Abstract
Previous work shows that medial prefrontal cortex (mPFC) cells exhibit spatio-selective activity at a goal location when rats are trained in a goal-oriented navigation task. Damaging the ventral and intermediate hippocampal regions severely disrupts both mPFC goal firing and behavioral performance in the same task. Additionally, hippocampal place cells tend to develop a secondary place field at the goal location, suggesting that goal locations can be encoded by local changes in firing rate, within an otherwise stable spatial representation. Therefore, it has been suggested that the coordinated activity of a large fraction of hippocampal cells at the goal location may interact with the mPFC to compute accurate planning trajectories, relying on both precise location-specific firing of place cells and the coarse-coded, goal-trajectory planning function of the prefrontal cortex. To test this hypothesis, we inactivated the mPFC and recorded hippocampal place cell activity while animals were performing the navigation task. The results show that post-training inactivation of the prefrontal cortex does not affect behavioral performance, suggesting that this structure is no longer required when animals are overtrained. The goal-related activity of place cells was not affected at either single unit or local field potential level. Conversely, profound modifications of place cell firing variability (overdispersion) were observed after suppression of prefrontal input, suggesting a possible mechanism underlying behavioral flexibility.
Introduction
As the recipient of input from a number of structures involved in learning and memory, or encoding reward and reward cues (Stefani and Moghaddam, 2006), the medial prefrontal cortex (mPFC) is well placed to integrate affective information for the production of adaptive behavior (Morgane et al., 2005). In particular, strong excitatory connections exist between the hippocampus and the mPFC (Ferino et al., 1987; Jay et al., 1995). Thus, there is a significant direct input to the mPFC deriving from the hippocampus, and more specifically from its ventral and intermediate regions (Cenquizca and Swanson, 2007). In contrast, the mPFC projections back to the hippocampus are only indirect in particular through the entorhinal cortex and the thalamic nucleus reuniens (Vertes, 2004).
Previously, we found mPFC neurons to express sustained activity at the goal zone in a goal-oriented navigation task (Hok et al., 2005). Ventral and intermediate hippocampal lesions (Burton et al., 2009) considerably degraded this activity along with the behavioral performance, which was interpreted as evidence for spatial coding of behaviorally relevant places by mPFC.
In contrast to the hippocampal-prefrontal projections, much less is known about the significance of the indirect afferents from the mPFC to the hippocampus. In the rat, the hippocampus has been shown to support flexible navigation (Renaudineau et al., 2007). This capacity is thought to be mediated by a cognitive spatial mapping process supported by hippocampal “place cells”—cells that fire only when the animal enters cell-specific locales, or “place fields,” in the environment (O'Keefe and Nadel, 1978). In addition to their place fields, hippocampal place cells exhibit temporally modulated activity while the animal waits at the goal location in a navigation task (Hok et al., 2007a). This finding raises the possibility that hippocampal place cell goal-related firing might actually rely on the goal activity found for mPFC cells (Gaussier et al., 2002; Hok et al., 2005). In support of the view that mPFC contributes to this hippocampal function, lesion of mPFC or disconnection of mPFC from hippocampus impairs performance of spatial learning tasks (Wang and Cai, 2006). However, these impairments appear to be greater when task difficulty is enhanced (Granon and Poucet, 1995). Furthermore, mPFC lesions make hippocampal unit place fields less stable over time and more reactive to changes in the local environment (Kyd and Bilkey, 2003, 2005).
To help clarify the functional relationship between the mPFC and hippocampus, we examined the effects of mPFC inactivation on activity of rat hippocampal cells as the animal performed the navigation task already used to study both areas separately. Our main aim was to establish whether hippocampal place cell activity and goal-related firing require mPFC functional integrity.
Materials and Methods
Many of the procedures used here have been described previously. For further details, the reader is referred to Hok et al. (2005, 2007a, 2012). All procedures complied with both European (European Community Council Directive 86/609/EEC) and French (Council directive no. 87848, permission no. 13.76 to BP) institutional guidelines.
Subjects.
Male Long–Evans rats (Centre d'Élevage Janvier, St.-Berthevin, France), weighing 300–350 g, were housed in separate cages at 20°C under a 12 h light/dark cycle. After being handled for 2 weeks, animals were food deprived to 85% of their bodyweight and trained in the navigation task.
Apparatus and behavioral procedures.
The recording arena consisted of a dark gray cylindrical wall, 50 cm high and 76 cm in diameter, placed on a gray wooden floor. Attached to the wall was a white card, 50 cm high and covering 100° of arc, thus providing an orienting cue for spatial navigation. The arena was surrounded by ceiling-high curtains and was dimly lit from above.
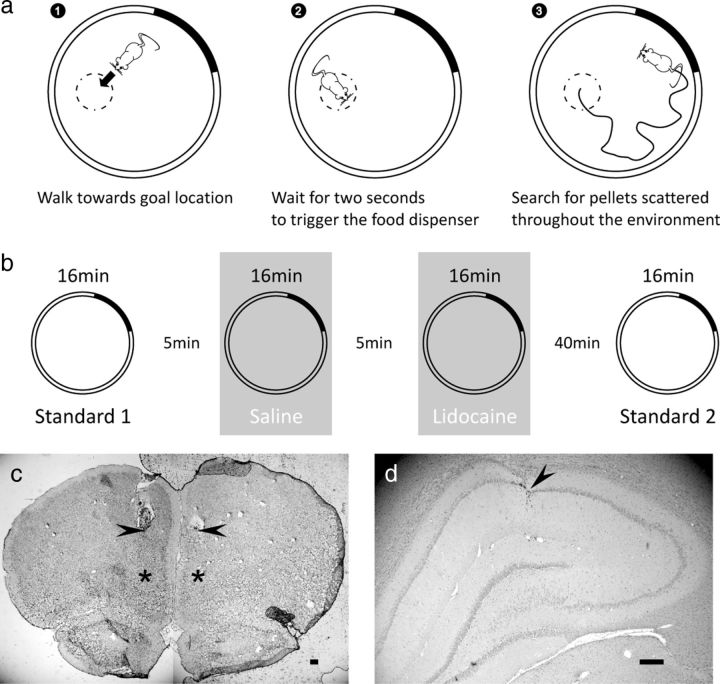
Rats were trained to perform a navigation task in which a 20 mg food pellet would be released from an overhead dispenser if the animal waited for 2 s in a 10 cm radius “goal ” zone (Fig. 1a). Because the pellet could go anywhere in the cylinder, the rat had to forage over the entire area to find it. After the rat spent at least a further 1 s outside the goal zone, the dispenser would be re-primed for a second release, thus forcing alternating episodes of foraging and goal-oriented navigation. Rats were scored on the rate per minute at which the food dispenser was triggered within each session. Training was similar to that described by Hok et al. (2005). Well-trained rats could reliably attain scores of >2 pellets per minute. Following behavioral training, rats were implanted with recording electrodes and cannulae. After 2 weeks of postsurgery recovery, training in the task was resumed until recordable hippocampal neurons were found.
Figure 1.
