Abstract
Background:
We have previously reported that physicians under-recognize smoking as a chronic pancreatitis (CP) risk factor. We hypothesized that availability of empiric data will influence physician recognition of this relationship.
Methods:
We analyzed data from 508 CP patients prospectively enrolled in the North American Pancreatitis Study-2 Continuation and Validation (NAPS2-CV) or NAPS2-Ancillary (AS) studies (2008–2014) from 26 US centers who self-reported ever-smoking. Information on smoking status, physician-defined etiology and identification of smoking as a CP risk factor was obtained from structured patient and physician questionnaires. We compared how often physician identified smoking as a CP risk factor in NAPS2-CV/ NAPS2-AS studies with NAPS2-original study (2000–2006).
Results:
Enrolling physician identified smoking as a risk factor in significantly (all p<0.001) greater proportion of patients in NAPS2-CV/AS studies when compared with NAPS2-original study among ever (80.7 vs. 45.3%), current (91.3 vs. 53%), past (60.3 vs. 30.2%) smokers, in those who smoked ≤1 pack/day (79.3 vs. 39.5%) or ≥1 packs/day (83 vs. 49.8%). In multivariable analyses, the enrolling physician was 3.32–8.49 times more likely to cite smoking as a CP risk factor in the NAPS2-CV/NAPS2-AS studies based on smoking status and amount after controlling for age, sex, race and alcohol etiology. The effect was independent of enrolling site in sub-analysis limited to sites participating in both phases of enrollment.
Conclusions:
Availability of empiric data likely enhanced physician recognition of the association between smoking and CP. Wide-spread dissemination of this information could potentially curtail smoking rates in subjects with and those at risk of CP.
Keywords: Pancreatitis, etiology, risk factor, smoking
Introduction
Chronic pancreatitis (CP) is a chronic fibroinflammatory disorder of the pancreas which is characterized by varying combination of features during its clinical course, including abdominal pain, one or more attacks of acute pancreatitis (AP), diabetes, malabsorption, morphological changes in the pancreas, local complications, and pancreatic cancer.1 While heavy alcohol consumption is the most common cause of CP, there is increasing appreciation of the role of other factors – such as smoking, genetic, autoimmune, and structural abnormalities in disease causation.2 The complexity of CP pathogenesis and contribution of multiple factors was recognized in the recently proposed definition by an international group of experts.3
Several clinical studies have defined and quantified the risk of the different forms of pancreatitis with smoking.4 In the large, prospective, multicenter, cross-sectional North American Pancreatitis Study-2 (NAPS2), we reported a dose-dependent association of smoking with CP.5 Smoking is also associated with progression of AP or recurrent AP (RAP) to CP, and few data also suggest that smoking cessation after the onset of disease may slow disease progression.6,7 The mechanistic basis by which smoking increases the risk of pancreatitis and affects disease progression is only beginning to be understood.8–12 These findings underscore the need for physicians to incorporate an assessment of smoking during evaluation of patients with CP, and if indicated, to provide appropriate counseling for smoking cessation. In the aforementioned NAPS2 study, we found that physicians frequently failed to recognize smoking as a CP risk factor, even in patients who were self-reported heavy smokers.13 There was also a high degree of variability between physicians in citing smoking as a CP risk factor.
Since our initial study, two additional NAPS2 studies have prospectively recruited additional patients for genome wide association study and for focused analyses in African-American subjects. We analyzed these data to determine if our publications on the role of smoking in CP influenced the enrolling physicians’ perception of this association. We hypothesized that demonstration of the strong association between smoking and CP will result in physician identifying smoking as a risk factor in a greater fraction of self-reported smokers.
METHODS
Study Population
The NAPS2 Group prospectively enrolled patients with RAP or CP, and controls from 26 centers in three sequential cross-sectional studies, namely original NAPS2 (2000–2006)14, the NAPS2 continuation and validation study (NAPS2-CV) (2008–2012)15 and the NAPS2 Ancillary Study (NAPS2-AS) (2011–2014)16. The purpose of the original NAPS2 study was to study the role of genetic and environmental factors in pancreatitis risk; for the NAPS2-CV study was to ascertain a replication cohort for genome wide association study, and for the NAPS2-AS study was to exclusively ascertain African-American subjects, who were under-represented in the first two studies. Each study was approved by the Institutional Review Boards of individual participating centers and all study subjects signed an informed consent form before enrollment.
