Abstract
Introduction
Antiretroviral therapy (ART) has reduced morbidity and mortality in sub‐Saharan Africa, but the burden of coexistent cardiopulmonary disease in perinatally HIV‐positive adolescents on antiretroviral therapy (ART) has not been well described. The aim of this study was to investigate the prevalence and associations of cardiopulmonary dysfunction in adolescents with perinatally acquired HIV on ART.
Methods
For this cross‐sectional analysis, 515 perinatally HIV‐positive adolescents ages 9 to 14 years on ART for at least six months, and a comparator group of 110 age‐matched HIV‐uninfected adolescents were tested between August 2013 and April 2015 using echocardiography, six‐minute walk test (6MWT) and spirometry. Those with either abnormal spirometry or abnormal 6MWT and any right or left systolic or diastolic dysfunction or abnormal mean pulmonary arterial pressure were considered as having impaired cardiopulmonary function. Logistic regression was used to investigate determinants of impaired cardiopulmonary function.
Results
Overall, 474 adolescents with perinatally acquired HIV (mean [SD] age, 12 [1.6] years; median [IQR] ART duration, 7 [4.6 to 9.3] years; median [IQR] CD4 count, 712 [571 to 959] cell/mm3) and 109 HIV‐uninfected adolescents mean (SD) age 11.8 (1.8) years, had successful cardiac and lung function testing. Impaired cardiopulmonary function was detected in 13% of adolescents with perinatally acquired HIV and 8% of HIV‐uninfected adolescents, p = 0.136. Among adolescents with perinatally acquired HIV, those with low tricuspid annular plane systolic excursion (TAPSE) had significantly lower mean FEV1, 1.5 L versus 1.6 L, p = 0.011. Height (OR 0.7, 95%CI 0.5 to 0.9), body mass index (OR 0.7, 95%CI 0.5 to 0.9) and past pulmonary tuberculosis (OR 2.3, 95%CI 1.2 to 4.4) were significantly associated with a low cardiopulmonary function.
Conclusions
Despite being on ART, cardiopulmonary dysfunction occurs in an appreciable proportion of perinatally HIV‐infected adolescents but no significant difference to uninfected controls. This finding requires further exploration. Factors associated with dysfunction may be amenable to public health interventions to reduce cardiopulmonary disease in this population.
Keywords: perinatal HIV infection, antiretroviral therapy, cardiopulmonary dysfunction, adolescents, HIV care continuum, South Africa
1. Introduction
With improved survival of adolescents with perinatally acquired HIV on antiretroviral therapy (ART), HIV has become a chronic disease, with perinatally infected children surviving into adolescence and adulthood 1. HIV‐related cardiac disease 2, 3, 4 and/or chronic lung disease has been reported in sub‐Saharan Africa 5, 6. Symptoms which can be attributed to either cardiac or lung disease like tachypnoea or dyspnoea can overlap posing a diagnostic challenge.
In the pre‐ART era, a high prevalence of cardiac abnormalities especially left ventricular dysfunction and cardiomyopathy were reported 7, but a sharp decline has been found with the use of ART 8, 9. Although ART has reduced the incidence and severity of acute pulmonary infections, lung function abnormalities are still highly prevalent 10, 11. Right heart dysfunction may be secondary to chronic lung disease. Miller et al. 4 reported a 29% prevalence of right ventricle dilatation in a Zimbabwe cohort of perinatally HIV‐positive adolescents (71% on ART, median duration of ART 20 months). Nearly 50% of this population had chronic lung disease.
ART also has an impact on exercise tolerance, which is a marker of cardiopulmonary function status. HIV‐positive children on ART in Malawi had better exercise tolerance compared with HIV‐positive ART‐naive children 12. ART‐naive HIV‐positive children had worse symptoms of cough, dyspnoea, hypoxaemia and low exercise tolerance compared with those on ART 13. Although ART is reported to improve cardiac function, exercise tolerance and lung function, children on ART still have lower lung function 11, lower exercise tolerance 12 and lower cardiac function 14 compared to HIV‐uninfected children. Most of these published studies were from the era when ART access was not universal, was initiated based on clinical and immunological severity; hence the need for studies from well‐established ART cohorts.
Risk factors for cardiopulmonary dysfunction may include HIV immunosuppression, opportunistic infections, poor ART adherence, late age at initiation of ART, malnutrition or smoking. Other risk factors may be specific to cardiac dysfunction like dyslipidaemia. Furthermore, transplacental exposure to drugs like zidovudine has been reported to impact foetal cardiac development 15. Lung disease may lead to right heart strain and pulmonary hypertension progressing to cor pulmonale 16. Conversely, cardiac dysfunction as measured by a reduced left ventricular ejection fraction may result in fluid overload causing pulmonary oedema and subsequent poor lung compliance. HIV has also been documented to cause primary pulmonary hypertension in adults 17 and adolescents 18.
The aim of this study was to investigate the prevalence and determinants of cardiopulmonary dysfunction in perinatally HIV‐positive adolescents on ART.
2. Methods
A prospective study, the Cape Town Adolescent Anti‐retroviral cohort (CTAAC), previously described 11, enrolled 515 perinatally HIV‐positive adolescents on ART and 110 age‐matched HIV‐uninfected adolescents. Patients were enrolled from August 2013 to April 2015 and followed six monthly at the Research Centre for Adolescent and Child Health at Red Cross War Memorial Children's Hospital, South Africa.
Participants were eligible for the study if they were adolescents aged 9 to 14 years, with perinatal HIV infection, had been on ART for at least six months and knew their HIV status. Informed parental consent and participant assent were obtained. Age‐matched HIV‐uninfected adolescents without known pre‐existing lung or cardiac disease were enrolled from Masiphumelele Youth Centre in Cape Town, South Africa. Perinatal HIV exposure of the HIV‐uninfected participants was unknown, but all tested negative for HIV prior to enrolment in the study. Ethical approval was obtained from the Human Research Ethics Committee of the University of Cape Town.
