Abstract
Objective:
The life expectancy of women increases with the advancement in modern medicine, leading them to spent about two decades in menopausal state along with its associated complications. The process of aging triggers a deleterious cascade of physiological changes in the body. Hence, the aim of our study is to determine the effects of both aerobic and anaerobic exercises on estrogen level in postmenopausal osteoporotic females.
Methodology:
A randomized control trial was conducted among 94 postmenopausal osteoporotic females, randomly divided into two equal groups. The participants in Group A were assigned to perform aerobic exercise, whereas Group B performed resistance exercises. Each group performed exercises for 12 weeks, whereas reading was collected for blood estrogen level, fat mass, and muscle mass before exercise training and after 12 weeks of training.
Results:
Twelve weeks of aerobic and anaerobic exercise program based on the American College of Sports Medicine (ACSM); frequency, intensity, time, and type protocol has beneficial effect on estradiol level and lean mass, whereas inversely correlated with fat mass of postmenopausal osteoporotic female.
Conclusion:
The study concluded that 12 weeks of exercise programs was found to be effective in improving estradiol level of postmenopausal osteoporotic female. The efficacy of anaerobic exercise was found to be more potent on estradiol level and lean mass than aerobic exercises as only 36 sessions of resisted exercises performed during a period of 12 weeks provided more significant result than 72 session of aerobic exercises.
Keywords: Aerobic exercise, estrogen, osteoporosis, postmenopausal
Introduction
Menopause is an inevitable event that causes plenty of physiological variation in female body.[1] The global prevalence of natural menopause varies significantly in developed countries with an average age of 51 years.[2,3] Globally, it has been estimated that around 25 million women reach menopause and the number is expected to be doubled by the late 2020. Moreover, in the U.S.A. and Europe, 51 and 50.7 years are average ages of the onset of menopause documented, respectively,[4,5] whereas in Pakistan 49.3,[6] India 45.0,[7] UAE 48.6,[8] and Saudi Arabia it is found to be 48.9 years.[9]
Women spend one-third of their life span in the postmenopausal state with its associated symptoms[10] such as hot flushes (65%),[11-14] mood swing (42.6%),[14] vaginal dryness (34%),[14,15] sleep problems (47.4%),[12-14] night sweats (44%),[12,14] memory loss (32.3%),[14,16] urinary symptoms (18.3%),[14,17] osteoporosis(12.6%),[18] anxiety (5.8%),[14,19] joint and muscle pain (40.1%),[13] depression (27.5%),[20,21] and irritability (5.9%)[14] have been documented.
It is well evident that during the process of aging and menopause, the level of anabolic hormones, especially estrogen hormone, changes significantly causing both physiological and psychological changes in the body.[22] Recent study suggests that menopause transition is associated with changes in body composition that increases in body fat (B.F), central adiposity[23-25] and promotes a loss of fat-free mass potentially a decline in skeletal muscle mass[23,24] that may be related to a decline in energy expenditure,[13,25] a loss of muscular strength,[26] and a decline in physical activity.[27] The bone loss experienced in the initial 6 years after menopause is estimated to be approximately 15% imposing a considerable risk for the development of osteoporosis.[28]
These postmenopausal endocrinal changes cause systematic skeletal changes characterized by changes in bone mass and microarchitectural deterioration of the bone,[29] as the ovaries stop producing estrogen hormone, leading to primary osteoporotic changes.[30] However, osteoporosis is often a silent disease without specific symptoms and detected in the connection with a fracture of a bone mass test called bone densitometer.[31,32] The measurement value obtained from a bone densitometer is compared to young reference material (T-score), whereas a T-score <−2.5 classifies as osteoporosis.[31,33]
Studies conducted overtime have suggested exercises as a potential intervention strategy in controlling and preventing the menace of osteoporosis and also in overcoming the postmenopausal complications due to change in the level of anabolic hormones, particularly estrogen.[34] Exercise training plays an important role in improving the muscle strength,[28,35] increasing bone metabolism,[34,35] functional capacity,[36] and decreasing obesity,[35,36] thus may lead to improve in the quality of life.[37] However, researches conducted until date is insufficient in estimating the effects of particular prescription of different types of exercises such as aerobic and anaerobic in the management of osteoporosis with postmenopausal complication among women.[38]