CP was defined by the presence of characteristic changes on any of the following – cross-sectional abdominal imaging (computerized tomography [CT] scan, magnetic resonance imaging [MRI]/magnetic resonance cholangiopancreatography[MRCP]), endoscopic retrograde cholangiopancreatography [ERCP], endoscopic ultrasound [EUS] presence of ≥5 findings or presence of calcifications]) or histology. Detailed protocol of the NAPS2 studies have been published previously.14–16
We have previously reported on physician perception of smoking as a risk factor for CP using data from the original NAPS2 study.13 In this analysis, we performed a similar analysis in CP patients who were enrolled in the NAPS2-CV and NAPS2-AS studies and compared data with the original NAPS2 study.
Patient and Physician Questionnaires
Data was collected in the NAPS2 studies using two comprehensive questionnaires, one completed by patients with assistance of a clinical research coordinator, and the other by the enrolling physician investigator14–16. Patient questionnaire collected information on demographics, exposure to risk factors, personal and family history, clinical symptoms, hospitalizations and emergency room visits, medication use, and quality of life. Physician questionnaire collected information on disease phenotype, etiology and risk factors, exocrine and endocrine insufficiency, findings on imaging studies, treatments tried, and their perceived effectiveness.
Self-Reported Smoking Status
Each enrolled subject was asked to report on whether he/she ever smoked (smoked >100 cigarettes during lifetime), age at smoking initiation, age at smoking cessation (if applicable) and the average number of cigarettes smoked in a day14. Using this information, patients were stratified into never or ever smokers, and the latter further stratified into current or past smokers. The amount of smoking was stratified as <1 packs per day or ≥1 packs per day, or as pack years <12 pack years, 12–35 pack years, and >35 pack years. In this study, we only included patients who were self-reported ever smokers.
Physician-defined etiology and smoking as a risk factor
The enrolling physician was asked to identify the most likely etiology that may explain the patient’s CP from a list of options including alcohol, genetic, idiopathic, obstructive, autoimmune, hyperlipidemia, gallstones, medications, and other (with space provided to specify ‘other’). In a separate question, the enrolling physicians was also asked to identify whether the patient had one or more of the well-recognized risk factors for pancreatitis using the TIGAR-O risk factor classification.1 This list contained smoking as one of the choices in the Toxic-Metabolic category in both original and the follow-up studies. Information from these two questions was used to identify whether the enrolling physician identified alcohol as the etiology and identified smoking as a risk factor for the patient’s CP. In the original NAPS2 study, one check box was provided for each risk factor for physician to indicate if the risk factor was present. In the NAPS2-CV and NAPS2-AS studies, physician was asked to choose a “yes” or “no” box for each risk factor.
Data Analysis
For the purposes of this analysis, we combined data from the NAPS2-CV and NAPS2-AS studies. We initially performed a descriptive analysis of the NAPS2-CV and NAPS2-AS studies to evaluate how often physicians identified smoking as a risk factor in patients who were self-reported ever smokers, overall and after stratification by smoking status (ever, past, current) and amount (<1 or ≥1 packs per day) or as pack years (<12, 12–35, >35). Comparison within each smoking category was then made based on whether the enrolling physician identified alcohol as the etiology of the patient’s CP. We then compared data on how often the enrolling physician identified smoking as a risk factor in the NAPS2-CV or NAPS2-AS studies with the original NAPS2 study, in each smoking category, overall and after stratification based on physician-defined alcohol etiology.
To better understand the independent effect of the study (NAPS2-CV or NAPS2-AS vs. original NAPS2 study) on physician perception, we performed multivariable logistic regression analyses separately for all patients and for each smoking category (ever smoker, past smoker, current smoker, <1 packs per day, ≥1 packs per day) where the outcome was physician identification of smoking as a CP risk factor and the independent variables were study type (NAPS2-CV or NAPS2-AS vs. original NAPS2 study) and other potential confounders (age, sex, race and physician-defined alcohol etiology).
