Abstract
Parvoviruses are a diverse group of viruses that infect a wide range of animals and humans. In recent years, advances in molecular techniques have resulted in the identification of several novel parvoviruses in swine. In this study, porcine parvovirus 7 (PPV7) isolates from clinical samples collected in Guangxi, China, were examined to understand their molecular epidemiology and co-infection with porcine circovirus type 2 (PCV2). In this study, among the 385 pig serum samples, 105 were positive for PPV7, representing a 27.3% positive detection rate. The co-infection rate of PPV7 and PCV2 was 17.4% (67/385). Compared with the reference strains, we noted 93.9%-97.9% similarity in the NS1 gene and 87.4%-95.0% similarity in the cap gene. Interestingly, compared with the reference strains, sixteen of the PPV7 strains in this study contained an additional 3 to 15 nucleotides in the middle of the cap gene. Therefore, the Cap protein of fourteen strains encoded 474 amino acids, and the Cap protein of the other two strains encoded 470 amino acids. However, the Cap protein of the reference strain PPV7 isolate 42 encodes 469 amino acids. This is the first report of sequence variation within the cap gene, confirming an increase in the number of amino acids in the Cap protein of PPV7. Our findings provide new insight into the prevalence of PPV7 in swine in Guangxi, China, as well as sequence data and phylogenetic analysis of these novel PPV7 isolates.
Introduction
The family Parvoviridae is classified into two subfamilies, Parvovirinae and Densovirinae, whose hosts are vertebrates and arthropods, respectively [1,2]. Most members of the subfamily Parvovirinae cause only mild clinical symptoms, but a small number are causative agents of important diseases, for example, goose parvovirus (geese: Gosling Plague), porcine parvovirus 1 (pigs: mainly reproductive disorders) and parvovirus B19 (humans: infectious erythema) [2,3]. Parvoviruses are small, single-stranded linear, non-enveloped DNA viruses with a genome of approximately 4–6 kb [2]. The genome contains two major open reading frames (ORFs) [4]. ORF1 encodes non-structural proteins (NS) involved in viral replication, while ORF2 encodes structural (Cap) proteins [5]. An additional ORF, ORF3, encodes nuclear phosphoproteins (NP) and is located in the middle of ORF1 and ORF2. It is characteristic of members of the Bocaparvovirus genus [6,7].
To date, six porcine parvovirus (PPV) genotypes (PPV1-6) have been discovered [8,9]. According to the amino acid similarity in the NS1 protein, these viruses are taxonomically divided into three genera [2]: Protoparvovirus (PPV1), Tetraparvovirus (PPV2-3), and Copiparvovirus (PPV4-6) [10]. Recently, a new species of the PPV genotypes, PPV7, was first detected in healthy adult pigs in the USA, and the complete genome sequence of PPV7 isolate 42 was obtained [11]. The amino acid similarity of the NS1 protein between PPV7 and other porcine parvoviruses is less than 30%. Therefore, a new genus, Chapparvovirus, has been established for PPV7 (over 30% amino acid identity within a genus) [11].
PPV1 is one of the major causative agents of reproductive failure syndromes in pigs and is characterized by infertility, mummified foetuses, early embryonic death, and stillbirths [12]. This virus is also known to contribute to the development of porcine circovirus-associated disease (PCVAD) [13,14]. PPV6 was first identified in aborted pig foetuses in China in 2014 and was subsequently reported to be co-infected with porcine reproductive and respiratory syndrome virus (PRRSV) in the USA [15,16]. The impact of other PPVs on pig health remains unknown. However, recent research has indicated an association of PPV2, PPV4 and PPV6 with PCV2 infection [8,14]. Furthermore, the presence of PPV4 and PPV6 was detected in foetal tissues [15]. PPV is considered to be a co-factor of PCV2, and concurrent infection with PCV2 and PPV increases disease and lesion severity compared to mono-infection with PCV2 [17,18]. Previous studies have reported PPV3 and PCV2 co-infections in Chinese swine populations and PPV2 and PPV4 co-infection in wild boars in Europe [19]. Recent studies report that at least 3 countries have found PPV7 in their porcine populations, including America, Poland and Korea [11,20,21]. In China, PPV7 was first reported in Guangdong and Anhui provinces in 2017 [22,23]. Interestingly, the PPV7 prevalence of 65.5% on PCV2-positive farms was significantly higher than on PCV2-negative farms, indicating that PPV7 might be associated with PCV2 infection [23].
