Abstract
Background: Maternal protein restriction in rats increases the risk of adult offspring arterial hypertension through unknown mechanisms.
Objectives: The aims of the study were to evaluate the effects of a low-protein (LP) diet during pregnancy and lactation on baseline sympathetic and respiratory activities and peripheral chemoreflex sensitivity in the rat offspring.
Methods: Wistar rat dams were fed a control [normal-protein (NP); 17% protein] or an LP (8% protein) diet during pregnancy and lactation, and their male offspring were studied at 30 d of age. Direct measurements of baseline arterial blood pressure (ABP), heart rate (HR), and respiratory frequency (Rf) as well as peripheral chemoreflex activation (potassium cyanide: 0.04%) were recorded in pups while they were awake. In addition, recordings of the phrenic nerve (PN) and thoracic sympathetic nerve (tSN) activities were obtained from the in situ preparations. Hypoxia-inducible factor 1α (HIF-1α) expression was also evaluated in carotid bifurcation through a Western blotting assay.
Results: At 30 d of age, unanesthetized LP rats exhibited enhanced resting Rf (P = 0.001) and similar ABP and HR compared with the NP rats. Despite their similar baseline ABP values, LP rats exhibited augmented low-frequency variability (∼91%; P = 0.01). In addition, the unanesthetized LP rats showed enhanced pressor (P = 0.01) and tachypnoeic (P = 0.03) responses to peripheral chemoreflex activation. The LP rats displayed elevated baseline tSN activity (∼86%; P = 0.02) and PN burst frequency (45%; P = 0.01) and amplitude (53%; P = 0.001) as well as augmented sympathetic (P = 0.01) and phrenic (P = 0.04) excitatory responses to peripheral chemoreflex activation compared with the NP group. Furthermore, LP rats showed an increase of ∼100% in HIF-1α protein density in carotid bifurcation compared with NP rats.
Conclusion: Sympathetic-respiratory overactivity and amplified peripheral chemoreceptor responses, potentially through HIF-1α–dependent mechanisms, precede the onset of hypertension in juvenile rats exposed to protein undernutrition during gestation and lactation.
Keywords: protein undernutrition, hypertension, sympathetic overactivity, peripheral chemoreflex, hypoxia-inducible factor 1 α
Introduction
Arterial hypertension is a major risk factor for cardiovascular dysfunction, which affects almost 1 billion people and is recognized as a major cause of morbidity and mortality worldwide (1). However, the underlying cause of hypertension has been difficult to identify due to its multifactorial nature. Hypertension may arise from a combination of genetic factors and lifestyle-related behaviors (1). In addition, adverse events experienced in utero or during perinatal life (gestation, lactation, and early infancy) can affect the development of physiologic systems, leading to increased risk of hypertension and metabolic diseases later in life (2, 3).
For example, maternal undernutrition has been associated with low nephron number, kidney disease, insulin resistance, and obesity because of rapid weight gain in childhood or adolescence (4, 5). The biological phenomenon underlying these associations is known as phenotypic plasticity, which refers to the ability of a single genotype to produce variable behavioral, morphologic, and/or physiologic phenotypes (6) in individuals in response to different environmental circumstances encountered during development.
The offspring of rat dams subjected to a maternal low-protein (LP)10 diet is a model that is often used to study the mechanisms of maternal undernutrition–related hypertension (6–8), which has been suggested to be associated with changes in the functioning of the sympathetic nervous system (9, 10). However, there is no direct evidence demonstrating that sympathetic vasoconstrictor tonus is elevated in rats subjected to a perinatal LP diet.
We recently showed that juvenile rats subjected to protein undernutrition during gestation and lactation exhibit increased baseline respiratory frequency associated with amplified ventilatory responses to hypoxia and hypercapnia (7). These findings indicated that the functioning of the respiratory network, in addition to that of the sympathetic nervous system, is affected by an LP diet during gestation and lactation, likely through a common excitation mechanism. In this regard, afferent inputs from peripheral chemoreceptors to the central nervous system, which are mainly generated in response to hypoxic stimuli, evoke reflex responses of sympatho-excitation and tachypnoea (11). Therefore, changes in the functioning of the peripheral chemoreflex may be involved in the exaggerated sympathetic and respiratory responses observed in the offspring of protein-restricted rats.
It has been shown that the sensitization of peripheral chemoreceptors is a risk factor for sympathetic overactivity and the development of arterial hypertension (12, 13). An important molecular mechanism involved in the enhanced sensory activity of peripheral chemoreceptors is the activation of hypoxia-inducible factor (HIF) (14, 15). Indeed, there is evidence that high expression of HIF-1α during early life is associated with an increased risk of developing hypertension (16, 17).
In this context, in the present study, we hypothesized that juvenile rats from dams subjected to protein undernutrition during pregnancy and lactation would exhibit enhanced baseline sympathetic and inspiratory motor activities associated with amplified respiratory and sympathetic responses to peripheral chemoreflex activation and enhanced HIF-1α concentrations in the carotid body peripheral chemoreceptors. This hypothesis was investigated in the offspring of protein-restricted dams before the onset of hypertension (7) to verify whether these changes are the cause or consequence of an increase in arterial pressure.
Methods
The experimental protocol was approved by the Ethical Committee of the Biological Sciences Center (protocol 044454/2010–94) at the Federal University of Pernambuco and by the Animal Experimentation Ethics Committee of the School of Dentistry of Araraquara at São Paulo State University (protocol 21/2012), Brazil. All efforts were made to minimize animal discomfort and the number of rats used; in addition, we followed the Guidelines for the Care and Use of Laboratory Animals.
Animals and experimental groups.
Virgin female albino Wistar rats (Rattus norvegicus) were obtained from the Academic Center of Vitoria de Santo Antão, Federal University of Pernambuco, Brazil. The rats were maintained in a room with a temperature of 22 ± 1°C and a controlled light-dark cycle (dark: 1800–0600 h). Standard laboratory feed pellets (52% carbohydrate, 21% protein, and 4% lipids, Labina; Purina Agriband) and water were consumed ad libitum up to the 3-mo point, when the rats were mated (2 females for 1 male). The day on which spermatozoa were identified in a vaginal smear was considered the date of conception, and pregnant rats were transferred to individual cages. Two experimental groups were designated according to diet manipulation: dams fed a 17% casein diet [normal-protein (NP) group; n = 6] and dams fed a 8% casein diet (LP group; n = 6) and water ad libitum.