Finally, to understand whether the effect on physician perception of smoking as a CP risk factor was limited to specific centers, we analyzed data for centers who participated in either the NAPS2-CV or NAPS2-AS studies and were also a participant of the original NAPS2 study, and where the enrolling physician identified smoking as a risk factor in <70% of self-reported ever-smokers in the original NAPS2 study. Although arbitrary, we chose this cut-off to be able to evaluate a positive impact on physician recognition, i.e. an increase in recognition in the NAPS2-CV/AS studies when compared to the original NAPS2 study.
For all comparisons, a p-value of <0.05 was considered significant. Data were analyzed using SAS 9.4 (SAS Institute, Inc., Cary, NC).
RESULTS
Demographics, self-reported smoking status and alcohol etiology in NAPS2-CV and NAPS2-AS studies
In the NAPS2-CV and NAPS2-AS studies, ever smoking was self-reported by 508/655 (78%) CP patients, who formed the study population for this analysis. The median age at the time of enrollment was 53.2 years (IQR, 44.9, 60), 306 (60.2%) were male, and 331 (65.2%) were white, 170 (33.5%) African-American, and the remaining 7 (1.4%) were of other race. About two-thirds of patients (334, 65.8%) were current smokers, while the rest reported past smoking (174, 34.3%). The amount of smoking was roughly distributed equally in terms of packs per day (275, 54.1% <1 packs per day; 229, 45.1% ≥1 packs per day). The amount of smoking was <12 pack years in 160 (31.5%), 12–35 packs years in 212 (41.7%) and >35 pack years in 131 (25.8%) patients. The enrolling physician identified alcohol to be the etiology in 315 (62%) of patients.
Physician recognition of smoking as a CP risk factor in NAPS2-CV or NAPS2-AS studies
Physicians identified smoking as a risk factor in 410/508 (80.7%) of self-reported ever smokers with CP (Table 1). Physicians were more likely to cite smoking as a risk factor when they consider alcohol as the etiology of CP when compared with those in whom they identified other etiologies (89.8% vs. 65.8%, p<0.001).
Table 1.
Self-reported smoking status and probability of physician citing smoking as a chronic pancreatitis risk factor in CP in NAPS-CV or NAPS2-AS Study
| Number | Physician identified smoking as a CP risk factor |
% |
p |
|
|---|---|---|---|---|
| Ever Smoker | 508 | 410 | 80.7 | |
| Alcohol etiology | 315 | 283 | 89.8 | <0.001 |
| No alcohol etiology | 193 | 127 | 65.8 | |
| Smoking status | ||||
| Current Smoker | 334 | 305 | 91.3 | <.0001 |
| Past Smoker | 174 | 105 | 60.3 | |
| Amount of smoking | ||||
| ≤ 1 PPD | 275 | 218 | 79.3 | 0.31 |
| ≥ 1 PPD | 229 | 190 | 83.0 | |
| Smoking in pack years | ||||
| <12 | 160 | 110 | 68.8 | <.0001 |
| 12 – 35 | 212 | 180 | 84.9 | |
| >35 | 131 | 117 | 89.3 | |
Alcohol etiology was based on physician assessment
Missing data: smoking status (4), pack years (5)
Physicians were significantly more likely to cite smoking as a risk factor in current smokers when compared with past smokers (91.3 vs 60.3%, p<0.001). Interestingly, the proportion of patients in whom physician identified smoking as a risk factor was similar based on packs/day (79.3% for <1 packs per day vs. 83% for ≥1 packs per day, p=0.31). However, the intensity of smoking did influence physician interpretation, as reflected by responses to pack years of smoking. Physicians were more likely to identify smoking as a risk factor in patients who smoked 12–35 pack years (84.9%) and >35 pack years (89.3%) when compared to those who smoked <12 pack years (68.8%, p<0.001).
