15, 581–588 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Nies AT, Koepsell H, Damme K & Schwab M Organic cation transporters (OCTs, MATEs), in vitro and in vivo evidence for the importance in drug therapy. Handb. Exp. Pharmacol 201, 105–167 (2011). [DOI] [PubMed] [Google Scholar]
- 127.Sato T, Yamaguchi H, Kogawa T, Abe T & Mano N Organic anion transporting polypeptides 1B1 and 1B3 play an important role in uremic toxin handling and drug-uremic toxin interactions in the liver. J. Pharm. Pharm. Sci 17, 475–484 (2014). [DOI] [PubMed] [Google Scholar]
- 128.Katsube Y et al. Cooperative inhibitory effects of uremic toxins and other serum components on OATP1B1-mediated transport of SN-38. Cancer Chemother. Pharmacol 79, 783–789 (2017). [DOI] [PubMed] [Google Scholar]
- 129.Fu ZD, Selwyn FP, Cui JY & Klaassen CD RNA-seq profiling of intestinal expression of xenobiotic processing genes in germ-free mice. Drug Metab. Dispos 45, 1225–1238 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Selwyn FP, Cheng SL, Klaassen CD & Cui JY Regulation of hepatic drug-metabolizing enzymes in germ-free mice by conventionalization and probiotics. Drug Metab. Dispos 44, 262–274 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Selwyn FP et al. Developmental regulation of drug-processing genes in livers of germ-free mice. Toxicol. Sci 147, 84–103 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Claus SP et al. Colonization-induced host–gut microbial metabolic interaction. mBio 2, e00271–10 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Meinl W, Sczesny S, Brigelius-Flohe R, Blaut M & Glatt H Impact of gut microbiota on intestinal and hepatic levels of phase 2 xenobiotic-metabolizing enzymes in the rat. Drug Metab. Dispos 37, 1179–1186 (2009). [DOI] [PubMed] [Google Scholar]
- 134.Jansen J, Jankowski J, Gajjala PR, Wetzels JFM & Masereeuw R Disposition and clinical implications of protein-bound uremic toxins. Clin. Sci 131, 1631–1647 (2017). [DOI] [PubMed] [Google Scholar]
- 135.Manley HJ et al. Medication prescribing patterns in ambulatory haemodialysis patients: comparisons of USRDS to a large not-for-profit dialysis provider. Nephrol. Dial. Transplant 19, 1842–1848 (2004). [DOI] [PubMed] [Google Scholar]
- 136.Clayton TA, Baker D, Lindon JC, Everett JR & Nicholson JK Pharmacometabonomic identification of a significant host-microbiome metabolic interaction affecting human drug metabolism. Proc. Natl Acad. Sci. USA 106, 14728–14733 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Kong F et al. Increased plasma exposures of conjugated metabolites of morinidazole in renal failure patients: a critical role of uremic toxins. Drug Metab. Dispos 45, 593–603 (2017). [DOI] [PubMed] [Google Scholar]
- 138.Cihlar T & Ho ES Fluorescence-based assay for the interaction of small molecules with the human renal organic anion transporter 1. Anal. Biochem 283, 49–55 (2000). [DOI] [PubMed] [Google Scholar]
- 139.Khamdang S et al. Interactions of human organic anion transporters and human organic cation transporters with nonsteroidal anti-inflammatory drugs. J. Pharmacol. Exp. Ther 303, 534–539 (2002). [DOI] [PubMed] [Google Scholar]
- 140.Yu CP et al. Effects of nonsteroidal anti-inflammatory drugs on the renal excretion of indoxyl sulfate, a nephro-cardiovascular toxin, in rats. Eur. J. Pharm. Sci 101, 66–70 (2017). [DOI] [PubMed] [Google Scholar]
- 141.Morrissey KM, Stocker SL, Wittwer MB, Xu L & Giacomini KM Renal transporters in drug development. Annu. Rev. Pharmacol. Toxicol 53, 503–529 (2013). [DOI] [PubMed] [Google Scholar]
- 142.DiNatale BC et al. Kynurenic acid is a potent endogenous aryl hydrocarbon receptor ligand thatsynergistically induces interleukin-6 in the presence of inflammatory signaling. Toxicol. Sci 115, 89–97 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Wang J et al. Kynurenic acid as a ligand for orphan G protein-coupled receptor GPR35. J. Biol. Chem 281, 22021–22028 (2006). [DOI] [PubMed] [Google Scholar]