Data presented here are from the enrolment visit. Data on demography, self‐reported smoking history and cardiorespiratory symptoms, ART duration and adherence, previous pulmonary tuberculosis (PTB) and other lower respiratory tract infections (LRTI) were collected by validated questionnaires. A history of previous PTB or LRTI was extracted from hospital records and supplemented by participant or caregiver report. Respiratory symptoms of wheeze, shortness of breath were self‐reported. Blood was taken for CD4 count (Beckman Coulter®, Fullerton, CA, USA) and HIV viral load (Roche COBAS Ampliprep, Mannheim, Germany). Adherence to ART was self‐reported and measured using any missed doses in the last 30 days.
2.1. Lung function testing
Lung function testing 11 included spirometry measuring forced expiratory volume in one second (FEV1), forced vital capacity (FVC) and FEV1/FVC as measures of dynamic lung volumes and airflow obstruction; and the six‐minute walk test measuring effort tolerance. Lung function testing was deferred if the participant had an acute respiratory illness. Spirometry was done using the NDD Easyone Pro LAB (NDD, Zurich, Switzerland). All testing adhered to the American Thoracic Society/European Respiratory Society (ATS/ERS) guidelines 19, 20, 21. We reported the highest FVC or FEV1 from any of three acceptable spirometric attempts. The lower limit of normal (LLN) for spirometry outcome variables was calculated using the African American reference cohort in Global Lung Initiative (GLI) software, −1.64 standard deviations (SD) below the mean 22.
For the six‐minute walk test, the participant was instructed to walk for six minutes, between two marked cones placed 30 m apart, as per standardized recommendations 21. Heart rate, respiratory rate, blood pressure, Borg scale for dyspnoea and oxygen saturation were recorded before and at the end of testing. Distance covered in metres was recorded at the end of the test, with published reference data used as normative values 23.
Spirometry patterns were used to define abnormal lung function. Abnormal lung function was defined as abnormal spirometry with obstructive (FEV1/FVC less than the lower limit of normal (LLN), restrictive (FVC<LLN with normal FEV1/FVC) or mixed pattern spirometry (FEV1<LLN, FEV1/FVC<LLN and FVC<LLN).
2.2. Cardiac function testing
Cardiac function testing 24 was assessed by echocardiography, performed by a trained research echocardiographer using either a Philips IE33 or CX50 echo machines (Phillips, Netherlands) using standardized techniques 25, 26. All echocardiographs were interpreted by a single cardiologist. A random subset of 10% was also read by a second blinded cardiologist. Both cardiologists were blinded to the HIV status of the participant. Inter‐reader disagreements were resolved by consensus.
Right ventricular (RV) systolic function was determined by calculating the percentage fractional area change (FAC) and the volumetric RV ejection fraction that is, tricuspid annular plane systolic excursion (TAPSE). TAPSE was measured using M Mode echo 27 and FAC was measured by a two‐dimensional technique for tracing the area during systole and diastole by using the formula = (RV end‐diastolic area − RV end‐systolic area)/RV end‐diastolic area × 100 28. Pulmonary artery pressures (systolic and diastolic) were estimated using standard continuous and pulse wave Doppler methods. Cardiac dimensions were assessed in the standard manner either using direct measurement of 2‐D images or M Mode recordings. TAPSE is a global parameter for right ventricular function which describes apex‐to‐base shortening 29, 30. TAPSE has been found to be highly specific and easy method to estimate the right ventricular ejection fraction 31, 32. FAC has better correlation with cardiac MRI‐derived RV systolic dysfunction 33.
Left ventricular (LV) systolic function was determined by measuring shortening fraction (M‐mode) and deriving ejection fraction using the Teichholz method 34 and the modified Simpson's method 35. Left ventricular diastolic function was measured using Doppler assessment of mitral inflow. Tissue Doppler techniques were used to measure mitral annular velocity.
The following terminologies were used to define abnormal findings:
Right ventricular systolic dysfunction was defined as low TAPSE or low FAC. TAPSE z‐score <2 (z‐score was calculated based on published normal values) 36. Z‐scores were normalized to body surface area. 37. A fractional area change measurement of the RV (FAC) ≤34% was considered abnormal 38.
Pulmonary hypertension: Mean pulmonary arterial pressure (mPAP) was calculated using the Chemla equation. 2, 39 (mPAP = (0.61 × PAPs) + 2 mmHg) was normal if less than 25 mmHg 2.
LV systolic dysfunction: Left ventricular shortening fraction (LVSF) ≤25% 27.
LV diastolic dysfunction: E‐wave/A‐wave normal range was calculated according to age as per Eidem et al. 40.
Cardiopulmonary dysfunction was defined as any right ventricle or left ventricle systolic or diastolic dysfunction or abnormal mean pulmonary arterial pressure and abnormal spirometry or abnormal 6MWT
2.3. Data analysis
Descriptive statistics were used to describe characteristics of the study population and to summarize cardiopulmonary outcomes by HIV status. Comparison of cardiopulmonary and clinical outcomes by HIV status was compiled using the two‐sample test of proportions. Independent two‐sample t‐test was used to compare lung function in the adolescents with perinatally acquired HIV between those with low TAPSE and normal TAPSE. A new variable, cardiopulmonary status was generated; those with either obstructive or restrictive or mixed spirometry or abnormal 6MWT and any right or left systolic or diastolic dysfunction or abnormal mean pulmonary arterial pressure were considered as an impaired cardiopulmonary function. Univariate and multivariate logistic regression was done using the cardiopulmonary function as the outcome variable.
3. Results
Five hundred and fifteen adolescents with perinatally acquired HIV and 110 HIV‐uninfected controls had lung function testing. Four hundred and seventy‐four adolescents with perinatally acquired HIV and 109 HIV‐uninfected completed echocardiogram testing; 478 HIV‐positive adolescents, 104 uninfected adolescents completed six‐minute walk test, Figure 1. Mean (SD) age was 12 (1.6) years and 50% were male. Median (IQR) duration of ART was 7.6 (4.6 to 9.3) years. Median (IQR) CD4 count was 712 (571 to 959) cells/mm3, 77.9% had viral load <50 copies/mL, Table 1. Sixty per cent of the participants were on two nucleoside‐reverse transcriptase inhibitors (NRTI), 75% on abacavir and one non‐nucleoside‐reverse transcriptase inhibitors (NNRTI), 98% were on efavirenz. Median age at ART initiation was 4.4 (2.0 to 7.0) years, with 29.7% initiating ART at <2 years of age. Cough and digital clubbing were more common in the adolescents with perinatally acquired HIV, p = 0.05 for both, Table 1. History of shortness of breath occurred rarely in less than 5% of participants, Table 1.