The diets were mixed at the Laboratory of Experimental Nutrition–Academic Center of Vitoria de Santo Antão, Federal University of Pernambuco, according to the American Institute of Nutrition–AIN-93 diet (18, 19). The casein was previously analyzed and found to be 85% pure (85 g of protein for each 100 g of casein). The diets were isoenergetic and were fed during pregnancy and lactation. Both diets presented the same amount of vitamin and mineral mix. Only the amount of protein and carbohydrate was changed (18). Offspring were standardized as litters of 8 pups 48 h after birth. Male offspring were used in each litter and females were used only to standardize the size of each litter to 8 pups (7). At weaning, 3 or 4 male offspring from each litter were randomly housed in collective cages and received a standard diet ad libitum. At least 2 or 3 male offspring from each litter were used to compose the NP or LP groups and to perform the experimental protocol. All of the experiments were performed in 30-d-old juvenile rats.
Cardiovascular and respiratory evaluations in vivo.
One day before the experiments, the NP (n = 8) and LP (n = 11) rats were anesthetized with ketamine (80 mg/kg) and xylazine (10 mg/kg), and the femoral artery and vein were cannulated [Polyethylene Tubing (PE)-50 connected to PE-10; Clay Adams]. The catheters were filled with heparinized saline (NaCl 0.9%), tunneled subcutaneously, and exteriorized through the back of the neck. After surgery, the rats were administered an injection of ketoprofen (5 mg/kg, intraperitoneally), and a period of 24 h was allowed to pass until the rats had fully recovered from the surgical and anesthetic procedures. The next day, the mean arterial pressure (MAP) and heart rate (HR) of unanesthetized freely moving rats were recorded by connecting the arterial catheter to a pressure transducer. The signals were amplified (ML866/P, Power Lab; ADInstruments), sampled at 2 kHz, and digitalized by using appropriate software (LabChart7 Pro; ADInstruments). Recordings of the baseline pulsatile arterial pressure, MAP, and HR were made for 30–50 min. After 50 min of acclimatization and cardiovascular recordings, measurements of the respiratory frequency (Rf) were also performed by using the whole-body plethysmography method (20). Before recording baseline data, the rats were placed into a Plexiglas chamber (5 L) that was flushed with humidified room air and maintained at a temperature of 25°C. After this acclimatization period, the Rf was recorded as the airflow was suspended for short periods (3 min), and the pressure oscillations caused by breathing were captured by a pressure differential transducer connected to a signal amplifier (ML141 Spirometer, PowerLab; ADInstruments). The signals were then captured by an acquisition system and data analysis was performed (PowerLab; ADInstruments). All of the data were analyzed off-line with the use of appropriate software (LabChart 7 Pro; ADInstruments).
After the baseline recordings of Rf and arterial pressure, the peripheral chemoreflex was activated through the intravenous injection of potassium cyanide (KCN; 40 μg/100 μL per rat; Merck) in accordance with previous reports (11, 21). At the end of the experiments, the rats were killed with a 1-mL overdose of a mixture of ketamine (80 mg/kg, intraperitoneally) and xylazine (10 mg/kg, intraperitoneally).
An indirect evaluation of the autonomic modulation of vascular resistance and cardiac function was performed through an analysis of the variability in the arterial pressure and HR in the frequency domain (22). Oscillations of arterial pressure and HR at the low-frequency (LF) range are representative of the modulatory effects of sympathetic activity controlling vascular tonus and heart activity, whereas oscillations at the high-frequency (HF) range are associated with a respiratory or parasympathetic modulation of blood vessels or the heart, respectively (22–24). To reach this goal, a beat-by-beat time series of the systolic arterial pressure (SAP) and HR were extracted from the baseline cardiovascular recordings (10-min epochs) of the pulsatile arterial pressure of the NP and LP rats (Chart Pro; ADInstruments), and the overall variability of these series was assessed through fast Fourier transformation infrared spectroscopy (Cardioseries software, version 2.4) (25). The power of the oscillatory components obtained from the rats belonging to the NP and LP groups was quantified in 2 frequency bands: LF (0.20–0.75 Hz) and HF (0.75–3.0 Hz) (22, 26).
In situ working heart-brainstem preparation.
Juvenile rats at 30 d of age [NP (n = 6) and LP (n = 8)] were deeply anesthetized with halothane (Astra Zeneca), such that the withdrawal responses to noxious pinching of the tail and paw were absent. The rats were then transected caudally to the diaphragm and submerged in cooled Ringer solution (in mM: 125 NaCl, 24 NaHCO3, 3 KCl, 2.5 CaCl2, 1.25 MgSO4, 1.25 KH2PO4, and 10 dextrose). They were made insentient by decerebration at the precollicular level, skinned, and had the descending aorta isolated. Preparations were then transferred to a recording chamber, where the descending aorta was cannulated and perfused retrogradely with modified Ringer solution containing lactate (2 mM), an oncotic agent (1.25% polyethylene glycol; Sigma), and a neuromuscular blocker (vecuronium bromide, 3–4 μg/mL; Cristalia) by using a roller pump (Watson-Marlow 502s) via a double-lumen cannula. Perfusion pressure was maintained in the range of 50–70 mm Hg by adjusting the rate flow between 21 and 25 mL/min and by adding vasopressin to the perfusate (6–12 nM; Sigma), as previously described (27). Electrical activity in all nerves was recorded by using glass suction bipolar electrodes held by a micromanipulator (Narishige). Left phrenic nerve (PN) discharges were recorded from the central end and its rhythmic ramping activity gave a continuous physiologic index of preparation viability. Thoracic sympathetic nerve (tSN) activity was recorded from the thoracic sympathetic chain at the level of T10–T12. All of the signals were amplified, band-pass filtered (0.05–5 kHz), and acquired with an A/D converter (CED 1401; Cambridge Electronic Design) on a computer using Spike2 software (version 7; Cambridge Electronic Design). Peripheral chemoreceptors were stimulated by injections of KCN (0.05%, 50 μL) into the descending aorta of the working heart-brainstem preparation via the perfusion cannula, as previously described (28).
All of the analyses of rectified and integrated (50-ms) signals were performed off-line by using the Spike 2 software with custom-written scripts. Before analyses, PN and tSN recordings were subtracted from the electrical noise obtained after the death of the working heart-brainstem preparation (induced by turning the pump off). For baseline measurements, PN activity was assessed by its frequency (cycles per minute), amplitude (μV), burst duration (inspiratory time, s), and burst interval (expiratory time, s). tSN activity was assessed by its mean activity (μV) and by the amplitude of inspiratory-related bursts (μV), which was calculated by the value difference between the maximal and lowest activity observed during inspiratory and postinspiratory phases. With respect to the changes induced by peripheral chemoreflex activation, the phrenic frequency reflex response was assessed by the difference between the baseline frequency and the peak of response observed after the stimulus (∆PN; expressed in cycles per minute). The sympathetic response was assessed by the measurement of the AUC and expressed as percentage change (∆tSN; in %) in relation to the activity before the stimulus.
