Abstract
Background.
The aims of this study were to investigate temporal patterns and potential risk factors for severe hyposalivation (xerostomia) after intensity-modulated radiotherapy (IMRT) for head and neck cancer (HNC), and to test the two QUANTEC (Quantitative Analysis of Normal Tissue Effects in the Clinic) guidelines.
Patients and Methods.
Sixty-three patients treated at the Memorial Sloan Kettering Cancer Center between 2006–2015, who had a minimum of three stimulated whole mouth saliva flow measurements (WMSFM) conducted at a median follow-up time of 11 (range: 3–24) months were included. Xerostomia was defined as WMSFM ≤25% compared to relative pre-radiotherapy. Patients were stratified into three follow-up groups: 1: <6 months; 2: 6–11 months; and 3: 12–24 months. Potential risk factors were investigated (Mann-Whitney U test), and relative risks (RRs) assessed for the two QUANTEC guidelines.
Results.
The incidence of xerostomia was 27%, 14% and 17% at follow-up time points 1, 2 and 3, respectively. At <6 months, the mean dose to the contralateral and the ipsilateral parotid glands (Dmeancontra, Dmeanipsi) was higher among patients with xerostomia (Dmeancontra: 25Gy vs. 15Gy; Dmeanipsi: 44Gy vs. 25Gy). Patients with xerostomia had higher pre-RT WMSFM (3.5g vs. 2.4g), and had been treated more frequently with additional chemotherapy (93% vs. 63%; all 4 variables: p<0.05). At 6–11 months, Dmeancontra among patients with xerostomia was higher compared to patients without (26Gy vs. 20Gy). The RR as specified by the one- and two-gland QUANTEC guideline was 2.3 and 1.4 for patients with <6 months follow-up time, and 2.0 and 1.2 for patients with longer follow-up (6–11 + 6–24 months).
Conclusion.
Xerostomia following IMRT peaks within six months post-radiotherapy and fades with time. Limiting the mean dose to both parotid glands (ipsilateral <25 Gy, contralateral <25 Gy) and reducing the use of chemotherapy will likely decrease the rate of xerostomia. Both QUANTEC guidelines are effective in preventing xerostomia.
Keywords: Xerostomia, Severe hyposalivation, IMRT, Head and neck cancer, QUANTEC
1. Introduction
Intensity modulated-radiation therapy (IMRT) is currently the standard of care for the treatment of head and neck cancer (HNC), and enables delivery of highly conformal dose distributions. Xerostomia is the most common long-term side effect observed in HNC patients following RT (Bjordal et al., 1994; Jensen et al., 1994; Wijers et al., 2002). Patients typically describe the condition as dry mouth, altered taste, and/or a reduction in salivary flow, and that it limits their quality of life in terms of poor oral hygiene, halitosis, dental caries, and difficulty with speech, mastication, and/or swallowing. Based on animal models, the pathophysiology of RT-induced xerostomia has been described by an early phase starting at the end of RT and lasting up to two months post-RT, and by a late phase between around two months up to eight months post-RT (Coppes et al., 2001). Furthermore, the early phase may be attributed to apoptosis or membrane damage-induced dysfunction, and the late phase to an inability of replacing the apoptotic acinar cells due to radiation-induced reduction of the stem/progenitor cells (Coppes et al., 2001; Vissink et al., 2010; Jensen et al., 2010; Konings et al., 2005; Lombaert et al., 2008). The study by Belli et al. showed that parotid gland volume and density changes occurred early during treatment and accurately correlated to acute xerostomia (Belli et al., 2014). The parotid glands produce up to 70% of the total stimulated saliva (Dawes and Wood, 1973; Humphrey and Williamson, 2001; Sreebny, 2000). Clinically significant hyposalivation is commonly defined as Grade 4 xerostomia according to the LENT-SOMA tables (LENT-SOMA, 1995) i.e. a preserved stimulated salivary function ≤25% post- relative to pre-RT. In two randomized controlled phase III trials, IMRT has been found to significantly reduce the risk of severe hyposalivation (xerostomia) compared with conventional RT (Nutting et al., 2011; Kam et al., 2007). However, limiting the dose to the surrounding critical structures completely, without compromising treatment efficacy can be challenging.
Previous studies aiming to establish a dose-response relationship for xerostomia have reported that parotid gland dysfunction is minimal at a mean dose (Dmean)<10 Gy, that the gland function is further reduced as Dmean increases to ~40 Gy, and that a Dmean>40 Gy typically involves complete parotid gland dysfunction (Chao et al., 2001; Blanco et al., 2005; Leslie and Dische, 1994). Within the Quantitative Analysis of Normal Tissue Effects in the Clinic (QUANTEC) report devoted to salivary gland function post-RT, two guidelines to minimize the risk of salivary gland dysfunction in patients with HNC treated with RT were proposed: long-term severe xerostomia would be reduced if: i.) One of the parotid glands receives a Dmean<~20 Gy, or ii.) Both parotid glands receive a Dmean<~25 Gy (Deasy et al., 2010). It has previously been demonstrated that the one-gland guideline is effective in order to reduce the number of patients who would otherwise experience moderate to severe xerostomia (Moiseenko et al., 2012) (Beetz et al., 2014). However, since the two-gland guideline has been studied to a much lesser extent, its applicability for preventing xerostomia and how it compares to the one-gland guideline remains unclear.
