Abstract
Quorum sensing was first described as the communication process bacteria employ to coordinate changes in gene expression and therefore, their collective behavior in response to population density. Emerging new evidence suggests that quorum sensing can also contribute to the regulation of immune cell responses. Quorum sensing might be achieved by the ability of immune cells to perceive the density of their own populations or those of other cells in their environment; responses to alterations in cell density might then be coordinated via changes in gene expression and protein signaling. Quorum sensing mechanisms can regulate T and B cell as well as macrophage function. We posit that perturbations in quorum sensing may undermine the balance between diverse immune cell populations, and predisposing a host to immune abnormalities.
Keywords: quorum sensing, cytokines, nitric oxide, lymphocytes, macrophages
The quorum sensing concept: from bacteria to immunity
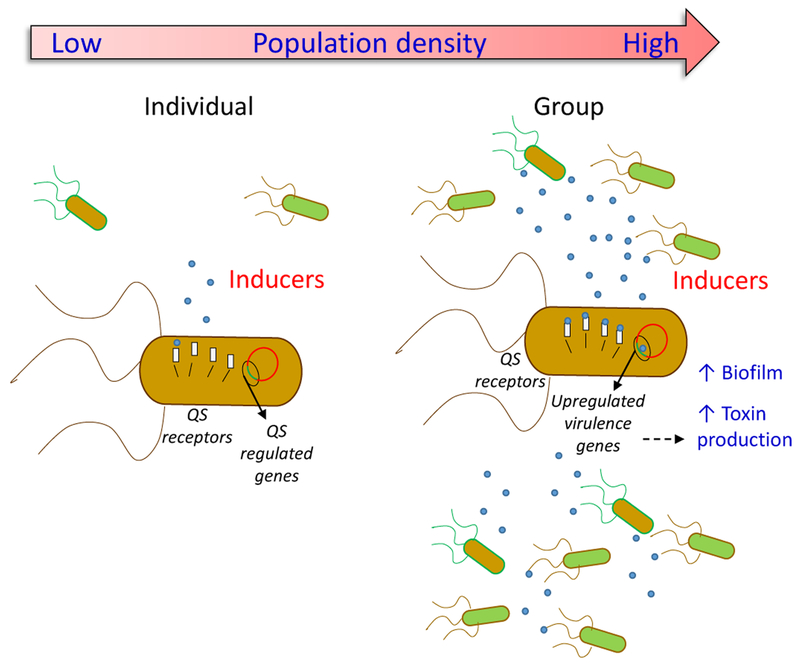
Bacterial quorum sensing encapsulates the notion that cooperative ventures among bacterial cells will not be worthwhile until a sufficient number of cells are present, or in other words, until a threshold of density is reached [1, 2]. Bacterial quorum sensing (Box 1) relies on extracellular mediators secreted by the bacteria, named autoinducers. Once autoinducer concentrations reach a critical threshold in a local environment, they can lead to a coordinated change in gene expression in bacteria in the local environment, resulting in coordinated alterations in “bacterial group behaviors”, such as biofilm formation, motility, and virulence factor expression (Figure 1) [1, 2].
Box 1. Bacterial quorum sensing.
Quorum sensing is a mechanism of bacterial communication where bacteria alter their gene expression based on the density of their populations [52]. Quorum sensing is now appreciated to not only occur in bacteria of the same species, but also in groups of heterogenous microorganisms that have evolved together, each one with adaptations tethered to the biology of the others. In bacteria, quorum sensing establishes evolutionarily stable interactions aimed at shaping and maintaining the equilibrium of the entire population inhabiting a niche [53, 54]. The achievement of this “peaceful coexistence” is the result of an intricate cell-to-cell communication network, where bacteria, through constant monitoring of the density of their populations perform synchronized specific behaviors. This process of network-like cell-to-cell communication is also a major feature of quorum sensing [1].
Despite the variations in the regulatory signals and in the molecular mechanisms, all known systems based on the quorum sensing principle depend on three basic criteria [55]: i) the members of the community produce signaling molecules called autoinducers or inducers; ii) such autoinducers or inducers are detected by receptors expressed on the cell surface (membrane receptors) or in the cells (cytoplasmic receptors); iii) the autoinducers or inducers play a critical role in regulating gene expression, which will facilitate cooperative behaviors, as well as in activating the production autoinducers or inducers, thus creating a feed-forward autoinduction loop which promotes the synchrony of bacterial populations [55].
Figure 1. Bacterial quorum sensing.
Bacteria use signaling molecules (inducers) that are released into the environment, to communicate with each other. The term ‘quorum sensing’ (QS) describes the phenomenon through which signaling molecules enable a single cell to sense the number of bacteria in its environment (cell density) and to adjust their behavior, helping to shape and maintain the behavior of the entire bacterial population.
Several features of cellular behavior similar to those underlying bacterial quorum sensing have recently been demonstrated in the mammalian immune system. Similar to bacterial quorum sensing, immune quorum sensing is a non-local, population-level communication on a length scale that is much larger than the length scale of the cell. This typically occurs because each cell secretes and then senses immune autoinducers whose concentration is typically too low for the cell to detect and respond to. But when there is a high enough density of the autoinducer-secreting cells, there can be a correspondingly high enough concentration of the autoinducer (above some threshold) to activate or repress certain genes in each cell, leading to a population level effect. So, quorum sensing is distinct from local, cell-to-cell communication, such as paracrine signaling between two cells that are a few cell-lengths apart. It is collective but at the same time, nonspecific – one cell cannot signal to another, specific cell that is nearby; either ‘everyone’ responds or ‘no one’ responds.