Details of the procedure. a, Schematic representation of the goal-oriented navigation task. The rat must enter an unmarked goal zone (1) and stay there for 2 s to release a pellet from an overhead feeder (2). To find and eat a food pellet, the rat has to forage around the cylinder (3). The solid line shows the foraging path taken after pellet release. b, Schematic showing the different phases of the procedure over the course of the experiment. c, Histological verification of cannulae placement showing a representative coronal section of the mPFC of the rat brain. The lowest visible tissue damage indicates the tip of the guide cannulae (black arrows); asterisks indicate the putative center of the perfusate (tip of the injector). Scale bar, 250 μm. d, Representative electrode location in dorsal hippocampal area CA1. Recording sites were identified by following the electrode tracks (arrowheads) through serial cresyl violet stained sections. Scale bar, 250 μm.
Electrode and cannula implantation.
Each rat was implanted with an array of 16 formvar-insulated 25 μm nichrome wires under general anesthetic (pentobarbital, 40 mg/kg, i.p.). Details of electrode design and general surgical procedures are provided by Hok et al. (2007a). The tip of the electrode bundle was placed over hippocampal CA1, 3.8 mm posterior and 2.5 mm lateral to bregma and 1.5 mm below skull surface (Paxinos and Watson, 2007). During the same surgery, rats were also implanted bilaterally with 23 gauge guide cannulae aimed at the prelimbic area of mPFC (anteroposterior +3.7 mm; lateral ± 0.5 mm from bregma). The tips of the guide cannulae were left 1.5 mm above the target. Cannulae were plugged with 30 gauge removable stylets preventing clogging of the guide cannulae. The cannulae and electrode headstage were anchored to the skull using miniature screws embedded in dental resin cement.
Following surgery, all animals received an intramuscular injection of antibiotic (terramycin, 60 mg/kg) and were allowed 1 week to recover. Recording followed a further 2 weeks of retraining. At the end of the experiment, rats were decapitated under deep anesthetic (pentobarbital) and their brains were removed. Brains were frozen at −80°C and then sectioned, mounted, and stained with cresyl violet to verify the positions of both electrodes and cannulae (Fig. 1c,d).
Recording techniques and inactivation protocol.
When recordable hippocampal neurons were found, rats were subjected to several 16 min recording sessions in a row as they performed the task. A first standard session (Standard 1) allowed the experimenter to adjust the recording parameters and to check that the rat was performing the behavioral task correctly. Following this, the rat received a bilateral injection of saline in mPFC and a second recording session was run to establish the basic characteristics of place cell firing. Then the mPFC was inactivated with lidocaine during a third recording session. Finally, a fourth recording session (Standard 2) was conducted to establish recovery from mPFC inactivation and signal stability between standard sessions. The animal was removed from the apparatus and the floor was cleaned and wiped with alcohol between sessions (Fig. 1b).
Before Sessions 2 and 3, animals received a bilateral injection of 0.5 μl saline or a dose of 10 μg of lidocaine dissolved in 0.5 μl saline per side in mPFC. Lidocaine was chosen over muscimol as it shows a faster wash-out time course (Martin, 1991; Tehovnik and Sommer, 1997; Malpeli, 1999) and has been used at similar dosages to successfully inactivate various brains regions in our past work (Poucet et al., 1991; Thinus-Blanc et al., 1991; Poucet and Buhot, 1994). After removal of the stylets, injection needles (30 gauge) were inserted into the guide cannulae such that the tip protruded about 1.5 mm below the opening. Agents were delivered in a volume of 0.5 μl at a constant rate of 0.25 μl/min through a Harvard pump. An additional 2 min were allowed for further diffusion of the perfusate.
Recording procedures.
Initial amplification of extracellular voltage was performed by a headstage connected to the electrode housing. A cable carried the signals to a commutator above the arena, allowing free turning movement of the animal. From there, the signals were relayed to a second amplifier in the adjacent room. Waveforms were amplified 10,000 times and bandpass was filtered between 0.3 and 10 kHz and saved to computer at 40 kHz. Two light emitting diodes (LEDs) situated on the top of the headstage were used for tracking the rat's head position and direction and were monitored at 50 Hz by an overhead camera and a digital spot follower. A red LED was positioned on the midline ∼1 cm above the head and somewhat forward of the rat's eyes, whereas a green LED was set ∼5 cm behind the red LED. The red LED coordinates were used to track the animal's head position while performing the task and therefore to trigger pellet delivery. Along with unit and position data, the system also stored the times of food dispenser activation and hippocampal CA1 pyramidal local field potentials (LFPs; filtered between 1 and 125 Hz) recorded from two electrodes and sampled at 256 Hz.
Data analyses.
Spikes from different cells recorded on the same electrode were isolated from noise and sorted off-line using DataWave Discovery and Offline Sorter (Plexon). Briefly, we used the following methods to assess unit isolation. First, autocorrelation histograms were calculated for each unit, and the unit was removed from further analysis if the histogram revealed the existence of correlations within the first 2 ms (refractory period), inconsistent with good unit isolation. Autocorrelograms were plotted between −10 and +10 ms, with a bin width of 100 μs. Second, for cells recorded simultaneously on the same electrode, we measured the degree to which the selected unit clusters are separated in the multidimensional cluster views as determined by a multivariate ANOVA (MANOVA) test (Offline Sorter). Sorted clusters were kept for analysis only if MANOVA tests yielded a p value <0.001. Last, we used two additional sort quality metrics provided by Offline Sorter software, to further assess the compactness of isolated clusters: (1) the J3 statistic, a nonparametric measure of the quality of sorting and (2) the Pseudo-F statistic, which is J3 that has been adjusted for the number of waveforms and the number of units. Firing rate maps were constructed at a pixel resolution of 2.5 cm according to the autoscaling scheme of Muller et al. (1987). The proficiency of spatial coding was measured using the information index (Skaggs et al., 1993) and spatial coherence (Muller and Kubie, 1987). The effects of mPFC inactivation on various statistics were assessed using paired t tests. To assess the stability of spatial firing following mPFC inactivation, a between-session spatial correlation was used as an index of spatial similarity. Other analyses of cell firing are presented directly along with the results.
To quantify theta activity, we analyzed mean LFP power in the theta (5–10 Hz) and delta (2–4 Hz) bands and calculated the theta ratio (θ/(θ + δ); Csicsvari et al., 1999; Harris et al., 2002) for the 2 s goal period. Theta peak frequency was taken as the frequency with peak power in the 5–10 Hz band.
