Abstract
Introduction:
There is limited evidence of studying the associated factors of acute kidney injury (AKI) among patients with influenza A (H1N1) virus infection pandemic in 2009. AKI is one of the most prevalent complications in the intensive care unit. Its incidence is associated with high mortality and negative impacts on long-term survival. The aim of this narrative review was to determine the prevalence and mortality due to AKI, among patients admitted with the H1N1 virus.
Materials and Methods:
A narrative review of studies reporting about treatment measures and mortality associated with AKI during the H1N1 pandemic over a 10-year period (from September 2009 to August 2018), was performed. We searched the following databases; EMBASE, Medline/PubMed, NHS evidence, Google Scholar, and the Cochrane Library. Our inclusion revealed 20 studies of patients (n = 3579) who were admitted with H1N1 infection and developed AKI.
Results:
In this study, 33% of patients (n = 1164) who were admitted with H1N1 infection had developed AKI. Within the first 5 years (2009 to 2013), 36% of patients (n = 1013) developed AKI. Within the next 5 years (2014–2018), 812 patients were admitted with the H1N1 infection and 18% of patients (n = 150) had developed AKI. Over the 10 years, there was a 50% reduction in the number of patients who developed AKI (2009 and 2018) (P < 0.0001).
Conclusion:
Patients showed varied responses to treatment measures, depending on their geographical location, comorbidities, and other characteristics. Despite a reasonable prevalence of AKI among patients with the H1N1 virus infection, the mortality over the last 10 years was reduced, with renal replacement therapy as a common therapy in most studies.
Keywords: Acute kidney injury, influenza A, mortality, review, treatment measures
Introduction
In June 2009, an influenza pandemic by a novel swine-origin influenza A virus (H1N1) was declared by the World Health Organization.[1] This pandemic leads to thousands of deaths, and hospitals and intensive care units (ICUs) admissions around the world. The mortality rate among those admitted to ICUs was about 16% with a high incidence of acute lung injury. Moreover, acute respiratory distress syndrome (ARDS) was recorded in some cases which required supportive extracorporeal membrane oxygenation.[2] At the same time, acute kidney injury (AKI) is a debilitating condition that can lead to a series of complications, including those that require some type of renal replacement. As there can be a causal link between patients with H1N1 infection and those who develop AKI, there is a clear need to summarize the correlates between these two conditions.
Duggal et al. conducted a systematic study with meta-regression to develop a guide of reporting outcomes during disease outbreaks. This study showed a global mortality rate variability for H1N1-related critical illness during the 2009 pandemic.[3] Duggal et al. reviewed 226 studies from 50 countries and showed 31% (95% confidence interval [CI] 28–34) global mortality rate among patients with H1N1-related critical illness. Mortality rates in South Asia (61% [95% CI 50–71]) and sub-Saharan Africa (53% [95% CI 29–75]) were the highest compared to North America (25% [95% CI 22–27]), Western Europe (25% [95% CI 22–30]), and Australia (15% [95% CI 13–18]) (P < 0.0001). In addition, this study showed significant lower mortality rates in high-income countries compared with upper- and lower-middle-income countries (P < 0.0001).[3] The authors concluded that there are multiple factors that may vary substantially mortality following outbreaks and pandemics such as patient characteristics, the number of patients, as well as the location and economic status of the affected country.[3]
Multiple studies have demonstrated comorbid or coexisting conditions among patients who were affected with H1N1 virus infection in 2009, such as acute lung injury and, in some cases, ARDS. However, there is limited evidence about the associated factors of AKI among infected patients. AKI is one of the most prevalent complications in the ICU. Its incidence is associated with high mortality and negative impacts on long-term survival.[4,5]
Aim of the review
Based on the aforementioned published material, the aim of this narrative review is two-fold. We aim to assess the prevalence of AKI in patients who were admitted with 2009 H1N1 virus infection. In addition, we aim to identify effective treatment measures and to document the mortality rates among infected patients with AKI.