The purpose of this study was to evaluate the prevalence and diversity of PPV7 in Guangxi, China. The availability of novel porcine parvoviruses allowed us to conduct a comprehensive genetic evolution analysis based on the NS1 and Cap proteins and examine the diversification of these novel viruses.
Materials and methods
Sample collection
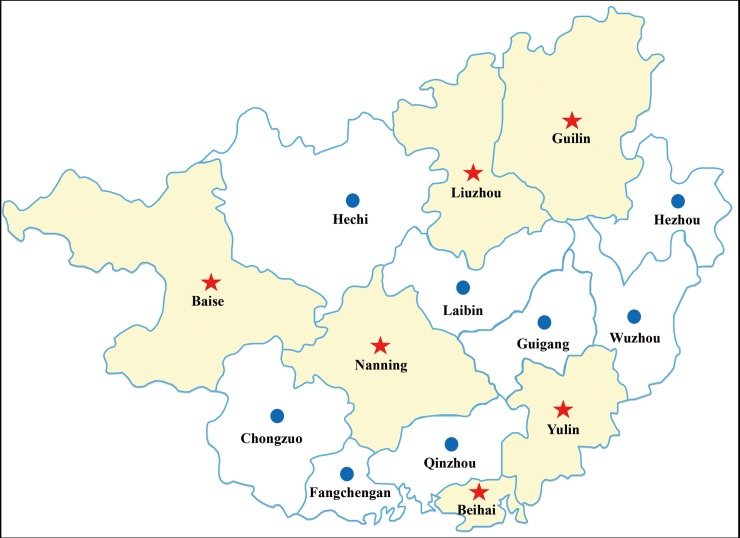
From 2015 to 2017, serum samples (n = 385) of pigs were collected from 11 pig farms in Guilin (N25°17′, E110°17′), Baise (N23° 54′, E106° 36′), Yulin (N22° 39′, E110° 10′), Nanning (N22° 49′, E108° 21′), Liuzhou (N24° 19′, E109° 24′) and Beihai (N21° 29′, E109° 06′) in Guangxi, China (Fig 1). Serum samples used in this study were obtained from the Guangxi Center for Animal Disease Control and Prevention and stored at −20°C. The experiment was approved by the Animal Welfare and the Animal Experimental Ethical Committee (Guangxi University, No. Xidakezi2000138).
Fig 1. Geographical information for serum samples collected in Guangxi, China.
Red stars indicate the geographical location of the sample.
DNA extraction and polymerase chain reaction (PCR)
Total DNA was isolated from tissue samples using the TIANamp Genomic DNA Kit (Tiangen Biotech, China). Four primer pairs were designed based on the reference sequences of isolate 42 (GenBank No. KU563733), and published primers and protocols were used to detect PCV-2 and PPV6 (Table 1). The PCR mixture contained 2 μL of extracted DNA, 2 μL of primer pairs (10 μM), 25 μL of 2×Phanta Max Master Mix (Vazyme, Nanjing, China), and 21 μL of DNase/RNase-free water. The PCR amplification conditions were as follows: predenaturation for 3 minutes at 95°C, followed by 35 cycles of 15 seconds at 95°C, 15 seconds at 62°C, an extension for 1 minute at 72°C, and a final extension for 5 minutes at 72°C. Subsequently, the PCR products were separated using 1.2% agarose gel electrophoresis and cloned into a pMD18-T vector (Takara Co. Dalian). The recombined vectors were amplified in Escherichia coli (E. coli, DH5α) for sequencing.
