Abstract
Previously several functional magnetic resonance imaging (fMRI) studies point toward the role of perceptual expectations in determining adaptation or repetition suppression (RS) in humans. These studies showed that the probability of repetitions of faces within a block influences the magnitude of adaptation in face-related areas of the human brain (Summerfield et al., 2008). However, a current macaque single-cell/local field potential (LFP) recording study using objects as stimuli found no evidence for the modulation of the neural response by the repetition probability in the inferior temporal cortex (Kaliukhovich and Vogels, 2010). Here we examined whether stimulus repetition probability affects fMRI repetition suppression for nonface object stimuli in the human brain. Subjects were exposed to either two identical [repetition trials (RTs)] or two different [alternation trials (ATs)] object stimuli. Both types of trials were presented blocks consisting of either 75% [repetition blocks (RBs)] or 25% [alternation blocks (ABs)] of RTs. We found strong RS, i.e., a lower signal for RTs compared to ATs, in the object sensitive lateral occipital cortex as well as in the face-sensitive occipital and fusiform face areas. More importantly, however, there was no significant difference in the magnitude of RS between RBs and ABs in each of the areas. This is in agreement with the previous monkey single-unit/LFP findings and suggests that RS in the case of nonface visual objects is not modulated by the repetition probability in humans. Our results imply that perceptual expectation effects vary for different visual stimulus categories.
Introduction
In functional magnetic resonance imaging (fMRI) experiments stimulus repetition leads to the attenuation of the blood oxygenation level-dependent (BOLD) signal when compared to alternating stimuli (Henson and Rugg, 2003), a phenomenon called fMRI adaptation (fMRIa), which is currently considered as the neuroimaging equivalent of the neural repetition suppression (RS) that is observed in extracellular single-cell recording experiments (Gross et al. 1967, 1969). While different variations of adaptation paradigms are used widely (Krekelberg et al., 2006) (for review, see Grill-Spector et al., 2006), the neural mechanisms of the effect are still unclear.
One explanation suggests that RS is related to the local alteration of the synaptic inputs/spike frequency of the neurons (Priebe et al., 2002; Kohn and Movshon, 2003; Sawamura et al., 2006; De Baene and Vogels, 2010). Alternatively, adaptation has been related to predictive coding (PC) (Rao and Ballard, 1999): repeating a stimulus leads to its increased expectation or a reduction of the prediction error (i.e., the neural activity signaling the mismatch between top-down and bottom-up events), leading to RS. Indeed, Summerfield et al. (2008) showed that the probability of stimulus repetition determines the degree of RS: fMRIa was significantly larger in the fusiform face area (FFA) in blocks with a high probability of face repetitions than in blocks where repetitions were less frequent. Later, Larsson and Smith (2012) also found that expectation influences fMRIa in several visual areas, provided the subjects attended the face stimuli. Kovács et al. (2012), using faces, found that repetition probability influenced fMRIa equally for overlapping and nonoverlapping peripheral stimulus arrangements in the FFA and the occipital face area (OFA), and tended to do so in the lateral occipital cortex (LO). A previous dynamic causal modeling study (Friston et al., 2003) suggested that changes of the top-down connections play a critical role in RS within the body-sensitive network as well (Ewbank et al., 2011).
However, the role of stimulus expectations in generating RS is questioned by a previous study of the macaque inferior temporal cortex (IT). Kaliukhovich and Vogels (2010) used the same paradigm as Summerfield et al. (2008) and measured spiking activity and local field potentials (LFPs) for natural stimuli and fractal patterns. Unlike for the human fMRI studies, the RS was not modulated by repetition probability for either neural measure. Theoretically, it is possible that the opposite results of the fMRIa and macaque IT experiments are due to the different experimental species, stimulus sets, neural measures, or tasks (monkeys were performing a passive fixation task, unlike the active stimulus discrimination tasks in the human fMRI studies). Hence, we combined the methods, paradigm, and task of Summerfield et al. (2008) with the stimuli of Kaliukhovich and Vogels (2010) in human subjects and tested whether the repetition probability of nonface stimuli affects fMRIa in LO, a proposed homolog of the macaque IT (Denys et al., 2004) as well as in OFA and FFA.
Materials and Methods
Subjects.
Eleven healthy university students participated in the first experiment (8 females; mean age, 24.4 years; SD, 3.6 years) and another 11 subjects took part in the second experiment (10 females; mean age, 22.9 years; SD, 3.5 years; one subject was excluded from all analyses due to excessive head movements). All participants had normal or corrected-to-normal vision, and they provided their written consent in accordance with the protocols approved by the Ethical Committee of the University of Regensburg.
Stimulation and procedure.
Stimuli of the first experiment were identical to the natural images in the study by Kaliukhovich and Vogels (2010), except that any stimuli depicting human or animal faces, bodies, or body parts were removed from the stimulus pool (for stimulus examples, see Fig. 1C, top). Stimuli of the second experiment were images of chairs, downloaded from the public domain of the worldwide web. To minimize the interstimulus variance, we selected only full-front images of chairs (for random stimulus examples, see Fig. 1C, middle). Images were converted into grayscale, isolated from their original background, resized, and matched in luminance to the average luminance of the object stimuli.
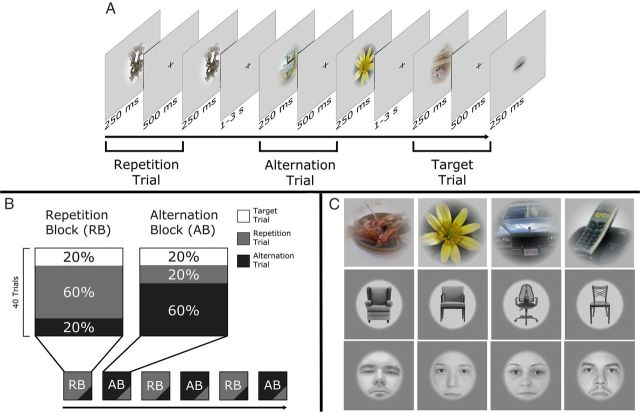
Figure 1.