Evaluation of HIF-1α protein density.
Under normoxic conditions, separate groups of NP (n = 6) and LP (n = 7) rats that were not subjected to any surgical procedure were killed by an overdose of ketamine (80 mg/kg, intraperitoneally) and xylazine (10 mg/kg, intraperitoneally) for collection of the carotid bifurcation at 30 d of age. The tissues were flash-frozen in liquid nitrogen and stored at −80°C until use. The carotid bifurcation samples were pooled respectively to obtain a sufficient amount of protein. The samples were then sonicated, and protein extracts were obtained in radioimmunoprecipitation assay buffer (50 mM Tris pH 7.6, 150 mM NaCl, 1% SDS, 0.5% sodium deoxycholate, 0.5% NP-40) with protease and phosphatase inhibitor cocktails (Sigma-Aldrich). Protein concentration was determined by using the Bradford method (Bio-Rad Laboratories). Ninety micrograms of protein was submitted to 8% SDS-PAGE and transferred to a polyvinylidene difluoride membrane (GE HealthCare). The membranes were blocked for 1 h by using Tris-buffered saline containing 10% Tween 20 (TBS-T) and 5% (wt:vol) nonfat dry milk (blocking buffer). Antibodies against HIF-1α (mouse monoclonal H1α67, GR45835-1; AbCam) and GAPDH (2118S; Cell Signaling) were used. The antibody against HIF-1α was used diluted 1:500 in TBS-T with 5% of BSA (Sigma-Aldrich). Anti-mouse secondary antibody was diluted 1:5000 in blocking buffer. Blots were developed by using the chemiluminescent ECL Western Blotting System (GE HealthCare). The bands were quantified by densitometry with the use of Image J software (NIH; http://rsbweb.nih.gov/ij/), and the relative densities of HIF-1α were normalized by their respective controls.
Statistical analysis.
Each experimental group included at least 2 rats from each litter. Bartlett's test was performed to evaluate data homogeneity of the respiratory and sympathetic variables, and statistical results supported the use of a parametric test. Thus, the significance of the difference between groups was assessed by unpaired Student's t test. The significance level was fixed to P < 0.05. The data are expressed as means with associated SEs. Statistical analysis was performed by using GraphPad Prism 5.0 software.
Results
Ponderal gain.
Pups from mothers subjected to an LP diet had lower birth weight than did the NP group (NP vs. LP: 6.3 ± 0.1 vs. 5.3 ± 0.2 g; P = 0.04). Furthermore, the reduced body weight of the LP group was maintained until 30 d of age (NP vs. LP: 86.3 ± 2.9 vs. 62.5 ± 2.6 g; P = 0.01).
Conscious rats.
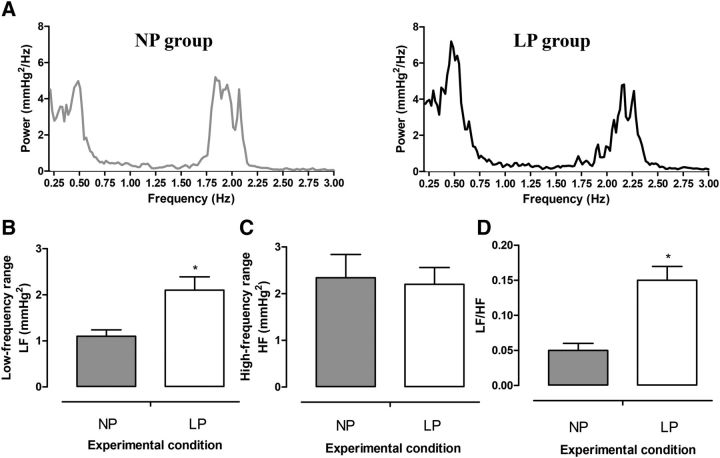
Representative baseline and chemoreflex-evoked changes in MAP, HR, and Rf of unanesthetized rats at 30 d of age are shown in Supplemental Figures 1 and 2. The baseline MAP (P = 0.83; Figure 1A) and HR (P = 0.95; Figure 1B) were similar between LP and NP rats. However, baseline Rf was higher in the LP group than in the NP group (P = 0.001; Figure 1C). Despite the similar baseline values, the autonomic modulation of arterial pressure and HR was altered in LP rats. As can be observed in the representative spectra of SAP (Figure 2A), LP rats exhibited an augmented magnitude of oscillation at the LF range (P = 0.01; Figure 2B) but not at the HF range (P = 0.75; Figure 2C) compared with NP rats. In relation to pulse interval, the LF:HF ratio (an index of sympathetic:parasympathetic balance to the heart) was enhanced in the LP group (P = 0.001; Figure 2D).
FIGURE 1.
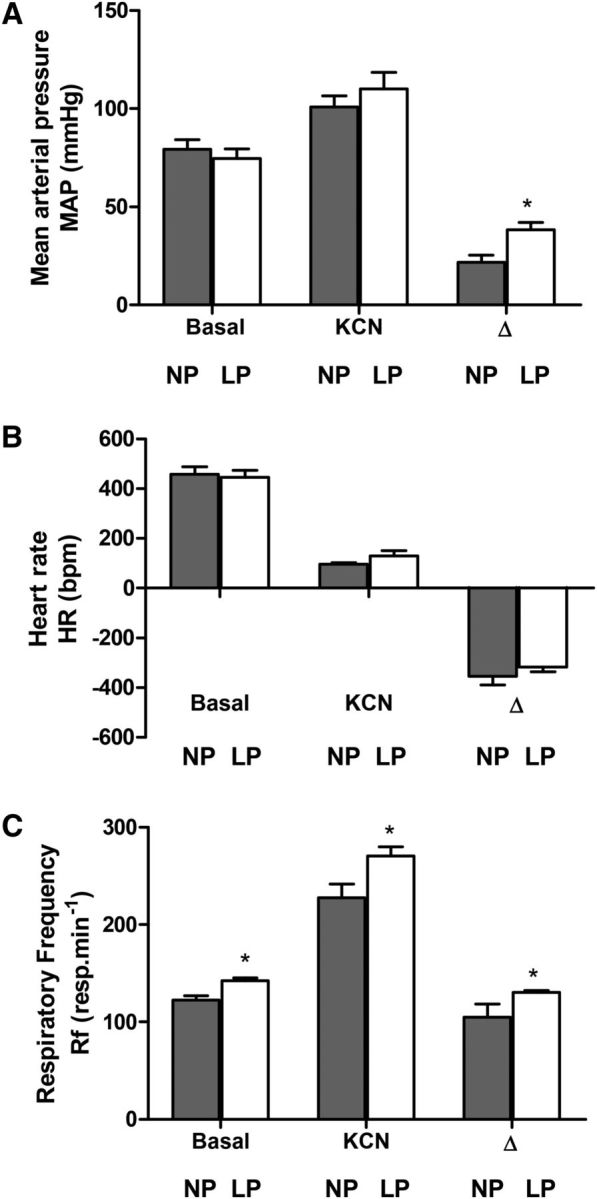
MAP (A), HR (B), and Rf (C) of 30-d-old male rat pups of dams fed an NP or an LP diet during pregnancy and lactation. Values are means ± SEMs, n = 8–11. *Different from NP, P < 0.05 (unpaired Student's t test). bpm, beats per minute; HR, heart rate, KCN, potassium cyanide; LP, offspring of experimental rat dams fed a low-protein diet (8% protein); MAP, mean arterial pressure; NP, offspring of control rat dams fed a normoproteic diet (17% protein); resp, respirations; Rf, respiratory frequency.
