Abstract
Objective
Breast cancer is a heterogenous disease, and genetic profiling helps to individualize adjuvant treatment. The Oncotype DX is a validated test to predict benefit of adjuvant systemic treatment. The aims of this study are to determine the costs of chemotherapy in government hospitals in Turkey and evaluate the cost-effectiveness of the Oncotype DX from the national insurance perspective.
Materials and Methods
A Markov model was developed to make long term projections of distant recurrence, survival, quality adjusted life expectancy, and direct costs for patients with ER+, HER2-, node-negative or up to 3 node-positive early stage breast cancer. Turkish decision impact study patient data were captured for model reference. In that study, ten academic centers across Turkey participated in a prospective trial. Of 165 patients with pT1–3, pN0-N1mic, ER-positive, and HER-2 negative tumors, 57% had low recurrence score (RS), 35% had intermediate RS, and 8% had high RS, respectively. The overall rate of change in chemotherapy treatment decisions following Oncotype DX was 33%.
Results
The cost of adjuvant chemotherapy in public hospitals was estimated at $3.649, and Oncotype Dx test was $5.141. Based on the cost-effectiveness analysis, Oncotype DX testing was estimated to improve life expectancy (+0.86 years) and quality-adjusted life expectancy (+0.68 QALYs) versus standard care. The incremental cost-effectiveness ratio (ICERs) of Oncotype DX was estimated to be $7207.9 per QALY gained and $5720.6 per LY gained versus current clinical practice.
Conclusion
As Oncotype DX was found both cost-effective and life-saving from a national perspective, the test should be introduced to standard care in patients with ER+, HER-2 negative early-stage breast cancer in Turkey.
Keywords: Early breast cancer, genetic profiling, oncotype-Dx, cost, markov model
Introduction
Invasive breast carcinoma is the most commonly seen malignancy and leading cause of cancer related death in Turkish women. An analysis of 13.240 patients in the National Breast Cancer Database established within the Turkish Federation of Breast Diseases Societies showed that 50% patients had N0 and 27% had Stage I breast cancer, respectively. Overall, 80.7% of patients had luminal molecular subtype
Despite available data on chemotherapy efficacy in locally advanced and metastatic breast cancer, it is not possible to predict who will benefit from adjuvant treatment in early stage breast cancer based on traditional clinical pathological features. The major pathological and clinical features including age, menopausal status, tumor size, histologic grade, ki 67 proliferative index, estrogen (ER) and progesteron receptor (PR) and human epidermal growth factor receptor 2 (HER-2) expression are commonly used by clinicians to guide chemotherapy treatment decisions; however, in cases with equivocal features, the decision to recommend adjuvant chemotherapy may be uncertain. Furthermore, challenges remain regarding the inter- and intra-laboratory standardization of a number of clinical risk factors.
As early stage breast cancer incidence is increasing with improved cancer screening methods, with half of breast cancer cases presenting as stage pN0 in Turkey, suboptimal evaluation for treatment planning may lead to many patients unnecessarily exposed to chemotherapy and associated toxicity and may increase health expenses. Besides, patients who may derive a substantial benefit from chemotherapy to prevent distant recurrence may not receive chemotherapy.
The emergence of genomics and transcriptomics techniques and the ability to measure various genes led to the identification of tumor-biology based prognostic and predictive determination. The Oncotype DX RS is one of the best-validated prognostic assays and may identify patients who are most and least likely to derive benefit from adjuvant chemotherapy (1, 2).
The Oncotype-DX test is validated for patients with node-negative early breast cancer as well as limited node involvement (pNmic/pN1), ER(+), HER-2(−) negative breast cancer to identify whether a patient who will receive at least a five-year course of endocrine therapy is likely to derive benefit from chemotherapy. (3, 4). The validity of Oncotype DX has been demonstrated in several studies both for prognosis and prediction of adjuvant chemotherapy (5, 6).
In two different analyses from the same patient cohort from 10 academic centers in Turkey, we demonstrated that only high Ki67 (>14%) and low PR (20%) levels were correlated with high Oncotype DX-RS in multivariate analysis, and Oncotype-DX RS may further change physician decisions for adjuvant treatment (7, 8). In a Turkish Oncotype-Dx Decision Impact Study involving patients with T1–3, ER+, HER-2(−), N0–1mic breast cancer, adjuvant chemotherapy treatment recommendations of enrolled patients were collected before and after availability of the RS. Changes in treatment decisions based on the information provided by the RS were then analysed. Of 165 patients; 57% had low RS, 35% had intermediate RS, and 8% had high RS, respectively. The overall rate of change in chemotherapy treatment decisions was found to be 33%. For the most part, recommendations changed from chemotherapy plus hormonotherapy to hormonotherapy alone, resulting in 19% absolute reduction in chemotherapy use (8).