A, Stimulation parameters and arrangements. A repetition trial (gray), an alternation trail (black), and a target trial (white). B, The composition of the repetition and alternation blocks. During a run, RBs and ABs were given randomly, each repeated three times. C, Random sample stimuli (objects, Experiment 1; chairs and faces, Experiment 2).
Altogether, we used 360 images of objects and 360 images of chairs (mean luminance, 18 cd/m2; radius, 5.5°), displayed centrally on a gray background.
The face stimuli (Fig. 1C, bottom) of the second experiment were identical to those of our previous study (Kovács et al., 2012) (360 full-front grayscale faces fit behind a circular mask; 50% female).
Stimuli were backprojected via an LCD video projector (JVC; DLA-G20; 72 Hz, 800 × 600 resolution) onto a translucent circular screen (diameter, 30°) placed inside the scanner bore at 63 cm from the observer. Stimulus presentation was controlled via Matlab (MathWorks), using Psychtoolbox (version 3.0.8). The stimulation paradigm and the structure of trials and blocks were identical to those used by Kovács et al. (2012) and Summerfield et al. (2008) with the exception of the number of trials per block, which were twice as many (40) in our study compared to (20) in the study by Summerfield et al. (2008). Note that this increase in the number of trials per block can, if anything, only increase the repetition probability effect. Stimuli were presented foveally for 250 ms each, pairwise, separated by a 500 ms interstimulus interval and followed by a 1- to 3-s-long intertrial interval (Fig. 1A). The first object (S1) could either be identical to [repetition trials (RTs)] or different from [alternation trials (ATs)] the second object (S2). To minimize spatially local feature adaptation and apparent motion cues, the size of S1 or S2 was reduced by 18% (radius, 4.5°). One trial lasted 1 s in each condition. Novel object images were presented on each trial.
Two different types of blocks were given to the subjects, each repeated three times within a single run (240 trials). The blocks (40 trials each) were separated from each other by a 4-s-long pause during which the words “New Block” appeared centrally on a black background. In the repetition blocks (RBs), 60% of the trials were RTs, while 20% were ATs. In the alternation blocks (ABs), 60% were ATs, and 20% were RTs. The remaining 20% were in both blocks target trials. With the exception of the first two trials of a block, which were always the more frequent trials of that specific block (RTs in RBs and ATs in ABs), RTs and ATs were mixed randomly within a block. The order of the blocks was randomized across subjects. The participant's task was to maintain central fixation throughout a trial and to signal the occurrence of a target trial, where the size difference between S1 and S2 was 55% (radius, 2.5°; smaller image was randomly assigned to S1 or S2 with equal probability), by pressing a button. Participants were briefly explained the task without mentioning different presentation probabilities of repetition and alternation trials in the two types of blocks and were given a 1 min presentation to demonstrate the size difference of the target trials before scanning. Next, three runs with object stimuli were presented one after the other during the first experiment (39 min) or two runs of chairs, followed by one run with faces in the second experiment (39 min), for a total of 720 trials. The design of the runs and the structure of the different blocks are presented schematically in Figure 1B.
Scanning parameters and data analysis.
Imaging was performed using a 3-Tesla MR head scanner (Siemens). For the functional series, we continuously acquired images (34 slices; 10° tilted relative to axial; T2*-weighted EPI sequence; TR, 2000 ms; TE, 30 ms; flip angle, 90°; 64 × 64 matrices; in-plane resolution, 3 × 3 mm; slice thickness, 3 mm). High-resolution sagittal T1-weighted images were acquired using a magnetization EPI sequence (MP-RAGE; TR, 2250 ms; TE, 2.6 ms; 1 mm isotropic voxel size) to obtain a 3D structural scan. Details of preprocessing and statistical analysis were described previously (Kovács et al., 2008, 2012; Cziraki et al., 2010). Briefly, the functional images were corrected for acquisition delay, realigned, normalized to the MNI-152 space, resampled to 2 × 2 × 2 mm resolution, and spatially smoothed with a Gaussian kernel of 8 mm FWHM (SPM8, Wellcome Department of Imaging Neuroscience, London, UK). Region of interest (ROI) analysis was based on the results of separate functional localizer runs (488 s long; 17 s epochs of faces, everyday objects, and their Fourier randomized versions interleaved with 17 s of blank periods; 2 Hz; 300 ms exposition time; 200 ms blank) and analyzed using MarsBaR 0.42 toolbox for SPM (Brett et al., 2002).
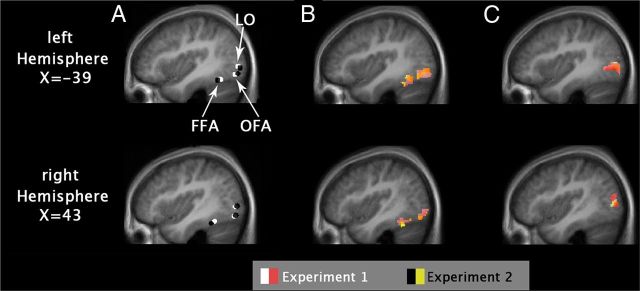
The locations of face-responsive areas (Fig. 2) were determined individually as areas responding more strongly to faces than to objects and to Fourier noise images in the functional localizer scans (puncorrected < 10−6; t = 4.86, df = 273). For the FFA in Experiment 1 (n = 11), average MNI coordinates (±SE) were as follows: left hemisphere, −39 (±1), −57 (±2), −18 (±1); right hemisphere, 40 (±1), −49 (±2), −19 (±1). In Experiment 2 (n = 10), coordinates (±SE) were as follows: left hemisphere, −38 (±1), −51 (±3), −19 (±1); right hemisphere, 39 (±1), −44 (±2), −18 (±1)]. These coordinates are very similar to those reported by Summerfield et al. (2008) for the FFA of that study and correspond to the more anterior part [FFA-2 in the study by Pinsk et al. (2009); middle fusiform gyrus in the study by Weiner and Grill-Spector (2010)] of the traditional FFA (Grill-Spector et al., 2004). For the OFA in Experiment 1 (n = 8), average MNI coordinates (±SE) were as follows: left hemisphere, −41 (±1), −77 (±1), −11 (±1); right hemisphere, 43 (±1), −76 (±2), −12 (±1). In Experiment 2 (n = 10), coordinates (±SE) were as follows: left hemisphere, −38 (±2), −80 (±3), −11 (±2); right hemisphere, 41 (±1), −78 (±2), −11 (±1). Areas selectively responding to objects were determined by functional localizer scans comparing the activity for objects versus their Fourier randomized versions and faces (puncorrected <10−6; t = 4.86; df = 273). For area LO (corresponding to the caudal–dorsal part of the lateral occipital complex) (Grill-Spector et al. 1999; Halgren et al. 1999) in Experiment 1 (n = 10), coordinates (±SE) were as follows: left hemisphere, −41 (±2), −81 (±2), 0 (±2); right hemisphere, 44 (±1), −77 (±1), 1 (±2). In Experiment 2 (n = 10), coordinates (±SE) were as follows: left hemisphere, −38 (±2), −84 (±2), −1 (±2); right hemisphere, 41 (±3), −79 (±3), 0 (±2). The ROIs were selected individually on the single-subject level from the thresholded t maps. Areas lying closest to the corresponding reference cluster (based on the results of the previous literature as well as on the results of the random-effects analysis for differential contrasts; puncorrected < 0.0001; t = 6.4) were considered as their appropriate equivalents on the single-subject level. A time series of the mean voxel value within an 4 mm radius sphere around the local maximum of the areas of interest was calculated and extracted from our event-related sessions using finite impulse response (FIR) models (Ollinger et al., 2001). The convolution of a reference hemodynamic response function with boxcars, representing the onsets and durations of the experimental conditions, was used to define the regressors for a general linear model analysis of the data.
