Abstract
Background: There is increasing interest in the extraskeletal effects of vitamin D, particularly in the obese state with regard to the development of insulin resistance and diabetes.
Objective: The objective of the study was to determine the effect of 2 doses of cholecalciferol (vitamin D3) supplementation on insulin action (Si) and pancreatic β-cell function in obese adolescents.
Methods: We performed a 12-wk double-blind, randomized comparison of the effect of vitamin D3 supplementation on Si and β-cell function in obese Caucasian adolescents (body mass index > 95th percentile). The subjects were randomly assigned to receive either 400 IU/d (n = 25) or 2000 IU/d (n = 26) of vitamin D3. Each subject underwent a 7-sample 75 g oral glucose tolerance test, with glucose, insulin, and C-peptide measurements, to calculate Si and β-cell function as assessed by the disposition index (DI), with use of the oral minimal model before and after supplementation. A total of 51 subjects aged 15.0 ± 1.9 y were enrolled. Included for analysis at follow-up were a total of 46 subjects (20 male and 26 female adolescents), 23 in each group.
Results: Initial serum 25-hydroxyvitamin D [25(OH)D] was 24.0 ± 8.1 μg/L. There was no correlation between 25(OH)D concentrations and Si or DI. There was a modest but significant increase in 25(OH)D concentration in the 2000 IU/d group (3.1 ± 6.5 μg/L, P = 0.04) but not in the 400 IU/d group (P = 0.39). There was no change in Si or DI following vitamin D3 supplementation in either of the treatment groups (all P > 0.10).
Conclusions: The current study shows no effect from vitamin D3 supplementation, irrespective of its dose, on β-cell function or insulin action in obese nondiabetic adolescents with relatively good vitamin D status. Whether obese adolescents with vitamin D deficiency and impaired glucose metabolism would respond differently to vitamin D3 supplementation remains unclear and warrants further studies. This trial was registered at clinicaltrials.gov as NCT00858247.
Keywords: vitamin D supplementation, insulin action, insulin secretion, obese adolescents, disposition index
Introduction
Vitamin D deficiency is highly prevalent in children and adolescents worldwide (1–4). Growing evidence suggests that obesity is a major risk factor for vitamin D insufficiency and deficiency in children and adolescents (5–10). Childhood obesity predisposes people to insulin resistance, subsequent β-cell failure, and ultimate development of type 2 diabetes (11–14). There is increasing interest in the extraskeletal effects of vitamin D, particularly in the obese state with regards to the development of insulin resistance and diabetes. Circulating concentration of 25-hydroxyvitamin D [25(OH)D]11 is frequently considered to be an indicator of vitamin D nutritional status, and several cross-sectional studies in children have reported inverse correlations between 25(OH)D concentrations and plasma glucose (15, 16) and surrogate markers of insulin resistance, such as the HOMA-IR (17, 18). Interestingly, studies in which hyperinsulinemic-euglycemic and hyperinsulinemic-hyperglycemic clamp approaches were used could not find such relations among 25(OH)D concentrations, glucose homeostasis, or in vivo insulin secretion and action in obese, or across the glucose tolerance groups, in children or youth (19, 20). A recent systematic review underscores the lack of clear evidence of the beneficial effects of vitamin D supplementation in improving glycemia or insulin resistance in patients with normal or impaired fasting glucose or diabetes (21).
Theoretic and mechanistic evidence implies that vitamin D status may affect both insulin secretion and sensitivity (22). Such a relation between vitamin D status and glucose homeostasis may involve the expression of vitamin D receptors in pancreatic β-cells, skeletal muscle, and adipose tissue (23, 24). These nonskeletal effects of vitamin D may result from its autocrine/paracrine actions in these insulin target tissues. Further, low 25(OH)D concentrations are also reported to be associated with low HDL cholesterol concentrations in children (16).
It also remains less clear whether vitamin D supplementation in obese, nondiabetic children can influence insulin action (Si) or insulin secretion rates (ISRs). Therefore, given the inconsistencies in the data on the relation between vitamin D status and insulin sensitivity and the paucity of studies on the potential effects of supplementation of vitamin D on Si and insulin secretion, there is a need for further studies of obese adolescents. The objectives of the present prospective, randomized, controlled study in obese adolescents were to investigate 1) the relation between vitamin D status and measures of insulin secretion and action by use of the oral glucose tolerance test (OGTT) and oral minimal model approach, and 2) the effect of 2 different doses of vitamin D3 supplementation (a low dose of 400 IU/d or a higher dose of 2000 IU/d) for 12 wk on concentrations of 25(OH)D and its effect on insulin sensitivity and pancreatic β-cell function, derived from an OGTT minimal model approach in obese children.
Methods
Participants.
Obese but otherwise healthy adolescents aged 12–18 y were recruited from 1 June 2009 through 31 May 2012. Subjects were considered to be obese if their BMI was ≥ the 95th percentile for age and gender, by use of age- and sex-specific BMI percentiles derived from the 2000 CDC growth charts (25). Exclusion criteria included a serum 25(OH)D concentration > 100 μg/L; serum calcium concentration > 10.8 mg/dL; hepatic or renal disorders; type 1 or type 2 diabetes mellitus; and malabsorption syndromes, such as celiac disease, as well as use of glucocorticoids or antiseizure medications in the preceding 6 mo and those subjects currently using multivitamin supplementation, insulin, metformin, or oral hypoglycemic medications. In addition, subjects with poor intravenous access were not enrolled. Informed consent and assents were obtained from participants and parents. The study protocol was approved by the Institutional Review Board at Mayo Clinic, Rochester, MN, and is registered at clinicaltrials.gov as NCT00858247.
Study design.