Currently Oncotype DX is not frequently used by Turkish Physicians due to its prohibitive cost for patients and also it is not currently reimbursed by the Turkish Social Security Administration. The cost-effectiveness is a matter of policy interest. Several developed countries have revealed the cost-effectiveness of testing based on analyses of the local use and impact of the test. Although the benefit was clearly established in these trials, in some European countries, Oncotype DX reimbursement is limited to selected patients. The question remains as to the optimal approach to implementing Oncotype DX testing.
In this study, we aimed to evaluate the cost-effectiveness of Oncotype DX in a developing country using our patient population as a model reference.
Materials and Methods
Model overview
The model used in this analysis is generated via local adaption of a Markov model, that was developed in Microsoft Excel, based on an original model by Hornberger, to evaluate the long-term costs and clinical outcomes associated with introducing Oncotype DX testing to inform decisions about adjuvant chemotherapy for patient with ER+, node-negative or single node positive early-stage breast cancer for an analysis for England and Wales (9). The model made projections of life expectancy, quality-adjusted life expectancy and direct costs, based on recurrence rates for low, intermediate and high-risk patients as well as country-specific mortality data. The risk was adjusted by reference models as demonstrated on Table 1, 2 and 3.
Table 1.
Summary of changes in adjuvant therapy recommendations with Oncotype DX testing in the modelling analysis
| Recurrence Score | Initial recommendation | Post Oncotype DX net change in CT use HT+CT (%) |
|
|---|---|---|---|
|
| |||
| HT (%) | HT+CT (%) | ||
| Low | 30.9 (51/165) | 25.5 (42/165) | −21.0 |
| Intermediate | 11.5 (19/165) | 23.6 (39/165) | 1.9 |
| High | 1.8 (3/165) | 6.7 (11/165) | 4.8 |
| Total | 44.2 (73/165) | 55.8 (92/165) | |
CT: chemotherapy; HT: hormone therapy
Table 2.
Summary of clinical variables in the cost-effectiveness modelling analysis
| Variable | p | Reference |
|---|---|---|
| Age (years) | 49.9 | Turkish Oncotype-Dx Decision Impact Study8 |
| Net change in chemotherapy use with low RS (%) | −20.9 | Holt et al. 201113 |
| Net change in chemotherapy use with intermediate RS (%) | 1.90 | Holt et al. 201113 |
| Net change in chemotherapy use with high RS (%) | 4.76 | Holt et al. 201113 |
| 10-year risk of recurrence (low RS) on HT (%) | 3.20 | Paik et al. 20063 |
| 10-year risk of recurrence (intermediate RS) on HT (%) | 9.10 | Paik et al. 20063 |
| 10-year risk of recurrence (high RS) on HT (%) | 39.5 | Paik et al. 20063 |
| RRR with chemotherapy (low RS) (%) | 0 | Assumed based on Paik et al. 20063 |
| RRR for chemotherapy (intermediate RS) (%) | 39.0 | Paik et al. 20063 |
| RRR for chemotherapy (high RS) (%) | 74.0 | Paik et al. 20063 |
| Post-recurrence survival (years) | 3.3 | Thomas et al. 200912 |
| Mortality rates | - | TÜİK (2013)11 |
HT: hormone/endocrine therapy; RRR: relative risk reduction; RS: Recurrence Score
Table 3.