FIGURE 2.
Representative spectra of SAP (A), average magnitudes of LF (B) and HF (C) components of SAP, and the LF/HF index of PIs (D) of 30-d-old male rat pups of dams fed an NP or an LP diet during pregnancy and lactation. Values are means ± SEMs, n = 8–11. *Different from NP, P < 0.05 (unpaired Student's t test). HF, high-frequency band; LF, low-frequency band; LF/HF, index of sympathetic/parasympathetic balance to the heart; LP, offspring of experimental rat dams fed a low-protein diet (8% protein); NP, offspring of control rat dams fed a normoproteic diet (17% protein); PI, pulse interval; SAP, systolic arterial pressure.
Peripheral chemoreflex activation with KCN (intravenous) produced pressor, bradycardic, and tachypnoeic responses in both NP and LP groups. The increase in MAP (P = 0.01; Figure 1A) and Rf (P = 0.03; Figure 1C) were significantly greater in the LP group than in the NP group. In contrast, the magnitude of decrease in HR of both groups were similar (P = 0.32; Figure 1B).
tSN and PN activities in situ.
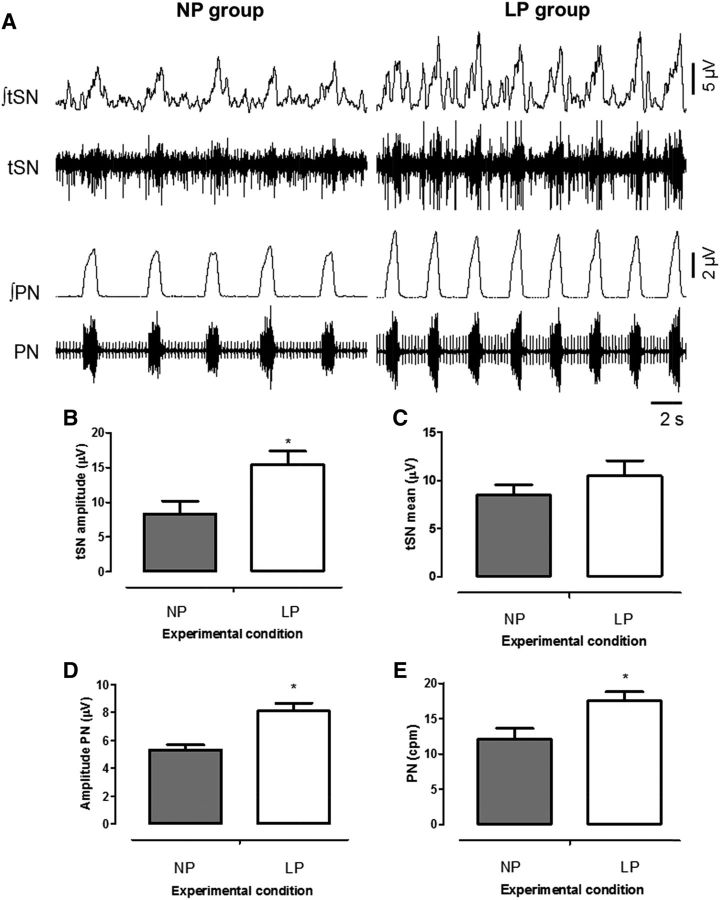
Baseline recordings of the PN and tSN activities of representative 30-d-old rats are shown in Figure 3A. The LP rats presented higher levels of tSN amplitude than did NP rats (P = 0.02; Figure 3B). Average tSN levels were not significantly different between groups (P = 0.35; Figure 3C). Furthermore, LP rats exhibited a larger PN burst frequency (P = 0.01; Figure 3E) and amplitude (P = 0.001; Figure 3D) in comparison to NP rats. The LP rats also showed shorter inspiratory (NP vs. LP: 1.4 ± 0.1 vs. 1.1 ± 0.1 s; P = 0.04) and expiratory (NP vs. LP: 4.3 ± 0.6 vs. LP: 2.6 ± 0.2 s; P = 0.01) times compared with the NP group. Together, these findings showed augmented sympathetic and inspiratory motor activities for LP rats at baseline conditions.
FIGURE 3.
Representative tracings showing raw and integrated PN and tSN activities (A), average of baseline tSN amplitude (B), tSN mean (C), PN amplitude (D), and PN mean (E) for 30-d-old male rat pups of dams fed an NP or an LP diet during pregnancy and lactation. Values are means ± SEMs, n = 6–8. *Different from NP, P < 0.05 (unpaired Student's t test). cpm, cycles per minute; LP, offspring of experimental rat dams fed a low-protein diet (8% protein); NP, offspring of control rat dams fed a normoproteic diet (17% protein); PN, phrenic nerve; tSN, thoracic sympathetic nerve; ∫, integrated activity.