There are conflicting results regarding the impact of physical exercise on anabolic hormones among the postmenopausal women. A study conducted in 2015 by Ketabipoor and Jahromi revealed that estrogen level increased significantly after the exercise program.[38] The results of this study were not in accordance with the results of Chan et al., 2007, his study conducted to review association between physical activity and androgenic hormones in postmenopausal women showed that increase in physical activity is associated with a significant decrease in estradiol level.[39] Body fat in postmenopausal women may have an influence on the metabolism of estrogen. Furthermore, low levels of aromatase activity in abdominal fat may be effective in reducing estrogen.[40] On the other hand, endurance exercises increase the level of testosterone, dehydroepiandrosterone, estradiol, cortisol, and growth hormone, whereas the effects of resistance exercises are only limited to estradiol and growth hormone.[41] There is a conflicting result showing a negative effect of aerobic exercises on estrogen level, whereas the effect of resistance exercise is unknown.[42] Postmenopausal females suffer from activity limitation and participation restriction, thus increasing the socioeconomic burden both nationally and internationally. Hence, the aim of the present study is to provide baseline evidence regarding the impact of aerobic and anaerobic exercises protocol that may help in increasing the activities of daily living and functional independence while reducing the health-care costs and improving health-related quality of life in older adults.
Methodology
The study was a randomized control trial comparing the effect of 12 weeks of aerobic exercises intervention versus anaerobic exercises on estradiol, fat mass, and muscle mass measured at the baseline and after 12 weeks of intervention. The study was conducted according to the guidelines of Belmont Report for ethical decision-making related to all the trials that include human subjects. The Ethical Review Committee of Ziauddin University approved the protocol (Ref # 0180617AFMPT).
Participants
All females fulfilling the inclusion criteria were recruited and screened on the basis of Physical Activity Readiness-Questionnaire and YOU form. Randomization was performed by a random number generation and group name was placed in a sealed envelope that was only opened at the time of performing exercises.
Assessment parameters
Estradiol blood test
Estradiol blood test of the participants was done by a laboratory assistant to evaluate the estrogen level of the participants’ pre- and post-exercise intervention that is at 0 week and after 12 weeks of training protocol.
Fat mass calculation
Skinfold thickness method was used to calculate fat mass according to National Health and Nutrition Examination Survey,[43] before exercise training and after 12 weeks of training. The measurement was usually performed at specific site on the right side of the body, the participants stood up, with relaxed shoulder and arms hanging freely at the sides. The measurement was taken using a caliper where the reliability and validity of it have previously been documented.[44] The performer pinched the tested site of skin from thumb and index finger away from underlying muscle so only skin and fat tissue were calculated. Three measurements were recorded and averagewas taken.[45] The value was taken from abdomen, suprailiac, and triceps using the following formula:
%Body fat = (0.41563 × sum of three skinfolds) – (0.00112 × [sum of three skinfolds] × 2 + (0.03661 × age) + 4.03653
Fat mass = Total body weight × Body Fat%
Muscle mass calculation
The muscle mass of the body was calculated by subtracting the fat mass from total body mass Muscle mass =Total body weight−Fat mas.[46]
The muscle mass was calculated before the 1st week of training and after the completion of 12 weeks of training.
Exercise intervention
The study was conducted on 94 postmenopausal osteoporotic females and randomized using an enveloped method in Group A (n = 47) and Group B (n = 47) to receive the intervention based on aerobic and anaerobic exercise protocols in accordance with the guidelines of the American College of Sports Medicine using the frequency, intensity, time, and type (FITT) protocol.[47]
Group A was given an intervention based on aerobic exercises protocol, whereas Group B was given anaerobic exercises protocol.
Warm up
Ten min of warm up[48] exercises was performed before the start of the training session on a prescribed criterion of +10 resting heartbeats. The warm session included initial stretching exercises of calf and hamstring for 5 min and walking on a treadmillwith intensity where heart rate increases to +10 beats from the resting level afterward conditioning was performed based on anaerobic and aerobic protocol.