Summary of cost variables in the cost-effectiveness modelling analysis
| Item | Mean cost (USD) | Reference |
|---|---|---|
| Oncotype DX test | 5141 | Genomic Health Ltd. Turkey branch |
| Endocrine therapy (years 1–5) | 256.5 | Turkish Cost-Effectiveness Analysis of Screening in Breast Cancer |
| Endocrine therapy (years 6–8) | 289.6 | Turkish Cost-Effectiveness Analysis of Screening in Breast Cancer |
| Chemotherapy | 1436 | Turkish Oncotype-Dx Decision Impact Study |
| Distant recurrence (monthly) | 98.08 | Turkish Oncotype-Dx Decision Impact Study and Turkish Cost-Effectiveness Analysis of Screening in Breast Cancer |
| Chemotherapy adverse events | 468.5 | Turkish Oncotype-Dx Decision Impact Study |
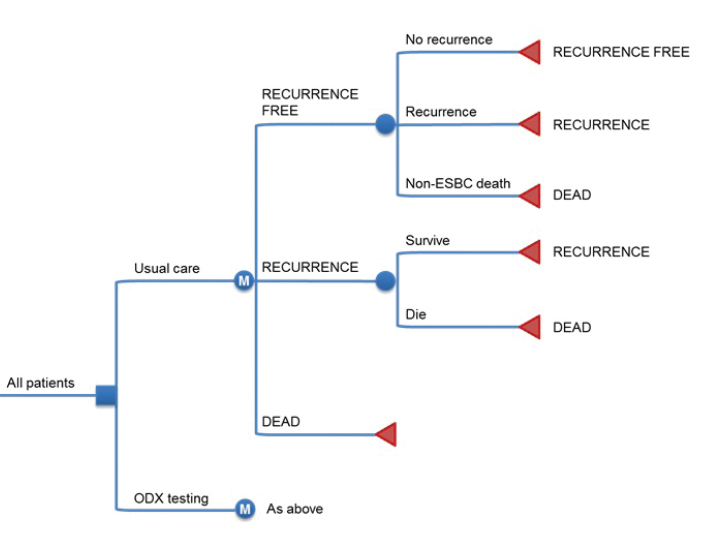
The model structure is outlined in Figure 1. There are three states in the model: recurrence-free (in which all patients start the simulation), recurrence (following a distant recurrence event) and dead (following a mortality event). The model had a 1-year cycle length. The base case time horizon was set to 30 years to capture long-term recurrence risk. All patients start the simulation in the recurrence-free state. In each 1-year cycle of the simulation, patients are exposed to the risk of competing mortality and recurrence. Patients who have a mortality event transition to the dead state, who experience a distant recurrence event transition to the recurrence state occurs, where they are exposed to the risk of breast cancer mortality in each subsequent year of the simulation. All cost-analyses were analysed according to Social Security Instution of Turkey (SGK).
Figure 1.
Overview of the Oncotype DX cost-effectiveness model structure
Clinical parameters
To ensure that the modelling analysis was in line with the standard clinical care pathways in Turkey, patients were assumed to receive standard endocrine therapy and chemotherapy regimens in line with local practices evidenced in the Turkish Oncotype-Dx Decision Impact Study and the Turkish Cost-Effectiveness Analysis of Screening in Breast Cancer (8, 10). The model cohort age was assumed to be 49.9 years based on the mean age from Turkish Oncotype-Dx Decision Impact Study cohort (8). In this patient group, 108 patients (65.4%) had pT1 tumors, and the median tumor size was 2 cm. Only 11 (6.7%) patients had micrometastasis in axillary lymph nodes (pN1mic). The majority (53.5%) of the patients had a ki67 score of <20%, 60.4% patients were considered to have luminal B molecular type. The change in the chemotherapy decision between pre and post RS assay treatment plans was analysed using McNemar’s test (Table 1).
In each cycle of the model, the risk of recurrence was evaluated for each simulated patient based on their RS defined category of low, intermediate or high risk as reported for the NSABP B-20 cohort (4) (Table 2). Risk was adjusted based on whether patients were receiving chemotherapy as per the initial recommendations (in the usual care arm) and based on the Oncotype DX Recurrence Score (in the Oncotype DX arm). Non-breast cancer death was captured as a competing risk in the model, based on Turkey life tables (Turkish Statistical Institute) for females in 2013 (11). For patients experiencing distant recurrence, survival was assumed to be 3.3 years (12).
Costs of treatment
In the cost-effectiveness model the costs of endocrine therapy, chemotherapy, adverse events associated with chemotherapy and the cost of distant recurrence were accounted. All costs were taken from Turkey-specific sources as Turkish lira, converted and expressed as dollars in the analysis, using the currency conversion rate as of February 2016 when the data collection was conducted. A summary of cost variables used in the model is provided in Table 3.
All the medicine costs, follow-up costs, mammogram costs and other cost items are taken from Turkish Cost-Effectiveness Analysis of Screening in Breast Cancer (10).
The cost of endocrine therapy is incurred over 8 years, at different rates over the initial 5 years and later 3 years to reflect varying treatment patterns. For those patients receiving chemotherapy, the costs of chemotherapy and endocrine therapy are both incurred in the first year. Based on 4–6 cycles of chemotherapy, there is thus an overlap of costs of approximately 3.5 months.