Materials and Methods
First, we conducted a scoping review to examine the suitability of conducting a systematic review and meta-analysis. We found marked heterogeneity in the available studies of the interventions developed to address AKI among patients diagnosed with the H1N1 virus infection. Therefore, a narrative literature review, over a 10-year period, from September 2009 to August 2018, was undertaken using electronic databases. We focused on the period after 2009, since the H1N1 virus gained universal awareness that year, while AKI began attracting critical media and medical attention.[6] We searched EMBASE, PubMed/Medline, NHS evidence, Google Scholar, and the Cochrane Library. In addition, we reviewed the relevant references in the selected papers. The selected studies included only adult patients (since no study conducted on children was found) and had defined intervention and methodology for patients who were diagnosed with the H1N1 virus infection (and who also developed AKI). The studies also reported a treatment measure. Regarding outcomes, studies reported clear measurement assessment such as creatinine change, dialysis, progression of AKI, incidence of AKI, mortality, or renal morbidity.
We used the following search keywords: “H1N1 virus and AKI,” “H1N1 and AKI,” “H1N1 virus and AKI,” “influenza A H1N1 and AKI,” and “2009 H1N1 pandemic and AKI.”
We initially screened papers by title to exclude any duplicates or unrelated papers. We then reviewed papers by abstracts to determine their relevance. We included all types of study designs. The final list of potentially eligible papers was then reviewed and judged against the following inclusion criteria: (1) Included only patients over the age of 18, (2) written in English, (3) from any country; (4) published from 2009–2018, (5) full-text published in peer-reviewed journals; and (6) at least one reported AKI outcome (treatment measure, creatinine change, dialysis, progression of AKI, incidence of AKI, mortality, or renal morbidity).
Figure 1 presents the total number of relevant articles at each stage of the review process.
Figure 1.
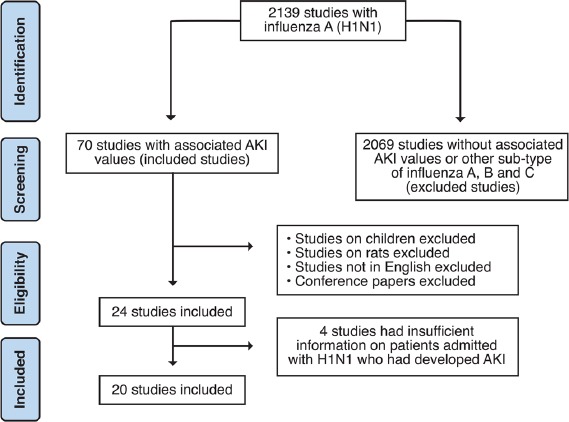
Flowchart of the included studies using PRISMA guidelines
Exclusion criteria included (1) studies that had been conducted on animals, (2) articles not written in English, (3) conference papers, and (4) studies on children or minors. We excluded four studies because they did not have insufficient information on patients admitted with the H1N1 virus who had developed AKI.
Study flow
Initially, our title review search resulted in 2139 studies with influenza A H1N1. After abstract review, we found 70 studies that included patients who were admitted with the H1N1 virus and who further developed AKI. Based on the above reasons for exclusion, 20 studies were included in the final review. Table 1 summarizes the breakdown of these studies by sample size, geographical region, and continent location. Overall, these 20 studies included those with sample sizes from <10 to >600, spanned 12 countries, surveyed three income brackets [Table 1], and covered a 10-year time frame [Table 2]. The individual papers that ended up in this survey are listed in Table 3.
Table 1.
Study-based characteristics and hierarchical model described in 20 studies
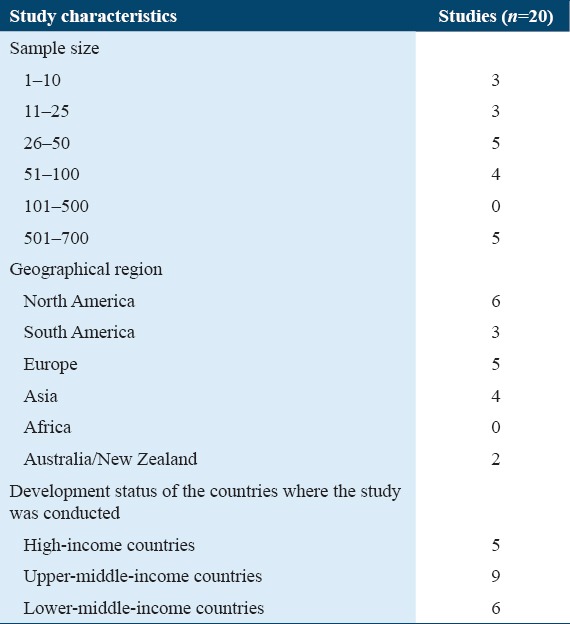
Table 2.