Table 1. List of primer sequences used in this study.
| Primer | Sequence (5’-3’) | Amplicon length (bp) |
|---|---|---|
| PPV7-30-F | GGAACGACAAGGACGACACTT | 504 |
| PPV7-533-R | CTTGAGGCTCTGGTATCTTATTGC | |
| PPV7-417-F | AGCGGGTTCACGGTGGGTAATGCTCTGGG | 1320 |
| PPV7-1736-R | TGATGGGTGTTCTCGGCAGGT | |
| PPV7-1692-F | CGGCCAAGTACAAGAAACCGCAGGACCT | 1452 |
| PPV7-3158-R | GGCCAGGTTGTGCCTGCTGTTGGATACG | |
| PPV7-3115-F | CCGTATCCAACAGCAGGCACAACCTGGCCACA | 899 |
| PPV7-4013-R | TGGCGTTGAGAAGACACTGGTTTAG | |
| PCV-2-F | GGACCCCAACCACATAAAA | 555 |
| PCV-2-R | CCCTAACCTATGACCCCTATGT |
Phylogenetic analysis
Sequences were assembled using SeqMan software (DNASTAR Inc., Madison, Wisconsin, USA) and aligned using MegAlign (DNASTAR Inc., Madison, Wisconsin, USA) with the Clustal W alignment method for genomic similarity analysis. The phylogenetic tree was calculated using the maximum likelihood method (LG+G+I model) with 1,000 bootstrap replicates and constructed on the aligned data set using the MEGA7 program.
Results
Detection of PPV7 and PCV2
PPV7 was detected in the six cities. The positive rates of PPV7 and PCV2 in these samples were 27.3% (105/385) and 36.4% (140/385), respectively. The co-infection rate of PPV7 and PCV2 was 17.4% (67/385). Interestingly, the positive rate of PPV7 ranged from 16.3 to 33.3%, with the highest rate recorded in Liuzhou, and the lowest in Yulin (Table 2).
Table 2. Frequency and distribution of PPV7 and PCV2 detected by PCR in samples from six cities in Guangxi, China.
| Prefecture | Number | PPV7 | PCV2 | Co-infection |
|---|---|---|---|---|
| Guilin | 57 | 15 | 24 | 11 |
| Baise | 34 | 9 | 17 | 5 |
| Yulin | 49 | 8 | 6 | 4 |
| Nanning | 104 | 29 | 26 | 19 |
| Liuzhou | 63 | 21 | 18 | 11 |
| Beihai | 78 | 23 | 39 | 17 |
| Total(%) | 385 | 105(27.3%) | 140(36.4%) | 65(17.4%) |
Multiple sequence alignment and phylogenetic analysis
Seventeen nearly complete PPV7 genome sequences were amplified by PCR. The two major ORFs, ORF1 (encoding NS1) and ORF2 (encoding Cap), were identified in the 17 sample sequences. Based on nucleotide similarity analysis of the complete coding region, the 17 sequences shared 94.1%-100% similarity, with 94.8%-100% similarity in the NS1 gene and 90.3%-100% similarity in the cap gene. In addition, the 17 sample sequences shared 93.9%-97.9% similarity in NS1 and 87.4%-95.0% similarity in the cap gene compared with the reference strain.
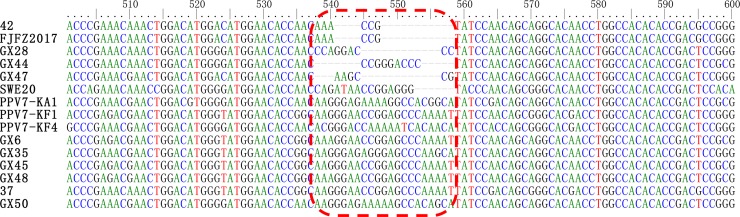
Of note, the PPV7 cap gene has a length of 1410 nt or 1401 nt; however, in this study, 14 strains with a cap region of 1425 nt and two sequences (Gx28 and Gx44) with a cap length of 1413 nt were identified. Only one strain (Gx47) was found to have a cap gene with a length of 1410 nt. Based on these findings, the sequences in our study contained an additional 3 to 15 nucleotides in the middle of the cap gene (Fig 2).