Costs of all drugs related with treatment and toxicities, costs of follow-up (mammography, ultrasound, biopsy, CT-scans etc.) are taken from Turkish Cost-Effectiveness Analysis of Bahcesehir Breast Cancer Screening Program (10). The cost of chemotherapy evaluated the chemotherapy regimens, number of cycles, doses of chemotherapeutics, concomitant medications used to prevent or treat adverse events, diagnostics etc; the frequency and duration. Adverse events associated with chemotherapy; the cost of screening, diagnostics, treatment and follow-up for adverse events associated with treatment were based on the Turkish Oncotype-Dx Decision Impact Study cohort (8).
Risk of recurrence associated with endocrine therapy and relative risk reduction associated with chemotherapy were both taken from the Paik et al. (4) NSABP B-20 study of Oncotype DX. Local recurrences are not captured in the model. The cost of recurrence was generated from Bahcesehir Breast Cancer Screening Program (11).
Rate of non-cancer related death is taken from Turkish life-tables.
Quality of life
Quality of life utility scores were based on the published literature. Patients that were in the recurrence-free state and in the recurrence state accrued utility scores. Health utility scores range from death (0) to perfect health (1) and quantify the particular health situation. Published utility scores were used, with a disutility of 0.07 was applied to capture the health-related QALY (14). and annual utility scores of 0.60 and 0.78 were applied for patients with and without recurrence respectively (15, 16) Health utility associated with one year in the recurrence free state was assumed to be the same during and after endocrine therapy (16).
Endocrine therapy costs
In the model, all endocrine regimens were considered, consistent with current practices in Turkey: tamoxifen for 5 years, AI for 5 years, tamoxifen and AI sequential use and extended adjuvan treatment beyond 5 years.
The probability of treatment with each regimen was derived from the Turkish Cost-Effectiveness Analysis of Screening in Breast Cancer, with pharmacy costs for all interventions, follow up and mammograms were taken from the SGK Appendixes. In the model the annual per patient cost of treatment and follow up was calculated to be $256.5 for the first 5 years and $289.6 for years 5–8.
Adverse event rates and costs for endocrine therapy were not included in the model.
Chemotherapy costs and adverse events
The chemotherapy regimens, number of cycles, doses of chemotherapeutics, concomitant medications used to prevent or treat adverse events and diagnostic tests etc were taken from Turkish Oncotype-Dx Decision Impact Study cohort.
Chemotherapy-related adverse event rates were generated from Turkish Oncotype-Dx Decision Impact Study cohort (Table 4).
Table 4.
Frequencies and costs of chemotherapy-related adverse events
| Adverse event | Maximum frequency in various chemotherapy regimens (% per cycle) Cost per event (USD) | |||
|---|---|---|---|---|
|
| ||||
| Grade 3 | Grade 4 | Grade 3 | Grade 4 | |
| Anaemia | 12.9% | 21.0% | 477.57 | 920.94 |
| Neutropenia | 36.5% | 19.4% | 391.05 | 393.12 |
| Febrile neutropenia | 32.8% | 5.9% | 1475.55 | 2099.04 |
| Infection | 8.7% | 4.6% | 615.58 | 1707.98 |
| Thrombocytopenia | 8.0% | 1.4% | 77.75 | 155.51 |
| Nausea/vomiting | 39.4% | 12.5% | 80.69 | 412.55 |
| Diarrhea | 25.1% | 50.3% | 63.0 | 413.08 |
| Motor neuropathy | 8.0% | 0% | 230.62 | 308.03 |
| Cardiac toxicity | 8.0% | 2.0% | 71.42 | 615.9 |
The majority of costs associated with chemotherapy are due to adverse event and monitoring rather than the acquisition costs of chemotherapy agents.
The total cost of chemotherapy drugs, administration and monitoring was $1436.07 ($507.7+$432.3+$495.9).
Sensitivity analyses
A series of one-way sensitivity analyses were performed to identify key drivers of model outcomes. Most clinical and cost parameters inputs the model were varied by +/− 25%. ICERs were reported for all one-way sensitivity analyses.
Results
Base-case analysis
Oncotype DX was projected to cost an additional $1.492 per patient compared with current clinical practice over a 30-year time horizon ($5.141 versus $3.649) (Table 5). The increase in costs was associated with an improvement in life expectancy of 0.86 years (24.84 years versus 25.70 years) and an increase in quality-adjusted life expectancy of 0.68 QALYs (19.26 QALYs versus 19.94 QALYs). The incremental cost-effectiveness ratio (ICERs) was estimated to be $7207.9 per QALY gained and $5720.6 per LY gained for Oncotype DX versus current clinical practice in Turkey.
Table 5.