Figure 1. Flow diagram for the study population.
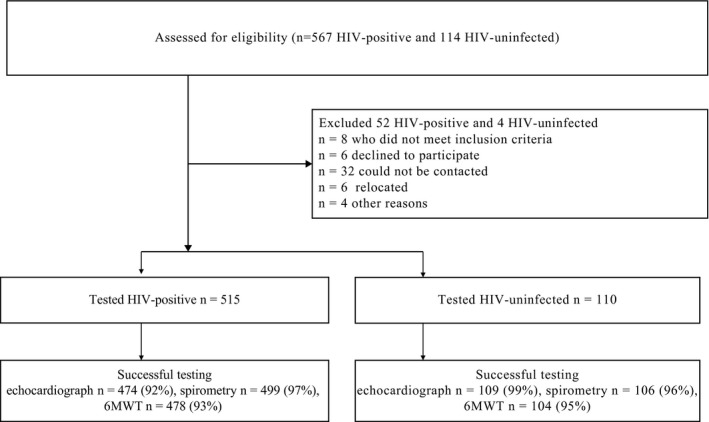
6MWT, six‐minute walk test.
Table 1.
Baseline characteristics of study population
| Variable | HIV‐positive n = 474 | HIV‐uninfected n = 109 | p value* |
|---|---|---|---|
| Age, years | 12.0 (1.6) | 11.8 (1.8) | 0.257 |
| Male, n % | 247 (51.7) | 47 (45.2) | 0.231 |
| Height z‐score | −1.3 (1.1) | −0.5 (1.0) | <0.001 |
| Respiratory rate, breaths/min | 21.5 (3.5) | 20.8 (5.1) | 0.082 |
| Viral load category, copies/mL | – | ||
| <50, n (%) | 369 (77.9) | ||
| 50 to 1000, n (%) | 46 (9.7) | ||
| 1001 to 10,000, n (%) | 32 (6.8) | ||
| >10,000, n (%) | 26 (5.5) | ||
| CD4 count, cells/mm3 | 712 (571 to 959) | – | |
| WHO HIV staging at HIV diagnosis, n (%) | |||
| I | 34 (7.2) | ||
| II | 47 (9.9) | ||
| III | 266 (56.1) | ||
| IV | 105 (22.2) | ||
| ART used | |||
| NNRTI+2NRTI | 282 (59.5) | ||
| PI+2NRTI | 175 (36.9) | ||
| Others | 9 (1.9) | ||
| Previous PTB, n (%) | 287 (58.5) | 2 (0) | <0.001 |
| Previous severe LRTI, n (%) | 134 (28.3) | 1 (0.9) | <0.001 |
| Tobacco smoke exposure, n (%) | 119 (25.0) | 22 (20.9) | 0.279 |
| Poor ART adherence, n (%) | 111 (23.4) | – | |
| ART duration, years, n (%) | 7.0 (3.0) | – | |
| Age at ART initiation, years | 4.4 (2.0 to 7.0) | ||
| Shortness of breath, n (%) | 16 (3.4) | 2 (1.8) | 0.402 |
| History of wheeze, n (%) | 51 (10.8) | 6 (5.5) | 0.096 |
| History of cough, n (%) | 69 (14.6) | 8 (7.3) | 0.045 |
| History of doctor‐diagnosed asthma, n (%) | 57 (12.0) | 6 (5.5) | 0.048 |
| Finger clubbing, n (%) | 16 (3.5) | 0 | 0.052 |
ART, antiretroviral therapy; LRTI, lower respiratory tract infections; NNRTI, non‐nucleoside reverse transcriptase inhibitor; NRTI, nucleoside reverse transcriptase inhibitor; PI, protease inhibitor; PTB, pulmonary tuberculosis; WHO, World Health Organization.
Values are mean/SD except for age at ART initiation, viral load and CD4 which are median (IQR). *p values derived from chi‐square or two sample t‐test.
Adolescents with perinatally acquired HIV with mixed pattern spirometry had a higher rate of RV dysfunction, Figure 2. None of the uninfected adolescents had mixed pattern spirometry. Obstructive and mixed pattern spirometry was reported in 5% of the HIV‐positive adolescents, Table 2. Right ventricle dysfunction (32%) was more common than left ventricular dysfunction (7%) in the adolescents with perinatally acquired HIV but not significantly different to uninfected adolescents (26.6% of whom had RV dysfunction and 5.5% LV dysfunction), Table 2. Thirteen per cent of perinatally HIV‐positive and 8.3% of uninfected adolescents had impaired cardiopulmonary function, Table 2. Sixteen per cent of HIV‐infected and 5% of uninfected adolescents had FEF25 to 75 less than the lower limit of normal for age, sex and height, Table 2. A pictorial diagram that shows the proportions of the various cardiac and lung function abnormalities in the HIV‐infected cohort is presented, Figure 3. Only two HIV ‐infected children had pulmonary hypertension, Table 2. Among adolescents with perinatally acquired HIV those with low TAPSE had significantly lower mean FEV1, 1.5 L versus 1.6 L, p = 0.011, Table 3.
Figure 2. Spirometry pattern and right ventricle function.
