Abstract
Objective
Breast cancer is the most common type of cancer and the leading cause of cancer-related deaths in women in Turkey. This study presents the characteristics of patients registered in National Breast Cancer Registry Program of Turkish Federation of Breast Diseases Societies.
Materials and Methods
The registry contains 242 variables under 10 categories and 699 questions. Patients were recorded (online and offline) from nationwide breast centers around Turkey.
Results
Twenty-thousand patients were registered between May 2005 and April 2017 at 36 centers. After data cleaning, 19,503 women were included in the study. The median age at diagnosis was 51 [14–97]; 17.2% were younger than 40 and 37.2% were premenopausal; 13.6% were nulliparous. Breast conserving surgery rate was 39.3%. Histopathology was invasive ductal cancer in 77%. Majority of patients had stage II cancer (48.3%). Estrogen, progesterone and HER-2 receptor positivity rates in invasive breast cancer were 72.5%, 62.5% and 21.8%, respectively. The mean tumor diameter was 2.5±1.7 cm. During the mean 51.6 months of follow-up, the local/regional and systemic recurrence rates were 3.7% and 5.2%, respectively; five and 10-year overall survival rates were 86% and 76%.
Conclusion
Despite increasing number of screening centers and free-of-charge mammography (ages 40 to 69) and mobile screening systems in recent years, a significant portion of patients were diagnosed at advanced stage due to lack of breast cancer awareness. In contrast with the study published 5 years ago, there was a decrease in the rate of pre-menopausal women and an increase in the breast conserving surgery.
Keywords: Breast cancer, demography, reproductive functions, pathology, survival, Turkey
Introduction
Worldwide, there will be about 2.1 million newly diagnosed female breast cancer (BC) cases in 2018, accounting for almost 1 in 4 cancer cases among women (1). The disease is the most frequently diagnosed cancer in the vast majority of the countries (154 of 185) and is also the leading cause of cancer death in over 100 countries. Its incidence and mortality rates have been increasing mostly in developing countries including Turkey (2). Breast cancer incidence has increased almost two times in the last two years (24/100,000 in 1994 and 43.8/100,000 in 2015) in Turkey (3, 4). According to the Cancer Report 2015 data published by the Cancer Registry Unit of the Cancer Control Department, 17,183 women diagnosed with breast cancer in 2015 (4).
Maintaining an accurate and complete cancer registry program is one of the most important factors to implement national cancer control programs and assess the outcomes of screening, diagnosis and treatment. Unless accurate data are obtained and statistically assessed, prioritization cannot be achieved and sound decisions cannot be taken in the development of national health policies, national cancer strategic plans and utilization of limited resources. The Turkish Federation of Breast Diseases Societies (TFBDS) is the largest and single association of national breast societies and implementing an active specific registry in this sense; namely, the National Breast Cancer Registry Program (NBCRP). The program has been collecting data since 2005 from 36 centers scattered throughout the country. The medical secretaries at these centers were trained periodically for online and offline data accumulation and data cleaning has been performed regularly. The first results of breast cancer registry program were published in 2014 including demographic, clinical and pathological characteristics of 13.184 patients with breast cancer. This study aimed to evaluate the demographic, clinical, reproductive and histopathologic features and also survival results of 20.000 female BC patients registered into the program, and to compare them with data from other developed or developing countries.
Materials and Methods
Data recorded in NBCRP between May 1, 2005 to April 17, 2017 were evaluated. The primary outcome included overall demographic, clinical, reproductive, pathological characteristics and overall survival data of around 20,000 patients diagnosed with breast cancer in Turkey. In the scope of analysis there were 242 different variables covered under 10 main categories. The main categories were identity, history, clinical information, reproductive functions, histologic diagnosis, surgical treatment, postoperative pathology, chemotherapy, radiotherapy, hormonotherapy and follow-up. Modified Scarff Bloom Richardson histological grading was used in the morphological assessment of the degree of differentiation in breast cancer (5, 6).