This 12-wk, randomized, prospective, double-blind study consisted of 2 data collection points: at baseline and at 12 wk. A balanced block randomization scheme, without any stratification factors, was prepared by the study statistician and used for random assignment. Participants, as well as investigators and associated research and laboratory personnel, remained blinded to patient allocation until after study completion.
Intervention.
Subjects were randomly assigned to receive either 400 IU/d or 2000 IU/d of cholecalciferol (vitamin D3) (BioTech Pharmacal) for a period of 12 wk. Compliance was assessed at the 12-wk visit by counting the number of pills remaining in the container.
Measurements.
Weight, height, and blood pressure measurements were taken with the use of standard protocols and equipment. After ≥12 h of fasting, blood samples were drawn both at study entry and after the 12-wk supplementation. All subjects also underwent a 3-h OGTT at baseline and after the 12-wk supplementation. After subjects received a 75 g oral glucose load, blood samples were obtained at 0, 10, 20, 30, 60, 90, 120, and 180 min for measurements of plasma glucose, C-peptide, and plasma insulin concentrations.
Plasma and serum samples were placed on ice, centrifuged at 1750 × g for 15 min at 4°C, separated, and stored at −80°C until assayed. Plasma glucose concentrations were measured with the use of a glucose oxidase method (Yellow Springs Instruments); plasma insulin with the use of a chemiluminescence assay (Access Assay; Beckman); plasma C-peptide by radioimmunoassay (Linco Research) and serum 25(OH)D by LC-tandem MS (LC-MS/MS) (26). An internally developed and validated method in a single laboratory (Mayo Medical Laboratories) was used in order to ensure uniform measurements (26). The total 25(OH)D concentration of each sample was calculated by summing the measured values of 25-hydroxyergocalciferol and 25-hydroxycholecalciferol [25(OH)D3]. Serum total cholesterol, HDL cholesterol, and TG concentrations were measured by an enzymatic colorimetric assay (Roche Diagnostics). Non-HDL cholesterol was calculated as total cholesterol − HDL cholesterol. LDL cholesterol was calculated through use of the following equation: LDL = Total cholesterol − HDL cholesterol − (TGs/5).
The HOMA-IR index was calculated as HOMA-IR = fasting serum glucose in milligrams per deciliter × fasting insulin in micro units per milliliter/405. The quantitative insulin sensitivity check index was derived by use of the inverse of the sum of the logarithms of the fasting insulin and fasting glucose: 1/log (fasting insulin in micro units per milliliter) + log (fasting glucose in milligrams per deciliter).
Si was calculated from plasma glucose and insulin concentrations measured during the 3-h OGTT with the use of the oral glucose minimal model (27–31). Si measures the overall effect of insulin on stimulating glucose disposal and inhibiting glucose production. This index has been validated against the euglycemic clamp, showing a correlation of 0.81 (P = 0.001) (28).
The β-cell responsivity indices were calculated from plasma glucose and C-peptide concentrations measured during the OGTT with the use of the oral C-peptide minimal model incorporating age-associated changes in C-peptide kinetics as measured by Van Cauter et al. (20). The calculation of insulin secretion rate is essentially the calculation of C-peptide secretion rate as previously described through use of standardized clearance rates for C-peptide (20). The model assumes that β-cell responsiveness (ϕtotal) is composed of 2 components: a dynamic component (ϕd), which is proportionate to the rate of rise of glucose, and a static component (ϕs) that represents the response to the increment in glucose above the basal concentration (27, 29, 31).
The appropriateness of insulin secretion for the prevailing degree of Si is assessed by calculating the total disposition index (DI), which expresses β-cell responsivity for the prevailing Si (ϕtotal × Si).
Power calculation.
In a similar experiment conducted in 14 adults with type 2 diabetes, we previously observed an overall mean ± SD change in the DI of 120 ± 138 · 10−14 dL · kg−1 · min−1 · pmol−1 · L−1 (32). Assuming similar variation in the changes from study 1 to study 2 (within-subject CV = 43% defined as the SD of the deltas divided by the overall mean DI), 20 subjects per group would provide ∼80% power (at a 2-sided 0.05 α level), to detect a difference in mean ∆DI values of 128 · 10−14 dL · kg−1 · min−1 · pmol−1 · L−1 between groups (i.e., a 40% difference in mean ∆DI values relative to the overall mean of 321 · 10−14 dL · kg−1 · min−1 · pmol−1 · L−1 observed in the previous study) (32). Such a difference would be clinically important in light of our previous studies observing similar-size treatment effects for a pharmacologic intervention (dipeptidyl peptidase-4 inhibition) (32). The present study was powered to detect similar differences with 20 subjects per group.
Study outcomes.
The primary study outcome was the effect of vitamin D3 supplementation on Si and pancreatic β-cell function (via DI) in obese adolescents, derived from the 3-h OGTT. Secondary outcome measures were changes in ISR and lipid profile (total cholesterol, TGs, and HDL cholesterol).
Statistical analysis.
Baseline data were descriptively summarized for the 2 treatment arms. The relation between 25(OH)D concentrations and other baseline measurements was assessed graphically with the use of scatter plots. Depending on the distribution of the measurements, correlations were estimated with the use of the Pearson or Spearman rank correlation coefficients. Two separate analyses were performed for each of the outcome measures. First, within each treatment arm, the changes between the baseline and 12-wk measurements were summarized and compared with the use of the paired t test if the distributions were normally distributed; otherwise the Wilcoxon signed rank test was used. Second, for each outcome, the 12-wk measurements were compared between the 2 treatment arms, based on an ANCOVA model, after adjusting for the corresponding baseline measurement. A logarithmic transformation was applied to both the baseline and 12-wk measurements before fitting the ANCOVA models. All tests were 2-sided and α was set at 0.05. Analyses were performed by use of SAS version 9.2 software package (SAS Institute). Values in the text were reported as means ± SDs or medians (IQR) as denoted.