Summary of cost-effectiveness results for the base case analysis
| Usual care | Oncotype DX testing | Difference | |
|---|---|---|---|
| Cost | $3649.3 | $8568.6 | $4919.3 |
| Life Expectancy (years) | 24.84 LY | 25.70 LY | 0.86 LY |
| Quality-Adjusted-Life Expectancy (QALYs) | 19.26 QALY | 19.94 QALY | 0.68 QALY |
| ICER (USD per life year gained) | $5720.6 per LY gained | ||
| ICER (USD per QALY gained) | $7207.9 per QALY gained |
ICER: incremental cost-effectiveness ratio; QALYs: quality-adjusted life years; LYs: life years
Sensitivity analyses
One-way sensitivity analysis showed that the base case outcomes were most sensitive to variation in patient age, the cost of Oncotype DX testing and the change in chemotherapy recommendations for low risk patients (Table 6). Increasing the baseline age for patients in the simulation by 25% increased the ICER for Oncotype DX testing versus current care to $7971.72 per LY gained. This was due to competing mortality, which meant that patients were not alive long enough to accumulate the full benefit of Oncotype DX testing. In contrast, reducing the baseline age improved the cost-effectiveness of Oncotype DX ($5213.7 per LY gained).
Table 6.
Summary of one-way sensitivity analysis results
| Parameter/scenario | ICER($perLYgained) for Oncotype DX testing versus usual care |
|
|---|---|---|
|
| ||
| −25% | +25% | |
| Base case | 5720.6 | |
| Cohort | ||
| Age | 5213.7 | 7971.7 |
| Cost | ||
| Cost of chemotherapy treatment | 5780.3 | 5661.0 |
| Cost of recurrence | 5725.5 | 5715.8 |
| Cost of Oncotype DX testing | 4226.2 | 7215.5 |
| Clinical parameters | ||
| Post-recurrence survival | 5705.1 | 5736.5 |
| Net change in the use of chemotherapy in the low Recurrence Score group | 8521.7 | 4330 |
| Net change in the use of chemotherapy in the intermediate Recurrence Score group | 5742.4 | 5699.6 |
| Net change in the use of chemotherapy in the high Recurrence Score group | 5836.8 | 4615.8 |
ICER: incremental cost-effectiveness ratio
Discussion and Conclusion
Contrary to developed countries, the rate of breast cancer incidence and mortality has been increasing in Turkey and other developing countries due to changing life style, ageing, increase in population size and mammography screening (17). Breast cancer incidence has more than doubled in last two decades in Turkey (1). In our breast cancer registry database, nearly half of the patients had node negative disease and 76.9% had ER positive breast cancer at diagnosis, making these patients good candidates for molecular testing to potentially spare them from unnecessary adjuvant chemotherapy (1). Overtreatment is a big problem due to chemotherapy toxicity and its cost to breast cancer patients (18). Gene expression profiling assays may provide an emerging paradigm to predict chemotherapy benefit based on expression levels of specific tumors. Several multigene assays are currently available for early breast cancer patients, of which Oncotype DX has the most compelling evidence of adding value to standard prognostic factors regarding the benefit of adjuvant chemotherapy for patients with early breast cancer (2).
The MINDACT trial revealed that Mammaprint (70 gene signature test) may identify subsets of patients who have a low likelihood of distant recurrence despite high-risk clinical features. In this trial, 6693 women, approximately 80 percent of whom had lymph node-negative disease, underwent risk assessment by clinical criteria (using Adjuvant! Online) and by the 70-genetic profile. Patients with discordant clinical and genomic predictions were randomly assigned to receive or not receive adjuvant chemotherapy. Among patients in the intention-to-treat population who had a high clinical risk of recurrence but a low risk by Mammaprint, a non-significant benefit of chemotherapy with respect to distant metastasis-free survival (DMFS) and a significant benefit of chemotherapy with respect to DFS were seen (19).
The TAILORx trial was designed to determine whether Oncotype DX that analyzes the expression of genes that are associated with risk of recurrence among women with early stage breast cancer could be used to assign patients to the most appropriate treatment choice. In the lowest risk group, the TAILORx trial provided prospective evidence that patients with RS 0–10 may be spared chemotherapy. Among these patients who were uniformly treated with ET, rates of distant recurrence at 5 and 9 years were <1% and 3% respectively. Furthermore, adjuvant endocrine therapy and chemoendocrine therapy was shown to have similar efficacy in women with hormone-receptor-positive, HER2-negative, axillary node-negative breast cancer who had a midrange 21-gene recurrence score (RS 11–25), although benefit of chemotherapy was found in some women 50 years of age or younger (20).