Physician recognition of smoking as a CP risk factor in NAPS-2 CV or NAPS2-AS studies vs. original NAPS2 study
The prevalence of ever smoking was higher by a few percentage points in the NAPS2-CV or NAPS2-AS studies when compared with the original NAPS2 study (508/655, 78% vs. 382/535, 71.4%, p=0.02). The proportion of ever smokers in whom the enrolling physician identified alcohol as the most likely etiology in the NAPS2-CV or NAPS2-AS studies was similar to the NAPS2 original study (315/508, 62% vs. 222/382, 58.1%, p=0.27). The enrolling physician identified smoking as a risk factor for CP in a significantly greater proportion of patients (all p<0.001) in the NAPS2-CV and NAPS2-AS studies when compared with the NAPS2 original study, including ever smokers (80.7% vs. 45.3%), past (60.3% vs. 30.2%) or current (91.3% vs. 53%) smokers, and those who reported smoking <1 packs per day (79.3% vs. 39.5%) or ≥1 packs per day (83% vs. 49.8%).These differences were preserved in each subgroup when data was analyzed after stratification by alcohol etiology (Table 2 and Figure 1).
Table 2.
Comparison of physician identification of smoking as a chronic pancreatitis risk factor based on smoking status and alcohol etiology in the NAPS2 studies
| Self-reported smoking status |
Alcohol etiology |
NAPS2-CV or NAPS2-AS Study |
NAPS2 Original Study | ||
|---|---|---|---|---|---|
| N | Physician identified smoking as a CP risk factor n (%) |
N | Physician identified smoking as a CP risk factor n (%) |
||
| Ever Smoked | 508 | 410 (80.7) | 382 | 173 (45.3) | |
| Yes | 315 | 283 (89.8) | 222 | 121 (54.5) | |
| No | 193 | 127 (65.8) | 160 | 52 (32.5) | |
| Current Smoker | 334 | 305 (91.3) | 253 | 134 (53.0) | |
| Yes | 236 | 220 (93.2) | 177 | 98 (55.4) | |
| No | 98 | 85 (86.7) | 76 | 36 (47.4) | |
| Past Smoker | 174 | 105 (60.3) | 129 | 39 (30.2) | |
| Yes | 79 | 63 (79.8) | 45 | 23 (51.1) | |
| No | 95 | 42 (44.2) | 84 | 16 (19.0) | |
| ≥1 PPD | 229 | 190 (83.0) | 213 | 106 (49.8) | |
| Yes | 142 | 126 (88.7) | 127 | 74 (58.3) | |
| No | 87 | 64 (73.6) | 86 | 32 (37.2) | |
| ≤ 1PPD | 275 | 218 (79.3) | 147 | 58 (39.5) | |
| Yes | 170 | 155 (91.2) | 85 | 42 (49.4) | |
| No | 105 | 63 (60.0) | 62 | 16 (25.8) | |
Alcohol etiology was based on physician assessment
All comparisons between NAPS2-CV or NAPS2-AS studies vs. NAPS2 original study were ≤0.001.
Figure 1:
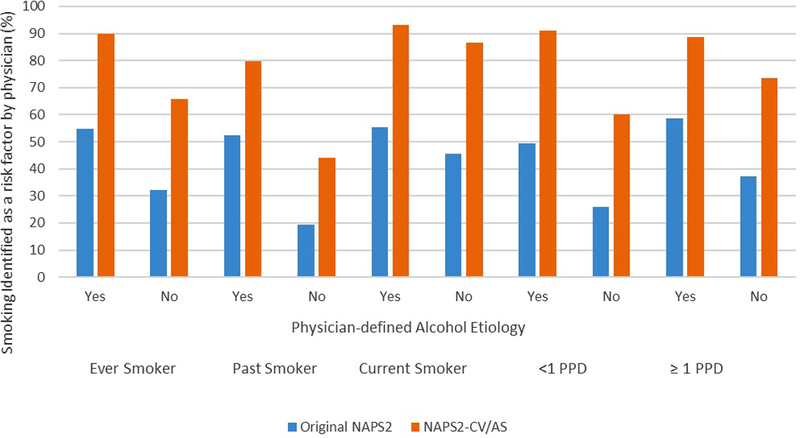
Physician identification of smoking as a risk factor in the NAPS2-CV or NAPS2-AS studies when compared with NAPS2 original study.