Fig 2. Alignment of nucleotide acid sequences of nucleotide acids representing different isolates of PPV7.
The red box highlights the sequence region where additional nucleotides were identified.
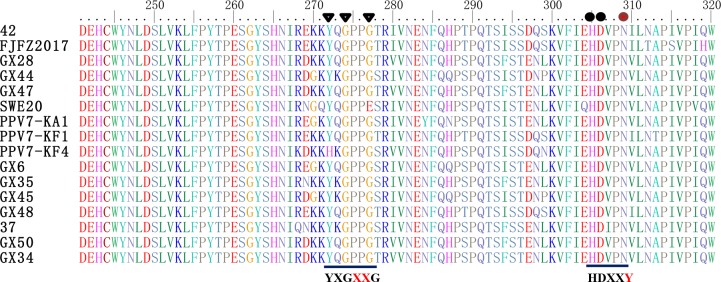
The Ca2+ binding loop (YXGXG) is present in the capsid proteins of PPV1, PPV2, PPV3 and PPV5 [2,9]. The amino acid sequence of the Ca2+ binding loop was “YXGXR” in PPV6 [15]. However, Ca2+ binding loops are absent in PPV4. In this study, the conserved amino acid sequence of the Ca2+ binding loop is the “YXGXXG” motif in PPV7, rather than the “YXGXR” or “YXGXG” motif found in other parvoviruses (Fig 3). On the other hand, a single amino acid mutation was present at 304 aa (Y to N) in the VP1 protein of all PPV7 strains. Therefore, the catalytic residues (HDXXY) of the putative secretory phospholipase A2 (PLA2) are lacking in PPV7 [9].
Fig 3. Sequence alignment of the putative phospholipase A2 motif of PPV7 with other parvoviruses.
The conserved amino acids of the Ca2+ binding loop (YXGXXG) and the catalytic residues (HDXXY) are indicated at the bottom of the alignment. Black circles represent the conserved amino acids of the catalytic residue, and brown circles represent the amino acid mutation sites of the catalytic residues. Black triangles represent the conserved amino acids of the Ca2+ binding loop.
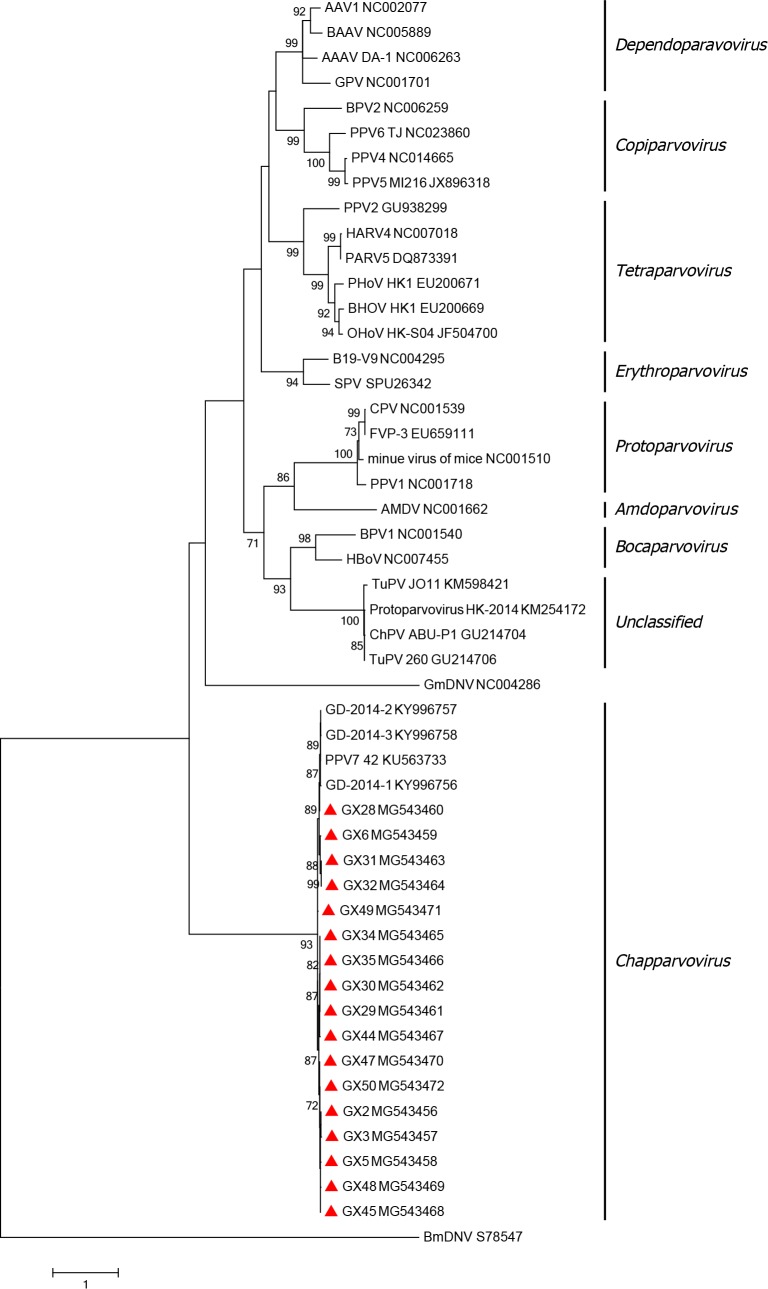
To better understand the genetic relationship between the strains identified in this study, a phylogenetic tree was constructed using the maximum likelihood method comparing the NS1 amino acid sequences from our strains and 33 reference strains of Parvoviridae family members downloaded from GenBank. Phylogenetic analyses of the amino acid sequences of NS1 revealed that all strains used in this study were in the same branch as PPV7 isolate 42, with all strains belonging to the Chapparvovirus genus (Fig 4).