Results
Baseline measurements.
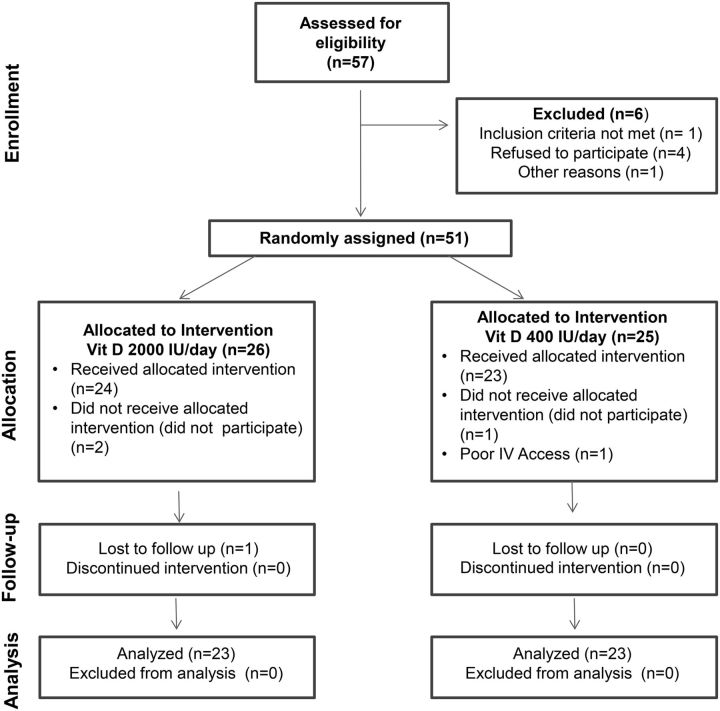
The Consolidated Standards Of Reporting Trials flow diagram for this study is shown in Figure 1. Of the 57 subjects assessed for eligibility for the study, 6 had to be excluded, leaving 51 subjects to be randomly assigned. After excluding those who subsequently decided not to participate (n = 3) or were found to have poor intravenous access for purposes of OGTT (n = 1), 47 subjects received the vitamin D3 supplement (n = 24 in the 2000 IU/d arm and n = 23 in the 400 IU/d arm). Of these 47 subjects, 20 (42.6%) were male, aged 15.0 ± 1.9 y (range: 12.7–18.7 y) (Table 1). The study cohort consisted of predominantly (45 out of the total 47) postpubertal (Tanner V) adolescents. The remaining 2 participants were in Tanner stage II. The majority of the 47 subjects entered into the study during the spring season (10 in the 400 IU/d group and 11 in the 2000 IU/d group), followed by summer (7 in each treatment group), winter (4 in each treatment group), and fall (2 in each treatment group). Serum 25(OH)D was 24.0 ± 8.1 μg/L and the 25(OH)D concentration was <30 μg/L for 37 (78.7%) subjects. Among the 23 patients randomly assigned to 400 IU/d, 6 had 25(OH)D concentrations <20 μg/L and 1 subject had a 25(OH)D concentration <12 μg/L. Among the 24 patients randomly assigned to 2000 IU/d, 7 had 25(OH)D concentrations <20 μg/L and 1 subject had a 25(OH)D concentration <12 μg/L. A higher proportion of the patients randomly assigned to 400 IU/d were male, compared with the other treatment arm (60.9% vs. 25.0%, P = 0.013). The rest of the baseline characteristics of the randomly assigned patients were similar in the 2 treatment arms (Table 1). There was no correlation between baseline measurements of 25(OH)D and indices of β-cell function, insulin action, or lipids. The relations between the 25(OH)D and the other markers were similar in the groups with 25(OH)D concentrations <20 μg/L and ≥20 μg/L.
FIGURE 1.
Consolidated Standards of Reporting Trials Diagram: Enrollment, random assignment, and follow-up of subjects. Vit, vitamin.
TABLE 1.
Comparison of baseline measurements between the 2 treatment groups1
| Characteristic or Measurement | 400 IU/d vitamin D3(n = 23) | 2000 IU/d vitamin D3(n = 24) |
|---|---|---|
| Males,2n (%) | 14 (60.9) | 6 (25.0) |
| Age at enrollment, y | 15.0 ± 1.9 | 15.1 ± 1.9 |
| BMI percentile | 98.0 ± 1.4 | 98.3 ± 1.0 |
| Body weight, kg | 96.6 ± 22.7 | 96.2 ± 16.9 |
| Height, cm | 171 ± 8.7 | 168 ± 8.2 |
| 25(OH)D, μg/L | 24.4 ± 7.7 | 23.5 ± 8.5 |
| Subjects with 25(OH)D < 20 μg/L, n | 6 | 7 |
| Subjects with 25(OH)D < 12 μg/L, n | 1 | 1 |
| HOMA-IR3 | 3.8 ± 1.8 | 4.7 ± 4.8 |
| QUICKI4 | 0.32 ± 0.02 | 0.32 ± 0.03 |
| LDL cholesterol, mg/dL | 93.3 ± 29.2 | 92.0 ± 19.8 |
| HDL cholesterol, mg/dL | 37.9 ± 8.0 | 40.3 ± 8.2 |
| Non-HDL cholesterol, mg/dL | 123 ± 40.5 | 113 ± 24.0 |
| Total cholesterol, mg/dL | 158 ± 40.2 | 153 ± 22.6 |
| TGs, mg/dL | 141 ± 77.6 | 103 ± 55.9 |
| Fasting glucose, mg/dL | 96.8 ± 5.4 | 98.5 ± 7.3 |
| Fasting insulin,5 μIU/mL | 15.9 ± 7.6 | 18.7 ± 16.8 |
| Si,6 10−4 dL · kg−1 · min−1 · μU−1 · mL−1 | 8.4 ± 6.1 | 12.3 ± 16.5 |
| Dynamic ISR,6 10−9 min−1 | 1339 ± 625 | 1105 ± 544 |
| Total ISR,6 10−9 min−1 | 106 ± 49 | 157 ± 248 |
| DI, 10−14 dL · kg−1 · min−2 · pmol−1 · L−1 | 1314 ± 929 | 2742 ± 6253 |
Values are means ± SDs, unless otherwise indicated. Glucose, insulin, and C-peptide were measured in plasma samples; 25(OH)D, total cholesterol, HDL cholesterol, and TGs were measured in serum. DI, disposition index; ISR, insulin secretion rate; QUICKI, quantitative insulin sensitivity check index; Si, insulin action; 25(OH)D, 25-hydroxyvitamin D.
