Abstract
Although training-based auditory cortical plasticity in the adult brain has been previously demonstrated in multiparametric sound domains, neurochemical mechanisms responsible for this form of plasticity are not well understood. In this study, we trained adult rats to identify a target sound stimulus at a specific azimuth angle by using a reward-contingent auditory discrimination task. We found that auditory spatial discrimination training significantly enhanced representation of sound azimuths in the primary auditory cortex, as shown by sharper azimuth-selective curves and more evenly distributed best angles of cortical neurons. Training also facilitated long-term potentiation of field potentials in the primary auditory cortex induced by theta burst stimulation of the white matter. In parallel, there were significant alterations in expression levels of certain cortical GABAA and NMDA receptor subunits, resulting in a marked decrease in the level of GABAA relative to NMDA receptors. These changes in the expression profile of inhibitory and excitatory neurotransmitter receptor subunits might enhance synaptic transmission, thereby facilitating training-induced cortical plasticity in the spatial domain.
Introduction
Sound localization is one of the most important tasks performed by the auditory system. Behavioral studies indicate that plasticity of auditory localization in the adult animal model, like other auditory functions, can be induced by using task-dependent behavioral training (Kacelnik et al., 2006). It is conceivable that neural processing of auditory spatial cues within the auditory pathway might undergo rapid adjustments in response to training-induced plasticity in spatial hearing. The auditory cortex, as demonstrated by many lesion studies in various species, plays a critical role in spatial information processing of sound (Jenkins and Merzenich, 1984; Kavanagh and Kelly, 1987; Heffner and Heffner, 1990; Nodal et al., 2010, 2012). However, few studies to date have determined whether behavioral training alters neuronal representation of sound locations in the adult auditory cortex of a normally reared animal model (Lee and Middlebrooks, 2011).
Although the neurochemical mechanisms underlying training-induced auditory cortical plasticity are not well understood, changes in synaptic function involving GABA and NMDA receptors have been strongly implicated (Feldman et al., 1996; Sun et al., 2005; Guo et al., 2012). For example, it has been shown that increased auditory cortical plasticity beyond the critical period epoch involves altered protein expression levels of certain GABAA receptor subunits (α1 and β2/3) and NMDA receptor subunits (NR2a and NR2b) (Zhou et al., 2011) and that the auditory discrimination task decreases cortical gene expression of NR2a and NR2b (Sun et al., 2005).
In this study, we trained rats at 8 weeks of age to identify a target sound stimulus at a specific azimuth angle that changed randomly in each trial. The spatial response characteristics of neurons in the primary auditory cortex (A1) were determined electrophysiologically to reflect the training-induced plasticity in cortical processing of spatial information of sound. In addition, shifts in long-term potentiation (LTP) magnitudes induced in A1 were assessed and changes in inhibitory GABAA receptor subunits (α1, α3, β2, and β3) and excitatory NMDA receptor subunits (NR2a and NR2b) were measured as a consequence of behavioral training to examine the synaptic and neurochemical mechanisms underlying training-based auditory cortical plasticity.
Materials and Methods
Procedures.
All experimental procedures were approved by the animal care and use committees at the East China Normal University.
Subjects.
Female Sprague Dawley rats, 8 weeks of age, were randomly divided into three groups: (1) experimental (EXP) rats, which were trained to discriminate a target sound stimulus at a specific azimuth to receive a water reward for ∼4 weeks; (2) passively exposed (PE) rats, which were exposed passively to training sounds that were identical to sounds delivered to EXP rats but not required to identify the sound azimuth for a water reward across the same epoch; and (3) age-matched naive rats, which were raised in a normal environment until experiments were conducted. The researcher was kept blind to the group identity of the animals.
Behavioral training.
Training was conducted in a sound-attenuated chamber, as in our earlier studies (Pan et al., 2011). The training apparatus was made of wood, had a radius of 150 cm, and its interior was covered with black polyethylene sponge. At the front of the apparatus was a starting box (20 × 7 × 8 cm, length × width × height) that allowed rats to run forward. Speakers were installed in the interior circular wall at 10° intervals with a water spout under each one. Water delivery was triggered by an automatic lick detection circuit. During training, a white noise burst (30 ms duration with 3 ms rise-decay time) was randomly emitted from one of speakers in each trail under the control of a computer. The intensity of the noise burst was 70 dB sound pressure level.
Rats assigned to the EXP group had ad libitum access to food but were deprived of water. Their body weights were maintained at ∼90% of ad libitum body weight during the training period (Rutkowski and Weinberger, 2005). Before the real training phase, they were habituated for 40 min/d to the training apparatus for ∼1 week, during which time the rats were allowed to move freely within the behavioral apparatus. They also learned to approach a water spout and lick it to obtain water rewards after presentation of a sound stimulus at a fixed azimuth.
During the real training phase, rats were in the starting box with their heads pointing to the frontal auditory space. They left the starting box after presentation of the sound stimulus from one of the speakers. As long as they licked any of these spouts, the trial finished. Only if they approached the source of the sound (i.e., the target speaker from which the stimulus was emitted) and licked the corresponding spout did they receive several drops of water as a reward and a trial was scored as a hit. The rats then returned to the starting box to initiate the next trial. If animals approached the incorrect azimuth and licked the spout beneath, a trial was scored as an error. In this case, the stimulus was presented again from the same speaker as a corrective mechanism to guide the animal to the target location, but these data were not used in the calculation of the training parameters.