Number of H1N1 patients who developed AKI over a 10-year period (2009–2013 and 2014–2018)
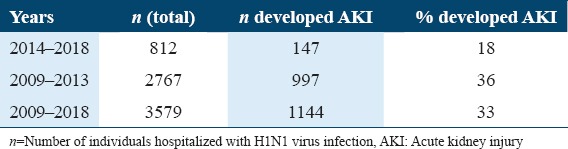
Table 3.
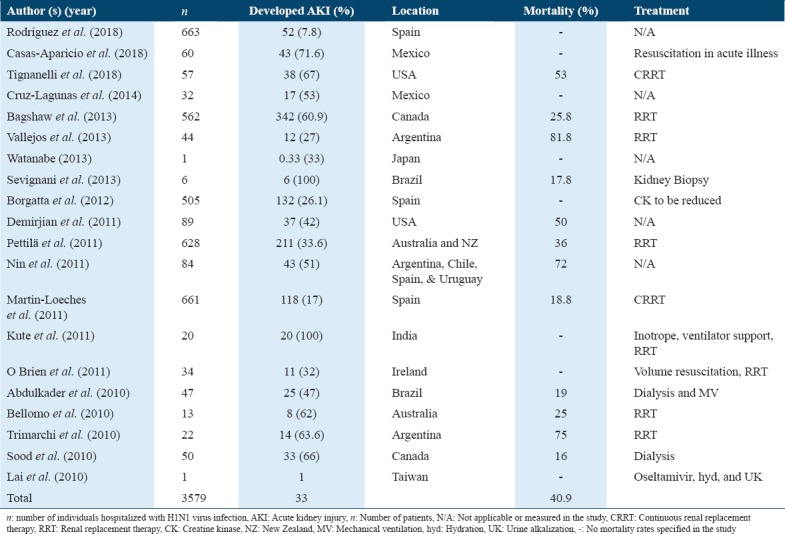
Treatment measures and outcomes for patients who developed AKI from between 2009 and 2018
Results
After reviewing the 20 included studies, we found that 33% of patients (n = 1164) who were admitted with H1N1, had developed AKI. Between 2009 and 2013, 2767, H1N1-infected patients were admitted to hospitals. In these first 5 years, 36% of patients (n = 1013) had developed AKI, whereas in the next 5 years, from 2014 to 2018, 812 patients had been admitted with the H1N1 virus infection and 18% of the patients (n = 150) had developed AKI. Over the 10 years, there was a 50% reduction in the number of patients who developed AKI (from 2009 to 2018, P < 0.0001) [Table 3]. This can be possibly attributed to the fact that there was an increase in knowledge by practitioners and medical centers for preventative and treatment measures. Other possible reasons could be that the virus was detected earlier and, therefore, further complications (i.e. AKI and ARDS) were prevented; as well as a reduction in the number of cases between 2014 and 2018. In addition, a high percentage of AKI during the first 5 years could also have been a result of poor treatment strategy due to the scarcity of information regarding the association of AKI with influenza A H1N1 virus. Furthermore, it could have been due to the inherent property of the H1N1 strain that was widely circulating in the first 5 years.
Discussion
Aside from the prevalence of AKI in patients who were admitted with the H1N1 virus infection, it is also noteworthy to discuss treatment measures for AKI and a measured outcome (creatinine change, dialysis, progression of AKI, incidence of AKI, mortality, or renal morbidity). The following studies were compared, contrasted, and discussed.