Finally, place cell firing variability (overdispersion) was measured as described byFenton and Muller (1998). Briefly, passes through the firing field were defined as the time series of positions starting when the rat entered the field and ending when the rat left the field. To ensure the reliability of firing rate estimates, passes were studied only if they met the following two criteria: (1) each pass had to last at least 1 s and (2) the pass had to go through the field center. The observed number of spikes fired during a pass was compared against the number of spikes predicted from the session-averaged positional firing rate distribution. The predicted activity during a pass depends only on the specific pixels visited and the time spent in those pixels without regard to the sequence of positions. For a given pass, the expected number of spikes is given by the following:
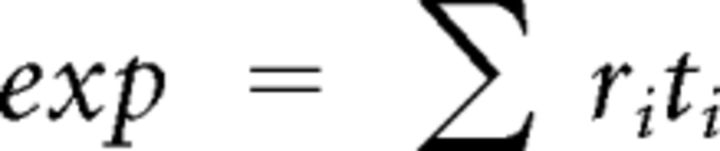 |
where ri is the time-averaged firing rate at position i in the pass through the field, and ti the time spent in location i during the pass. According to the Poisson assumption, the standard deviation of the expected numbers of spikes is equal to . Thus Z is a standard-normal deviate that measures the standardized deviation of the observed discharge (obs) from this expectation for each pass, and is calculated as follows:
 |
Overdispersion was then measured as the variance of the distribution of Z values computed for a set of passes. Similar to previous studies (Fenton et al., 2010; Hok et al., 2012), place cells were selected for analysis if their spatial firing patterns were location specific (coherence ≥ 0.25; spatial information ≥ 0.5 bits/action potential) and robust (average activity ≥ 0.25 Hz).
Results
Fifty-three well isolated (J3 statistic: 3.09 ± 0.46; Pseudo-F statistic: 7360.22 ± 1402.97) hippocampal place cells (average number of neurons per electrode: 1.47 ± 0.09) were recorded in six rats (range of number of neurons: 2—30) that performed the navigation task. In total, 19 blocks of four sessions were recorded, with an average of 25.8 ± 2.96 correct responses per session, comparable to what has been reported previously (Hok et al., 2005). On average, each animal received 3.17 ± 0.65 injections of lidocaine separated by a minimum of 5 d.
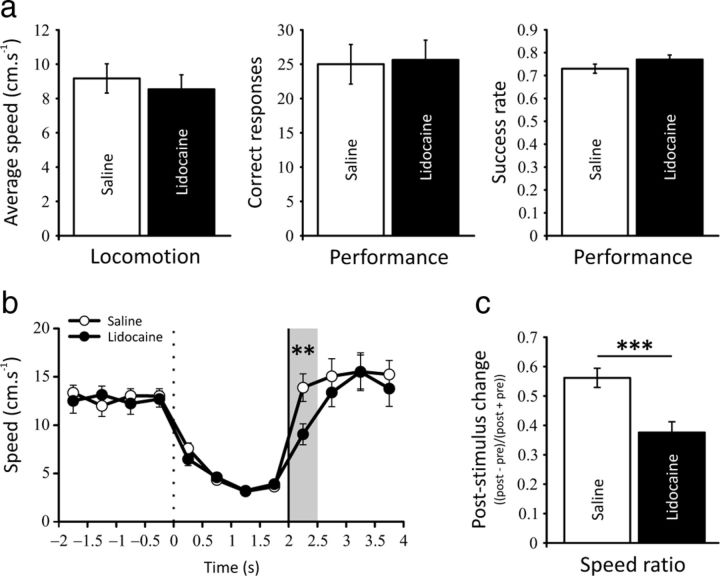
Behavioral analyses revealed no effect of prefrontal inactivation on locomotion (paired t test, t(18) = 0.958, NS) nor task performance (t(18) = 0.289, NS; Fig. 2a). The proportion of successful trials over the number of attempts (defined as goal visits lasting ≥ 1 s and < 2 s) was also unaffected (success rate: t(18) = 1.274, NS; Fig. 2a). Given the evidence suggesting a role of the mPFC in the preparatory processes during reaction time performance (Risterucci et al., 2003; Narayanan et al., 2006; Kim et al., 2009), we sought to analyze the instantaneous speed of the animals around the triggering stimulus (activation of the food dispenser) indicating the end of the delay period. The speed profiles revealed no differences between saline and lidocaine conditions before the onset of the stimulus, but a marked decrease in locomotion could be seen just after (t(18) = 3.680, p < 0.01; Fig. 2b). To further assess the behavioral differences in response to the auditory stimulus between saline and lidocaine conditions, we calculated, for each session, a poststimulus change ratio based on locomotion ((post − pre)/(post + pre)). This analysis confirmed the increased reaction time of prefrontal inactivated rats compared with saline conditions (t(18) = 4.395, p < 0.001; Fig. 2c). This behavioral effect supports the idea that the dosage of lidocaine used in the present study is effective.
Figure 2.
a, Main behavioral results during saline and lidocaine injections in the prefrontal cortex. No modification is seen in locomotion during the prefrontal inactivation. The behavioral performances (correct responses and success rate) are also not affected, suggesting that the prefrontal cortex is no longer required during the execution of this goal-oriented navigation task when animals are overtrained. b, Comparison of speed profiles in the saline and lidocaine conditions at 0.5 s resolution starting 2 s before the goal period and ending 2 s after the food dispenser has been activated (vertical black line). Goal zone entry is at t = 0 s (vertical dotted line). Several behavioral features are visible including a marked slowing of running speed just after goal zone entry, quite similar speeds in the first and second halves of the goal period, and very similar profiles for the two conditions before the auditory stimulus (food dispenser activation). Lidocaine injection induces a marked decrease in locomotion just after the food dispenser activation (shaded area; **p < 0.01). Error bars indicate SEM. c, To further assess the differences of the reaction time during saline and lidocaine sessions, we calculated, for each session, a poststimulus change ratio based on locomotion ((post − pre)/(post + pre)). This analysis confirmed the increased reaction time of prefrontal inactivated rats compared with saline conditions (***p < 0.001).
Kyd and Bilkey (2005) reported modifications of hippocampal place cell waveform characteristics after mPFC lesions (spike amplitude and spike width were greater in frontal lesioned animals). We therefore sought to analyze these parameters in saline and lidocaine conditions. None of the waveform's main features were changed after mPFC inactivation, suggesting that previous results obtained by Kyd and Bilkey (2005) were long-term adaptation to lesion effects.
We further analyzed the spatial characteristics of place cells in the different conditions. We found no difference between the two standard sessions (Standard 1 and Standard 2) regarding firing activity, spatial selectivity, information content, spatial coherence, and place field size, thus indicating a good stability of the signals through time and the absence of long-term effects of lidocaine injections (Table 1). When performing the gist comparison between the saline and lidocaine sessions (Table 2), we found an effect of mPFC inactivation resulting in an increase of place cell overall firing (t(52) = 2.340, p < 0.05) largely caused by an elevated peak firing rate within the field (t(52) = 3.047, p < 0.01). Along with this local change in place field firing, spatial coherence was significantly increased during lidocaine session (t(52) = 2.821, p < 0.05). Note, however, that prefrontal inactivation did not increase place cell excitability as measured by the propensity to burst (proportion of spikes occurring at intervals of 2–10 ms) for these cells (t(52) = 0.766, NS). Finally, prefrontal inactivation did not affect spatial stability of place cell firing as shown by the absence of a significant difference between the spatial similarity scores computed for the Standard 1/saline session pair and the saline/lidocaine session pair (0.47 ± 0.03 vs 0.49 ± 0.03, t(52) = 0.522, NS).
Table 1.