The PE rats were put in the behavioral apparatus and passively exposed to training sounds that were identical to sounds delivered to EXP rats in the same time period, but they were not engaged in the above-described task.
Cortical response recording procedure.
Cortical recordings were conducted under pentobarbital anesthesia (50 mg/kg body weight) as described previously (Cai et al., 2010). Pure tone stimuli (50 ms duration with 5 ms rise-decay time) were delivered from a speaker positioned 34 cm from the rat's head. The speaker could be placed at any specific azimuth in the frontal auditory space using a remote control system driven by two small electric motors. Once a single unit was isolated, its characteristic frequency (CF) and minimum threshold (MT) were determined. The number of impulses discharged to CF sound (set at 10 dB above each neuron's MT) at 10° increments between contralateral 90° relative to the recording site (abbreviated as c90°) and ipsilateral 90° (abbreviated as i90°) was then recorded. The CF sound was presented 32 times at each azimuth angle and the neuron's azimuth-selectivity curve was plotted by using the total number of impulses in response to CF sounds (after correction for the spontaneous activity) against azimuth angles.
All azimuth-selectivity curves recorded can be described as azimuth-selective, hemifield, multipeak, or nonselective based on their shapes (Fig. 2B). An azimuth-selective curve has a clear peak at a certain azimuth angle (referred to as the best angle [BA]) that is at least 50% greater than the minimum obtained at both lateral angles. A hemifield curve ascends from an ipsilateral angle by >50% and either reaches a plateau or declines by <50% over a wide range of contralateral angles. A multipeak curve has two peaks that are greater than the minimum obtained at the trough and at lateral angles by at least 50%. A nonselective curve does not show any clear peak and the number of impulses obtained from all angles tested never differs by >50%.
Figure 2.
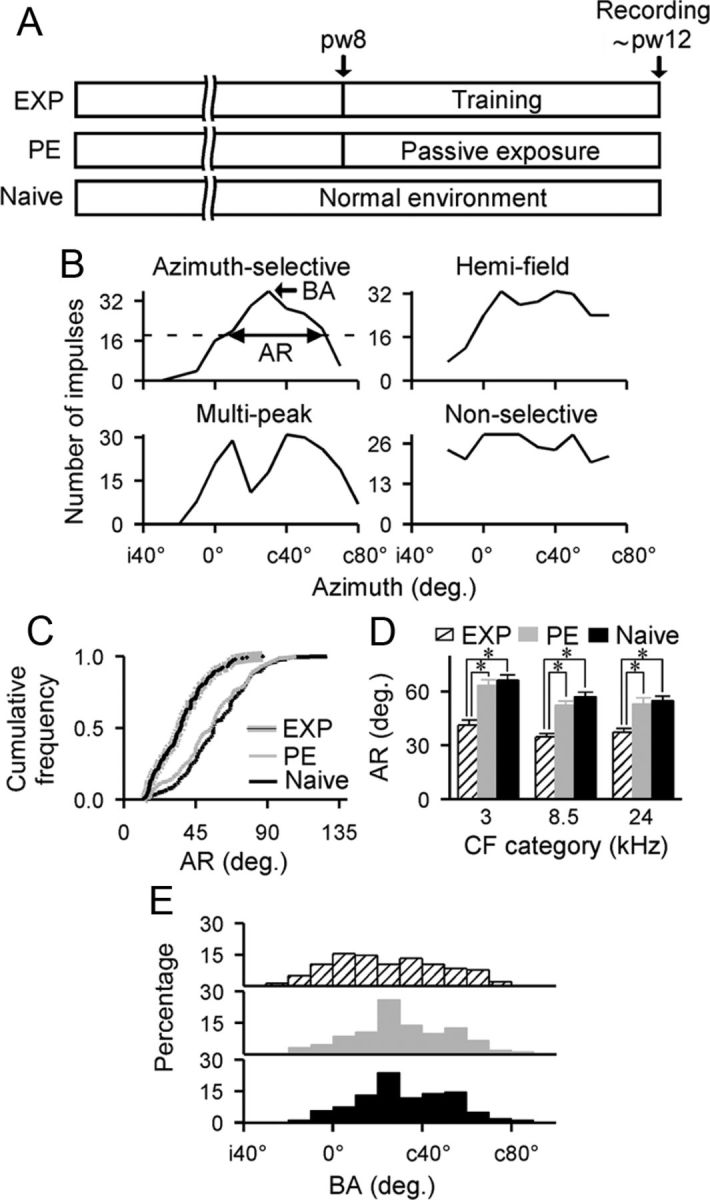
Posttraining effects on cortical azimuth selectivity. A, Experimental time line. Pw indicates postnatal week. B, Representative azimuth-selectivity curves recorded from naive rats. These curves were categorized as azimuth-selective, multipeak, hemifield, or nonselective. The sharpness of an azimuth-selective curve is defined as the AR at 50% below the maximal response. The azimuth angle that has the maximal response (i.e., BA) is also shown. Horizontal dashed line indicates the 50% maximal response. c and i indicate contralateral and ipsilateral relative to the recording site, respectively. C, Cumulative frequency histograms of ARs recorded from EXP (n = 143), PE (n = 150), and naive (n = 159) rats. D, Average AR for each of three CF ranges. Values shown are mean ± SE. *p < 0.001. E, Distributions of BAs recorded from different rat groups.