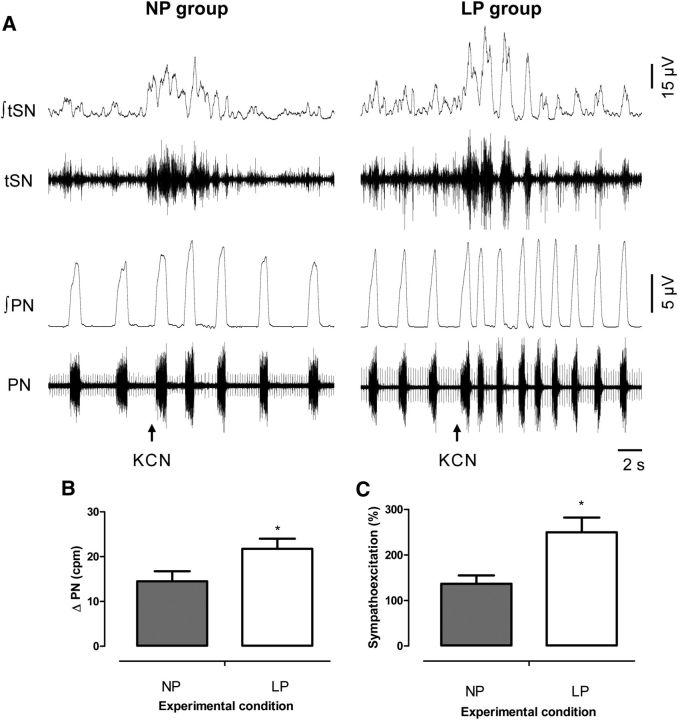
The activation of peripheral chemoreceptors elicited responses of sympatho-excitation and increased PN burst frequency in the in situ preparations of 30-d-old rats from the NP and LP groups, as shown in Figure 4A. We verified that LP rats exhibited greater increases in PN burst frequency (P = 0.04; Figure 4B) and higher sympatho-excitatory responses (P = 0.01; Figure 4C) than did NP rats.
FIGURE 4.
Representative tracings showing raw and integrated PN and tSN activities during peripheral chemoreflex activation (A) and averages of percentage tSN (B) and PN (C) during peripheral chemoreflex activation in 30-d-old male rat pups of dams fed an NP or an LP diet during pregnancy and lactation. Values are means ± SEMs, n = 6–8. *Different from NP, P < 0.05 (unpaired Student's t test). cpm, cycles per minute; KCN, potassium cyanide; LP, offspring of experimental rat dams fed a low-protein diet (8% protein); NP, offspring of control rat dams fed a normoproteic diet (17% protein); PN, phrenic nerve; tSN, thoracic sympathetic nerve; ∫, integrated activity.
HIF-1α expression in carotid bifurcation.
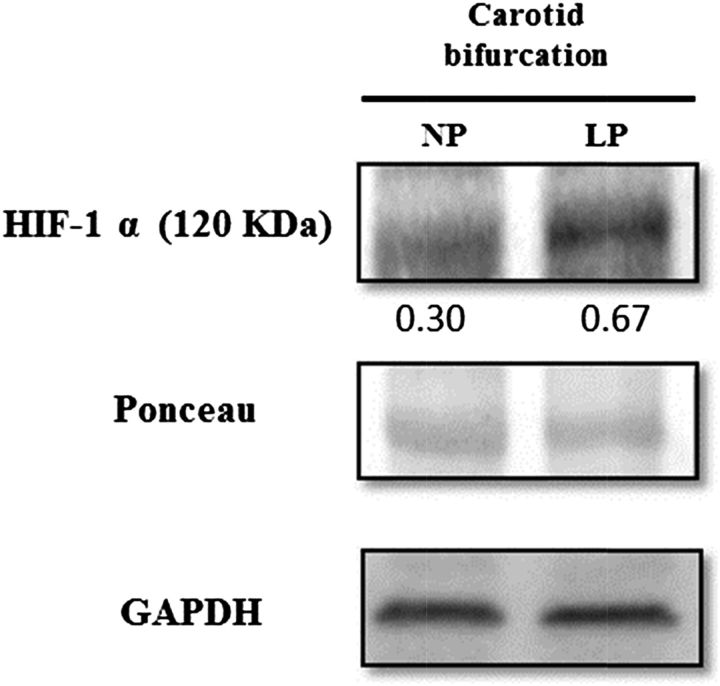
Bands corresponding to HIF-1α were observed at 120 kDa in samples of carotid bifurcations from NP and LP rats. HIF-1α protein density, which was normalized by GAPDH density, was 119% higher (NP vs. LP: 0.30 vs. 0.67 arbitrary units) in carotid bifurcation samples from LP rats compared with the samples from NP rats, as shown in Figure 5.
FIGURE 5.
Western blot assay for expression of HIF-1α showing results presented to confirm equal loading of the protein samples. Relative densities of HIF-1α were normalized by the respective amounts of Ponceau S red in 30-d-old male rat pups of dams fed an NP or an LP diet during pregnancy and lactation. HIF-1α, hypoxia-inducible factor 1α LP, offspring of experimental rat dams fed a low-protein diet (8% protein); NP, offspring of control rat dams fed a normoproteic diet (17% protein).
Discussion
In the present study, we investigated the effects of perinatal protein restriction on baseline and chemoreflex-evoked control of arterial pressure, Rf, sympathetic and phrenic activities, and HIF-1α expression in the carotid bodies. The main findings of this study showed that protein restriction during perinatal development produced in 30-d-old rats 1) increased baseline ventilation, as evidenced by greater respiratory frequency in vivo and higher phrenic burst frequency and amplitude in situ; 2) showed normal arterial pressure but augmented sympathetic activity at rest, as indicated by enhanced LF modulation of arterial pressure in unanesthetized rats and elevated levels of thoracic sympathetic activity in in situ preparations; and 3) amplified sympatho-excitatory and tachypnoeic responses to peripheral chemoreceptor stimulation combined with enhanced HIF-1α expression in the carotid bodies, suggesting a sensitization of the carotid peripheral chemoreceptors. Altogether, our data bring new insights into the etiologic mechanisms underlying the development of arterial hypertension in protein-restricted rats, highlighting a critical role of the sympathetic nervous system and peripheral chemoreceptors.
Although a relation between maternal protein restriction during pregnancy and lactation and the development of arterial hypertension in offspring during adult life has been previously described (6, 7, 29, 30), its underlying mechanisms are poorly understood. We recently reported that rat offspring subjected to protein undernutrition during pregnancy and lactation exhibited reduced ponderal gain and higher baseline arterial pressure at 90 d but not at 30 d of age. Despite the lack of augmented baseline arterial pressure, 30-d-old rats subjected to a perinatal LP diet exhibited an increased baseline respiratory frequency and enhanced respiratory responses to hypoxia and hypercapnia, suggesting that changes in the mechanisms controlling respiratory motor activity and/or increased oxygen/carbon dioxide chemosensitivity occur before the development of hypertension (7). Accordingly, in the present study, juvenile offspring from protein-restricted dams showed increased Rf in both the conscious state and in the in situ preparation. It was clearly shown that protein-restricted rats exhibited higher PN burst frequency and amplitude, indicating an increased central inspiratory activity at rest. Therefore, our data strongly support the notion that an LP diet during gestation and lactation elicits changes in the functioning of the central respiratory pattern and rhythm generator that precede the development of arterial hypertension.