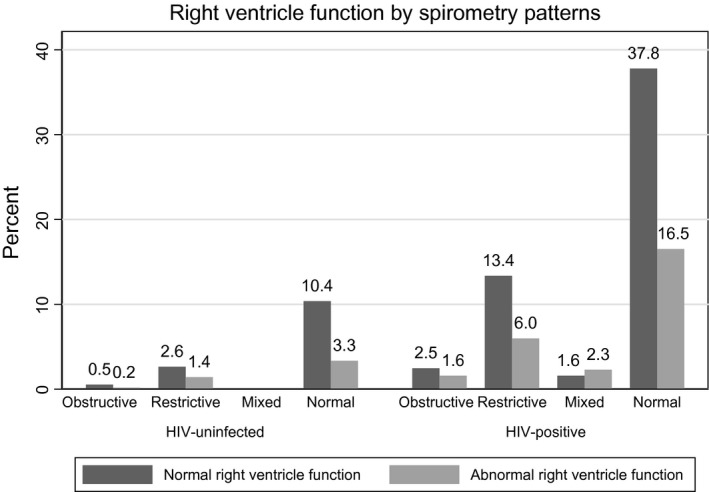
Table 2.
Cardiopulmonary measurements by HIV status
| Variable, n (%) | n | HIV‐positive | n | HIV‐uninfected | p value* |
|---|---|---|---|---|---|
| Restrictive spirometry | 474 | 110 (23.2) | 109 | 23 (21.1) | 0.637 |
| Obstructive spirometry | 474 | 23 (4.9) | 109 | 4 (3.4) | 0.596 |
| Mixed pattern spirometry | 474 | 22 (4.6) | 109 | 0 | 0.022 |
| FEF25 to 75 below LLN | 474 | 76 (16.0) | 109 | 6 (5.5%) | 0.004 |
| Right ventricular dysfunction (low TAPSE and low FAC)* | 474 | 154 (32.5) | 109 | 29 (26.6) | 0.232 |
| Pulmonary hypertension | 474 | 2 (0.46) | 109 | 0 | 0.476 |
| Left ventricular diastolic dysfunction | 474 | 36 (7.6) | 109 | 6 (5.5) | 0.447 |
| Left ventricle systolic dysfunction | 474 | 1 (0.2) | 109 | 0 | 0.631 |
| Cardiopulmonary function (impaired)* | 474 | 64 (13.5) | 109 | 9 (8.3) | 0.136 |
| Pulse before walk (mean/SD) | 478 | 78.6 (12.5) | 104 | 82.9 (14.2) | 0.002 |
| Pulse after walk (mean/SD) | 478 | 82.9 (13.8) | 104 | 87.1 (15.0) | 0.004 |
| Oxygen saturation before walk (mean/SD) | 478 | 98.4 (2.0) | 104 | 98.6 (0.8)) | 0.315 |
| Oxygen saturation after walk (mean/SD) | 478 | 98.3 (2.7) | 104 | 98.4 (1.4) | 0.675 |
| MAP before 6MWT (mean/SD) | 478 | 79.2 (7.7) | 104 | 82.6 (8.1) | <0.001 |
| MAP after 6MWT | 478 | 83.0 (8.7) | 104 | 86.6 (8.5) | 0.001 |
| Borg scale before 6MWT (mean/SD) | 478 | 0.1 (0.2) | 104 | 0.04 (0.2) | 0.577 |
| Borg scale after 6MWT (mean/SD) | 478 | 1.3 (0.6) | 104 | 1.3 (0.7) | 0.737 |
| Distance walked in 6 min (mean/SD) | 478 | 437.8 (60.4) | 104 | 443.8 (60.7) | 0.380 |
6MWT, six‐minute walk test; FAC, fractional area change, Borg scale (perceived exertion scale); FEF25 to 75, forced expiratory flow at 25% and 75% of forced vital capacity; LLN, lower limit of normal calculated from African American reference values 22; MAP, mean arterial pressure (calculated from blood pressure); TAPSE, tricuspid annular plane systolic excursion.
p value derived from chi‐square or two sample t‐test.
Figure 3. Lung and cardiac functional abnormalities in perinatally HIV‐positive adolescents.
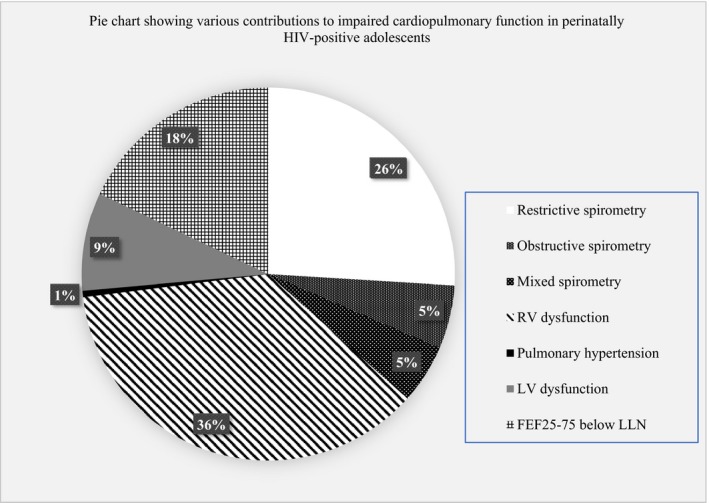
FEF25‐75, forced expiratory flow at 25% and 75% of forced vital capacity; LLN, lower limit of normal calculated from African‐American reference values 22 ; LV, left ventricle; RV, right ventricle.
Table 3.
Lung function by tricuspid annular plane systolic excursion (TAPSE) index in HIV‐positive adolescents
| Lung function | n | Low TAPSE | n | Normal TAPSE | p value* |
|---|---|---|---|---|---|
| Mean/SD | Mean/SD | ||||
| FEV1 (L) | 114 | 1.5 (0.5) | 350 | 1.6 (0.4) | 0.011 |
| FVC (L) | 114 | 1.7 (0.5) | 350 | 1.8 (0.5) | 0.022 |
| FEF25 to 75 (L) | 114 | 2.0 (0.9) | 350 | 2.2 (0.8) | 0.041 |
| FEV1/FVC | 114 | 0.9 (0.1) | 350 | 0.9 (0.1) | 0.165 |
FEF25 to 75, forced expiratory flow at 25 to 75% of vital capacity; FEV1, forced expiratory volume in 1 sec; FVC, forced vital capacity; TAPSE, tricuspid annular plane systolic excursion.
p value from two sample t‐test.