The goals of the current study were: 1.) To objectively evaluate the occurrence and rate of severe hyposalivation (xerostomia) following IMRT in HNC patients with a particular focus on temporal patterns; 2.) To investigate potential risk factors for its development, and; 3.) To explore the applicability of the two guidelines provided in the QUANTEC salivary gland function-specific summary.
2. Patients and methods
2.1. Patient cohort
The Institutional Review Board approved this retrospective study including all HNC patients treated with IMRT at the Memorial Sloan Kettering Cancer Center between March 2006 and March 2015. Whole mouth saliva flow measurements (WMSFM) were prospectively collected from HNC patient. To further qualify for inclusion, the following criteria applied: a minimum of three stimulated WMSFM [g/5 mins], WMSFM >1g/5mins assessed pre-RT to exclude potential predisposition of xerostomia pre-RT, reasonably high RT prescription dose (≥50.4 Gy) to the tumor site, and at least two WMSFM assessed within 24 months post-RT. In total, 63 patients fulfilled these inclusion criteria. Flow chart of the patients is presented in Figure 1. During the study period, the standard of care was to systematically avoid Dmean>26 Gy to the contralateral and the ipsilateral parotid glands (Dmeancontra, Dmeanipsi).
Figure 1.
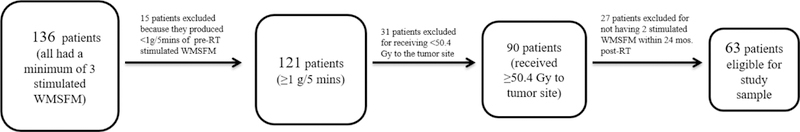
Shows the flowchart of patients
2.2. Xerostomia definition and stimulated saliva measurements
Patients refrained from eating and drinking at a minimum of one hour prior to WMSFM. Saliva was collected in a pre-weighed plastic cup, and patients were asked to spit into the cup every minute for five minutes after the administration of a citrate solution to both sides of the tongue every 30 seconds during a two-minute period. Xerostomia was defined as Grade 4 according to the LENT SOMA tables (LENT-SOMA, 1995) i.e. WMSFM ≤25% post- relative to pre-RT
2.3. Statistical analysis
Patients were stratified into three follow-up groups: 1: <6 months; 2: 6–11 months; and 3: 12–24 months. In each of these groups, patient- (gender (binary), N-stage T-stage, tumor site (categorical), age, and pre-RT WMSFM (continuous)), and treatment-related (concurrent chemotherapy, involved neck RT, surgery (binary), histology (categorical), Dmeancontra and Dmeanipsi (continuous)) characteristics were compared between patients with and without xerostomia using a Mann-Whitney U test. The number of patients fulfilling/violating the QUANTEC guidelines was recorded, and the relative risk (RR) was assessed for each guideline (Eq.1):
| (Eq.1) |
3. Results
The mean (±SD) age for the 63 included patients was 57 (±10) years, of which the majority were men (81%), diagnosed with squamous cell carcinoma (81%), treated to 70.0–70.2 Gy (65%) with involved neck RT (73%) for tumors of stage T1-T2 (65%), and nodal spread of disease of stage N1-N2 (75%).
3.1. Xerostomia <6m post-RT is associated with chemotherapy use and tumor site
The incidence of xerostomia was 27% (n=15), 14% (n=5), and 17% (n=5) at <6 months, 6–11 months, and 12–24 months, respectively. For the shortest follow-up time, the use of concurrent chemotherapy and WMSFM pre-RT were significantly higher among patients with xerostomia (chemotherapy: 93% vs. 63%, p=0.02; WMSFM: 3.5±1.5g vs. 2.4±0.8g, p=0.01; Table 1). At this follow-up time, the tumor site was significantly different between patients with xerostomia and those without xerostomia (p=0.03) with tumors of unknown primary being present only among xerostomia patients, and tumors of the buccal mucosa, floor of mouth, larynx, nasal cavity, sinus, submandibular gland, retromolar trigone, and thyroid being present only in patients without xerostomia. At the <6 months follow-up there was a tendency of xerostomia patients being women to a larger extent (33% vs. 13%; p=0.08), and treated to a lesser extent with surgery (7% vs. 30%; p=0.06). Within the two later follow-up times, none of the investigated patient characteristics were significantly different between patients with and without xerostomia. However, a trend towards significantly higher WMSFM pre-RT values for patients with xerostomia at 12–24 months was observed (3.8±1.7g vs. 2.5±1.1g; p=0.06). Since the two later follow-up times consisted of considerably fewer patients than in the <6 months follow-up group (n=34 and 35 vs. n=55), we combined the patients within the two later follow-up groups into a 6–24 months group (n=53). In this new follow-up group, none of the investigated characteristics were significantly different between patients with and without xerostomia, but WMSFM pre-RT tended to be higher for patients with xerostomia (3.4±1.4g vs. 2.6±1.2g; p=0.06).
