Abstract
Intramammary metastasis from ovarian cancer is rare. It is usually associated with poor prognosis. We present a 56-year-old female with advanced ovarian cancer in whom a metastatic intramammary lymph node was discovered after finishing the first line of neoadjuvant chemotherapy.
Keywords: Breast neoplasms, ovarian cancer, metastasis, lymph nodes
Introduction
Breast metastasis from solid tumors is a rare event. Melanoma, lung cancer, soft tissue sarcoma and ovarian carcinoma are the most common primaries. Intramammary metastases do not show a unique clinical or radiological pattern. Yet, accurate diagnosis and differentiation from primary breast tumors is very important to choose the appropriate treatment strategy and to avoid unnecessary interventions (1).
Case Presentation
A 56-year-old female, diabetic & hypertensive, with previous surgical history of appendectomy, complained from progressive abdominal enlargement. Pelvi-abdominal ultrasonographic (US) evaluation revealed bilateral adnexal neoplastic soft tissue masses with internal vascularity. The right one was seen inseparable from the surrounding intestinal loops, it measured 8×13 cm. The left one was seen inseparable from the uterus with the possibility of infiltration, it measured 10×14 cm. Multiple hyperechoic subcapsular hepatic peritoneal deposits were detected, the largest one measured 2.3 cm in diameter. Also amalgamated intestinal loops with sheet like omental cake and moderate ascites were observed. Serum carbohydrate antigen 125 (CA 125) value was 195 U/mL. Post contrast Magnetic Resonance Imaging (MRI) of the abdomen and pelvis showed mildly enlarged liver with non-enhanced focal lesion at segment VII of right lobe, it measured 1.5 cm in diameter. Large heterogeneously enhanced pelvic soft tissue mass was seen infiltrating the uterus and compressing posterior wall of the urinary bladder as well as recto-sigmoid colon with no line of separation in-between, it measured 10.5×14.5×10.5 cm in APXTRXH respectively. Another similar soft tissue mass was seen at right lower abdominal cavity, compressing and displacing surrounding intestinal loops, it measured 8×13×10 cm. Multiple variable sized enhanced nodular and sheet-like peritoneal deposits were seen scattered in the abdomen as well, the largest deposit measured 3.5×4 cm. Neoplastic lower para aortic and left iliac lymph nodes were also noted, the largest lymph node diameter was 2.5cm. The patient underwent US guided aspiration from the ascitic fluid. Microscopic examination of the aspirated sample (Figure 1) revealed clusters of rounded or hyperchromatic nuclei with overlapping in background of Red Blood Cells (RBCs). Some cells exhibited cytoplasmic vacuoles. The cell block revealed papillary & acinar structures with thin vascular connective tissue and covered by cuboidal cells showing rounded vesicular nuclei. Psammoma bodies were seen as well. This led to the diagnosis of metastatic papillary carcinoma mostly of ovarian origin. Immunohistochemical staining (IHC) showed positive nuclear staining for Estrogen Receptor (ER), negative staining for tumor protein P53, and focal nuclear positivity for Wilms tumor gene product (WT1). These results confirmed the diagnosis of metastatic papillary carcinoma of ovarian origin. The patient received 6 Taxol & Carboplatin cycles as neoadjuvant therapy with stationary course of the disease. Upon breast sonomammographical evaluation (Figure 2), the left breast showed an oval hypoechoic lesion with foci of calcification measuring 11×4.3 mm at 12 o’clock in zone C, which was reported to be likely a suspicious intramammary lymph node. US guided core needle biopsy from the breast lesion was performed. Microscopic examination (Figure 3) revealed cores of lymphoid tissue showing infiltration by papillary structures covered by atypical epithelial cells showing mild degree of atypia and pleomorphism. IHC studies for WT1, Cytokeratin 7 (CK7) and ER showed positive reaction in atypical cells, while (Progesterone Receptor) PR, mammaglobin and P53 showed negative reaction in tumor cells. This supported the diagnosis of metastatic ovarian carcinoma. The patient was prepared for receiving second line neoadjuvant therapy. Informed consent was obtained from the patient reported in this study.