There were three studies conducted in 2018 by Rodríguez et al., Casas-Aparicio et al., and Tignanelli et al. [7-9] Rodríguez et al.[7] investigated the relationship between AKI and serum procalcitonin (PCT) in patients with H1N1-related critical illness. The study found that out of 663 patients studied, 62 (9.8%) developed AKI who were older with more comorbidities, and they had a more critical illness. Casas-Aparicio et al.[8] demonstrated that the risk of AKI and mortality was increased with aggressive fluid accumulation in a group of patients with H1N1-induced severe pneumonia. About 72% (43/60) of patients developed AKI. There was significant association between mortality and older age (P = 0.009), higher pneumonia severity index score on admission (112 vs. 76, P = 0.008), and nephrotoxic drugs (P = 0.034).[8] Tignanelli et al.[9] also investigated the outcomes of AKI in H1N1-infected patients with severe ARDS in 2009. Among the 57 patients, most of them presented with Stage III AKI. The mortality rate was higher (53% vs. 0%) in patients who required continuous renal replacement therapy (CRRT) compared with patients who did not require CRRT or intermittent hemodialysis. In addition, the survival was not improved with increased duration of CRRT. In this study, 15 CRRT patients transitioned to intermittent hemodialysis. Renal recovery was reached in 94% of patients with CRRT who survived. Seventeen patients required extracorporeal membrane oxygenation, and 15 of them undergone CRRT.[9]
From a lifestyle perspective, Cruz-Lagunas et al.[10] studied patients presented with AKI with H1N1 influenza A and ARDS to assess associated obesity and pro-inflammatory mediators. AKI patients with ARDS had higher body mass index (BMI) with elevated levels of leptin, C-peptide, insulin, serum amyloid A, and PCT compared to patients with ARDS only.[10] Bagshaw et al.[11] conducted a cohort study on AKI among patients with pH1N1-related critical illness in Canada. The study included 562 patients (479 with a confirmed diagnosis and 83 with probable diagnosis). The common comorbid conditions included chronic obstructive pulmonary disease, obesity, and diabetes. The study found that AKI occurred in 60.9% in patients. In addition, 23% presented with injury category and 37.9% with failure category of the risk, injury, failure, loss of kidney function, and end-stage kidney disease (RIFLE) categories.[11] Obesity, acute physiology, and chronic health evaluation (APACHE) II score, chronic kidney disease, and P(a)O2/F(i)O2 ratio were the main independent predictors of AKI. RRT was required in 24.9% (85/342) of patients with AKI. APACHE II score, obesity, day 1 mechanical ventilation, and day 1 creatinine were the main independent predictors of the requirement of RRT. Mortality occurred in 25.8% (85/329) of patients. It was concluded that AKI development was not independently associated with hospital mortality.[11]
Vallejos et al.[1] investigated dialysis for AKI associated with influenza A H1N1 infection. The study reported the data of 44 “first wave” patients of the pandemic. Similarly, about 70.5% of patients showed comorbid conditions (obesity, chronic respiratory diseases, hypertension, diabetes, pregnancy, and chronic renal disease).[1] Critical patients were admitted to the ICU, especially patients with severe acute respiratory failure. The initiation of RRT after the admission to the ICU occurred after an average time of 3.16 ± 2.6 days. The majority of patients required mechanical ventilation at the initiation of RRT. Most of the patients (75%) were observed for 3 weeks. Mortality related to use of inotropics, doubling of alanine aminotransferase, and respiratory failure was 81.8%. The study concluded a high mortality rate in H1N1-infected patients with AKI who required RRT, in the context of multi-organ failure. In addition, it recommended the mandatory development of strategies to limit potential renal complications with future H1N1 pandemics.[1]
Based on the above, complications are certainly evident among patients who were admitted with the H1N1 virus infection. Watanabe[12] conducted a study to assess renal complications associated with influenza A virus of seasonal and pandemic infections. The study showed uncommon incidence among H1N1-infected patients. However, when it occurs, it can deteriorate the patient’s condition including AKI in critically ill patients, hemolytic uremic syndrome, rhabdomyolysis, disseminated intravascular coagulation (DIC), Goodpasture’s syndrome, acute glomerulonephritis, and acute tubulointerstitial nephritis. The study found that clinical characteristics of AKI in patients with H1N1-related critical illness in 2009 pandemic were similar to the uninfected patients. In addition, AKI was associated with older age, obesity, diabetes mellitus, history of asthma, pregnancy, and chronic kidney disease. The histological assessment of the kidneys of H1N1-infected patients after death showed acute tubular necrosis (ATN), DIC, and myoglobin pigments. The virus was detected in the kidneys of some patients. Similar to the findings in our study, Watanabe observed that AKI occurs in approximately one-third of patients with rhabdomyolysis and can lead to kidney complications due to influenza A.[12]. Lai et al.[13] also demonstrated that pandemic H1N1 infection may be presented with mild rhabdomyolysis and AKI.
