Abstract
Nitrification in terrestrial soils is one of the major processes of emission of nitrous oxide (N2O), a potent greenhouse gas and stratospheric-ozone-depleting substance. We assessed the fraction of N2O emission associated with nitrification in soil through a meta-analysis and sensitivity analysis using a process-based model. We corrected observational values of gross nitrification and associated N2O emission rates from 71 records for various soils in the world spanning from 0.006% to 29.5%. We obtained a median value of 0.14%, and then assessed how the nitrification-associated N2O emission fraction has been considered in terrestrial nitrogen cycle models. Using a process-based biogeochemical model, we conducted a series of sensitivity analyses for the effects of different values of nitrification-associated N2O emission fraction on soil N2O emission. Using an empirical relationship between soil pH and nitrification-associated N2O emission fraction, the model well simulated global emission patterns (global total in the 2000s, 16.8 Tg N2O yr–1). Differences in the nitrification-associated N2O emission fraction caused differences in total N2O emission of as much as 2.5 Tg N2O yr–1. Therefore, to obtain reliable estimation of soil N2O emission for nitrogen and climate management, it is important to constrain the parameterization in models by ensuring extensive and accurate observations.
Introduction
Nitrous oxide (N2O) is the third important long-lived greenhouse gas next to carbon dioxide (CO2) and methane (CH4) [1] and is the most important substance depleting stratospheric ozone [2]. To reach the overarching mitigation targets of the Paris Agreement [3] we need to suppress the growth of atmospheric N2O concentration, to which anthropogenic emissions contribute at a level comparable to that from natural sources [4, 5]. Also, assessment and regulation of N2O emission contribute to management of nitrogen cycle, which is closely related to many issues of human sustainability, such as food production and sanitation [6]. Nevertheless, there remain serious uncertainties in our understanding and predictability of N2O dynamics.
Terrestrial soils—both natural and agricultural—are a prevailing source of N2O in the atmosphere [7, 8], but spatial heterogeneity and temporal variability of the N2O flux make it difficult to quantify broad-scale budgets. Most of the N2O released from the soil surface is produced by two separate microbial processes, nitrification and denitrification, which differ in terms of active microbes, substrates, and environmental responsiveness [9, 10]. There are still serious knowledge gaps and difficulties in using models to predict soil N2O emissions in a quantitative manner.
Nitrification by ammonia oxidizers is the primary process of N2O production in oxic (aerobic) soils and is thought to be more ubiquitous than denitrification, which occurs in anaerobic wet soils. Recent studies have revealed the contributions of different soil microbes, such as ammonia-oxidizing archaea and bacteria, to nitrification [11, 12]. In nitrification, most of the oxidized ammonia is turned into nitrate (NO3–) via nitrite (NO2–), and a certain (usually small) fraction of nitrogen is released as N2O. The fraction of nitrification-associated N2O emission (fN2Onit) and its regulation mechanism are important but barely understood. Although fN2Onit is critically important to predict soil N2O emission, a few studies have investigated the responses of fN2Onit and the corresponding nitric oxide (NO) emission fraction to soil temperature and moisture conditions [13, 14]. However, observational data and knowledge are still insufficient to evaluate broad-scale emissions, including from a variety of soils. Farquharson (2016) [15] conducted a systematic analysis of fN2Onit from agricultural soils in Australia. He found that 0.03% to 1% of nitrogen is released as N2O associated with nitrification in soils and found no strong relationship with environmental factors such as soil moisture. For a broad range of natural soils, and in other regions, we have found no systematic analysis on fN2Onit.
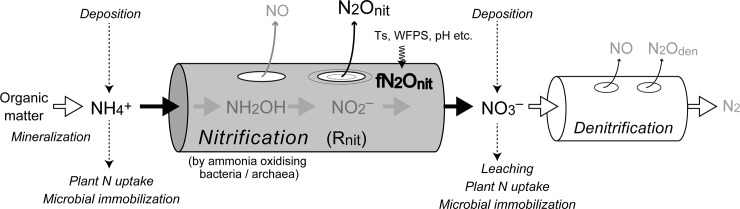
fN2Onit should be an important parameter in biogeochemical models that aim to simulate nitrogen cycles and predict N2O emissions from land. Many terrestrial nitrogen cycle and N2O emission models have been developed, from simple box-flow models (e.g., the Terrestrial Ecosystem Model [16]) to more mechanistic ones (e.g., the Denitrification Decomposition [DNDC] model [17]). In these models, fN2Onit or a similar parameter (i.e., the total N2O and NO emission fraction) has been determined in a simplified empirical manner. In the well-recognized “hole in the pipe” or “leaky pipe” concept of soil nitrogenous gas emission [18, 19], fN2Onit represents the size of the N2O hole in the nitrification pipe (Fig 1). Note that the nitrification rate (as defined by pipe diameter and flow velocity) varies also with the environmental conditions, so it is possible to regard fN2Onit as a constant or as an independent variable that changes with environmental condition. In the latter case, empirical parameterizations have been adopted to determine the N2O emission fraction, using a limited amount of observational data. As a consequence, these emissions could have a considerable range of bias and error due to the uncertainty of fN2Onit values.
Fig 1. Conceptual diagram of nitrification and the “holes-in-a-pipe” concept.
Ts, soil temperature; WFPS, water-filled pore space.
Here, we focused on fN2Onit from the perspective of the global N2O budget, aiming at better predictability of emission by biogeochemical models. Our focal research questions are as follows. (1) What is the feasible range of fN2Onit in the terrestrial ecosystems? (2) How does fN2Onit vary in the field in response to environmental conditions? (3) Can we attain a better parameterization of fN2Onit, applicable to global scale, on the basis of present data? First, to clarify the range of variability and the broad-scale trends in these values, we conducted a meta-analysis of the observed values of fN2Onit for both natural and agricultural soils. Second, we surveyed how fN2Onit was included in current terrestrial N2O estimation models. Third, we used the results of the meta-analysis and model survey to conduct a series of sensitivity analyses of fN2Onit and N2O emissions by using our biogeochemical model. Finally, we discuss how we can reduce the range of estimation uncertainty in both experimental and modeling studies. Note that this study focuses on nitrification as the first step and that other important processes such as denitrification, nitrifier denitrification, and abiotic production [20] are not explicitly addressed here. We intend to address these other processes in a forthcoming study using a similar approach.
Methods
Meta-analysis
Overview
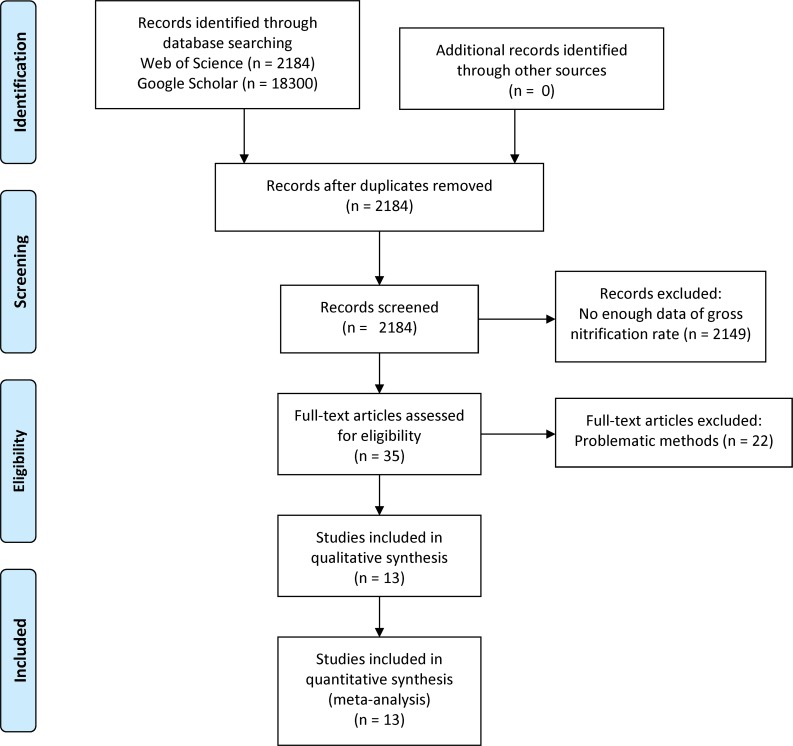
A meta-analysis was conducted to reveal the range, frequency, and tendencies of soil fN2Onit reported in the literature. The results were reported following the Preferred Reporting Items for Systematic Review and Meta-Analyses protocols (PRISMA) [21] protocol (S1 Table). To obtain information on the general properties of the parameter, we gathered observational values reported from a wide range of studies. By using Web of Science (Thomson Reuters, New York, NY, USA) and Google Scholar (Alphabet, Mountain View, CA, USA), we searched papers and reports that included data on nitrogenous gas exchange and soil biogeochemistry. We used combinations of three terms (each from #1 to #3) in S2 Table to search candidate papers; for example, “nitrous oxide flux” and “nitrification rate” and “soil surface”. No date and time limitations were applied to harvest from the maximum extent of the literature. Also, we examined the reference lists in each paper to find additional literature that did not appear in the web searches.