Although arterial pressures were similar between the NP and LP groups at 30 d old, our data indicate that the sympathetic vasoconstrictor tonus of LP rats is enhanced. In unanesthetized rats, we verified that the variability at an LF range of SAP and pulse inteval, which are correlated with sympathetic drive to blood vessels and to the heart (23, 31), was significantly enhanced in the LP group. These observations suggest that maternal protein restriction can lead to augmented sympathetic modulation of the cardiovascular system in juvenile offspring. We also verified that in situ preparation of LP rats presented increased levels of sympathetic nerve activity, supporting the notion that baseline sympathetic activity is indeed elevated in rats subjected to an LP diet during the perinatal period. This higher level of sympathetic activity observed before the onset of hypertension in LP rats suggests that dysfunction of the sympathetic nervous system, culminating in enhanced baseline sympathetic activity, contributes to the development of hypertension in these rats. These findings are in agreement with those observed in spontaneously hypertensive rats, which exhibit an elevated sympathetic activity in early life, just before the development of arterial hypertension (32, 33).
The role of the sympathetic nervous system in the generation of neurogenic hypertension has been convincingly supported with different experimental models (22, 32, 34–36). Because high levels of tSN activity in protein-restricted rats were associated with elevated Rf and increased PN burst frequency and amplitude, we hypothesize that the increased sympathetic drive of LP rats is linked to the increased baseline inspiratory activity. It is well established that the respiratory system markedly modulates sympathetic nerve discharge at rest (27, 34, 35, 37), introducing phasic bursts in sympathetic activity, mainly during the inspiratory/postinspiratory phases (27, 28, 35, 38). This respiratory modulation of sympathetic activity is consequent to synaptic interactions between respiratory and sympathetic neurons of the brainstem (37, 39–41). Presympathetic neurons that exhibit an inspiratory-modulated pattern of activity with increased frequency of discharge during inspiration have been identified within the rostral ventrolateral medulla (41).
Therefore, because baseline central inspiratory activity is enhanced in protein-restricted rats, we theorize that perinatal protein restriction increases the activity of inspiratory neurons that, in turn, send excitatory inputs to presympathetic neurons of the rostral ventrolateral medulla, thereby enhancing sympathetic activity primarily during inspiration (41). However, this hypothesis still requires further experimental verification.
In addition to baseline cardiorespiratory changes, the sympathetic and inspiratory reflex responses to peripheral chemoreceptor stimulation were also amplified in LP rats. These data are in agreement with our previous observation that the ventilatory responses to hypoxia are enhanced in unanesthetized LP rats at the same age (7), suggesting that peripheral chemoreflex is sensitized in rats subjected to perinatal protein restriction. In agreement with this hypothesis, we verified that the expression of HIF-1α is enhanced in the carotid bodies of these rats, revealing that this transcriptional factor, which is related to the response of cell to reduced oxygen and energy availabilities (42–44), may be involved in the adaptive responses elicited by protein restriction during the perinatal period (45) and mediates, at least in part, the cardiorespiratory changes observed in protein-restricted rats.
Recent experimental and clinical evidence indicates that dysfunction of carotid body chemoreceptors plays a critical role in the progression of cardiorespiratory morbidities associated with baseline sympathetic overactivity, including sleep disorders, congestive heart failure, chronic pulmonary obstructive disease, and hypertension (9, 12, 13, 27, 46–48). In agreement with this notion, we hypothesize that the enhanced baseline sympathetic and inspiratory motor activities of juvenile offspring from dams subjected to protein restriction during pregnancy and lactation, before the development of hypertension, are dependent, at least in part, on the sensitization of the peripheral chemoreflex. These represent new insights into the mechanisms underlying the arterial blood pressure control of rats that have undergone perinatal protein restriction.
In conclusion, the present study suggests that juvenile offspring from protein-restricted dams exhibit enhanced baseline sympathetic and inspiratory motor activities combined with amplified ventilatory and autonomic responses to peripheral chemoreflex activation before the establishment of hypertension. These changes are apparently associated with a high HIF-1α concentration in carotid body peripheral chemoreceptors. These findings can aid in understanding why blood pressure increases in individuals subjected to protein undernutrition during a critical period of life.
Supplementary Material
Representative tracing of pulsatile arterial pressure (PAP),mean arterial pressure (MAP) and heart rate (HR) at rest and during peripheral chemoreflex activation (KCN, 0.04%) of 30-day-old rats from mothers subjectedto a normoproteic diet (NP, 17% of protein) or low protein diet (LP, 8% of protein) during pregnancy and lactation.
Representative tracing of ventilation before and after peripheral chemoreflex activation (KCN, 0.04%) of 30-day-old rats from mothers subjected to a normoproteic diet (NP, 17% of protein) or low protein diet (LP, 8% of protein) during pregnancy and lactation.
Acknowledgments
JLdBA, CGL, DBZ, and JHC-S designed the research; JLdBA, VON, MPCN, DBZ, and JHC-S conducted the research; JLdBA, VON, MPCN, AML, CC, DSAC, EC, AGW, CGL, DBZ, and JHC-S analyzed the data and performed the statistical analysis; and JLdBA, DBZ, and JHC-S had primary responsibility for the final content. All authors read and approved the final manuscript.
Abbreviations
- HF
high frequency
- HIF
hypoxia-inducible factor
- HR
heart rate
- KCN
potassium cyanide
- LF
low frequency
- LP
low protein
- MAP
mean arterial pressure
- NP
normal protein
- PN
phrenic nerve
- Rf
respiratory frequency
- SAP
systolic arterial pressure
- tSN
thoracic sympathetic nerve
Footnotes
Supported by the Pernambuco Research Foundation (FACEPE; grant 1365-2.07/10), National Counsel of Technological and Scientific Development (CNPQ; grants 484452/2011-8 and 478640/2013-7), and São Paulo Research Foundation (FAPESP; grants 2009/54888-7 and 2011/20040-1).