LTP induction.
Cortical slices were prepared as in our earlier studies (Mao et al., 2006). The location of A1 was determined according to Paxinos and Watson's (1998) atlas and studies by Polley et al. (2007). The electrode for electrical stimulation (twisted 70 μm nichrome wires insulated except at the tips) was placed on the border between the white matter (WM) and cortical layer VI and that for recording (a glass pipette electrode with tip diameter of 1.0–1.5 μm) was placed in layer III/IV (Fig. 3A). After a 20–30 min stable baseline, theta burst stimulation (TBS; 5 pulses at 100 Hz, 11 pulse trains at 5 Hz, repeated 3 times at 0.2 Hz) was applied to the WM and the amplitudes of the field potentials were recorded every 4 min for 2 h after the cessation of TBS.
Figure 3.
LTP induced in the cortical field A1. A, Schematic model showing the stimulating and recording locations. B, Examples of field potentials (FPs) evoked before (black) and after (gray) the TBS (top) and time courses of WM-LTPs for EXP (n = 12), PE (n = 15), and naive (n = 12) rats (bottom). Values shown are mean ± SE.
Quantitative immunoblots.
Auditory cortices (Paxinos and Watson, 1998; Polley et al., 2007) were dissected under pentobarbital anesthesia (75 mg/kg body weight), and were homogenized immediately in ice-cold homogenization buffer.
Quantitative immunoblotting assays were performed as described previously (Cai et al., 2010; Guo et al., 2012). Briefly, the concentration of total proteins was first determined using the bicinchoninic acid assay. Proteins were separated on a 7.5% SDS-polyacrylamide gel and then transferred to a nitrocellulose membrane. After both primary and secondary antibody incubations, proteins were visualized using enhanced chemiluminescence followed by exposure to the x-ray film. Primary antibodies used included anti-GABAAα1, anti-GABAAα3, anti-GABAAβ2, and anti-GABAAβ3 (Millipore); anti-NMDA NR2a and NR2b (Millipore); and anti-β-actin (Millipore).
The density of each band on Western blotting was measured and the relative level of each protein was calculated as the ratio of target protein band compared with the β-actin loading control band.
Results
Sound-azimuth discrimination performance
Rats assigned to the EXP group were trained to identify a specific azimuth angle where a speaker emitted a sound stimulus and then lick the associated water spout to receive a water reward. As shown in Figure 1A, the percent correct of these EXP rats in the sound-azimuth discrimination task dramatically increased as training progressed. It took an average of 15 ± 0.4 d for them to achieve at least 80% accuracy for three consecutive days while conducting the task. We also defined the azimuth deviation (i.e., the angle difference between the spout that the rat licked and where the stimulus was presented during the training) as a performance index for error trials. As expected, azimuth deviation decreased significantly with training (Fig. 1B). The percent decrease of average azimuth deviation (compared with that recorded in the first training day) was 54.2% after ∼3 weeks of training. These data indicate a successful task acquisition for EXP rats.
Figure 1.
Sound-azimuth discrimination performance. A, Correct scores on the sound-azimuth discrimination task for each of EXP rat (n = 10; black lines) and group values (mean ± SE; gray line). B, Azimuth deviation of error trials. Graph plots the median, 10th, 25th, 75th, and 90th percentiles as vertical boxes with error bars.
Sound-azimuth selectivity of A1 neurons
After the cessation of training, azimuth-selective curves (i.e., the number of neuronal responses against azimuth angles) of cortical neurons were measured at 10 dB above MT for EXP rats and were compared with those of PE and age-matched naive rats (Fig. 2A). Data were recorded in the middle cortical layers from 170 single neurons in eight EXP rats, 215 single neurons in eight PE rats, and 237 single neurons in 10 naive rats. CFs of these neurons ranged from 1.6 to 27.8 kHz. No significant differences in the distribution of CFs were found among the three rat groups (Kolmogorov–Smirnov test, all p > 0.25 with Bonferroni correction).
As described in the Materials and Methods, all azimuth-selectivity curves recorded were categorized as either azimuth-selective, hemifield, multipeak, or nonselective (Fig. 2B). In EXP rats, more neurons displayed azimuth-selective curves, but fewer neurons displayed hemifield curves than those in naive rats (84% vs 67% azimuth-selective and 8% vs 15% hemifield). The distribution of azimuth-selectivity curves for PE rats, however, was much like that of naive rats (70% vs 67% azimuth-selective and 15% vs 15% hemifield).
We used an angular range (AR; Fig. 2B, double arrows) at 50% below the maximum to describe the sharpness of an azimuth-selective curve. An AR is an indication of the width of an azimuth-selective curve; a small AR represents sharp azimuth-selective tuning. As shown in Figure 2C, there was a significantly leftward shift of AR distribution for EXP compared with naive and PE rats (Kolmogorov–Smirnov test, both p < 0.0001 with Bonferroni correction), indicating enhanced sharpness of azimuth-selective tuning resulting from training. The AR distribution for PE rats, however, was comparable to that of naive rats (Kolmogorov–Smirnov test, p > 0.35 with Bonferroni correction).
We also compared ARs obtained from different rat groups by binning their CF values into three categories (Fig. 2D). As expected, average ARs were significantly smaller for EXP than for naive and PE rats across all CF ranges (ANOVA with post hoc Student-Newman–Keuls test, all p < 0.001). The average ARs for PE rats were again substantially similar to those of naive rats (ANOVA with post hoc Student-Newman–Keuls test, all p > 0.05).