Fig 4. Phylogenetic analysis of viruses in the Parvoviridae family.
Phylogenetic reconstruction of amino acid sequences of NS1 by the ML method with the LG+F+I model of sequence evolution with 1,000 bootstrap resampling. The strains identified in this study are represented with a triangle (▲). Scale bars indicate the number of substitutions per site. Only bootstrap support values of >70% are indicated.
Discussion
A high level of PCV2 and PPV co-infection in pigs is common in most pig-producing countries [8]. Previous reports revealed that the prevalence of PPV1 ranges from 25.8% to 71.88% [8,17,24]. PPV6 was reported to be co-infected with multiple viruses and associated with abortion in pregnant sows [13,14]. Recently, a new species of the Parvoviruses genus, PPV7, was discovered in rectal swabs from adult pigs [11] and subsequently in Poland and Korea [20,21]. In addition, this virus has become prevalent in Guangdong and Anhui provinces in China [23]. PPV2 and PCV2 are commonly present with PPV7. In this study, we noted a higher PPV7 prevalence in serum samples than in other studies.
PPV7 is 4103 nt in length and contains two major ORFs encoding proteins 672 and 469 amino acids in length [11]. In this study, we noted that the majority of the isolates contained additional nucleotides in the middle of the cap gene. Sequence comparison revealed that within nucleotide residues 541–557 at the 5’ end of the cap gene, 14 strains had an additional 15 nucleotides, while two strains had an additional three nucleotides, leading to five additional amino acids (within residues 181–186) or one additional amino acid (within residues 181–182). Because of the increased number of amino acids, it may have an effect on the structure and function of the protein. Therefore, the influence of this change on PPV7 requires further study.