Figure 2.
Functional region localization. A, Average location of ROIs (spheres with 4 mm radius) in Experiments 1 and 2 as identified in the individual localizer scans. B, Face-selective activation in the functional localizer runs as an overlay of Experiments 1 and 2. C, Object-selective activation in the functional localizer runs. Activation maps from B and C show group-level maps from the functional localizer runs. All maps were thresholded at p < 0.0001 (uncorrected) and overlaid with anatomical ROI masks.
RTs and ATs were analyzed and modeled at the onset of the S1 stimuli separately (duration, 1 s), following the methods of other studies (Murray and Wojciulik, 2004; Summerfield et al., 2008). Only the nontarget trials were modeled and included in the statistical analysis. The peak of the event-related averages at 6 s after stimulus onset was used as an estimate of the magnitude of the response and was averaged across observers. For the first and second experiments, we performed three-way within-subject ANOVAs on the peaks with hemisphere (2), block type (2; RB, AB), and trial type (2; RT, AT) as factors for each area. Post hoc analysis was performed by Fisher LSD test. For the second experiment we also performed a four-way within-subject ANOVA with stimulus category (2), hemisphere (2), block type (2), and trial type (2) to test the effect of stimulus category on the fMRIa as well.
Results
Experiment 1: objects
Participants detected the occurrence of target trials on average with 93.8 and 95.6% (1.0 and 1.3% ±SE) accuracy in the ABs and RBs, respectively. Informal questioning of the subjects after the experiments revealed that none of them was aware of the manipulation of the repetition probability between blocks.
fMRI results
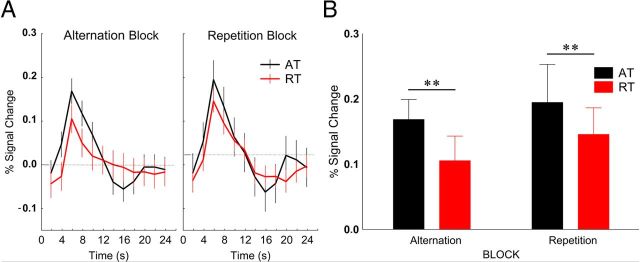
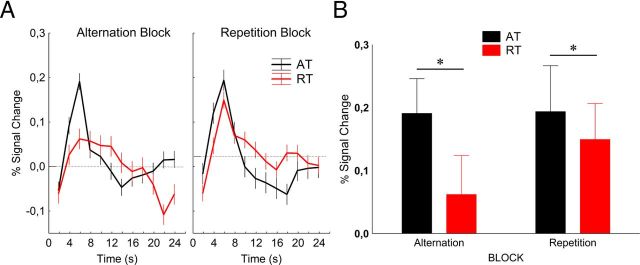
In the lateral occipital cortex (Fig. 3) we found a significant main effect of trial type (F(1,9) = 11.4, p < 0.01, ηp2 = 0.56), which was due to the larger BOLD response during ATs than during RTs [response reduction, 27.6 ± 8.3%, mean ± SE; a value similar to what was found by Summerfield et al. (2008) for the ABs in FFA]. However, neither the main effect of block (F(1,9) = 1.1, p = 0.3, ηp2 = 0.1), nor its interaction with trial type (F(1,9) = 0.1, p = 0.8, ηp2 = 0.01) proved to be significant (response reduction for ABs and RBs, 30.8 ± 10.1% and 22.3 ± 14.5%, respectively; mean ± SE). Fisher's post hoc analysis showed that neither the ATs nor the RTs differed between the two blocks (p > 0.25 for both trial types). In addition, neither the main effect of recording hemisphere nor any of its interactions were significant, implying similar effects in the two hemispheres. Differences in baseline were significant in neither block (p > 0.40). These data suggest that, just like in the case of the single-cell recordings of the macaque IT (Kaliukhovich and Vogels, 2010), repetition probability of natural objects also has no effect on the fMRIa of LO.
Figure 3.
A, Time course (mean ± SE) of fMRI activity in the lateral occipital cortex. Data were derived from an FIR model with 2 s time bins. B, Average peak activation profiles (±SE) of the LO for ATs and RTs. **p < 0.001 (Fisher's post hoc comparisons).