We further evaluated the distribution of BAs (Fig. 2B, single arrow) for azimuth-selective curves recorded from different rat groups. As shown in Figure 2E, BAs recorded from PE or naive rats were mostly clustered between c20° and c60°. After training, however, BAs were more evenly distributed across the azimuth range with no specific clustering on azimuth angles. Quantitative comparison of the BA distribution from EXP rats revealed a significant difference compared with that of naive and PE rats (Kolmogorov–Smirnov test, both p < 0.007 with Bonferroni correction). As expected, BA distribution from PE rats was not different from that recorded in naive rats (Kolmogorov–Smirnov test, p > 0.6 with Bonferroni correction).
LTP induced in cortical field A1
LTP of field potentials was successfully induced in cortical field A1 by TBS of the WM (Fig. 3A) in all three rat groups. As shown in Figure 3B, field potential amplitude averaging 145% of baseline was observed for naive rats during the whole recording period after TBS. A similar magnitude of LTP was observed in PE rats under the same recording protocol, which showed an increase to 142% of baseline. EXP rats, however, showed significantly greater synaptic enhancement compared with naive and PE rats (ANOVA with post hoc Student-Newman–Keuls test, all p < 0.001), with levels of LTP at 183% of baseline after TBS of the WM.
Expression of cortical GABAA and NMDA receptor subunits
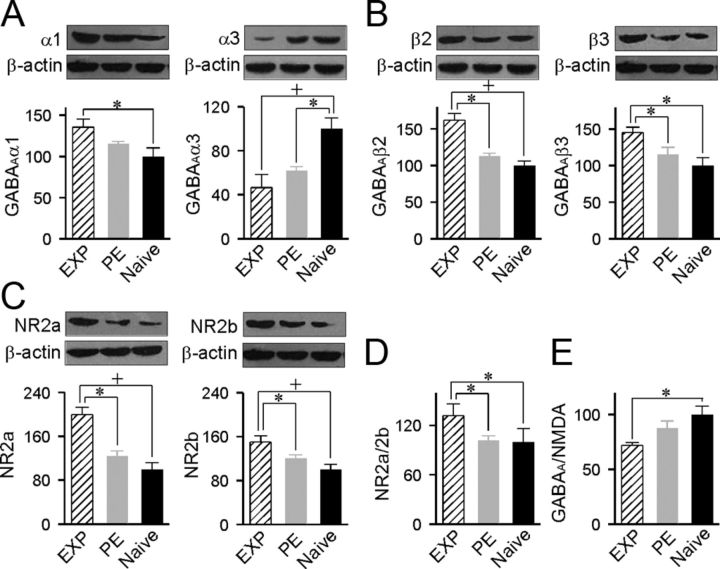
To begin documenting the molecular changes that paralleled the training-induced changes in azimuth selectivity of cortical neurons, levels of cortical inhibition and excitation were examined in EXP rats by using quantitative immunoblotting. The data were then compared with those obtained in PE and naive rats. Antibodies recognizing the α1, α3, β2, and β3 subunits of GABAA receptors and the NR2a and NR2b subunits of NMDA receptors were used to assay changes in cortical inhibition and excitation, respectively.
As shown in Figure 4A, quantitative immunoblotting revealed a significantly higher expression level of GABAAα1 but a lower expression level of GABAAα3 for EXP compared with naive rats (ANOVA with post hoc Student-Newman–Keuls test, p < 0.05–0.01). It should be noted that although the expression level of GABAAα1 for PE rats was not different from that of naive rats (ANOVA with post hoc Student-Newman–Keuls test, p > 0.05), that of GABAAα3 was markedly lower (ANOVA with post hoc Student-Newman–Keuls test, p < 0.05).
Figure 4.
Cortical molecular changes induced by training. A, Expression levels of the GABAAα1 and GABAAα3 subunits for different rat groups measured by using quantitative immunoblotting. The insets show representative Western blots. Values shown are mean ± SE. n = 8 for both subunits for all rat groups. *p < 0.05; +p < 0.01. B, Expression levels of the GABAAβ2 and GABAAβ3 subunits. n = 8 for β2 and n = 7 for β3 for all rat groups. C, Expression levels of the NMDA NR2a and NR2b subunits. n = 8 for NR2a and n = 10 for NR2b for all rat groups. D, NR2a/2b ratio for the different rat groups. E, GABAA/NMDA ratio for the different rat groups.
Moreover, the expression levels of GABAAβ2 and GABAAβ3 for EXP rats were both higher than those of naive and PE rats (Fig. 4B; ANOVA with post hoc Student-Newman–Keuls test, p < 0.05–0.01). As expected, expression levels of both GABAAβ2 and GABAAβ3 for PE rats were not different from that of naive rats (ANOVA with post hoc Student-Newman–Keuls test, both p > 0.05).