Table 1.
Patient and treatment-related characteristics stratified by patients with xerostomia and no xerostomia for the studied follow-up times.
Note: the right-most column is a fusion of the two adjacent follow-up times.
| <6m | 6–11m | 12–24m | 6–24m | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Xerostomia | No Xerostomia |
Xerostomia | No Xerostomia |
Xerostomia | No Xerostomia |
Xerostomia | No Xerostomia |
|||||
| (n=15) | (n=40) | (n=5) | (n=30) | (n=5) | (n=29) | (n=10) | (n=43) | |||||
| Mean±SD | Mean±SD | p | Mean±SD | Mean±SD | p | Mean±SD | Mean±SD | p | Mean±SD | Mean±SD | p | |
| Age [y] | 56±7 | 57±10 | 0.89 | 51±9 | 58±9 | 0.20 | 54±10 | 57±12 | 0.69 | 53±9 | 58±10 | 0.20 |
| Dmeancontra [Gy] | 24.9±12.1 | 15.3±8.2 | 0.002* | 26.4±2.7 | 19.5±10.7 | 0.01* | 22.8±6.0 | 15.5±9.4 | 0.08 | 24.6±4.8 | 17.4±10.7 | 0.003* |
| Dmeanipsi [Gy] | 43.8±20.3 | 25.1±12.6 | <0.0001* | 38.6±14.9 | 29.2±17.1 | 0.06 | 41.0±14.3 | 27.6±15.6 | 0.10 | 39.8±13.8 | 29.4±17.3 | 0.02* |
| WMSFM Pre-RT | 3.5±1.5 | 2.4±0.8 | 0.01* | 3.0±1.2 | 2.7±1.3 | 0.40 | 3.8±1.7 | 2.5±1.1 | 0.06 | 3.4±1.4 | 2.6±1.2 | 0.06 |
| N (%) | N (%) | N (%) | N (%) | N (%) | N (%) | N (%) | N (%) | |||||
|
Chemotherapy N/A |
14 (93) | 25 (63) | 0.02* | 5 (100) | 19 (63) | 0.24 | 4 (80) | 14 (48) | 0.60 | 9 (90) | 26 (60) | 0.22 |
| 1 (7) | 4 (10) | - | 5 (17) | - | 2 (7) | - | 6 (14) | |||||
| 1 (7) | 4 (10) | - | 5 (17) | - | 2 (7) | - | 6 (14) | |||||
| T-stage | 0.17 | 0.77 | 0.18 | 0.63 | ||||||||
| 1 | 4 (27) | 15 (38) | 1 (20) | 12 (40) | 1 (20) | 11 (38) | 2 (20) | 18 (42) | ||||
| 2 | 4 (27) | 13 (33) | 2 (40) | 7 (23) | 3 (60) | 7 (24) | 5 (50) | 12 (28) | ||||
| 3 | 1 (7) | 4 (10) | - | 5 (17) | 1 (20) | 1 (3) | 1 (10) | 4 (9) | ||||
| 4 | 2 (13) | 7 (18) | 1 (20) | 5 (17) | - | 2 (7) | 1 (10) | 6 (14) | ||||
| N/A | 4 (27) | 1 (3) | 1 (20) | 1 (3) | - | 3 (10) | 1 (10) | 3 (7) | ||||
| Tumor site | 0.03* | 0.60 | 0.79 | 0.19 | ||||||||
| Base of tongue | 2 (13) | 7 (18) | 1 (20) | 6 (20) | 1 (20) | 8 (28) | 2 (20) | 8 (19) | ||||
| Buccal mucosa | - | 2 (5) | - | 1 (3) | 1 (20) | - | 1 (10) | 1 (2) | ||||
| Floor of mouth | - | 1 (3) | - | 1 (3) | - | - | - | 1 (2) | ||||
| Larynx | - | 6 (15) | 1 (20) | 4 (13) | - | - | 1 (10) | 5 (11) | ||||
| Max sinus | - | 1 (3) | - | 1 (3) | - | - | - | 1 (2) | ||||
| Nasal cavity | - | 1 (3) | - | - | - | 1 (3) | - | 1 (2) | ||||
| Nasopharynx | 3 (20) | 5 (13) | 2 (40) | 4 (13) | 2 (40) | 3 (10) | 4 (40) | 5 (12) | ||||
| Oral tongue | 2 (13) | 5 (13) | 1 (20) | 4 (13) | 1 (20) | 2 (7) | 2 (20) | 5 (12) | ||||
| Parotid | 1 (7) | 1 (3) | - | - | - | 3 (10) | - | 3 (7) | ||||
| Submandibular gland | - | 1 (3) | - | - | - | - | - | 1 (2) | ||||
| Retromolar trigone | - | 1 (3) | - | - | - | 1 (3) | - | - | ||||
| Thyroid | - | 1 (3) | - | - | - | - | - | - | ||||
| Tonsil | 4 (27) | 8 (20) | - | 8 (27) | - | 2 (7) | - | 10 (43) | ||||
| Unknown primary | 3 (20) | - | - | 1 (3) | - | 1 (3) | - | 2 (5) | ||||
3.2. Mean dose to the contralateral and the ipsilateral parotid gland predicts Xerostomia <6m post-RT
For patients with a follow-up time <6 months, both Dmeancontra and Dmeanipsi were significantly higher among patients with xerostomia (Dmeancontra: 25±2Gy vs. 15±8Gy, p=0.002; Dmeanipsi: 44±20Gy vs. 25±13Gy, p<0.0001; Table 1). A similar trend was observed when combining patients in the two later follow-up groups (Dmeancontra: 25±5Gy vs. 17±11Gy, p=0.003; Dmeanipsi: 40±14Gy vs. 29±17Gy, p=0.02). At 6–11m, Dmeancontra was significantly higher among patients with than without xerostomia (26±3Gy vs. 20±11Gy, p=0.01).
