Abstract
The perirhinal cortex (PRh) and basolateral amygdala (BLA) appear to mediate distinct aspects of learning and memory. Here, we used rats to investigate the involvement of the PRh and BLA in acquisition and extinction of associations between two different environmental stimuli (e.g., a tone and a light) in higher-order conditioning. When both stimuli were neutral, infusion of the GABAA, muscimol, or the NMDA receptor (NMDAR) antagonist ifenprodil into the PRh impaired associative formation. However, when one stimulus was neutral and the other was a learned danger signal, acquisition and extinction of the association between them was unaffected by manipulations targeting the PRh. Temporary inactivation of the BLA had the opposite effect: formation and extinction of an association between two stimuli was spared when both stimuli were neutral, but impaired when one stimulus was a learned danger signal. Subsequent experiments showed that the experience of fear per se shifts processing of an association between neutral stimuli from the PRh to the BLA. When training was conducted in a dangerous environment, formation and extinction of an association between neutral stimuli was impaired by BLA inactivation or NMDAR blockade in this region, but was unaffected by PRh inactivation. These double dissociations in the roles of the PRh and BLA in learning under different stimulus and environmental conditions imply that fear-induced activation of the amygdala changes how the brain processes sensory stimuli. Harmless stimuli are treated as potentially harmful, resulting in a shift from cortical to subcortical processing in the BLA.
Introduction
Fear conditioning in laboratory rats is widely used to study how neutral stimuli acquire motivational value. In one protocol, rats exposed to pairings of a tone and shock exhibit fear when subsequently tested with the tone. These fear responses extinguish when the tone is repeatedly presented in the absence of shock. Both forms of learning critically depend on the amygdala, specifically its basolateral nucleus [i.e., basolateral amygdale (BLA)]: BLA infusion of the GABAA agonist muscimol (MUS) or the NMDA receptor (NMDAR) antagonist ifenprodil (IFEN) impairs both the acquisition and extinction of so-called first-order fear to the tone (Wilensky et al., 1999; Rodrigues et al., 2001; Sotres-Bayon et al., 2007; Herry et al., 2008). Rats also learn associations between neutral stimuli and learned danger signals (e.g., the tone). Pairings of a neutral light with the now dangerous tone result in fear of the light; this fear again extinguishes when the light is repeatedly presented in the absence of its tone associate. The BLA is also critical for both forms of learning about the light. Temporary inactivation of the BLA or disruption of NMDAR transmission in the BLA impairs both the acquisition and extinction of so-called second-order fear of the light (Gewirtz and Davis, 1997; Parkes and Westbrook, 2010).
Rats also form associations between two neutral stimuli. Here, the association that results from paired presentations of a neutral light and a neutral tone is revealed once the tone is paired with shock: as a consequence of the tone–shock pairing, the light elicits fear. This association can also be broken through presentations of the neutral light in the absence of its neutral tone associate. However, in contrast to the acquisition and extinction of associations involving either innate (first-order) or learned (second-order) sources of danger, the BLA is not required for the acquisition or extinction of an association between a neutral light and a neutral tone (Parkes and Westbrook, 2010). Some evidence suggests that the perirhinal cortex (PRh) may mediate formation of this association: rats with pretraining PRh lesions are impaired in the expression of fear to the light after conditioning of the tone (Nicholson and Freeman, 2000). However, the use of permanent lesions in this study leaves open the possibility that the formation of an association between neutral stimuli is coded elsewhere in the brain and that the PRh is simply required for its retrieval during testing.
The present study had two aims. The first was to identify the brain region that supports the formation and extinction of an association between a neutral light and a neutral tone. The second aim was to determine whether the brain region that codes the association between the light and the tone is determined by the emotional state of the rat. We show here that the formation and extinction of an association between two neutral stimuli requires the PRh, not the BLA, but only when rats are not afraid; the formation and extinction of this association requires the BLA, not the PRh, when rats are afraid.
Materials and Methods
Subjects.
Subjects were experimentally naive, male, outbred Wistar rats (280–350 g) obtained from a commercial supplier (Animal Resources Centre). They were housed in plastic boxes (67 cm length × 40 cm width × 22 cm height) with food and water continuously available. There were eight rats per box. The boxes were located in a climate-controlled colony room (lights on at 7:00 A.M.). All experimental procedures were approved by the Animal Care and Ethics Committee at the University of New South Wales and in accordance with the National Institutes of Health Guidelines for the Care and Use of Laboratory Animals (revised 1996).
Surgery and drug infusions.
Before behavioral testing, rats were implanted with guide cannulae directed toward the BLA or PRh. Rats were injected intraperitoneally with 1.3 ml/kg ketamine, an anesthetic (Ketapex; Apex Laboratories), at a concentration of 100 mg/ml and 0.3 ml/kg xylazine, a muscle relaxant (Rompun; Bayer), at a concentration of 20 mg/ml. Anesthetized rats were then mounted on a stereotaxic apparatus (David Kopf Instruments), and 26 gauge guide cannulae (Plastics One) were implanted through holes drilled in both hemispheres of the skull. The tips of the guide cannulae were aimed bilaterally at one of two sites: BLA (anteroposterior: −2.6 mm; mediolateral: +4.9 mm; dorsoventral: −8.0 mm); or PRh (anteroposterior: −4.08 mm; mediolateral: +5.00 mm; dorsoventral: −8.0; angled at 10°; Paxinos and Watson, 1997). The guide cannulae were maintained in position with dental cement, and dummy cannulae were kept in each guide at all times except during infusions. Immediately after the surgical procedure, rats were injected intraperitoneally with a prophylactic (0.4 ml) dose of a 300 mg/kg solution of procaine penicillin. Rats were allowed 7 d to recover from surgery, during which time they were handled and weighed daily.
Muscimol, ifenprodil, or vehicle (VEH) was infused bilaterally into the BLA or PRh by inserting a 33 gauge internal cannula into the guide cannula. The internal cannula was connected to a 25 μl glass syringe attached to an infusion pump (Harvard Apparatus) and was projected an additional 1 mm ventral to the tip of the guide cannula. A total volume of 0.3 μl (BLA) or 0.5 μl (PRh) was delivered to both sides at a rate of 0.1 μl/min. The internal cannula remained in place for an additional 1 min after the infusions and was then removed. One day before infusions, the dummy cannula was removed, and the infusion pump was turned on for 3–5 min to familiarize the rats with the procedure and thereby minimize stress on the infusion day.
Drugs.
The GABAA agonist muscimol (Sigma) was dissolved in nonpyrogenic saline (0.9% w/v) to obtain a final concentration of 1 μg/μl. Nonpyrogenic saline was used as a vehicle for experiments studying the effects of muscimol. Ifenprodil, a selective antagonist that blocks the NR2B subunit of NMDAR (Sigma), was dissolved in a solution of 0.9% nonpyrogenic saline (w/v) containing 5% (2-hydrocypropryl)-β-cyclodextrin (Sigma) adjusted to pH 7. This latter solution was used as a vehicle for experiments studying the effects of ifenprodil. Ifenprodil was microinjected into the BLA or PRh at a final concentration of 3.33 μg/μl. The intervals between drug administration and behavior were 20 and 15 min, respectively, for muscimol and ifenprodil. These intervals were selected on the basis of previous experiments reported by Parkes and Westbrook (2010).
Histology.
Subsequent to behavioral testing, subjects received a lethal dose of sodium pentobarbital. The brains were removed and sectioned coronally at 40 μm through the BLA or PRh. Every second section was collected on a slide and stained with cresyl violet. The location of the cannula tip was determined under a microscope by a trained observer, unaware of the subject's group designations, using the boundaries defined by the atlas of Paxinos and Watson (1997). Subjects with inaccurate cannula placements or with extensive damage were excluded from the statistical analysis.
Behavioral apparatus.
Training and testing took place in eight chambers. The side walls and ceiling of each chamber (30 cm height × 27 cm length × 30 cm width) were made of aluminum, and the back and front walls were made of clear plastic. The side walls and ceiling were painted black. The floor was made of stainless steel rods, 2 mm in diameter, spaced 13 mm apart, center to center. A tray below the floor contained bedding material. Each chamber was enclosed in a sound- and light-attenuating shell. A white fluorescent tube and speaker mounted on the back wall of each shell were used, respectively, for the presentation of a light conditioned stimulus (CS; ∼57 lux measured at the center of the chamber) flashing at a rate of 3.5 Hz and a 620 Hz square-wave tone CS measuring 70 dB (A scale) against a background noise of ∼45 dB measured by a digital sound level meter (Dick Smith Electronics). The physical identity of all CSs was fully counterbalanced. The levels of freezing to the CSs did not differ as a function of their physical identity in any experiment.
A custom-built constant-current shock generator, capable of delivering an unscrambled alternating current 50 Hz shock to the floor of each chamber, was used for the presentation of a 0.5 s duration shock at 0.8 mA intensity, unless specified otherwise. The floor of each chamber was cleaned with water after removal of each rat at the end of a session. Illumination for each chamber was provided by an infrared light source (940 ± 25 nm). A camera mounted on the back wall of each shell recorded the behavior of each rat. Each camera was connected to a monitor and a DVD recorder located in another room of the laboratory. This room contained the computer that controlled stimulus presentations via the appropriate software (LabView; National Instruments).
Context exposure.
On the first 2 d of each experiment, rats received two 30 min exposures to the conditioning chamber, separated by a minimum interval of 2 h.
Sensory preconditioning.
Sensory preconditioning involved eight presentations of the visual and auditory stimuli in a 1 h session. Five minutes after placement in the chamber, the stimulus designated as S2 was presented. The duration of each S2 presentation was 30 s, whereas the duration of the stimulus designated as S1 was 10 s. The offset of S2 co-occurred with the onset of S1 in groups receiving paired presentations, whereas S2 and S1 were presented separately for groups receiving unpaired presentations. The intertrial intervals (ITIs) were 6 and 3 min, respectively, for paired and unpaired presentations. Rats remained in the conditioning chamber for 1 min following the final stimulus presentation.