The quantitative composition of immune cell populations, shaped in the early phases of mammalian life, is typically preserved throughout adulthood [3], and quorum sensing appears to contribute to this composition. In addition, quorum sensing might also be essential for optimizing the repertoire (e.g., antibody-isotype and cytokine secretion) of immune-competent cells and the capacity of immune cells to respond to exogenous antigens, as well as to maintain self-tolerance [4-6].
Quorum sensing in the immune system may be mostly based on the release of soluble signals [i.e. interleukins, chemokines, cell metabolites, exosomes] , whose role is similar to bacterial autoinducers, as the concentration of these soluble signals can be indicative of cell density [7, 8]. Similar to heterogenous bacterial communities, inducers in the immune system can act on several different cell types, and their action on target cells may be mediated indirectly through intervening cell types [4, 6, 9].
In the current Opinion piece, we discuss recent advances on the emerging role of quorum sensing in regulating B cell, as well as CD4+ and CD8+ T cell numbers and function. In addition, we particularly focus on how macrophage quorum sensing and densities might regulate inflammation, bacterial infection, and potentially tissue repair. We posit that quorum sensing, which is a newly discovered mechanism for communication among immune cells, serves to facilitate the success of immune cell development and immune response in mammalian organisms.
Quorum sensing by CD4+ T cells
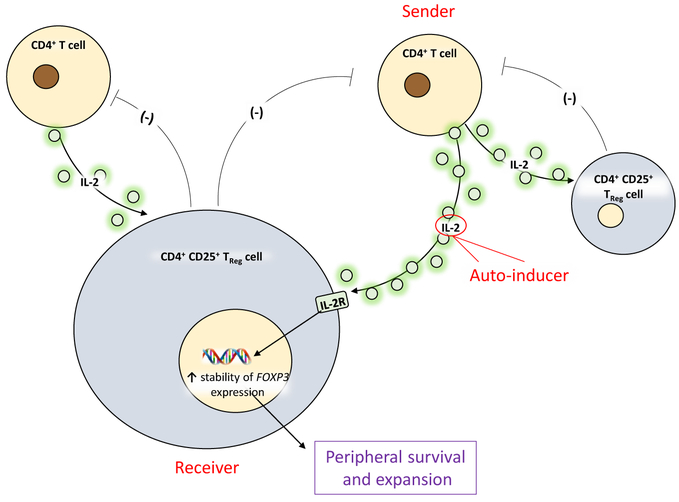
The control of CD4+ T cell numbers has been long thought to be achieved by cellular competition for limited amounts of resources, e.g., trophic factors (e.g., IL-2, IL-7, and IL-15) and/or by the size of the niche required for survival [10-12]. However, it has not been clear what mechanisms are used to control lymphocyte numbers in situations where resources are not limiting, e.g., in excess of self-antigens or cytokines, or during the course of an immune response. It is now becoming clear that CD4+ T cell homeostasis also relies on the ability of various immune cell populations to perceive and respond to fluctuations in their densities, and quorum sensing has newly emerged as a mechanism by which homeostasis can be attained. The most important quorum sensing autoinducer in T cells is IL-2 [13]. IL-2 has a critical role in mediating T cell homeostasis by regulating T cell survival, proliferation, and differentiation [14]. At steady state and in the absence of an immune response, IL-2 is produced mainly by endogenously activated αβ CD4+ T cells in secondary lymphoid organs[14, 15]. In humans and mice, these IL-2-producing T cells are “master regulators” of CD4+CD25+ TReg cells, as they have been deemed essential for the maintenance of IL-2-dependent TReg populations (themselves unable to produce IL-2) (Figure 2)[14, 15]. There is a negative feedback loop between TRegs and IL-2-producing T cells, as TRegs are known to negatively control the number of IL-2-producing T cells. This reciprocal regulation of IL-2-producing T cells and TRegs at the steady state ensures that the two cell populations are in equilibrium (Figure 2) – an important characteristic for the prevention of autoimmunity while enabling the immune response to develop in a controlled manner if and when antigen is encountered. A question then arises: if IL-2-producing αβ CD4+ T cells are central to the homeostasis of both themselves and that of TRegs, how are their numbers regulated? The answer to this question is at least in part, that this regulation can be based on a form of quorum sensing. By using IL-2 reporter mice and IL-2 deficient mice, one study demonstrated that TRegs can perceive the density of IL-2-producing αβ CD4+ T cells based on the IL-2 concentrations they encounter, which are a function of the number of αβ CD4+ T cells secreting IL-2 [16]. Once IL-2 concentrations that are sensed by TRegs reach a certain threshold, TRegs can adapt their collective behavior to control the number of IL-2-producing T cells [4]. Thus, IL-2 appears to be a bona fide autoinducer in the immune system, capable of signaling αβ CD4+ T cell density to TRegs in order to control the numbers of the latter.
Figure 2. IL-2 as a critical player in immune quorum sensing.