The precision medicine achievable via the use of molecular analysis for early breast cancer patients has been shown to change treatment recommendations. Oncotype DX testing was associated with a notable change in treatment recommendations based on the data reported by Holt et al. (13), with approximately half of all patients originally recommended chemotherapy being recommended endocrine therapy after Oncotype DX testing. In our study, overall adjuvant treatment decisions changed for 33% of patients after Oncotype Dx RS results were discussed in multidisciplinary tumor conference.
The present study is the first multicenter analysis to demonstrate cost-effectiveness of Oncotype DX in Turkey using a Markov model. Real-world patient data was used in the model based on the Turkish Oncotype-Dx decision impact study (8).
In several developed countries, Oncotype DX cost-effectiveness has been demonstrated in the early stage breast cancer setting. All studies concluded that Oncotype DX has an ICER less than $100,000 per QALY, however the results were disparate with each other (21).
A study which looked at use of the test in a community “real-world setting,” found that the likely cost-effectiveness ratio for Oncotype DX testing was higher than the ratios for the most commonly accepted diagnostic and preventive interventions. Their simulation model compared 25-year incremental costs and quality-adjusted life-years (QALYs) for Oncotype DX use in the community from 2005 to 2012 with costs and QALYs of usual care in the time period before testing (2000 to 2004). The patients who underwent testing were younger and were most likely to have stage l than stage ll disease. Patients who underwent testing and who were younger than age 50 years had lower chemotherapy rates than patients in the same age group who were not tested (53.0% vs 63.6%). In contrast, older patients who were tested had higher rates of chemotherapy compared with the untested cohort (age 50 to 64 years: 36.5% vs 30.8%; age ≥ 65 years: 17.6% vs 8.2%) (22).
In a recent analysis reviewing multiple clinical studies simulation models, demonstrated that cost-effectivity studies has a wide range of heterogenity in terms of model structure. Some studies did not use the real-world RS distributions and rely on database, some did not evaluate the patients’ risk status independent of Oncotype DX. When cost of chemotherapy were used in simulation models, treatment related toxicity were ignored in some studies. Despite the heterogenity of these trials, the simulation model revealed that the problematic issues that were identified in the analyses do not change the conclusion that Oncotype DX is cost-effective for the clinically intermediate or high-risk group but not for the clinically low-risk groups (21).
Cost-effectiveness analysis (CEA) is increasingly important in public health decision-making especially in low- and middle-income countries. When cost-effectiveness is evaluated for developing countries, willingness to pay may be at lower ICER thresholds, with many health interventions deemed cost-effective but not accepted as affordable by local authorities. This multicenter prospective trial showed that Oncotype DX is cost-effective and improves QALY in a developing country model.
By leading to changes in adjuvant chemotherapy decision and modifying long-term risk of distant recurrence, Oncotype DX was projected to improve life expectancy (+0.86 years) and quality-adjusted life expectancy (+0.68 QALYs) versus standard care. The incremental cost-effectiveness ratio (ICERs) of Oncotype DX was estimated to be $7207.9 per QALY gained and $5720.6 per LY gained versus current clinical practice. Sensitivity analysis showed that the cost-effectiveness of Oncotype DX testing was not sensitive to variations in several clinical and economic parameters. In all sensitivity analyses, Oncotype DX was associated with ICERs in the range that would be considered cost-effective by commonly quoted standards.
Oncotype DX was estimated to improve quality-adjusted life expectancy versus standard care, due to chemotherapy avoidance in low-risk patients in addition to survival benefits in high-risk patients. In this analysis, data of patients who were recruited from state hospital and academic centers were taken into account where all costs are reimbursed by general health insurance. However, there are considerable amount of patients who apply to private hospitals and take the Oncotype DX with their personal expense. If it was possible to add these patients’ data to the analysis, we believe that the cost-effectiveness of the test would be more favorable.
Oncotype DX provides additional information to improve personalized chemotherapy treatment in early stage breast cancer patients and changed adjuvant chemotherapy treatment decisions in 33% of patients. The test was found cost-effective from a national perspective, with improvements in quality of life and may be introduced to routine clinical practice in patients with ER+, HER-2 negative early-stage breast cancer in Turkey.
Footnotes
Ethics Committee Approval: Ethics committee approval was received for this study from the Ethics Committee of İstanbul University İstanbul School of Medicine (2014/800; 09.05.2014-09).
Informed Consent: Written informed consent was obtained from patient who participated in this study.
Peer-review: Externally peer-reviewed.