Study selection
We carefully selected source data of fN2Onit for the meta-analysis, particularly taking into account the consistency between N2O emission and nitrification rates. First, we removed papers that addressed non-soil N2O emissions (i.e., from ponds, landfills, animal slurry, etc.). For quantitative consistency, we selected papers including data on gross nitrification (i.e., NH4+ consumption) and associated N2O emission. Therefore, several papers that reported only net nitrification (i.e., NO3– production) rates, potential emission rates, and data under oxygen-free condition, were carefully removed from the meta-analysis. Also, we focused on daily or longer phenomena, so that rates of N2O emission from the soil surface to the atmosphere could be adequately approximated to N2O production rates within the soil (i.e., the vertical diffusion time lag was negligible). As a result, papers reporting only instantaneous (i.e., for seconds to minutes) measurements were excluded; in general, these instantaneous measurement data show extremely wide ranges of variability, making a robust analysis difficult. Finally, we selected the source papers by measurement method used in each study, because several methods could give biased values under certain conditions (e.g., DMPP inhibition slows greatly at >25°C [22]). We confirmed that the method-based selection had a small impact on the analysis results. The paper selection was made by two authors and discrepancies were resolved by discussion.
Data extraction
Data on fN2Onit values and associated properties were extracted: soil pH, solvent of pH, soil temperature, soil moisture content with units, soil texture, clay / silt / sand composition, latitude, longitude, land-cover type, and soil-type classification. Few papers provide values of fN2Onit directly, and therefore, if applicable, we calculated the values from gross nitrification and associated N2O emission rates measured under the same condition.
Data analyses
A statistical software R [23] was used to calculate the statistical metrics for the records: i.e., mean, median, maximum, minimum, standard deviation [σ], kurtosis, skewness, and quartiles. A few extreme values can, in most cases harmfully, affect the results of statistical metrics. To assess the influences of outliers, these statistical metrics were also calculated after removing top and bottom outliers (10% from all the records). Note that we used both datasets with and without outlier values in the following analyses and model simulations. Furthermore, to reduce the size effect of different sample numbers (i.e., weights) among papers, these metrics were also calculated using the mean values for each paper.
Parameterization of fN2Onit in other models
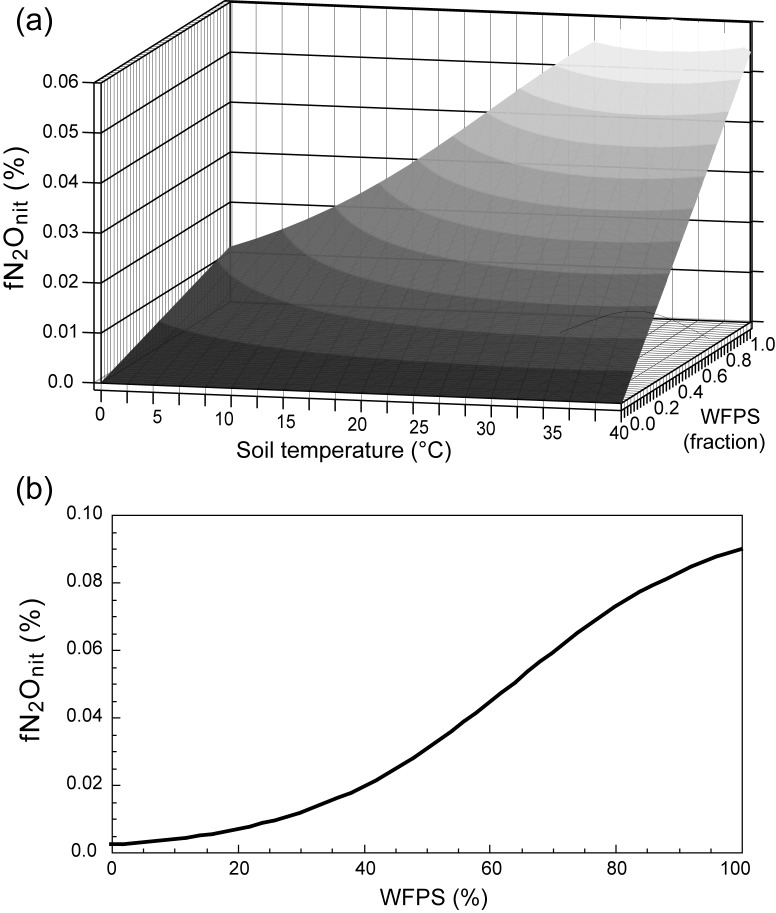
We then assessed how fN2Onit has been parameterized in other terrestrial nitrogen models and evaluated the influence on N2O emission estimation. According to a review by Frolking et al. (1998) [24], former models have adopted different constant fN2Onit values, namely 0.5% in the ExpertN model and 2% in the CENTURY model. These values were examined in the sensitivity simulations mentioned below. In later models, fN2Onit was parameterized as a function of environmental conditions in different manners. The modified DNDC model [25] (their Table 3, Eq 8) adopted the following parameterization:
| (1) |
| (2) |
where Ft is a scholar function of soil temperature (Ts,°C) and WFPS is water-filled pore space (fraction). This parameterization gives a peak value, about 0.06%, at about 35°C under saturated soil water conditions (Fig 2A). The Dynamic Land Ecosystem Model [26] (DLEM) parameterizes fN2Onit as a function of WFPS (%):
| (3) |
Fig 2. Relationship between the fraction of nitrification-associated N2O emission (fN2Onit) assumed in the models.
(a) DNDC and (b) DLEM. WFPS, water-filled pore space.
In this parameterization, the fN2Onit value increases with increasing soil water content (Fig 2B) and does not exceed 0.1%. The Community Land Model [27] and O-CN model [28] adopted a version of the DNDC parameterization. In the recent paper on N2O model intercomparison [29], an elaborate table summarizes how nitrification and N2O emission are formulated in contemporary land nitrogen models.
Description of the N2O simulation model
To assess the range of estimations associated with variations in fN2Onit, we conducted a series of simulations using a process-based model, namely, Vegetation Integrative SImulator for Trace gases (VISIT [30, 31]). This model was selected, because it has an intermediate complexity among the global N2O models and gave moderate results in the model intercomparison project [29]. Also, the model was used for a regional evaluation of soil N2O emission in East Asia, one of the highly human-influenced regions, demonstrating the credibility for broad-scale applications [32]. Briefly, the model consists of water, carbon, and nitrogen cycling schemes for terrestrial ecosystems and is aimed at assessing atmosphere–ecosystem exchange of greenhouse gases and trace gases. The nitrogen cycle is fully included, from inputs (atmospheric deposition, biological fixation, and fertilizer input) to outputs (leaching, ammonia volatilization, and nitrogenous gas emissions through nitrification and denitrification). Intra-ecosystem dynamics of nitrogen among plant, soil, and microbe is simulated in an explicit manner. The model was validated by comparing various biogeochemical aspects with observational data [33–35]. In the VISIT model, fN2Onit is assumed to be a universal, constant value (1% of gross nitrification).
A brief description of the method used to estimate fN2Onit is given below. In the VISIT model, soil N2O production through the nitrogen cycle is conceptualized by using the “hole in the pipe” scheme (see Fig 1). Gross nitrification (Rnit) and associated N2O emission (N2Onit) are related as follows:
| (4) |
Nitrification rate and its environmental dependencies were derived from the NGAS scheme developed by Parton et al. (1996) [36], as follows:
| (5) |
where pWFPS-nit, ppH, and pTs denote the environmental scalar functions derived from observations (see ref. [36]) of water-filled pore space (WFPS), soil pH, and soil temperature (Ts), respectively. K denotes the coefficient of N turnover, taking values from 3.5 of natural soils to 12.0 of agricultural soils. Fmax is the maximum nitrification-associated gas flux coefficient and NH4+ is the soil ammonium content. WFPS and NH4+ were simulated by VISIT and so varies temporally and spatially.