To assess whether increased recognition of smoking as a risk factor for CP in the NAPS2-CV or NAPS2-AS studies was confounded by other potential factors, we performed a logistic regression analyses which in addition to the study type, also included age, sex, race and etiology of CP based on physician assessment (alcohol vs. no alcohol) in the model. As shown in Table 2, after controlling for these confounding factor, the odds for enrolling physician to cite smoking as a risk factor for CP was 3.32 to 8.49 folds greater in the different self-reported smoking categories in the NAPS2-CV and NAPS2-AS studies when compared with the original NAPS2 study.
Evaluation of center effect in physician recognition of smoking as a CP risk factor
To assess whether increased recognition of smoking as a risk factor for CP was limited to certain centers, we performed a subset analysis of seven centers who participated in NAPS2-CV and/or NAPS2-AS studies as well as the original NAPS2 study, and where the enrolling physicians identified smoking as a risk factor for CP in <70% of ever smokers in the original NAPS2 study. This analysis was restricted to subjects with self-reported ever smoking due to small sample sizes for other smoking categories (e.g. past/current smoking, amount of smoking). As shown in figure 2, at each of these centers, a significant increase in physician recognition of smoking as a risk factor for CP was noted at each center.
Figure 2:
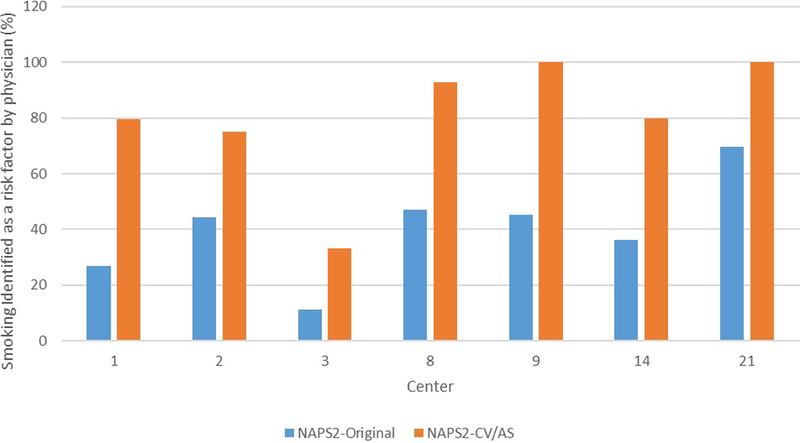
Physician identification of smoking as a risk factor in the NAPS2-CV or NAPS2-AS studies when compared with NAPS2 original study. Only centers that participated in the NAPS2 original study and either one or both of the NAPS2-CV or NAPS2-AS studies and in whom the enrolling physician identified smoking as a risk factor in <70% ever smokers in the NAPS2 original study were included in this analysis
Discussion
In this large study we demonstrate that availability of empiric data and increase in awareness led to a significant increase in physician recognition of smoking as a risk factor for CP. This was reflected by physicians identifying smoking as a risk factor for a patients’ CP more often in NAPS2-CV/AS studies when compared with the original NAPS2 study. This observation was noted among current and past smokers, as well as when stratified based on the amount of smoking. Furthermore, increased recognition persisted after controlling for the effect of confounding factors and appears to be independent of the enrolling site.
Although first reported in 198217, it is only in the past few years that smoking has been widely accepted to be an independent risk factor for susceptibility and progression of pancreatitis. This change in perception is due to availability of empiric data from multiple studies4,6, and has led to investigations into the mechanistic role of effect of smoking on pancreatitis.