The cardiopulmonary functional status as measured by distance walked in six minutes, Borg scale and oxygen saturation were not different between the two groups, Table 2. Mean Borg scale was 1.3 in both groups, Table 2. Mean pulse rate and mean arterial pressures were lower in the adolescents with perinatally acquired HIV compared to the HIV‐uninfected, p < 0.05 for all, Table 2.
Height (OR 0.7, 95% CI 0.5 to 0.9), body mass index (OR 0.7, 95%CI 0.5 to 0.9) and past pulmonary tuberculosis (OR 2.3, 95%CI 1.2 to 4.4) were significantly associated with low cardiopulmonary function, Table 4. Those with digital clubbing had significantly higher odds for impaired cardiopulmonary function, OR 4.5, 95% CI 1.6 to 12.3, adjusted for age, sex, height and HIV status, Table 5.
Table 4.
Associations of impaired cardiopulmonary function (n = 569) in perinatally HIV‐positive and uninfected adolescents
| Variable | Univariate Odds ratio | p value* | 95% CI | Multivariate Odds ratio | p value | 95% CI |
|---|---|---|---|---|---|---|
| Age | 1.1 | 0.365 | 0.9 to 1.2 | – | ||
| z‐height | 0.8 | 0.017 | 0.6 to 0.9 | 0.7 | 0.010 | 0.5 to 0.9 |
| z‐bmi | 0.7 | <0.001 | 0.5 to 0.8 | 0.7 | 0.009 | 0.5 to 0.9 |
| ETS exposure | 0.8 | 0.545 | 0.5 to 1.5 | – | ||
| Sex | 0.8 | 0.419 | 0.5 to 1.3 | – | ||
| Past LRTI | 1.6 | 0.124 | 0.9 to 2.7 | 1.4 | 0.321 | 0.7 to 2.5 |
| Past PTB | 2.1 | 0.013 | 1.2 to 3.9 | 2.3 | 0.017 | 1.2 to 4.4 |
| Viral load copies/mL | – | |||||
| 50 to 1000 | 0.8 | 0.677 | 0.3 to 2.2 | |||
| 1001 to 10,000 | 1.9 | 0.171 | 0.8 to 4.6 | |||
| >10,000 | 1.7 | 0.331 | 0.6 to 4.6 | |||
| ART duration | 1.1 | 0.062 | 1.0 to 1.2 | 1.1 | 0.228 | 0.9 to 1.2 |
ART, antiretroviral therapy; BMI, body mass index; ETS, environmental tobacco smoke; LRTI, lower respiratory tract infection; PTB, pulmonary tuberculosis.
Adjusted for HIV status. Logistic regression.
Table 5.
Association of respiratory symptoms/signs with impaired cardiopulmonary function (n = 569) in perinatally HIV‐positive and uninfected adolescents
| Symptom | Univariate | Multivariateb | ||||
|---|---|---|---|---|---|---|
| OR | 95% CI | p value | OR | 95% CI | p value | |
| Wheeze | 1.5 | 0.7 to 3.2 | 0.249 | 1.4 | 0.7 to 3.0 | 0.369 |
| Shortness of breath | 0.9 | 0.2 to 3.8 | 0.831 | 0.8 | 0.2 to 3.7 | 0.808 |
| Cough | 1.6 | 0.8 to 3.0 | 0.180 | 1.5 | 0.8 to 2.9 | 0.253 |
| Digital clubbing | 5.2 | 1.9 to 14.0 | 0.001 | 4.5 | 1.6 to 12.3 | 0.004 |
| Asthmaa | 1.5 | 0.7 to 3.2 | 0.249 | 1.3 | 0.6 to 2.7 | 0.445 |
Symptoms are self‐reported except digital clubbing aphysician‐diagnosed asthma; badjusted for HIV status, age, sex, height. Logistic regression.
4. Discussion
This study provides comprehensive lung function and cardiac function data on a large cohort of perinatally HIV‐positive South African adolescents on ART showing that a proportion of HIV‐positive adolescents had cardiopulmonary dysfunction despite being on long‐term ART and having well‐controlled HIV disease. However, the prevalence of cardiopulmonary dysfunction was similar to the uninfected adolescents. This may be due to lack of validated local African reference values for cardiac function parameters or measurement error in the echocardiography measurements.
Low FEF25 to 75, a marker of small airways disease, was significantly higher in the HIV‐positive group, consistent with studies of HIV‐positive adolescents in sub‐Saharan Africa that have reported a predominance of small airways lung disease 6, 41. However, we did not include low FEF25 to 75 in the definition of impaired cardiopulmonary function but used FEV1 or FVC as the standard measurement of clinically relevant lung disease 42.
Height, body mass index and previous history of pulmonary tuberculosis were associated with impaired cardiopulmonary function; and digital clubbing had higher odds of low cardiopulmonary function. Those with low TAPSE had lower lung function.
Contrary to the few published studies 43, 44, 45 that showed reduced exercise capacity as measured by treadmill or six‐minute walk test in HIV‐positive adolescents, this study did not show any differences in distance walked nor oxygen saturation post‐exercise between perinatally HIV‐positive and uninfected adolescents. This may reflect differences in study populations, as in the current study, the perinatally HIV‐positive adolescents were relatively well established on ART and had good control of HIV disease. It may also be due to differences in the exercise test modalities or outcome measured; the current study reported distance walked and oxygen saturation before and after the test, while the outcome measure was peak oxygen consumption (VO2max) in other studies 44, 45 43. Furthermore, the six‐minute walk test is an insensitive measure of mild cardiopulmonary compromise. It is a submaximal test and less predictive of VO2 max compared to the shuttle‐walk test 46. The lower mean heart rate and arterial pressure observed in adolescents with perinatally acquired HIV requires further study.