Statistical Analysis
Data were analyzed using the Statistical Package for Social Sciences for Windows 20.0 (IBM Corp.; Armonk, NY, USA) program. Categorical variables were compared using chi-square analysis and presented as frequencies and percentage. Kolmogorov-Smirnov test was used to evaluate the distribution of continuous variables. Data were presented as mean and standard deviation or median and range. For non-parametric data, Mann-Whitney U test was used to compare means of two independent groups, and Kruskall-Wallis test was used to compare means of more than two independent groups. Where appropriate, continuous variables were regrouped and separately analyzed based on their cutoff points and groupings. The correlation between categorical variables was assessed using the chi-squared test, and data presented as frequencies and percentage. The comparisons between the survival times in subtypes were made through Log-Rank test. Survival rates as well as mean survival times and 95% confidence interval of averages were demonstrated in the corresponding rows for the groups compared in the tables. A p value lower than 0.05 was considered as statistically significant. The survival curves of the groups found to be statistically significant in the analysis were drawn.
Results
After data cleaning, the number of female BC was reduced to 19,503. The estimated age-adjusted breast cancer risk in women according to the 2013 census data is displayed in Figure 1. The median age of patients at diagnosis was 51 [14–97]. The age group 45–49 was the most populated group (16.5%) in the cohort for 5-year age intervals. At the time of diagnosis, 37.5% of patients were premenopausal. The mean menarche age was 13.4 (±1.0) years, the mean breastfeeding duration was 24.2 (±17.7) months, missed abortion rate was 19.4%, induced abortion rate was 29.9%, the rate of oral contraceptive use was 14.7%, and child delivery rate was 86.3% (mean number of births given was 2.4±1.8). Only 8.2% of the patients diagnosed with breast cancer received hormone replacement therapy (HRT) on a regular basis, while 27% of them received HRT for more than 5 years. 33.9% of the patients had a family history of any cancer, and 15.8% of them had a family history of breast. 3.3% of them had family history of ovarian cancer, respectively. Patients and tumor characteristics are displayed in Table 1.
Figure 1.
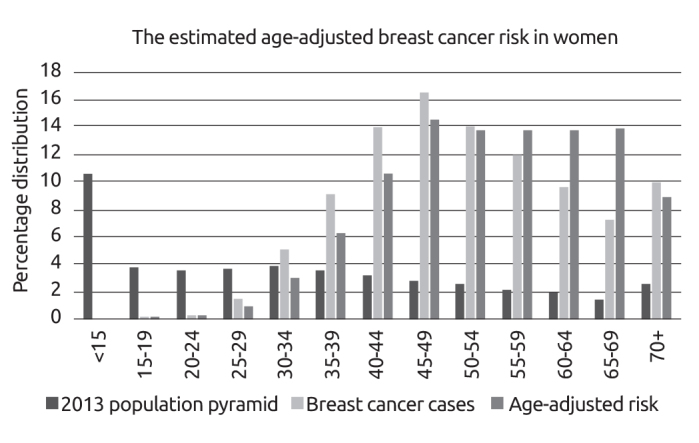
The estimated age-adjusted breast cancer risk in women (with 5-year intervals) according to the 2013 census data
Table 1.
Patients and tumor characteristics
| Number of patients | 19503 |
| Mean age (±SD) | 51.8 (±12.6) |
| <40 years old | 3101 (16.6%) |
| ≥40 years old | 15604 (83.4%) |
| Histopathologic subtype | |
| Invasive ductal cancer | 7726 (76.9%) |
| Invasive lobular cancer | 649 (6.5%) |
| Invasive mixed type | 425 (4.2%) |
| Other | 1241 (12.4%) |
| Histologic grade | |
| I | 562 (7.7%) |
| II | 3416 (46.8%) |
| III | 3320 (45.5%) |
| Receptor status | |
| ER | 5745 (72.5%) |
| PR | 4736 (62.5%) |
| HER-2 | 1659 (21.8%) |
| Ki-67 (≤%14) | 378 (%35.0) |
| Pathologic stages at the time of diagnosis | |
| Stage I+II | 4990 (76.8%) |
| Stage III+IV | 1728 (23.2%) |
| Obstetric history | |
| Nulliparous | 1281 (13.6%) |
| Monoparous | 1227 (13.1%) |
| Spontaneous abortion | 1494 (19.3%) |
| Induced abortion | 2326 (30.0%) |
| Molecular subtypes | |
| Luminal A | 3326 (57.7 %) |
| Luminal B | 1187 (50.3 %) |
| HER-2 | 555 (12.2 %) |
| TNG | 695 (8.1 %) |
| 5-year overall survival | 3555 (85.8%) |
| 10-year overall survival | 4826 (75.7%) |
ER: Estrogen; PR: Progesterone; TNG: triple negative
Breast conserving surgery rate was 39.3%, and mastectomy rate was 60.7%. Sentinel lymph node biopsy was performed for the patients who were negative for axillary involvement in clinical examination (69.4%); 55.3% of the patients underwent axillary lymph node dissection (ALND). When the surgical interventions are divided into groups regarding years before and after 2000, breast conserving surgery (BCS) rate was 25% before and 45% after the year 2000. As a result, the rate of patients with mastectomy decreased from 75% to 60.7% in the last 15 years.