Sevignani et al.[14] conducted a correlation study between the clinical and histological findings in a number of patients with AKI who were infected with H1N1. The study revealed that AKI clinical and laboratory evidence was detected in all cases except one case that did not have oliguria. Borgatta et al.[15] reported bad outcomes in 2009 H1N1-infected patients who had an elevation in creatine kinase (CK) levels. The study included 505 patients and found that the global ICU mortality rate was 17.8% without differences between breakpoints. Renal dysfunction was higher in a patient with CK level ≥500 UI/L, which was documented in 23.8% of ICU admissions. The study found that the incidence of AKI was high (26.1%), with 11% of patients required RRT. The study concluded that CK level ≥1000 UI/L was associated with 5 extra days of hospital and ICU stay.[15]
Bellomo et al.[2], Demirjian et al.,[16] and Pettilä et al.[17] investigated AKI and the 2009 H1N1-related critical illness.
The study by Bellomo et al.[2] surveyed several case series in different locations, and reflected that in some cases, a number of patients developed AKI, which complicated their clinical course and, in some patients, required support with RRT. A study from Mexico reported roughly 30% incidence rate of severe AKI.[2] Similarly, a report from Texas reported eight of 13 patients had developed AKI, with three being classified into the failure category of the RIFLE classification. Hospital mortality was approximately 25% among the AKI patients. Of the AKI patients, three (37.5%) received RRT, and, among these, one died.[2]
Demirjian et al.[16] investigated the incidence, risk factors, and complications of the 2009 influenza A infection and AKI. The study included 89 patients who tested positive for influenza A from August to December 2009. AKI occurred in 37 (42%) patients with the majority (24/37) of patients in a critically ill state. Chronic kidney disease, elevated CK, and obesity were the risk factors for AKI. However, there was a lower AKI risk among positive influenza A patients compared to seronegative subjects. Mortality was 50% in infected patients with AKI requiring dialysis.[16]
In another study by Pettilä et al.[17] for assessment of AKI in 628 H1N1 infected adult patients, 211 (33.6%) had AKI. Out of these 211 AKI patients, 76 (0.036%) died at the hospital.[17] RRT was required in 33 AKI. Thirteen (39.4%) of the RRT-treated patients died. Contrary to the findings of Bagshaw et al.,[11] it was also shown that mechanical ventilation, age, AKI, and any severe comorbidity were independently associated with hospital mortality.[17]
Nin et al.[18] conducted an observational study on AKI among patients with H1N1-related critical illness in 2009. About half (43/84) of patients developed AKI early AKI in 28 (33%) and late AKI in 15 (18%). RRT was required in 20 (24%) patients. AKI was associated with higher APACHE II score and ICU mortality (72% vs. 39%, P < 0.01). Compared with patients without AKI, patients with early AKI had higher APACHE II score and marked organ dysfunction on admission. However, late AKI had high ICU mortality compared with early AKI (93% vs. 61%, P < 0.001). Using multivariate analysis, the study found that only late AKI and APACHE II score were associated with mortality.[18]
Martin-Loeches et al.[19] also investigated AKI among patients with H1N1-related critical illness. The study involved 661 patients and found that 118 (17.7%) patients developed AKI. Regarding stages of AKI, the study included 37 (31.4%) patients with AKI I, 15 (12.7%) with AKI II, and 66 (55.9%) with AKI III. CRRT was needed in 50 (75.7%) patients.[19] AKI was associated with higher SOFA scores, higher APACHE II scores, a greater incidence of shock and multi-organ dysfunctional syndrome, more need for mechanical ventilation and a greater incidence of coinfection.[19] In survivors, AKI patients required longer support on mechanical ventilation. In addition, they had longer ICU and hospital stay compared with patients without AKI. The overall mortality rate was 18.8%, which was significantly higher with AKI (44.1% vs. 13.3%; P < 0.01). Using logistic regression analysis, the study found that only AKI III patients were associated with higher ICU mortality.[19]
Kute et al.[20] showed high mortality in patients with H1N1-related critical illness. It was shown that H1N1 infected patients may complicate with pneumonia and AKI. The study examined 20 confirmed patients with pneumonia and AKI with a mean age of 42.8±18.2 years. All patients had pneumonia and AKI with increased serum lactate dehydrogenase levels.[20] Mechanical ventilation was required in 15 (75%) patients and 14 (70%) patients died. All healthcare worker received oseltamivir prophylaxis and no one developed the influenza-like illness. Mortality was associated with higher Multiple Organ Dysfunction Score (MODS), SOFA, APACHE II, and X-ray chest scores. In addition, mortality was associated with the requirement of an inotrope, RRT, ventilator support, and the presence of underlying risk factors for severe disease.[20]