Aerobic protocol
The aerobic exercise protocol included cross trainer. The participants were informed about the cardiac prodromal symptoms such as shortness of breath, dizziness, chest discomfort, or palpitation that might develop during exercise and were advised to immediately inform these symptoms to the physical therapist. The patient’s vitals (oxygen saturation, blood pressure, and pulse rate) were monitored before, during, and after exercise through pulse oximeter and sphygmomanometer.
The details of the FITT protocol for aerobic group according to the American College of Sports Medicine (ACSM) guidelines are given in Table 1.
Table 1.
The protocol of aerobic group (Group A) FITT protocol given according to the American College of Sports Medicine guideline[47]

Anaerobic protocol
The protocol was based on strengthening exercises of 10 major muscle groups. The intensity of the weight-bearing exercises was calculated using one repetition maximum method[49] which involved the following protocol:
The patient was asked to lift a weight of 3 kg and perform 10 repetitions
If the patient completed 10 repetitions, then 1 kg of weight was added and the same protocol was repeated
In case, the patient fails to complete 10 repetitions of any given weight, then 60–80% of that weight was calculated and used for training purpose.
The detail of the FITT protocol for anaerobic group according to the ACSM guidelines, to be performed is as under Table 2.
Table 2.
The protocol of anaerobic group (Group B) FITT protocol given according to the American College of Sports Medicine guidelines[47]
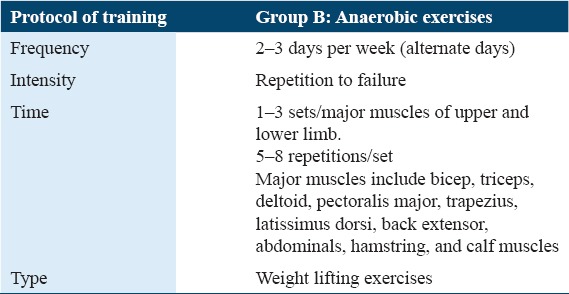
Cool down
The process involved a post-conditioning resting period of 10 min during which the patients were asked to sit on a chair and performed deep breathing exercises. During a cool down process, patient vitals; pulse rate, oxygen saturation, and blood pressures were monitored. The session of cool down continued until the patient pulse rate reaches to the resting pulse rate that was recorded before the start of the training.
Exercise termination criteria
On the occurrence of any one of the following events at any time during the session, the exercises were prematurely terminated:
Inclusion criteria
The following criteria were included in the study:
Exclusion criteria
The following criteria were excluded from the study:
Results
Demographic representation
A total of 94 participants were recruited in the study; the mean age of the participants, weight, and BMI measurement at the baseline are illustrated in Table 3.
Table 3.
Demographic characteristics of postmenopausal osteoporotic female
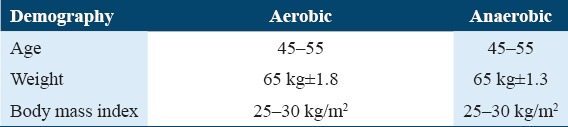
At baseline, the participants in both the groups have similar demographic characteristics in terms of age, weight, and BMI.
Level of significance
The level of significance within the group was calculated at 95% of C.I using a paired t-test (two tailed) statistics. Table 4 depicts the values obtained after 12 weeks of interventional strategies on estradiol and bone mass density (BMD).
Table 4.
The pre- and post-values of estradiol and BMD among the postmenopausal osteoporotic women
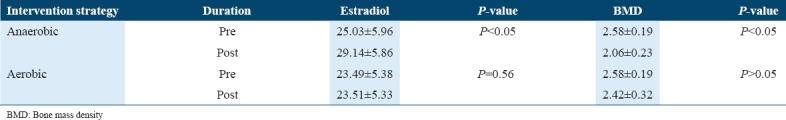
It was analyzed from above table that the pre-post mean difference after intervention of 12 weeks from baseline in anaerobic group shows significant improvement in the estradiol level and BMD, whereas the impact of aerobic exercises was found to be non-significant on BMD and estradiol levels of postmenopausal osteoporotic females.
The changes in fat mass and bone mass using both the intervention strategies are clearly revealed in Table 5.
Table 5.