Despite the lower absolute signal magnitudes, essentially the same results were found for the face-sensitive areas of the occipitotemporal cortex (Fig. 4). A significant main effect of trial type was found for both the FFA (F(1,10) = 8.98, p < 0.05, ηp2 = 0.47) and OFA (F(1,7) = 6.8, p < 0.05, ηp2 = 0.49) without a significant main effect of block or its interaction with trial type [FFA, main effect of block, F(1,10) = 0.17, p = 0.7, ηp2 = 0.02; block by trial type, F(1,10) = 0.15, p = 0.7, ηp2 = 0.01; response reduction for ABs and RBs (mean ± SE), 61.6 ± 18% and 24.4 ± 18.1%, respectively; OFA, main effect of block, F(1,7) = 0.47, p = 0.5, ηp2 = 0.06; block by trial type, F(1,7) = 0.19, p = 0.67, ηp2 = 0.03; response reduction for ABs and RBs (mean ± SE), 57.3 ± 22% and 35.5 ± 21.1%, respectively].
Figure 4.
A, B, Average peak activation profiles (±SE) of the FFA (A) and OFA (B) for ATs and RTs. *p < 0.05; **p < 0.001 (Fisher's post hoc comparisons).
Experiment 2: chairs and faces
The stimuli of the Experiment 1 (Kaliukhovich and Vogels, 2010) depicted a large variety of colorful natural and man-made scenes, objects, plants, animals, and everyday objects, mostly on complex backgrounds. In contrast, all previous studies that found an effect of stimulus probability on the RS of neurons used grayscale frontal views of faces, cropped, resized, and against a uniform gray background, making the stimuli very similar to each other. Could the lack of a repetition probability modulation in Experiment 1 [and in the study by Kaliukhovich and Vogels (2010)] be explained by the large variability of these object stimuli? To test this hypothesis, we repeated the first experiment, but instead of a large variety of objects, we chose images of a single category, chairs, as stimuli (Fig. 1C). Chairs are known to activate the LO (Ishai et al., 1999) as well as the FFA (Pourtois et al., 2008; Sadeh et al., 2010), and are widely used in both neuroimaging and human electrophysiological studies as a stimulus category.
In addition, in Experiment 2 we tested whether the lack of any effect of repetition probability on the magnitude of RS is indeed due to the applied stimulus category. To this end, in a separate run we also presented face stimuli, identical to those used by Kovács et al. (2012), foveally. Besides the use of different stimuli, Experiments 1 and 2 were identical.
Similarly to Experiment 1, participants detected the occurrence of target trials, on average, with 99.1 and 98.3% (±0.3 and ±0.5% SE, respectively) accuracy in the ABs and RBs, respectively, for chair stimuli, and 96.2 and 96.6% (±1.7 and ±2.3% SE, respectively) for face stimuli. Furthermore, informal questioning of the subjects after the Experiment 2 revealed that none of them was aware of the manipulation of the repetition probability between blocks.
Comparison of chair and face stimuli
We first tested explicitly whether stimulus category (chairs vs faces) had an effect on the pattern of results using a four-way within-subject ANOVA (see Materials and Methods). In the FFA, we found a main effect of stimulus type (F(1,9) = 54.48, p < 0.001, ηp2 = 0.86), with larger responses to faces compared to chairs. Importantly, the interaction of stimulus type, block type, and trial type was significant (F(1,9) = 14.13, p = 0.004, ηp2 = 0.61), indicating that the block-type-dependent fMRIa depends on the stimulus category in the FFA. We also found a significant main effect of stimulus type in the OFA (F(1,9) = 13.22, p = 0.005, ηp2 = 0.59), with higher responses to faces than to chairs, without any significant interactions. In LO we did not find any significant effect. For the sake of easier comparison of the current and previous studies, we also analyzed the data obtained with chair and face stimuli separately.
fMRI results for chairs
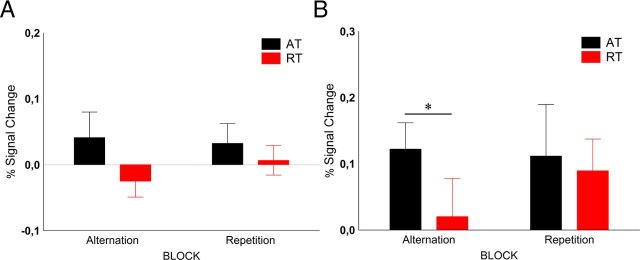
The results of Experiment 2 (Fig. 5) were very similar to those of Experiment 1. There was a significant main effect of trial type in the lateral occipital cortex (F(1,9) = 13.32, p < 0.005, ηp2 = 0.60), which was due to the larger BOLD response during ATs than during RTs (response reduction, 46.2 ± 18.3%, mean ± SE). Also, there was a main effect of block (F(1,9) = 5.50, p = 0.044, ηp2 = 0.38), indicating higher overall responses in RBs, but no interaction of block type and trial type (F(1,9) = 3.96, p = 0.1, ηp2 = 0.31) proved to be significant. Fisher's post hoc analysis showed that neither the ATs nor the RTs differed between the two blocks (p > 0.1 for both trial types). In addition, neither the main effect of recording hemisphere nor any of its interactions was significant, implying similar effects in the two hemispheres. These data confirm the results of Experiment 1 in the sense that repetition probability of natural objects has no effect on the fMRIa of LO.
Figure 5.
A, B, Time course (A) and average peak activations (B) of the LO (mean ± SE) for Experiment 2 with chair stimuli. *p < 0.05 (Fisher's post hoc comparisons).
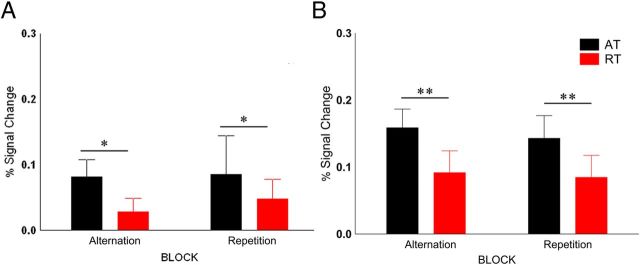
We observed low activations in the FFA (Fig. 6A) to the chair stimuli, whose peak in the ATs was nevertheless significantly different from baseline (Fisher's post hoc test for 0 vs 6 s; p = 0.005). Only the main effect of trial type was significant (F(1,9) = 8.14, p = 0.019, ηp2 = 0.47), with lower responses for RTs than for ATs (response reduction, 29.1 ± 20.8%, mean ± SE). The OFA showed a significant main effect of trial type (Fig. 6B; F(1,9) = 6.3, p = 0.034, ηp2 = 0.25) without any other significant main effect or interaction (response reduction, 49 ± 13.6%, mean ± SE), a result very similar to that of Experiment 1.