References
- 1. Hedner T, Kjeldsen SE, Narkiewicz K. State of global health–hypertension burden and control. Blood Press 2012;21(Suppl 1):1–2. [DOI] [PubMed] [Google Scholar]
- 2. Gluckman PD, Hanson MA. The developmental origins of the metabolic syndrome. Trends Endocrinol Metab 2004;15:183–7. [DOI] [PubMed] [Google Scholar]
- 3. Jia Y, Cong R, Li R, Yang X, Sun Q, Parvizi N, Zhao R. Maternal low-protein diet induces gender-dependent changes in epigenetic regulation of the glucose-6-phosphatase gene in newborn piglet liver. J Nutr 2012;142:1659–65. [DOI] [PubMed] [Google Scholar]
- 4. Barker DJ, Bull AR, Osmond C, Simmonds SJ. Fetal and placental size and risk of hypertension in adult life. BMJ 1990;301:259–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Fowden AL, Giussani DA, Forhead AJ. Intrauterine programming of physiological systems: causes and consequences. Physiology (Bethesda) 2006;21:29–37. [DOI] [PubMed] [Google Scholar]
- 6. Langley-Evans SC, Welham SJ, Jackson AA. Fetal exposure to a maternal low protein diet impairs nephrogenesis and promotes hypertension in the rat. Life Sci 1999;64:965–74. [DOI] [PubMed] [Google Scholar]
- 7. de Brito Alves JL, Nogueira VO, de Oliveira GB, da Silva GS, Wanderley AG, Leandro CG, Costa-Silva JH. Short- and long-term effects of a maternal low-protein diet on ventilation, O2/CO2 chemoreception and arterial blood pressure in male rat offspring. Br J Nutr 2014;111:606–15. [DOI] [PubMed] [Google Scholar]
- 8. Langley-Evans SC, Gardner DS, Jackson AA. Maternal protein restriction influences the programming of the rat hypothalamic-pituitary-adrenal axis. J Nutr 1996;126:1578–85. [DOI] [PubMed] [Google Scholar]
- 9. Gomide JM, de Menezes RC, Fernandes LG, Silva FC, Cardoso LM, Miranda PH, da Silva LG Jr, Lima MP, Pesquero JL, Foureaux G, et al. Increased activity of the renin-angiotensin and sympathetic nervous systems is required for regulation of the blood pressure in rats fed a low-protein diet. Exp Physiol 2013;98:57–66. [DOI] [PubMed] [Google Scholar]
- 10. Barros MA, de Brito Alves JL, Nogueira VO, Wanderley AG, Costa-Silva JH. Maternal low-protein diet induces changes in the cardiovascular autonomic modulation in male rat offspring. NMCD 2015;25:123–30. [DOI] [PubMed] [Google Scholar]
- 11. Franchini KG, Krieger EM. Cardiovascular responses of conscious rats to carotid body chemoreceptor stimulation by intravenous KCN. J Auton Nerv Syst 1993;42:63–9. [DOI] [PubMed] [Google Scholar]
- 12. McBryde FD, Abdala AP, Hendy EB, Pijacka W, Marvar P, Moraes DJ, Sobotka PA, Paton JF. The carotid body as a putative therapeutic target for the treatment of neurogenic hypertension. Nat Commun 2013;4:2395. [DOI] [PubMed] [Google Scholar]
- 13. Abdala AP, McBryde FD, Marina N, Hendy EB, Engelman ZJ, Fudim M, Sobotka PA, Gourine AV, Paton JF. Hypertension is critically dependent on the carotid body input in the spontaneously hypertensive rat. J Physiol 2012;590:4269–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Nanduri J, Vaddi DR, Khan SA, Wang N, Makerenko V, Prabhakar NR. Xanthine oxidase mediates hypoxia-inducible factor-2alpha degradation by intermittent hypoxia. PLoS ONE 2013;8:e75838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Semenza GL. Oxygen homeostasis. Wiley Interdiscip Rev Syst Biol Med. 2010;2:336–61. [DOI] [PubMed] [Google Scholar]
- 16. Ito T, Funamoto K, Sato N, Nakamura A, Tanabe K, Hoshiai T, Suenaga K, Sugawara J, Nagase S, Okamura K, et al. Maternal undernutrition induces the expression of hypoxia-related genes in the fetal brain. Tohoku J Exp Med 2012;226:37–44. [DOI] [PubMed] [Google Scholar]
- 17. Lee SH, Wolf PL, Escudero R, Deutsch R, Jamieson SW, Thistlethwaite PA. Early expression of angiogenesis factors in acute myocardial ischemia and infarction. N Engl J Med 2000;342:626–33. [DOI] [PubMed] [Google Scholar]
- 18. Reeves PG. Components of the AIN-93 diets as improvements in the AIN-76A diet. J Nutr 1997;127(Suppl):838S–41S. [DOI] [PubMed] [Google Scholar]
- 19. Reeves PG, Nielsen FH, Fahey GC Jr. AIN-93 purified diets for laboratory rodents: final report of the American Institute of Nutrition ad hoc writing committee on the reformulation of the AIN-76A rodent diet. J Nutr 1993;123:1939–51. [DOI] [PubMed] [Google Scholar]
- 20. Malan A. Ventilation measured by body plethysmography in hibernating mammals and in poikilotherms. Respir Physiol 1973;17:32–44. [DOI] [PubMed] [Google Scholar]
- 21. Machado BH, Bonagamba LG. Antagonism of glutamate receptors in the intermediate and caudal NTS of awake rats produced no changes in the hypertensive response to chemoreflex activation. Auton Neurosci 2005;117:25–32. [DOI] [PubMed] [Google Scholar]
- 22. Zoccal DB, Bonagamba LG, Paton JF, Machado BH. Sympathetic-mediated hypertension of awake juvenile rats submitted to chronic intermittent hypoxia is not linked to baroreflex dysfunction. Exp Physiol 2009;94:972–83. [DOI] [PubMed] [Google Scholar]
- 23. Malliani A, Pagani M, Lombardi F, Cerutti S. Cardiovascular neural regulation explored in the frequency domain. Circulation 1991;84:482–92. [DOI] [PubMed] [Google Scholar]
- 24. Bernardi L, Porta C, Gabutti A, Spicuzza L, Sleight P. Modulatory effects of respiration. Auton Neurosci 2001;90:47–56. [DOI] [PubMed] [Google Scholar]
- 25. Tezini GC, Dias DP, Souza HC. Aerobic physical training has little effect on cardiovascular autonomic control in aging rats subjected to early menopause. Exp Gerontol 2013;48:147–53. [DOI] [PubMed] [Google Scholar]
- 26. Cerutti C, Gustin MP, Paultre CZ, Lo M, Julien C, Vincent M, Sassard J. Autonomic nervous system and cardiovascular variability in rats: a spectral analysis approach. Am J Physiol 1991;261:H1292–9. [DOI] [PubMed] [Google Scholar]