Each first-order conditioning session involved two pairings of the 10 s S1 and the unconditioned stimulus (US; 0.5 s, 0.8 mA footshock). Five minutes after placement in the chamber, S1 was presented. The final 0.5 s of S1 overlapped with the US in groups receiving paired presentations, whereas S1 and the US were presented separately in groups receiving unpaired presentations. The average ITI between paired S1–US presentations was 12 min, and 10 min for unpaired presentations. Rats remained in the chamber for 1 min following the final stimulus presentation. The interval between the two first-order conditioning sessions was 2 h.
Context extinction.
Twenty-four hours after first-order conditioning, rats received two 30 min context extinction sessions, one in the morning and the other in the afternoon. This was done to extinguish any freezing elicited by context and thereby to provide a measure of the freezing elicited by the CSs per se.
Second-order conditioning.
Second-order conditioning consisted in four presentations each of S2 and S1. Five minutes after placement in the chamber, S2 was presented. The offset of the 30 s S2 co-occurred with the onset of the 10 s S1 in paired groups. The ITI between the paired presentations was 5 min. S2 and S1 were presented separately in unpaired groups. The average ITI between presentations was 6 min. Rats remained in the chamber for 1 min following the final stimulus presentation.
Sensory preconditioning extinction.
Five minutes after placement in the chambers, rats received eight S2-alone presentations with an ITI of 3 min. These S2-alone presentations occurred before S1–US pairings (Experiments 3 and 6), so-called pre-extinction (PE; Coppock, 1958), or after S1–US pairings conditioning (Experiments 4A and 4B).
Testing.
Five minutes after placement in the chambers, rats received eight S2-alone presentations, and on the following day eight S1-alone presentations. The ITI was 3 min in each test session.
Data analysis.
Freezing was used to assess conditioned fear. It was defined as the absence of all movement except those related to breathing (Fanselow, 1980). Each rat was observed every 2 s and scored as either “freezing” or “not freezing” by two observers, one of whom was naive to group allocation. A percentage score was calculated for the proportion of the total observations scored as freezing for each rat. There was a high degree of agreement between the two observers, with a Pearson product moment correlation >0.90. Any disagreement was resolved in favor of the score by the naive observer. Data were analyzed with a planned, orthogonal contrast procedure controlling the per contrast error rate (Hays, 1963). Significance was set at α = 0.05.
Results
Experiment 1A: demonstration of sensory preconditioned fear
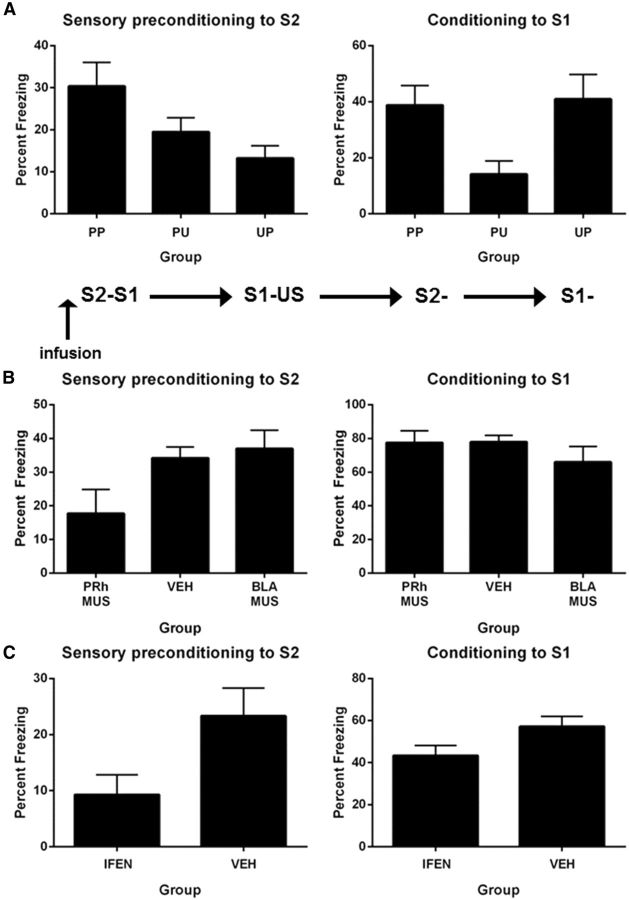
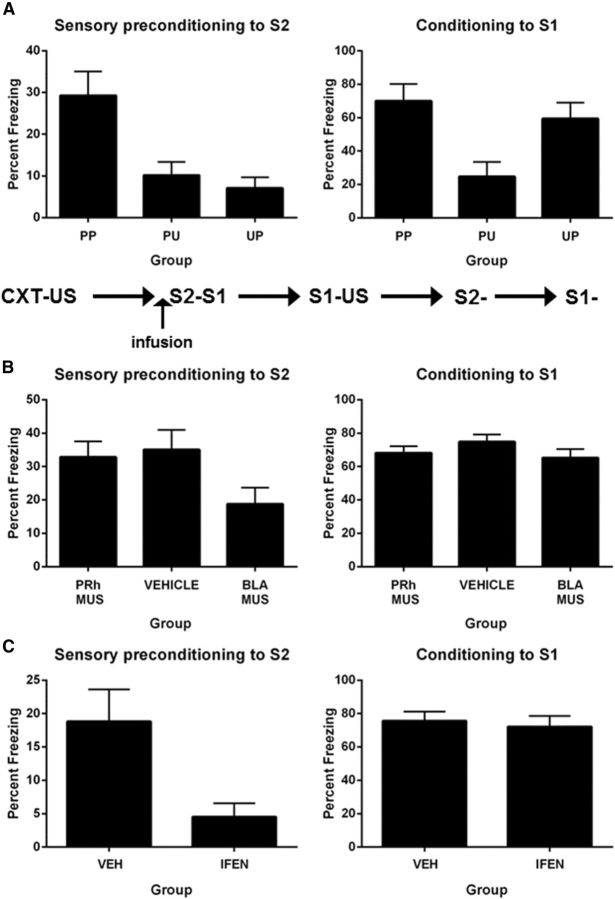
The aim of Experiment 1A was to show that freezing to S2 on test was due to its pairing with the neutral S1 and to the subsequent pairings of S1 and the US. The design is shown in Table 1. On Days 1 and 2, all rats received twice-daily pre-exposure to the context. On Day 3, rats in Groups PP (paired–paired presentation) and PU (paired–unpaired presentation) were exposed to pairings of S2 and S1, whereas Group UP (unpaired–paired presentation) received unpaired presentations of S2 and S1. On Day 4, rats in Groups PP and UP were exposed to pairings of S1 and the US, while Group PU was exposed to unpaired presentations of S1 and the US. Freezing to the context was extinguished in two 30 min sessions on Day 5. All rats were tested with S2 on Day 6 and with S1 on Day 7.
Table 1.
Behavioral demonstration of sensory preconditioned fear
| SPC | First-order | S2 test | S1 test | |
|---|---|---|---|---|
| Group PP | S2 − S1 | S1 − US | S2− | S1− |
| Group PU | S2 − S1 | S1/US | S2− | S1− |
| Group UP | S2/S1 | S1 − US | S2− | S1− |
S1 and S2 were a tone and flashing light stimulus (counterbalanced), and the US was electric footshock. A minus sign (−) between two events denotes that the events were paired; a virgule (/) denotes that the two events were explicitly unpaired; A minus sign (−) following one event denotes that the event was presented alone (in the absence of shock).
One rat did not receive the shock during first-order conditioning and was excluded from the statistical analysis. Groups exposed to pairings of S1 and shock (PP and UP) did not differ in their levels of freezing to S1 (F values <1), but froze significantly more than Group PU, which received unpaired presentations of S1 and shock (F(1,20) = 6.45; p = 0.02). There was a linear increase in freezing across the four conditioning trials (F(1,20) = 38.22; p < 0.05) but no significant linear × group interactions (F(1,20) values <1; p > 0.05).
Figure 1A, left, shows the mean (±SEM) levels of freezing to S2 at test, averaged across the eight presentations. Group PP froze significantly more to S2 than Groups PU and UP (F(1,20) = 7.71; p < 0.05), who did not differ from each other in their levels of freezing (F(1,20) < 1.3; p > 0.05). Figure 1A, right, shows the mean (±SEM) levels of freezing to S1 at test, averaged across the eight presentations. Groups PP and UP, which received S1–US pairings, did not differ from each other in their levels of freezing to S1 (F(1,20) < 1; p > 0.05) but froze significantly more to S1 than the group that received unpaired presentations of S1 and the US (F(1,20) = 11.73; p < 0.05).
Figure 1.
Sensory preconditioning of an association between S2 and a neutral S1. A, Behavioral demonstration of sensory preconditioning (Experiment 1A: Group PP, n = 7; Group PU, n = 8; and Group UP, n = 8). Left, Freezing to S2 during the test for sensory preconditioned fear. Right, Freezing to S1 during the test for first-order conditioned fear. B, The roles of the PRh and BLA in the acquisition of a sensory preconditioned association (Experiment 1B: Group PRh-VEH, n = 10; Group PRh-MUS, n = 7; Group BLA-VEH, n = 8; Group BLA-MUS, n = 8). Left, Freezing to S2 during the test for sensory preconditioned fear. Right, Freezing to S1 during the test for first-order conditioned fear. C, The involvement of PRh NMDAR in acquisition of a sensory preconditioned association (Experiment 1C: Group VEH, n = 14; Group IFEN, n = 14). Left, Freezing to S2 during the test for sensory preconditioned fear. Right, Freezing to S1 during the test for retention of first-order conditioned fear. Data shown are means ± SEM.
These results show that freezing to S2 was contingent on its pairings with S1 and on the subsequent pairings of S1 and the US. Thus, freezing to S2 was due to its association with S1, rather than a generalization of freezing to S2 from S1, and to the association between S1 and the US, rather than to any intrinsic ability of S1 to condition freezing to S2.