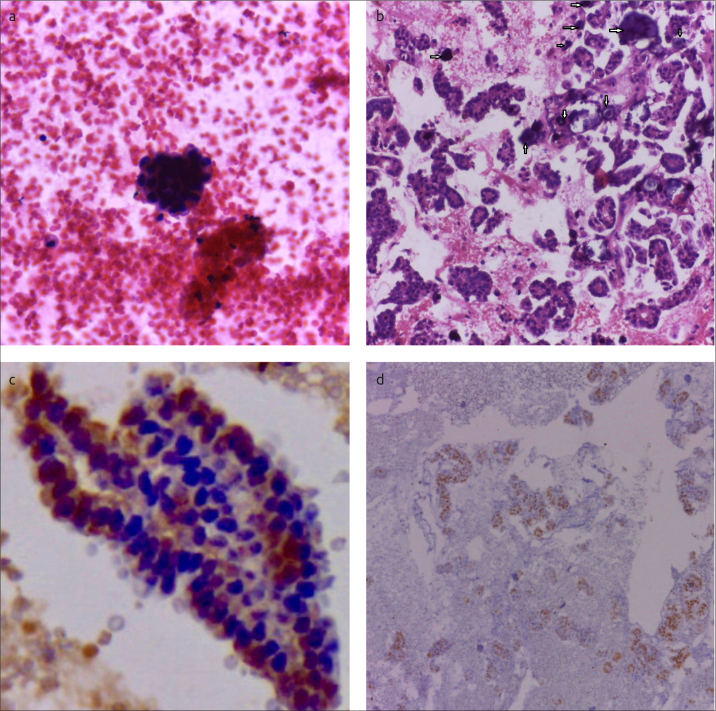
Figure 1. a–d.
Microscopic examination of aspirate from ascites. Smear showing a 3D appearance of a morula of malignant epithelial cells (Hx&E, ×400) (a). Highly cellular cell block showing papillary structures lined by malignant epithelial cells with evident Psammoma bodies (marked by arrows) (Hx&E, ×200) (b). Cytoplasmic and focal nuclear staining of tumor cells for WT1 (×400) (c). Nuclear ER staining of tumor cells (×100) (d)
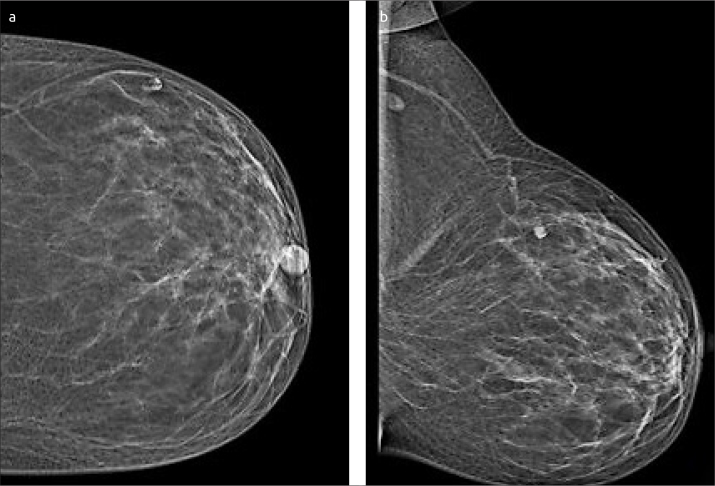
Figure 2. a, b.
Left breast mammogram. Craniocaudal and mediolateral (a, b) mammogram of asymptomatic 56-year-old patient shows oval dense indistinct mass at upper outer quadrant of the left breast, no related architectural distortion nor overlying skin thickening. Oval left axillary LN with lucent center
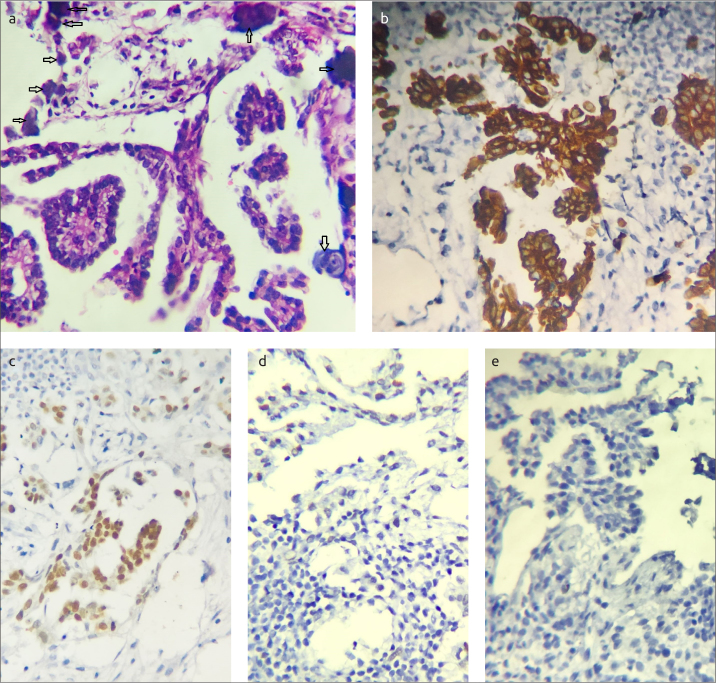
Figure 3. a–e.