Percentages are based on the chi-square test. The proportion of males was significantly different between the 2 treatment arms (60.9% vs. 25.0%, P = 0.013). All other P values were >0.05.
HOMA-IR = fasting serum glucose in milligrams per deciliter × fasting insulin in micro units per milliliter/405.
QUICKI = 1/log(fasting insulin in micro units per milliliter) + log(fasting glucose in milligrams per deciliter).
n = 44 participants.
n = 42 participants for variables derived from the oral glucose tolerance test.
Follow-up measurements.
One subject in the 2000 IU/d group was lost to follow-up after the initial OGTT. Within each treatment arm at follow-up (23 subjects in each group), the changes between the baseline and 12-wk measurements were evaluated (Table 2). BMI and weight did not change during the study period in either group. There was a modest but statistically significant increase in 25(OH)D concentration in the 2000 IU/d arm (3.1 ± 6.5 μg/L, P = 0.04), but not in the 400 IU/d group (P = 0.39). Of note, only 4 participants in the 400 IU/d group and 6 participants in the 2000 IU/d group achieved 25(OH)D concentrations of at least 30 μg/L. There was an inverse relation between increase in 25(OH)D concentrations and initial 25(OH)D concentration in both treatment arms (Spearman correlation = −0.25 and −0.60 in the 400 IU/d and 2000 IU/d treatment arms, respectively).
TABLE 2.
Summary of changes within participants and comparisons between participants from the 400 IU/d group and the 2000 IU/d group1
| Change (postsupplementation – presupplementation) within each treatment group | Comparison of presupplementation vs. postsupplementation2 | ||||||
|---|---|---|---|---|---|---|---|
| 400 IU/d vitamin D3 | 2000 IU/d vitamin D3 | 400 IU/d vitamin D3, P value | 2000 IU/d vitamin D3, P value | Comparison between the 2 arms,3P value | |||
| Measurement | n | Value | n | Value | |||
| Weight, kg | 23 | −1.1 ± 2.8 | 23 | −1.6 ± 3.4 | 0.2 | 0.1 | 0.7 |
| BMI, kg/m2 | 23 | 0.2 ± 0.9 | 23 | −0.3 ± 1.3 | 0.4 | 0.44 | 0.3 |
| 25(OH)D, μg/L | 21 | 0.8 ± 4.2 | 23 | 3.1 ± 6.5 | 0.39 | 0.04* | 0.17 |
| 0.0 (−1.0, 3.0) | 5.0 (−3.0, 8.0) | ||||||
| Fasting glucose, mg/dL | 23 | −0.1 ± 5.6 | 23 | −1.1 ± 5.8 | 0.93 | 0.37 | 0.96 |
| −2.0 (−4.0, 2.5) | −1.0 (−5.0, 2.0) | ||||||
| Fasting insulin, mIU/mL | 21 | 0.2 ± 5.7 | 21 | −0.4 ± 17.8 | 0.67* | 0.58* | 0.96 |
| −1.0 (−3.6, 2.9) | 1.2 (−2.8, 3.1) | ||||||
| HOMA-IR | 21 | 0.01 ± 1.3 | 21 | −0.3 ± 4.9 | 0.98 | 0.67* | 0.95 |
| 0.1 (−1.3, 0.6) | 0.4 (−0.8, 0.7) | ||||||
| QUICKI | 21 | −0.002 ± 0.015 | 21 | −0.001 ± 0.021 | 0.47 | 0.89 | 0.99 |
| 0 (−0.009, 0.009) | −0.004 (−0.011, 0.006) | ||||||
| Si, 10−4 dL · kg−1 · min−1 · μU−1 · mL−1 | 20 | 0.42 ± 4.29 | 20 | −4.57 ± 15.9 | 0.81* | 0.28* | 0.12 |
| 0.17 (−1.46, 2.70) | −0.60 (−5.32, 1.10) | ||||||
| Dynamic ISR, 10−9 min−1 | 18 | −165 ± 457 | 18 | 79.3 ± 751 | 0.14 | 0.66 | 0.48 |
| −191 (−334, −21.6) | 32.7 (−193, 603) | ||||||
| Static ISR, 10−9 min−1 | 18 | 1.2 ± 26.1 | 18 | −1.4 ± 29.7 | 0.85 | 0.58* | 0.59 |
| −1.2 (−13.1, 11.9) | 1.9 (−7.5, 15.1) | ||||||
| Total ISR, 10−9 min−1 | 18 | −1.0 ± 46.9 | 18 | −75.8 ± 269 | 0.61* | 0.26* | 0.61 |
| −9.5 (−40.2, 22.5) | −10.2 (−50.6, 13.4) | ||||||
| DI, 10−14 dL · kg−1 · min−1 · pmol−1 · L−1 | 18 | 274 ± 825 | 17 | −1865 ± 6833 | 0.44* | 0.22* | 0.20 |
| 59.4 (−273, 579) | −41.1 (−738, 178) | ||||||
| Total cholesterol, mg/dL | 23 | 4.7 ± 24.3 | 23 | 3.5 ± 16.80 | 0.36 | 0.32 | 0.80 |
| 1.0 (−12.0, 18.0) | 0 (−11.0, 14.0) | ||||||
| LDL cholesterol, mg/dL | 23 | 4.6 ± 22 | 23 | 1.3 ± 15.6 | 0.33 | 0.69 | 0.65 |
| 2.0 (−8.0, 16) | 2.0 (−14.0, 11.0) | ||||||
| HDL cholesterol, mg/dL | 23 | 1.3 ± 6.8 | 23 | 1.9 ± 5.7 | 0.38 | 0.13 | 0.68 |
| 2.0 (−4.0, 5.0) | 1.0 (−2.0, 7.0) | ||||||