Global simulations by using the VISIT model were conducted with the common protocol and initial and boundary conditions. Namely, they were conducted at a spatial resolution of 0.5° x 0.5° for latitude and longitude, during the period from January 1901 to December 2016. Historical climate data from CRU TS3.25 [37] (temperature, precipitation, vapor pressure, and cloudiness) and land-use data [38] were used to drive the model. Historical changes in atmospheric nitrogen deposition were derived from Galloway et al. (2004) [39], and in croplands, input of nitrogen fertilizer was determined on a country-basis by using FAOSTAT (http://www.fao.org/faostat). The amount of national fertilizer use was divided by total cropland area and allocated to each grid cell. For each grid, a spin-up calculation was conducted for 300 to 2000 years, depending on case, under stationary conditions until a stable-state carbon budget was reached, before starting the historical experiment.
Sensitivity simulations
All sensitivity simulations were conducted by VISIT using the common forcing dataset and protocols; only the fN2Onit value was changed. First, to simply assess the sensitivity of N2O emission estimation to fN2Onit, we halved (i.e., 0.5%) and doubled (2%) the parameter value (originally 1% in VISIT) and compared the results. We then changed the fN2Onit values to those obtained by the meta-analysis mentioned above, that is, mean and median values for all data and several subsets. In these simulations, constant fN2Onit values were applied to all grids. Second, the fN2Onit value was replaced by those of DNDC and DLEM described above, using the same temperature and moisture conditions. Third, finally, we derived an empirical relationship between soil pH and fN2Onit value from the meta-analysis data. Such a relationship was shown in laboratory studies [40] and previous meta-analysis [15], but has not been examined by models at the global scale. Here, we used the global soil pH map (S1 Fig) produced by the Global Soil Data Task of the International Geosphere-Biosphere Programme [41].
Results and discussion
We obtained 71 records from 13 studies in the published literature (Table 1; Fig 3), covering a wide range of different ecosystems and soil texture types; see S3 Table for the data extracted. Although no date and time limitation were applied, the data were obtained from 1985 to 2013. Although a large number of papers addressed the nitrification and N2O emission (2184 papers), we found that only a small number of papers (35 papers) contain the data on gross nitrification rate for a sufficiently long period. Other 2149 papers were rejected, although they contained partial data on nitrification-associated N2O emission. Furthermore, many of the measurements (22 out of 35) were made using problematic methods or conditions and so removed. As a result of data selection, measurements in the literature used in this study were made mainly by using two methods of soil incubation: the inhibitor (C2H2, N-serve, and NaClO3) treatment and the stable carbon isotope (15N labelling) method [10, 42]. Most of the measurements were conducted in the laboratory: only one study was done in the field. The record number is not so abundant, but the dataset covers a wide variety of ecosystems and soils such as forest, grassland, and cropland. Therefore, we used this dataset for following analyses and model assessments.
Table 1. List derived from literature search of methods to measure nitrification-associated N2O flux.
| References | Method | fN2Onit | Note |
|---|---|---|---|
| mean (max ~ min), % | |||
| Ambus (2005) ref. [43] | 15N labelling | 0.046 (0.046 ~ 0.046) | Land cover (sward), soil type (Typic Hapludult) |
| Bateman and Baggs (2005) ref. [14] | 15N labelling, C2H2 inhibition | 0.011 (0.006 ~ 0.014) | Land cover (agriculture), soil type (Cambion) |
| Carter (2007) ref. [44] | C2H2 inhibition, field measurement (15N) | 0.020 (0.01 ~ 0.029) | Land cover (sward) |
| Garrido et al. (2002) ref. [45] | C2H2 inhibition | 0.30 (0.028 ~ 0.48) | Land cover (agriculture), soil type (HypercalcareousRendosol, RedoxicLuvisol, PachicCalcisol, Neoluvisol) |
| Khalil et al. (2004) ref. [46] | 15N labelling | 0.77 (0 ~ 1.57) | Land cover (agriculture), soil type (Orthic Luvisol) |
| Klemedtsson et al. (1988) ref. [47] | C2H2 inhibition | -0.49 (-9.62 ~ 7.5) | Land cover (arable land) |
| Maag and Vinther (1996) ref. [48] | C2H2 inhibition | 0.36 (0.28 ~ 0.48) | |
| Martikainen (1985) ref. [40] | C2H2 inhibition, N-serve inhibition | 28.3 (27.0 ~ 29.4) | Land cover (forest) |
| Mathieu et al. (2006) ref. [49] | 15N labelling | 1.23 (0.13 ~ 2.32) | Land cover (agriculture), soil type (Gleyie luvisol) |
| Mørkved et al. (2007) ref. [50] | 15N labelling, C2H2 inhibition | 0.79 (0.018 ~ 7.62) | Land cover (meadow, agriculture), soil type (sapric histosol, Stagnic Albeluvisol) |
| Mørkved et al. (2006) ref. [51] | 15N labelling, C2H2 inhibition | 27 (27 ~ 27) | Land cover (agriculture), soil type (mollic gleysol) |
| Tortoso and Hutchinson (1990) ref. [52] | N-serve inhibition, NaClO3 inhibition | 0.068 (0.068 ~ 0.068) | Land cover (agriculture) |
| Zhu et al. (2013) ref. [20] | 15N labelling, 18O, C2H2 inhibition | 2.35 (0 ~ 8.3) | Land cover (agriculture), soil type (Fine-silty mixed, nonacid, thermic Typic Xerorthent) |
N-Serve, 2-Chloro-6-(trichloromethyl)-pyridine
Fig 3. PRISMA flow diagram.
PRISMA, Preferred Reporting Items for Systematic Reviews and Meta-Analysis.
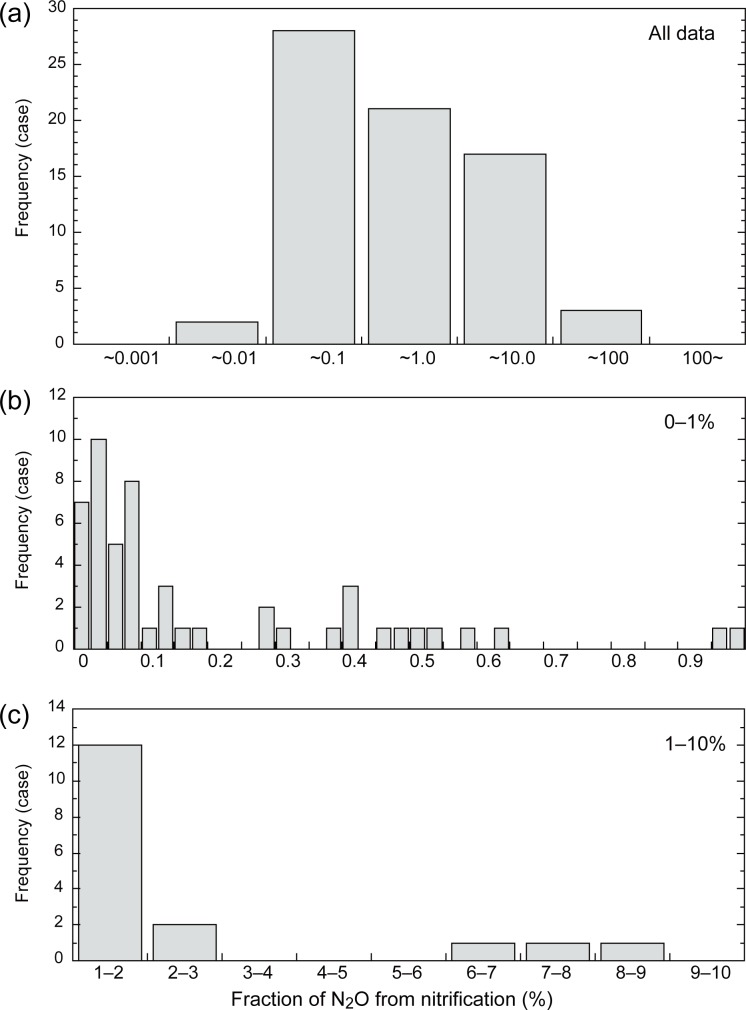
The observational fN2Onit values (n = 71) were distributed widely from 0.006% to 29.4%, with a mean of 1.92% and a median of 0.19% (first column of Table 2). The high mean value was likely attributable to the presence of a few anomalous values in the dataset. A histogram of the observed fN2Onit values (Fig 4) showed a concentrated and skewed distribution with a clear peak around the median value. Additionally, with removal of 10% outliers (top 4 and bottom 3 records, second column of Table 2) the mean (0.43%) and median (0.14%) values became lower. Notably, standard deviation narrowed greatly after the removal of outliers, and both the maximum value and minimum value were obtained when the C2H2 inhibition method was used. When publication-based data (i.e., the means of the values reported in each paper) were used, the mean and median value became higher (5.17% and 0.57%, respectively; third column of Table 2).