3.3. The QUANTEC guidelines are effective in preventing xerostomia
In total, xerostomia was observed among 10% and 4% of the patients that met the one-gland QUANTEC guideline (Dmeancontra<20 Gy) at <6 months, and at 6–24 months, respectively, while the analogous rate of xerostomia for patients violating this guideline was 46% and 54% (Table 2). Seven and 8% of the patients that fulfilled the two-gland guideline (Dmeancontra and Dmeanipsi<25 Gy) experienced xerostomia at <6 months, and at 6–24 months, respectively; the corresponding figure for patients violating this guideline was 34% and 22%, respectively (Table 2). These findings indicate that both guidelines are effective in order to prevent RT-induced xerostomia regardless of follow-up time. The reason for patients violating the two-gland guideline was primarily due to both glands presenting Dmean >25 Gy, except for patients without xerostomia at <6m where the violation was instead explained primarily by Dmeanipsi only being >25 Gy. The RR for the one-gland guideline was 2.3 (95%CI: 1.4–3.7) at <6 months, and 2.0 (95%CI: 1.4–3.0) at 6–24 months, and was considerably lower for the two gland guideline, and not statistically significantly higher relative to pre-RT at 6–24 months (<6 months: RR=1.4 (95%CI: 1.1–1.8); 6–24 months: RR=1.2 (95%CI: 0.9–1.6)). This indicates that violation of the one-gland guideline is riskier with respect to the development of xerostomia than violation of the two-gland guideline. A 3D representation of the relationship between WMSFM post-RT/pre-RT, Dmeancontra, and Dmeanipsi, including also patients not adhering to any of the two QUANTEC guidelines, is given in Figure 2.
Table 2.
The number (%) of patients with and without xerostomia fulfilling / violating the two criteria provided by the QUANTEC guidelines, and the related mean dose to the contralateral and the ipsilateral parotid glands (Dmeancontra, Dmeanispi), as well as the relative risks (RRs), 95% confidence intervals (95%CIs), and p-values for the two guidelines.
| Dmeancontra≤20 Gy | Dmeancontra>20 Gy | |||||||
| Follow-up | N (%) Fulfilled | Dmeancontra [Gy] | N (%) Not fulfilled | Dmeancontra [Gy] | RR | 95% CI | p | |
| <6m | Xerostomia | 3 (10) | 11.2±6.7 | 12 (46) | 28.3±10.7 | 2.3 | 1.4–3.7 | 0.001* |
| No Xerostomia | 26 (90) | 10.6±6.0 | 14 (54) | 24.2±2.5 | ||||
| 6–24m | Xerostomia | 1 (4) | 12.7±0.0 | 9 (32) | 25.9±2.5 | 2.0 | 1.4–3.0 | 0.0004* |
| No Xerostomia | 24 (96) | 10.5±6.0 | 19 (68) | 26.1±8.9 | ||||
| Dmeancontra≤25Gy, Dmeanipsi≤25 Gy | Dmeancontra>25Gy, Dmeanipsi>25 Gy | |||||||
| Follow-up | N (%) Fulfilled | Dmeanipsi [Gy] | N (%) Not fulfilled | Dmeanipsi [Gy] | RR | 95% CI | p | |
| <6m | Xerostomia | 1 (7) | 23.2±0.0 | 14 (34) | 45.3±20.2 | 1.4 | 1.1–1.8 | 0.01* |
| No Xerostomia | 13 (93) | 13.5±8.9 | 27 (66) | 31.2±9.6 | ||||
| 6–24m | Xerostomia | 1 (8) | 24.5±0.0 | 9 (22) | 41.5±13.5 | 1.2 | 0.9–1.6 | 0.13 |
| No Xerostomia | 12 (92) | 13.8±8.1 | 32 (78) | 35.3±16.2 | ||||
Figure 2.