For excitatory receptor subunits, auditory discrimination training resulted in a significant increase in the expression of both NMDA receptor NR2a and NR2b subunits in EXP compared with naive and PE rats (Fig. 4C; ANOVA with post hoc Student-Newman–Keuls test, p < 0.05–0.01). The percent increases for NMDA receptor NR2a and NR2b versus those of control rats were 99.4% and 50.1%, respectively. Therefore, the ratio of NMDA receptor NR2a/2b for the EXP group also significantly increased compared with naive rats (Fig. 4D; ANOVA with post hoc Student-Newman–Keuls test, p < 0.05). Similarly, the NMDA receptor NR2a/2b ratio for the EXP group was significantly higher than that in PE rats (Fig. 4D; ANOVA with post hoc Student-Newman–Keuls test, p < 0.05). The expression levels of both NMDA receptor subunits NR2a and NR2b for PE rats, however, were comparable to those of naive rats (Fig. 4C; ANOVA with post hoc Student-Newman–Keuls test, both p > 0.05).
Last, we calculated the ratio of GABAA receptor subunits (α1, α3, β2, and β3) versus NMDA receptor subunits (NR2a and NR2b) as an index of the balance between inhibitory and excitatory receptor subunit expression. As shown in Figure 4E, the GABAA/NMDA ratio for EXP rats was significantly lower than that of naive rats (ANOVA with post hoc Student-Newman–Keuls test, p < 0.05). Again, there was no difference in the GABAA/NMDA ratio between PE and naive rats (ANOVA with post hoc Student-Newman–Keuls test, p > 0.05).
Discussion
Training-based cortical plasticity in multiparametric acoustic domains is typically expressed as changes in the receptive field bandwidth and/or the preferential tuning to reinforced or nonreinforced stimuli (Bakin and Weinberger, 1990; Recanzone et al., 1993; Fritz et al., 2003; Bao et al., 2004; Polley et al., 2004, 2006; Zhou and Merzenich, 2007, 2009). In this study, we extended findings from prior research by showing that sound-azimuth discrimination training markedly improved the cortical representation of sound azimuths, manifesting in decreased ARs and more evenly distributed BAs of azimuth-selective curves of cortical neurons. We also showed that training facilitated LTP of field potentials in A1 induced by TBS of the WM. This increased LTP required that previous acoustic experiences carried some behavioral significance to the animals, because the effects were absent in PE rats.
Earlier studies using adult animal models indicated that changes in synaptic efficacy, including LTP, were associated with learning (training) processes (Rioult-Pedotti et al., 2000; Whitlock et al., 2006; Sale et al., 2007; Hager and Dringenberg, 2010; Gagolewicz and Dringenberg, 2011). For example, environmental enrichment resulted in an enhancement of WM-stimulation-induced LTP recorded in rat visual cortex in vitro (Sale et al., 2007). Similar findings have recently been obtained in adult rats in vivo, showing that visual discrimination learning altered the plasticity properties of neurons in the visual cortex by facilitating LTP. Observed LTP enhancement was suggested to reflect an increase in the dynamic range of synaptic strength (Hager and Dringenberg, 2010; Gagolewicz and Dringenberg, 2011). Conversely, occlusion of LTP in the motor cortex of adult rats after motor learning was also reported by Rioult-Pedotti et al. (2000). Although mechanisms underlying this discrepancy between studies that were conducted on different model systems—sensory (auditory or visual) versus motor systems—are currently unknown, these findings highlight the potential of the mature cortex to express heightened levels of plasticity after training or manipulations of sensory experiences. They also indicate that LTP can serve as a mechanism mediating the training/experience-dependent plasticity in the mature brain.
To date, the neurochemical factors underlying the training-induced cortical plasticity remain to be fully determined. Although it has been proposed that this form of plasticity might result from posttraining changes in the expression of certain molecules, the identity of these putative molecular determinants is far from established. NMDA receptors (particularly the subunits NR2a and NR2b) have been implicated in various training/experience-dependent changes in the mature cortex (Quinlan et al., 2004; Sun et al., 2005; He et al., 2006; Yashiro and Philpot, 2008; Gagolewicz and Dringenberg, 2011). For example, visual deprivation in adulthood induced a significant increase in the expression level of the NMDA receptor subunit NR2b relative to that of NR2a in rat visual cortex (He et al., 2006). A recent study also showed that local application of an antagonist of the NMDA receptor NR2b subunit in the visual cortex reversed the training-induced LTP enhancement (Gagolewicz and Dringenberg, 2011). Consistent with these previous results, we found here that auditory discrimination training upregulated expression of both the NR2a and NR2b NMDA receptor subunits in A1. In addition, training increased the expression level of GABAAα1 but decreased that of GABAAα3. Expression levels of both GABAAβ2 and GABAAβ3 also increased as a result of training. Involvement of inhibitory GABAergic activity as a potent regulator of plasticity in mature auditory cortex has been previously indicated in a study showing long-term exposure of adult rats to auditory inputs that lack instructive salient patterns reduced expression levels of the GABAAα1 and GABAAβ2/3 (Zhou et al., 2011). Therefore, expression levels of certain NMDA and GABAA receptor subunits may be affected by behaviorally relevant auditory experiences in the adult auditory cortex, underscoring the important role of these molecules in mediating synaptic cortical plasticity.
However, we cannot exclude the contribution of other molecules such as acetylcholine (ACh) to the induction and facilitation of plasticity in the mature cortex (Kilgard and Merzenich, 1998; Rasmusson, 2000; Gu 2003; Weinberger, 2003, 2004; Tsui and Dringenberg, 2013). Indeed, pairing the release of endogenous ACh with a tonal stimulus of a specific frequency was sufficient to induce the overrepresentation of the tone in A1 (Kilgard and Merzenich, 1998). A similar ACh-dependent effect was also reported in the auditory cortex after auditory discrimination training (Weinberger, 2003, 2004).