Table 2. Summary of statistics on measured nitrification N2O emission ratio.
| All data | Excluding outlier |
Aggregated by paper |
|
|---|---|---|---|
| Sample no. | 71 | 64 | 12 |
| Mean (%) | 1.922 | 0.426 | 5.167 |
| Standard deviation (%) | 5.702 | 0.526 | 10.526 |
| Kurtosis (-) | 15.255 | 1.191 | 0.498 |
| Skewness (-) | 4.006 | 1.369 | 1.549 |
| Maximum (%) | 29.445 | 2.320 | 28.234 |
| 75% quartile (%) | 1.084 | 0.605 | 1.703 |
| Median (%) | 0.192 | 0.139 | 0.573 |
| 25% quartile (%) | 0.053 | 0.048 | 0.063 |
| Minimum (%) | 0.006 | 0.006 | 0.011 |
Fig 4. Histogram of the N2O emission associated with nitrification, obtained by a meta-analysis of 71 observations.
(a) All data, (b) data of 0–1%, and (c) data of 1–10%.
In comparison with the study of Australian agricultural soils by Farquharson (2016), typical values of fN2Onit obtained by our meta-analysis seem comparable. That study found that fN2Onit values varied from 0.03% to 1%, with a typical value of 0.2%, that falls between the mean and median values obtained in the present study for all data. When removing outlier values, our results of mean and median became even closer to the result of Farquharson (2016). Namely, we examined anomalous values removed from the present meta-analysis, such as zero to negative and extremely high, 100% values. Several these values were obtained by Ambus (1998) [53] in the only study that conducted C2H2 treatment in the field; this probably led to a larger fluctuation in values than in the laboratory studies. Negative values of fN2Onit are attributable to net N2O uptakes, which are sometimes observed but are usually small [54]. The fN2Onit values used by emission models assuming a constant N2O emission fraction (0.5% to 2.0%) fell within the range of observed values. For example, the constant fN2Onit value used in the original VISIT model, 1%, did not differ significantly from the mean value of the observed dataset (Student’s t-test, t = 1.36, p = 0.177). However, it should be noted that the median observed value (0.19%) was much lower than the model-assumed value.
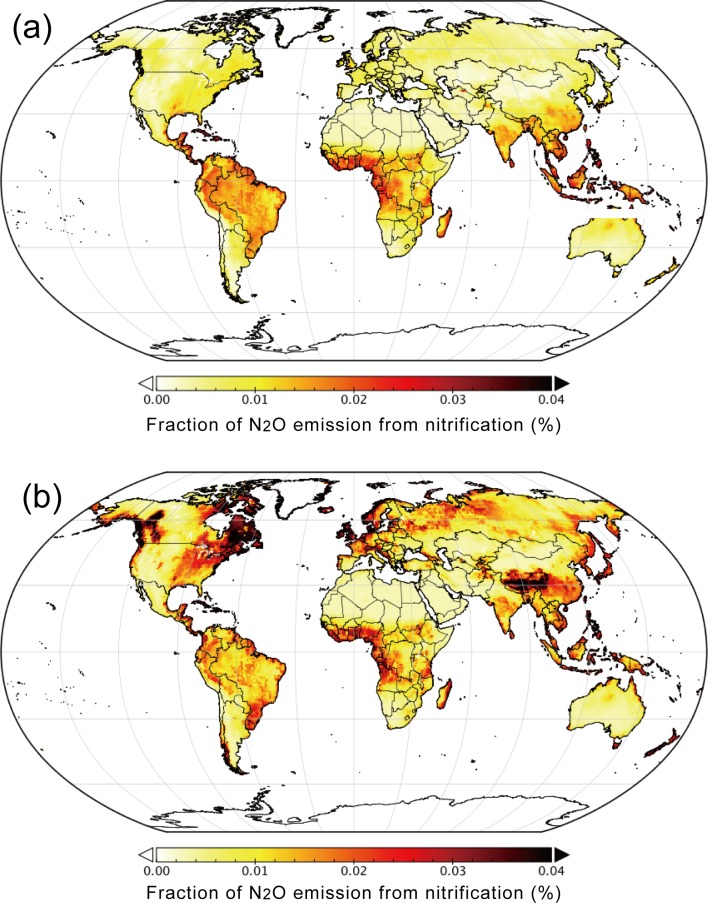
The fN2Onit values used in the DNDC and DLEM models including the environmental variability of the N2O emission fraction were generally lower than those used in the models assuming constant values. As shown in Fig 2, these models assume the maximum fN2Onit values of 0.06 to 0.1%. However, these fN2Onit values were still within the range of observed values. We examined the spatial distribution of mean fN2Onit values estimated by the DNDC (Fig 5A) and DLEM (Fig 5B). When the DNDC parameterization was used, higher fN2Onit values were estimated mainly in the humid tropics. In contrast, when the DLEM parameterization was used, higher fN2Onit values were obtained in humid temperate to boreal regions such as Europe, eastern and western North America, and the Tibetan Plateau. Note again that a common soil temperature and moisture (after the VISIT simulation) were used in this comparison, and the differences among the results were caused exclusively by differences in the fN2Onit parameterizations. The difference of fN2Onit indicated here may account for a part of outcomes of the N2O model intercomparison project [29]. The project showed that the existing models differ in global soil N2O emission by about 20%, and our study implies that fN2Onit is one of the key parameters to reduce the estimation uncertainty.
Fig 5. Distributions of estimated fraction of nitrification-associated N2O emission, fN2Onit.
(a) DNDC and (b) DLEM parameterizations.
Sensitivity of N2O flux to fN2Onit
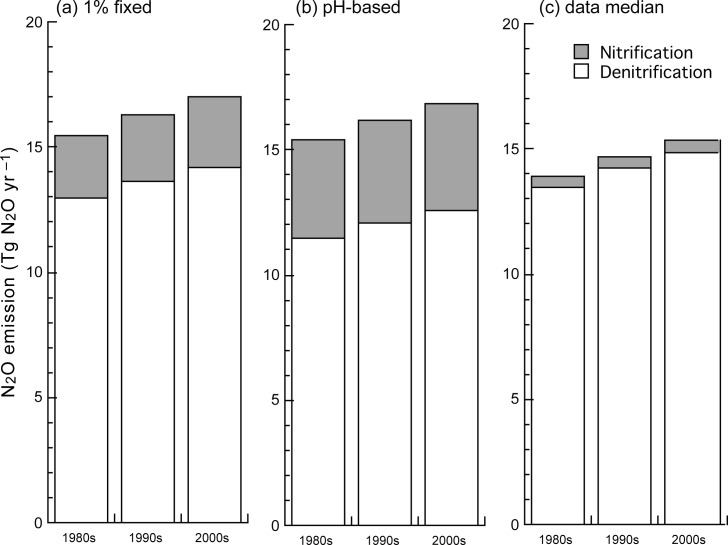
The default VISIT model with a constant fN2Onit of 1% estimated total N2O emission from terrestrial soils as 15.47 ± 0.52, 16.32 ± 0.98, and 17.03 ± 0.73 Tg N2O yr–1 (average ± s.d. of interannual variability) in the 1980s, 1990s, and 2000s, respectively (Fig 6). When converted into nitrogen weight (multiplied by 28/44), these values correspond to 9.85 to 10.84 Tg N yr–1. These estimates are close to previous estimations: e.g., 11.1 Tg N yr–1 by IPCC (2013) [1] and 11.4 Tg N yr–1 by Syakila and Kroeze (2011) [55] for emissions from natural vegetation, agriculture, and deposition on land. In the 2000s simulation, 16.5% of total N2O emission was from nitrification and 84% was from denitrification. About 32% and 68% of N2O emission occurred in agricultural and natural ecosystem soils, respectively. During the simulation period, total N2O emission increased from 12.11 Tg N2O yr–1 in 1901 to 18.60 Tg N2O yr–1 in 2016 as a result of the increase of fertilizer and deposition inputs. See our resent study [32] on the temporal change and its driver of the regional N2O emissions.