| Non-HDL cholesterol, mg/dL | 23 | 0.50 ± 24.1 | 23 | 1.70 ± 15.3 | 0.80* | 0.61 | 0.99 |
| 1.0 (−8.0, 10) | 1.0 (−11, 12.0) | ||||||
| TGs, mg/dL | 23 | −4.70 ± 56.7 | 23 | 1.7 ± 43.5 | 0.69 | 0.85 | 0.40 |
| −1.0 (−32, 36) | −12.0 (−20.0, 26.0) | ||||||
Values are means ± SDs or medians (IQRs). Glucose, insulin, and C-peptide were measured in plasma samples; 25(OH)D, total cholesterol, HDL cholesterol, and TGs were measured in serum. DI, disposition index; ISR, insulin secretion rate; QUICKI, quantitative insulin sensitivity check index; Si, insulin action; 25(OH)D, 25-hydroxyvitamin D.
Evaluated with the use of the paired t test or Wilcoxon signed rank test (denoted by *).
Comparison of the postsupplementation values between each treatment arm, after adjusting for presupplementation differences, was made by fitting a 1-factor ANCOVA model to the measurements after applying a logarithmic transformation.
No change in Si was noted within either group (P = 0.28 in the 2000 IU/d arm and P = 0.81 in the 400 IU/d group); nor did it change between groups (P = 0.12). Likewise, the DI did not change within either group (P = 0.22 in the 2000 IU/d arm, P = 0.44 in the 400 IU/d group) nor did the DI differ between groups (P = 0.20). No significant differences were observed for any of the other measurements within each of the treatment groups after supplementation, or between the 2 groups. Insulin secretion rates did not differ between the 2 arms (Table 2).
Compliance could not be assessed in 8 subjects (5 in the 2000 IU/d group and 3 in the 400 IU/d group) because they did not bring their pill bottles back for a pill count. In the 2000 IU/d group, 1 subject missed 21 doses and another missed 24 doses, whereas 6 subjects in the group were noted to have missed between 2 and 6 doses of vitamin D3. In the 400 IU/d group, 5 subjects missed between 2 and 9 pills.
Discussion
The results of the current study suggest that, in Caucasian adolescents with uncomplicated obesity, there is no independent relation between plasma 25(OH)D concentrations and Si and β-cell function as assessed by OGTT, with the use of the minimal model approach. There was also no relation between 25(OH)D concentrations and lipid profile in the obese adolescents studied. Vitamin D3 supplementation for 12 wk enhanced circulating 25(OH)D concentrations only in the cohort that received the higher dose of 2000 IU/d. But the modest enhancement of vitamin D status in this cohort of obese nondiabetic adolescents with relatively good vitamin D status appears insufficient to impart beneficial changes in Si, β-cell function, and lipid variables.
The lack of an independent relation between 25(OH)D concentrations and Si observed in the current study is in agreement with the findings of Rajakumar and colleagues (19) in healthy and obese white and black youth, in which in vivo insulin sensitivity and β-cell function were measured by hyperinsulinemic-euglycemic and hyperinsulinemic-hyperglycemic clamp approaches. Additionally, de las Heras et al. (33) noted no differences in plasma 25(OH)D between obese youth across the spectrum of glucose tolerance from normal to prediabetes to type 2 diabetes. Of note, reports on the relation between 25(OH)D and insulin resistance in children are mostly based on indices derived from fasting insulin and glucose values, such as HOMA-IR and the quantitative insulin sensitivity check index (34–38). The discrepancy in data between these studies and our study may be due to various factors, including differences in the methods of measurement of insulin resistance or sensitivity and 25(OH)D (immunoassays vs. LC-MS/MS). Potential differences in the ethnicity and age of the study population, geographic location, and spectrum of weight status may also have contributed to this inconsistency.