IL-2-producing T cells are “master regulators” of CD4+CD25+ TReg cells, as they are essential for the maintenance of IL-2-dependent TReg populations. Quorum-sensing is mediated by a negative feedback loop between TRegs and IL-2-producing T cells, as TRegs curb the number of IL-2-producing T cells. IL-2 is the quorum sensing autoinducer in this system.
In addition to the two-cell quorum sensing circuit between IL-2-producing αβ CD4+ T cells and Tregs, recent studies have highlighted additional quorum sensing mechanisms in simpler systems. Computational simulation has demonstrated that in cultured T cells, the density of TEff cells appears to be critical in maintaining IL-2-mediated signal transducer and activator of transcription (STAT) 5 phosphorylation, and thus, TEff expansion [17]. In a similar vein, using live cell imaging of murine T cells in a synthetic microenvironment, a recent study demonstrated that the differentiation of precursor memory T cells could dramatically increase above a certain density (> 30 cells) of interacting cells [18]. In this case, the inducers were shown to be IL-2 and IL-6 [18].
While the clinical relevance of CD4+ T cell quorum sensing has not been addressed, we speculate that impaired access or response to IL-2 can cause quorum sensing abnormalities resulting in the overwhelming expansion of one cell subtype over others, thus leading to an onset of pathophysiological conditions. This possibility is highlighted by the multi-organ autoimmune disease known to develop in the absence of IL-2 signaling in mice and humans [19-21].
Quorum sensing by CD8+ T cells
Recent data showed that quorum sensing mechanisms might also be operational in CD8+ T cells[22]. Specifically, antigen-experienced murine CD8+ memory T cells were found to interact with naive CD8+ T cells during priming in a way that resulted in the accelerated differentiation of naïve CD8+ T cells into more differentiated T effector memory (TEM) cells at the expense of less differentiated T stem cell memory (Tscm) and T central memory (Tcm) cells when memory and naïve T cells were cocultured in vitro. The extent of accelerated naïve CD8+ T cell differentiation caused by their interaction with CD8+ memory T cells was dependent on the density of the two subsets, suggesting that the two subsets synchronized their function in a manner resembling quorum sensing [22]. Mechanistically, using both anti-FasL antibody and cells genetically lacking Fas, the authors demonstrated that FasL, expressed on memory T cells but not naïve T cells (following activation), acutely mediated the precocious differentiation of naïve CD8+ T cells. Although FasL is not an autoinducer in the classical sense (it is not free to diffuse as it is a cell surface protein), it still seems able to coordinate the function of a large group of cells, perhaps by creating cell networks and allowing transcellular signaling [22], although this remains to be further investigated.
The quorum-sensing behavior of CD8+ T cells might have clinical implications. For instance, in B16 melanoma mouse models, adoptive cell transfer of less-differentiated CD8+ T cells has been shown to result in superior persistence and anti-tumor immunity compared with adoptive transfer of more differentiated TEM cells [23, 24]. This suggests that the quorum sensing-mediated interaction between the less- and more-differentiated cell populations during adoptive cell transfer might hamper the efficacy of adoptive therapy, which opens exciting new lines of future investigation.
Quorum sensing by B cells
Studies using adoptive B cell transfer into Rag2-deficient mice indicate that the IgM-secreting B cell pool size and plasma IgM concentrations may be stably maintained through a quorum sensing mechanism[6, 25]. Specifically, homeostasis of the innate IgM-secreting B cell pool might be attained through a mechanism whereby total B cell populations can regulate the number of activated IgM-secreting B cells through IgG secretion, in turn controlling innate IgM-secreting B cells [6]. Studies with adoptive transfer of FcγRIIB-deficient B cells into Rag2-deficient mice showed that this negative feedback effect of IgG on the number and/or activation of innate IgM-secreting B cells was mediated by FcγRIIB, a SH2 domain-containing inositol 5′-phosphatase (SHIP)-coupled low-affinity IgG receptor that acts as a negative regulator of B cell activation [6]. Therefore, in this scenario, IgG might act as the quorum sensing autoinducer that reflects the density of the B cell population [6]. These results suggest a new mechanism of homeostatic regulation of B cell numbers, which is not dependent on competition for resources or niche [6], but which is based on sensing cell density, analogous to bacterial quorum sensing. Based on these results, these studies speculated that the therapeutic efficacy of intravenous IgG in autoimmune diseases might also be mediated by IgG interfering with B cell quorum sensing and homeostasis resulting in decreased autoimmunity [6]. Additional studies are warranted to better elucidate the putative mechanisms involved in the regulation of quorum cells in B-cell mediated immune responses.
Quorum sensing by myeloid cells
Regulation of macrophage density
Macrophages are able to maintain stable cell population densities in organs at steady state[26, 27]. This might occur through distinct mechanisms based on their anatomical location. For instance, in the murine intestinal lamina propria and dermis, there is continuous input of circulating monocytes that differentiate into macrophages [26, 27] [28, 29]. This continuous input may be required to compensate for macrophage death and keep macrophage numbers constant. Other macrophage populations seem to self-maintain without input from circulating precursors by continuous proliferation or by being particularly long-lived [30]. For example, on the one hand, a multi-color “confetti” fate-mapping imaging study revealed that murine epidermal Langerhans cells in the skin could divide extensively in the steady-state, yielding clusters of uni-color Langerhans cells [30]. Constant proliferation might be occurring to offset the continuous migration of cells to the skin-draining lymph node, thereby maintaining Langerhans cell population density [30]. On the other hand, another study reported that in the brain cortex at steady state, microglia were mostly long-lived cells which formed little uni-color “confetti” clusters, suggetsing that only marginal proliferation of cells occurred [31]. Thus, these studies highlight the differential regulation of quorum sensing based on anatomical location.