Parvoviruses are rapidly evolving viruses with high sequence diversity [2,25]. Frequent recombination between different parvoviruses has long been observed [26]. Several novel porcine parvoviruses have already spread worldwide and show some geographic variation [2,8]. To further study porcine parvoviruses, several studies have attempted to establish cell culture models for virus propagation in different cell types, including porcine kidney (PK-15 and PK-13) cells, swine testicular cells and African green monkey kidney (Vero) cells [15,27,28]. Unfortunately, PPV7 has not yet been successfully isolated.
PCV2 is the main causative agent of PCVAD [29]. Co-infection with PCV2 and other viruses (for example, PCV3, PPV or PRRSV) [18], may lead to a secondary infection following the PCV2-induced depletion of lymphocytes and aggravate clinical symptoms [30]. Some studies have found that co-infection with PCV2 and PPV4 causes more severe disease and lesions than pigs infected with PCV2 alone [14,18]. Allan etc. suggested that PPV-induced immune dysfunction promotes enhanced replication of PCV2 [14]. In this study, nearly one-third of clinical samples were PPV7-positive. Interestingly, the PCV2-positive rate was significantly higher in the PPV7-positive samples than in the non-PPV7 samples, and the difference was extremely significant (P<0.01). The results suggest that PPV7 is likely a significant co-factor in porcine circovirus-associated disease; however, further investigation is still needed to confirm this. PCV2 and PPV contribute to severe disease. Further research is needed to determine if there is any clinical significance associated with novel porcine PPV7 infection.
Conclusion
In this study, we investigated the prevalence of PPV7 in Guangxi province and conducted genome sequencing of the PPV7 strains found in this province. The high prevalence of PPV7 and high co-infection rate with PCV2 suggests that PPV7 might be co-transmitted with PCV2. Analysis of the Cap protein showed that the protein has significant variability compared with the reference isolate. To date, the number of studies focused on PPV7 is limited. Co-infection with PCV2 and the effects of Cap protein mutations on the virus should be considered in subsequent studies.
Supporting information
(DOCX)
(DOCX)
(A) Homologies of the complete sequences of PPV7 isolates. (B) Homologies of the NS1 gene of PPV7 isolates. (C) Homologies of the cap gene of PPV7 isolates.
(DOCX)
Acknowledgments
We would like to thank Ningning Hu and Jin Zhao for their assistance with reagent preparation.
Data Availability
All relevant data are within the paper and its Supporting Information files. The genome sequences of PPV7 obtained in this study have been deposited in GenBank under the accession numbers MG543456–MG543472.
Funding Statement
This work was supported by the following Grants: National Key Research and Development Program of China (Grant numbers 2017YFD0500101, 2018YFD0500803, and 2018YFD0500104), and National Natural Science Foundation of Zhejiang Province (LQ19C180001). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Phan TG, Gulland F, Simeone C, Deng X, Delwart E. Sesavirus: prototype of a new parvovirus genus in feces of a sea lion. Virus Genes. 2015; 50:134–136. 10.1007/s11262-014-1123-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cotmore SF, Agbandje-McKenna M, Chiorini JA, Mukha DV, Pintel DJ, Qiu J, et al. The family Parvoviridae. Arch Virol. 2014; 159:1239–1247. 10.1007/s00705-013-1914-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cui J, Biernacka K, Fan J, Gerber PF, Stadejek T, Opriessnig T. Circulation of Porcine Parvovirus Types 1 through 6 in Serum Samples Obtained from Six Commercial Polish Pig Farms. Transbound Emerg Dis. 2017; 64:1945–1952. 10.1111/tbed.12593 [DOI] [PubMed] [Google Scholar]