Experiment 1B: the association between S2 and a neutral S1 requires the PRh but not the BLA
Experiment 1B examined the roles of the PRh and the BLA in coding the association between S2 and a neutral S1. Rats were implanted with bilateral cannulae targeting the PRh or BLA and were allowed 5 d for recovery. On Days 1 and 2, rats received twice-daily exposures to the chambers. On Day 3, rats in Groups PRh-MUS and BLA-MUS received an infusion of muscimol in the PRh or BLA, respectively, whereas those in Groups PRh-VEH and BLA-VEH were infused with VEH in the PRh and BLA, respectively. Twenty minutes later, all rats received eight paired presentations of S2 and S1. On Day 4, all rats received two S1–US pairings in the morning session and two pairings in the afternoon session. On Day 5, all rats received a context extinction session in the morning and another in the afternoon. On Days 6 and 7, rats were tested with S2 and S1, respectively.
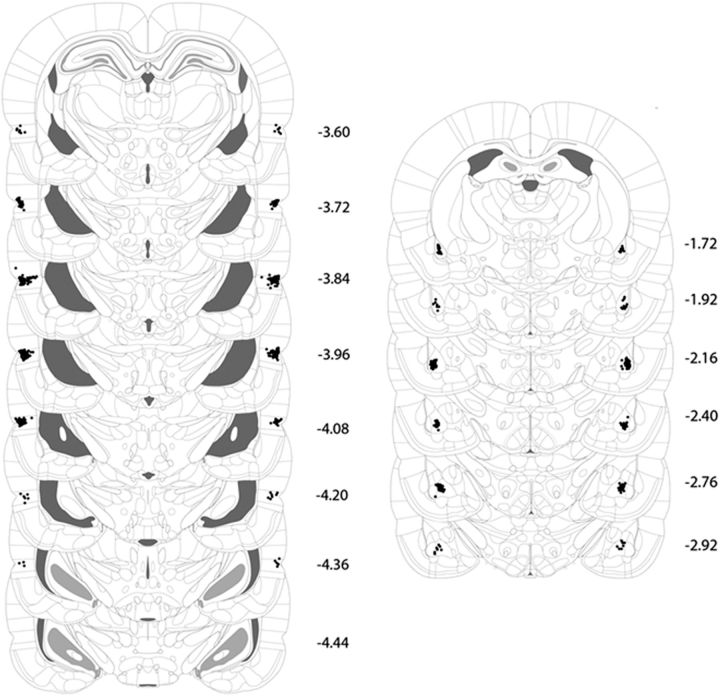
Histology
Figure 2 shows the locations of injection cannulae tips for rats in this and the remaining experiments. Plotted points represent the ventral point of the cannula track. Five rats in Experiment 1B were excluded due to misplaced cannulae, resulting in the following group sizes: Group PRh-VEH, n = 10; Group PRh-MUS, n = 7; Group BLA-VEH, n = 8; and Group BLA-MUS, n = 8.
Figure 2.
Microinfusion cannula placements as verified on Nissl-stained coronal sections for the PRh (left) and BLA (right). Sections are based on the atlas of Paxinos and Watson (1997).
Behavior
As there were no differences between PRh and BLA rats infused with saline at any stage of the experiment, the data for these rats were combined to form a single control group (Group VEH). There were no differences among the groups in acquisition of conditioned fear (F(1,30) values <2.2; p > 0.05). The mean (±SEM) levels of freezing on the final S1 trial were 72 ± 6.7% in Group VEH, 68 ± 7.5% in Group PRh-MUS, and 68 ± 6.3% in Group BLA-MUS. Figure 1B, left, shows the mean (±SEM) levels of freezing to S2 at test, averaged across the eight presentations. The PRh was critical for the association between S2 and the neutral S1 as rats infused with muscimol into the PRh before the S2–S1 pairings froze significantly less to S2 than Groups VEH and BLA-MUS combined (F(1,30) = 7.32; p < 0.05). In contrast, the association between S2 and the neutral S1 did not require the BLA as there was just as much freezing to S2 among rats in Group BLA-MUS as in Group VEH (F values <1). Figure 1B, right, shows the mean (±SEM) levels of freezing to S1 at test, averaged across the eight presentations. There were no significant differences between the groups (F(1,30) < 1.9; p > 0.05), showing that the deficit in sensory preconditioned freezing to S2 among rats in Group PRh-MUS was not due to impaired conditioning of S1.
Two additional groups were used to examine whether NMDAR transmission in the PRh was critical for the plasticity underlying the association between S2 and the neutral S1. The protocol was identical to that described except that S2–S1 pairings were conducted under a PRh infusion of IFEN (Group IFEN) or VEH (Group VEH). Seven rats were excluded due to misplaced cannulae, resulting in n = 14 in each group.
Groups VEH and IFEN did not differ in the acquisition of conditioned fear (F(1,26) < 2.4; p > 0.05). The mean (±SEM) levels of freezing on the final S1 trial were 57 ± 8.9% in Group VEH and 53 ± 6.9% in Group IFEN. Figure 1C shows mean (±SEM) test levels of freezing to S2, averaged across the eight presentations (Fig 1C, left), and S1, averaged across the eight presentations (Fig 1C, right). The association between S2 and the neutral S1 was impaired by an infusion of ifenprodil as Group IFEN froze significantly less when tested with S2 than Group VEH (F(1,26) = 5.15; p < 0.05). The difference between the levels of freezing to S2 was not due to differences in freezing to S1 as rats in both groups exhibited substantial and similar (F(1,26) = 3.97; p > 0.05) levels of freezing to S1.
Experiment 2: extinction of the association between S2 and the neutral S1 requires neuronal activity, specifically, NMDAR neurotransmission in the PRh
This experiment had two aims. The first was to show that S2-alone presentations interpolated between S2–S1 pairings and S1–US pairings extinguish the association that mediates fear responses to S2. The second aim was to show that neuronal activity in the PRh, specifically NMDAR neurotransmission, is critical for extinction of the association between S2 and the neutral S1.
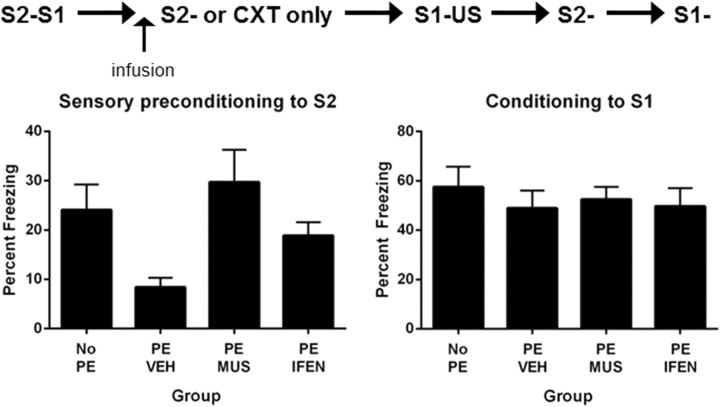
All rats were implanted with bilateral cannulae targeting the PRh and were allowed 5 d recovery. On Days 1 and 2, rats were exposed to the chambers. On Day 3, all rats received S2–S1 pairings, and on Day 4 three groups of rats were exposed to eight presentations of S2 alone. This so-called PE occurred under a PRh infusion of MUS (PE-MUS), IFEN (PE-IFEN), or VEH (saline for half the rats and saline plus cyclodextrin for the remainder; PE-VEH). A fourth group of rats (NO-PE) were exposed to the chambers but did not received S2-alone presentations. Equal numbers of rats in this group received a PRh infusion of muscimol, ifenprodil, or vehicle. On Day 5, all rats received S1–US pairings in the manner previously described, and on Day 6, two sessions of context extinction to reduce levels of freezing to the context alone. On Days 7 and 8, rats were tested with S2 and S1, respectively.
Histology
Four rats were excluded due to a misplaced cannula, yielding the following group sizes: PE-MUS, n = 9; PE-IFEN, n = 9; PE-VEH, n = 9; and NO-PE, n = 9.
Behavior
First-order conditioning was successful. All rats learned to fear S1, and there were no significant differences among the groups in rate of acquisition (F(1,32) values <1; p > 0.05) or overall levels of freezing (F(1,32) values <1; p > 0.05). The mean (±SEM) levels of freezing on the final S1 trial were 53 ± 8.0% in Group PE-MUS, 53 ± 8.0% in Group PE-IFEN, 60 ± 7.0% in Group PE-VEH, and 62 ± 9.0% in Group NO-PE.
Figure 3 shows the test levels of freezing (mean ± SEM) to S2 (Fig. 3, left) and S1 (Fig. 3, right). S2-alone presentations extinguished its association with S1 as Group PE-VEH froze significantly less to S2 than the other three groups combined (F(1,32) = 9.24; p < 0.05). There were no statistically significant differences between the levels of freezing to S2 in the group that was not extinguished to S2 (NO-PE) and the groups extinguished under muscimol or ifenprodil (F values <1), showing that inactivation of the PRh or disruption of NMDAR neurotransmission in the PRh impaired extinction of the association between S2 and the neutral S1. There were no statistically significant differences between the levels of freezing to S2 in groups PE-MUS and PE-IFEN (F(1,32) = 2.9; p > 0.05), indicating that extinction of the S2–S1 association had been equally impaired by these treatments. None of the differences in freezing to S2 were due to variations in conditioning to S1; freezing to S1 was substantial, and there were no statistically significant differences among the groups (F(1,32) values <1; p > 0.05). These results show that NMDAR neurotransmission in the PRh is critical for extinction of the association between S2 and the neutral S1, while previous results have shown that neuronal activity in the BLA is not required for extinction of this association (Parkes and Westbrook, 2010; Experiment 6).
Figure 3.