Similar to Kute et al.[20] and Trimarchi et al.[21] investigated the association between H1N1 infection and AKI in critically ill patients. The study included 22 patients with H1N1 pneumonia with a mean age of 52.91. Oseltamivir was given within 48 h to all patients once the diagnosis was established. AKI developed in 14 patients (63.6%)[21] with the mean of the highest creatinine levels of 2.74 ± 2.83 mg/dl. RRT was required for four patients (18.2%) with a mean duration of 15 ± 12 days. Recovery of renal function was achieved in six patients (42.9%). AKI was associated with immunosuppression, high APACHE, SOFA and Murray scores, pregnancy, and less time on mechanical ventilation assistance, hemodynamically instability, and thrombocytopenia. Between the two groups, mortality was associated with thrombocytopenia, higher APACHE, SOFA and Murray scores, lower oxygen inspired fraction/alveolar pressure ratio, a higher oseltamivir dose, hypoalbuminemia, oligoanuria, acute renal failure, and lack of recovery of renal function. Three out of four (75%) of the hemodialyzed patients died.[21]
Sood et al.[22] reported the experience of a Canadian Province showing that kidney injury, kidney failure, and the need for dialysis are common in patient with H1N1-related critical illness. In addition, the mortality and length of ICU admission were increased in these patients. The study reported the results of 50 patients with H1N1-related critical illness with the severe respiratory syndrome (47 confirmed cases and three probable).[22] The study found that kidney injury occurred in 66.7% of patients, while kidney failure, and the need for dialysis occurred in 66% and 11%, respectively. In addition, death was increased in kidney failure (OR, 11.29; 95% CI, 1.29-98.9), while the length of stay was increased in the patients who required dialysis (RR, 2.38; 95% CI, 2.13–25.75). The mortality rate was 16%. However, due to the small sample size, the results of this study have limited generalizability.[22]
O Brien et al.[23] outlined the Irish national tertiary referral center experience of the 2009 H1N1 pandemic with renal failure. The study reported 34 admitted patients with H1N1 infection with an average length of admission of 10 days (3–84). From the 34 patients, 11 (32%) developed AKI according to the RIFLE criteria (creatinine range 120–610).[23] In addition, four patients received RRT for a range of 10–52 days. AKI was developed in seven patients who responded to volume resuscitation. The study concluded that sepsis with ATN was the most common cause of AKI.[23] Abdulkader et al.[24] identified characteristics of AKI in H1N1-infected patients in 2009. About half (25/47) of the H1N1-infected patients developed AKI, which was associated with vasopressor use, high APACHE II scores, mechanical ventilation, and severe acidosis. In addition, it was associated with higher levels of lactic dehydrogenase and C-reactive protein upon ICU admission. The study found that eight patients (50%) needed dialysis, while 16 patients (64%) requested a nephrology consultation. Nine patients with AKI (19%) died. Mortality was associated with mechanical ventilation, high APACHE II score, vasopressor use, high bilirubin levels, dialysis, and a low RIFLE score at ICU admission.[24]
It was concluded that in patients with H1N1-related critical illness, the risk of renal impairment is high, which may require RRT in 18% of cases. Patients who required RRT were associated with an increased risk of death from multiple organ failure, acute renal injury, oligoanuria, and a failure of recovery from the renal impairment.
In summary, 12 of the 20 studies reported about mortality. An average of 40.9% of patients who were diagnosed with H1N1 and/or had developed AKI had died. These included patients who were treated with various measures (i.e. RRT and dialysis). It was also shown that among nine out of the 20 studies, RRT was the common treatment measure for patients who developed AKI from admission due to H1N1 virus infection [Table 3]. Based on this, it can be suggested that physicians should use RRT as a preferred treatment measure for such patients. However, such treatment preferences should also depend on a patient-to-patient basis and should not be adopted as a generic recommendation.
Limitations
There are multiple limitations to our study. One limitation is that the included studies were not primarily designed to assess whether the H1N1 virus had an effect on AKI. Therefore, a large diversity in studies, study designs, renal failure definitions, patient categories for different diagnoses, and severity of illness, were combined. Another limitation is that, in some cases, the presence of AKI at the start of the patient(s) admission for the 2009 H1N1 virus infection was hard to determine from the published materials. Some studies with low sample sizes were also included since they met our inclusion criteria.