Among patients with complete histopathologic diagnostic data, the majority (72.8%) of them had invasive ductal cancer (IDC); 10.1% of them had invasive lobular cancer (ILC) or invasive mixed cancers (IMC=IDC+ILC). A comparison between age groups and histopathological tumor types (IDC, ILC and IMC) revealed that the rate of IDC was 91.5% among young women (<40 years) while it was 87% for older women (≥40 years) (p<0.001).
The distribution of patients whose Modified Scarff Bloom Richardson HG were registered in the database was as follows: HG I 7.8%, HG II 46,8%, HG III 45.5%. Majority of the patients had HG II or III tumors, whereas HG declined with an inverse relation with the increase in the age at diagnosis (p<0.001). The histological grades of tumors (HG) were divided into two groups as HG I+II and HG III, and their associations with histological types (IDC and ILC/IMC) were studied. Nearly half of all patients (45.5%) had HG III. 46,4% of patients with IDC had HG III, while 37.3% of patients with ILC/IMC had HG III (p<0.001).
The distribution of the pathologic stages of these patients at diagnosis was as follows: Stage 0 (DCIS) 4.7%, Stage I 28.5%, Stage II 48.3%, Stage III 14.5% and Stage IV 4%. The rate of Stage I breast cancer was 21.9% in women aged ≤40 years; while it was 30.6% in the age group 50–59 (p<0.05). Percentage distribution of the pathological lymphatic stages according to different age groups are displayed in Figure 2. As for the association between pathologic stages and age groups, it was found that pathologic stage decreased with advancing age at diagnosis (Figure 3, p=0.002).
Figure 2.
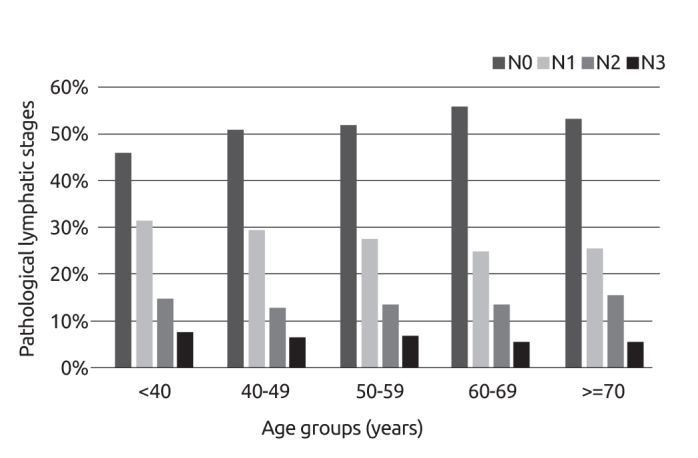
Pathological lymphatic stages according to different age groups
Figure 3.