Pre-extinction of a sensory preconditioned association requires activation of the PRh and activation of NMDAR containing the NR2B subunit (Experiment 2: PE-MUS, n = 9; PE-IFEN, n = 9; PE-VEH, n = 9; NO-PE, n = 9). Left, Freezing to S2 during the test for pre-extinction of a sensory preconditioned association. Right, Freezing to S1 during the test for retention of first-order conditioned fear. Data shown are means ± SEM.
Experiment 3A: demonstration of second-order conditioned fear
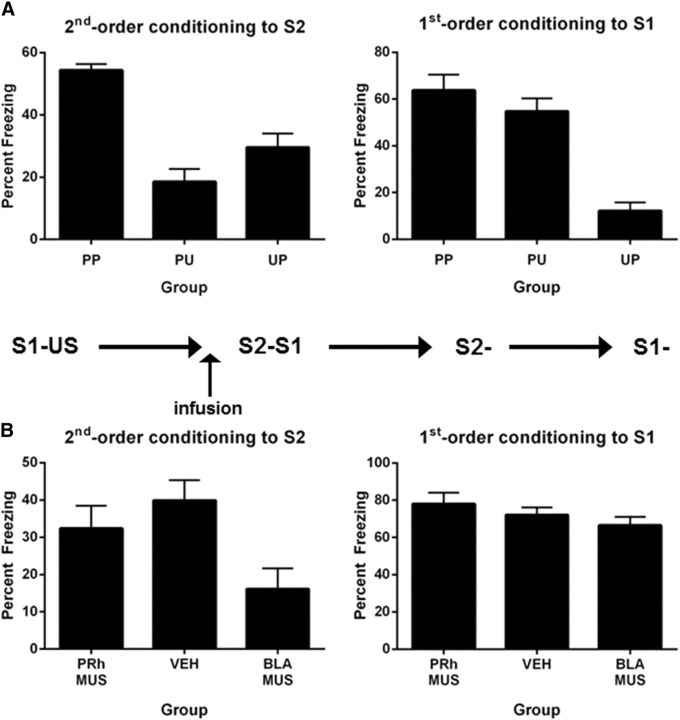
The aim of this experiment was to show that second-order conditioned fear was due to S1–US and S2–S1 pairings. The design is shown in Table 2. On Days 1 and 2, rats were exposed to the chambers. On Day 3, rats in Groups PP and PU received S1–US pairings, whereas Group PU was exposed to unpaired presentations of S1 and the US. On Day 4, freezing to the context was extinguished in two 30 min sessions, one in the morning and the other in the afternoon. On Day 5, Groups PP and UP were exposed to pairings of S2 and S1, whereas Group UP received unpaired presentations of S2 and S1. All rats received two sessions (morning and afternoon) of context extinction on Day 6, and were then tested for freezing to S2 on Day 7, and to S1 on Day 8.
Table 2.
Behavioral demonstration of second-order conditioned fear
| First-order | Second-order | S2 test | S1 test | |
|---|---|---|---|---|
| Group PP | S1 − US | S2 − S1 | S2− | S1− |
| Group PU | S1 − US | S2/S1 | S2− | S1− |
| Group UP | S1/US | S2 − S1 | S2− | S1− |
S1 and S2 were a tone and flashing light stimulus (counterbalanced), and the US was electric footshock. A minus sign (−) between two events denotes that the events were paired; a virgule (/) denotes that the two events were explicitly unpaired; a minus sign (−) following one event denotes that the event was presented alone (in the absence of shock).
First-order conditioning was successful. Rats in Groups PP and PU froze more to S1 than those in Group UP (F(1,21) = 17.24; p < 0.05) but did not differ from each other (F(1,21) = 3.06; p > 0.05). The mean (±SEM) level of freezing on the final S1 trial was 50 ± 8% in Group PP, 51 ± 10%) in Group PU, and 18 ± 8% in Group UP. Second-order conditioning was also successful in that rats in Group PP froze significantly more to S2 across its pairings with S1 than those in Groups PU and UP (F(1,21) = 11.42; p < 0.05), who did not differ from each other (F values <1). First-order conditioned freezing remained intact across the second-order conditioning stage: rats in Groups PP and PU did not differ in their levels of freezing to S1 (F values <1) but froze significantly more to S1 than those in Group UP (F(1,21) = 70.97; p < 0.05).
Figure 4A shows the test levels of freezing (mean ± SEM) to S2 (Fig 4A, left) and S1 (Fig 4A, right). Group PP froze significantly more to S2 than Groups PU and UP (F(1,21) = 45.25; p < 0.05), who did not differ from each other in their levels of freezing (F(1,21) = 4.5; p > 0.05), showing that freezing to S2 was due to its pairing with S1 and to the prior pairings of S1 with the US. Groups PP and PU did not differ from each other in their levels of freezing to S1 (F(1,21) < 1.4; p > 0.05) but froze significantly more to S1 than Group UP, who received unpaired presentations of S1 and the US (F(1,21) = 49.19; p < 0.05), confirming that first conditioning was intact and that generalization of freezing from S1 to S2 was negligible in Group PU.
Figure 4.
Second-order conditioning of an association between S2 and a dangerous S1. A, Behavioral demonstration of second-order conditioned fear (Experiment 3A: Group PP, n = 8; Group PU, n = 8; Group UP, n = 8). Left, Freezing to S2 during the test for second-order fear. Right, Freezing to S1 during the test for first-order conditioned fear. B, The roles of the PRh and BLA in acquisition of second-order conditioned fear (Experiment 3B: Group PRh-VEH, n = 8; Group PRh-MUS, n = 9; Group BLA-VEH, n = 8; Group BLA-MUS, n = 8). Left, Freezing to S2 during the test for second-order fear. Right, Freezing to S1 during the test for first-order conditioned fear. Data shown are means ± SEM.
Experiment 3B: the association between S2 and the conditioned S1 requires the BLA but not the PRh
Experiment 3B examined the roles of the PRh and the BLA in coding the association between S2 and the conditioned S1. Half of the rats in this experiment were implanted with bilateral cannulae targeting the PRh, while the remaining rats were implanted with bilateral cannulae targeting the BLA. Rats were allowed 5 d of recovery. All rats then received context familiarization (Days 1 and 2), first-order conditioning of S1 (Day 3), and context extinction (Day 4) in the manner described for Group PP in Experiment 3A. On Day 5, rats in Groups PRh-MUS and BLA-MUS received an infusion of muscimol, whereas rats in Groups PRh-VEH and BLA-VEH were infused with vehicle. Twenty minutes later, all rats received eight paired presentations of S2 and the first-order conditioned S1. On Day 6, rats received two sessions of context extinction. Rats were then tested with S2 on Day 7 and S1 on Day 8 in the manner described previously.
Histology
Eight rats were excluded from Experiment 3B due to misplaced cannulae. This resulted in the following group sizes: Group PRh-VEH, n = 8; Group PRh-MUS, n = 9; Group BLA-VEH, n = 8; and Group BLA-MUS, n = 8.
Behavior
As there were no differences between PRh and BLA rats infused with saline at any stage of the experiment, the data for these rats were combined to form a single control group (Group VEH). First-order conditioning was successful. All rats froze to S1, and there were no significant differences among the groups in overall levels of freezing to S1 (F(1,30) values <2.25; p > 0.05) or in the rate of its acquisition (F(1,30) values <2.44; p > 0.05). The mean (±SEM) levels of freezing on the final S1 trial were 70 ± 6% in Group VEH, 65 ± 7% in Group BLA-MUS, and 75 ± 5% in Group PRh-MUS. During second-order conditioning, infusion of muscimol into PRh and BLA depressed the freezing response: Group VEH froze significantly more to S2 and S1 than Groups PRh-MUS and BLA-MUS (F(1,30) < 23.11; p < 0.05). There were no differences in freezing between the latter groups (F(1,30) < 1.07; p < 0.05).
Figure 4B shows the test levels of freezing (mean ± SEM) to S2 (Fig. 4B, left) and S1 (Fig. 4B, right). Rats exposed to pairings of S2 and the conditioned S1 under vehicle or a PRh infusion of muscimol did not differ in their levels of freezing to S2 (F values <1), showing that the formation of this association was not dependent on the PRh. However, the levels of freezing to S2 in the vehicle- and PRh-infused groups were significantly greater than those exhibited by rats that received the pairings under a BLA infusion of muscimol (F(1,30) < 6.25; p < 0.05), showing that formation of the association between S2 and the fear-eliciting S1 was dependent on the BLA. Although muscimol infusion into both the PRh and BLA depressed freezing across the S2–S1 pairings, there were no long-term effects on freezing to S1 as there were no significant differences between muscimol- and vehicle-infused groups in the test levels of freezing to S1 (F(1,30) values <1.61; p > 0.05).
Together with the previous findings, these results show that the roles of the PRh and BLA in the formation of an association between S2 and S1 are doubly dissociable depending on the value of S1: the PRh supports associative formation when S1 is neutral but not when it has been conditioned; and the BLA supports associative formation when S1 has been conditioned but not when it is neutral.