Fig 6. Sensitivity analysis of global N2O emission to the fraction of nitrification-associated N2O emission, fN2Onit.
(a) Fixed 1%, (b) soil pH-based parameterization, and (c) median of meta-analysis records (0.139%, outliers removed). Each N2O flux was estimated by using the VISIT model. Decadal mean values for the 1980s, 1990s, and 2000s are shown.
The sensitivity analysis using different values of fN2Onit (constant) indicated that the simulated total N2O emission was sensitive to the assumed emission fraction. When fN2Onit = 0.5% was used, the total nitrification-associated N2O emission was reduced to 1.59 Tg N2O yr–1 (–43% in comparison with fN2Onit = 1% case) in the 2000s. Because of the smaller nitrogen loss by nitrification in these cases, N2O emission from denitrification increased slightly because of the use of excess inorganic nitrogen in the soils. As a result of compensation, total N2O emission was only slightly affected (–4.3%) by the halved fN2Onit value. When fN2Onit = 2% was used, total nitrification-associated N2O emission increased to 4.4 Tg N2O yr–1 (+56.6%). The asymmetric sensitivity of nitrification-associated N2O emission to the change in fN2Onit value is attributable to alteration of the nitrogen stock in the soils and the non-linear response of N2O emission to nitrogen availability [36]. Finally, when using the parameterizations of DNDC and DLEM models, lower total N2O emissions were estimated (15.0 Tg N2O yr–1) with lower contribution of nitrification-associated emission due to the generally low value of fN2Onit (data not shown).
When the median fN2Onit value of the meta-analysis (0.14%) was used in the VISIT simulation, the total N2O emission was estimated as 15.4 Tg N2O yr–1 in the 2000s; nitrification-associated N2O emission was largely reduced to 0.50 Tg N2O yr–1. In contrast, when the mean value of all data (fN2Onit = 1.92%) was used, higher rates of total and nitrification-associated N2O emission (17.5 and 4.3 Tg N2O yr–1) were estimated. Therefore, selection of representative fN2Onit value can affect the simulation result by as much as 2.1 Tg N2O yr–1 at the global scale. When including the difference in model parameterizations, the uncertainty becomes even larger to 2.5 Tg N2O yr–1.
These results confirmed that the estimated N2O emission was sensitive to the assumed fN2Onit value, which was poorly constrained in the present models and varied with selection of the metric from the observational data. Although observations implied that the fN2Onit can be variable in response to environmental conditions such as temperature and moisture, the scarcity of observational evidence has prevented us to use a standard parameterization and permitted us to assume constant values. Apparently, additional constraints and new parameterizations are required to obtain a reliable N2O budget and its flow components. Observational data and insights are accumulating with support of technical developments such as isotopic tracers, but it would take decades to obtain a comprehensive dataset with enough coverage. Next, we made an attempt to develop a new parameterization of fN2Onit applicable at the global scale.
Application of pH-based parameterization
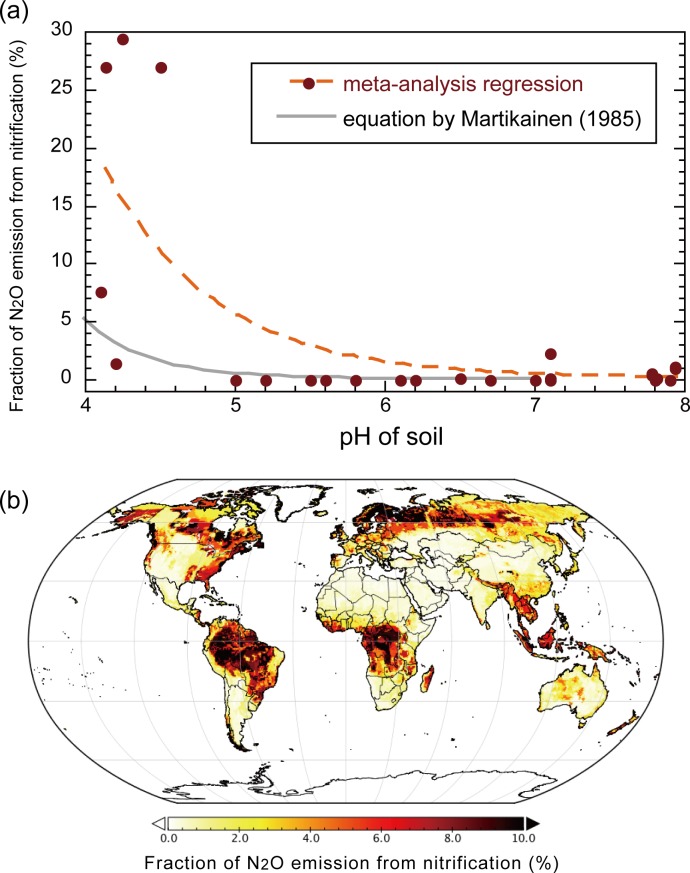
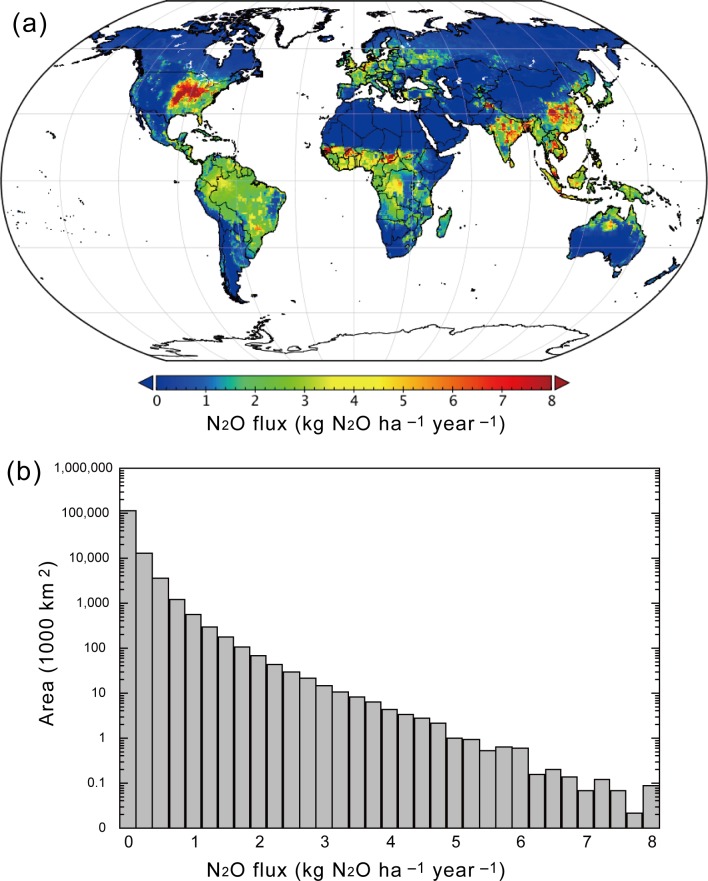
In 54 records of the meta-analysis data, soil pH condition was included, allowing us to relate with fN2Onit (Fig 7A). It was found that fN2Onit takes higher values at acidic soil conditions with pH below 5 and lower values under neutral to alkaline soil conditions. We obtained a regression curve using exponential function, which gives slightly higher fN2Onit values in comparison with the equation of Martikainen (1985) [40]. Using the soil pH map and the regression curve, global distribution of pH-based fN2Onit was obtained (Fig 7B). As expected from the pH pattern, boreal conifer forest soils and humid tropical soils show higher fN2Onit values. High fN2Onit in humid tropics estimated by the present study is consistent with those by DNDC and DLEM parameterizations, while the three maps differ largely in temperate regions. When using the pH-based parameterization, total N2O emission in the 2000s was estimated as 16.8 Tg N2O yr–1 (25.2% by nitrification and 74.8% by denitrification). Global distribution of soil N2O emission was reasonably simulated (Fig 8; see S2 Fig for seasonal change), in comparison with those obtained by atmospheric inversion studies [56]. For example, high emissions from temperate croplands and tropical forests were well captured.