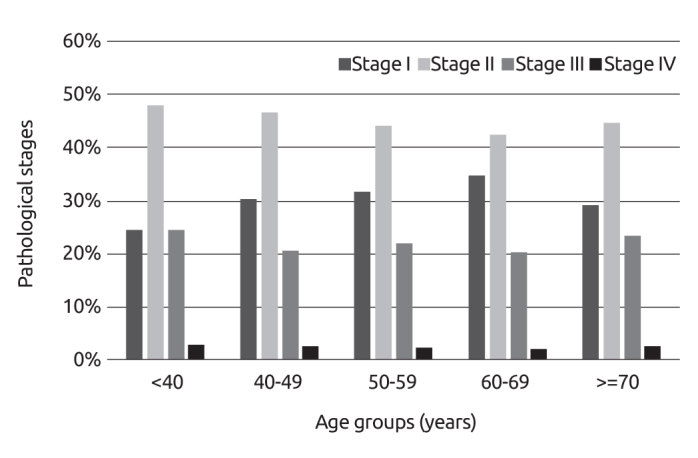
Pathological stages according to different age groups
The mean tumor diameter was 2.5±1.7 cm, while the median tumor size was 2 cm [0.1–25 cm]. The tumor diameter in young patients (<40 years) was significantly larger (mean diameter 2.8; median diameter 2.5 cm; p<0.001). Lymph node involvement rates of patients diagnosed with invasive breast cancer were as follows: pN0 51.4%; pN1 28.1%; pN2 13.9% and pN3 6.6%. The clinical stages of patients were Stage-0 2.3%, Stage I 29.7%, Stage II 44.2%, Stage III 21.4% and Stage IV 2.4%. Associations of pathological lymphatic stages with different tumor diameter groups are displayed in Figure 4.
Figure 4.
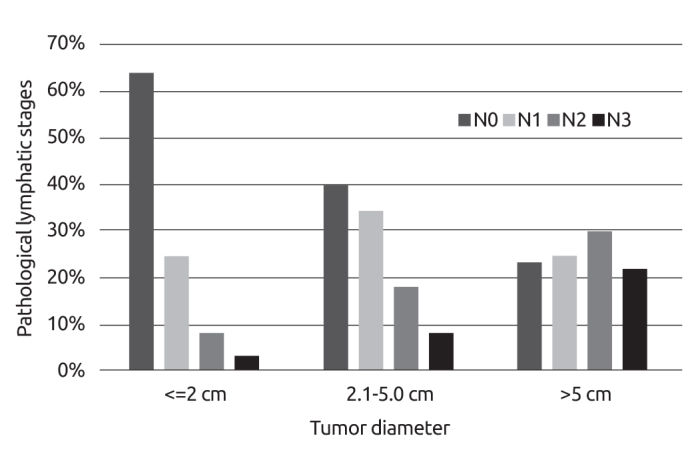
Associations of pathological lymphatic stages with different tumor diameter groups
Estrogen receptor positivity was (72.5%). The patients were divided into two age groups (<40 y/o and ≥40 y/o) to compare ER positivity between these groups. ER positivity was 65.9% in young (<40 y/o), and 73.8% in older patients (age ≥40 y/o, p<0.001). Progesterone receptor positivity was 62.3%. PR positivity rate was higher in older patients (≥40 y/o) (62.5% vs. 60.5%, p>0.05). HER-2 receptor expression was analyzed through immuno-histochemical (IHC) and fluorescence in situ hybridization method (FISH/SISH). It was found that 21.8% of the patients were HER-2 positive.
In the cohort, rate of Ki-67 value >14% was 62.7%, while the percentage of those with a Ki-67 value of >20% was 54.2%. 59% of the patients were positive for lymphovascular invasion. Estrogen (ER), progesterone (PR) and HER-2 receptor expression were positive in 76.9%, 65.8% and 21.8% of the patients, respectively.
The mean follow-up time was 51.6 months and loco-regional recurrence rate was 3.7% in this period. The overall survival rate was 85.8% for 5-years and 75.7% for 10-years. Young age (<40 y/o, p<0.049), small tumor size (<5 cm vs. ≥5 cm, p=0.017), high HG (I+II vs. III; p=0.003), PR status (positive or negative; p<0.001), pathologic stage (Stage I vs. III, p=0.036), and triple negative and HER-2 positive molecular subtypes (p<0.001; p<0.001) were found related with loco-regional recurrence. Overall survival rate and overall survival according to different molecular subtypes are displayed in Figure 5 and Figure 6, respectively.
Figure 5.