Microscopic examination of left breast mass core needle biopsy. Section in breast showing papillary structures lined by malignant epithelial cells, evident psammoma bodies in upper right (marked by arrows) (×400 Hx&E) (a). Cytoplasmic positivity of tumor cells in breast for ck7 (×400) (b). Nuclear staining of tumor cells for WT1 (×400) (c). Negative tumor cells for mammaglobin (×400) (d, e)
Discussion and Conclusion
Secondary tumors to the breast are rare. They account to 0.2–2% of all breast neoplasms. The most common primary sites are contralateral breast, melanoma, lung cancer and ovarian cancer. Diagnosis of intramammary metastasis is a diagnostic challenge as it may resemble primary breast tumors clinically, radiologically and even pathologically (2–5). Proper evaluation and accurate pathological examination is recommended to avoid the possibility of unneeded breast intervention (e.g., mastectomy) (4, 6–10).
Most of intramammary metastasis present as palpable breast swellings. Only about 14% are discovered by radiological evaluation –such as our presented case. The majority of these tumors present as solitary masses but 8–25% present as multifocal or bilateral breast masses (3) There are no specific radiologic criteria for intramammary metastasis which may be misinterpreted as benign or malignant primary breast masses on ultrasound or mammography. The most common pattern of presentation after being incidentally discovered during breast radiological evaluation is the presence of well-defined, hypoechoic oval or rounded mass with regular margins that does not show microcalcifications, speculations or posterior shadowing. (1, 3, 5)
Pathologically, there are no specific criteria for diagnosis of such cases, especially in the absence of well-known history of prior malignancy. Yet, common histologic findings could be identified such as the presence of peritumoral fibrous pseudo-capsule and the absence of in situ component (4).
Incidence of intramammary metastasis from ovarian carcinoma is very rare. It constitutes about 0.03%–0.6% of all breast tumors. It usually occurs 2 years after the primary presentation –In our patient, it was discovered 8 months after the initial presentation-. The most common histologic subtype is ovarian serous carcinoma. Those patients usually present with advanced stage with heavy peritoneal infiltration – like the case presented in this report-. Eighty-five percent of ovarian serous tumors are limited to the peritoneal cavity. The liver, lung and pleura are the most common site for metastases followed by the spleen, central nervous system, bone and skin. Yet, breast metastasis from ovarian carcinoma is associated with poorer prognosis with reported short term survival after the incidence of metastasis (1, 7–9, 11).
Accurate analysis of clinical, radiological and pathological features is required to differentiate such patients from those with primary breast carcinoma associated with Krukenburg ovarian tumors due to overlapping of pathologic features. Histopathological classification of breast secondary tumors and the differentiation from primary breast neoplasms is not an easy job and is based on the interpretation of both morphological and IHC features. The majority of intramammary metastases are characterized by the presence of papillary clusters and solid areas with slit-like spaces. Those spaces are composed of cells showing marked nuclear atypia. The adjacent breast tissue shows no pathological changes or intraductal component. IHC plays a very important role in such scenarios especially WT-1, the Wilms tumor gene product –which shows nuclear expression in about 95% of serous papillary carcinomas and only in less than 10% of breast cancers-, Gross cystic disease fluid protein (GCDFP-15), Paired-box gene 8 (PAX8) and mesothelin, which is expressed in more than 90% of ovarian serous papillary carcinoma. However, it is weakly expressed in 3 to 14% of primary breast malignancies (4, 7, 9, 11, 12)
It is to be noted that breast and epithelial ovarian cancers share some similar hereditary and risk factors which can explain the co-incidence of primary breast and ovarian cancers. That is why patients with epithelial ovarian cancer are usually subjected to thorough physical, radiological & serological investigations raising the possibility for detection any breast masses (8).
In conclusion, intramammary metastasis from ovarian cancer is a rare condition that carries a poor prognosis. Despite having no pathognomonic clinical or radiological characteristics, thorough analysis of clinical, radiological and pathological data is required so as not to miss the diagnosis.