Quorum sensing can also control macrophage numbers following bacterial infection and injury. For example, Listeria monocytogenes infection results in the death of Kupffer cells (KC) that have phagocytosed bacteria in mice [32, 33]. This decrease in KC numbers was found to be countered by increased monocyte recruitment and differentiation into KCs in such a way that the density of KCs returned back to steady-state levels 4 weeks following infection [32]. Similar findings were observed in a mouse model of paracetamol-induced liver injury[34]. However, in this latter study, the main repopulation mechanism appeared to constitute a temporary increase in the proliferation of remaining KCs relative to the proliferation rate observed pre-depletion [35]. Specifically, when murine KCs were partially depleted using Clec4F-DTR, a repopulation of KCs was observed in the liver. This was deemed to occur via two mechanisms in parallel: proliferation of remaining KCs and recruitment of monocytes differentiating into KCs [36]. Regardless of the mechanism used for repopulation, KC density per gram of liver returned to pre-injury levels, suggesting a need for keeping KC density constant for liver homeostasis. Brain injury in mice, such as by facial nerve axotomy, has also resulted in massive proliferation of resident microglia at the lesion site [31]. However, once the mice have recovered from the injury, the population of microglia has been reported to return to normal density[31]. Altogether, these studies suggest that the density of the macrophage population is tightly controlled and that potentially, stable quorum sensing mechanisms might be implicated.
Implications of Macrophage Densities in Tissues
Macrophages can mediate quorum sensing of other cells during tissue regeneration. Indeed, macrophages were recently proposed to contribute to the coordinated hair regeneration that occurs after plucking across neighboring hair follicles by spreading among follicles, and locally inducing hair regeneration by TNF-α-mediated activation of stem cells in mice[48]. Of note, histological evidence in general has pointed to an inverse correlation between the density of macrophages infiltrating tumors and clinical outcomes in human cancer patients [49, 50]. It is thus reasonable to speculate that the immunosuppressive functions of macrophages in tumors might also potentially depend to some extent on quorum sensing, although evidence supporting this possibility is unavailable at this time.
Mechanisms of macrophage quorum sensing
CSF1-CSF1-R Signaling
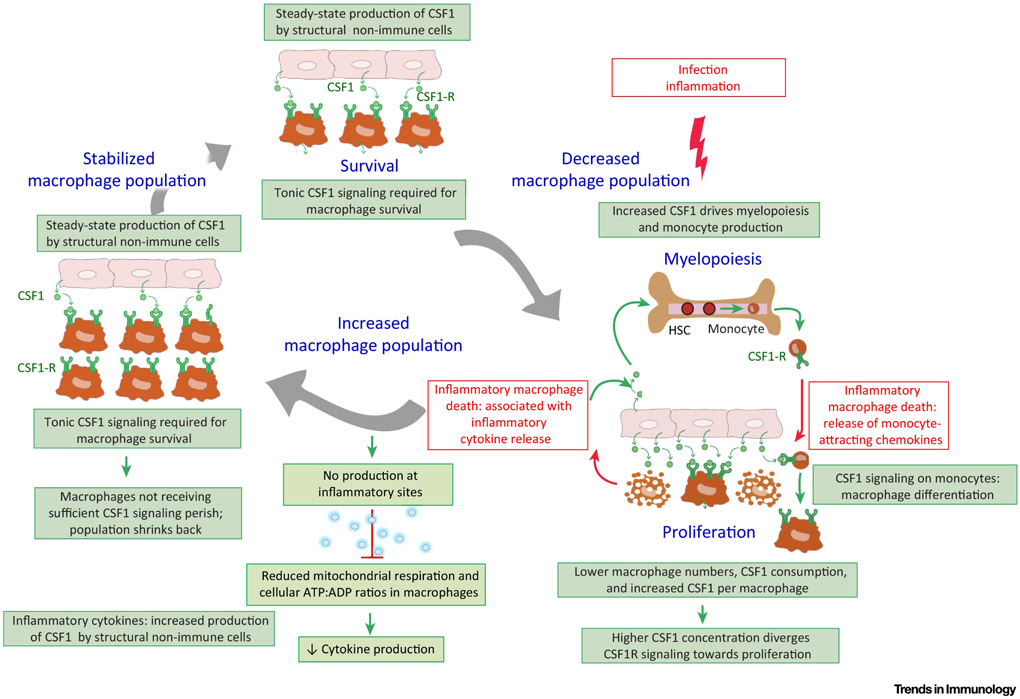
The cellular and molecular mechanisms controlling quorum sensing in macrophages are only beginning to be revealed, and one potential pathway points towards a central role for colony stimulating factor (CSF)1-receptor (CSF1-R) signaling because it controls the survival and proliferation of macrophages at steady state [37]. Specifically, Binding of CSF1 to CSF1-R on macrophages leads to its internalization and degradation in the lysosome through ubiquitination of the CSF1-R in mice[38]. This effectively depletes CSF1 from the milieu and creates competition of macrophages for the available CSF1. Csf1r−/− mice lack most tissue resident macrophages [39] and treatment of adult animals with CSF1-R blocking antibodies or pharmacological blockade of CSF1-R signaling can induce generalized death of macrophages across tissues, suggesting that CSF1-R signaling is essential for macrophage survival and therefore, a likely regulatory of population density [40].