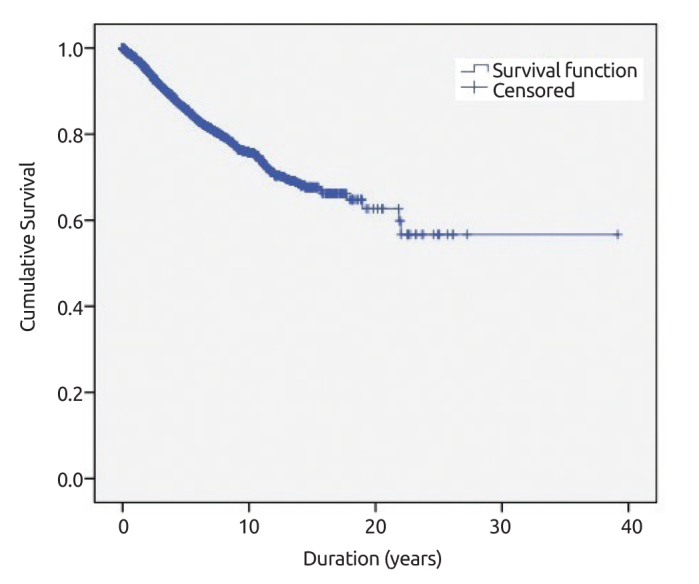
Overall survival rate of breast cancer patients
Figure 6.
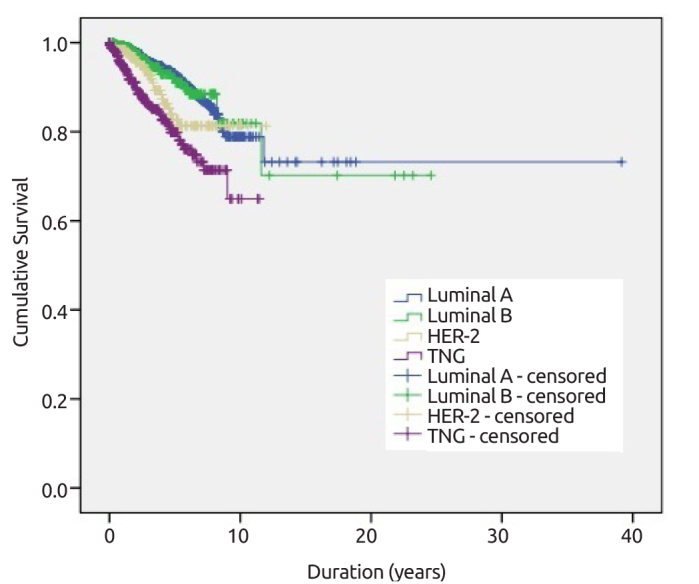
Overall survival according to different molecular subtypes
Distant metastasis rate was 9% in 4.3 years of follow-up period. Young age (<40 vs ≥40; p<0.001), type of surgery (mastectomy vs. BCS; p<0.001), pathologic lymphatic stage (pN0 vs pN1–3, p<0.001), menopausal status (pre- vs post-menopausal; p=0.013), tumor size (<2 cm vs. ≥2 cm p<0.001), histopathological type (IDC vs ILC or IMC, p=0.002), HG (I+II vs III, p=0.002), and molecular subtypes (Luminal A vs. others p<0.001; Luminal A/B vs. HER-2/TNG p<0.001; TNG vs. others p<0.001) were found to be associated with distant metastasis.
When we compared these results with our previous study, the rate of premenopausal women decreased from 45% to 37.7%, the rates of pathologic Stage I breast cancer and breast conserving surgery (BCS) increased 3.1% and 4% respectively (Table 2). The rates of DCIS and tumor size of invasive breast cancer did not change in this 5-year period.
Table 2.
Comparison of data of breast cancer patients in 2012 and 2017
| TFBDS Registry 2005–2010 13.120 patients |
TFBDS Registry 2005–2015 20.000 patients |
Difference in 5-years | |
|---|---|---|---|
| Mean age, years | 51.6±12.6 | 51.8±12.6 | 0.2 |
| <40 years, % | 17.1 | 16.5 | 0.6 |
| >70 years, % | 10 | 10 | - |
| Pre-menopausal, % | 45 | 37.7 | 7.3 |
| DCIS, % | 4.9 | 4.7 | 0.2 |
| pN0, % | 49.8 | 51.4 | 1.6 |
| Mean tumor size (cm) | 2.5±1.6 | 2.5±1.7 | - |
| pStage I, % | 26.6 | 29.7 | 3.1 |
| BCS, % | 35 | 39 | 4 |
| 5-year overall survival, % | - | 86 |
DCIS: Ductal carcinoma in situ, BCS: Breast conserving surgery, pStage: Pathologic stage
Discussion and Conclusion
Although breast cancer mortality rates are decreasing in most high-income countries, incidence and mortality rates are increasing particularly in rapidly developing countries (7). Such increase is explained by changes in life styles (changes in the reproductive functions such as early menarche, nulliparity, delivery after 35 years old, less breast feeding, late menopause, etc.), nutritional habits (obesity, inactivity etc.), increased population growth, aging and increasing opportunistic screening (8). Although it is a possibility that improvements in access to medical care over time, might have resulted in inclusion of patients with milder disease in estimates, the reported incidence of breast cancer in Turkey increased more than 2-folds from 24/100.000 in 1993 to 50/100.000 in 2017.