The pre- and post-values of fat mass and muscle mass among the postmenopausal osteoporotic women
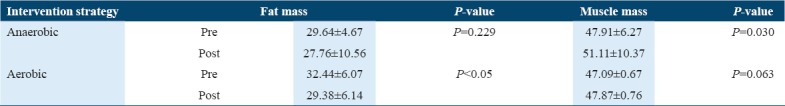
The values observed in Table 5 showed that anaerobic exercises have positive effects on the level of muscle mass; however, its impact on fat mass was found to be non-significant. Whereas on the other hand, the impact of aerobic exercises on fat mass was significant; however, no significant effects had been observed in muscle mass.
Table 6 illustrates that the effects of anaerobic exercises were significant in comparison to the aerobic exercises P < 0.05 in improving the estradiol levels and reducing the BMD the participants P < 0.05
Table 6.
P-values of estradiol and BMD among the postmenopausal osteoporotic women

P-values of fat mass and muscle mass among the postmenopausal osteoporotic women after 12-week intervention are elaborated in Table 7.
Table 7.
P-values of fat mass and muscle mass among the postmenopausal osteoporotic women

Table 7 shows that the no significant mean difference in pre-post fat mass in anaerobic group had been observed favoring aerobic exercises over anaerobic intervention, whereas between-group comparisons in the two groups for muscle mass were found to be weekly significant favoring anaerobic over aerobic.
Discussion
Our results of the 12 weeks randomized control trial study suggest that exercise intervention based on aerobic and anaerobic exercise does improve the level of circulating estrogen in the overweight postmenopausal osteoporotic women. Besides that, it also shows that resisted exercises were found to be effective in increasing the muscle mass and decreasing the fat mass and ultimately improving health-related quality of life of postmenopausal osteoporotic women. The finding of this study was according to the result of the previous study conducted by Moghadasi and Siavashpour, 2013, suggested that 12 weeks of resistance training significantly increase in the growth hormone, estrogen, parathyroid hormone, and testosterone.[53] Another study conducted by Ketabipoor and Jahromi, in 2015, where aerobic exercise reduced the BMI causing increase estrogen level, despite in reduction of fat tissue that is considered as a source of estrogen secretion.[38] On the other hand, Kenney et al., 2015, emphasized that several exercise programs are also incorporated in the fitness training to burn fat, resulting in reducing the fat mass ultimately leading to a decrease in the estrogen level.[54] Moreover, in another RCT, the fitness level and quality of life of postmenopausal women were improved through a regular controlled exercise program of 6 weeks.[55]
There are conflicting results about the impact of different physical activity programs on estrogen level of postmenopausal females. Some studies have found a negative association between physical activity and fat mass and also in the estradiol levels, whereas others revealed the positive association. A study conducted in 2016 by Wint et al. revealed that 3 h of moderate exercise per week decrease the estrogen level. However study conducted in 2015 by Hackney concluded that aerobic training decreases the estrogen hormone, whereas the effect of anaerobic exercise was unknown.[42]
Several prior reports have also evaluated association among body composition and estradiol level in the body, but such association does not compare according to the dose of exercise.
Rossi et al., in 2015, suggest that aerobic exercise decreases the adiposity, whereas resistance exercise improving the muscle mass. Another study conducted in 2015 concludes that exercise helps to burn more fat and reduces fat mass, hence, helps in decreasing the estrogen level.[56]
The findings of our study indicate that despite decreasing fat mass, physical exercises increase the estradiol level significantly when 12 weeks of exercise protocol is incorporated among the postmenopausal females. The individuality of this study is based on the concept of exercises as medicine, in which the 12 weeks of training protocol was used. Besides, determining the effect of exercise regimes as a medicine, the study also aimed to identify the efficacy of exercises applied in the management of postmenopausal symptoms and for that purpose efforts had been made in the process of data collection where the data had been taken twice from the patient once before the session and after 12 weeks of intervention. The study was unique in its approach as exercises based on the prescribed criteria of FITT as per the guidelines of the American College of Sports Medicine, 2013, were used to quantify the dosage of exercises. The results showed that exercises were not only found to be effective in the management of the primary outcome measure, i.e. estradiol level but indeed it was also revealed that out of the two groups, the anaerobic exercises had more causal effects on lean mass and BMD, in comparison to aerobic exercises. This may be due to the criteria of anaerobic exercises that were performed only 3 days per week, whereas aerobic exercises were performed for 5 days per week making a total dosage of anaerobic for 36 days and aerobic for 60 days, thus establishing a fact that anaerobic regime of exercises was time and cost effective than aerobic for the management of postmenopausal osteoporotic female. A few limitations of the study need to be overcome where only 12 weeks of protocol was used and no residual effects were checked as a follow-up of next 6 months was to be checked. However, this is beyond the scope of our study.