Right ventricular dysfunction was more common than left ventricular dysfunction in these data. However, this finding must be interpreted cautiously as the normal reference ranges used were from North America and symptoms of RV dysfunction in this cohort were minimal. Miller et al. 4 in a Zimbabwe cohort reported 29% of right ventricular dysfunction in a cohort that had a high rate of abnormal lung function 6. The prevalence of restrictive spirometry pattern in this cohort, Table 2, was unexpected and requires further exploration in an ongoing longitudinal study.
The finding that body mass index, height and past pulmonary tuberculosis were associated with impaired cardiopulmonary dysfunction may reflect the fact that stunting and opportunistic infections affect lung function and may lead to subsequent cardiac dysfunction. Pulmonary TB has also been associated with chronic obstructive pulmonary disease in adults with HIV 47. Previous data 11 reported that PTB and previous pneumonia were more prevalent in HIV‐positive adolescents and were both associated with low lung function. In addition, the higher likelihood of impaired cardiopulmonary dysfunction in those with digital clubbing may reflect lung disease leading to consequent heart dysfunction. Digital clubbing is well known to be associated with chronic cardiac or lung disease 48. Similarly, nail clubbing was common in a Malawi cohort of HIV‐positive adolescents with a high prevalence of chronic lung disease 49.
The strengths of this study include a large sample size of perinatally HIV‐positive adolescents on ART with a comparative group of HIV‐uninfected adolescents and the comprehensive lung and cardiac function tests. Our study was limited by the independent assessment of lung and cardiac function rather than a combined measure of cardiopulmonary function such as formal cardiopulmonary exercise testing. This would measure oxygen consumption and carbon dioxide excretion by sampling inspired and expired gas during exercise 50. Such technology is expensive and inaccessible in most paediatric centres in South Africa. However, we used a broader definition of impaired cardiopulmonary function to include both heart and lung function parameters used in our study. The cardiac function measures, TAPSE and FAC, are limited by the absence of African reference parameters; normative values were derived from a Caucasian population. Nevertheless, the uninfected control group served as a comparator.
5. Conclusions
This study indicates that cardiopulmonary dysfunction occurs in an appreciable proportion of African adolescents with perinatally acquired HIV despite being on ART and having well‐controlled HIV disease. However, the prevalence of cardiopulmonary dysfunction was similar in HIV‐uninfected adolescents, which may reflect lack of validated local African reference values for echocardiography measurements. In turn, this study highlights the need for development of more sensitive markers of lung or heart disease in adolescents with perinatally acquired HIV, given the minimal symptoms that participants reported. The study identified risk factors for cardiopulmonary dysfunction such as prior PTB and impaired nutrition highlighting areas that may be amenable to public health interventions to optimize health.
Competing interests
None.
Authors’ contributions
LG: data collection, statistical analysis and wrote the manuscript.
SM: analysed cardiac function data and wrote the section on cardiac function testing.
LZ: read all the echocardiography, critically reviewed the manuscript and provided comments which were incorporated into this manuscript.
JL: advised on data analysis, critically reviewed the manuscript and provided comments which were incorporated into this manuscript.
DG: data collection, critically reviewed the manuscript and provided comments which were incorporated into this manuscript.
LM: conception and designing of study, obtained funding, advised on data analysis, critically reviewed the manuscript and provided comments which were incorporated into this manuscript.
HZ: conception and designing of study, obtained funding, advised on data analysis and wrote the manuscript.
Acknowledgements
We thank the CTAAC study staff for patient assessment, recruitment and data management and the study participants for making themselves available for this project.
Funding
NIH R01HD074051, Medical Research Council SA, African Partnership for Chronic Diseases Research, Astra Zeneca ‐ South African Thoracic Society Grant, LZ is funded by the Medical Research Council SA and the National Research Foundation of South Africa.
Githinji, L. N. , Mahtab, S. , Zühlke, L. , Lawrenson, J. , Myer, L. , Gray, D. and Zar, H. Cardiopulmonary dysfunction in perinatally HIV‐infected South African adolescents on antiretroviral therapy: baseline findings from the Cape Town Adolescent Antiretroviral Cohort.J Int AIDS Soc. 2019; 22(7):e25340
References
- 1. Lowenthal ED, Bakeera‐Kitaka S, Marukutira T, Chapman J, Goldrath K, Ferrand RA. Perinatally acquired HIV infection in adolescents from sub‐Saharan Africa: a review of emerging challenges. Lancet Infect Dis. 2014;14:627–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Chelo D, Wawo E, Siaha V, Anakeu A, Ndongo FA, Ndombo POK, et al. Cardiac anomalies in a group of HIV‐infected children in a pediatric hospital: an echocardiographic study in Yaounde, Cameroon. Cardiovasc Diagno Ther. 2015;5(6):444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Okoromah CA, Ojo OO, Ogunkunle OO. Cardiovascular dysfunction in HIV‐infected children in a sub‐Saharan African country: comparative cross‐sectional observational study. J Trop Pediatr. 2012;58(1):3–11. [DOI] [PubMed] [Google Scholar]