Experiment 4A: extinction of sensory preconditioned fear does not require the PRh or NMDAR neurotransmission in the PRh
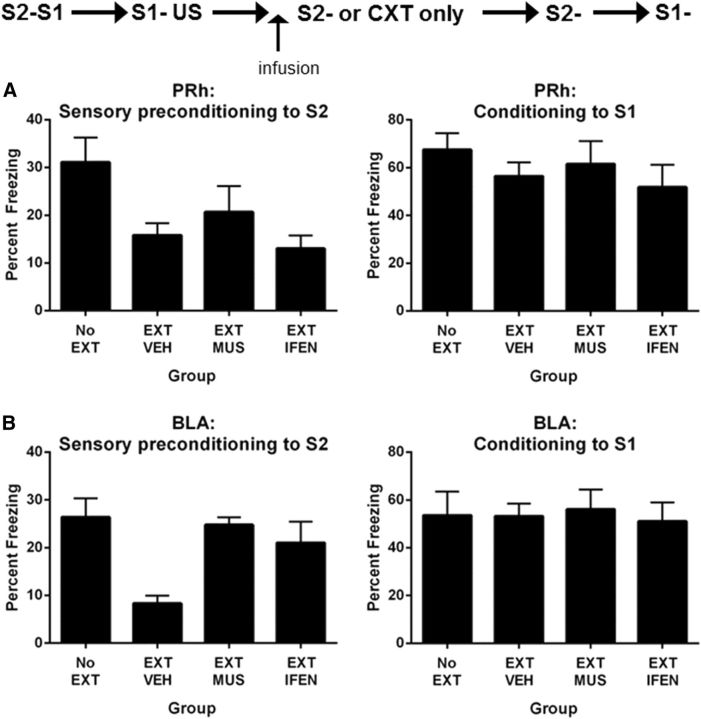
Neuronal activity, specifically NMDAR neurotransmission, in the PRh was critical for the extinction of the association between S2 and the neutral S1. The present experiment examined the role of the PRh in extinction of the fear elicited by S2. All rats were implanted with cannulae in the PRh. After recovery and familiarization with the chambers (Days 1 and 2), all rats received S2–S1 pairings on Day 3 and S1–US pairings on Day 4, and two sessions of context extinction (morning and afternoon) on Day 5. On Day 6, three groups of rats were exposed to eight presentations of S2 alone. Before this session, rats received a PRh infusion of muscimol (Group EXT-MUS), ifenprodil (Group EXT-IFEN), or vehicle (saline for half the rats and saline plus cyclodextrin for the remainder; Group EXT-VEH). A fourth group of rats, Group NO-EXT, were exposed to the chambers but did not receive S2-alone presentations. Rats in Group NO-EXT received an infusion of muscimol, ifenprodil, or vehicle. On Days 6 and 7, rats were tested with S2 and S1, respectively, in the manner described previously.
Histology
One rat was excluded from Experiment 4A due to a misplaced cannula, yielding the following group sizes: EXT-MUS, n = 8; EXT-IFEN, n = 8; EXT-VEH, n = 8; and NO- EXT, n = 7.
Behavior
First-order conditioning was successful. All rats learned to fear S1, and there were no differences among the groups in the overall levels of freezing to S1 (F(1,27) values <1; p > 0.05) or in the rate at which freezing was acquired (F(1,27) values <1; p > 0.05). The mean (±SEM) levels of freezing on the final S1–US trial were 68 ± 8% in Group EXT-VEH, 63 ± 10% in Group EXT-MUS, 65 ± 9% in Group EXT-IFEN, and 60 ± 8% in Group NO-EXT. During the extinction of sensory preconditioned fear, the freezing elicited by S2 declined linearly across the S2-alone presentations (F(1,21) = 12.63; p < 0.05). The PRh infusion of muscimol depressed freezing to S2, as rats in Group EXT-MUS froze significantly less often than those in Groups EXT-VEH and EXT-IFEN (F(1,21) = 14.24; p < 0.05), who did not differ from each other (F(1,21) < 1; p > 0.05). The linear × group interactions were not significant (largest: F(1,21) = 2.42; p > 0.05).
Figure 5A shows test levels of freezing (mean ± SEM) to S2 (Fig. 5, left) and S1 (Fig. 5, right). Rats that did not receive the S2-alone presentations (Group NO-EXT) froze significantly more to S2 than the other three groups that received these presentations (F(1,27) = 8.44; p < 0.05). Group EXT-VEH did not differ in their levels of freezing to S2 compared with Groups EXT-MUS and EXT-IFEN combined (F(1,27) < 1), and freezing to S2 did not differ between the latter groups (F(1,27) < 1.67; p > 0.05), showing that extinction had not been impaired by temporary inactivation of the PRh or by disruption of NMDA neurotransmission in this region. Infusion of muscimol or ifenprodil before extinction of S2 had no effect on retention of first-order conditioned fear as there were no statistically significant differences among the groups in the test levels of freezing to S1 (F(1,27) values <1.08; p > 0.05).
Figure 5.
The roles of the PRh and BLA in extinction of sensory preconditioned fear. A, Extinction of sensory preconditioned fear does not require activation of the PRh or NMDAR in the PRh (Experiment 4A: EXT-MUS, n = 8; EXT-IFEN, n = 8; EXT-VEH, n = 8; NO-EXT, n = 7). Left, Freezing to S2 during the test for extinction of sensory preconditioned fear. Right, Freezing to S1 during the test for retention of first-order conditioned fear. B, Extinction of sensory preconditioned fear requires activation of the BLA and NMDAR in the BLA (Experiment 4B: EXT-MUS, n = 8; EXT-IFEN, n = 8; EXT-VEH, n = 9; NO-EXT, n = 9). Left, Freezing to S2 during the test for extinction of sensory preconditioned fear. Right, Freezing to S1 during the test for retention of first-order conditioned fear. Data shown are means ± SEM.
Experiment 4B: extinction of sensory preconditioned fear requires the BLA and NMDAR neurotransmission in the BLA
The aim of this experiment was to show that the extinction of the fear elicited by the sensory preconditioned S2 was dependent on neuronal activity in the BLA, specifically NMDAR neurotransmission. All rats were implanted with bilateral cannulae targeting the BLA and were allowed 5 d of recovery. The procedure was identical to that in the previous experiment. Rats were familiarized with the chamber on Days 1 and 2; were exposed to S2–S1 pairings on Day 3, and first-order conditioning of S1 on Day 4; and were subjected to extinction of context-conditioned freezing on Day 5. On Day 6, rats received a BLA infusion of muscimol (EXT-MUS), ifenprodil (EXT-IFEN), or vehicle (EXT-VEH) followed 20 min later by S2-alone presentations. Rats in Group NO-EXT were infused with muscimol, ifenprodil, or vehicle into the BLA 20 min before exposure to the context alone. On Days 7 and 8, rats were tested with S2 and S1, respectively, in the manner described previously.
Histology
Two rats were excluded due to misplaced cannulae yielding the following group sizes: EXT-MUS, n = 8; EXT-IFEN, n = 8; EXT-VEH, n = 9; and NO-EXT, n = 9.
Behavior
First-order conditioning was successful. The rate of acquisition of freezing responses across S1–US pairings did not differ between groups (F(1,30) values <1; p > 0.05). The mean (±SEM) levels of freezing on the final S1–US trial were 58 ± 9% in Group EXT-VEH, 55 ± 8% in Group EXT-MUS, 53 ± 6% in Group EXT-IFEN, and 62 ± 10% in Group NO-EXT. Sensory preconditioned freezing declined linearly across the S2-alone presentations (F(1,21) = 12.63; p < 0.05). Muscimol in the BLA depressed freezing, as rats in Group EXT-MUS froze less to S2 than those in Groups EXT-VEH and EXT-IFEN (F(1,22) = 13.12; p < 0.05), who did not differ from each other (F(1,22) = 2.43; p > 0.05). Figure 5B shows test levels of freezing (mean ± SEM) to S2 (Fig. 5B, left) and S1 (Fig. 5B, right). Extinction of sensory preconditioned fear was successful: Group NO-EXT froze significantly more to S2 than the other three groups combined (F(1,30) = 5.39; p < 0.05). Muscimol or ifenprodil infusion into the BLA impaired extinction of sensory preconditioning freezing: rats in Groups EXT-MUS and EXT-IFEN did not differ in their levels of freezing to S2 (F(1,30) < 1; p > 0.05), but these levels were significantly less than those by rats in Group EXT-VEH (F(1,30) = 14.39; p < 0.05). In the test of S1, a mechanical failure of the DVD caused the loss of data from four rats (two rats from the EXT-VEH Group, one rat from the EXT-MUS Group, and one rat from the EXT-IFEN Group). Analysis of the data from the remaining rats showed that infusion of muscimol or ifenprodil before extinction of the sensory preconditioned S2 had no effect on retention of first-order conditioned fear as there were no statistically significant differences among the groups in the test levels of freezing to S1 (F(1,26) values <1; p > 0.05).
Together with our previous findings, these results show that the roles of the PRh and BLA in the extinction of an association between S2 and S1 are also doubly dissociable depending on the value of S1: the PRh supports extinction when S1 is neutral but not when it has been conditioned; and the BLA supports extinction when S1 has been conditioned but not when it is neutral (Parkes and Westbrook, 2010).
Experiment 5A: demonstration of sensory preconditioned fear in a dangerous context
The protocols used in the previous experiments exposed rats to S1–US pairings and then S2–S1 pairings, or to S2–S1 pairings and then to S1–US pairings. The rats in each of these protocols thus differed in their history: the former rats had been subjected to fear conditioning before they received the S2–S1 pairings, whereas the latter rats had not been subjected to such a history. The remaining experiments examine the brain regions that code associations between S2 and a neutral S1 but in rats that had been previously subjected to fear conditioning, specifically, context fear conditioning.
The aim of Experiment 5A was to show that paired presentations of S2 and a neutral S1 result in sensory preconditioned fear of S2 when the pairings occurred in a dangerous context. The design is shown in Table 3. On Days 1 and 2, rats were exposed to the chambers. On Day 3, they were placed in the chambers for 5 min and shocked twice, after 3 and 4 min. Each shock was 0.5 mA of 0.5 s duration. Three hours later, rats in Groups PP and PU received paired presentations of S2 and S1, whereas those in Group UP received unpaired presentations of S2 and S1. On Day 4, rats in Groups PP and UP received paired presentations of S1 and the US, whereas those in Group PU received unpaired presentations of S1 and the US. On Day 5, rats received a context extinction session in the morning and a second extinction session in the afternoon. On Days 6 and 7, rats were tested with S2 and S1, respectively, in the manner described previously.
Table 3.