Main electrophysiological and behavioral characteristics in standard conditions
| Standard 1 | Standard 2 | |
|---|---|---|
| Waveform characteristics | ||
| Spike amplitude (μV) | 155.8 ± 9.7 | 152.3 ± 8.8 |
| Spike height (μV) | 240.3 ± 14.0 | 234.8 ± 12.8 |
| Spike width (μs) | 230.9 ± 4.4 | 230.6 ± 4.6 |
| Behavioral characteristics | ||
| Correct responses | 25.8 ± 3.0 | 24.3 ± 3.0 |
| Success rate | 0.70 ± 0.03 | 0.68 ± 0.04 |
| Average speed (cm . s−1) | 10.3 ± 0.9 | 8.8 ± 1.0 |
| Poststimulus change | 0.56 ± 0.04 | 0.51 ± 0.04 |
| Firing rate (Hz) | ||
| Overall | 1.48 ± 0.14 | 1.51 ± 0.18 |
| Infield (GR) | 2.92 ± 0.23 | 3.16 ± 0.32 |
| Infield (CR) | 9.83 ± 0.92 | 9.49 ± 0.96 |
| Outfield | 0.39 ± 0.05 | 0.37 ± 0.04 |
| Spikes with 2–10 ms ISI (%) | 14.5 ± 1.18 | 14.8 ± 1.10 |
| Spatial characteristics | ||
| Spatial selectivity | 0.95 ± 0.05 | 0.94 ± 0.05 |
| IC (bits/spike) | 0.72 ± 0.06 | 0.80 ± 0.07 |
| Spatial coherence | 0.46 ± 0.03 | 0.45 ± 0.03 |
| Field size (pixels) | 200.4 ± 23.7 | 171.8 ± 22.1 |
Spike amplitude: peak relative to resting potential. Spike height: peak relative to the most negative voltage reached during the afterhyperpolarization immediately after the spike. Spike width: width at half-maximal spike amplitude. CR, center rate; GR, grand rate; IC, information content; ISI, interspike interval. Averages are given ± SEM (n = 53).
Table 2.
Main electrophysiological and behavioral characteristics in experimental conditions
| Saline | Lidocaine | |
|---|---|---|
| Waveform characteristics | ||
| Spike amplitude (μV) | 154.2 ± 9.4 | 154.5 ± 8.9 |
| Spike height (μV) | 237.4 ± 13.0 | 239.1 ± 12.5 |
| Spike width (μs) | 231.3 ± 4.4 | 230.2 ± 4.5 |
| Behavioral characteristics | ||
| Correct responses | 25.0 ± 2.9 | 25.6 ± 2.9 |
| Success rate | 0.73 ± 0.02 | 0.77 ± 0.02 |
| Average speed (cm . s−1) | 9.2 ± 0.9 | 8.5 ± 0.9 |
| Poststimulus change | 0.56 ± 0.03 | 0.38 ± 0.04††† |
| Firing rate (Hz) | ||
| Overall | 1.21 ± 0.12 | 1.42 ± 0.16† |
| Infield (GR) | 2.37 ± 0.16 | 2.60 ± 0.22 |
| Infield (CR) | 7.07 ± 0.64 | 8.89 ± 0.93†† |
| Outfield | 0.43 ± 0.05 | 0.42 ± 0.05 |
| Spikes with 2–10 ms ISI (%) | 14.6 ± 1.05 | 15.2 ± 1.22 |
| Spatial characteristics | ||
| Spatial selectivity | 0.81 ± 0.04 | 0.83 ± 0.05 |
| IC (bits/spike) | 0.68 ± 0.05 | 0.70 ± 0.06 |
| Spatial coherence | 0.36 ± 0.03 | 0.41 ± 0.02†† |
| Field size (pixels) | 128.1 ± 14.8 | 146.2 ± 17.4 |
†p < 0.05;
††p < 0.01,
†††p < 0.001, compared to saline sessions (n = 53). CR, Center rate; GR, grand rate; IC, information content; ISI, interspike interval. Averages are given ± SEM.
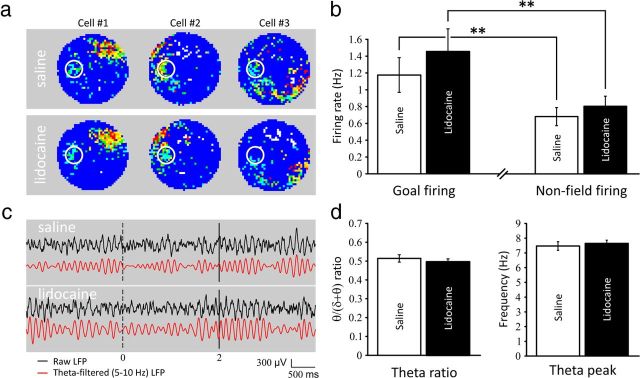
Out of 53 place cells, 22 cells had their main place field away from the goal location and a clear firing activity at the goal location. Twenty-four cells had their main field encroaching the goal zone (comprising two cells that had a field centered on the goal location, therefore precluding accurate measurement of goal-related firing). Seven cells did not show any goal-related activity. These proportions are in line with previous results obtained in the same conditions (Hok et al., 2007a). Figure 3a shows three representative place cells with goal-related activity during the control session (saline). This goal-related activity seemed preserved during prefrontal inactivation (lidocaine). A comparison of firing activity at the goal location during the 2 s delay period for the two sessions (Fig. 3b), revealed no significant effect of prefrontal inactivation (1.17 ± 0.21 vs 1.46 ± 0.27 Hz, t(21) = 1.619, NS). The firing activity for still episodes (speed < 3 cm.s−1) recorded outside the main place field and excluding the goal location was also unaffected by suppression of prefrontal input (0.68 ± 0.11 vs 0.80 ± 0.12 Hz, t(21) = 1.390, NS). These results suggest that prefrontal inactivation alters preferentially hippocampal place cell firing when activity is at its maximum (i.e., in the place field center). In addition to single unit activity, we looked for network-wide modifications that might have been induced by prefrontal inactivation during the delay period at the goal location. We therefore analyzed both power and peak frequency of LFPs in the theta band (5–10 Hz) for saline and lidocaine sessions. Consistent with previous results (Hok et al., 2007a) theta rhythm was still present during the 2 s delay period at the goal location (Fig. 3c). No difference was seen regarding the theta ratio (0.51 ± 0.02 vs 0.50 ± 0.02, t(16) = 1.151, NS) and theta peak (7.47 ± 0.29 vs 7.65 ± 0.22 Hz, t(16) = 0.793, NS; Fig. 3d). To further analyze the spatial properties of the goal-related firing, we sought to estimate the spatial distribution of this signal by looking at the number of active pixels over the total number of pixels comprised in a 15 cm radius circular zone centered on the goal zone. We then extracted the mean firing activity and calculated the spatial coherence and information content from this specific region, in both experimental conditions. We found no difference in terms of number of active pixels within the region of interest (45.05 ± 4.93 vs 45.85 ± 5.17 pixels, t(21) = 0.267, NS), or in terms of firing rate (1.00 ± 0.21 vs 1.22 ± 0.33 Hz, t(21) = 1.100, NS). Measures of spatial coherence (0.54 ± 0.04 vs 0.53 ± 0.04, t(21) = 0.148, NS) and information content (1.67 ± 0.24 vs 3.12 ± 0.99, t(21) = 1.574, NS) also failed to reveal any modification of goal-related firing induced by prefrontal inactivation. Taken together, these results show that mPFC inactivation does not alter the average goal-related activity of hippocampal place cells.