Footnotes
Informed Consent: Written informed consent was obtained from patientspatient who participated in this study.
Peer-review: Externally peer-reviewed.
Author Contributions: Concept - O.H.; Design - O.H.; Supervision - M.Z.; Resources - O.H., F.S., G.S.; Materials - O.H., F.S., G.S.; Data Collection and/or Processing - O.H., F.S., G.S.; Analysis and/or Interpretation - O.H., F.S., G.S.; Literature Search - O.H., F.S.; Writing Manuscript - O.H., F.S., G.S.; Critical Review - M.Z.
Conflict of Interest: The authors have no conflicts of interest to declare.
Financial Disclosure: The authors declared that this study has received no financial support.
References
- 1.Antuono L, Angela F, Luca N, Giovanni M, Enrico C. Breast metastasis from ovarian cancer: A case report. Radiol Case Rep. 2018;13:1166–1169. doi: 10.1016/j.radcr.2018.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Della Corte L, Giampaolino P, Fabozzi A, Cieri M, Zizolfi B, Morra I, Bifulco G. Breast metastasis two years after pelvic surgery and adjuvant chemotherapy for serous ovarian cancer. Gynecol Endocrinol. 2019;35:211–213. doi: 10.1080/09513590.2018.1521795. [DOI] [PubMed] [Google Scholar]
- 3.Cimino-Mathews A, Harvey SC, Argani P. Metastases to the Breast. In: Shin SJ, editor. A Comprehensive Guide to Core Needle Biopsies of the Breast [Internet] Cham: Springer International Publishing; 2016. pp. 819–51. Available from: URL: [DOI] [Google Scholar]
- 4.DeLair DF, Corben AD, Catalano JP, Vallejo CE, Brogi E, Tan LK. Non-mammary metastases to the breast and axilla: a study of 85 cases. Mod Pathol. 2013;26:343–349. doi: 10.1038/modpathol.2012.191. [DOI] [PubMed] [Google Scholar]
- 5.Surov A, Fiedler E, Holzhausen H-J, Ruschke K, Schmoll H-J, Spielmann R-P. Metastases to the Breast from Non-mammary Malignancies: Primary Tumors, Prevalence, Clinical Signs, and Radiological Features. Acad Radiol. 2011;18:565–574. doi: 10.1016/j.acra.2010.12.009. [DOI] [PubMed] [Google Scholar]
- 6.Luo Y, Xu B, Li Q, Zhang P, Yuan P, Wang J, et al. Clinicopathological features and prognosis of metastases to the breast from extramammary solid tumors. Zhonghua Zhong Liu Za Zhi. 2014;36:453–456. [PubMed] [Google Scholar]
- 7.Demir L, Erten C, Yigit SC, Can A, Dirican A, Bayoglu V, Kucukzeybek Y, Somali I, Tarhan MO. Intramammary lymph node metastasis in a patient with ovarian carcinoma and a brief review of the literature. Contemp Oncol (Pozn) 2012;16:108–110. doi: 10.5114/wo.2012.28789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Karam AK, Stempel M, Barakat RR, Morrow M, Gemignani ML. Patients with a history of epithelial ovarian cancer presenting with a breast and/or axillary mass. Gynecol Oncol. 2009;112:490–495. doi: 10.1016/j.ygyno.2008.11.006. [DOI] [PubMed] [Google Scholar]
- 9.Recine MA, Deavers MT, Middleton LP, Silva EG, Malpica A. Serous carcinoma of the ovary and peritoneum with metastases to the breast and axillary lymph nodes: a potential pitfall. Am J Surg Pathol. 2004;28:1646–1651. doi: 10.1097/00000478-200412000-00015. [DOI] [PubMed] [Google Scholar]
- 10.Schneuber SE, Scholz HS, Regitnig P, Petru E, Winter R. Breast metastasis 56 months before the diagnosis of primary ovarian cancer: a case study. Anticancer Res. 2008;28:3047–3050. [PubMed] [Google Scholar]
- 11.Tempfer CB, El Fizazi N, Ergonenc H, Solass W. Metastasis of ovarian cancer to the breast: A report of two cases and a review of the literature. Oncol Lett. 2016;11:4008–4012. doi: 10.3892/ol.2016.4514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lee AHS. The histological diagnosis of metastases to the breast from extramammary malignancies. J Clin Pathol. 2007;60:1333–1341. doi: 10.1136/jcp.2006.046078. [DOI] [PMC free article] [PubMed] [Google Scholar]