- 4.Glasa M, Palkovics L, Kominek P, Labonne G, Pittnerova S, Kudela O, et al. Geographically and temporally distant natural recombinant isolates of Plum pox virus (PPV) are genetically very similar and form a unique PPV subgroup. J Gen Virol. 2004; 85:2671–2681. 10.1099/vir.0.80206-0 [DOI] [PubMed] [Google Scholar]
- 5.Oh WT, Kim RY, Nguyen VG, Chung HC, Park BK. Perspectives on the Evolution of Porcine Parvovirus. Viruses. 2017; 9: 10.3390/v9080196 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Huang L, Zhai SL, Cheung AK, Zhang HB, Long JX, Yuan SS. Detection of a novel porcine parvovirus, PPV4, in Chinese swine herds. Virol J. 2010; 7:333 10.1186/1743-422X-7-333 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cheung AK, Wu G, Wang D, Bayles DO, Lager KM, Vincent AL. Identification and molecular cloning of a novel porcine parvovirus. Arch Virol. 2010; 155:801–806. 10.1007/s00705-010-0646-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Opriessnig T, Xiao CT, Gerber PF, Halbur PG. Identification of recently described porcine parvoviruses in archived North American samples from 1996 and association with porcine circovirus associated disease. Vet Microbiol. 2014; 173:9–16. 10.1016/j.vetmic.2014.06.024 [DOI] [PubMed] [Google Scholar]
- 9.Xiao CT, Gimenez-Lirola LG, Jiang YH, Halbur PG, Opriessnig T. Characterization of a novel porcine parvovirus tentatively designated PPV5. PLoS One. 2013; 8:e65312 10.1371/journal.pone.0065312 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cui J, Fan J, Gerber PF, Biernacka K, Stadejek T, Xiao CT, et al. First identification of porcine parvovirus 6 in Poland. Virus Genes. 2017; 53:100–104. 10.1007/s11262-016-1386-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Palinski RM, Mitra N, Hause BM. Discovery of a novel Parvovirinae virus, porcine parvovirus 7, by metagenomic sequencing of porcine rectal swabs. Virus Genes. 2016; 52:564–567. 10.1007/s11262-016-1322-1 [DOI] [PubMed] [Google Scholar]
- 12.Mayr A, Bachmann PA, Siegl G, Mahnel H, Sheffy BE. Characterization of a small porcine DNA virus. Arch Gesamte Virusforsch. 1968; 25:38–51. [DOI] [PubMed] [Google Scholar]
- 13.Opriessnig T, Halbur PG. Concurrent infections are important for expression of porcine circovirus associated disease. Virus Res. 2012; 164:20–32. 10.1016/j.virusres.2011.09.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Allan GM, Kennedy S, McNeilly F, Foster JC, Ellis JA, Krakowka SJ, et al. Experimental reproduction of severe wasting disease by co-infection of pigs with porcine circovirus and porcine parvovirus. J Comp Pathol. 1999; 121:1–11. 10.1053/jcpa.1998.0295 [DOI] [PubMed] [Google Scholar]
- 15.Ni J, Qiao C, Han X, Han T, Kang W, Zi Z, et al. Identification and genomic characterization of a novel porcine parvovirus (PPV6) in China. Virol J. 2014; 11:203 10.1186/s12985-014-0203-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Schirtzinger EE, Suddith AW, Hause BM, Hesse RA. First identification of porcine parvovirus 6 in North America by viral metagenomic sequencing of serum from pigs infected with porcine reproductive and respiratory syndrome virus. Virol J. 2015; 12:170 10.1186/s12985-015-0401-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Csagola A, Lorincz M, Cadar D, Tombacz K, Biksi I, Tuboly T. Detection, prevalence and analysis of emerging porcine parvovirus infections. Arch Virol. 2012; 157:1003–1010. 10.1007/s00705-012-1257-3 [DOI] [PubMed] [Google Scholar]