Fig 7. Parameterization of nitrification-associated N2O emission fraction, fN2Onit as a function of soil pH.
(a) Relationships in the meta-analysis data. Orange dashed curve is obtained by Gauss-Newton non-linear regression of an exponential function: fN2Onit = 47.59 exp(–1.345 · pH) (R2 = 0.557). Grey curve shows an empirical function by Martikainen (1985) for reference. (b) Global distribution of fN2Onit estimated using the regression curve and soil pH map (S1 Fig).
Fig 8. Simulated distribution of N2O emission using VISIT model with the pH-based fN2Onit parameterization.
(a) Global map for the 2000s and (b) frequency distribution of N2O emission intensity; note the log scale of y-axis.
Impacts on global N2O budget
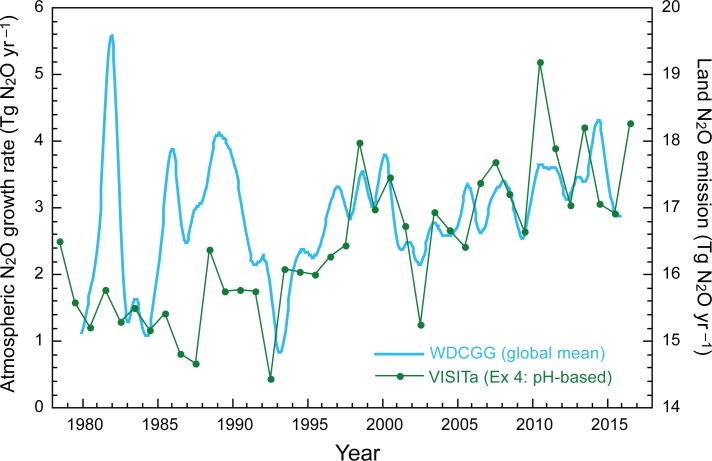
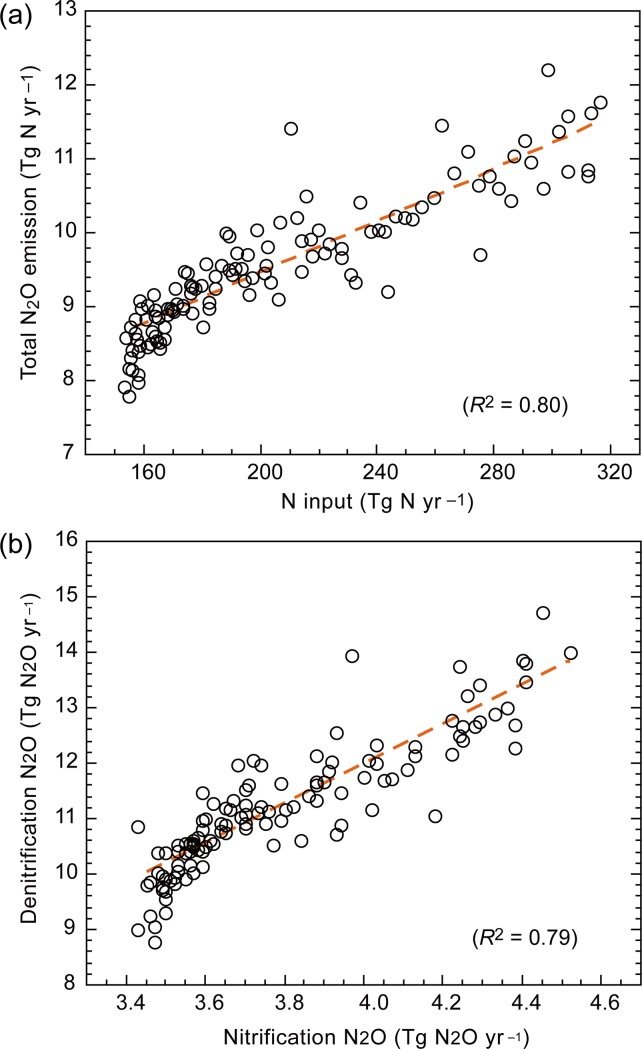
The simulated N2O emission account for important features of the global budget. For example, interannual variability in the total N2O emission was comparable with the atmospheric growth rate especially after 1990, i.e. the period when ample observational data became available (Fig 9). The decline after the Mt. Pinatubo eruption in 1991 and following increase were well captured, implying the major impact of soil emission on the atmospheric N2O variability in recent decades. As clearly shown in the relationship between nitrogen input and N2O emission (Fig 10A), the historical increase of N2O emission in recent decades is mainly attributable to increased land N inputs by atmospheric deposition and fertilizer use. The slope, so-called emission factor, was estimated as 1.75%. This is a bit higher than the typical emission factor value of IPCC guideline [57], 1%, but note that the present estimate includes the effects of climate and land-use changes. In the model simulation, N2O emissions from nitrification and denitrification increased in parallel, as shown by the linear relationship between the two emissions (Fig 10B). Validating the N2O production scheme at broad scales is difficult even by comparing with inversion studies. In forthcoming studies, appropriate observations of N2O isotopomers may provide supporting evidences [58, 59].
Fig 9. Time-series of total soil N2O emissions estimated using VISIT model with the pH-based fN2Onit parameterization.
Observed global mean growth rate of atmospheric N2O by the World Data Center for Greenhouse Gases (https://ds.data.jma.go.jp/gmd/wdcgg/wdcgg.html) are shown for reference.
Fig 10. Relationships in the simulated global nitrogen budget by VISIT model with the pH-based parameterization of fN2Onit.
(a) Total N2O emission related to nitrogen input by biological fixation, atmospheric deposition, and fertilizer. Orange dashed line shows liner regression: N2O emission = 0.0175 N-input + 5.94 (R2 = 0.804). (b) Relationship between nitrification- and denitrification-associated N2O emission: N2O-denitrification = 3.57 N2O-nitrification– 2.33 (R2 = 0.794). Simulation data from 1901 to 2015 were used.
Our meta-analysis and model simulations suggest the importance and uncertainty of fN2Onit values in the evaluation of global N2O budget. Variability in a single parameter could cause a difference in total N2O emission of as much as 2.5 Tg N2O yr–1 (in the 2000s, 15.0 to 17.5 Tg N2O yr–1)—equivalent to the variability of 0.2 Pg CO2-C yr–1 (based on a global warming potential of 298 for N2O with 100-yr horizon [1]). The magnitude of the estimated N2O emission increase from the beginning to the end of the simulation was about 3.9 Tg N2O yr–1 in the simulations with different fN2Onit values (comparable with a simulation by O-CN model [60] and a global synthesis [55]). Because the global nitrogen cycle would be further perturbed by human activities and climate change [5], the uncertainty in the present models can be a critical limiting factor for environmental management. Although global N2O budget may be constrained by using atmospheric observational data to some extent, in-depth understanding of flow components and their environmental regulations is essential to conduct effective nitrogen and climate managements.
Concluding remarks
To our knowledge, this is the first study to have focused on fN2Onit in a comprehensive manner. We should pay attention to the fact that this study used a limited number of observational data and extrapolated them to the global scale. Nevertheless, the dataset covering a variety of ecosystems and soils and the process-based model assessment gave us clues to better understanding of N2O cycle. Our findings gives an explanation for the results of the N2O model intercomparison project, which shows 20% of global soil N2O emission difference among terrestrial models [61]. In our analysis, selection of fN2Onit affected the estimation of global N2O emission by about 15% (2.5/16 Tg N2O yr–1). Because of the scarcity of reliable observational data, our meta-analysis did not give a conclusive value or equation for fN2Onit. Most of observed values were low (<1%) but neglecting high values may result in underestimation of nitrification-associated N2O emission at broad scales. We found a potential and representative range of fN2Onit values and a useful pH-based empirical model covering both natural and agricultural soils. This result should encourage extensive observations of nitrification-associated N2O emission and fN2Onit by using a standardized protocol especially in the field. Although the present meta-analysis showed that the majority of empirical data were obtained by the C2H2 inhibition or isotopic tracer methods, further discussions on effective measurement strategy (e.g., spatial and temporal coverage and representativeness) are required for field and model researchers to improve model accuracy. Because of extreme complexity of the soil biogeochemical processes, it is inevitable to use simplified schemes like the ‘hole-in-a-pipe’ concept and bulk parameters like fN2Onit to conduct simulations at broad scales. Because N2O has a high global warming potential, a small difference in estimated N2O emission can considerably influence the total greenhouse gas budget, as shown by our sensitivity simulations. To develop a better parameterization of fN2Onit and other, related properties, further observations—especially in the field—and process studies of the nitrogen cycle and greenhouse gas emissions are critically important.
