Abstract
Objective
Metastases from prostate cancer to the brain are very unusual and very few case series have been reported in the literature. Present study was performed to assess the proportion of brain metastasis from prostate cancer among other brain metastasis in men, to evaluate the distribution, pattern and magnetic resonance imaging (MRI) appearance of these metastatic lesions, and prognosis of brain metastasis in patients with prostate cancer.
Material and methods
Between January 2010 and November 2016, 339 males who had received radiotherapy at our department were retrospectively reviewed. After the first evaluation of patients data, we reviewed only the patients with brain metastases from prostate cancer. We evaluated MRI characteristics of metastatic brain lesions and characteristics of the patients, tumor and treatment modalities.
Results
Ten of 339 patients (2.9%) had brain metastases from prostate cancer. Sixty percent of the patients had pure intraparenchymal metastasis, 20% of the patients had pure extensive dural metastasis and 20% of them had both. Seventy-five percent of the patients with intraparenchymal metastasis had multiple metastatic lesions. The median prostate specific antigen (PSA) level was 49.40 ng/mL and the Gleason score was ≥7 in all patients. Sixty percent of the patients had distant metastasis at the time of the diagnosis of prostate cancer. Median survival time in patients with brain metastasis was 4.5 months.
Conclusion
Lesions of brain metastasis from prostate cancer had a large variety of imaging presentation and it is very difficult to distinguish them from the other brain metastasis originating from other types of cancer. Presence of a disseminated disease, high PSA level and high Gleason score can be useful parameters for the prediction of brain metastasis from prostate cancer.
Keywords: Brain metastasis, magnetic resonance imaging, prostate cancer, radiotherapy, survival analysis
Introduction
Brain metastasis is a common challenge in oncology and the incidence of brain metastasis varies according to types of tumor.[1] Every year, an estimated 24%–45% of all patients with cancer develop brain metastasis.[2,3] Prostate cancer is the second most common cancer type for men in Turkey and the most common metastatic sites of prostate cancer are bone and distant lymph nodes.[4,5] Metastases from prostate cancer to the brain are very unusual and very few case series have been reported in the literature.[6,7] Approximately, 0.63% of prostate cancer patients had brain metastasis in the largest case series from M.D. Anderson Cancer Center.[7] Estimated rates of brain metastases have varied from 1% to 6% in post-mortem studies.[8–12] The current standard of care for patients with brain metastasis consists of neurosurgical resection and/or stereotactic radiosurgey and/or whole brain radiotherapy (WBRT).[2,3] Hormonal manipulation and chemotherapy are the other treatment options for brain metastases from prostate cancer.[13] Magnetic resonance imaging (MRI) is the gold standard to evaluate the location, number, size and pattern of brain metastases but there is not any evidence about specific MRI characteristics of brain metastases from prostate cancer. Herein, we report the proportion of brain metastases of prostate cancer among other brain metastases in men and the radiological findings, treatment modalities, outcomes and survival of 10 prostate cancer patients with brain metastasis who were treated with WBRT at our department.
Material and methods
Patient population
Between January 2010 and November 2016, the patients with brain metastases who had received radiotherapy at our department were evaluated in this retrospective study. After evaluation of patients’database, we reviewed only the patients with brain metastases of ostate cancer. All patients who were included in this case series had received diagnosis of prostate cancer based on pathology report and they were staged with imaging modalities. Also, all patients had at least one radiologically proven diagnosis of brain metastasis based on brain MRI. The patients with second primary cancers were excluded from the study.
The study was confirmed by the board of our university and conducted according to the ethical principles of the latest version of the Ethical Principles for Medical Research Involving Human Subjects.
Treatment
All patients underwent our department’s routine procedures for the management of brain metastasis. First, the patients underwent a treatment-planning computed tomography (CT) scan with images obtained at 3 mm slice intervals. The RT planning was based on these transverse CT scans. The required CT images were sent directly to the 3D planning system (Eclipse version 8.2, Varian, USA). All patients received RT using 3D conformal technique with fraction doses of 3 Gy, five days a week, for a total dose of 30 Gy to the brain.
The endpoints
The first primary endpoint of this study was to assess the proportion of brain metastasis from prostate cancer among the other brain metastasis from all cancer types in men and to evaluate the distribution, pattern and MRI appearance of these metastatic lesions. The second primary endpoint of this study was to evaluate the prognosis of brain metastases in patients with prostate cancer who were treated at our department.