Cancer registry is the starting point of cancer control. Unless accurate data are obtained and statistically assessed, prioritization cannot be achieved and sound decisions cannot be made for development of national health policies, strategic plans and utilization of limited resources. Currently, there are around 200 population-based registries maintained across the world. Under the light of these registries, IARC (International Agency for Research on Cancer) under the World Health Organization (WHO) publish global health statistics covering all countries in the world. The incidence and mortality analyses on cancer that is performed every 3–5 years are published as GLOBOCAN for the use of the scientific community. One of the most popular registries is the SEER Program (The Surveillance, Epidemiology, and End Results). It is affiliated to the National Cancer Institute (NCI) and started collecting data in 1973 as the official source of data in the USA on cancer incidence and survival (9). On the European side, in 1995, the European Commission has supported the European Network of Cancer Registries (ENCR) which was set up in 1990 (10). In Turkey, the first cancer registry was kicked off in 1982. In the late 90’s, İzmir Cancer Registry became a member of the IARC, and of the ENCR. In 2002 and 2008, IARC used İzmir Cancer Registry data for GLOBOCAN, which served as an endorsement of the quality of data from the province of İzmir (11). Eventually, local initiatives created a stronger national emphasis and National Breast Cancer Database (NBCD) was launched for use in 2005. The ultimate aim is to contribute to the national cancer registry program by revealing very different characteristics of a large patient population.
Today we understand that the rapid westernization especially in the younger population, increases the breast cancer incidence in Turkey. The rate of young female (<40) and premenopausal patients with invasive breast cancer were 16.6% and 37.5%, respectively, in our study. Complete prevalence distributions of patients (with invasive breast cancer) younger than 40 and 50 years old were 1.1% and 7.5%, respectively, on January 1, 2015 according to the SEER database (12). The Ministry of Health of the Republic of Turkey has changed the screening period from 50–69 to 40–69 years of age due to the NBCD results revealing that the distribution of breast cancer cases under 50 years of age constitutes 47% of all cases in Turkey.
Breast cancer is most frequently diagnosed among women aged 55–64 in developed countries; median age at diagnosis is 62 in USA. The most populated group is 45–49 in Turkey, where the median age is 51. This can be explained by the young population age. In the United States, DCIS accounts for 20% of all newly diagnosed breast cancers (13). In Turkey, DCIS patients constituted only 5% of all patients diagnosed with breast cancer. The mean tumor diameter was 2.5±1.7 cm. It can be thought that this situation is related to the lack of community-based screening, lack of education and low breast cancer awareness. In order to demonstrate the feasibility of well-organized, continuous and invitation-driven community-based screening program in line with the social, cultural, educational and economic structure of Turkey, “Bahçeşehir Community-Based Mammographic Screening Project” was launched in Bahçeşehir for up to 10 years (2008–2018). In this Project, among patients diagnosed with breast cancer, 19% had ductal carcinoma in situ (DCIS), and 55% had stage I invasive breast cancer (14). These results indicate the possibility of a successful community-based screening in our country.
The molecular sub-type analysis of the tumors in this study showed that 57.7% of the tumors were luminal A (ER and/or PR positive, HER-2 negative), 20.6% were luminal B (ER and/or PR positive, HER-2 positive), 9.6% were HER-2 type (ER and PR negative, HER-2 positive) and 12.1% were triple negative. Studies comparing the patients with breast cancer detected through screening and symptomatic breast cancer cases found that tumor diameter is smaller and the rate of luminal A subtype of breast cancers is higher in patients diagnosed with breast cancer through screening (15, 16). In 2011, St. Gallen International Expert Consensus on the Primary Therapy of Early Breast Cancer recommended the clinical use of Ki-67 index (cut-off value >14%), to distinguish luminal A and B molecular subtypes (17). Many specialized breast centers updated their data based on modern definitions of molecular subtypes and some centers reported their updated results. For example, the molecular subtypes of regularly followed-up 2032 patients from Istanbul Florence Nightingale Breast Center were as follows; Luminal A and B (Ki67 >14%, HG=3) were 30,4% and 50,3%, respectively; HER-2 positive group was 8,1%; triple negative breast cancer was 11,2% (18, 19).