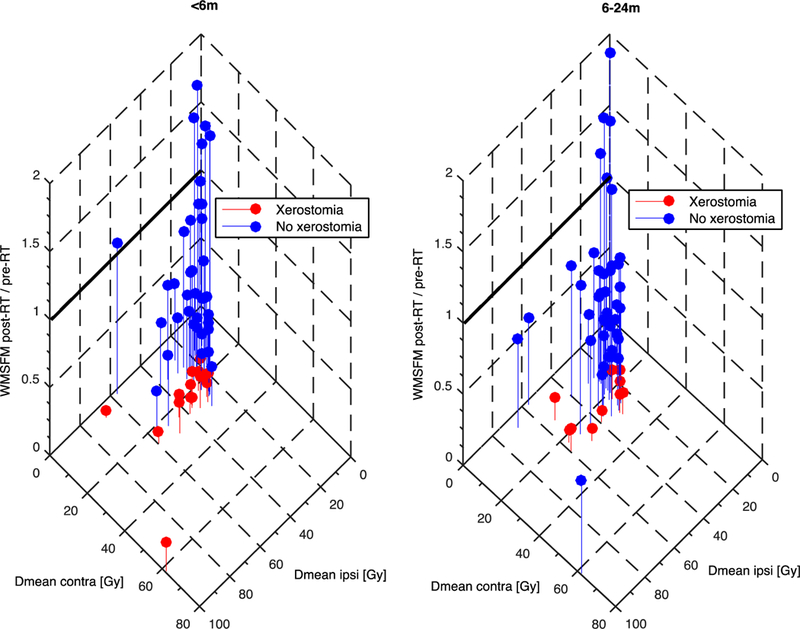
A three-dimensional scatter plot illsutrating the relationship between the ratio of WMSFM pre- and post-RT (z-axis), Dmeancontra (y-axis), and Dmeanipsi (x-axis) for patients experiencing (red), and not experiencing (blue) xerostomia for a <6 months follow-up (left), and a 6 – 24 months follow-up (right).
Note: Patients fulfilling the one-gland QUANTEC guideline are located left of the dashed 20 Gy y-axis line, and patients with a WMSFM post-RT/pre-RT ˃1.0 are located above the solid z-axis line.
4. Discussion
Based on stimulated whole mouth saliva flow measurements (WMSFM) acquired for 63 patients pre- and post-IMRT for head and neck cancer (HNC), our data suggest that xerostomia is most prevalent within the six first months after RT. Furthermore, our findings suggest that dose to the parotid glands and the use of chemotherapy contributed to xerostomia.
We found that the rate of xerostomia decreased with follow-up time, and was around twice as high within six months post-RT relative to follow-up times beyond this. A similar temporal pattern of recovery from xerostomia, has been observed previously by both Beetz et al, where a significant recovery in patient-reported xerostomia was identified between six and 24 months post-RT (Beetz et al., 2014), and by Moiseenko et al. where patients were found to recover from xerostomia (using the same endpoint as investigated in this study) in a time frame between three and 12 months (Moiseenko et al., 2012). The observed temporal recovery from xerostomia is also in agreement with previous findings based on animal studies where a recovery has been observed at around eight months after RT (Lombaert et al., 2008). A temporal recovery pattern was also reflected in the mean dose to the contralateral and the ipsilateral parotid gland with both being significantly higher among patients with xerostomia compared to patients without xerostomia at <6 months, which was also true but less distinct at the 6–24 months follow-up. Refining the 6–24 months follow-up group into a 6–11 months, and a 12–24 months resulted in considerably smaller number of patients than in the <6 months follow-up group and only the mean dose to the contralateral parotid gland was significantly higher among patients with xerostomia at the 6–11 months follow-up. A complete understanding of the exact temporal recovery of xerostomia between six and 24 months after RT, thus, remains unclear, and would likely require a much larger population than studied here. Higher RT doses to the parotid glands predispose patients to xerostomia, although some patients gradually recover within 24 months and may experience complete recovery (Chao et al., 2001; Blanco et al., 2005; Eisbruch et al., 2001; Maes et al., 2002; Li et al., 2007; Moiseenko et al., 2012; Beetz et al., 2014). In summary, lowering the mean dose to both parotid glands is likely to reduce the number of patients experiencing xerostomia after head and neck RT.