Conclusion
The finding of our study shows that 12 weeks of anaerobic exercise training based on the ACSM FITT protocol is found to be effective in the improvement of estradiol level and BMD in postmenopausal osteoporotic female. Moreover, it was also concluded that anaerobic exercise is associated with increasing the lean mass, whereas aerobic exercise plays a key role in reducing the fat mass; hence, we concluded that quality of life of postmenopausal osteoporotic female can be improved by anaerobic exercise program of 12 weeks.
References
- 1.Topçuoglu A, Uzun H, Aydin S, Kahraman N, Vehid S, Zeybek G, et al. The effect of hormone replacement therapy on oxidized low density lipoprotein levels and paraoxonase activity in postmenopausal women. Tohoku J Exp Med. 2005;205:79–86. doi: 10.1620/tjem.205.79. [DOI] [PubMed] [Google Scholar]
- 2.Gold EB, Bromberger J, Crawford S, Samuels S, Greendale GA, Harlow SD, et al. Factors associated with age at natural menopause in a multiethnic sample of midlife women. Am J Epidemiol. 2001;153:865–74. doi: 10.1093/aje/153.9.865. [DOI] [PubMed] [Google Scholar]
- 3.Sowers MR, La Pietra MT. Menopause:Its epidemiology and potential association with chronic diseases. Epidemiol Rev. 1995;17:287–302. doi: 10.1093/oxfordjournals.epirev.a036194. [DOI] [PubMed] [Google Scholar]
- 4.Fleming LE, Levis S, LeBlanc WG, Dietz NA, Arheart KL, Wilkinson JD, et al. Earlier age at menopause, work, and tobacco smoke exposure. Menopause. 2008;15:1103–8. doi: 10.1097/gme.0b013e3181706292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dratva J, Gómez Real F, Schindler C, Ackermann-Liebrich U, Gerbase MW, Probst-Hensch NM, et al. Is age at menopause increasing across Europe? Results on age at menopause and determinants from two population-based studies. Menopause. 2009;16:385–94. doi: 10.1097/gme.0b013e31818aefef. [DOI] [PubMed] [Google Scholar]
- 6.Nisar N, Sohoo NA. Severity of menopausal symptoms and the quality of life at different status of menopause:A community based survey from rural Sindh, Pakistan. Int J Collab Res Intern Med Public Health. 2010;2:118. [Google Scholar]
- 7.Kapur P, Sinha B, Pereira BM. Measuring climacteric symptoms and age at natural menopause in an Indian population using the greene climacteric scale. Menopause. 2009;16:378–84. doi: 10.1097/gme.0b013e31818a2be9. [DOI] [PubMed] [Google Scholar]
- 8.Jassim GA, Al-Shboul Q. Attitudes of Bahraini women towards the menopause:Implications for health care policy. Maturitas. 2008;59:358–72. doi: 10.1016/j.maturitas.2008.03.014. [DOI] [PubMed] [Google Scholar]
- 9.Greer W, Sandridge AL, Chehabeddine RS. The frequency distribution of age at natural menopause among Saudi Arabian women. Maturitas. 2003;46:263–72. doi: 10.1016/s0378-5122(03)00215-9. [DOI] [PubMed] [Google Scholar]
- 10.Vaze N, Joshi S. Yoga and menopausal transition. J Midlife Health. 2010;1:56–8. doi: 10.4103/0976-7800.76212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hunter MS, Gentry-Maharaj A, Ryan A, Burnell M, Lanceley A, Fraser L, et al. Prevalence, frequency and problem rating of hot flushes persist in older postmenopausal women:Impact of age, body mass index, hysterectomy, hormone therapy use, lifestyle and mood in a cross-sectional cohort study of 10,418 British women aged 54-65. BJOG. 2012;119:40–50. doi: 10.1111/j.1471-0528.2011.03166.x. [DOI] [PubMed] [Google Scholar]