The mortality rates of breast cancer cases in USA decreased from 24.1 in 2005 to 21.5 in 2010 and to 20.3 in 2015; and overall 5-year survival rate is 90.9%. The 5-year survival rate was found to be 86% in this study. Thus, it is imperative that increased use of screening programs and increasing awareness will result in a reduction in the tumor diameter and regional lymphatic involvement in breast cancer patients.
This study highlights the impact of mammographic screening and the benefits of a structured national breast cancer registry. The NBCRP registry, constituting a national framework for breast cancer control, facilitates the evaluation of the improvement in screening, ongoing awareness, education and training. Despite increasing number of screening centers and free-of-charge mammography (ages 40 to 69) and mobile screening systems in recent years, a significant portion of patients were diagnosed at advanced stage due to lack of breast cancer awareness. In contrast with the study published 5 years ago, there was a decrease in the rate of pre-menopausal women and an increase in the breast conserving surgery.
Footnotes
Ethics Committee Approval: N/A
Informed Consent: Informed consent was not taken due to retrospective design of the study.
Peer-review: Externally peer-reviewed.
Author Contributions: Concept - V.Ö., T.Ö., V.D.; Design - V.Ö., T.Ö., V.D.; Supervision - V.Ö.; Resources - V.Ö., T.Ö., V.D.; Materials – V.Ö.; Data Collection and/or Processing - V.Ö., T.Ö., V.D.; Analysis and/or Interpretation - V.Ö., T.Ö., V.D.; Literature Search - V.Ö., T.Ö., V.D.; Writing Manuscript - V.Ö., T.Ö., V.D.; Critical Review - V.Ö., T.Ö., V.D.
Conflict of Interest: The authors have no conflicts of interest to declare.
Financial Disclosure: The authors declared that this study has received no financial support.
References
- 1.Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68:394–424. doi: 10.3322/caac.21492. [DOI] [PubMed] [Google Scholar]
- 2.Ferlay J, Colombet M, Soerjomataram I, Mathers C, Parkin DM, Piñeros M, Znaor A, Bray F. Estimating the global cancer incidence and mortality in 2018: GLOBOCAN sources and methods. Int J Cancer. 2019;144:1941–1953. doi: 10.1002/ijc.31937. [DOI] [PubMed] [Google Scholar]
- 3.Ozmen V. Breast Cancer in Turkey: Clinical and Histopathological Characteristics (Analysis of 13.240 Patients) J Breast Health. 2014;10:98–105. doi: 10.5152/tjbh.2014.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ozmen V, Boylu S, Ok E, Canturk NZ, Celik V, Kapkac M, Girgin S, Tireli M, Ihtiyar E, Demircan O, Baskan MS, Koyuncu A, Tasdelen I, Dumanli E, Ozdener F, Zaborek P. Factors affecting breast cancer treatment delay in Turkey: a study from Turkish Federation of Breast Diseases Societies. Eur J Public Health. 2015;25:9–14. doi: 10.1093/eurpub/cku086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bloom HJ, Richardson WW. Histological grading and prognosis in breast cancer; a study of 1409 cases of which 359 have been followed for 15 years. Br J Cancer. 1957;11:359–377. doi: 10.1038/bjc.1957.43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Elston CW, Ellis IO. Pathological prognostic factors in breast cancer. I. The value of histological grade in breast cancer: experience from a large study with long-term follow-up. Histopathology. 1991;19:403–410. doi: 10.1111/j.1365-2559.1991.tb00229.x. [DOI] [PubMed] [Google Scholar]