Conclusion
About 33% of the patients who were admitted for H1N1 infection had developed AKI. In general, variable treatment measures do not seem to modify the risk of AKI. However, in this review, it was shown that in nine out of the 20 studies, RRT was the most common treatment measure administered to patients admitted with the H1N1 virus infection, to prevent AKI development. The later the detection and treatment of AKI, the higher the chances of mortality. In addition, chances of mortality were also higher among AKI patients compared to patients who did not develop AKI. Despite a reasonable prevalence of AKI in patients after being admitted with the H1N1 virus infection, the mortality over the last 10 years decreased with RRT as a common therapy in most of the studies.
Interestingly, there were no studies conducted in Africa. Future research is therefore required to understand the prevalence of AKI in patients after being admitted with the H1N1 virus infection, in this continent. It must also be noted that patients might have varied responses to treatment measures, depending on their geographical location, the economic status of the country, comorbidities, other associated complications, BMI, age, and other patient characteristics. Based on these factors, the prevalence of AKI in H1N1-infected patients, and treatment measures could vary considerably.
References
- 1.Vallejos A, Arias M, Cusumano A. Dialysis for acute kidney injury associated with influenza a (H1N1) infection. Saudi J Kidney Dis Transpl. 2013;24:527–33. doi: 10.4103/1319-2442.111045. [DOI] [PubMed] [Google Scholar]
- 2.Bellomo R, Pettilä V, Webb SA, Bailey M, Howe B, Seppelt IM, et al. Acute kidney injury and 2009 H1N1 influenza-related critical illness. Contrib Nephrol. 2010;165:310–4. doi: 10.1159/000313771. [DOI] [PubMed] [Google Scholar]
- 3.Duggal A, Pinto R, Rubenfeld G, Fowler RA. Global variability in reported mortality for critical illness during the 2009-10 influenza A(H1N1) pandemic:A systematic review and meta-regression to guide reporting of outcomes during disease outbreaks. PLoS One. 2016;11:e0155044. doi: 10.1371/journal.pone.0155044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Singbartl K, Kellum JA. AKI in the ICU:Definition, epidemiology, risk stratification, and outcomes. Kidney Int. 2011;81:819–25. doi: 10.1038/ki.2011.339. [DOI] [PubMed] [Google Scholar]
- 5.van den Akker JP, Egal M, Groeneveld AB. Invasive mechanical ventilation as a risk factor for acute kidney injury in the critically ill:A systematic review and meta-analysis. Crit Care. 2013;17:1–9. doi: 10.1186/cc12743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sykes L, Nipah R, Kalra P, Green D. A narrative review of the impact of interventions in acute kidney injury. J Nephrol. 2018;31:523–35. doi: 10.1007/s40620-017-0454-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rodríguez A, Reyes LF, Monclou J, Suberviola B, Bodí M, Sirgo G, et al. On behalf of GETGAG study group. Relationship between acute kidney injury and serum procalcitonin (PCT) concentration in critically ill patients with influenza infection. Med Intensiva. 2018;42:399–408. doi: 10.1016/j.medin.2017.12.004. [DOI] [PubMed] [Google Scholar]
- 8.Casas-Aparicio GA, León-Rodríguez I, Hernández-Zenteno RJ, Castillejos-López M, Barrera C, Ormsby CE, et al. Aggressive fluid accumulation is associated with acute kidney injury and mortality in a cohort of patients with severe pneumonia caused by influenza A H1N1 virus. PLoS One. 2018;15:e0192592. doi: 10.1371/journal.pone.0192592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tignanelli CJ, Wiktor AJ, Vatsaas CJ, Sachdev G, Heung M, Park PK, et al. Outcomes of acute kidney injury in patients with severe ARDS Due to influenza A(H1N1) pdm09 virus. Am J Crit Care. 2018;27:67–73. doi: 10.4037/ajcc2018901. [DOI] [PubMed] [Google Scholar]