Figure 3.
Goal-related electrophysiological modulations induced by prefrontal inactivations. a, Color-coded rate maps of three representative hippocampal place cells during control (saline) and inactivation (lidocaine) sessions (16 min each). Rate map is coded on a color scale from blue (silent) to red (maximum rate) with cyan, green, yellow, and orange pixels as intermediate firing rates from low to high; the number of pixels of a given color is set to 0.8 times the number of pixels in the next lower color. Note the presence of secondary firing centered on the goal location in both conditions. b, Prefrontal inactivation spares goal-related firing. Goal-related firing is observed in both conditions as the difference between goal firing and firing outside the main place field (nonfield firing) remains significant (**p < 0.01). c, Dynamic analysis of changes in theta activity. In this representative example, the top insert shows the average unfiltered LFP (black line) and the theta-filtered trace (5–10 Hz; red line) during one saline session made up of 30 trials. The bottom insert shows the same traces during prefrontal inactivation (39 trials). The dashed and solid lines indicate the delay period at the goal location. d, No difference is seen while the prefrontal cortex is inactivated regarding the theta ratio and the theta peak analyzed during the 2 s delay period, indicating that suppression of prefrontal input does not affect synaptic integration at the CA1 level (at least in the theta band).
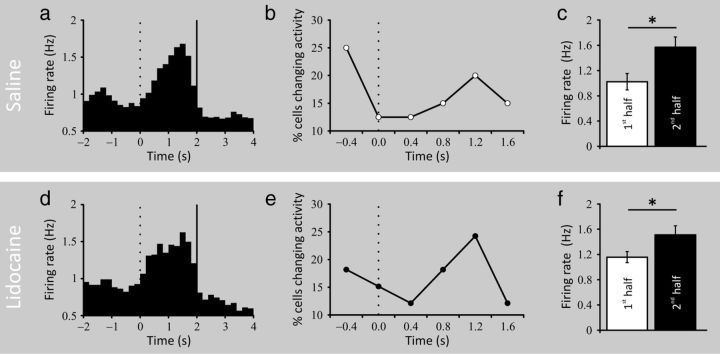
Although no differences were seen on average goal-firing activity between the two experimental conditions, a temporal analysis of the place cell firing activity centered on the delay period might have revealed subtle modulations under prefrontal inactivation. Therefore, we made a cumulative peri-event time histogram (PETH) for all trials from all cells after normalizing the activity of each cell separately, using feeder operation at the end of a successful 2 s period stay in the goal zone as the alignment event. In this PETH, t = 0 s is when the rat entered the goal zone and t = 2 s is when the dispenser was activated. Analysis was confined to 6 s intervals starting at t = −2 s and ending at 4 s. Consistent with our previous results (Hok et al., 2007a,b), the average pattern seen in Figure 4a is a gradual activity increase that peaks ∼1 s after goal zone entry. This pattern of rate change was confirmed by a nonparametric analysis that determined the first moment at which the smoothed PETH for each cell exceeded the half-height of the peak of the PETH (Gawne et al., 1996; Friedman and Priebe, 1998). As shown in Figure 4b, the modal time of rate increase was during the second half of the delay period. This result was further confirmed when comparing the firing rate during the first half of the delay period to the activity during the second half (1.02 ± 0.13 vs 1.57 ± 0.16 Hz, t(21) = 2.341, p < 0.05; Fig. 4c). Prefrontal inactivation did not change the overall firing pattern during the delay period (Fig. 4d) nor the precise timing at which the majority of cells increase their firing (Fig. 4e). Comparing the firing rate between the two halves of the delay period still revealed a significant difference (1.16 ± 0.09 vs 1.51 ± 0.15 Hz, t(21) = 2.390, p < 0.05; Fig. 4f).
Figure 4.
Characteristics of population activity during the goal period. a, Smoothed cumulative PETH for place cells showing goal-related firing in the saline condition (n = 22). The 2 s goal period (0–2 s) is bracketed by vertical lines (200 ms bins). Goal zone entry is at t = 0 s (vertical dotted line) and food dispenser activation is at t = 2 s (vertical black line). The activity of each cell was normalized before summation was done over the sample. Note that the mean peak activity is delayed ∼1 s after goal arrival. b, Percentage of cells whose activity showed the greatest increase at different times during the goal period of the place task. Entry into the goal zone is at t = 0 s. The delay suggested in the average of the cumulative PETH is seen here as a rise near the middle of the goal period. c, Bar graph showing significant differences in firing rate between the first half and the second half of the delay period (*p < 0.05). d, Smoothed PETH (same convention as in a) for place cells showing goal-related firing in lidocaine condition. Although broader in shape, the goal firing activity is still present when the prefrontal cortex is inactivated and its dynamic is unaltered as can be seen in e (same convention as in b). f, Significant differences in firing rate between the two halves of the delay period are still present under prefrontal inactivation (*p < 0.05).
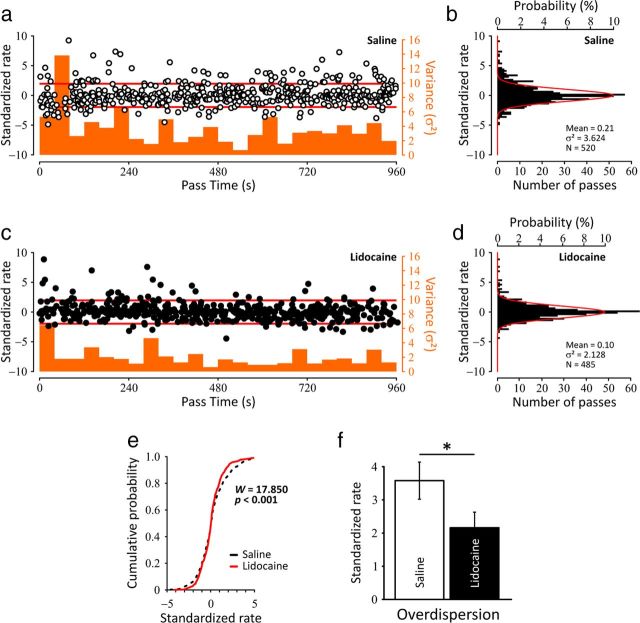
To further characterize the modifications observed in the center of the place fields during mPFC inactivation we sought to quantify the degree of firing variability within the place fields (i.e., overdispersion). This measure is thought to reflect network-wide fluctuations and has been correlated to various cognitive components in spatial tasks (Jackson and Redish, 2007; Fenton et al., 2010; Hok et al., 2012). Figure 5a shows the plot of the time series of standardized firing rates for the observed firing on all passes through the center of the field of 36 place cells that qualified for this analysis (see Materials and Methods), recorded for 16 min. Consistent to what is expected from normal place cells (Fenton and Muller, 1998), there was no trend for the observed Z-scores to increase or decrease with time in the saline condition (Pearson's correlation coefficient, r2 = 0.007, NS). In a consistent manner, the instantaneous measure of variance (Fig. 5a, orange histogram) indicated no trend to increase or decrease over time. The distribution of standardized firing rates obtained from all the place cells recorded during saline is indicated in Figure 5b (σ2 = 3.624, μ = 0.21, number of passes = 520). Similar to the saline session, negative and positive Z-scores follow each other and do so over the whole recording time (r2 = 0.002, NS), indicating a good stability of lidocaine effect over time (Fig. 5c). Figure 5d shows the distribution of the standardized firing rates for the lidocaine session (σ2 = 2.128, μ = 0.10, Npasses = 485), which was significantly less dispersed than the saline distribution (Levene's test for equality of variances, W = 17.850, p < 0.001) as highlighted by the clear dissociation of the two cumulative probability functions presented in Figure 5e. This result was further confirmed by taking into account each cell of the two experimental conditions as an individual estimate of the variability (t(35) = 2.048, p < 0.05; Fig. 5f), indicating therefore a decrease of overdispersion induced by prefrontal inactivation.