Multiple stromal cells, including endothelial cells, epithelial cells, fibroblasts, keratinocytes and neurons can produce CSF1 or IL-34 (the only other CSF1-R binding cytokine)[41]. Macrophages are the main cells expressing high concentrations of CSF1-R. And, although high CSF1 concentrations activate macrophage proliferation, low CSF1 concentrations are required for macrophage survival; thus, macrophage density may be controlled by two factors: (i) CSF1 production by stromal cells and (ii) CSF1 consumption by the macrophage population as a whole [37]. Recently, a two-cell circuit based model of reciprocal growth factor exchange was described between CSF1-R-expressing murine macrophages secreting PDGF, and PDGF-R-expressing murine stromal cells secreting CSF1[42]. Specifically, stromal cells producing CSF1 controlled the macrophage population density, while macrophages in turn controlled the stromal cell population density by producing PDGF, the growth factor for stromal cells [42]. Co-culturing varying numbers of stromal cells and macrophages resulted in a stable ratio of stromal cell vs. macrophage numbers, which was independent of the start ratio. This appeared to be due to the fact that CSF1 production by a given number of stromal cells could only support the survival of a fixed number of macrophages and vice versa[42]. This two-cell system appears to be resilient to perturbations: the addition of exogenous CSF1 transiently yielded more macrophages when compared to non-treated macrophages, but once excess CSF1 was consumed by the macrophages, the population stabilized again at the same macrophage-stromal cell ratio in vitro. This model suggests that stable macrophage densities following perturbations might form the basis of in vivo quorum sensing; it might contribute to explaining how macrophage populations might return to normal densities in response to perturbed homeostasis in vivo, following hypothetically, an acute infection. We speculate that infection-induced death of macrophages might not only decrease their CSF1 consumption, but also be accompanied by the release of inflammatory mediators which induce a higher expression of CSF1 by stromal cells relative to macrophages. This might further increase the concentrations of available CSF1 for the remaining macrophages, inducing their proliferation. Of note, inflammation is also associated with monocyte recruitment, leading cells to differentiate into additional macrophages. As a result of monocyte recruitment, macrophage expansion after inflammation might result in the overshoot of the original number of macrophages present in the tissue. However, once inflammation resolves, the concentrations of CSF1 may decrease because i) the production of CSF1 by stromal cells can return to its steady-state level and ii) because of the high CSF1 consumption by an oversized macrophage population. It’s plausible to reason that once the concentration of CSF1 per macrophage drops under the concentration required for macrophage survival, a contraction of the macrophage population may be induced up to the point of a stabilized macrophage population density, corresponding to the stable macrophage-stromal-cell ratio. Thus, we propose that macrophage quorum sensing might mainly occur indirectly through a cell-cell circuit involving macrophages and CSF1-producing stromal cells where the macrophage population density is controlled by the number CSF1-producing stromal cells and their concentration of CSF1 production at any given time (Figure 3). Future investigations are aimed at further testing this model.
Figure 3. Quorum sensing of tissue-resident macrophages in homeostasis.
Competition for CSF1 controls the population density, as low CSF1 signaling is required for macrophage survival, while high CSF1 signaling drives macrophage proliferation. Higher concentrations of circulating CSF1 also drive myelopoiesis in the bone-marrow. Macrophage activation states can spread within the macrophage network through paracrine signaling of activating and de-activating cytokines such as TNF/IL-1β and IL-10, respectively. At inflammatory sites, nitric oxide (NO) can reduce mitochondrial respiration and cellular ATP:ADP ratios. The density of NO-producing cells can control immune cell activity at the tissue level; thus, NO production can act as a quorum sensing mechanism to help terminate inflammation.
TLR Signaling
Macrophages might also function as a socially integrated population during bacterial infections. Macrophages produce many inflammatory cytokines but also express various cytokine receptors, resulting in both autocrine and paracrine signaling. Macrophages phagocytosing bacteria are activated by TLR-signaling, leading to nuclear factor-κB (NF-κB) activation and the production of inflammatory cytokines such as IL1-β or tumor necrosis factor (TNF)[43]. Paracrine signaling will permit neighboring macrophages, or recently recruited monocyte-derived macrophages that have not sensed bacteria yet, to become activated by IL-1-receptor and TNF-receptor signaling, leading to NF-κB activation, ‘spreading’ the activation state within the population [44]. When isolated, TLR-stimulated monocytes and macrophages produce lower amounts of cytokines and chemokines per cell than monocytes and macrophages within groups [45, 46]; therefore, we suggest that macrophage quorum sensing can regulate the magnitude of the inflammatory response. Indeed, TNF-α and IL-10 have been identified as the primary positive, abd the primary negative contributors, respectively, capable of mediating an increased production of inflammatory cytokines by macrophages in groups vs. in isolation [45, 46], (Figure 3). In contrast to TLR stimulation in vitro, in one study where macrophages were infected with live Leishmania major in mice, the increasing macrophage density inhibited cytokine production by the same macrophages, and the negative quorum sensing signal was reported to be nitric oxide [9]. Approximately 5,000 nitric oxide-producing macrophages per mm3 were required for substantial cytokine inhibition in this study. Thus, in addition to regulating inflammation, macrophage coordination can also regulate the spread of bacterial infection, as macrophages need to be present at a critical density to control mycobacterial proliferation [47].