- 18.Ritzmann M, Wilhelm S, Zimmermann P, Etschmann B, Bogner KH, Selbitz HJ, et al. [Prevalence and association of porcine circovirus type 2 (PCV2), porcine parvovirus (PPV) and porcine reproductive and respiratory syndrome virus (PRRSV) in aborted fetuses, mummified fetuses, stillborn and nonviable neonatal piglets]. Dtsch Tierarztl Wochenschr. 2005; 112:348–351. [PubMed] [Google Scholar]
- 19.Sun J, Huang L, Wei Y, Wang Y, Chen D, Du W, et al. Prevalence of emerging porcine parvoviruses and their co-infections with porcine circovirus type 2 in China. Arch Virol. 2015; 160:1339–1344. 10.1007/s00705-015-2373-7 [DOI] [PubMed] [Google Scholar]
- 20.Milek D, Wozniak A, Stadejek T. The detection and genetic diversity of novel porcine parvovirus 7 (PPV7) on Polish pig farms. Res Vet Sci. 2018; 120:28–32. 10.1016/j.rvsc.2018.08.004 [DOI] [PubMed] [Google Scholar]
- 21.Ouh IO, Park S, Lee JY, Song JY, Cho IS, Kim HR, et al. First detection and genetic characterization of porcine parvovirus 7 from Korean domestic pig farms. J Vet Sci. 2018; 19:855–857. 10.4142/jvs.2018.19.6.855 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wang Y, Yang KK, Wang J, Wang XP, Zhao L, Sun P, et al. Detection and molecular characterization of novel porcine parvovirus 7 in Anhui province from Central-Eastern China. Infect Genet Evol. 2019; 71:31–35. 10.1016/j.meegid.2019.03.004 [DOI] [PubMed] [Google Scholar]
- 23.Xing X, Zhou H, Tong L, Chen Y, Sun Y, Wang H, et al. First identification of porcine parvovirus 7 in China. Arch Virol. 2018; 163:209–213. 10.1007/s00705-017-3585-9 [DOI] [PubMed] [Google Scholar]
- 24.Saekhow P, Kishizuka S, Sano N, Mitsui H, Akasaki H, Mawatari T, et al. Coincidental detection of genomes of porcine parvoviruses and porcine circovirus type 2 infecting pigs in Japan. J Vet Med Sci. 2016; 77:1581–1586. 10.1292/jvms.15-0167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mira F, Canuti M, Purpari G, Cannella V, Di Bella S, Occhiogrosso L, et al. Molecular Characterization and Evolutionary Analyses of Carnivore Protoparvovirus 1 NS1 Gene. Viruses. 2019; 11: 10.3390/v11040308 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Shackelton LA, Hoelzer K, Parrish CR, Holmes EC. Comparative analysis reveals frequent recombination in the parvoviruses. J Gen Virol. 2007; 88:3294–3301. 10.1099/vir.0.83255-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zeng S, Wang D, Fang L, Ma J, Song T, Zhang R, et al. Complete coding sequences and phylogenetic analysis of porcine bocavirus. J Gen Virol. 2011; 92:784–788. 10.1099/vir.0.028340-0 [DOI] [PubMed] [Google Scholar]
- 28.Heldt CL, Hernandez R, Mudiganti U, Gurgel PV, Brown DT, Carbonell RG. A colorimetric assay for viral agents that produce cytopathic effects. J Virol Methods. 2006; 135:56–65. 10.1016/j.jviromet.2006.01.022 [DOI] [PubMed] [Google Scholar]
- 29.Ouyang T, Zhang X, Liu X, Ren L. Co-Infection of Swine with Porcine Circovirus Type 2 and Other Swine Viruses. Viruses. 2019; 11: 10.3390/v11020185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Andersson M, Ahlberg V, Jensen-Waern M, Fossum C. Intestinal gene expression in pigs experimentally co-infected with PCV2 and PPV. Vet Immunol Immunopathol. 2011; 142:72–80. 10.1016/j.vetimm.2011.04.012 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOCX)
(DOCX)
(A) Homologies of the complete sequences of PPV7 isolates. (B) Homologies of the NS1 gene of PPV7 isolates. (C) Homologies of the cap gene of PPV7 isolates.
(DOCX)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files. The genome sequences of PPV7 obtained in this study have been deposited in GenBank under the accession numbers MG543456–MG543472.