In the original NAPS2 study, we demonstrated an independent and dose-dependent association of smoking with the risk of CP5. Interestingly, in this study, we also observed a high variability in physician recognition of smoking as a risk factor13. In the current analysis, we evaluated how often physicians identified smoking as a risk factor for CP in the two subsequently recruited NAPS2 cohorts. When compared with the original NAPS2 study, the enrolling physician identified smoking to be a risk factor in a much greater proportion of ever, past or current smokers, as well as the based on the amount of smoking. The results were similar after stratification of patients by alcohol etiology. In multivariable analyses, physicians were 3–8 times more likely to cite smoking as a risk factor in the NAPS2-CV and NAPS2-AS studies when compared with the original NAPS2 study, after controlling for the effect of age, sex and alcohol etiology. Furthermore, this increased physician recognition seemed to be independent of participating center. This observation is also not explained by the possibility of a change in the composition of patient population – the proportion of patients in whom the enrolling physician considered alcohol as the most likely etiology was similar, and while the prevalence of self-reported smoking was higher in the NAPS2-CV or NAPS2-AS studies when compared with the NAPS2 original study (78 vs 71.4%), this difference was not clinically significant to have accounted for the observations. Finally, at the majority of centers participating, the physician investigators remained the same during the different phases of the NAPS2 studies.
There are numerous compounds in cigarette smoke. Of these, the effect of two key components, i.e. nicotine and tobacco-specific nitrosamine 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone (NNK) have been evaluated in experimental studies. These compounds (either or both) have a range of effects on pancreatic structure and function, including acinar cell secretion, zymogen activation, pro- and anti-inflammatory effects, and thiamine deficiency which can lead to abnormal mitochondrial function8 Studies focusing on the effect of cigarette smoke have demonstrated it to cause alteration in the function of acinar and duct cells, and promote pancreatic fibrosis by its effect on stellate cells9–11. A recent study performed in-vitro and in-vivo experiments to evaluate the synergistic role of smoking and alcohol in pancreatitis. Cigarette smoke was found to suppress the adaptive and protective mechanisms of the unfolded protein response (UPR) that prevent alcohol by itself from causing pancreatic injury. This suppression of the protective UPR by cigarette smoke resulted in unimpeded endoplasmic reticulum stress leading to acinar cell death and pancreatitis12. Cigarette smoke may also result in inhibiting CFTR activity in the pancreatic ducts resulting in decreased pancreatic fluid secretion, and reduced levels of antioxidants (glutathione) which in part is dependent on CFTR activity.18
The U.S. Preventive Services Task Force (USPSTF) recommends that clinicians direct patients who smoke tobacco to cessation interventions.19 Physicians have many opportunities to counsel patients about smoking cessation. As evident from this study, a greater awareness of gastroenterologists led to an increase in their recognition of the relationship between smoking and CP. Smoking cessation can be a daunting task for the patient, and as demonstrated by a recent small study, can be extremely challenging in CP patients20. Therefore, how much of this increased physician recognition will affect reduction on smoking rates by patients cannot be answered by our study. However, dissemination of this information on increased physician recognition would prompt not only gastroenterologists, but also general internists, primary care physicians, family practitioners and other specialists to ask the patients about smoking, counsel them about the association between smoking and pancreatitis, and encourage them to consider smoking cessation programs. This would lead to physician adherence to U.S. Public Health Service recommendation to use the five A’s model (Ask, Advise, Assess, Assist, and Arrange) model when treating CP patients with smoking history21.
A potential limitation of our study is the enrollment of patients from specialized centers limiting the generalizability of results. The enrolling physicians in the NAPS2 studies were gastroenterologists with an interest in pancreatic diseases. We believe that increasing physician awareness led to the higher citation of smoking as a CP risk factor and this approach will apply toall gastroenterologists and physicians in other specialties. However, it is also possible that other factors may have contributed to this observation. Although we did not specifically ask physicians for what led to their increased recognition of smoking as a CP risk factor, we were able to evaluate for several potential factors that could have confounded our findings. In a stratified univariate analysis, the increase in physician recognition was noted irrespective of a centers’ participation in the original NAPS2 study (data not shown). The prevalence of self-reported smoking and physician-defined alcohol etiology were generally similar across the NAPS2 studies. Moreover, we were able to control for the effect of age, sex and physician-defined alcohol etiology in multivariable analyses.
In conclusion, our results suggest that increased awareness of physicians, and possibly all health care professionals, would increase in recognition of smoking as a major risk factor for CP. Increased recognition could potentially culminate in counseling and smoking cessation among CP patients.