- 27. Zoccal DB, Simms AE, Bonagamba LG, Braga VA, Pickering AE, Paton JF, Machado BH. Increased sympathetic outflow in juvenile rats submitted to chronic intermittent hypoxia correlates with enhanced expiratory activity. J Physiol 2008;586:3253–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Costa-Silva JH, Zoccal DB, Machado BH. Glutamatergic antagonism in the NTS decreases post-inspiratory drive and changes phrenic and sympathetic coupling during chemoreflex activation. J Neurophysiol 2010;103:2095–106. [DOI] [PubMed] [Google Scholar]
- 29. Elmes MJ, Gardner DS, Langley-Evans SC. Fetal exposure to a maternal low-protein diet is associated with altered left ventricular pressure response to ischaemia-reperfusion injury. Br J Nutr 2007;98:93–100. [DOI] [PubMed] [Google Scholar]
- 30. Bertram C, Khan O, Ohri S, Phillips DI, Matthews SG, Hanson MA. Transgenerational effects of prenatal nutrient restriction on cardiovascular and hypothalamic-pituitary-adrenal function. J Physiol 2008;586:2217–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Oliveira LR, de Melo VU, Macedo FN, Barreto AS, Badaue-Passos D Jr, Viana dos Santos MR, Dias DP, Sluka KA, DeSantana JM, Santana-Filho VJ. Induction of chronic non-inflammatory widespread pain increases cardiac sympathetic modulation in rats. Auton Neurosci 2012;167:45–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Simms AE, Paton JF, Pickering AE, Allen AM. Amplified respiratory-sympathetic coupling in the spontaneously hypertensive rat: does it contribute to hypertension? J Physiol 2009;587:597–610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Simms AE, Paton JF, Allen AM, Pickering AE. Is augmented central respiratory-sympathetic coupling involved in the generation of hypertension? Respir Physiol Neurobiol 2010;174:89–97. [DOI] [PubMed] [Google Scholar]
- 34. Dick TE, Hsieh YH, Morrison S, Coles SK, Prabhakar N. Entrainment pattern between sympathetic and phrenic nerve activities in the Sprague-Dawley rat: hypoxia-evoked sympathetic activity during expiration. Am J Physiol Regul Integr Comp Physiol 2004;286:R1121–8. [DOI] [PubMed] [Google Scholar]
- 35. Malpas SC. The rhythmicity of sympathetic nerve activity. Prog Neurobiol 1998;56:65–96. [DOI] [PubMed] [Google Scholar]
- 36. Toney GM, Pedrino GR, Fink GD, Osborn JW. Does enhanced respiratory-sympathetic coupling contribute to peripheral neural mechanisms of angiotensin II-salt hypertension? Exp Physiol 2010;95:587–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Haselton JR, Guyenet PG. Central respiratory modulation of medullary sympathoexcitatory neurons in rat. Am J Physiol 1989;256:R739–50. [DOI] [PubMed] [Google Scholar]
- 38. Gilbey MP, Numao Y, Spyer KM. Discharge patterns of cervical sympathetic preganglionic neurones related to central respiratory drive in the rat. J Physiol 1986;378:253–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Mandel DA, Schreihofer AM. Central respiratory modulation of barosensitive neurones in rat caudal ventrolateral medulla. J Physiol 2006;572:881–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Moraes DJ, Bonagamba LG, Costa KM, Costa-Silva JH, Zoccal DB, Machado BH. Short-term sustained hypoxia induces changes in the coupling of sympathetic and respiratory activities in rats. J Physiol 2014;592:2013–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Moraes DJ, da Silva MP, Bonagamba LG, Mecawi AS, Zoccal DB, Antunes-Rodrigues J, Varanda WA, Machado BH. Electrophysiological properties of rostral ventrolateral medulla presympathetic neurons modulated by the respiratory network in rats. J Neurosci 2013;33:19223–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Yuan G, Peng YJ, Reddy VD, Makarenko VV, Nanduri J, Khan SA, Garcia JA, Kumar GK, Semenza GL, Prabhakar NR. Mutual antagonism between hypoxia-inducible factors 1alpha and 2alpha regulates oxygen sensing and cardio-respiratory homeostasis. Proc Natl Acad Sci USA 2013;110:E1788–96. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 43. Manalo DJ, Rowan A, Lavoie T, Natarajan L, Kelly BD, Ye SQ, Garcia JG, Semenza GL. Transcriptional regulation of vascular endothelial cell responses to hypoxia by HIF-1. Blood 2005;105:659–69. [DOI] [PubMed] [Google Scholar]
- 44. Prabhakar NR. Sensing hypoxia: physiology, genetics and epigenetics. J Physiol 2013;591:2245–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Ito T, Tanabe K, Nakamura A, Funamoto K, Aoyagi A, Sato K, Hoshiai T, Suenaga K, Sugawara J, Nagase S, et al. Aberrant expression of hypoxia-inducible factor 1alpha in the fetal heart is associated with maternal undernutrition. Tohoku J Exp Med 2011;224:163–71. [DOI] [PubMed] [Google Scholar]
- 46. Costa-Silva JH, Zoccal DB, Machado BH. Chronic intermittent hypoxia alters glutamatergic control of sympathetic and respiratory activities in the commissural NTS of rats. Am J Physiol Regul Integr Comp Physiol 2012;302:R785–93. [DOI] [PubMed] [Google Scholar]
- 47. Silva AQ, Schreihofer AM. Altered sympathetic reflexes and vascular reactivity in rats after exposure to chronic intermittent hypoxia. J Physiol 2011;589:1463–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Marcus NJ, Del Rio R, Schultz EP, Xia XH, Schultz HD. Carotid body denervation improves autonomic and cardiac function and attenuates disordered breathing in congestive heart failure. J Physiol 2014;592:391–408. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Representative tracing of pulsatile arterial pressure (PAP),mean arterial pressure (MAP) and heart rate (HR) at rest and during peripheral chemoreflex activation (KCN, 0.04%) of 30-day-old rats from mothers subjectedto a normoproteic diet (NP, 17% of protein) or low protein diet (LP, 8% of protein) during pregnancy and lactation.
Representative tracing of ventilation before and after peripheral chemoreflex activation (KCN, 0.04%) of 30-day-old rats from mothers subjected to a normoproteic diet (NP, 17% of protein) or low protein diet (LP, 8% of protein) during pregnancy and lactation.