Author Contributions: Concept - M.Ö., O.D., N.G.; Design - V.Ö., O.D.; Supervision - E.G., E.O.; Resources - V.Ö., A.I., E.O.; Materials - P.S., N.G.; Data Collection and/or Processing - B.Ç., M.Ö.; Analysis and/or Interpretation - C.U., E.O.; Literature Search - A.I., B.Ç.; Writing Manuscript - B.Ç., E.G.; Critical Review - V.Ö., B.Ç., E.G., M.Ö., N.G., C.U., E.O., O.D., A.I., P.S.
Conflict of Interest: The authors have no conflicts of interest to declare.
Financial Disclosure: The authors declared that this study has received no financial support.
References
- 1.Ozmen V. Breast Cancer in Turkey: Clinical and Histopathological Characteristics (Analysis of 13.240 Patients) J Breast Health. 2014;10:98–105. doi: 10.5152/tjbh.2014.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Markopoulos C, van de Velde C, Zarca D, Ozmen V, Masetti R. Clinical evidence supporting genomic tests in early breast cancer: Do all genomic tests provide the same information? Eur J Surg Oncol. 2017;43:909–920. doi: 10.1016/j.ejso.2016.08.012. [DOI] [PubMed] [Google Scholar]
- 3.Paik S, Shak S, Tang G, Kim C, Baker J, Cronin M, Baehner FL, Walker MG, Watson D, Park T, Hiller W, Fisher ER, Wickerham DL, Bryant J, Wolmark N. A multigene assay to predict recurrence of tamoxifen-treated, node-negative breast cancer. N Engl J Med. 2004;351:2817–2826. doi: 10.1056/NEJMoa041588. [DOI] [PubMed] [Google Scholar]
- 4.Paik S, Tang G, Shak S, Kim C, Baker J, Kim W, Cronin M, Baehner FL, Watson D, Bryant J, Costantino JP, Geyer CE, Jr, Wickerham DL, Wolmark N. Gene expression and benefit of chemotherapy in women with node-negative, estrogen receptor-positive breast cancer. J Clin Oncol. 2006;24:3726–3734. doi: 10.1200/JCO.2005.04.7985. [DOI] [PubMed] [Google Scholar]
- 5.Albain KS, Barlow WE, Shak S, Hortobagyi GN, Livingston RB, Yeh IT, Ravdin P, Bugarini R, Baehner FL, Davidson NE, Sledge GW, Winer EP, Hudis C, Ingle JN, Perez EA, Pritchard KI, Shepherd L, Gralow JR, Yoshizawa C, Allred DC, Osborne CK, Hayes DF. Prognostic and predictive value of the 21-gene recurrence score assay in postmenopausal women with node-positive, oestrogen-receptor-positive breast cancer on chemotherapy: a retrospective analysis of a randomised trial. Lancet Oncol. 2010;11:55–65. doi: 10.1016/S1470-2045(09)70314-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dowsett M, Cuzick J, Wale C, Forbes J, Mallon EA, Salter J, Quinn E, Dunbier A, Baum M, Buzdar A, Howell A, Bugarini R, Baehner FL, Shak S. Prediction of risk of distant recurrence using the 21-gene recurrence score in node-negative and node-positive postmenopausal patients with breast cancer treated with anastrozole or tamoxifen: a TransATAC study. J Clin Oncol. 2010;28:1829–1834. doi: 10.1200/JCO.2009.24.4798. [DOI] [PubMed] [Google Scholar]
- 7.Özmen V, Atasoy A, Gökmen E, Özdoğan M, Güler N, Uras C, Ok E, Demircan O, Işıkkdoğan A, Cabioğlu N, Şen F, Saip P. Correlations Between Oncotype DX Recurrence Score and Classic Risk Factors in Early Breast Cancer: Results of A Prospective Multicenter Study in Turkey. J Breast Health. 2016;12:107–111. doi: 10.5152/tjbh.2016.2874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ozmen V, Atasoy A, Gokmen E, Ozdogan M, Guler N, Uras C, Ok E, Demircan O, Isikdogan A, Saip P. Impact of Oncotype DX Recurrence Score on Treatment Decisions: Results of a Prospective Multicenter Study in Turkey. Cureus. 2016;8:e522. doi: 10.7759/cureus.522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hornberger J, Cosler LE, Lyman GH. Economic analysis of targeting chemotherapy using a 21-gene RT-PCR assay in lymph-node-negative, estrogen-receptor-positive, early-stage breast cancer. Am J Manag Care. 2005;11:313–324. [PubMed] [Google Scholar]