Studies on the effect of vitamin D supplementation on measurements of Si and secretion are scarce (19, 39, 40). Unlike in other studies, we determined the effect of 2 different doses, a low and a higher, of vitamin D3 supplementation on Si and β-cell function as assessed by DI with the use of the OGTT minimal model approach. Neither the low nor the higher dose of vitamin D3 supplementation for 12 wk resulted in significant improvements in measurements of Si and β-cell function. To our knowledge, there are no published studies on the effect of vitamin D3 supplementation on Si and disposition index in the pediatric age group. Similar to our study, in overweight and obese African American adults with prediabetes or early diabetes, Harris et al. (39) also noted that supplementation with 4000 IU/d vitamin D3 increased 25(OH)D concentrations but had divergent effects on insulin secretion and sensitivity with no overall effect on disposition index or glycemia. Belenchia et al. (40), through the use of a placebo-controlled randomized study in obese adolescents, also found no effect of vitamin D3 supplementation for 12 wk on fasting plasma glucose, fasting insulin, and surrogate markers of insulin resistance. However, they noted a decrease in fasting insulin and HOMA-IR at 6 mo in the vitamin D3–supplemented group compared with placebo. The doses and duration of vitamin D3 in our study were half of that used in the study by Belenchia et al. (40). The increment in circulating 25(OH)D concentrations in response to the supplementation in our study was rather modest compared with the increment in the other study. This may be related to relatively normal 25(OH)D concentrations at baseline in our study subjects, because the increment in 25(OH)D for a given dose of vitamin D is known to be inversely related to the initial concentration of 25(OH)D (39). There is no clear consensus on the definition for optimal concentrations of circulating 25(OH)D. However, Ashraf et al. (37) have proposed a cutoff of 15 μg/L 25(OH)D, below which vitamin D deficiency confers profound negative effects on insulin sensitivity. Based on this cutoff, the potential difference in the baseline 25(OH)D concentrations in our study (23.5 μg/L) and that by Belenchia et al. (19.5 μg/L) may also have contributed to disparity in the data from the 2 studies (40). The participants in our study were ethnically homogeneous and consisted of predominantly Caucasians. The association between vitamin D status and measures of insulin resistance appears to be dependent on ethnicity. A significant inverse correlation between 25(OH)D and hemoglobin A1c in Caucasian adolescents, but not in African-Americans or Hispanics, has been reported (38).
Although studies on the effect of vitamin D3 supplementation on lipid variables in children and adolescents are limited, the data from adult studies on its lipid-modulating effects are divergent (26, 41). In the current study there was no improvement in any of the lipid variables in either of the groups. Future studies should shed more light on whether correcting for vitamin D deficiency will in fact translate into clinically meaningful improvements in lipid variables in children and adolescents with a combination of vitamin D deficiency and hyperlipidemia.
The study has several strengths and limitations that should be considered when interpreting the data. We used the OGTT minimal model approach, as opposed to other surrogate measures of insulin resistance, such as insulin levels and HOMA-IR. The measurement of 25(OH)D concentrations was performed with the use of the LC-MS/MS technique. Further, we included 2 doses of vitamin D3 in a prospective randomized and double-blind fashion to understand its potential dose effect on Si and β-cell function in obese adolescents. A limitation of our study is the small sample size, which decreases the power to detect small differences, if any, between the 2 treatment arms. However, the data from the current study should help in the design of future studies exploring potentially small effects of vitamin D supplementation on β-cell function. The obese adolescents in the current study had relatively adequate vitamin D status with a mean value of 24.4 μg/L, i.e., almost 5 μg/L above the level that is considered to meet the requirements of at least 97% of the North American population (40). The good vitamin D status of the subjects is likely due to the fact that the participants were Caucasians and the least number of subjects were recruited during the winter (6, 7). Although the participants in our study were obese, they had no signs of prediabetes and/or diabetes. Additionally, the small increase in 25(OH)D concentration of 3.1 μg/L in the group treated with 2000 IU/d of vitamin D3 compared with the 0.8 μg/L increase in the group treated with 400 IU/d may not be sufficient for generating remarkable changes in insulin sensitivity. This study, however, supports the need for higher doses of vitamin D3 in obese adolescents for quantifiable outcomes (42). The poor response to vitamin D3 supplementation in obese adolescents is likely secondary to a greater degree of sequestration of lipid-soluble vitamin D in their adipose tissue, as well as the potential differences in vitamin D metabolism in the obese state (43, 44). The small sample size precluded us from conducting subgroup analysis to determine whether those with 25(OH)D concentrations <15 μg/L or <20 μg/L had an improvement in any of the variables. The number of patients recruited in each season was too small to allow us to determine the effect of season on baseline 25(OH)D or response to vitamin D3 supplementation. Further, the LC-MS/MS method we used for the measurement of 25(OH)D could not separate 3-epi-25(OH)D3 from regular 25(OH)D3. We could not delineate the role of other potential confounders, such as sun exposure, dietary intake of calcium and vitamin D, and physical activity.
In conclusion, our data suggest that vitamin D supplementation, taken even at a dose of 2000 IU/d by nondiabetic and vitamin D–replete obese adolescents for 12 wk, has no effect on insulin sensitivity and pancreatic β-cell function, or on lipid profile. Yet, future larger randomized controlled studies are needed to address the effect of vitamin D supplementation in sufficient dose and duration in severely vitamin D–deficient and insulin-resistant adolescents. The potential relation between vitamin D status and other obesity-related cardiometabolic risk factors, and the effect of vitamin D supplementation in attenuating these risk factors, remain poorly understood in children and adolescents.
Acknowledgments
AV, PRF, and SK designed the research; PDG and JML conducted the research; ALW, FP, CDM, and CC analyzed the data; AJ, PBB, ALW, and SK interpreted the data and wrote the paper; and SK had primary responsibility for the final content. All authors read and approved the final manuscript.
Abbreviations
- DI
disposition index
- ISR
insulin secretion rate
- LC-MS/MS
liquid chromatography-tandem mass spectrometry
- OGTT
oral glucose tolerance test
- Si
insulin action
- 25(OH)D
25-hydroxyvitamin D
Footnotes
Funding support for this research was provided by the Thrasher Research Foundation.