Figure 5.
Dynamics of the spike firing variability in both conditions. a, Plot of the time series of standardized firing rates (left ordinate) for the observed firing on all passes through the center of the field of 36 place cells recorded for 960 s (16 min). The red lines indicate the 0.05 probability tails of the normal distribution (Z = 1.96 and Z = −1.96). Consistent to what is expected from normal place cells (Fenton and Muller, 1998), there is no trend for the observed Z-scores to increase or decrease with time in the saline condition (Pearson's correlation coefficient, r2 = 0.007, NS). The histogram in orange shows the instantaneous measure of variance (bin width = 40 s; right ordinate) and indicates no trend to increase or decrease over time. b, Histogram showing the distribution of standardized firing rates for saline (N = 520 passes through firing fields; lower ordinate). The expected distribution is the unit normal distribution drawn in red (upper ordinate). c, Similar to the saline session, negative and positive Z-scores follow each other and do so over the whole recording time (r2 = 0.002, NS), indicating a good stability of lidocaine effect over time. d, Histogram showing the distribution of standardized firing rates for lidocaine session (Npasses = 485). Consistent with previous findings (Jackson and Redish, 2007; Fenton et al., 2010; Hok et al., 2012) the variance is well above the unitary value expected from a normal distribution for both conditions, but the distribution from lidocaine sessions is significantly narrower than the one based on the saline sessions, as can be seen with the clear dissociation of the two cumulative probability functions (Levene's test for equality of variances, W = 17.850, p < 0.001) in e. f, Overdispersion was also characterized by analyzing the firing variance in the set of passes through individual cells firing field. This analysis confirmed overdispersion was reduced during inactivation of the prefrontal cortex. (*p < 0.05).
Discussion
Our results shed new light on the role exerted by the prefrontal cortex in controlling behavior and place cell activity while animals perform reliably in a navigation task. First, prefrontal inactivation does not alter the behavioral performance (number of correct responses and number of attempts) of well trained animals (Fig. 2a) even though a lidocaine effect could be observed on reaction time. Based on previous work, a severe disruption of task performance following mPFC inactivation was not expected, as this would have precluded any analysis of goal-related firing. Second, the goal-related activity of hippocampal place cells previously observed in this task (Hok et al., 2007a) is unaffected by suppression of prefrontal input at both single unit and LFP levels (Fig. 3b,d). Third, inactivating the prefrontal cortex increases firing activity in the place field center while sparing place cell excitability as measured by its propensity to burst (Table 2). Finally, suppression of the prefrontal control over hippocampal formation does not increase place cell firing variability (overdispersion) but produces the exact reverse effect (Fig. 5f). Together, these results show that PFC inactivation focally modulates hippocampal place cell spatial activity by selectively altering their place field center (i.e., the smallest active portion used to define the existence of a place field). Furthermore, these data provide evidence for a dissociation between overdispersion modulation and behavioral performance in a task that has been previously deemed to affect place cell firing variability given its cognitive demands (Fenton et al., 2010).
Behavioral control by prefrontal cortex in the navigation task
While recording prefrontal cortex cells during task performance, we found clustering of place fields in the immediate vicinity of a fixed goal region (Hok et al., 2005) suggesting that they were tied to the motivational salience of this specific place. This type of coding is consistent with models in which spatial planning relies on the activity of a prefrontal network that associates places with their motivational salience (Banquet et al., 2002), a key component necessary for computing optimal paths in the environment (Poucet et al., 2004). Despite its role in planning (Granon and Poucet, 1995) and its strong (albeit indirect) connections to the hippocampus (Vertes, 2004), prefrontal inactivation did not affect behavioral performances in the navigation task (Fig. 2a). The present results suggest that the prefrontal cortex is no longer required when animals are well trained (>2 months of training) and that the spatio-selective activity found previously (Hok et al., 2005) may be instrumental in the acquisition of the task during earlier phases.
Hippocampal goal representation is not affected by suppression of the prefrontal input
Hippocampal goal signals have been suggested to complement coarse encoding of goal location provided by mPFC cells, and thus to participate in path planning (Hok et al., 2005, 2007a). Modulation of goal signals by the prefrontal cortex could be achieved through indirect connections via cortical (e.g., perirhinal and entorhinal cortices) or subcortical structures (Vertes, 2004). We found no evidence of alteration of goal-related firing during prefrontal inactivation, whether considering average single unit activity, LFP (theta band), or a more subtle temporal analysis of place cells activity (Figs. 3, 4). While ruling out the possibility of a prefrontal origin to this goal-related signal, these results cannot permit us to dissociate whether such signal is generated outside the hippocampus or internally.
Prefrontal inactivation focally modulates hippocampal place cell firing activity
Few studies explored the effects of prefrontal lesions on electrophysiological properties of hippocampal formation neurons. Zironi et al. (2001) reported, for example, that perirhinal cortex units in prefrontal-lesioned animals had more discrete location-related properties compared with controls. It has been proposed that the prefrontal cortex modulates neural activity in posterior cortex via inhibitory mechanisms. As a result, damage to the former area may produce disinhibition in posterior regions and increase sensitivity to extraneous information. Results from Kyd and Bilkey (2003, 2005) are consistent with this hypothesis. Place cells were recorded while rats foraged in a circular arena with access to both local and distal cues. Although the position of place fields in lesion-group cells was not excessively tied to local cues, a greater proportion of the fields lost their spatial selectivity following a rotation of these cues. In the current study, prefrontal inactivation did not affect spatial selectivity per se, but had some effects on the internal organization of the place field (i.e., spatial coherence). The major effect of prefrontal inactivation can be seen on peak firing activity within the place field. As pointed out by Kyd and Bilkey (2005) possible mechanisms of prefrontal modulation on hippocampal activity include the prefrontal pyramidal cells that regulate activity of dopaminergic neurons in the ventral tegmental area (VTA; Sesack et al., 1989; Taber et al., 1995). Prefrontal inactivation would therefore reduce the inhibitory influence of dopaminergic projections from VTA to CA1 inhibitory input. Alternatively, prefrontal inactivation effects on inhibitory activity could be mediated via the perirhinal—entorhinal–hippocampal pathway as there is some evidence to suggest that a portion of the perirhinal and entorhinal input to CA1 projects directly onto inhibitory interneurons (Empson and Heinemann, 1995). Consistent with this hypothesis, our results suggest that control exerted by the prefrontal cortex over place cell activity is limited to a fine time window resulting in subtle changes of the transient structural dynamic of firing.