Supporting information
(DOC)
(XLSX)
(XLSX)
(TIF)
(a) Norther winter (DJF: December, January, and February), (b) northern spring (MAM: March, April, and May), (c) northern summer (JJA: June, July, and August), and (d) northern autumn (SON: September, October, and November).
(TIF)
Data Availability
All relevant data are within the manuscript and its Supporting Information files.
Funding Statement
AI was supported by KAKENHI grant (no. 26281014 and 17H01867) from the Japan Society for Promotion of Science. URL: https://www.jsps.go.jp/. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Intergovernmental Panel on Climate Change (IPCC). Climate Change 2013: The Physical Science Basis. 2013:996. [Google Scholar]
- 2.Ravishankara AR, Daniel JS, Portmann RW. Nitrous oxide (N2O): The dominant ozone-depleting substance emitted in the 21st century. Science 2009; 326:123–5. 10.1126/science.1176985 [DOI] [PubMed] [Google Scholar]
- 3.Rogelj J, den Elzen M, Höhne N, Fransen T, Fekete H, Winkler H, et al. Paris Agreement climate proposals need a boost to keep warming well below 2°C. Nature 2016; 534: 631–9. 10.1038/nature18307 [DOI] [PubMed] [Google Scholar]
- 4.Bouwman AF, Fung I, Matthews E, John J. Global analysis of the potential for N2O production in natural soils. Global Biogeochem Cycles 1993; 7(3): 557–97. [Google Scholar]
- 5.Galloway JN, Townsend AR, Erisman JW, Bekunda M, Cai Z, Freney JR, et al. Transformation of the nitrogen cycle: recent trends, questions, and potential solutions. Science 2008; 320: 889–92. 10.1126/science.1136674. [DOI] [PubMed] [Google Scholar]
- 6.Davidson EA. The contribution of manure and fertilizer nitrogen to atmospheric nitrous oxide since 1860. Nature Geoscience 2009; 2: 659–62. 10.1038/NGEO608 [DOI] [Google Scholar]
- 7.Gruber N, Galloway JN. An Earth-system perspective of the global nitrogen cycle. Nature 2008; 451: 293–6. 10.1038/nature06592 [DOI] [PubMed] [Google Scholar]
- 8.Tian H, Lu C, Ciais P, Michalak AM, Canadell JG, Saikawa E, et al. The terrestrial biosphere as a net source of greenhouse gases to the atmosphere. Nature 2016; 531: 225–8. 10.1038/nature16946 [DOI] [PubMed] [Google Scholar]
- 9.Barnard R, Leadley PW, Hungate BA. Global change, nitrification, and denitrification: A review. Global Biogeochem Cycles 2005; 19(GB1007). 10.1029/2004GB002282GB1007 [DOI] [Google Scholar]
- 10.Butterbach-Bahl K, Baggs EM, Dannenmann M, Kiese R, Zechmeister-Boltenstern S. Nitrous oxide emissions from soils: how well do we understand the processes and their controls. Phil Trans Royal Soc 2013; 2013 (20130122). 10.1098/rstb.2013.0122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bollmann A, Conrad R. Influence of O2 availability on NO and N2O release by nitrification and denitrification in soils. Global Change Biol 1998; 4: 387–96. [Google Scholar]
- 12.Hink L, Nocol GW, Prosser JI. Archaea produce lower yields of N2O than bacteria during aerobic ammonia oxidation in soil. Environmental Microbiology 2017; 19(12): 4829–37. 10.1111/1462-2920.13282 [DOI] [PubMed] [Google Scholar]
- 13.Stark JM. Modeling the temperature response of nitrification. Biogeochemistry 1996; 35: 433–45. [Google Scholar]
- 14.Bateman EJ, Baggs EM. Contributions of nitrification and denitrification to N2O emissions from soils at different water-filled pore space. Biol Fertil Soils 2005; 41: 379–88. [Google Scholar]
- 15.Farquharson R. Nitrification rates and associated nitrous oxide emissions from agricultural soils–a synopsis. Soil Research 2016; 54: 469–80. 10.1071/SR15304 [DOI] [Google Scholar]
- 16.Raich JW, Rastetter EB, Melillo JM, Kicklighter DW, Grace AL, Moore III B, et al. Potential net primary productivity in South America: application of a global model. Ecol Appl 1991; 1: 399–429. 10.2307/1941899 [DOI] [PubMed] [Google Scholar]
- 17.Li C, Frolking S, Frolking TA. A model of nitrous oxide evolution from soil driven by rainfall events: 1. model structure and sensitivity. J Geophys Res 1992; 97: 9759–76. [Google Scholar]
- 18.Firestone MK, Davidson EA. Microbiological basis of NO and N2O production and consumption in soil In: Andreae MO, Schimel DS, editors. Exchange of Trace Gases between Terrestrial Ecosystems and the Atmosphere: John Wiley and Sons Ltd; 1989. p. 7–21. [Google Scholar]
- 19.Davidson EA, Keller M, Erickson HE, Vershot LV, Veldkamp E. Testing a conceptual model of soil emissions of nitrous and nitric oxides. BioScience 2000; 50(8): 667–80. [Google Scholar]
- 20.Zhu X, Burger M, Doane TA, Horwath WR. Ammonia oxidation pathways and nitrifier denitrification are significant sources of N2O and NO under low oxygen availability. Proc Nat Acad Sci USA 2013; 110(16): 6328–33. 10.1073/pnas.1219993110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic review and meta-analyses: PRISMA statement. BMJ 2009; 339: b2535 10.1136/bmj.b2535 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chen D, Suter HC, Islam A, Edis R. Influence of nitrification inhibitors on nitrification and nitrous oxide (N2O) emission from a clay loam soil fertilized with urea. Soil Biol Biochem 2010; 42: 660–4. 10.1016/j.soilbio.2009.12.014 [DOI] [Google Scholar]
- 23.R-Core-Team. R version 3.4.2. 2017.