- 12.Couzi RJ, Helzlsouer KJ, Fetting JH. Prevalence of menopausal symptoms among women with a history of breast cancer and attitudes toward estrogen replacement therapy. J Clin Oncol. 1995;13:2737–44. doi: 10.1200/JCO.1995.13.11.2737. [DOI] [PubMed] [Google Scholar]
- 13.Pérez JA, Garcia FC, Palacios S, Pérez M. Epidemiology of risk factors and symptoms associated with menopause in Spanish women. Maturitas. 2009;62:30–6. doi: 10.1016/j.maturitas.2008.10.003. [DOI] [PubMed] [Google Scholar]
- 14.Asadi M, Jouyandeh Z, Nayebzadeh F. Prevalence of menopause symptoms among Iranian women. J Fam Reprod Health. 2012;6:1–3. [Google Scholar]
- 15.Schnatz PF, Serra J, O'Sullivan DM, Sorosky JI. Menopausal symptoms in hispanic women and the role of socioeconomic factors. Obstet Gynecol Surv. 2006;61:187–93. doi: 10.1097/01.ogx.0000201923.84932.90. [DOI] [PubMed] [Google Scholar]
- 16.Henderson VW. Aging, estrogens, and episodic memory in women. Cogn Behav Neurol. 2009;22:205–14. doi: 10.1097/WNN.0b013e3181a74ce7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jackson RA, Vittinghoff E, Kanaya AM, Miles TP, Resnick HE, Kritchevsky SB, et al. Urinary incontinence in elderly women:Findings from the health, aging, and body composition study. Obstet Gynecol. 2004;104:301–7. doi: 10.1097/01.AOG.0000133482.20685.d1. [DOI] [PubMed] [Google Scholar]
- 18.Geusens P, Autier P, Boonen S, Vanhoof J, Declerck K, Raus J, et al. The relationship among history of falls, osteoporosis, and fractures in postmenopausal women. Arch Phys Med Rehabil. 2002;83:903–6. doi: 10.1053/apmr.2002.33111. [DOI] [PubMed] [Google Scholar]
- 19.Iatrakis G, Haronis N, Sakellaropoulos G, Kourkoubas A, Gallos M. Psychosomatic symptoms of postmenopausal women with or without hormonal treatment. Psychother Psychosom. 1986;46:116–21. doi: 10.1159/000287971. [DOI] [PubMed] [Google Scholar]
- 20.Erbil N. Attitudes towards menopause and depression, body image of women during menopause. Alex J Med. 2018;54:241–6. [Google Scholar]
- 21.Freeman EW. Associations of depression with the transition to menopause. Menopause. 2010;17:823–7. doi: 10.1097/gme.0b013e3181db9f8b. [DOI] [PubMed] [Google Scholar]
- 22.Blumel JE, Castelo-Branco C, Binfa L, Gramegna G, Tacla X, Aracena B, et al. Quality of life after the menopause:A population study. Maturitas. 2000;34:17–23. doi: 10.1016/s0378-5122(99)00081-x. [DOI] [PubMed] [Google Scholar]
- 23.Messier V, Rabasa-Lhoret R, Barbat-Artigas S, Elisha B, Karelis AD, Aubertin-Leheudre M, et al. Menopause and sarcopenia:A potential role for sex hormones. Maturitas. 2011;68:331–6. doi: 10.1016/j.maturitas.2011.01.014. [DOI] [PubMed] [Google Scholar]
- 24.Toth MJ, Tchernof A, Sites CK, Poehlman ET. Menopause-related changes in body fat distribution. Ann N Y Acad Sci. 2000;904:502–6. doi: 10.1111/j.1749-6632.2000.tb06506.x. [DOI] [PubMed] [Google Scholar]
- 25.Heymsfield SB, Gallagher D, Poehlman ET, Wolper C, Nonas K, Nelson D, et al. Menopausal changes in body composition and energy expenditure. Exp Gerontol. 1994;29:377–89. doi: 10.1016/0531-5565(94)90018-3. [DOI] [PubMed] [Google Scholar]
- 26.Maltais ML, Desroches J, Dionne IJ. Changes in muscle mass and strength after menopause. J Musculoskelet Neuronal Interact. 2009;9:186–97. [PubMed] [Google Scholar]
- 27.Wasalathanthri S. Menopause and exercise:Linking pathophysiology to effects. Arch Med. 2015;28:1–7. [Google Scholar]