Table 3.
Logistic regression analyses evaluating the independent effect of study type (NAPS2-CV or NAPS2-AS vs. NAPS2 Original) in physician identification of smoking as a chronic pancreatitis risk factor
| Self-reported smoking status | NAPS2-CV or NAPS2-AS Study vs. NAPS2 Original |
|---|---|
| Odds ratio (95% Confidence Interval) | |
| Ever Smoked | 5.06 (3.67, 6.99) |
| Current Smoker | 8.49 (5.32, 13.56) |
| Past Smoker | 3.32 (1.95, 5.65) |
| ≤ 1PPD | 6.56 (3.98, 10.82) |
| ≥1 PPD | 4.88 (3.08, 7.75) |
Adjusted for sex, age, race and alcohol etiology
Acknowledgements:
This study was presented as a Poster at the Digestive Disorders Week 2015 and published in an abstract form in Gastroenterology April 2015, Vol 148, Supp 1, Page S-909 as “Increased Awareness Enhances Physician Recognition of the Role of Smoking on Chronic Pancreatitis (CP).”
The authors acknowledge the Epidemiology Data Center, Michael O’Connell, PhD Division of Gastroenterology & Hepatology at the University of Pittsburgh for data management of NAPS2-CV and NAPS2-AS studies, Kim Stello and Danielle Dwyer for genotyping and laboratory management, John Baillie MD, Department of Medicine, Virginia Commonwealth University, Richmond, VA and other members of the NAPS2 consortium.
Grant Support: This research was supported by the National Institute of Health under award numbers DK061451 (DCW), DK077906 (DY), UO1 DK108327 (DC), UO1 DK108320 (CEF), U01 DK108306 (DCW, DY), and UL1 RR024153 and UL1TR000005 (PI—Steven E Reis, MD). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Abbreviations used in this paper:
- AP
(acute pancreatitis)
- CP
(chronic pancreatitis)
- CT
(computerized tomography)
- CFTR
(cystic fibrosis transmembrane conductance regulator)
- EUS
(endoscopic ultrasound)
- MRCP
(magnetic resonance cholangiopancreatography)
- NNK
(nicotine-derived nitrosamine ketone )
- NAPS2
(North American Pancreatitis Studies)
- NAPS2-CV
(North American Pancreatitis Studies- continuation and validation study)
- NAPS2-AS
(North American Pancreatitis Studies- Ancillary Study)
- RAP
(recurrent acute pancreatitis)
- UPR
(unfolded protein response)
- USPSTF
(United States Preventive Services Task Force)
Footnotes
Disclosure: The authors declare no relevant conflicts to disclose.
References:
- 1.Etemad B, Whitcomb DC. Chronic pancreatitis: diagnosis, classification, and new genetic developments. Gastroenterology. 2001;120(3):682–707. [DOI] [PubMed] [Google Scholar]
- 2.Yadav D, Lowenfels AB. The epidemiology of pancreatitis and pancreatic cancer. Gastroenterology. 2013;144(6):1252–1261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Whitcomb DC, Frulloni L, Garg P, et al. Chronic pancreatitis: An international draft consensus proposal for a new mechanistic definition. Pancreatology : official journal of the International Association of Pancreatology (IAP) [et al]. 2016;16(2):218–224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ye X, Lu G, Huai J, Ding J. Impact of smoking on the risk of pancreatitis: a systematic review and meta-analysis. PloS one. 2015;10(4):e0124075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yadav D, Hawes RH, Brand RE, et al. Alcohol consumption, cigarette smoking, and the risk of recurrent acute and chronic pancreatitis. Archives of internal medicine. 2009;169(11):1035–1045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sankaran SJ, Xiao AY, Wu LM, Windsor JA, Forsmark CE, Petrov MS. Frequency of progression from acute to chronic pancreatitis and risk factors: a meta-analysis. Gastroenterology. 2015;149(6):1490–1500.e1491. [DOI] [PubMed] [Google Scholar]
- 7.Talamini G, Bassi C, Falconi M, et al. Smoking cessation at the clinical onset of chronic pancreatitis and risk of pancreatic calcifications. Pancreas. 2007;35(4):320–326. [DOI] [PubMed] [Google Scholar]