Figure 6.
A, B, Average peak activation of FFA (A) and OFA (B) for chair stimuli, respectively. *p < 0.05 (Fisher's post hoc comparisons).
fMRI results for faces
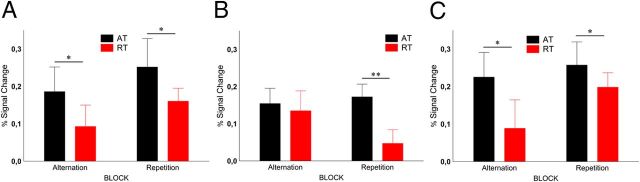
With respect to face stimuli, our results are similar to those of previous papers (Summerfield et al., 2008; Kovács et al., 2012; Larsson and Smith, 2012). For the FFA (Fig. 7A), we found significantly larger BOLD signal for ATs compared to RTs (main effect of trial type, F(1,9) = 10.22, p = 0.01, ηp2 = 0.61). The magnitude of repetition suppression, however, differed between the two blocks (block type by trial type interaction, F(1,9) = 12.45, p < 0.006, ηp2 = 0.61). RS was more pronounced in the RBs (Fisher's post hoc test, p < 0.001; response reduction, 73.1 ± 14.1%, mean ± SE) than in the ABs (Fisher's post hoc test: p = 0.38; response reduction, 18.1 ± 11.5%, mean ± SE). This effect was largely due to the fact that RTs showed lower overall responses than ATs in RBs (post hoc test for RTs vs ATs in RBs, p < 0.00005), which is similar to the results of Summerfield et al. (2008), who also reported different activations evoked by RTs in RBs and ABs. While both LO (response reduction, 20.1 ± 29.5%, mean ± SE) and OFA (response reduction, 31.1 ± 16.4%, mean ± SE) showed significant repetition suppression for faces (Fig. 7B,C; main effect of trial type, F(1,9) > 6, p < 0.05 for both areas), this effect was independent of the repetition probability (block type by trial type interaction, F(1,9) < 2.5, p > 0.16 for both areas).
Figure 7.
A–C, Average peak activation of LOC (A), FFA (B), and OFA (C) for face stimuli, respectively. *p < 0.05; **p < 0.001 (Fisher's post hoc comparisons).
Whole-brain analyses
To test whether other areas reflect the repetition probability modulation effects as well, we also performed whole-brain random-effects analyses separately for the object stimuli of Experiment 1 and for the chair and face stimuli of Experiment 2. Testing the main effect of trial type (AT vs RT) and the main effect of block type (AB vs RB) led to no significant activations in additional brain regions even at the liberal threshold of puncorrected < 0.0001. The contrast testing the interaction of trial type × block type for face stimuli [(ATAB vs RTAB) vs (ATRB vs RTRB)] led to one cluster of voxels in the left middle frontal gyrus (MNI coordinates, x = −28, y = 26, z = 52; cluster size, 29 voxels; threshold, puncorrected < 0.0001), which, however, did not survive error correction. No voxels showing an interaction between block and trial type at uncorrected levels were present for objects in Experiment 1 and chairs in Experiment 2.
Discussion
Contrary to studies that used faces as stimuli, we did not observe repetition probability effects on the magnitude of RS of the BOLD signal evoked by natural images in the occipitotemporal areas of the human brain. However, note that with an identical paradigm, repetition probability effects were demonstrated in the FFA for faces. The results of the current human fMRIa experiment are in agreement with those of the macaque single-unit/LFP recording experiments of Kaliukhovich and Vogels (2010) in the sense that repetition probability had no effect on the observed RS for objects. We suggest that the previous discrepant results regarding stimulus probability modulations in fMRIa and single-cell studies were not due to the different tasks, species, or neural measures. Since attention is an important factor in the generation of repetition probability effects (Larsson and Smith, 2012), one possible factor that could have explained the discrepancy is the differences in attention to the stimuli in the macaque and human studies: passive fixation in the macaque study and a stimulus-related task in the human study. However, in our current experiment, we used a size-deviant stimulus detection task identical to that of Summerfield et al. (2008) and also did not observe repetition probability effects. This excludes differences in attentional factors as a potential explanation.
Another potential explanation of the discrepant results could have been that the previously observed repetition probability effects are area specific, being restricted to the FFA. This is unlikely, since predictive coding models suggest that such modulations should occur in every processing stage of the visual cortex (Rao and Ballard, 1999). Indeed, for face stimuli previous fMRIa studies demonstrated repetition probability modulations in several areas upstream of the FFA (Kovács et al., 2012; Larsson and Smith, 2012), including LO. While Larsson and Smith (2012) used retinotopic mapping and did not publish the stereotaxic coordinates of their LO1/2, our own previous study (Kovács et al., 2012), which demonstrated repetition probability effects using faces, applied the same functional localizer as the current study and found LO (Grill-Spector et al., 1999; Halgren et al., 1999) at similar coordinates as well. This suggests that differences in area localization are unlikely as an explanation of the different results.
This leaves us only with one possible explanation of the discrepancy: the different stimulus sets. Whereas all previous studies demonstrating repetition probability modulations of the fMRIa, used faces, we used the natural stimuli of Kaliukhovich and Vogels (2010).
LO is considered primarily as an object-selective area, but shows elevated activation for faces as well (Malach et al., 1995; Puce et al., 1995; Grill-Spector et al., 1998; Lerner et al., 2001), especially for inverted ones (Yovel and Kanwisher, 2005; Aguirre et al., 1999; Haxby et al., 1999; Epstein et al., 2006). Indeed, previous studies of repetition probability modulations found a significant BOLD response in LO for faces (Kovács et al., 2012; Larsson and Smith, 2012). Thus, it is possible that the PC model applies only to the category of faces and not for other objects. This would not be the first effect suggesting that faces are special in neural mechanisms (Tovée, 1998).