- 24.Frolking SE, Mosier AR, Ojima DS, Li C, Parton WJ, Potter CS, et al. Comparison of N2O emissions from soils at three temperate agricultural sites: simulations of year-round measurements by four models. Nutr Cycl Agroecosys 1998; 52: 77–105. [Google Scholar]
- 25.Li C, Aber J, Stange F, Butterbach-Bahl K, Papen H. A process-oriented model of N2O and NO emissions from forest soils: 1. model development. J Geophys Res 2000; 105: 4369–84. [Google Scholar]
- 26.Tian H, Xu X, Liu M, Ren W, Zhang C, Chen G, et al. Spatial and temporal patterns of CH4 and N2O fluxes in terrestrial ecosystems of North America during 1979–2008: application of a global biogeochemistry model. Biogeosci. 2010; 7: 2673–94. 10.5194/bg-7-2673-2010 [DOI] [Google Scholar]
- 27.Saikawa E, Schlosser CA, Prinn RG. Global modeling of soil nitrous oxide emissions from natural processes. Global Biogeochem Cycles 2013; 27: 972–89. 10.1002/gbc.20087 [DOI] [Google Scholar]
- 28.Zaehle S, Friend AD. Carbon and nitrogen cycle dynamics in the O-CN land surface model: 1 Model description, site-scale evaluation, and sensitivity to parameter estimates. Global Biogeochem Cycles 2010; 24 (GB1005). 10.1029/2009GB003521. [DOI] [Google Scholar]
- 29.Tian H, Yang J, Lu C, Xu R, Canadell JG, Jackson RB, et al. The global N2O Model Intercomparison Project. Bull Am Meteorol Soc 2018; 99(6): 1231–51. 10.1175/BAMS-D-17-0212.1 [DOI] [Google Scholar]
- 30.Inatomi M, Ito A, Ishijima K, Murayama S. Greenhouse gas budget of a cool temperate deciduous broadleaved forest in Japan estimated using a process-based model. Ecosystems 2010; 13: 472–83. 10.1007/s10021-010-9332-7 [DOI] [Google Scholar]
- 31.Ito A, Inatomi M. Use of a process-based model for assessing the methane budgets of global terrestrial ecosystems and evaluation of uncertainty. Biogeosci 2012; 9: 759–73. 10.5194/bg-9-759-2012 [DOI] [Google Scholar]
- 32.Ito A, Nishina K, Ishijima K, Hashimoto S, Inatomi M. Emissions of nitrous oxide (N2O) from soil surfaces and their historical changes in East Asia: a model-based assessment. Prog Earth Planet Sci 2018; 5(55): 10.1186/s40645-018-0215-4 [DOI] [Google Scholar]
- 33.Ito A, Oikawa T. A simulation model of the carbon cycle in land ecosystems (Sim-CYCLE): A description based on dry-matter production theory and plot-scale validation. Ecol Model 2002; 151: 147–79. [Google Scholar]
- 34.Ito A. The regional carbon budget of East Asia simulated with a terrestrial ecosystem model and validated using AsiaFlux data. Agr For Meteorol 2008; 148: 738–47. 10.1016/j.agrformet.2007.12.007 [DOI] [Google Scholar]
- 35.Ito A, Nishina K, Reyer CPO, François L, Henrot A-J, Munhoven G, et al. Photosynthetic productivity and its efficiencies in ISIMIP2a biome models: benchmarking for impact assessment studies. Env Res Lett 2017; 12 (085001). 10.1088/1748-9326/aa7a19 [DOI] [Google Scholar]
- 36.Parton WJ, Mosier AR, Ojima DS, Valentine DW, Schimel DS, Weier K, et al. Generalized model for N2 and N2O production from nitrification and denitrification. Global Biogeochem Cycles 1996; 10: 401–12. [Google Scholar]
- 37.Harris I, Jones PD, Osborn TJ, Lister DH. Updated high-resolution grids of monthly climatic observations–the CRU TS3.10 Dataset. Int J Climatol 2014; 34: 623–42. 10.1002/joc.3711 [DOI] [Google Scholar]
- 38.Hurtt GC, Chini LP, Frolking S, Betts RA, Feddema J, Fischer G, et al. Harmonization of land-use scenarios for the period 1500–2100: 600 years of global gridded annual land-use transitions, wood harvest, and resulting secondary lands. Clim Chan 2011; 109: 117–61. 10.1007/s10584-011-0153-2 [DOI] [Google Scholar]
- 39.Galloway JN, Dentener FJ, Capone DG, Boyer EW, Howarth RW, Seitzinger SP, et al. Nitrogen cycles: past, present, and future. Biogeochem 2004; 70: 153–226. [Google Scholar]
- 40.Martikainen PJ. Nitrous oxide emission associated with autotrophic ammonium oxidation in acid coniferous forest soil. Appl Env Microbiol 1985; 50(6): 1519–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.IGBP-DIS. Global Soil Data Products CD-ROM. Oak Ridge, Tennessee, U.S.A.: Oak Ridge National Laboratory, 2000. [Google Scholar]
- 42.Mosier AR, Duxbury JM, Freney JR, Heinemeyer O, Minami K. Nitrous oxide emissions from agricultural fields: Assessment, measurement and mitigation. Plant Soil 1996; 181: 95–108. [Google Scholar]
- 43.Ambus P. Relationship between gross nitrogen cycling and nitrous oxide emission in grass-clover pasture. Nutr Cycl Agroecosys 2005; 72: 189–99. 10.1007/s10705-005-1269-4 [DOI] [Google Scholar]
- 44.Carter MS. Contribution of nitrification and denitrification to N2O emissions from urine patches. Soil Biol Biochem 2007; 39: 2091–102. 10.1016/j.soilbio.2007.03.013 [DOI] [Google Scholar]
- 45.Garrido F, Hénault C, Gaillard H, Pérez S, Germon JC. N2O and NO emissions by agricultural soils with low hydraulic potentials. Soil Biol Biochem 2002; 34: 559–75. [Google Scholar]
- 46.Khalil K, Mary B, Renault P. Nitrous oxide production by nitrification and denitrification in soil aggregates as affected by O2 concentration. Soil Biol Biochem 2004; 36: 687–99. [Google Scholar]
- 47.Klemedtsson L, Svensson BH, Rosswall T. A method of selective inhibition to distinguish between nitrification and denitrification as sources of nitrous oxide in soil. Biol Fertil Soils 1988; 6: 112–9. [Google Scholar]
- 48.Maag M, Vinther FP. Nitrous oxide emission by nitrification and denitrification in different soil types and at different soil moisture contents and temperatures. Appl Soil Ecol 1996; 4: 5–14. [Google Scholar]
- 49.Mathieu O, Hénault C, Lévêque J, Baujard E, Milloux M-J, Andreux MF. Quantifying the contribution of nitrification and denitrification to the nitrous oxide flux using 15N tracers. Env Pollut 2006; 144: 933–40. 10.1016/j.envpol.2006.02.005 [DOI] [PubMed] [Google Scholar]
- 50.Mørkved PT, Dörsch P, Bakken LR. The N2O product ratio of nitrification and its dependence on long-term changes in soil pH. Soil Biol Biochem 2007; 39: 2048–57. [Google Scholar]
- 51.Mørkved PT, Dörsch P, Henriksen TM, Bakken LR. N2O emissions and product ratios of nitrification and denitrification as affected by freezing and thawing. Soil Biol Biochem 2006; 38: 3411–20. 10.1016/j.soilbio.2006.05.015 [DOI] [Google Scholar]
- 52.Tortoso AC, Hutchinson GL. Contributions of autotrophic and heterotrophic nitrifiers to soil NO and N2O emissions. Appl Env Microbiol 1990; 56(6): 1799–805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ambus P. Nitrous oxide production by denitrification and nitrification in temperate forest, grassland and agricultural soils. Eur J Soil Sci 1998; 49: 495–502. [Google Scholar]
- 54.Chapuis-Lardy L, Wrage N, Metay A, Chotte J-L, Bernoux M. Soils, a sink for N2O? A review. Global Change Biol 2007; 13: 1–17. [Google Scholar]
- 55.Syakila A, Kroeze C. The global nitrous oxide budget revisited. Greenhouse Gas Measur Manag 2010; 1: 17–26. 10.3763/ghgmm.2010.0007 [DOI] [Google Scholar]
- 56.Thompson RL, Patra PK, Ishijima K, Saikawa E, Corazza M, Karstens U, et al. TransCom N2O model inter-comparison–Part 1: Assessing the influence of transport and surface fluxes on tropospheric N2O variability. Atm Chem Phys 2014; 14: 4349–68. 10.5194/acp-14-4349-2014 [DOI] [Google Scholar]
- 57.Intergovernmental Panel on Climate Change (IPCC). 2006 IPCC Guidance for National Greenhouse Gas Inventories: Cambridge University Press; 2006. [Google Scholar]
- 58.Yoshida N, Toyoda S. Constraining the atmospheric N2O budget from intramolecular site preference in N2O isotopomers. Nature 2000; 405: 330–4. 10.1038/35012558 [DOI] [PubMed] [Google Scholar]
- 59.Park S, Pérez T, Boering KA, Trumbore SE, Gil J, Marquina S, et al. Can N2O stable isotopes and isotopomers be useful tools to characterize sources and microbial pathways of N2O production and consumption in tropical soils? Global Biogeochem Cycles 2011; 25(GB1001). 10.1029/2009GB003615 [DOI] [Google Scholar]
- 60.Zaehle S, Ciais P, Friend AD, Prieur V. Carbon benefits of anthropogenic reactive nitrogen offset by nitrous oxide emissions. Nature Geosci. 2011; 4: 601–5. 10.1038/NGEO1207 [DOI] [Google Scholar]
- 61.Tian H, Yang J, Xu R, Lu C, Canadell JG, Davidson EA, et al. Global soil nitrous oxide emissions since the pre-industrial era estimated by an ensemble of terrestrial biosphere models: Magnitude, attribution and uncertainty. Global Change Biol 2019; 25: 640–59. 10.1111/gcb.14514 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOC)
(XLSX)
(XLSX)
(TIF)
(a) Norther winter (DJF: December, January, and February), (b) northern spring (MAM: March, April, and May), (c) northern summer (JJA: June, July, and August), and (d) northern autumn (SON: September, October, and November).
(TIF)
Data Availability Statement
All relevant data are within the manuscript and its Supporting Information files.