Concluding remarks
Traditionally, local cellular competition for trophic survival factors, space limitations, and confluence restriction have been considered as major mechanisms shaping immune cell homeostasis [51]. The past few years have seen the emergence of a new mechanism contributing to immune homeostasis, immune quorum sensing. Quorum sensing can enable the immune system to coordinate the behavior of its various parts through synchronization, conferring a robust response to perturbations to ensure greater stability of the system. Various mechanisms can work together to create immune cell homeostasis and may share signaling molecules and pathways. For example, as discussed earlier, IL-2 is a prime driver of T cell homeostasis and IL-2 signaling is regulated both through its consumption and removal by target cells and through IL-2-producing cell density-dependent availability. The mechanisms mediating quorum sensing in various immune populations are only beginning to emerge, as are the mechanisms coordinating quorum sensing and other forms of homeostatic regulation (see Outstanding Questions). We posit that quorum sensing, an ancient signaling mechanism originally used by unicellular organisms to coordinate the function and increase the health of cell populations contained within a limited niche, is also employed by cells of the immune systems in mammalian organisms rendering the immune system, a robust and well-controlled apparatus that is able to fight intruders both from outside and inside of cells.
Outstanding Questions Box.
How and to what extent does the structure of lymphoid organs participate in determining quorum sensing?
How accurately can populations engaged in quorum sensing measure their densities?
Does quorum sensing exist beyond the cell types mentioned in this article?
What ecological pressures favor particular quorum-sensing regulatory strategies?
Highlights.
Immune homeostasis relies on the ability of various immune cell populations to perceive and respond to changes in their densities, and thus, quorum sensing is an emerging mechanism by which homeostasis can be attained.
Autoinducers are signaling molecules reporting cell density. One major autoinducer for CD4+ T cells is IL-2. IgG is a major autoinducer for B cells. Autoinducers for macrophages include TNF-α and nitric oxide.
Disruption of immune quorum sensing may trigger a dysregulation in the size of various immune cell pools (e.g., TEff and TReg cells) and may potentially predispose to the development of immune-related disorders.
Impaired access or response to inducers may cause quorum sensing abnormalities, potentially leading to the onset of pathophysiological conditions
Acknowledgements
This work was supported by National Institutes of Health grants R01GM066189 (G.H.) and R01DK113790 (G.H.); the Intramural Research Program of the National Institutes of Health, National Institute on Alcohol Abuse and Alcoholism (P.P.).
Glossary
- Colony stimulating factor (CSF)1-receptor
(or macrophage colony-stimulating factor receptor (M-CSFR), CD115 (Cluster of Differentiation 115)), is a cell surface protein encoded, in humans, by the CSF1R gene (known also as c-FMS). It acts as the receptor for colony stimulating factor 1, a cytokine which controls the production, differentiation, and function of macrophages
- FcγRIIB
inhibitory receptor critically involved in regulating the generation of autoantibodies and their effector functions, which include activation of innate immune cells and the cellular arm of the adaptive immune system, via effects on antigen presentation to CD4+ T cells.
- Homeostasis
can be defined as the stable state of an organism and its internal environment
- Kupffer cells
critical components of the mononuclear phagocytic system. They are pivotal to both the hepatic and systemic response to pathogens.
- Langerhans cells
are dendritic cells (antigen-presenting immune cells) of the skin and mucosae, and contain organelles called Birbeck granules. They are present in all layers of the epidermis and are prominently expressed in the stratum spinosum
- Nuclear factor-kB (NF-kB)
protein complex that controls transcription of DNA, cytokine production and cell survival.
- Priming
phase of the first contact between, for example, a T or B cell with its specific antigen, causing their differentiation into effector T or B cells (cytotoxic T cells, cells producing cytokines, and in the case of B cells, producing antibodies)
- TLR-signaling
Toll-like receptor (TLR) signaling plays an essential role in the innate immune response. Activation of TLR signaling through recognition of pathogen-associated molecular patterns leads to the transcriptional activation of genes coding for pro-inflammatory cytokines, chemokines and co-stimulatory molecules.
- T central memory cells
co-express L-selectin and chemokine CCR7; they provide central immunosurveillance by patrolling lymph nodes draining from peripheral tissues.
- TEff cells
group of cells that include several T cell subsets hat actively respond to a stimulus, such as co-stimulation. (e.g. CD4+, CD8+ T cells, Treg cells)
- TReg cells
subpopulation of T cells that modulate the immune system, maintain tolerance to self-antigens, and contribute to preventing autoimmune diseases.
- T stem memory cells
rare subset of memory lymphocytes endowed with the stem cell–like ability to self-renew and the multipotent capacity of reconstituting the overall spectrum of memory and effector T cell subsets.