The expression level of GABAAα3 for PE rats was found to be significantly lower than that in naive rats. The expression level of GABAAα1 for PE rats was also slightly higher than naive rats, although no significant difference was found. How passive exposure to irrelevant sound results in changes in these GABAA receptor subunits remain unknown. In addition, no significant differences were observed in the expression levels of either the GABAAα1 or the GABAAα3 subunit between EXP and PE rats. Further work is necessary to characterize the neurochemical systems involved in the training-based cortical plasticity seen here and previously.
One remaining question is how parallel changes in GABAA and NMDA receptors after auditory training might translate into altered synaptic function. It has been suggested that the balance between inhibition and excitation (I/E) is a primary determinant of the level of synaptic plasticity within sensory cortices (Huang et al., 1999; Fagiolini and Hensch, 2000; Rozas et al., 2001; He et al., 2006; Morishita et al., 2010). For example, an increase in the strength of inhibition relative to excitation over the course of postnatal development resulted in a decrease of the ocular dominance plasticity in the visual cortex (Huang et al., 1999; Rozas et al., 2001). Visual deprivation in adulthood induced a significant decrease in the expression level of GABAAβ2/3 relative to AMPA GluR2 while reactivating ocular dominance plasticity (He et al., 2006). Given the fact that cortical I/E balance has been implicated in mediating activity-dependent synaptic plasticity in both juvenile and adult animals, we hypothesized that the level of I/E in adult auditory cortex would change significantly after sound azimuth discrimination training. In addition, regulation of NMDA receptor subunit composition (i.e., the NR2a/2b ratio) significantly affected characteristics of NMDA-receptor-dependent synaptic plasticity (Yashiro and Philpot, 2008). Increases in the NMDA receptor NR2a/2b ratio during development have also been associated with decreases in cortical plasticity (Hsieh et al., 2002; Yashiro and Philpot, 2008) and refinement of cortical receptive fields (Zhang et al., 2001). It is thus conceivable that relative levels of NR2a and NR2b in the auditory cortex may also be affected by behavioral training in adult rats.
Our above hypotheses were confirmed: cortical I/E balance as indexed by expression levels of GABAA receptor subunits versus that of NMDA receptor subunits decreased, but the relative expression level of NR2a/2b increased after azimuth discrimination training. In the visual cortex, the ratio of synaptic NR2a-containing to NR2b-containing NMDA receptors has been suggested to determine the threshold for NMDA-receptor-dependent LTP (Quinlan et al., 1999). Application of a selective antagonist of the NR2b subunit completely reversed the training-induced LTP enhancement recorded in the visual cortex (Gagolewicz and Dringenberg, 2011). In the auditory cortex, chronic blockade of NR2b also led to a decline in the LTP amplitude (Mao et al., 2006). It is possible that the sound azimuth discrimination training applied here caused long-term effects on the ratio of NR2a-containing and NR2b-containing NMDA receptors in the auditory cortex. Therefore, LTP, a common measurement of synaptic efficacy, was enhanced and cortical azimuth selectivity was refined.
It should be noted that the relative expression level of I/E for EXP rats was only slightly lower than that of PE rats and did not reach statistical significance. However, physiological data recorded from EXP rats (i.e., azimuth selectivity and LTP facilitation) were significantly different from that of PE rats. Nevertheless, our studies indicate that training-based plastic changes in the adult auditory cortex are associated with altered I/E balance and NR2a/2b ratio. These changes in both the absolute and relative expression levels of GABAA and NMDA receptor subunits after sound-azimuth discrimination training likely regulate cortical plasticity locally at individual synapses and globally at neural circuits, as observed in the present study.
Footnotes
This work was supported by the National Nature Science Foundation of China (Grant #31271178). We thank Dr. Michael Merzenich for assistance with data analysis and Drs. Dan Darcy, Daniel Polley, Elizabeth Quinlan, and Hans Dringenberg for reading an earlier version of this paper.