- 10.Cruz-Lagunas A, Jiménez-Alvarez L, Ramírez G, Mendoza-Milla C, García-Sancho MC, Avila-Moreno F, et al. Canadian critical care trials group H1N1 collaborative. Obesity and pro-inflammatory mediators are associated with acute kidney injury in patients with A/H1N1 influenza and acute respiratory distress syndrome. Exp Mol Pathol. 2014;97:453–7. doi: 10.1016/j.yexmp.2014.10.006. [DOI] [PubMed] [Google Scholar]
- 11.Bagshaw SM, Sood MM, Long J, Fowler RA, Adhikari NK, et al. Canadian Critical Care Trials Group H1N1 Collaborative. Acute kidney injury among critically ill patients with pandemic H1N1 influenza a in Canada:Cohort study. BMC Nephrol. 2013;14:123. doi: 10.1186/1471-2369-14-123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Watanabe T. Renal complications of seasonal and pandemic influenza a virus infections. Eur J Pediatr. 2013;172:15–22. doi: 10.1007/s00431-012-1854-x. [DOI] [PubMed] [Google Scholar]
- 13.Lai CC, Wang CY, Lin HI. Rhabdomyolysis and acute kidney injury associated with 2009 pandemic influenza a (H1N1) Am J Kidney Dis. 2010;55:615. doi: 10.1053/j.ajkd.2010.01.002. [DOI] [PubMed] [Google Scholar]
- 14.Sevignani G, Soares MF, Marques GL. Acute kidney injury in patients infected by H1N1:Clinical histological correlation in a series of cases. J Bras Nefrol. 2013;35:185–90. doi: 10.5935/0101-2800.20130030. [DOI] [PubMed] [Google Scholar]
- 15.Borgatta B, Pérez M, Rello J, Vidaur L, Lorente L, Socías L, et al. Elevation of creatine kinase is associated with worse outcomes in 2009 pH1N1 influenza A infection. Intensive Care Med. 2012;38:1152–61. doi: 10.1007/s00134-012-2565-5. [DOI] [PubMed] [Google Scholar]
- 16.Demirjian SG, Raina R, Bhimraj A, Navaneethan SD, Gordon SM, Schreiber MJ, Jr, et al. 2009 influenza a infection and acute kidney injury:Incidence, risk factors, and complications. Am J Nephrol. 2011;34:1–8. doi: 10.1159/000328386. [DOI] [PubMed] [Google Scholar]
- 17.PettiläV Webb SA, Bailey M, Howe B, Seppelt IM, Bellomo R, et al. Acute kidney injury in patients with influenza A (H1N1) 2009. Intensive Care Med. 2011;37:763–7. doi: 10.1007/s00134-011-2166-8. [DOI] [PubMed] [Google Scholar]
- 18.Nin N, Lorente JA, Soto L, Ríos F, Hurtado J, Arancibia F, et al. Acute kidney injury in critically ill patients with 2009 influenza A (H1N1) viral pneumonia:An observational study. Intensive Care Med. 2011;37:768–74. doi: 10.1007/s00134-011-2167-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Martin-Loeches I, Papiol E, Rodríguez A, Diaz E, Zaragoza R, Granada RM, et al. Acute kidney injury in critical ill patients affected by influenza A (H1N1) virus infection. Crit Care. 2011;15:R66. doi: 10.1186/cc10046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kute VB, Godara SM, Goplani KR, Gumber MR, Shah PR, Vanikar AV, et al. High mortality in critically ill patients infected with 2009 pandemic influenza A (H1N1) with pneumonia and acute kidney injury. Saudi J Kidney Dis Transpl. 2011;22:83–9. [PubMed] [Google Scholar]
- 21.Trimarchi H, Greloni G, Campolo-Girard V, Giannasi S, Pomeranz V, San-Roman E, et al. H1N1 infection and the kidney in critically ill patients. J Nephrol. 2010;23:725–31. [PubMed] [Google Scholar]
- 22.Sood MM, Rigatto C, Zarychanski R, Komenda P, Sood AR, Bueti J, et al. Acute kidney injury in critically ill patients infected with 2009 pandemic influenza A (H1N1):Report from a Canadian province. Am J Kidney Dis. 2010;55:848–55. doi: 10.1053/j.ajkd.2010.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.O Brien FJ, Jairam SD, Traynor CA, Kennedy CM, Power M, Denton MD, et al. Pandemic H1N1 (2009) and renal failure:The experience of the Irish national tertiary referral center. Ir J Med Sci. 2011;180:135–8. doi: 10.1007/s11845-010-0617-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Abdulkader RC, Ho YL, de Sousa Santos S, Caires R, Arantes MF, Andrade L, et al. Characteristics of acute kidney injury in patients infected with the 2009 influenza A (H1N1) virus. Clin J Am Soc Nephrol. 2010;5:1916–21. doi: 10.2215/CJN.00840110. [DOI] [PMC free article] [PubMed] [Google Scholar]