Place cell firing variability is decreased when the prefrontal cortex is inactivated
The levels of place cell spiking variability (overdispersion) are thought to be modulated by cognitive demands (Jackson and Redish, 2007; Fenton et al., 2010; Hok et al., 2012). Previous studies showed that the collective activity of large place cell ensembles is separable into two alternating place codes and that individual place cells have distinct spatial firing patterns and rates in the two place codes (Jackson and Redish, 2007; Fenton et al., 2010). The switching between place codes would correspond to transitions between distinct attentional states (Fenton et al., 2010). Furthermore, the reconstruction accuracy of the rat's position was improved when taking into account the ensemble state. Prefrontal inactivation might therefore alter the switching process between these two states, with place cells having a tendency to dwell longer using the preferred place code to solve the task. This bias toward a specific state could then generate higher firing rates without affecting place cell excitability and increase the internal organization of the place field, reflecting in this way a better accuracy of the place cell signal. Massive parallel recording techniques thus appear instrumental in helping to decode the multiple states that place cells might express while animals are solving the navigation task and in testing effects of prefrontal inactivation on those states. Further experiments are therefore needed to fully investigate the behavioral performance of the animals when some degree of behavioral flexibility is required (like changing the goal location for instance) that could help us to highlight a prefrontal involvement in monitoring task contingencies.
Footnotes
Support for this work was provided by the Centre National de la Recherche Scientifique, Ministère de I'Éducation Nationale et de la Recherche et Technologie, Agence Nationale de la Recherche (ANR-10-BLAN-0217), and the Fondation pour la Recherche Médicale (Grant FDT-2006-1208711).
The authors declare no competing financial interests.
References
- Banquet JP, Gaussier P, Quoy M, Revel A, Burnod Y. Cortico-hippocampal maps and navigation strategies in robots and rodents. In: Hallam B, Floreano D, Hallam J, Hayes G, Meyer JA, editors. From animals to animats 7. Boston: MIT; 2002. pp. 141–150. [Google Scholar]
- Burton BG, Hok V, Save E, Poucet B. Lesion of the ventral and intermediate hippocampus abolishes anticipatory activity in the medial prefrontal cortex of the rat. Behav Brain Res. 2009;199:222–234. doi: 10.1016/j.bbr.2008.11.045. [DOI] [PubMed] [Google Scholar]
- Cenquizca LA, Swanson LW. Spatial organization of direct hippocampal field CA1 axonal projections to the rest of the cerebral cortex. Brain Res Rev. 2007;56:1–26. doi: 10.1016/j.brainresrev.2007.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Csicsvari J, Hirase H, Czurkó A, Mamiya A, Buzsáki G. Oscillatory coupling of hippocampal pyramidal cells and interneurons in the behaving Rat. J Neurosci. 1999;19:274–287. doi: 10.1523/JNEUROSCI.19-01-00274.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Empson RM, Heinemann U. Perforant path connections to area CA1 are predominantly inhibitory in the rat hippocampal-entorhinal cortex combined slice preparation. Hippocampus. 1995;5:104–107. doi: 10.1002/hipo.450050203. [DOI] [PubMed] [Google Scholar]
- Fenton AA, Muller RU. Place cell discharge is extremely variable during individual passes of the rat through the firing field. Proc Natl Acad Sci U S A. 1998;95:3182–3187. doi: 10.1073/pnas.95.6.3182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fenton AA, Lytton WW, Barry JM, Lenck-Santini PP, Zinyuk LE, Kubík S, Bures J, Poucet B, Muller RU, Olypher AV. Attention-like modulation of hippocampus place cell discharge. J Neurosci. 2010;30:4613–4625. doi: 10.1523/JNEUROSCI.5576-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferino F, Thierry AM, Glowinski J. Anatomical and electrophysiological evidence for a direct projection from Ammon's horn to the medial prefrontal cortex in the rat. Exp Brain Res. 1987;65:421–426. doi: 10.1007/BF00236315. [DOI] [PubMed] [Google Scholar]
- Friedman HS, Priebe CE. Estimating stimulus response latency. J Neurosci Methods. 1998;83:185–194. doi: 10.1016/s0165-0270(98)00075-2. [DOI] [PubMed] [Google Scholar]
- Gaussier P, Revel A, Banquet JP, Babeau V. From view cells and place cells to cognitive map learning: processing stages of the hippocampal system. Biol Cybern. 2002;86:15–28. doi: 10.1007/s004220100269. [DOI] [PubMed] [Google Scholar]
- Gawne TJ, Kjaer TW, Richmond BJ. Latency: another potential code for feature binding in striate cortex. J Neurophysiol. 1996;76:1356–1360. doi: 10.1152/jn.1996.76.2.1356. [DOI] [PubMed] [Google Scholar]
- Granon S, Poucet B. Medial prefrontal lesions in the rat and spatial navigation: evidence for impaired planning. Behav Neurosci. 1995;109:474–484. doi: 10.1037//0735-7044.109.3.474. [DOI] [PubMed] [Google Scholar]
- Harris KD, Henze DA, Hirase H, Leinekugel X, Dragoi G, Czurkó A, Buzsáki G. Spike train dynamics predicts theta-related phase precession in hippocampal pyramidal cells. Nature. 2002;417:738–741. doi: 10.1038/nature00808. [DOI] [PubMed] [Google Scholar]
- Hok V, Save E, Lenck-Santini PP, Poucet B. Coding for spatial goals in the prelimbic/infralimbic area of the rat frontal cortex. Proc Natl Acad Sci U S A. 2005;102:4602–4607. doi: 10.1073/pnas.0407332102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hok V, Lenck-Santini PP, Roux S, Save E, Muller RU, Poucet B. Goal-related activity in hippocampal place cells. J Neurosci. 2007a;27:472–482. doi: 10.1523/JNEUROSCI.2864-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hok V, Lenck-Santini PP, Save E, Gaussier P, Banquet JP, Poucet B. A test of the time estimation hypothesis of place cell goal-related activity. J Integr Neurosci. 2007b;6:367–378. doi: 10.1142/s0219635207001611. [DOI] [PubMed] [Google Scholar]