Behavioral demonstration of sensory preconditioning in a dangerous context
| CXT cond | SPC | First-order | S2 test | S1 test | |
|---|---|---|---|---|---|
| Group PP | CXT − US | S2 − S1 | S1 − US | S2− | S1− |
| Group PU | CXT − US | S2 − S1 | S1/US | S2− | S1− |
| Group UP | CXT − US | S2/S1 | S1 − US | S2− | S1− |
S1 and S2 were a tone and flashing light stimulus (counterbalanced), and the US was electric footshock. A minus sign (−) between two events denotes that the events were paired; a virgule (/) denotes that the two events were explicitly unpaired; a minus sign (−) following one event denotes that the event was presented alone (in the absence of shock). SPC, Sensory preconditioning; CXT cond, context conditioning.
None of the rats froze in the context before the shocks on Day 3, and all of them froze after the shocks. Freezing increased linearly across exposure to the context (F(1,24) = 155.07; p < 0.05), but there were no differences among the groups (F(1,24) values <1.81; p > 0.05), and the group × linear trend interactions were not significant (F(1,24) values <2.33; p > 0.05). Freezing declined linearly across exposure to the context in the afternoon session in which S2 and S1 were presented (F(1,24) = 16.18; p < 0.05), but there were no differences among the groups (F(1,24) values <2.31; p > 0.05), and the group × linear trend interactions were not significant (F(1,24) values <1; p > 0.05).
First-order conditioning on Day 4 was successful. Freezing increased linearly across the four S1–shock pairings (F(1,24) = 30.38; p < 0.05). Groups PP and UP froze more to S1 than Group PU, but this difference only approached significance (F(1,24) = 4.01; p < 0.06), probably because the shocks interacted with the previous context conditioning to increase freezing levels among all rats. The linear × group interactions were not significant (F(1,24) values <3.09; p > 0.05). The mean (±SEM) levels of freezing on the final S1 presentation were 53 ± 10% in Group PP, 50 ± 11% in Group UP, and 22 ± 11% in Group PU. Figure 6A shows test levels (mean ± SEM) of freezing to S2 (Fig. 6A, left) and S1 (Fig. 6A, right). Group PP froze significantly more to S2 than Groups PU and UP (F(1,24) = 15.81; p < 0.05), but freezing in the latter groups did not differ (F values <1). Finally, Group PU froze less to S1 than Groups PP and UP combined (F(1,24) = 11.84; p < 0.05), but freezing in the latter groups did not differ (F values <1). These results show that pairings of S2 and the neutral S1 in a dangerous context followed by pairings of S1 and shock resulted in sensory preconditioned fear of S2.
Figure 6.
Sensory preconditioning in a dangerous context. A, Behavioral demonstration of acquisition of a sensory preconditioned association in a dangerous context (Experiment 5A: Group PP, n = 9; Group PU, n = 8; Group UP, n = 8). Left, Freezing to S2 during the test for sensory preconditioning. Right, Freezing to S1 during the test for first-order conditioned fear. Data shown are means ± SEM. B, Acquisition of a sensory preconditioned association in a dangerous context requires activation of the BLA but not the PRh (Experiment 5B: BLA-MUS, n = 8; VEH, n = 10; PRh-MUS, n = 7). Left, Freezing to S2 during the test for sensory preconditioning. Right, Freezing to S1 during the test for first-order conditioned fear. C, Acquisition of a sensory preconditioned association in a dangerous context requires activation of NMDAR in the BLA (Experiment 5C: Group VEH, n = 8, Group IFEN, n = 7). Left, Freezing to S2 during the test for sensory preconditioning. Right, Freezing to S1 during the test for first-order conditioned fear. Data shown are means ± SEM.
Experiment 5B: in a dangerous context, the association between S2 and a neutral S1 requires the BLA but not the PRh
Experiment 5B examined the roles of the PRh and BLA in the formation of an association between S2 and a neutral S1 in a dangerous context. Rats were implanted with cannulae targeting the PRh or BLA in both hemispheres and were allowed 7 d for recovery. The procedure was that used for Group PP in Experiment 5A. All rats received context exposure on Days 1 and 2, context conditioning in the morning session of Day 3, and S2–S1 pairings in the afternoon session. Twenty minutes before the afternoon session, half of the PRh rats and half of the BLA rats received an infusion of muscimol, while the remaining rats received an infusion of saline. All rats received pairings of S1–US on Day 4, and context extinction in the morning and afternoon of Day 5. Rats were tested with S2 on Day 6 and S1 on Day 7.
Histology
Three rats were excluded due to misplaced cannulae, yielding the following group sizes: BLA-VEH, n = 5; BLA-MUS, n = 8; PRh-VEH, n = 5; and PRh-MUS, n = 7.
Behavior
As there were no differences between PRh and BLA rats infused with saline at any stage of the experiment, the data for these rats were combined to form a single control group. All rats acquired fear to the context during the morning session of Day 3, in which they were placed in the context and shocked (F(1,22) = 148.77; p < 0.05). There was no overall difference between the groups (F values <1) and no group × linear trend interaction (F values <1). During the afternoon session in which S2 was paired with S1, Group VEH froze more than Groups PRh-MUS and BLA-MUS combined (F(1,22) = 32.75; p < 0.05), and the latter groups did not differ (F(1,22) = 1.98; p > 0.05). There was a linear decline in freezing across the session (F(1,22) = 11.97; p < 0.05), but the linear × group interactions were not significant (F(1,22) values <2.34; p > 0.05).
First-order conditioning was successful. The rate of acquisition of first-order fear did not differ between groups (F(1,22) values <1; p > 0.05). The mean (±SEM) levels of freezing on the final S1 → shock trial were 50 ± 7% in Group VEH, 51 ± 0% in Group PRh-MUS, and 58 ± 9.6% in Group BLA-MUS. Figure 6B shows test levels (mean ± SEM) of freezing to S2 (Fig. 6B, left) and S2 (Fig. 6B, right). Group BLA-MUS froze significantly less to S2 than Groups VEH and PRh-MUS (F(1,22) = 4.89; p < 0.05), and the latter groups did not differ from each other (F(1,22) < 1; p > 0.05). In contrast, there were no differences between the groups in freezing to S1 at test (F(1,22) < 2.1; p > 0.05). Thus, inactivation of the BLA impaired formation of an association between S2 and a neutral S1 in the dangerous context, and this effect was not due differences in conditioning of S1.
Two additional groups were used to examine whether NMDAR transmission in the BLA was critical for the plasticity underlying the association between S2 and a neutral S1 in the dangerous context. The protocol was identical to that described above except that S2–S1 pairings were conducted under a BLA infusion of ifenprodil (Group IFEN) or vehicle (Group VEH). One rat was excluded due to a misplaced cannula, resulting in eight rats in Group VEH and seven rats in Group IFEN.
Rats acquired fear in response to the context during the morning session of Day 3 in which they were placed in the context and shocked (F(1,13) = 64.93; p < 0.05). There was no difference between the groups (F values <1) and no group × linear trend interaction (F values <1). In the afternoon session in which S2 was paired with S1, context freezing declined linearly across the session (F(1,13) = 68.84; p < 0.05), but the overall effect of group (F values <1) and the group × linear trend interaction (F values <1) were not significant.
First-order conditioning was successful. The rate of acquisition of fear to S1 did not differ among groups (F(1,13) < 1.03; p > 0.05). The mean (±SEM) levels of freezing in the final S1–US trial were 75 ± 10% in Group VEH and 89 ± 9% in Group IFEN. Figure 6C shows test levels (mean ± SEM) of freezing to S2 (Fig. 6C, left) and S1 (Fig. 6C, right). Group VEH froze significantly more to S2 than Group IFEN (F(1,13) = 6.64; p < 0.05), showing that, in a dangerous context, NMDAR neurotransmission in the BLA is required for formation of an association between S2 and a neutral S1. The difference in freezing to S2 was not due to differences in first-order conditioning, as levels of freezing to S1 did not differ between groups (F values <1). Together with the earlier results, these findings show that the roles of the PRh and BLA in the formation of an association between S2 and S1 are doubly dissociable depending on the value of the training context: the PRh supports associative formation in a safe or neutral context but not in a dangerous one; in contrast, the BLA supports associative formation in a dangerous context but not in a neutral one.
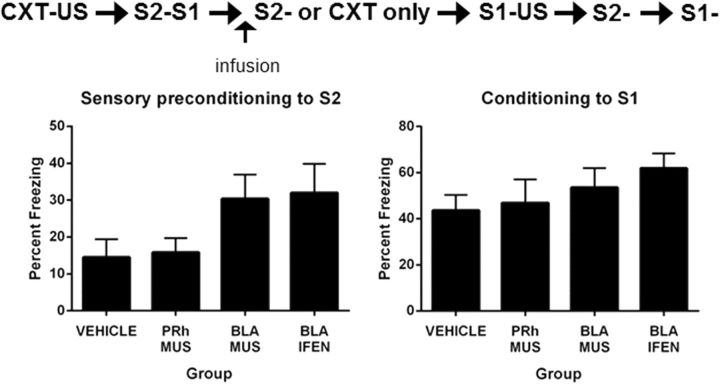
Experiment 6: in a dangerous context, extinction of an association between S2 and a neutral S1 requires NMDAR neurotransmission in the BLA but does not require the PRh
Experiment 6 examined the roles of the PRh and BLA in the extinction of an association between S2 and a neutral S1 in a dangerous context. Rats in this experiment were surgically prepared and trained in the manner described for Experiment 5B. Specifically, rats received 2 d of context exposure, context conditioning in the morning of Day 3 followed by S2–S1 pairings in the afternoon. On Day 4, some of the PRh rats received an infusion of muscimol, while the remaining rats received an infusion of saline; some of the BLA rats received an infusion of muscimol, others received an infusion of ifenprodil, and the remaining rats received an infusion of vehicle. Twenty minutes after the infusion, rats were placed in the conditioning chambers, where they were exposed to eight presentations of S2 alone. Subsequent to first-order conditioning on Day 5 and two sessions of context extinction on Day 6, rats were tested with S2 on Day 7 and with S1 on Day 8.