References
- Bakin JS, Weinberger NM. Classical conditioning induces CS-specific receptive field plasticity in the auditory cortex of the guinea pig. Brain Res. 1990;536:271–286. doi: 10.1016/0006-8993(90)90035-A. [DOI] [PubMed] [Google Scholar]
- Bao S, Chang EF, Woods J, Merzenich MM. Temporal plasticity in the primary auditory cortex induced by operant perceptual learning. Nat Neurosci. 2004;7:974–981. doi: 10.1038/nn1293. [DOI] [PubMed] [Google Scholar]
- Cai R, Zhou X, Guo F, Xu J, Zhang J, Sun X. Maintenance of enriched environment-induced changes of auditory spatial sensitivity and expression of GABAA, NMDA, and AMPA receptor subunits in rat auditory cortex. Neurobiol Learn Mem. 2010;94:452–460. doi: 10.1016/j.nlm.2010.08.008. [DOI] [PubMed] [Google Scholar]
- Fagiolini M, Hensch TK. Inhibitory threshold for critical-period activation in primary visual cortex. Nature. 2000;404:183–186. doi: 10.1038/35004582. [DOI] [PubMed] [Google Scholar]
- Feldman DE, Brainard MS, Knudsen EI. Newly learned auditory responses mediated by NMDA receptors in the owl inferior colliculus. Science. 1996;271:525–528. doi: 10.1126/science.271.5248.525. [DOI] [PubMed] [Google Scholar]
- Fritz J, Shamma S, Elhilali M, Klein D. Rapid task-related plasticity of spectrotemporal receptive fields in primary auditory cortex. Nat Neurosci. 2003;6:1216–1223. doi: 10.1038/nn1141. [DOI] [PubMed] [Google Scholar]
- Gagolewicz PJ, Dringenberg HC. NR2B-subunit dependent facilitation of long-term potentiation in primary visual cortex following visual discrimination training of adult rats. Eur J Neurosci. 2011;34:1222–1229. doi: 10.1111/j.1460-9568.2011.07842.x. [DOI] [PubMed] [Google Scholar]
- Gu Q. Contributions of acetylcholine to visual cortex plasticity. Neurobiol Learn Mem. 2003;80:291–301. doi: 10.1016/S1074-7427(03)00073-X. [DOI] [PubMed] [Google Scholar]
- Guo F, Zhang J, Zhu X, Cai R, Zhou X, Sun X. Auditory discrimination training rescues developmentally degraded directional selectivity and restores mature expression of GABA(A) and AMPA receptor subunits in rat auditory cortex. Behav Brain Res. 2012;229:301–307. doi: 10.1016/j.bbr.2011.12.041. [DOI] [PubMed] [Google Scholar]
- Hager AM, Dringenberg HC. Training-induced plasticity in the visual cortex of adult rats following visual discrimination learning. Learn Mem. 2010;17:394–401. doi: 10.1101/lm.1787110. [DOI] [PubMed] [Google Scholar]
- He HY, Hodos W, Quinlan EM. Visual deprivation reactivates rapid ocular dominance plasticity in adult visual cortex. J Neurosci. 2006;26:2951–2955. doi: 10.1523/JNEUROSCI.5554-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heffner HE, Heffner RS. Effect of bilateral auditory cortex lesions on sound localization in Japanese macaques. J Neurophysiol. 1990;64:915–931. doi: 10.1152/jn.1990.64.3.915. [DOI] [PubMed] [Google Scholar]
- Hsieh CY, Chen Y, Leslie FM, Metherate R. Postnatal development of NR2A and NR2B mRNA expression in rat auditory cortex and thalamus. J Assoc Res Otolaryngol. 2002;3:479–487. doi: 10.1007/s10162-002-2052-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang ZJ, Kirkwood A, Pizzorusso T, Porciatti V, Morales B, Bear MF, Maffei L, Tonegawa S. BDNF regulates the maturation of inhibition and the critical period of plasticity in mouse visual cortex. Cell. 1999;98:739–755. doi: 10.1016/S0092-8674(00)81509-3. [DOI] [PubMed] [Google Scholar]
- Jenkins WM, Merzenich MM. Role of cat primary auditory cortex for sound-localization behavior. J Neurophysiol. 1984;52:819–847. doi: 10.1152/jn.1984.52.5.819. [DOI] [PubMed] [Google Scholar]
- Kacelnik O, Nodal FR, Parsons CH, King AJ. Training-induced plasticity of auditory localization in adult mammals. PLoS Biol. 2006;4:e71. doi: 10.1371/journal.pbio.0040071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kavanagh GL, Kelly JB. Contribution of auditory cortex to sound localization by the ferret (Mustela putorius) J Neurophysiol. 1987;57:1746–1766. doi: 10.1152/jn.1987.57.6.1746. [DOI] [PubMed] [Google Scholar]
- Kilgard MP, Merzenich MM. Cortical map reorganization enabled by nucleus basalis activity. Science. 1998;279:1714–1718. doi: 10.1126/science.279.5357.1714. [DOI] [PubMed] [Google Scholar]
- Lee CC, Middlebrooks JC. Auditory cortex spatial sensitivity sharpens during task performance. Nat Neurosci. 2011;14:108–114. doi: 10.1038/nn.2713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mao Y, Zang S, Zhang J, Sun X. Early chronic blockade of NR2B subunits and transient activation of NMDA receptors modulate LTP in mouse auditory cortex. Brain Res. 2006;1073–1074:131–138. doi: 10.1016/j.brainres.2005.12.077. [DOI] [PubMed] [Google Scholar]
- Morishita H, Miwa JM, Heintz N, Hensch TK. Lynx1, a cholinergic brake, limits plasticity in adult visual cortex. Science. 2010;330:1238–1240. doi: 10.1126/science.1195320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nodal FR, Kacelnik O, Bajo VM, Bizley JK, Moore DR, King AJ. Lesions of the auditory cortex impair azimuthal sound localization and its recalibration in ferrets. J Neurophysiol. 2010;103:1209–1225. doi: 10.1152/jn.00991.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nodal FR, Bajo VM, King AJ. Plasticity of spatial hearing: behavioural effects of cortical inactivation. J Physiol. 2012;590:3965–3986. doi: 10.1113/jphysiol.2011.222828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan Y, Zhang J, Cai R, Zhou X, Sun X. Developmentally degraded directional selectivity of the auditory cortex can be restored by auditory discrimination training in adults. Behav Brain Res. 2011;225:596–602. doi: 10.1016/j.bbr.2011.08.033. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The rat brain in stereotaxic coordinate. New York: Academic; 1998. [Google Scholar]