- 7.DeSantis CE, Bray F, Ferlay J, Lortet-Tieulent J, Anderson BO, Jemal A. International Variation in Female Breast Cancer Incidence and Mortality Rates. Cancer Epidemiol Biomarkers Prev. 2015;24:1495–1506. doi: 10.1158/1055-9965.EPI-15-0535. [DOI] [PubMed] [Google Scholar]
- 8.Porter P. “Westernizing” women’s risks? Breast cancer in lower-income countries. N Engl J Med. 2008;358:213–216. doi: 10.1056/NEJMp0708307. [DOI] [PubMed] [Google Scholar]
- 9.Ries LG, Pollack ES, Young JL., Jr Cancer patient survival: Surveillance, Epidemiology, and End Results Program, 1973–79. J Natl Cancer Inst. 1983;70:693–707. [PubMed] [Google Scholar]
- 10.European Commission. European Network of Cancer Registries. Luxembourg: Office for Official Publications of the European Communities; 1995. [Google Scholar]
- 11.Tuncer M, Özgül N, Olcayto EÖ, Gültekin M, Erdin B. Ulusal Kanser Programı 2009–2015, TC Sağlık Bakanlığı Kanserle Savaş Dairesi başkanlığı. 2009. [Google Scholar]
- 12.Noone AM, Howlader N, Krapcho M, Miller D, Brest A, Yu M, Ruhl J, Tatalovich Z, Mariotto A, Lewis DR, Chen HS, Feuer EJ, Cronin KA. SEER Cancer Statistics Review, 1975–2015. National Cancer Institute; Bethesda, MD: https://seer.cancer.gov/csr/1975_2015/, based on November 2017 SEER data submission, posted to the SEER web site, April 2018. [Google Scholar]
- 13.Siegel RL, Miller KD, Jemal A. Cancer statistics. CA Cancer J Clin. 2016;66:7–30. doi: 10.3322/caac.21332. [DOI] [PubMed] [Google Scholar]
- 14.Kayhan A, Gurdal SO, Ozaydin N, Cabioglu N, Ozturk E, Ozcinar B, Aribal E, Ozmen V. Successful first round results of a Turkish breast cancer screening program with mammography in Bahcesehir, Istanbul. Asian Pac J Cancer Prev. 2014;15:1693–1697. doi: 10.7314/APJCP.2014.15.4.1693. [DOI] [PubMed] [Google Scholar]
- 15.Kim J, Lee S, Bae S, Choi MY, Lee J, Jung SP, Kim S, Choe JH, Kim JH, Kim JS, Lee JE, Nam SJ, Yang JH. Comparison between screen-detected and symptomatic breast cancers according to molecular subtypes. Breast Cancer Res Treat. 2012;131:527–540. doi: 10.1007/s10549-011-1836-0. [DOI] [PubMed] [Google Scholar]
- 16.Újhelyi M, Pukancsik D, Kelemen P, Kovács E, Kenessey I, Udvarhelyi N, Bak M, Kovács T, Mátrai Z. Does breast screening offer a survival benefit? A retrospective comparative study of oncological outcomes of screen-detected and symptomatic early stage breast cancer cases. Eur J Surg Oncol. 2016;42:1814–1820. doi: 10.1016/j.ejso.2016.06.403. [DOI] [PubMed] [Google Scholar]
- 17.Goldhirsch A, Wood WC, Coates AS, Gelber RD, Thürlimann B, Senn HJ. Strategies for subtypes - dealing with the diversity of breast cancer: highlights of the St. Gallen International Expert Consensus on the Primary Therapy of Early Breast Cancer 2011. Ann Oncol. 2011;22:1736–1747. doi: 10.1093/annonc/mdr304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Çelebi F, Pilancı KN, Ordu Ç, Ağacayak F, Alço G, İlgün S, Sarsenov D, Erdoğan Z, Özmen V. The role of ultrasonographic findings to predict molecular subtype, histologic grade, and hormone receptor status of breast cancer. Diagn Interv Radiol. 2015;21:448–453. doi: 10.5152/dir.2015.14515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Alco G, Bozdogan A, Selamoglu D, Pilanci KN, Tuzlali S, Ordu C, Igdem S, Okkan S, Dincer M, Demir G, Ozmen V. Clinical and histopathological factors associated with Ki-67 expression in breast cancer patients. Oncol Lett. 2015;9:1046–1054. doi: 10.3892/ol.2015.2852. [DOI] [PMC free article] [PubMed] [Google Scholar]