Footnotes
Competing interests statement
The authors declare no competing interests.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Waters CM and Bassler BL (2005) Quorum sensing: cell-to-cell communication in bacteria. Annu Rev Cell Dev Biol 21, 319–46. [DOI] [PubMed] [Google Scholar]
- 2.Ng WL and Bassler BL (2009) Bacterial quorum-sensing network architectures. Annu Rev Genet 43, 197–222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Simon AK et al. (2015) Evolution of the immune system in humans from infancy to old age. Proc Biol Sci 282 (1821), 20143085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Almeida AR et al. (2012) Quorum-Sensing in CD4(+) T Cell Homeostasis: A Hypothesis and a Model. Front Immunol 3, 125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Reynolds J et al. (2014) A mathematical perspective on CD4(+) T cell quorum-sensing. J Theor Biol 347, 160–75. [DOI] [PubMed] [Google Scholar]
- 6.Montaudouin C et al. (2013) Quorum sensing contributes to activated IgM-secreting B cell homeostasis. J Immunol 190 (1), 106–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.McCoy-Simandle K et al. (2016) Exosomes and nanotubes: Control of immune cell communication. Int J Biochem Cell Biol 71, 44–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Xie J et al. (2013) How the immune system talks to itself: the varied role of synapses. Immunol Rev 251 (1), 65–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Postat J et al. (2018) A Metabolism-Based Quorum Sensing Mechanism Contributes to Termination of Inflammatory Responses. Immunity 49 (4), 654–665 e5. [DOI] [PubMed] [Google Scholar]
- 10.de la Rosa M et al. (2004) Interleukin-2 is essential for CD4+CD25+ regulatory T cell function. Eur J Immunol 34 (9), 2480–8. [DOI] [PubMed] [Google Scholar]
- 11.Martin CE et al. (2017) Interleukin-7 Availability Is Maintained by a Hematopoietic Cytokine Sink Comprising Innate Lymphoid Cells and T Cells. Immunity 47 (1), 171–182 e4. [DOI] [PubMed] [Google Scholar]
- 12.Ranson T et al. (2003) IL-15 is an essential mediator of peripheral NK-cell homeostasis. Blood 101 (12), 4887–93. [DOI] [PubMed] [Google Scholar]
- 13.Benczik M and Gaffen SL (2004) The interleukin (IL)-2 family cytokines: survival and proliferation signaling pathways in T lymphocytes. Immunol Invest 33 (2), 109–42. [DOI] [PubMed] [Google Scholar]
- 14.Boyman O and Sprent J (2012) The role of interleukin-2 during homeostasis and activation of the immune system. Nat Rev Immunol 12 (3), 180–90. [DOI] [PubMed] [Google Scholar]
- 15.Almeida AR et al. (2006) Indexation as a novel mechanism of lymphocyte homeostasis: the number of CD4+CD25+ regulatory T cells is indexed to the number of IL-2-producing cells. J Immunol 177 (1), 192–200. [DOI] [PubMed] [Google Scholar]
- 16.Amado IF et al. (2013) IL-2 coordinates IL-2-producing and regulatory T cell interplay. J Exp Med 210 (12), 2707–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Feinerman O et al. (2010) Single-cell quantification of IL-2 response by effector and regulatory T cells reveals critical plasticity in immune response. Mol Syst Biol 6, 437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Polonsky M et al. (2018) Induction of CD4 T cell memory by local cellular collectivity. Science 360 (6394). [DOI] [PubMed] [Google Scholar]
- 19.Ballesteros-Tato A (2014) Beyond regulatory T cells: the potential role for IL-2 to deplete T-follicular helper cells and treat autoimmune diseases. Immunotherapy 6 (11), 1207–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Schimpl A et al. (2002) IL-2 and autoimmune disease. Cytokine Growth Factor Rev 13 (4-5), 369–78. [DOI] [PubMed] [Google Scholar]
- 21.Long SA et al. (2011) An autoimmune-associated variant in PTPN2 reveals an impairment of IL-2R signaling in CD4(+) T cells. Genes Immun 12 (2), 116–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Klebanoff CA et al. (2016) Memory T cell-driven differentiation of naive cells impairs adoptive immunotherapy. J Clin Invest 126 (1), 318–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hinrichs CS et al. (2009) Adoptively transferred effector cells derived from naive rather than central memory CD8+ T cells mediate superior antitumor immunity. Proc Natl Acad Sci U S A 106 (41), 17469–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cieri N et al. (2013) IL-7 and IL-15 instruct the generation of human memory stem T cells from naive precursors. Blood 121 (4), 573–84. [DOI] [PubMed] [Google Scholar]
- 25.Cabatingan MS et al. (2002) Naive B lymphocytes undergo homeostatic proliferation in response to B cell deficit. J Immunol 169 (12), 6795–805. [DOI] [PubMed] [Google Scholar]
- 26.Tamoutounour S et al. (2013) Origins and functional specialization of macrophages and of conventional and monocyte-derived dendritic cells in mouse skin. Immunity 39 (5), 925–38. [DOI] [PubMed] [Google Scholar]
- 27.Scott CL et al. (2014) Mononuclear phagocytes of the intestine, the skin, and the lung. Immunol Rev 262 (1), 9–24. [DOI] [PubMed] [Google Scholar]
- 28.Tamoutounour S et al. (2012) CD64 distinguishes macrophages from dendritic cells in the gut and reveals the Th1-inducing role of mesenteric lymph node macrophages during colitis. European journal of immunology. [DOI] [PubMed] [Google Scholar]