- 8.Greer JB, Thrower E, Yadav D. Epidemiologic and Mechanistic Associations Between Smoking and Pancreatitis. Current treatment options in gastroenterology. 2015;13(3):332–346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sliwinska-Mosson M, Milnerowicz H, Jablonowska M, Milnerowicz S, Nabzdyk S, Rabczynski J. The effect of smoking on expression of IL-6 and antioxidants in pancreatic fluids and tissues in patients with chronic pancreatitis. Pancreatology : official journal of the International Association of Pancreatology (IAP) [et al]. 2012;12(4):295–304. [DOI] [PubMed] [Google Scholar]
- 10.Dávid Tálas PP, Viktória Venglovecz, Eleonóra Gál, Krisztina Tóth, Andrea Schnúr, József Maléth, Dezső Csupor, Zoltán Rakonczay Jr., Péter Hegyi. Cigarette Smoke Extract Inhibits Fluid and HCO3- secretion and CFTR activity in guinea pig pancreatic ductal cells. Pancreatology : official journal of the International Association of Pancreatology (IAP) [et al]. 2017;17(3):S48. [Google Scholar]
- 11.Xue J, Zhao Q, Sharma V, et al. Aryl Hydrocarbon Receptor Ligands in Cigarette Smoke Induce Production of Interleukin-22 to Promote Pancreatic Fibrosis in Models of Chronic Pancreatitis. Gastroenterology. 2016;151(6):1206–1217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lugea A, Gerloff A, Su HY, et al. The Combination of Alcohol and Cigarette Smoke Induces Endoplasmic Reticulum Stress and Cell Death in Pancreatic Acinar Cells. Gastroenterology. 2017;153(6):1674–1686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yadav D, Slivka A, Sherman S, et al. Smoking is underrecognized as a risk factor for chronic pancreatitis. Pancreatology : official journal of the International Association of Pancreatology (IAP) [et al]. 2010;10(6):713–719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Whitcomb DC, Yadav D, Adam S, et al. Multicenter approach to recurrent acute and chronic pancreatitis in the United States: the North American Pancreatitis Study 2 (NAPS2). Pancreatology : official journal of the International Association of Pancreatology (IAP) [et al]. 2008;8(4–5):520–531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wilcox CM, Yadav D, Ye T, et al. Chronic pancreatitis pain pattern and severity are independent of abdominal imaging findings. Clinical gastroenterology and hepatology : the official clinical practice journal of the American Gastroenterological Association. 2015;13(3):552–560; quiz e528–559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wilcox CM, Sandhu BS, Singh V, et al. Racial Differences in the Clinical Profile, Causes, and Outcome of Chronic Pancreatitis. The American journal of gastroenterology. 2016;111(10):1488–1496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yen S, Hsieh CC, MacMahon B. Consumption of alcohol and tobacco and other risk factors for pancreatitis. American journal of epidemiology. 1982;116(3):407–414. [DOI] [PubMed] [Google Scholar]
- 18.Hegyi P, Wilschanski M, Muallem S, et al. CFTR: A New Horizon in the Pathomechanism and Treatment of Pancreatitis. Reviews of physiology, biochemistry and pharmacology. 2016;170:37–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Siu AL. Behavioral and Pharmacotherapy Interventions for Tobacco Smoking Cessation in Adults, Including Pregnant Women: U.S. Preventive Services Task Force Recommendation Statement. Annals of internal medicine. 2015;163(8):622–634. [DOI] [PubMed] [Google Scholar]
- 20.Han S, Kheder J, Bocelli L, et al. Smoking Cessation in a Chronic Pancreatitis Population. Pancreas. 2016;45(9):1303–1308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.A clinical practice guideline for treating tobacco use and dependence: 2008 update. A U.S. Public Health Service report. American journal of preventive medicine. 2008;35(2):158–176. [DOI] [PMC free article] [PubMed]