References
- 1. Gordon CM, DePeter KC, Feldman HA, Grace E, Emans SJ. Prevalence of vitamin D deficiency among healthy adolescents. Arch Pediatr Adolesc Med 2004;158:531–7. [DOI] [PubMed] [Google Scholar]
- 2. Rovner AJ, O'Brien KO. Hypovitaminosis D among healthy children in the United States: a review of the current evidence. Arch Pediatr Adolesc Med 2008;162:513–9. [DOI] [PubMed] [Google Scholar]
- 3. Looker AC, Pfeiffer CM, Lacher DA, Schleicher RL, Picciano MF, Yetley EA. Serum 25-hydroxyvitamin D status of the US population: 1988–1994 compared with 2000–2004. Am J Clin Nutr 2008;88:1519–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Harkness LS, Bonny AE. Calcium and vitamin D status in the adolescent: key roles for bone, body weight, glucose tolerance, and estrogen biosynthesis. J Pediatr Adolesc Gynecol 2005;18:305–11. [DOI] [PubMed] [Google Scholar]
- 5. Turer CB, Lin H, Flores G. Prevalence of vitamin D deficiency among overweight and obese US children. Pediatrics 2013;131:e152–61. [DOI] [PubMed] [Google Scholar]
- 6. Kumar J, Muntner P, Kaskel FJ, Hailpern SM, Melamed ML. Prevalence and associations of 25-hydroxyvitamin D deficiency in US children: NHANES 2001–2004. Pediatrics 2009;124:e362–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Holick MF. Vitamin D deficiency. N Engl J Med 2007;357:266–81. [DOI] [PubMed] [Google Scholar]
- 8. Hill TR, Cotter AA, Mitchell S, Boreham CA, Dubitzky W, Murray L, Strain JJ, Flynn A, Robson PJ, Wallace JM, et al. Vitamin D status and its determinants in adolescents from the Northern Ireland Young Hearts 2000 cohort. Br J Nutr 2008;99:1061–7. [DOI] [PubMed] [Google Scholar]
- 9. Houghton LA, Szymlek-Gay EA, Gray AR, Ferguson EL, Deng X, Heath AL. Predictors of vitamin D status and its association with parathyroid hormone in young New Zealand children. Am J Clin Nutr 2010;92:69–76. [DOI] [PubMed] [Google Scholar]
- 10. Smotkin-Tangorra M, Purushothaman R, Gupta A, Nejati G, Anhalt H, Ten S. Prevalence of vitamin D insufficiency in obese children and adolescents. J Pediatr Endocrinol Metab 2007;20:817–23. [DOI] [PubMed] [Google Scholar]
- 11. Dietz WH. Health consequences of obesity in youth: childhood predictors of adult disease. Pediatrics 1998;101:518–25. [PubMed] [Google Scholar]
- 12. Reilly JJ, Methven E, McDowell ZC, Hacking B, Alexander D, Stewart L, Kelnar CJ. Health consequences of obesity. Arch Dis Child 2003;88:748–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Cook S, Weitzman M, Auinger P, Nguyen M, Dietz WH. Prevalence of a metabolic syndrome phenotype in adolescents: findings from the third National Health and Nutrition Examination Survey, 1988–1994. Arch Pediatr Adolesc Med 2003;157:821–7. [DOI] [PubMed] [Google Scholar]
- 14. Weiss R, Dziura J, Burgert TS, Tamborlane WV, Taksali SE, Yeckel CW, Allen K, Lopes M, Savoye M, Morrison J, et al. Obesity and the metabolic syndrome in children and adolescents. N Engl J Med 2004;350:2362–74. [DOI] [PubMed] [Google Scholar]
- 15. Reis JP, von Muhlen D, Miller ER, 3rd, Michos ED, Appel LJ. Vitamin D status and cardiometabolic risk factors in the United States adolescent population. Pediatrics 2009;124:e371–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Johnson MD, Nader NS, Weaver AL, Singh R, Kumar S. Relationships between 25-hydroxyvitamin D levels and plasma glucose and lipid levels in pediatric outpatients. J Pediatr 2010;156:444–9. [DOI] [PubMed] [Google Scholar]
- 17. Olson ML, Maalouf NM, Oden JD, White PC, Hutchison MR. Vitamin D deficiency in obese children and its relationship to glucose homeostasis. J Clin Endocrinol Metab 2012;97:279–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Kelly A, Brooks LJ, Dougherty S, Carlow DC, Zemel BS. A cross-sectional study of vitamin D and insulin resistance in children. Arch Dis Child 2011;96:447–52. [DOI] [PubMed] [Google Scholar]
- 19. Rajakumar K, de las Heras J, Lee S, Holick MF, Arslanian SA. 25-hydroxyvitamin D concentrations and in vivo insulin sensitivity and beta-cell function relative to insulin sensitivity in black and white youth. Diabetes Care 2012;35:627–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Van Cauter E, Mestrez F, Sturis J, Polonsky KS. Estimation of insulin secretion rates from C-peptide levels. Comparison of individual and standard kinetic parameters for C-peptide clearance. Diabetes 1992;41:368–77. [DOI] [PubMed] [Google Scholar]
- 21. George PS, Pearson ER, Witham MD. Effect of vitamin D supplementation on glycaemic control and insulin resistance: a systematic review and meta-analysis. Diabet Med 2012;29:e142–50. [DOI] [PubMed] [Google Scholar]
- 22. Alvarez JA, Ashraf A. Role of vitamin d in insulin secretion and insulin sensitivity for glucose homeostasis. Int J Endocrinol. 2010;2010:351385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Johnson JA, Grande JP, Roche PC, Kumar R. Immunohistochemical localization of the 1,25(OH)2D3 receptor and calbindin D28k in human and rat pancreas. Am J Physiol 1994;267:E356–60. [DOI] [PubMed] [Google Scholar]