- 144.Wirthgen E, Hoeflich A, Rebl A & Gunther J Kynurenic acid: the Janus-faced role of an immunomodulatory tryptophan metabolite and its link to pathological conditions. Front. Immunol 8, 1957 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Moroni F, Cozzi A, Sili M & Mannaioni G Kynurenic acid: a metabolite with multiple actions and multiple targets in brain and periphery. J. Neural Transm 119, 133–139 (2012). [DOI] [PubMed] [Google Scholar]
- 146.Schwarcz R, Bruno JP, Muchowski PJ & Wu HQ Kynurenines in the mammalian brain: when physiology meets pathology. Nat. Rev. Neurosci 13, 465–477 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Fujigaki H, Yamamoto Y & Saito K L-Tryptophan kynurenine pathway enzymes are therapeutic target for neuropsychiatric diseases: focus on cell type differences. Neuropharmacology 112, 264–274 (2017). [DOI] [PubMed] [Google Scholar]
- 148.Schwarcz R & Stone TW The kynurenine pathway and the brain: challenges, controversies and promises. Neuropharmacology 112, 237–247 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Mandi Y & Vecsei L The kynurenine system and immunoregulation. J. Neural Transm 119, 197–209 (2012). [DOI] [PubMed] [Google Scholar]
- 150.Stone TW, Stoy N & Darlington LG An expanding range of targets for kynurenine metabolites of tryptophan. Trends Pharmacol. Sci 34, 136–143 (2013). [DOI] [PubMed] [Google Scholar]
- 151.Agudelo LZ et al. Kynurenic acid and Gpr35 regulate adipose tissue energy homeostasis and inflammation. Cell Metab 27, 378–392 (2018). [DOI] [PubMed] [Google Scholar]
- 152.Prentice KJ et al. CMPF, a metabolite formed upon prescription omega-3-acid ethyl ester supplementation, prevents and reverses steatosis. EBioMedicine 27, 200–213 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Tanaka H et al. p-Cresyl sulfate induces osteoblast dysfunction through activating JNK and p38 MAPK pathways. Bone 56, 347–354 (2013). [DOI] [PubMed] [Google Scholar]
- 154.Yang K et al. Indoxyl sulfate induces oxidative stress and hypertrophy in cardiomyocytes by inhibiting the AMPK/UCP2 signaling pathway. Toxicol. Lett 234, 110–119 (2015). [DOI] [PubMed] [Google Scholar]
- 155.Park JS, Choi HI, Bae EH, Ma SK & Kim SW Paricalcitol attenuates indoxyl sulfateinduced apoptosis through the inhibition of MAPK, Akt, and NF-kB activation in HK-2 cells. Korean J. Intern. Med 34, 146–155 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Pegg AE Functions of polyamines in mammals. J. Biol. Chem 291, 14904–14912 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Ramani D, De Bandt JP & Cynober L Aliphatic polyamines in physiology and diseases. Clin. Nutr 33, 14–22 (2014). [DOI] [PubMed] [Google Scholar]
- 158.Park MH, Nishimura K, Zanelli CF & Valentini SR Functional significance of eIF5A and its hypusine modification in eukaryotes. Amino Acids 38, 491–500 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Weiger TM & Hermann A Cell proliferation, potassium channels, polyamines and their interactions: a mini review. Amino Acids 46, 681–688 (2014). [DOI] [PubMed] [Google Scholar]
- 160.Tassone EJ et al. Uric acid impairs insulin signaling by promoting ENPP1 binding to insulin receptor in human umbilical vein endothelial cells. Front. Endocrinol 9, 98 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Spiga R et al. Uric acid is associated with inflammatory biomarkers and induces inflammation via activating the NF-κB signaling pathway in HepG2 cells. Arterioscler. Thromb. Vasc. Biol 37, 1241–1249 (2017). [DOI] [PubMed] [Google Scholar]
- 162.Liang WY et al. Uric acid promotes chemokine and adhesion molecule production in vascular endothelium via nuclear factor-κB signaling. Nutr. Metab. Cardiovasc. Dis 25, 187–194 (2015). [DOI] [PubMed] [Google Scholar]
- 163.Filiopoulos V, Hadjiyannakos D & Vlassopoulos D New insights into uric acid effects on the progression and prognosis of chronic kidney disease. Ren. Fail 34, 510–520 (2012). [DOI] [PubMed] [Google Scholar]