A previous electrophysiological study apparently argues against the face specificity of probability effects. Stefanics et al. (2011) used pairs of disks with identical or different colors with varying probability and measured the visual mismatch negativity (vMMN) (Czigler et al., 2002) elicited by the alternating colors. They found larger vMMN when the alternation probability was lower. However, the vMMN was analyzed in different time windows (130–160 ms after stimulus for the frequent and ∼100 ms later for the rare pairs), suggesting different neural mechanisms and making the interpretation difficult. Nevertheless, the relationship of the electrophysiologically observed vMMN with the RS of the BOLD signal certainly requires further investigation.
It seems that modulation of sensory cortical responses by expectations is not restricted to the visual modality. Previously, it has been shown that in higher-level voice-selective cortical regions, but not in the primary auditory cortex, fMRI RS evoked by voice stimuli is modulated by repetition probabilities (Andics et al., 2013). Furthermore, expectation effects within the acoustic and somatosensory modalities were also found previously (but for a different conclusion, see Valentini et al. (2011)). Todorovic et al. (2011) applied auditory stimulation and found that prior expectations increase the RS of both the evoked activity and gamma-band synchrony of the MEG signal. However, they either repeated the same tone twice or presented a single tone, a paradigm that manipulates the general occurrence rather than the expectation of an event (for discussion of the differential contribution of probability and predictability, see Valentini (2011)). Todorovic and de Lange (2012) presented pairs of (identical or different) sounds and manipulated expectation orthogonally. They found that the repetition and expectation of a stimulus attenuated separate, subsequent phases of the auditory response. Thus, theoretically it is possible that repetition probability has an effect for object stimuli as well, but similarly to auditory stimuli, with a distinct time course, and the lower temporal resolution of fMRI hinders the detection of such modulation. The negative results of the single-cell/LFP study of Kaliukhovich and Vogels (2010), however, argue against such a conclusion.
Our second experiment was designed to exclude another explanation that hides in the nature of the stimulus pool. The stimuli used by Kaliukhovich and Vogels (2010), used in Experiment 1 as well, depicted a large variety of colorful scenes, objects, plants, animals, and everyday objects, mostly on complex backgrounds. In contrast to this, all of the previously mentioned studies of repetition probability used grayscale frontal views of faces, cropped, resized, and against a uniform gray background. Thus, while the ATs always depicted two (very well matched) faces in the previous studies, they could depict very different objects or scenes in the monkey study and in our Experiment 1. This implies that, overall, during a given block, a rather selective and narrow-tuned neuronal population is activated for faces, while a distributed and more broadly tuned population responds for objects. It is then possible that repetition probability modulates the neural adaptation in both cases, but its effect is rendered invisible by the large variation of object stimuli in Experiment 1. However, the results of Experiment 2 make this explanation unlikely. The stimuli of Experiment 2 belonged to the same category, chairs, and they were matched in size, position, and viewpoint to each other, presented on a uniform background. This makes them, at least on a qualitative level, similarly homogenous to the stimuli of the previous studies with faces. The fact that we did not observe any modulation of RS for the more homogenous stimulus set suggests that the discrepant results regarding faces and objects is not due to the different variability inside the object categories.
Another possibility is that the effect of stimulus repetition probability shows regional differences across the ventral stream regions. Indeed, a previous high-resolution fMRI study (Weiner et al., 2010) showed differential RS across ventral stream regions with posterior face-selective regions having less suppression of the BOLD signal for repeated stimuli compared to anterior face regions (for object stimuli, see also Sawamura et al., 2006). However, in our study, the more posterior LO showed no probability modulation for its preferred (objects) or for its less-preferred stimuli (faces), whereas the more anteriorly localized FFA showed the modulation in a stimulus category-specific manner. This and the fact that Larsson and Smith (2012) did find probability modulations for face stimuli in a large range of areas, including portions of LO and FFA, makes the role of regional differences in determining probability effects unlikely. Nevertheless, this issue should be explored further, preferably by high-resolution fMRI and using different stimulus categories.
The neural mechanisms underlying the unexpected category specificity of the repetition probability effects on fMRIa are unclear. One possibility is that the modulation of repetition suppression by identity-dependent expectation is stronger for neurons preferring faces compared to neurons preferring nonfaces. A second possibility is that the modulation by expectation is stimulus-category specific, independent of the stimulus preference of the neurons. Indeed, top-down modulations might be stronger for faces compared to the ecologically less important object stimuli. Note that any stimuli depicting other ecologically important classes such as bodies or body parts were also excluded from our object stimulus sets.
Undoubtedly, further studies are necessary to test the validity of PC models on RS. The presence of adaptation in both monkey single-cell/LFP study and the present human fMRI study without any effect of repetition probability for nonface stimuli clearly demonstrates that the two phenomena can be independent.
In conclusion, we found no evidences of any modulatory effects of object stimulus repetition probability on adaptation of the BOLD signal, supporting the single-cell/LFP results of Kaliukhovich and Vogels (2010). This suggests that RS in the case of nonface visual objects is not modulated by the repetition probability. Our results imply that perceptual expectation effects vary for different visual stimulus categories.
Footnotes
This work was supported by the Deutsche Forschungsgemeinschaft (KO 3918/1-2; 2-1), Geconcerteerde onderzoeksactie (GOA/2010), Interuniversitaire Attractiepool (IUAP7), Programma Financiering KU Leuven (PF), and Fonds voor Wetenschappelijk Onderzoek Vlaanderen (G.0582.12).