Four rats were excluded from Experiment 6 due to misplaced cannulae. As there were no differences between PRh and BLA rats infused with vehicle at any stage of the experiment, the data for these rats were combined to form a single control group. Group sizes were as follows: VEH, n = 12; PRh-MUS, n = 8; BLA-MUS, n = 8; and BLA-IFEN, n = 7.
During the 5 min session of context conditioning on the morning of Day 3, there was a linear increase in freezing (F(1,32) = 112.02; p < 0.05), but no differences between the groups (F(1,32) values <1.40; p > 0.05) or group × linear trend interactions (F(1,32) < 1.00; p > 0.05). Freezing to the context declined across the afternoon session in which S2 was paired with S1. All rats initially showed a moderate level of freezing at the start of the session and little freezing at the end of the session (F(1,32) = 69.65; p < 0.05). There was no difference in context freezing between Groups VEH and PRh-MUS (F(1,32) = 2.05; p > 0.05), or between Groups BLA-MUS and BLA-IFEN (F values <1). There was no difference in context freezing between the former and latter groups (F values <1) and no significant group × linear trend interactions (F(1,32) values <1.10; p > 0.05). There was also freezing to the context on Day 4 when S2 was presented alone. This freezing declined across the session (F(1,32) = 17.46; p < 0.05). There were no statistically significant differences between the groups in the levels of freezing to the context (F(1,32) values <1.93; p > 0.05), and the group × linear interactions were not significant (F(1, 32) values <2.64; p > 0.05).
First-order conditioning was successful. The rate of acquisition of first-order fear did not differ between groups (F(1,32) values <1.69; p > 0.05). The mean (±SEM) levels of freezing on the final S1 → shock trial were 65 ± 8.0% in Group VEH, 63 ± 7.0% in Group PRh-MUS, 75 ± 8.% in Group BLA-MUS, and 69 ± 11% in Group BLA-IFEN. Figure 7 shows test levels (mean ± SEM) of freezing to S2 (Fig. 7, left) and S1 (Fig. 7, right). Groups BLA-MUS and BLA-IFEN froze significantly more to S2 than Groups VEH and PRh-MUS (F(1,32) = 7.18; p < 0.05). There were no significant differences in the levels of freezing to S2 between Groups BLA-MUS and BLA-IFEN (F values <1) or between Group VEH and PRh-MUS (F values <1). There were no differences between the groups in freezing to S1 (F(1,32) values <1.23; p > 0.05).
Figure 7.
Pre-extinction of a sensory preconditioned association in a dangerous context requires activation of the BLA but not the PRh, and activation of NMDAR in the BLA (Experiment 6: VEH, n = 12; PRh-MUS, n = 8; BLA-MUS, n = 8; BLA-IFEN, n = 7). Left, Freezing to S2 during the test for sensory preconditioning. Right, Freezing to S1 during the test for first-order conditioned fear. Data shown are means ± SEM.
These results stand in contrast to those obtained earlier: in a safe or neutral context, the extinction of an association between S2 and S1 required neuronal activity in the PRh, specifically, NMDAR neurotransmission; neuronal activity in the BLA is not required for the extinction of the S2–S1 association under these circumstances (Parkes and Westbrook, 2010). However, in a dangerous context, the extinction of the S2–S1 association requires neuronal activity in the BLA, specifically, NMDAR neurotransmission; neuronal activity in the PRh is not required for extinction under these circumstances.
Discussion
Recapitulation of the key results
This series of experiments studied the roles of the PRh and BLA in sensory preconditioned and second-order conditioned fear. The initial experiments demonstrated that neuronal activity in the PRh, specifically NMDAR neurotransmission, is critical for the association between S2 and the neutral S1 (Experiment 1B). These findings confirm results observed in rats with lesions of the PRh (Nicholson and Freeman, 2000), while additionally showing that the PRh is critical for associative formation rather than updating of the association across the S1–US pairings or retrieval of the association at test. In addition, this study has demonstrated for the first time that plasticity in the PRh is critical for the extinction of the association between S2 and the neutral S1 (Experiment 2). The PRh, but not the BLA (Parkes and Westbrook, 2010), is thus critical for the formation and extinction of the association between S2 and the neutral S1. In contrast, the BLA, but not the PRh, is critical not only for the association between S2 and the conditioned S1 (Experiment 3B), but also for the extinction of the fear responses elicited by the second-order or the sensory preconditioned S2 (but see Parkes and Westbrook, 2010; Experiment 4B). The present experiments confirmed this role for the BLA in the extinction of the fear responses elicited by the sensory preconditioned S2 and additionally demonstrated that the PRh is not involved in the extinction of this fear (Experiment 4A).
These results show that the brain regions coding the associations produced by paired presentations of S2 and S1 are determined by the emotional significance of S1: the BLA, but not the PRh, is critical for the association between S2 and a fear-eliciting S1, whereas the PRh, but not the BLA, is critical for the association between S2 and a neutral S1. Similarly, the brain regions that code the extinction of the associations between S2 and S1 are determined by the emotional significance of S2: the BLA, not the PRh, is critical for the extinction of the fear elicited by S2 (either second-order conditioned or sensory preconditioned), whereas the PRh, but not the BLA, is critical for extinction of the association between S2 and the neutral S1.
However, these conclusions regarding the roles of the PRh and BLA in forming and extinguishing associations between S2 and a neutral S1 only apply in a familiar, safe context. In a dangerous context, neuronal activity, specifically, NMDAR neurotransmission in the BLA, but not in the PRh, is critical for the association between S2 and a neutral S1 (Experiment 5B) as well as extinction of this association when S2 was presented in the absence of its neutral S1 associate (Experiment 6). Consequently, the roles of the PRh and BLA are again doubly dissociable with respect to acquisition and extinction of a neutral stimulus–stimulus association. Specifically, the roles of these structures in forming and extinguishing associations between S2 and a neutral S1 depend on the emotional significance of the context. In a safe context, the PRh, not the BLA is critical. However, in a dangerous context the PRh ceases to support associative formation. Instead, sensory representations of the paired auditory and visual stimuli converge in the BLA, where the formation and extinction of their association is encoded via NMDAR-mediated plasticity.
Interactions between the PRh and BLA
These findings raise two questions. The first is how the neutral S2–S1 association stored in the PRh results in expression of sensory preconditioned fear. Here, it is worth noting that expression of sensory preconditioned fear was impaired by muscimol-induced activation of both the PRh (Experiment 4A) and BLA (Experiment 4B). This suggests that activation of both brain regions is critical for expression of this fear. One possibility is that the PRh-mediated S2–S1 association makes contact with the BLA-mediated S1–US association. In this respect, there are strong reciprocal (glutamatergic) connections between the BLA and PRh (Shi and Cassell, 1999; Pitkanen et al., 2000); and other evidence shows that, under some circumstances, these brain areas interact in the service of motivated behavior (Paz et al., 2006, 2009; Bauer et al., 2007).
The second question is why the PRh does not compensate when the BLA is inactivated during second-order conditioning. To be sure, inactivation of the BLA eliminates fear responses, and, hence, S2 could not have associated with the fear elicited by S1 (there was none). Here, the PRh could have been recruited to encode the association between the sensory properties of S2 and S1, effectively transforming second-order conditioning into sensory preconditioning. However, no such compensation was observed: second-order fear conditioning to S2 was clearly impaired by inactivation of the BLA but was completely spared following inactivation of the PRh. One possibility is that, when activated, the BLA provides positive feedback to the thalamus, such that subsequent presentations of paired auditory and visual stimuli are processed in the BLA and not the PRh. Hence, the BLA mediates the formation and extinction of associations between a neutral stimulus and a learned danger signal, or between neutral stimuli in a dangerous environment; and critically, when the BLA is inactivated, the PRh does not compensate because the thalamus continues to route signals to the BLA and not the PRh.
The PRh and BLA are part of distinct neural pathways
Although the PRh and BLA interact under some circumstances, the fact that an association between innocuous stimuli can be processed in either the PRh or the BLA, depending on a subject's emotional state, suggests that these brain areas are also parts of distinct neural pathways. In this respect, several studies have examined the anatomical properties of pathways connecting the thalamus to structures in the medial temporal lobe (including the PRh and BLA) and their functional significance for auditory fear conditioning (Li et al., 1996; Weisskopf and LeDoux, 1999; Doron and Ledoux, 2000; Yaniv et al., 2000, 2001; Doyère et al., 2003; Sigurosson et al., 2010). These studies have identified two critical pathways: the first is a cortical pathway in which information is transmitted from specific thalamic nuclei (the dorsal and ventral medial geniculate) to the lateral and basolateral amygdala via primary auditory cortex and PRh; the second is a subcortical pathway from other thalamic nuclei (medial geniculate body, posterior intralaminar nucleus, and suprageniculate nucleus) to the lateral amygdala (LeDoux, 2003, 2007). It has been suggested that these two pathways have distinct roles in stimulus processing. Activation of the indirect (and therefore, slower) cortical pathway has been implicated in detailed processing of stimulus attributes (LeDoux, 2007; Sigurosson et al., 2010); thus, plasticity in this pathway may support the formation of an association between the sensory features of paired stimuli. In contrast, activation of the direct (and therefore, fast) subcortical pathway has been linked to rapid initiation of defensive reactions to danger (LeDoux, 2007; Sigurosson et al., 2010). Plasticity in this pathway may be necessary for learning about the motivational relevance of paired stimuli.