- Polley DB, Heiser MA, Blake DT, Schreiner CE, Merzenich MM. Associative learning shapes the neural code for stimulus magnitude in primary auditory cortex. Proc Natl Acad Sci U S A. 2004;101:16351–16356. doi: 10.1073/pnas.0407586101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polley DB, Steinberg EE, Merzenich MM. Perceptual learning directs auditory cortical map reorganization through top-down influences. J Neurosci. 2006;26:4970–4982. doi: 10.1523/JNEUROSCI.3771-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polley DB, Read HL, Storace DA, Merzenich MM. Multiparametric auditory receptive field organization across five cortical fields in the albino rat. J Neurophysiol. 2007;97:3621–3638. doi: 10.1152/jn.01298.2006. [DOI] [PubMed] [Google Scholar]
- Quinlan EM, Philpot BD, Huganir RL, Bear MF. Rapid, experience dependent expression of synaptic NMDA receptors in visual cortex in vivo. Nat Neurosci. 1999;2:352–357. doi: 10.1038/7263. [DOI] [PubMed] [Google Scholar]
- Quinlan EM, Lebel D, Brosh I, Barkai E. A molecular mechanism for stabilization of learning-induced synaptic modifications. Neuron. 2004;41:185–192. doi: 10.1016/S0896-6273(03)00874-2. [DOI] [PubMed] [Google Scholar]
- Rasmusson DD. The role of acetylcholine in cortical synaptic plasticity. Behav Brain Res. 2000;115:205–218. doi: 10.1016/S0166-4328(00)00259-X. [DOI] [PubMed] [Google Scholar]
- Recanzone GH, Schreiner CE, Merzenich MM. Plasticity in the frequency representation of primary auditory cortex following discrimination training in adult owl monkeys. J Neurosci. 1993;13:87–103. doi: 10.1523/JNEUROSCI.13-01-00087.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rioult-Pedotti MS, Friedman D, Donoghue JP. Learning-induced LTP in neocortex. Science. 2000;290:533–536. doi: 10.1126/science.290.5491.533. [DOI] [PubMed] [Google Scholar]
- Rozas C, Frank H, Heynen AJ, Morales B, Bear MF, Kirkwood A. Developmental inhibitory gate controls the relay of activity to the superficial layers of the visual cortex. J Neurosci. 2001;21:6791–6801. doi: 10.1523/JNEUROSCI.21-17-06791.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rutkowski RG, Weinberger NM. Encoding of learned importance of sound by magnitude of representational area in primary auditory cortex. Proc Natl Acad Sci U S A. 2005;102:13664–13669. doi: 10.1073/pnas.0506838102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sale A, Maya Vetencourt JF, Medini P, Cenni MC, Baroncelli L, De Pasquale R, Maffei L. Environmental enrichment in adulthood promotes amblyopia recovery through a reduction of intracortical inhibition. Nat Neurosci. 2007;10:679–681. doi: 10.1038/nn1899. [DOI] [PubMed] [Google Scholar]
- Sun W, Mercado E, 3rd, Wang P, Shan X, Lee TC, Salvi RJ. Changes in NMDA receptor expression in auditory cortex after learning. Neurosci Lett. 2005;374:63–68. doi: 10.1016/j.neulet.2004.10.032. [DOI] [PubMed] [Google Scholar]
- Tsui CK, Dringenberg HC. Role of cholinergic-muscarinic receptors in visual discrimination performance of rats: Importance of stimulus load. Behav Brain Res. 2013;238:23–29. doi: 10.1016/j.bbr.2012.10.005. [DOI] [PubMed] [Google Scholar]
- Weinberger NM. The nucleus basalis and memory codes: Auditory cortical plasticity and the induction of specific, associative behavioral memory. Neurobiol Learn Mem. 2003;80:268–284. doi: 10.1016/S1074-7427(03)00072-8. [DOI] [PubMed] [Google Scholar]
- Weinberger NM. Specific long-term memory traces in primary auditory cortex. Nat Rev Neurosci. 2004;5:279–290. doi: 10.1038/nrn1366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitlock JR, Heynen AJ, Shuler MG, Bear MF. Learning induces long-term potentiation in the hippocampus. Science. 2006;313:1093–1097. doi: 10.1126/science.1128134. [DOI] [PubMed] [Google Scholar]
- Yashiro K, Philpot BD. Regulation of NMDA receptor subunit expression and its implications for LTD, LTP, and metaplasticity. Neuropharmacology. 2008;55:1081–1094. doi: 10.1016/j.neuropharm.2008.07.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang LI, Bao S, Merzenich MM. Persistent and specific influences of early acoustic environments on primary auditory cortex. Nat Neurosci. 2001;4:1123–1130. doi: 10.1038/nn745. [DOI] [PubMed] [Google Scholar]
- Zhou X, Merzenich MM. Intensive training in adults refines A1 representations degraded in an early postnatal critical period. Proc Natl Acad Sci U S A. 2007;104:15935–15940. doi: 10.1073/pnas.0707348104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou X, Merzenich MM. Developmentally degraded cortical temporal processing restored by training. Nat Neurosci. 2009;12:26–28. doi: 10.1038/nn.2239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou X, Panizzutti R, de Villers-Sidani E, Madeira C, Merzenich MM. Natural restoration of critical period plasticity in the juvenile and adult primary auditory cortex. J Neurosci. 2011;31:5625–5634. doi: 10.1523/JNEUROSCI.6470-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]