References
- Aguirre GK, Singh R, D'Esposito M. Stimulus inversion and the responses of face and object-sensitive cortical areas. Neuroreport. 1999;10:189–194. doi: 10.1097/00001756-199901180-00036. [DOI] [PubMed] [Google Scholar]
- Andics A, Gál V, Vicsi K, Rudas G, Vidnyánszky Z. FMRI repetition suppression for voices is modulated by stimulus expectations. Neuroimage. 2013;69:277–283. doi: 10.1016/j.neuroimage.2012.12.033. [DOI] [PubMed] [Google Scholar]
- Brett M, Johnsrude IS, Owen AM. The problem of functional localization in the human brain. Nat Rev Neurosci. 2002;3:243–249. doi: 10.1038/nrn756. [DOI] [PubMed] [Google Scholar]
- Czigler I, Balázs L, Winkler I. Memory-based detection of task-irrelevant visual change. Psychophysiology. 2002;39:869–873. doi: 10.1111/1469-8986.3960869. [DOI] [PubMed] [Google Scholar]
- Cziraki C, Greenlee MW, Kovács G. Neural correlates of high-level adaptation-related aftereffects. J Neurophysiol. 2010;103:1410–1417. doi: 10.1152/jn.00582.2009. [DOI] [PubMed] [Google Scholar]
- De Baene W, Vogels R. Effects of adaptation on the stimulus selectivity of macaque inferior temporal spiking activity and local field potentials. Cereb Cortex. 2010;20:2145–2165. doi: 10.1093/cercor/bhp277. [DOI] [PubMed] [Google Scholar]
- Denys K, Vanduffel W, Fize D, Nelissen K, Peuskens H, Van Essen D, Orban GA. The processing of visual shape in the cerebral cortex of human and nonhuman primates: a functional magnetic resonance imaging study. J Neurosci. 2004;24:2551–2565. doi: 10.1523/JNEUROSCI.3569-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Epstein RA, Higgins JS, Parker W, Aguirre GK, Cooperman S. Cortical correlates of face and scene inversion: a comparison. Neuropsychologia. 2006;44:1145–1158. doi: 10.1016/j.neuropsychologia.2005.10.009. [DOI] [PubMed] [Google Scholar]
- Ewbank MP, Lawson RP, Henson RN, Rowe JB, Passamonti L, Calder AJ. Changes in “top-down” connectivity underlie repetition suppression in the ventral visual pathway. J Neurosci. 2011;31:5635–5642. doi: 10.1523/JNEUROSCI.5013-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friston KJ, Harrison L, Penny W. Dynamic causal modelling. Neuroimage. 2003;19:1273–1302. doi: 10.1016/S1053-8119(03)00202-7. [DOI] [PubMed] [Google Scholar]
- Grill-Spector K, Kushnir T, Edelman S, Itzchak Y, Malach R. Cue-invariant activation in object-related areas of the human occipital lobe. Neuron. 1998;21:191–202. doi: 10.1016/S0896-6273(00)80526-7. [DOI] [PubMed] [Google Scholar]
- Grill-Spector K, Kushnir T, Edelman S, Avidan G, Itzchak Y, Malach R. Differential processing of objects under various viewing conditions in the human lateral occipital complex. Neuron. 1999;24:187–203. doi: 10.1016/S0896-6273(00)80832-6. [DOI] [PubMed] [Google Scholar]
- Grill-Spector K, Knouf N, Kanwisher N. The fusiform face area subserves face perception, not generic within-category identification. Nat Neurosci. 2004;7:555–562. doi: 10.1038/nn1224. [DOI] [PubMed] [Google Scholar]
- Grill-Spector K, Henson R, Martin A. Repetition and the brain: neural models of stimulus-specific effects. Trends Cogn Sci. 2006;10:14–23. doi: 10.1016/j.tics.2005.11.006. [DOI] [PubMed] [Google Scholar]
- Gross CG, Schiller PH, Wells C, Gerstein GL. Single-unit activity in temporal association cortex of the monkey. J Neurophysiol. 1967;30:833–843. doi: 10.1152/jn.1967.30.4.833. [DOI] [PubMed] [Google Scholar]
- Gross CG, Bender DB, Rocha-Miranda CE. Visual receptive fields of neurons in inferotemporal cortex of the monkey. Science. 1969;166:1303–1306. doi: 10.1126/science.166.3910.1303. [DOI] [PubMed] [Google Scholar]
- Halgren E, Dale AM, Sereno MI, Tootell RB, Marinkovic K, Rosen BR. Location of human face-selective cortex with respect to retinotopic areas. Hum Brain Mapp. 1999;7:29–37. doi: 10.1002/(SICI)1097-0193(1999)7:1<29::AID-HBM3>3.0.CO;2-R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haxby JV, Ungerleider LG, Clark VP, Schouten JL, Hoffman EA, Martin A. The effect of face inversion on activity in human neural systems for face and object perception. Neuron. 1999;22:189–199. doi: 10.1016/S0896-6273(00)80690-X. [DOI] [PubMed] [Google Scholar]
- Henson RN, Rugg MD. Neural response suppression, haemodynamic repetition effects, and behavioural priming. Neuropsychologia. 2003;41:263–270. doi: 10.1016/S0028-3932(02)00159-8. [DOI] [PubMed] [Google Scholar]
- Ishai A, Ungerleider LG, Martin A, Schouten JL, Haxby JV. Distributed representation of objects in the human ventral visual pathway. Proc Natl Acad Sci U S A. 1999;96:9379–9384. doi: 10.1073/pnas.96.16.9379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaliukhovich DA, Vogels R. Stimulus repetition probability does not affect repetition suppression in macaque inferior temporal cortex. Cereb Cortex. 2010;21:1547–1558. doi: 10.1093/cercor/bhq207. [DOI] [PubMed] [Google Scholar]
- Kohn A, Movshon JA. Neuronal adaptation to visual motion in area MT of the macaque. Neuron. 2003;39:681–691. doi: 10.1016/S0896-6273(03)00438-0. [DOI] [PubMed] [Google Scholar]
- Kovács G, Cziraki C, Vidnyánszky Z, Schweinberger SR, Greenlee MW. Position-specific and position-invariant face aftereffects reflect the adaptation of different cortical areas. Neuroimage. 2008;43:156–164. doi: 10.1016/j.neuroimage.2008.06.042. [DOI] [PubMed] [Google Scholar]
- Kovács G, Iffland L, Vidnyánszky Z, Greenlee MW. Stimulus repetition probability effects on repetition suppression are position invariant for faces. Neuroimage. 2012;60:2128–2135. doi: 10.1016/j.neuroimage.2012.02.038. [DOI] [PubMed] [Google Scholar]