- 28.Moreira LD, Oliveira ML, Lirani-Galvão AP, Marin-Mio RV, Santos RN, Lazaretti-Castro M, et al. Physical exercise and osteoporosis:Effects of different types of exercises on bone and physical function of postmenopausal women. Arq Bras Endocrinol Metabol. 2014;58:514–22. doi: 10.1590/0004-2730000003374. [DOI] [PubMed] [Google Scholar]
- 29.Lindsay R. The menopause and osteoporosis. Obstet Gynecol. 1996;87:16S–9. doi: 10.1016/0029-7844(95)00430-0. [DOI] [PubMed] [Google Scholar]
- 30.Schiessl H, Frost HM, Jee WS. Estrogen and bone-muscle strength and mass relationships. Bone. 1998;22:1–6. doi: 10.1016/s8756-3282(97)00223-8. [DOI] [PubMed] [Google Scholar]
- 31.Al-Maatouq MA, El-Desouki MI, Othman SA, Mattar EH, Babay ZA, Addar M, et al. Prevalence of osteoporosis among postmenopausal females with diabetes mellitus. Saudi Med J. 2004;25:1423–7. [PubMed] [Google Scholar]
- 32.Arnold BA. Image Analysis Inc. Assignee. Automated X-ray Bone Densitometer. United States Patent US 6,320,931. 2001 [Google Scholar]
- 33.Zahoor S, Ayub U. Prevalence of osteoporosis in postmenopausal women visiting police and services hospital, PESHAWAR, NWFP. J Postgrad Med Inst. 2011;24:4–7. [Google Scholar]
- 34.Iwamoto J, Takeda T, Ichimura S. Effect of exercise training and detraining on bone mineral density in postmenopausal women with osteoporosis. J Orthop Sci. 2001;6:128–32. doi: 10.1007/s007760100059. [DOI] [PubMed] [Google Scholar]
- 35.Asikainen TM, Kukkonen-Harjula K, Miilunpalo S. Exercise for health for early postmenopausal women:A systematic review of randomised controlled trials. Sports Med. 2004;34:753–78. doi: 10.2165/00007256-200434110-00004. [DOI] [PubMed] [Google Scholar]
- 36.Schwartz RS, Evans WJ. Effects of exercise on body composition and functional capacity of the elderly. J Gerontol Ser A Biol Sci Med Sci. 1995;50:147–50. doi: 10.1093/gerona/50a.special_issue.147. [DOI] [PubMed] [Google Scholar]
- 37.Elavsky S, McAuley E. Physical activity, symptoms, esteem, and life satisfaction during menopause. Maturitas. 2005;52:374–85. doi: 10.1016/j.maturitas.2004.07.014. [DOI] [PubMed] [Google Scholar]
- 38.Ketabipoor SM, Jahromi MK. Effect of aerobic exercise in water on serum estrogen and C-reactive protien and body mass index level in obese and normal weight postmenopausal women. Womens Health Bull. 2015;2:e25048. [Google Scholar]
- 39.Chan MF, Dowsett M, Folkerd E, Bingham S, Wareham N, Lu S, et al. Usual physical activity and endogenous sex hormones in postmenopausal women:The European prospective investigation into cancer-norfolk population study. Cancer Epidemiol Biomarkers Prev. 2007;16:900–5. doi: 10.1158/1055-9965.EPI-06-0745. [DOI] [PubMed] [Google Scholar]
- 40.Atkinson C, Lampe JW, Tworoger SS, Ulrich CM, Bowen D, Irwin ML, et al. Effects of a moderate intensity exercise intervention on estrogen metabolism in postmenopausal women. Cancer Epidemiol Biomarkers Prev. 2004;13:868–74. [PubMed] [Google Scholar]
- 41.Copeland JL, Consitt LA, Tremblay MS. Hormonal responses to endurance and resistance exercise in females aged 19-69 years. J Gerontol A Biol Sci Med Sci. 2002;57:B158–65. doi: 10.1093/gerona/57.4.b158. [DOI] [PubMed] [Google Scholar]
- 42.Hackney AC. Exercise as a stressor to the human neuroendocrine system. Medicina (Kaunas) 2006;42:788–97. [PubMed] [Google Scholar]