Among the nine patient- and treatment-related characteristics investigated, we found that the use of concomitant chemotherapy increased the risk of xerostomia within six months post-RT, but not at the later follow-up times. Hey et al. similarly observed that patients treated with RT combined with chemotherapy experienced xerostomia to a larger extent at six months after treatment compared to patients treated with RT alone (Hey et al., 2009). Within the shortest follow-up time we also found that tumor site was significantly different between patients that experienced and not experienced xerostomia with tumors of unknown primary being present only among the former group, and tumors of the buccal mucosa/floor of mouth/larynx/nasal cavity/sinus/submandibular gland/retromolar trigone/thyroid being present only among the latter group. The tumor site finding can be directly translated as a dose dependency since the population average of both Dmeancontra and Dmeanipsi were up to twice as high for the tumor sites being present only/among patients with xerostomia compared to the population average of the corresponding Dmean for all tumor sites among patients without xerostomia.
In general, we found that the two QUANTEC guidelines prevented xerostomia both at the <6 months, and at the 6–24 months follow-up. For the one-gland guideline, the relative risks (RRs) were of the same magnitude regardless of follow-up time. The RRs for the two-gland guideline were considerably lower than that of the one-gland guideline, and not significantly different between patients that fulfilled and violated the two-gland guideline at the later follow-up time. Also, patients with xerostomia that violated the one-gland guideline had on average 2.5, and 2.0 times higher mean doses to the contralateral gland at <6 months, and 6–24 months, respectively, compared to patients that fulfilled this guideline, whereas the corresponding ratio for the mean dose to the ipsilateral gland was 2.0 and 1.7 at <6 months, and 6–24 months, respectively. This indicates that another cut-off value on the ipsilateral mean dose might be more suitable to prevent xerostomia within our dataset. Interestingly, in a recent study from our institution patient reported xerostomia outcome was better correlated with significant reduction in the mean radiation dose to the bilateral submandibular salivary glands, oral cavity, and contralateral parotid gland if using target delineation-sparing bilateral neck level 1B (Tam et al., 2015). The study by Tribius et al. showed that the incidence of xerostomia in patients with bilateral parotid gland sparing to <26 Gy was significantly lower when compared to patients with one parotid gland sparing to >26 Gy (Tribius et al., 2013). A recent study by Miah et al., also showed that patients treated with bilateral superficial lobe parotid-sparing IMRT reduces the risk of developing xerostomia compared to patients treated with contralateral parotid–sparing IMRT (Miah et al., 2016).
Also, the salivary function-specific QUANTEC summary demonstrated that the dose-response curve for xerostomia is shallow (Deasy et al., 2010), implicating that lowering the mean dose even further, without deteriorating the prescribed dose coverage of the tumor, than suggested in their two guidelines, should be a goal in routine treatment planning for HNC. Previous studies on this topic have primarily focused on the one-gland guideline. For instance, and for the same xerostomia definition as used in this study, Moiseenko et al. showed that less than 20% of the patients in their cohort developed xerostomia twelve months after RT if the mean dose of the less irradiated parotid gland was <20 Gy (Moiseenko et al., 2012). The corresponding rate in our study was much less than 20%: 10% at <6 months, and 4% at 6–24 months. Using patient-reported instruments, (Lee and Fang, 2013) also found that the rate of xerostomia for patients who met the one gland guideline decreased with follow-up time: 22% at three months post-RT, and 13% at twelve months post-RT. Regardless of xerostomia status and gland guideline, we found that in total 46% and 39.6% of the patients in our cohort adhered to the one gland guideline at <6 months, and at 6–24 months after RT. Based on patient-reported xerostomia, Beetz et al. recently reported a similar rate for the one gland guideline (32% of the patients met the guideline) following IMRT, but in that study it was also found that the likelihood to meet the guideline was higher following 3DCRT (47% of the patients met the guideline) (Beetz et al., 2014). Given the differences in dose distribution patterns between IMRT and 3DCRT, it may be needed to refine the QUANTEC guidelines, which were primarily suggested based on the experience from 3DCRT regimens, to also be valid for more novel RT planning and delivery technique such as IMRT.
Recently a number of studies have addressed intra-heterogeneity within the parotid glands, and in particular highlighted that sparing of primarily the gland region containing stem/progenitor cells from radiation is important (van Luijk et al., 2015; Pringle et al., 2013). To the best of our knowledge, the only related effort including actual patient data is the study by Clark et al. who, however, found that the mean dose to the entire gland predicted xerostomia to a same extent as did the mean dose to subsets of the parotid gland (Clark et al., 2015a). A more sophisticated approach to segment the region of the parotid gland that includes the stem cells, e.g. as outlined in the image-based pilot study on volunteers, is likely needed to further examine the dependence of a hypothesized intra-gland response of xerostomia (Clark et al., 2015b). A limitation to this study is that certain confounders such as smoking status and the use of medication was not taken into consideration in the evaluation of xerostomia.