- Krekelberg B, Boynton G, van Wezel RJ. Adaptation: from single cells to BOLD signals. Trends Neurosci. 2006;29:250–256. doi: 10.1016/j.tins.2006.02.008. [DOI] [PubMed] [Google Scholar]
- Larsson J, Smith AT. fMRI repetition suppression: neuronal adaptation or stimulus expectation? Cereb Cortex. 2012;22:567–576. doi: 10.1093/cercor/bhr119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lerner Y, Hendler T, Ben-Bashat D, Harel M, Malach R. A hierarchical axis of object processing stages in the human visual cortex. Cereb Cortex. 2001;11:287–297. doi: 10.1093/cercor/11.4.287. [DOI] [PubMed] [Google Scholar]
- Malach R, Reppas JB, Benson RR, Kwong KK, Jiang H, Kennedy WA, Ledden PJ, Brady TJ, Rosen BR, Tootell RB. Object-related activity revealed by functional magnetic resonance imaging in human occipital cortex. Proc Natl Acad Sci U S A. 1995;92:8135–8139. doi: 10.1073/pnas.92.18.8135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray SO, Wojciulik E. Attention increases neural selectivity in the human lateral occipital complex. Nat Neurosci. 2004;7:70–74. doi: 10.1038/nn1161. [DOI] [PubMed] [Google Scholar]
- Ollinger JM, Corbetta M, Shulman GL. Separating processes within a trial in event-related functional MRI. Neuroimage. 2001;13:218–229. doi: 10.1006/nimg.2000.0711. [DOI] [PubMed] [Google Scholar]
- Pinsk MA, Arcaro M, Weiner KS, Kalkus JF, Inati SJ, Gross CG, Kastner S. Neural representations of faces and body parts in macaque and human cortex: a comparative FMRI study. J Neurophysiol. 2009;101:2581–2600. doi: 10.1152/jn.91198.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pourtois G, Schwartz S, Spiridon M, Martuzzi R, Vuilleumier P. Object representations for multiple visual categories overlap in lateral occipital and medial fusiform cortex. Cereb Cortex. 2008;19:1806–1819. doi: 10.1093/cercor/bhn210. [DOI] [PubMed] [Google Scholar]
- Priebe NJ, Churchland MM, Lisberger SG. Constraints on the source of short-term motion adaptation in macaque area MT. I. the role of input and intrinsic mechanisms. J Neurophysiol. 2002;88:354–369. doi: 10.1152/.00852.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puce A, Allison T, Gore JC, McCarthy G. Face-sensitive regions in human extrastriate cortex studied by functional MRI. J Neurophysiol. 1995;74:1192–1199. doi: 10.1152/jn.1995.74.3.1192. [DOI] [PubMed] [Google Scholar]
- Rao RP, Ballard DH. Predictive coding in the visual cortex: a functional interpretation of some extra-classical receptive-field effects. Nat Neurosci. 1999;2:79–87. doi: 10.1038/4580. [DOI] [PubMed] [Google Scholar]
- Sadeh B, Podlipsky I, Zhdanov A, Yovel G. Event-related potential and functional MRI measures of face-selectivity are highly correlated: a simultaneous ERP-fMRI investigation. Hum Brain Mapp. 2010;31:1490–1501. doi: 10.1002/hbm.20952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sawamura H, Orban GA, Vogels R. Selectivity of neuronal adaptation does not match response selectivity: a single-cell study of the FMRI adaptation paradigm. Neuron. 2006;49:307–318. doi: 10.1016/j.neuron.2005.11.028. [DOI] [PubMed] [Google Scholar]
- Stefanics G, Kimura M, Czigler I. Visual mismatch negativity reveals automatic detection of sequential regularity violation. Front Hum Neurosci. 2011;5:1–9. doi: 10.3389/fnhum.2011.00046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Summerfield C, Trittschuh EH, Monti JM, Mesulam MM, Egner T. Neural repetition suppression reflects fulfilled perceptual expectations. Nat Neurosci. 2008;11:1004–1006. doi: 10.1038/nn.2163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Todorovic A, de Lange FP. Repetition suppression and expectation suppression are dissociable in time in early auditory evoked fields. J Neurosci. 2012;32:13389–13395. doi: 10.1523/JNEUROSCI.2227-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Todorovic A, van Ede F, Maris E, de Lange FP. Prior expectation mediates neural adaptation to repeated sounds in the auditory cortex: an MEG study. J Neurosci. 2011;31:9118–9123. doi: 10.1523/JNEUROSCI.1425-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tovée MJ. Is face processing special? Neuron. 1998;21:1239–1242. doi: 10.1016/S0896-6273(00)80644-3. [DOI] [PubMed] [Google Scholar]
- Valentini E. The role of perceptual expectation on repetition suppression: a quest to dissect the differential contribution of probability of occurrence and event predictability. Front Hum Neurosci. 2011;5:143. doi: 10.3389/fnhum.2011.00143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valentini E, Torta DME, Mouraux A, Iannetti GD. Dishabituation of laser-evoked EEG responses: dissecting the effect of certain and uncertain changes in stimulus modality. J Cog Neurosci. 2011;23:2822–2837. doi: 10.1162/jocn.2011.21609. [DOI] [PubMed] [Google Scholar]
- Weiner KS, Grill-Spector K. Sparsely-distributed organization of face and limb activations in human ventral temporal cortex. Neuroimage. 2010;52:1559–1573. doi: 10.1016/j.neuroimage.2010.04.262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiner KS, Sayres R, Vinberg J, Grill-Spector K. fMRI-adaptation and category selectivity in human ventral temporal cortex: Regional differences across time scales. J Neurophysiol. 2010;103:3349–3365. doi: 10.1152/jn.01108.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yovel G, Kanwisher N. The neural basis of the behavioral face-inversion effect. Curr Biol. 2005;15:2256–2262. doi: 10.1016/j.cub.2005.10.072. [DOI] [PubMed] [Google Scholar]