- 29.Bain CC et al. (2012) Resident and pro-inflammatory macrophages in the colon represent alternative context-dependent fates of the same Ly6C(hi) monocyte precursors. Mucosal immunology. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ghigo C et al. (2013) Multicolor fate mapping of Langerhans cell homeostasis. J Exp Med 210 (9), 1657–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tay TL et al. (2017) A new fate mapping system reveals context-dependent random or clonal expansion of microglia. Nat Neurosci 20 (6), 793–803. [DOI] [PubMed] [Google Scholar]
- 32.Bleriot C et al. (2015) Liver-resident macrophage necroptosis orchestrates type 1 microbicidal inflammation and type-2-mediated tissue repair during bacterial infection. Immunity 42 (1), 145–58. [DOI] [PubMed] [Google Scholar]
- 33.Ginhoux F et al. (2017) Dying for a Cause: Regulated Necrosis of Tissue-Resident Macrophages upon Infection. Trends Immunol 38 (10), 693–695. [DOI] [PubMed] [Google Scholar]
- 34.Zigmond E et al. (2014) Infiltrating monocyte-derived macrophages and resident kupffer cells display different ontogeny and functions in acute liver injury. J Immunol 193 (1), 344–53. [DOI] [PubMed] [Google Scholar]
- 35.Zigmond E et al. (2014) Infiltrating Monocyte-Derived Macrophages and Resident Kupffer Cells Display Different Ontogeny and Functions in Acute Liver Injury. Journal of immunology. [DOI] [PubMed] [Google Scholar]
- 36.Scott CL et al. (2016) Bone marrow-derived monocytes give rise to self-renewing and fully differentiated Kupffer cells. Nature communications 7, 10321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Jenkins SJ and Hume DA (2014) Homeostasis in the mononuclear phagocyte system. Trends Immunol 35 (8), 358–67. [DOI] [PubMed] [Google Scholar]
- 38.Lee PS et al. (1999) The Cbl protooncoprotein stimulates CSF-1 receptor multiubiquitination and endocytosis, and attenuates macrophage proliferation. EMBO J 18 (13), 3616–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Dai XM et al. (2002) Targeted disruption of the mouse colony-stimulating factor 1 receptor gene results in osteopetrosis, mononuclear phagocyte deficiency, increased primitive progenitor cell frequencies, and reproductive defects. Blood 99 (1), 111–20. [DOI] [PubMed] [Google Scholar]
- 40.Hamilton JA et al. (2016) Anti-colony-stimulating factor therapies for inflammatory and autoimmune diseases. Nat Rev Drug Discov 16 (1), 53–70. [DOI] [PubMed] [Google Scholar]
- 41.Stanley ER and Chitu V (2014) CSF-1 receptor signaling in myeloid cells. Cold Spring Harb Perspect Biol 6 (6). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zhou X et al. (2018) Circuit Design Features of a Stable Two-Cell System. Cell 172 (4), 744–757 e17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Takeda K and Akira S (2005) Toll-like receptors in innate immunity. Int Immunol 17 (1), 1–14. [DOI] [PubMed] [Google Scholar]
- 44.Kapetanovic R et al. (2007) Contribution of phagocytosis and intracellular sensing for cytokine production by Staphylococcus aureus-activated macrophages. Infect Immun 75 (2), 830–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Xue Q et al. (2015) Analysis of single-cell cytokine secretion reveals a role for paracrine signaling in coordinating macrophage responses to TLR4 stimulation. Sci Signal 8 (381), ra59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Caldwell AB et al. (2014) Network dynamics determine the autocrine and paracrine signaling functions of TNF. Genes Dev 28 (19), 2120–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Boechat N et al. (2001) Culture at high density improves the ability of human macrophages to control mycobacterial growth. J Immunol 166 (10), 6203–11. [DOI] [PubMed] [Google Scholar]
- 48.Chen CC et al. (2015) Organ-level quorum sensing directs regeneration in hair stem cell populations. Cell 161 (2), 277–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zhang QW et al. (2012) Prognostic significance of tumor-associated macrophages in solid tumor: a meta-analysis of the literature. PLoS One 7 (12), e50946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Fridman WH et al. (2017) The immune contexture in cancer prognosis and treatment. Nat Rev Clin Oncol 14 (12), 717–734. [DOI] [PubMed] [Google Scholar]
- 51.Hart Y et al. (2014) Paradoxical signaling by a secreted molecule leads to homeostasis of cell levels. Cell 158 (5), 1022–1032. [DOI] [PubMed] [Google Scholar]
- 52.Padder SA et al. (2018) Quorum sensing: A less known mode of communication among fungi. Microbiol Res 210, 51–58. [DOI] [PubMed] [Google Scholar]
- 53.Blaser MJ and Kirschner D (2007) The equilibria that allow bacterial persistence in human hosts. Nature 449 (7164), 843–9. [DOI] [PubMed] [Google Scholar]
- 54.Chow J et al. (2010) Host-bacterial symbiosis in health and disease. Adv Immunol 107, 243–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Rutherford ST and Bassler BL (2012) Bacterial quorum sensing: its role in virulence and possibilities for its control. Cold Spring Harb Perspect Med 2 (11). [DOI] [PMC free article] [PubMed] [Google Scholar]