5. Conclusion
This study demonstrates that the rate of RT-induced xerostomia is highest within the first half-year after treatment, and decreases considerably at longer follow-up times. Precaution should be made to patients with tumors of unknown primary, and when administrating chemotherapy since these two factors are likely to increase the risk of developing xerostomia. In general, we recommend the use of QUANTEC guidelines to prevent the development of xerostomia, but we also acknowledge that the rate of xerostomia is likely to decrease even further if reducing the mean dose to both parotid glands below 25 Gy, without deteriorating the delivery of the prescribed tumor dose.
Acknowledgments
The authors acknowledge the support of NIH/NCI Cancer Center Support Grant P30 CA008748.
Footnotes
Disclosure: The authors report no conflicts of interest, and they are solely responsible for the conduct of this study and the content of this paper.
References
- Beetz I, Steenbakkers RJ, Chouvalova O, Leemans CR, Doornaert P, van der Laan BF, et al. : The QUANTEC criteria for parotid gland dose and their efficacy to prevent moderate to severe patient-rated xerostomia. Acta Oncol 53(5): 597–604, 2014 [DOI] [PubMed] [Google Scholar]
- Belli ML, Scalco E, Sanguineti G, Fiorino C, Broggi S, Dinapoli N, et al. : Early changes of parotid density and volume predict modifications at the end of therapy and intensity of acute xerostomia. Strahlenther Onkol 190(11): 1001–1007, 2014 [DOI] [PubMed] [Google Scholar]
- Bjordal K, Kaasa S, Mastekaasa A: Quality of life in patients treated for head and neck cancer: a follow-up study 7 to 11 years after radiotherapy. Int J Radiat Oncol Biol Phys 28(4): 847–856, 1994 [DOI] [PubMed] [Google Scholar]
- Blanco AI, Chao KS, El Naqa I, Franklin GE, Zakarian K, Vicic M, et al. : Dose-volume modeling of salivary function in patients with head-and-neck cancer receiving radiotherapy. Int J Radiat Oncol Biol Phys 62(4): 1055–1069, 2005 [DOI] [PubMed] [Google Scholar]
- Chao KS, Deasy JO, Markman J, Haynie J, Perez CA, Purdy JA, et al. : A prospective study of salivary function sparing in patients with head-and-neck cancers receiving intensity-modulated or three-dimensional radiation therapy: initial results. Int J Radiat Oncol Biol Phys 49(4): 907–916, 2001 [DOI] [PubMed] [Google Scholar]
- Clark H, Hovan A, Moiseenko V, Thomas S, Wu J, Reinsberg S: Regional radiation dose susceptibility within the parotid gland: effects on salivary loss and recovery. Med Phys 42(4): 2064–2071, 2015a [DOI] [PubMed] [Google Scholar]
- Clark HD, Moiseenko VV, Rackley TP, Thomas SD, Wu JS, Reinsberg SA: Development of a method for functional aspect identification in parotid using dynamic contrast-enhanced magnetic resonance imaging and concurrent stimulation. Acta Oncol 54(9): 1686–1690, 2015b [DOI] [PubMed] [Google Scholar]
- Coppes RP, Zeilstra LJ, Kampinga HH, Konings AW: Early to late sparing of radiation damage to the parotid gland by adrenergic and muscarinic receptor agonists. Br J Cancer 85(7): 1055–1063, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dawes C, Wood CM: The contribution of oral minor mucous gland secretions to the volume of whole saliva in man. Arch Oral Biol 18(3): 337–342, 1973 [DOI] [PubMed] [Google Scholar]
- Deasy JO, Moiseenko V, Marks L, Chao KS, Nam J, Eisbruch A: Radiotherapy dose-volume effects on salivary gland function. Int J Radiat Oncol Biol Phys 76(3 Suppl): S58–63, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisbruch A, Kim HM, Terrell JE, Marsh LH, Dawson LA, Ship JA: Xerostomia and its predictors following parotid-sparing irradiation of head-and-neck cancer. Int J Radiat Oncol Biol Phys 50(3): 695–704, 2001 [DOI] [PubMed] [Google Scholar]
- Hey J, Setz J, Gerlach R, Vordermark D, Gernhardt CR, Kuhnt T: Effect of Cisplatin on parotid gland function in concomitant radiochemotherapy. Int J Radiat Oncol Biol Phys 75(5): 1475–1480, 2009 [DOI] [PubMed] [Google Scholar]
- Humphrey SP, Williamson RT: A review of saliva: normal composition, flow, and function. J Prosthet Dent 85(2): 162–169, 2001 [DOI] [PubMed] [Google Scholar]
- Jensen AB, Hansen O, Jorgensen K, Bastholt L: Influence of late side-effects upon daily life after radiotherapy for laryngeal and pharyngeal cancer. Acta Oncol 33(5): 487–491, 1994 [DOI] [PubMed] [Google Scholar]
- Jensen SB, Pedersen AM, Vissink A, Andersen E, Brown CG, Davies AN, et al. : A systematic review of salivary gland hypofunction and xerostomia induced by cancer therapies: management strategies and economic impact. Support Care Cancer 18(8): 1061–1079, 2010 [DOI] [PubMed] [Google Scholar]