- 10.Özmen V, Gürdal SÖ, Cabioğlu N, Özcinar B, Özaydın AN, Kayhan A, Arıbal E, Sahin C, Saip P, Alagöz O. Cost-Effectiveness of Breast Cancer Screening in Turkey, a Developing Country: Results from Bahçeşehir Mammography Screening Project. Eur J Breast Health. 2017;13:117–122. doi: 10.5152/ejbh.2017.3528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.TÜİK. Yaş ve cinsiyete göre yaşam tabloları. 2013. [Google Scholar]
- 12.Thomas RJ, Williams M, Marshall C, Glen J, Callam M. The total hospital and community UK costs of managing patients with relapsed breast cancer. Br J Cancer. 2009;100:598–600. doi: 10.1038/sj.bjc.6604911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Holt SDH, Pudney D, Rolles M. Results from a prospective clinical study on the impact of Oncotype DX® ® on adjuvant treatment decision and risk classification by Nottingham Prognostic Index(NPI) and Adjuvant! Online. Poster Presentation at the 12th St. Gallen International Breast Cancer Conference; 16–19 March, 2011; St. Gallen, Switzerland. [DOI] [Google Scholar]
- 14.Peasgood T, Ward SE, Brazier J. Health-state utility values in breast cancer. Expert Rev Pharmacoecon Outcomes Res. 2010;10:553–566. doi: 10.1586/erp.10.65. [DOI] [PubMed] [Google Scholar]
- 15.Milne RJ, Heaton-Brown KH, Hansen P, Thomas D, Harvey V, Cubitt A. Quality-of-life valuations of advanced breast cancer by New Zealand women. Pharmacoeconomics. 2006;24:281–292. doi: 10.2165/00019053-200624030-00007. [DOI] [PubMed] [Google Scholar]
- 16.Conner-Spady BL, Cumming C, Nabholtz JM, Jacobs P, Stewart D. A longitudinal prospective study of health-related quality of life in breast cancer patients following high-dose chemotherapy with autologous blood stem cell transplantation. Bone Marrow Transplant. 2005;36:251–259. doi: 10.1038/sj.bmt.1705032. [DOI] [PubMed] [Google Scholar]
- 17.Özmen V. A Patient Advocacy Group Summit, Cancer Care in Turkey and The Society of Breast Health. Eur J Breast Health. 2018;14:1–4. doi: 10.5152/ejbh.2017.1213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Katz SJ, Morrow M. Addressing overtreatment in breast cancer: The doctors’ dilemma. Cancer. 2013;119:3584–3588. doi: 10.1002/cncr.28260. [DOI] [PubMed] [Google Scholar]
- 19.Cardoso F, Van’t Veer L, Rutgers E, Loi S, Mook S, Piccart-Gebhart MJ. Clinical application of the 70-gene profile: the MINDACT trial. J Clin Oncol. 2008;26:729–735. doi: 10.1200/JCO.2007.14.3222. [DOI] [PubMed] [Google Scholar]
- 20.Sparano JA, Gray RJ, Makower DF, Pritchard KI, Albain KS, Hayes DF, Geyer CE, Jr, Dees EC, Goetz MP, Olson JA, Jr, Lively T, Badve SS, Saphner TJ, Wagner LI, Whelan TJ, Ellis MJ, Paik S, Wood WC, Ravdin PM, Keane MM, Gomez Moreno HL, Reddy PS, Goggins TF, Mayer IA, Brufsky AM, Toppmeyer DL, Kaklamani VG, Berenberg JL, Abrams J, Sledge GW., Jr Adjuvant Chemotherapy Guided by a 21-Gene Expression Assay in Breast Cancer. N Engl J Med. 2018;379:111–121. doi: 10.1056/NEJMoa1804710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wang SY, Dang W, Richman I, Mougalian SS, Evans SB, Gross CP. Cost-Effectiveness Analyses of the 21-Gene Assay in Breast Cancer: Systematic Review and Critical Appraisal. J Clin Oncol. 2018;36:1619–1627. doi: 10.1200/JCO.2017.76.5941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chandler Y, Schechter CB, Jayasekera J, Near A, O’Neill SC, Isaacs C, Phelps CE, Ray GT, Lieu TA, Ramsey S, Mandelblatt JS. Cost Effectiveness of Gene Expression Profile Testing in Community Practice. J Clin Oncol. 2018;36:554–562. doi: 10.1200/JCO.2017.74.5034. [DOI] [PMC free article] [PubMed] [Google Scholar]