- 43.Truesdale KP, Roberts A, Cai J, Berge JM, Stevens J. Comparison of eight equations that predict percent body fat using skinfolds in American youth. Child Obes. 2016;12:314–23. doi: 10.1089/chi.2015.0020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Aandstad A, Holtberget K, Hageberg R, Holme I, Anderssen SA. Validity and reliability of bioelectrical impedance analysis and skinfold thickness in predicting body fat in military personnel. Mil Med. 2014;179:208–17. doi: 10.7205/MILMED-D-12-00545. [DOI] [PubMed] [Google Scholar]
- 45.O'Donnell E, Kirwan LD, Goodman JM. Aerobic exercise training in healthy postmenopausal women:Effects of hormone therapy. Menopause. 2009;16:770–6. doi: 10.1097/gme.0b013e318198cddb. [DOI] [PubMed] [Google Scholar]
- 46.Peltz G, Aguirre MT, Sanderson M, Fadden MK. The role of fat mass index in determining obesity. Am J Hum Biol. 2010;22:639–47. doi: 10.1002/ajhb.21056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.American College of Sports of Medicine, editor. ACSM's Health-Related Physical Fitness Assessment Manual. Philadelphia, PA: Lippincott Williams and Wilkins; 2013. [Google Scholar]
- 48.Park HK, Jung MK, Park E, Lee CY, Jee YS, Eun D, et al. The effect of warm-ups with stretching on the isokinetic moments of collegiate men. J Exerc Rehabil. 2018;14:78–82. doi: 10.12965/jer.1835210.605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lauersen JB, Bertelsen DM, Andersen LB. The effectiveness of exercise interventions to prevent sports injuries:A systematic review and meta-analysis of randomised controlled trials. Br J Sports Med. 2014;48:871–7. doi: 10.1136/bjsports-2013-092538. [DOI] [PubMed] [Google Scholar]
- 50.Johnson MJ, Close L, Gillon SC, Molassiotis A, Lee PH, Farquhar MC, et al. Use of the modified borg scale and numerical rating scale to measure chronic breathlessness:A pooled data analysis. Eur Respir J. 2016;47:1861–4. doi: 10.1183/13993003.02089-2015. [DOI] [PubMed] [Google Scholar]
- 51.Guazzi M, Adams V, Conraads V, Halle M, Mezzani A, Vanhees L, et al. Clinical recommendations for cardiopulmonary exercise testing data assessment in specific patient populations. Eur Heart J. 2012;33:2917–27. doi: 10.1093/eurheartj/ehs221. [DOI] [PubMed] [Google Scholar]
- 52.Honisett SY, Tangalakis K, Wark J, Apostolopoulos V, Stojanovska L. The effects of hormonal therapy and exercise on bone turnover in postmenopausal women:A randomised double-blind pilot study. Pril (Makedon Akad Nauk Umet Odd Med Nauki) 2016;37:23–32. doi: 10.1515/prilozi-2016-0013. [DOI] [PubMed] [Google Scholar]
- 53.Moghadasi M, Siavashpour S. The effect of 12 weeks of resistance training on hormones of bone formation in young sedentary women. Eur J Appl Physiol. 2013;113:25–32. doi: 10.1007/s00421-012-2410-0. [DOI] [PubMed] [Google Scholar]
- 54.Kenney WL, Wilmore J, Costill D. Physiology of Sport and Exercise. 6th ed. Champaign, IL: Human Kinetics; 2015. [Google Scholar]
- 55.Teoman N, Ozcan A, Acar B. The effect of exercise on physical fitness and quality of life in postmenopausal women. Maturitas. 2004;47:71–7. doi: 10.1016/s0378-5122(03)00241-x. [DOI] [PubMed] [Google Scholar]
- 56.Rossi FE, Buonani C, Viezel J, Silva EP, Diniz TA, Santos VR, et al. Effect of combined aerobic and resistance training in body composition of obese postmenopausal women. Motriz. 2015;21:61–7. [Google Scholar]