- Kam MK, Leung SF, Zee B, Chau RM, Suen JJ, Mo F, et al. : Prospective randomized study of intensity-modulated radiotherapy on salivary gland function in early-stage nasopharyngeal carcinoma patients. J Clin Oncol 25(31): 4873–4879, 2007 [DOI] [PubMed] [Google Scholar]
- Konings AW, Cotteleer F, Faber H, van Luijk P, Meertens H, Coppes RP: Volume effects and region-dependent radiosensitivity of the parotid gland. Int J Radiat Oncol Biol Phys 62(4): 1090–1095, 2005 [DOI] [PubMed] [Google Scholar]
- Lee TF, Fang FM: Quantitative analysis of normal tissue effects in the clinic (QUANTEC) guideline validation using quality of life questionnaire datasets for parotid gland constraints to avoid causing xerostomia during head-and-neck radiotherapy. Radiother Oncol 106(3): 352–358, 2013 [DOI] [PubMed] [Google Scholar]
- LENT SOMA tables. Radiother Oncol 35: 17–60, 1995 [PubMed]
- Leslie MD, Dische S: The early changes in salivary gland function during and after radiotherapy given for head and neck cancer. Radiother Oncol 30(1): 26–32, 1994 [DOI] [PubMed] [Google Scholar]
- Li Y, Taylor JM, Ten Haken RK, Eisbruch A: The impact of dose on parotid salivary recovery in head and neck cancer patients treated with radiation therapy. Int J Radiat Oncol Biol Phys 67(3): 660–669, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lombaert IM, Brunsting JF, Wierenga PK, Faber H, Stokman MA, Kok T, et al. : Rescue of salivary gland function after stem cell transplantation in irradiated glands. PLoS One 3(4): e2063, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maes A, Weltens C, Flamen P, Lambin P, Bogaerts R, Liu X, et al. : Preservation of parotid function with uncomplicated conformal radiotherapy. Radiother Oncol 63(2): 203–211, 2002 [PubMed] [Google Scholar]
- Miah AB, Gulliford SL, Morden J, Newbold KL, Bhide SA, Zaidi SH, et al. : Recovery of Salivary Function: Contralateral Parotid-sparing Intensity-modulated Radiotherapy versus Bilateral Superficial Lobe Parotid-sparing Intensity-modulated Radiotherapy. Clin Oncol (R Coll Radiol), 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moiseenko V, Wu J, Hovan A, Saleh Z, Apte A, Deasy JO, et al. : Treatment planning constraints to avoid xerostomia in head-and-neck radiotherapy: an independent test of QUANTEC criteria using a prospectively collected dataset. Int J Radiat Oncol Biol Phys 82(3): 1108–1114, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nutting CM, Morden JP, Harrington KJ, Urbano TG, Bhide SA, Clark C, et al. : Parotid-sparing intensity modulated versus conventional radiotherapy in head and neck cancer (PARSPORT): a phase 3 multicentre randomised controlled trial. Lancet Oncol 12(2): 127–136, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pringle S, Van Os R, Coppes RP: Concise review: Adult salivary gland stem cells and a potential therapy for xerostomia. Stem Cells 31(4): 613–619, 2013 [DOI] [PubMed] [Google Scholar]
- Sreebny LM: Saliva in health and disease: an appraisal and update. Int Dent J 50(3): 140–161, 2000 [DOI] [PubMed] [Google Scholar]
- Tam M, Riaz N, Kannarunimit D, Pena AP, Schupak KD, Gelblum DY, et al. : Sparing bilateral neck level IB in oropharyngeal carcinoma and xerostomia outcomes. Am J Clin Oncol 38(4): 343–347, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tribius S, Sommer J, Prosch C, Bajrovic A, Muenscher A, Blessmann M, et al. : Xerostomia after radiotherapy. What matters--mean total dose or dose to each parotid gland? Strahlenther Onkol 189(3): 216–222, 2013 [DOI] [PubMed] [Google Scholar]
- van Luijk P, Pringle S, Deasy JO, Moiseenko VV, Faber H, Hovan A, et al. : Sparing the region of the salivary gland containing stem cells preserves saliva production after radiotherapy for head and neck cancer. Sci Transl Med 7(305): 305ra147, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vissink A, Mitchell JB, Baum BJ, Limesand KH, Jensen SB, Fox PC, et al. : Clinical management of salivary gland hypofunction and xerostomia in head-and-neck cancer patients: successes and barriers. Int J Radiat Oncol Biol Phys 78(4): 983–991, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wijers OB, Levendag PC, Braaksma MM, Boonzaaijer M, Visch LL, Schmitz PI: Patients with head and neck cancer cured by radiation therapy: a survey of the dry mouth syndrome in long-term survivors. Head Neck 24(8): 737–747, 2002 [DOI] [PubMed] [Google Scholar]