- 24. Pittas AG, Lau J, Hu FB, Dawson-Hughes B. The role of vitamin D and calcium in type 2 diabetes. A systematic review and meta-analysis. J Clin Endocrinol Metab 2007;92:2017–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Kuczmarski RJ, Ogden CL, Guo SS, Grummer-Strawn LM, Flegal KM, Mei Z, Wei R, Curtin LR, Roche AF, Johnson CL. CDC Growth Charts for the United States: methods and development. Vital Health Stat 11 2000;2002:1–190. [PubMed] [Google Scholar]
- 26. Nader NS, Aguirre Castaneda R, Wallace J, Singh R, Weaver A, Kumar S. Effect of vitamin D3 supplementation on serum 25(OH)D, lipids and markers of insulin resistance in obese adolescents: A prospective, randomized, placebo-controlled pilot trial. Horm Res Paediatr. 2014;82:107–12. [DOI] [PubMed] [Google Scholar]
- 27. Dalla Man C, Caumo A, Basu R, Rizza R, Toffolo G, Cobelli C. Minimal model estimation of glucose absorption and insulin sensitivity from oral test: validation with a tracer method. Am J Physiol Endocrinol Metab 2004;287:E637–43. [DOI] [PubMed] [Google Scholar]
- 28. Dalla Man C, Caumo A, Cobelli C. The oral glucose minimal model: estimation of insulin sensitivity from a meal test. IEEE Trans Biomed Eng 2002;49:419–29. [DOI] [PubMed] [Google Scholar]
- 29. Dalla Man C, Yarasheski KE, Caumo A, Robertson H, Toffolo G, Polonsky KS, Cobelli C. Insulin sensitivity by oral glucose minimal models: validation against clamp. Am J Physiol Endocrinol Metab 2005;289:E954–9. [DOI] [PubMed] [Google Scholar]
- 30. Steil GM, Hwu CM, Janowski R, Hariri F, Jinagouda S, Darwin C, Tadros S, Rebrin K, Saad MF. Evaluation of insulin sensitivity and beta-cell function indexes obtained from minimal model analysis of a meal tolerance test. Diabetes 2004;53:1201–7. [DOI] [PubMed] [Google Scholar]
- 31. Cobelli C, Dalla Man C, Toffolo G, Basu R, Vella A, Rizza R. The oral minimal model method. Diabetes 2014;63:1203–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Dalla Man C, Bock G, Giesler PD, Serra DB, Ligueros Saylan M, Foley JE, Camilleri M, Toffolo G, Cobelli C, Rizza RA, et al. Dipeptidyl peptidase-4 inhibition by vildagliptin and the effect on insulin secretion and action in response to meal ingestion in type 2 diabetes. Diabetes Care 2009;32:14–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. de las Heras J, Rajakumar K, Lee S, Bacha F, Holick MF, Arslanian SA. 25-Hydroxyvitamin D in obese youth across the spectrum of glucose tolerance from normal to prediabetes to type 2 diabetes. Diabetes Care 2013;36:2048–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Parikh S, Guo DH, Pollock NK, Petty K, Bhagatwala J, Gutin B, Houk C, Zhu H, Dong Y. Circulating 25-hydroxyvitamin D concentrations are correlated with cardiometabolic risk among American black and white adolescents living in a year-round sunny climate. Diabetes Care 2012;35:1133–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Ford ES, Zhao G, Tsai J, Li C. Associations between concentrations of vitamin D and concentrations of insulin, glucose, and HbA1c among adolescents in the United States. Diabetes Care 2011;34:646–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Delvin EE, Lambert M, Levy E, O'Loughlin J, Mark S, Gray-Donald K, Paradis G. Vitamin D status is modestly associated with glycemia and indicators of lipid metabolism in French-Canadian children and adolescents. J Nutr 2010;140:987–91. [DOI] [PubMed] [Google Scholar]
- 37. Ashraf A, Alvarez J, Saenz K, Gower B, McCormick K, Franklin F. Threshold for effects of vitamin D deficiency on glucose metabolism in obese female African-American adolescents. J Clin Endocrinol Metab 2009;94:3200–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Alemzadeh R, Kichler J, Babar G, Calhoun M. Hypovitaminosis D in obese children and adolescents: relationship with adiposity, insulin sensitivity, ethnicity, and season. Metabolism 2008;57:183–91. [DOI] [PubMed] [Google Scholar]
- 39. Harris SS, Pittas AG, Palermo NJ. A randomized, placebo-controlled trial of vitamin D supplementation to improve glycaemia in overweight and obese African Americans. Diabetes Obes Metab 2012;14:789–94. [DOI] [PubMed] [Google Scholar]
- 40. Belenchia AM, Tosh AK, Hillman LS, Peterson CA. Correcting vitamin D insufficiency improves insulin sensitivity in obese adolescents: a randomized controlled trial. Am J Clin Nutr 2013;97:774–81. [DOI] [PubMed] [Google Scholar]
- 41. Challoumas D. Vitamin D supplementation and lipid profile: what does the best available evidence show? Atherosclerosis 2014;235:130–9. [DOI] [PubMed] [Google Scholar]
- 42. Aguirre Castaneda R, Nader N, Weaver A, Singh R, Kumar S. Response to vitamin D3 supplementation in obese and non-obese Caucasian adolescents. Horm Res Paediatr. 2012;78:226–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Harel Z, Flanagan P, Forcier M, Harel D. Low vitamin D status among obese adolescents: prevalence and response to treatment. J Adolesc Health 2011;48:448–52. [DOI] [PubMed] [Google Scholar]
- 44. Wortsman J, Matsuoka LY, Chen TC, Lu Z, Holick MF. Decreased bioavailability of vitamin D in obesity. Am J Clin Nutr 2000;72:690–3. [DOI] [PubMed] [Google Scholar]