- 164.Kielstein JT, Fliser D & Veldink H Asymmetric dimethylarginine and symmetric dimethylarginine: axis of evil or useful alliance? Semin. Dial 22, 346–350 (2009). [DOI] [PubMed] [Google Scholar]
- 165.De Deyn PP, Vanholder R & D’Hooge R Nitric oxide in uremia: effects of several potentially toxic guanidino compounds. Kidney Int. Suppl 84, S25–S28 (2003). [DOI] [PubMed] [Google Scholar]
- 166.De Deyn PP, D’Hooge R, Van Bogaert PP & Marescau B Endogenous guanidino compounds as uremic neurotoxins. Kidney Int. Suppl 78, S77–S83 (2001). [DOI] [PubMed] [Google Scholar]
- 167.Chen JY et al. Nitric oxide bioavailability dysfunction involves in atherosclerosis. Biomed. Pharmacother 97, 423–428 (2018). [DOI] [PubMed] [Google Scholar]
- 168.Velenosi TJ et al. Untargeted plasma and tissue metabolomics in rats with chronic kidney disease given AST-120. Sci. Rep 6, 22526 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Hsiao EY et al. Microbiota modulate behavioral and physiological abnormalities associated with neurodevelopmental disorders. Cell 155, 1451–1463 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Lafaye A et al. Profiling of sulfoconjugates in urine by using precursor ion and neutral loss scans in tandem mass spectrometry. Application to the investigation of heavy metal toxicity in rats. J. Mass Spectrom 39, 655–664 (2004). [DOI] [PubMed] [Google Scholar]
- 171.John GK et al. Dietary alteration of the gut microbiome and its impact on weight and fat mass: a systematic review and meta-analysis. Genes 9, E167 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Yamaguchi J, Tanaka T & Inagi R Effect of AST-120 in chronic kidney disease treatment: still a controversy? Nephron 135, 201–206 (2017). [DOI] [PubMed] [Google Scholar]
- 173.Masereeuw R et al. The kidney and uremic toxin removal: glomerulus or tubule? Semin. Nephrol 34, 191–208 (2014). [DOI] [PubMed] [Google Scholar]
- 174.Lowenstein J & Grantham JJ The rebirth of interest in renal tubular function. Am. J. Physiol. Renal Physiol 310, F1351–F1355 (2016). [DOI] [PubMed] [Google Scholar]
- 175.Wang K & Kestenbaum B Proximal tubular secretory clearance: a neglected partner of kidney function. Clin. J. Am. Soc. Nephrol 13, 1291–1296 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Leong SC et al. Residual function effectively controls plasma concentrations of secreted solutes in patients on twice weekly hemodialysis. J. Am. Soc. Nephrol 29, 1992–1999 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Bhatnagar V et al. Analyses of 5′ regulatory region polymorphisms in human SLC22A6 (OAT1) and SLC22A8 (OAT3). J. Hum. Genet 51, 575–580 (2006). [DOI] [PubMed] [Google Scholar]
- 178.Xu G et al. Analyses of coding region polymorphisms in apical and basolateral human organic anion transporter (OAT) genes [OAT1 (NKT), OAT2, OAT3, OAT4, URAT (RST)]. Kidney Int 68, 1491–1499 (2005). [DOI] [PubMed] [Google Scholar]
- 179.Stanley LA in Pharmacognosy (ed. Delgoda R) 527–545 (Academic, 2017). [Google Scholar]
- 180.Almazroo OA, Miah MK & Venkataramanan R Drug metabolism in the liver. Clin. Liver Dis 21, 1–20 (2017). [DOI] [PubMed] [Google Scholar]
- 181.Ishikawa T The ATP-dependent glutathione S-conjugate export pump. Trends Biochem. Sci 17, 463–468 (1992). [DOI] [PubMed] [Google Scholar]
- 182.Döring B & Petzinger E Phase 0 and phase III transport in various organs: combined concept of phases in xenobiotic transport and metabolism. Drug Metab. Rev 46, 261–282 (2014). [DOI] [PubMed] [Google Scholar]
- 183.Petzinger E & Geyer J Drug transporters in pharmacokinetics. Naunyn Schmiedebergs Arch. Pharmacol 372, 465–475 (2006). [DOI] [PubMed] [Google Scholar]
- 184.Eraly SA et al. Multiple organic anion transporters contribute to net renal excretion of uric acid. Physiol. Genomics 33, 180–192 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Vallon V et al. A role for the organic anion transporter OAT3 in renal creatinine secretion in mice. Am. J. Physiol. Renal Physiol 302, F1293–F1299 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]