- 4. Miller RF, Kaski JP, Hakim J, Matenga J, Nathoo K, Munyati S, et al. Cardiac disease in adolescents with delayed diagnosis of vertically acquired HIV infection. Clin Infect Dis. 2013;56:576–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Rylance J, Mchugh G, Metcalfe J, Mujuru H, Nathoo K, Wilmore S, et al. Chronic lung disease in HIV‐infected children established on antiretroviral therapy. AIDS. 2016;30(18):2795–803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Ferrand RA, Desai SR, Hopkins C, Elston CM, Copley SJ, Nathoo K, et al. Chronic lung disease in adolescents with delayed diagnosis of vertically acquired HIV infection. Clin Infect Dis. 2012;55(1):145–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Lubega S, Zirembuzi G, Lwabi P. Heart disease among children with HIV/AIDS attending the paediatric infectious disease clinic at Mulago Hospital. Afr Health Sci. 2005;5(3):219–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Namuyonga J, Lubega S, Musiime V, Lwabi P, Lubega I. Cardiac dysfunction among Ugandan HIV‐infected children on antiretroviral therapy. Pediatr Infect Dis J. 2016;35:e85–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Lipshultz SE, Williams PL, Wilkinson JD, Leister EC, Van Dyke RB, Shearer WT, et al. Cardiac status of children infected with human immunodeficiency virus who are receiving long‐term combination antiretroviral therapy: results from the Adolescent Master Protocol of the Multicenter Pediatric HIV/AIDS Cohort Study. JAMA Pediatr. 2013;167(6):520–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Desai SR, Nair A, Rylance J, Mujuru H, Nathoo K, McHugh G, et al. HIV‐associated chronic lung disease in children and adolescents in Zimbabwe: chest radiographic and high‐resolution computed tomography findings. Clin Infect Dis. 2017;66(2):274–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Githinji LN, Gray DM, Hlengwa S, Myer L, Zar HJ. Lung function in South African adolescents infected perinatally with HIV and treated long‐term with antiretroviral therapy. Ann Am Thorac Soc. 2017;14(5):722–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Sims Sanyahumbi AE, Hosseinipour MC, Guffey D, Hoffman I, Kazembe PN, McCrary M, et al. HIV‐infected children in Malawi have decreased performance on the 6‐minute walk test with preserved cardiac mechanics regardless of antiretroviral treatment status. Pediatr Infect Dis J. 2017;36(7):659–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Rylance S, Rylance J, McHugh G, Mujuru H, Munyati S, Bandason T, et al. G276 (P) Chronic respiratory morbidity among HIV‐infected children in Zimbabwe; a comparison of ART naïve and treated cohorts. Arch Dis Child. 2016;101(Suppl 1):A156–7. [Google Scholar]
- 14. Idris NS, Cheung MM, Grobbee DE, Burgner D, Kurniati N, Uiterwaal CS. Cardiac effects of antiretroviral‐naive versus antiretroviral‐exposed HIV infection in children. PLoS ONE. 2016;11:e0146753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. García‐Otero L, López M, Gómez O, Goncé A, Bennasar M, Martínez JM, et al. Zidovudine treatment in HIV‐infected pregnant women is associated with fetal cardiac remodelling. AIDS (London, England). 2016;30(9):1393–401. [DOI] [PubMed] [Google Scholar]
- 16. Kolb TM, Hassoun PM. Right ventricular dysfunction in chronic lung disease. Cardiol Clin. 2012;30(2):243–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Barnett CF, Hsue PY. HIV‐associated pulmonary hypertension: a global perspective. Adv Pulm Hypertens. 2017;15(3):138–43. [Google Scholar]
- 18. Stepffer C, Gaynor E, Lopez M, Gonzalez NE, Arri M, De AD. Pulmonary hypertension associated with the human immunodeficiency virus in children: treatment with sildenafil. A case report. Arch Argent Pediatr. 2018;116:e437–41. [DOI] [PubMed] [Google Scholar]
- 19. Miller MR, Hankinson J, Brusasco V, Burgos F, Casaburi R, Coates A, et al. Standardisation of spirometry. Eur Respir J. 2005;26(2):319–38. [DOI] [PubMed] [Google Scholar]
- 20. Pellegrino R, Viegi G, Brusasco V, Crapo R, Burgos F, Casaburi REA, et al. Interpretative strategies for lung function tests. Eur Respir J. 2005;26(5):948–68. [DOI] [PubMed] [Google Scholar]
- 21. Laboratories ACoPSfCPF . ATS statement: guidelines for the six‐minute walk test. Am J Respir Crit Care Med. 2002;166(1):111. [DOI] [PubMed] [Google Scholar]
- 22. Quanjer PH, Stanojevic S, Cole TJ, Baur X, Hall GL, Culver BH, et al. Multi‐ethnic reference values for spirometry for the 3–95‐yr age range: the global lung function 2012 equations. Eur Respir J. 2012;40(6):1324–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Ulrich S, Hildenbrand FF, Treder U, Fischler M, Keusch S, Speich R, et al. Reference values for the 6‐minute walk test in healthy children and adolescents in Switzerland. BMC Pulm Med. 2013;13(1):49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Mahtab S LJ, Jamieson‐Luff N, Asafu‐Agyei NA, Zühlke L, Myer L, Zar HJ. Cardiac abnormalities in perinatally infected HIV+ South African adolescents on ART. Conference on Retroviruses and Opportunistic Infections CROI. Boston, Massachusetts.