- Hok V, Chah E, Reilly RB, O'Mara SM. Hippocampal dynamics predict interindividual cognitive differences in rats. J Neurosci. 2012;32:3540–3551. doi: 10.1523/JNEUROSCI.6449-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson J, Redish AD. Network dynamics of hippocampal cell-assemblies resemble multiple spatial maps within single tasks. Hippocampus. 2007;17:1209–1229. doi: 10.1002/hipo.20359. [DOI] [PubMed] [Google Scholar]
- Jay TM, Burette F, Laroche S. NMDA receptor-dependent long-term potentiation in the hippocampal afferent fibre system to the prefrontal cortex in the rat. Eur J Neurosci. 1995;7:247–250. doi: 10.1111/j.1460-9568.1995.tb01060.x. [DOI] [PubMed] [Google Scholar]
- Kim J, Jung AH, Byun J, Jo S, Jung MW. Inactivation of medial prefrontal cortex impairs time interval discrimination in rats. Front Behav Neurosci. 2009;3:38. doi: 10.3389/neuro.08.038.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kyd RJ, Bilkey DK. Prefrontal cortex lesions modify the spatial properties of hippocampal place cells. Cereb Cortex. 2003;13:444–451. doi: 10.1093/cercor/13.5.444. [DOI] [PubMed] [Google Scholar]
- Kyd RJ, Bilkey DK. Hippocampal place cells show increased sensitivity to changes in the local environment following prefrontal cortex lesions. Cereb Cortex. 2005;15:720–731. doi: 10.1093/cercor/bhh173. [DOI] [PubMed] [Google Scholar]
- Malpeli JG. Reversible inactivation of subcortical sites by drug injection. J Neurosci Methods. 1999;86:119–128. doi: 10.1016/s0165-0270(98)00161-7. [DOI] [PubMed] [Google Scholar]
- Martin JH. Autoradiographic estimation of the extent of reversible inactivation produced by microinjection of lidocaine and muscimol in the rat. Neurosci Lett. 1991;127:160–164. doi: 10.1016/0304-3940(91)90784-q. [DOI] [PubMed] [Google Scholar]
- Morgane PJ, Galler JR, Mokler DJ. A review of systems and networks of the limbic forebrain/limbic midbrain. Prog Neurobiol. 2005;75:143–160. doi: 10.1016/j.pneurobio.2005.01.001. [DOI] [PubMed] [Google Scholar]
- Muller RU, Kubie JL. The effects of changes in the environment on the spatial firing of hippocampal complex-spike cells. J Neurosci. 1987;7:1951–1968. doi: 10.1523/JNEUROSCI.07-07-01951.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narayanan NS, Horst NK, Laubach M. Reversible inactivations of rat medial prefrontal cortex impair the ability to wait for a stimulus. Neuroscience. 2006;139:865–876. doi: 10.1016/j.neuroscience.2005.11.072. [DOI] [PubMed] [Google Scholar]
- O'Keefe J, Nadel L. Hippocampus as a cognitive map. Oxford: Clarendon; 1978. [Google Scholar]
- Paxinos G, Watson C. The rat brain in stereotaxic coordinates. Ed 6. San Diego CA: Elsevier Academic; 2007. [Google Scholar]
- Poucet B, Buhot MC. Effects of medial septal or unilateral hippocampal inactivations on reference and working spatial memory in rats. Hippocampus. 1994;4:315–321. doi: 10.1002/hipo.450040315. [DOI] [PubMed] [Google Scholar]
- Poucet B, Herrmann T, Buhot MC. Effects of short-lasting inactivations of the ventral hippocampus and medial septum on long-term and short-term acquisition of spatial information in rats. Behav Brain Res. 1991;44:53–65. doi: 10.1016/s0166-4328(05)80239-6. [DOI] [PubMed] [Google Scholar]
- Poucet B, Lenck-Santini PP, Hok V, Save E, Banquet JP, Gaussier P, Muller RU. Spatial navigation and hippocampal place cell firing: the problem of goal encoding. Rev Neurosci. 2004;15:89–107. doi: 10.1515/revneuro.2004.15.2.89. [DOI] [PubMed] [Google Scholar]
- Renaudineau S, Poucet B, Save E. Flexible use of proximal objects and distal cues by hippocampal place cells. Hippocampus. 2007;17:381–395. doi: 10.1002/hipo.20277. [DOI] [PubMed] [Google Scholar]
- Risterucci C, Terramorsi D, Nieoullon A, Amalric M. Excitotoxic lesions of the prelimbic-infralimbic areas of the rodent prefrontal cortex disrupt motor preparatory processes. Eur J Neurosci. 2003;17:1498–1508. doi: 10.1046/j.1460-9568.2003.02541.x. [DOI] [PubMed] [Google Scholar]
- Sesack SR, Deutch AY, Roth RH, Bunney BS. Topographical organization of the efferent projections of the medial prefrontal cortex in the rat: an anterograde tract-tracing study with Phaseolus vulgaris leucoagglutinin. J Comp Neurol. 1989;290:213–242. doi: 10.1002/cne.902900205. [DOI] [PubMed] [Google Scholar]
- Skaggs WE, McNaughton BL, Gothard KM. An information theoretic approach to deciphering the hippocampal code. In: Hanson SJ, Cowan JD, Giles CL, editors. Advances in neural information processing systems. San Mateo, CA: Morgan Kaufmann; 1993. pp. 1030–1037. [Google Scholar]
- Stefani MR, Moghaddam B. Rule learning and reward contingency are associated with dissociable patterns of dopamine activation in the rat prefrontal cortex, nucleus accumbens, and dorsal striatum. J Neurosci. 2006;26:8810–8818. doi: 10.1523/JNEUROSCI.1656-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taber MT, Das S, Fibiger HC. Cortical regulation of subcortical dopamine release: mediation via the ventral tegmental area. J Neurochem. 1995;65:1407–1410. doi: 10.1046/j.1471-4159.1995.65031407.x. [DOI] [PubMed] [Google Scholar]
- Tehovnik EJ, Sommer MA. Effective spread and timecourse of neural inactivation caused by lidocaine injection in monkey cerebral cortex. J Neurosci Methods. 1997;74:17–26. doi: 10.1016/s0165-0270(97)02229-2. [DOI] [PubMed] [Google Scholar]
- Thinus-Blanc C, Save E, Poucet B, Buhot MC. The effects of reversible inactivations of the hippocampus on exploratory activity and spatial memory. Hippocampus. 1991;1:365–371. doi: 10.1002/hipo.450010404. [DOI] [PubMed] [Google Scholar]
- Vertes RP. Differential projections of the infralimbic and prelimbic cortex in the rat. Synapse. 2004;51:32–58. doi: 10.1002/syn.10279. [DOI] [PubMed] [Google Scholar]
- Wang G-W, Cai JX. Disconnection of the hippocampal-prefrontal cortical circuits impairs spatial working memory performance in rats. Behav Brain Res. 2006;175:329–336. doi: 10.1016/j.bbr.2006.09.002. [DOI] [PubMed] [Google Scholar]
- Zironi I, Iacovelli P, Aicardi G, Liu P, Bilkey DK. Prefrontal cortex lesions augment the location-related firing properties of area TE/perirhinal cortex neurons in a working memory task. Cereb Cortex. 2001;11:1093–1100. doi: 10.1093/cercor/11.11.1093. [DOI] [PubMed] [Google Scholar]