One implication of the present findings is that motivational state can bias activation of one pathway over the other. In the absence of fear, cortical processing (involving the PRh) dominates and supports learning about the relationship between sensory features of paired stimuli; however, when a state of fear prevails, the subcortical pathway (involving the BLA) is activated to facilitate appraisal of the need for fight or flight, and, as a consequence, it takes over processing of the same relationships. In light of the proposed distinction between information content conveyed by thalamo-cortical (sensory) and thalamo-amygdala (affective, motivational) pathways, a further implication of fear-induced activation of the latter may be a lower-resolution encoding of stimulus detail (Adolphs et al., 2001). This is relevant to anxiety disorders like post-traumatic stress, which have been associated with heightened arousal and amygdala hyperactivity (Goodman et al., 2012), as well as information-processing bias (Buckley et al., 2000; Weber, 2008) and specific deficits in episodic memory (Isaac et al., 2006). This suggests that encoding of general rather than specific detail in these disorders may occur as a consequence of the arousal or anxiety-induced transition from cortical to subcortical processing of the environment (Isaac et al., 2006). Impaired processing of detail may underlie enhanced generalization of fear and anxiety across a range of environmental stimuli reported in the context of these disorders (Kheirbek et al., 2012).
Summary
We have shown that how sensory stimuli are processed by the brain depends on an animal's emotional state at the time these stimuli are encountered. When the context is safe, sensory stimulation activates cortical pathways that permit detailed (or high-resolution) processing of stimulus attributes and their relations. However, when the context is dangerous (and thus, animals are in a state of fear), sensory stimulation bypasses the cortex and, instead, is routed directly to the amygdala so that responses relevant to the current motivational state can be quickly initiated. These findings support the view that thalamo-amygdala and thalamo-cortical pathways process functionally distinct aspects of stimuli and their relations. Pathological activation of these pathways may underpin aberrations in stimulus processing that characterize many psychopathological conditions, including clinical anxiety.
Footnotes
This research was supported by Australian Research Council Discovery Project Grant DP13013687 to R.F.W. and A.S.K. The authors thank Belinda Lay and Neda Assareh for assistance with histology and scoring; Dominic Tran for assistance with the figures; and Kelly Clemens for comments on this and previous versions of the manuscript.
The authors declare no competing financial interests.
References
- Adolphs R, Denburg NL, Tranel D. The amygdala's role in long-term declarative memory for gist and detail. Behav Neurosci. 2001;115:983–992. doi: 10.1037/0735-7044.115.5.983. [DOI] [PubMed] [Google Scholar]
- Bauer EP, Paz R, Paré D. Gamma oscillations coordinate amygdalo-rhinal interactions during learning. J Neurosci. 2007;27:9369–9379. doi: 10.1523/JNEUROSCI.2153-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckley TC, Blanchard EB, Neill WT. Information processing and PTSD: a review of the empirical literature. Clin Psychol Rev. 2000;20:1041–1065. doi: 10.1016/S0272-7358(99)00030-6. [DOI] [PubMed] [Google Scholar]
- Coppock WJ. Pre-extinction in sensory preconditioning. J Exp Psychol. 1958;55:213–219. doi: 10.1037/h0046379. [DOI] [PubMed] [Google Scholar]
- Doron NN, Ledoux JE. Cells in the posterior thalamus project to both amygdala and temporal cortex: a quantitative retrograde double-labeling study in the rat. J Comp Neurol. 2000;425:257–274. doi: 10.1002/1096-9861(20000918)425:2<257::AID-CNE8>3.0.CO;2-Y. [DOI] [PubMed] [Google Scholar]
- Doyère V, Schafe GE, Sigurdsson T, LeDoux JE. Long-term potentiation in freely moving rats reveals asymmetries in thalamic and cortical inputs to the lateral amygdala. Eur J Neurosci. 2003;17:2703–2715. doi: 10.1046/j.1460-9568.2003.02707.x. [DOI] [PubMed] [Google Scholar]
- Fanselow MS. Conditioned and unconditional components of post-shock freezing. Pavlov J Biol Sci. 1980;15:177–182. doi: 10.1007/BF03001163. [DOI] [PubMed] [Google Scholar]
- Gewirtz JC, Davis M. Second-order fear conditioning prevented by blocking NMDA receptors in amygdala. Nature. 1997;388:471–474. doi: 10.1038/41325. [DOI] [PubMed] [Google Scholar]
- Goodman J, Leong KC, Packard MG. Emotional modulation of multiple memory systems: implications for the neurobiology of post-traumatic stress disorder. Rev Neurosci. 2012;23:627–643. doi: 10.1515/revneuro-2012-0049. [DOI] [PubMed] [Google Scholar]
- Hays WL. Statistics for psychologists. New York: Holt, Rinehart and Winston; 1963. [Google Scholar]
- Herry C, Ciocchi S, Senn V, Demmou L, Müller C, Lüthi A. Switching on and off fear by distinct neuronal circuits. Nature. 2008;454:600–606. doi: 10.1038/nature07166. [DOI] [PubMed] [Google Scholar]
- Isaac CL, Cushway D, Jones GV. Is posttraumatic stress disorder associated with specific deficits in episodic memory? Clin Psychol Rev. 2006;26:939–955. doi: 10.1016/j.cpr.2005.12.004. [DOI] [PubMed] [Google Scholar]
- Kheirbek MA, Klemenhagen KC, Sahay A, Hen R. Neurogenesis and generalization: a new approach to stratify and treat anxiety disorders. Nat Neurosci. 2012;15:1613–1620. doi: 10.1038/nn.3262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LeDoux J. The emotional brain, fear, and the amygdala. Cell Mol Neurobiol. 2003;23:727–738. doi: 10.1023/A:1025048802629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LeDoux J. The amygdala. Curr Biol. 2007;17:R868–R874. doi: 10.1016/j.cub.2007.08.005. [DOI] [PubMed] [Google Scholar]
- Li XF, Stutzmann GE, LeDoux JE. Convergent but temporally separated inputs to lateral amygdala neurons from the auditory thalamus and auditory cortex use different postsynaptic receptors: in vivo intracellular and extracellular recordings in fear conditioning pathways. Learn Mem. 1996;3:229–242. doi: 10.1101/lm.3.2-3.229. [DOI] [PubMed] [Google Scholar]
- Nicholson DA, Freeman JH., Jr Lesions of the perirhinal cortex impair sensory preconditioning in rats. Behav Brain Res. 2000;112:69–75. doi: 10.1016/S0166-4328(00)00168-6. [DOI] [PubMed] [Google Scholar]
- Parkes SL, Westbrook RF. The basolateral amygdala is critical for the acquisition and extinction of associations between a neutral stimulus and a learned danger signal but not between two neutral stimuli. J Neurosci. 2010;30:12608–12618. doi: 10.1523/JNEUROSCI.2949-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The rat brain in stereotaxic coordinates. Ed 3. San Diego: Academic; 1997. [DOI] [PubMed] [Google Scholar]
- Paz R, Pelletier JG, Bauer EP, Paré D. Emotional enhancement of memory via amygdala-driven facilitation of rhinal interactions. Nat Neurosci. 2006;9:1321–1329. doi: 10.1038/nn1771. [DOI] [PubMed] [Google Scholar]
- Paz R, Bauer EP, Paré D. Measuring correlations and interactions among four simultaneously recorded brain regions during learning. J Neurophysiol. 2009;101:2507–2515. doi: 10.1152/jn.91259.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pitkanen A, Pikkarainen M, Nurminen N, Ylinen A. Reciprocal connections between the amygdala and the hippocampal formation, perirhinal cortex, and postrhinal cortex in rat. A review. Ann N Y Acad Sci. 2000;911:369–391. doi: 10.1111/j.1749-6632.2000.tb06738.x. [DOI] [PubMed] [Google Scholar]
- Rodrigues SM, Schafe GE, LeDoux JE. Intra-amygdala blockade of the NR2B subunit of the NMDA receptor disrupts the acquisition but not the expression of fear conditioning. J Neurosci. 2001;21:6889–6896. doi: 10.1523/JNEUROSCI.21-17-06889.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi CJ, Cassell MD. Perirhinal cortex projections to the amygdaloid complex and hippocampal formation in the rat. J Comp Neurol. 1999;406:299–328. doi: 10.1002/(SICI)1096-9861(19990412)406:3<299::AID-CNE2>3.0.CO;2-9. [DOI] [PubMed] [Google Scholar]
- Sigurosson T, Cain CK, Doyere V, LeDoux JE. Asymmetries in long-term and short-term plasticity at thalamic and cortical inputs to the amygdala in vivo. The Eur J Neurosci. 2010;31:250–262. doi: 10.1111/j.1460-9568.2009.07056.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sotres-Bayon F, Bush DE, LeDoux JE. Acquisition of fear extinction requires activation of NR2B-containing NMDA receptors in the lateral amygdala. Neuropsychopharmacology. 2007;32:1929–1940. doi: 10.1038/sj.npp.1301316. [DOI] [PubMed] [Google Scholar]
- Weber DL. Information processing bias in post-traumatic stress disorder. Open Neuroimag J. 2008;2:29–51. doi: 10.2174/1874440000802010029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weisskopf MG, LeDoux JE. Distinct populations of NMDA receptors at subcortical and cortical inputs to principal cells of the lateral amygdala. J Neurophysiol. 1999;81:930–934. doi: 10.1152/jn.1999.81.2.930. [DOI] [PubMed] [Google Scholar]
- Wilensky AE, Schafe GE, LeDoux JE. Functional inactivation of the amygdala before but not after auditory fear conditioning prevents memory formation. J Neurosci. 1999;19:RC48. doi: 10.1523/JNEUROSCI.19-24-j0006.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yaniv D, Schafe GE, LeDoux JE, Richter-Levin G. Perirhinal cortex and thalamic stimulation induces LTP in different areas of the amygdala. Ann N Y Acad Sci. 2000;911:474–476. doi: 10.1111/j.1749-6632.2000.tb06749.x. [DOI] [PubMed] [Google Scholar]
- Yaniv D, Schafe GE, LeDoux JE, Richter-Levin G. A gradient of plasticity in the amygdala revealed by cortical and subcortical stimulation, in vivo. Neuroscience. 2001;106:613–620. doi: 10.1016/S0306-4522(01)00312-8. [DOI] [PubMed] [Google Scholar]