- 25. Gottdiener JS, Bednarz J, Devereux R, Gardin J, Klein A, Manning WJ, et al. American Society of Echocardiography recommendations for use of echocardiography in clinical trials: a report from the american society of echocardiography's guidelines and standards committee and the task force on echocardiography in clinical trials. J Am Soc Echocardiogr. 2004;17(10):1086–119. [DOI] [PubMed] [Google Scholar]
- 26. Lang R, Bierig M, Devereux R, Flachskampf F, Foster E, Pellikka P, et al. Members of the Chamber Quantification Writing Group. Recommendations for chamber quantification: a report from the American Society of Echocardiography's Guidelines and Standards Committee and the Chamber Quantification Writing Group, developed in conjunction with the European Association of Echocardiography, a branch of the European Society of Cardiology. J Am Soc Echocardiogr. 2005;18(12):1440–63. [DOI] [PubMed] [Google Scholar]
- 27. Lopez L, Colan SD, Frommelt PC, Ensing GJ, Kendall K, Younoszai AK, et al. Recommendations for quantification methods during the performance of a pediatric echocardiogram: a report from the Pediatric Measurements Writing Group of the American Society of Echocardiography Pediatric and Congenital Heart Disease Council. J Am Soc Echocardiogr. 2010;23(5):465–95. [DOI] [PubMed] [Google Scholar]
- 28. Jone P‐N, Ivy DD. Echocardiography in pediatric pulmonary hypertension. Front Pediatr. 2014;2:124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Alam M, Wardell J, Andersson E, Samad BA, Nordlander R. Characteristics of mitral and tricuspid annular velocities determined by pulsed wave Doppler tissue imaging in healthy subjects. J Am Soc Echocardiogr. 1999;12(8):618–28. [DOI] [PubMed] [Google Scholar]
- 30. Ueti O, Camargo E, de A Ueti A, de Lima‐Filho E, Nogueira E. Assessment of right ventricular function with Doppler echocardiographic indices derived from tricuspid annular motion: comparison with radionuclide angiography. Heart. 2002;88(3):244–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Kaul S, Tei C, Hopkins JM, Shah PM. Assessment of right ventricular function using two‐dimensional echocardiography. Am Heart J. 1984;107(3):526–31. [DOI] [PubMed] [Google Scholar]
- 32. Lee S, Kamdar F, Madlon‐Kay R, Boyle A, Colvin‐Adams M, Pritzker M, et al. Effects of the HeartMate II continuous‐flow left ventricular assist device on right ventricular function. J Heart Lung Transpl. 2010;29(2):209–15. [DOI] [PubMed] [Google Scholar]
- 33. Lee JZ, Low S‐W, Pasha AK, Howe CL, Lee KS, Suryanarayana PG. Comparison of tricuspid annular plane systolic excursion with fractional area change for the evaluation of right ventricular systolic function: a meta‐analysis. Arch Dis Child. 2018;5:e000667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Teichholz LE, Kreulen T, Herman MV, Gorlin R. Problems in echocardiographic volume determinations: echocardiographic‐angiographic correlations in the presence or absence of asynergy. Am J Cardiol. 1976;37(1):7–11. [DOI] [PubMed] [Google Scholar]
- 35. Wyatt HL, Meerbaum S, Heng MK, Gueret P, Corday E. Cross‐sectional echocardiography III. Analysis of mathematic models for quantifying volume of symmetric and asymmetric left ventricles. Am Heart J. 1980;100(6):821–8. [DOI] [PubMed] [Google Scholar]
- 36. Koestenberger M, Ravekes W, Everett AD, Stueger HP, Heinzl B, Gamillscheg A, et al. Right ventricular function in infants, children and adolescents: reference values of the tricuspid annular plane systolic excursion (TAPSE) in 640 healthy patients and calculation of z score values. J Am Soc Echocardiogr. 2009;22(6):715–9. [DOI] [PubMed] [Google Scholar]
- 37. Núñez‐Gil IJ, Rubio MD, Cartón AJ, López‐Romero P, Deiros L, García‐Guereta L, et al. Determination of normalized values of the tricuspid annular plane systolic excursion (TAPSE) in 405 Spanish children and adolescents. Rev Esp Cardiol. 2011;64(8):674–80. [DOI] [PubMed] [Google Scholar]
- 38. Lai WW, Gauvreau K, Rivera ES, Saleeb S, Powell AJ, Geva T. Accuracy of guideline recommendations for two‐dimensional quantification of the right ventricle by echocardiography. Int J Cardiovasc Imaging. 2008;24(7):691–8. [DOI] [PubMed] [Google Scholar]
- 39. Chemla D, Castelain V, Provencher S, Humbert M, Simonneau G, Hervé P. Evaluation of various empirical formulas for estimating mean pulmonary artery pressure by using systolic pulmonary artery pressure in adults. CHEST J. 2009;135(3):760–8. [DOI] [PubMed] [Google Scholar]
- 40. Eidem BW, McMahon CJ, Cohen RR, Wu J, Finkelshteyn I, Kovalchin JP, et al. Impact of cardiac growth on Doppler tissue imaging velocities: a study in healthy children. J Am Soc Echocardiogr. 2004;17(3):212–21. [DOI] [PubMed] [Google Scholar]
- 41. Desai SR, Nair A, Rylance J, Mujuru H, Nathoo K, McHugh G, et al. Human immunodeficiency virus‐associated chronic lung disease in children and adolescents in Zimbabwe: chest radiographic and high‐resolution computed tomographic findings. Clin Infect Dis. 2018;66(2):274–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Culver BH, Graham BL, Coates AL, Wanger J, Berry CE, Clarke PK, et al. Recommendations for a standardized pulmonary function report. An Official American Thoracic Society technical statement. Am J Respir Crit Care Med. 2017;196(11):1463–72. [DOI] [PubMed] [Google Scholar]
- 43. Chisati EM, Vasseljen O. Aerobic endurance in HIV‐positive young adults and HIV‐negative controls in Malawi. Malawi Med J. 2015;27(1):5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Cade WT, Peralta L, Keyser RE. Aerobic capacity in late adolescents infected with HIV and controls. Pediatr Rehabil. 2002;5(3):161–9. [DOI] [PubMed] [Google Scholar]
- 45. Keyser RE, Peralta L, Cade WT, Miller S, Anixt J. Functional aerobic impairment in adolescents seropositive for HIV: a quasiexperimental analysis. Arch Phys Med Rehabil. 2000;81(11):1479–84. [DOI] [PubMed] [Google Scholar]
- 46. Morales FJ, Martínez A, Méndez M, Agarrado A, Ortega F, Fernández‐Guerra J, et al. A shuttle walk test for assessment of functional capacity in chronic heart failure. Am Heart J. 1999;138(2):291–8. [DOI] [PubMed] [Google Scholar]
- 47. Byrne AL, Marais BJ, Mitnick CD, Lecca L, Marks GB. Tuberculosis and chronic respiratory disease: a systematic review. Int J Infect Dis. 2015;32:138–46. [DOI] [PubMed] [Google Scholar]
- 48. Pasterkamp H, Zielinski D. The history and physical examination. Kendig's Disorders of the respiratory tract in children (Ninth Edition). Amsterdam: Elsevier; 2019. p. 2–25. [Google Scholar]
- 49. Mwalukomo T, Rylance SJ, Webb EL, Anderson S, O'Hare B, van Oosterhout JJ, et al. Clinical characteristics and lung function in older children vertically infected with human immunodeficiency virus in Malawi. J Pediatr Infect Dis Soc. 2016;5(2):161–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Albouaini K, Egred M, Alahmar A, Wright DJ. Cardiopulmonary exercise testing and its application. Postgrad Med J. 2007;83(985):675–82. [DOI] [PMC free article] [PubMed] [Google Scholar]