Statistical analysis
Characteristics of patients, disease and treatment modalities were analysed with descriptive statistics. Mean, median, proportion values with their ranges, and standard deviations (SDs) were calculated for descriptive statistics. Categorical variables were compared using Pearson’s Chi-square test and continuous variables with independent samples t-test and ANOVA test. Survival analysis was performed using the Kaplan-Meier survival analysis and survival curves of subgroups were compared with-two sided long-rank test. Overall survival time was described as the time from the diagnosis to the date of the patient’s death or last follow-up. Survival time for brain metastasis was defined as the time from radiological diagnosis of brain metastasis to death or last follow-up. The time to diagnosis of brain metastasis was defined as the period in months from the date of diagnosis of primary tumor to the date of the detection of brain metastasis. Statistical analysis was carried out using Statistical Package for Social Sciences software, version 13.0 (SPSS Inc.; Chicago, IL, USA). A p value of ≤0.05 was considered statistically significant.
Results
The proportion of brain metastases with prostate cancer
Three hundred and thirty-nine male patients who underwent WBRT between January 2010 and November 2016 were retrospectively reviewed. Of these patients, 10 (2.9%) had radiologically proven brain metastases from prostate cancer.
Characteristics of patients, tumors and treatment modalities
The median age of the patients at the diagnosis of brain metastasis was 73 (range; 50–85) years. Eight patients had adenocarcinoma subtype (n=8; 80%) and the remaining patients had small cell carcinoma subtype. At the time of diagnosis of prostate cancer, the patients had (n=7 patients: 70%) had T3-4 tumor, and stage N1 nodal stage (n=7; 70%). The median prostate specific antigen (PSA) level was 49.40 (range; 3–100) ng/mL, the Gleason score was ≥7 in all patients and ≥8 in 8 patients (80%) and also all patients were in the high risk group. Six patients (60%) had distant metastases and 4 patients (40%) had curative treatment for prostate cancer at the beginning of treatment. All patients received radiotherapy at our department. Characteristics of the patients, tumor and treatment modalities are summarized in Table 1.
Table 1.
Characteristics of the patients, tumors and treatments
| Variables | No. of patients (total:10) | % |
|---|---|---|
| Age (years) | ||
| Median | 73 | |
| Range | 50–85 | |
|
| ||
| Histopathological subtype | ||
| Adenocarcinoma | 8 | 80 |
| Small cell carcinoma | 2 | 20 |
|
| ||
| Initial T-stage | ||
| T1–2 | 3 | 30 |
| T3–4 | 7 | 70 |
|
| ||
| Initial N-stage | ||
| N0 | 3 | 30 |
| N1 | 7 | 70 |
|
| ||
| Initial M-stage | ||
| M0 | 4 | 40 |
| M1 | 6 | 70 |
|
| ||
| PSA (ng/mL) | ||
| Mean | 49.40 | |
| Range | 3–100 | |
|
| ||
| Gleason score | ||
| ≥7 | 10 | 100 |
| ≥8 | 8 | 80 |
|
| ||
| Curative prostate surgery | ||
| Yes | 4 | 40 |
| No | 6 | 60 |
|
| ||
| Postoperative prostate radiotherapy | ||
| Yes | 2 | 20 |
| No | 8 | 80 |
|
| ||
| Brain irradiation dose | ||
| 30 Gy | 5 | 50 |
PSA: prostate-specific antigen
Neurological findings
Seven patients (70%) had at least one neurological deficit. The most common clinical presentation of brain metastasis from prostate cancer was headache (40%) and the predominant focal neurologic presentation was hemiparesis (50%).
Radiological findings
Patients (n=6; 60%) had pure intraparenchymal metastasis, 2 patients (20%) pure extensive dural metastasis (n=2; 20%) and both (n=2; 20%). Six patients (75%) with intraparenchymal metastasis had multiple metastatic lesions in brain. 4 cases (40%) had supratentorial metastasis only, 1 patient (10%) had infratentorial lesions only and 5 patients (50%) had both.
Nine patients (90%) had solid metastases while partially cystic and solid metastasis was found in only 1 patient (10%). Seven patients (70%) had edema invoked by their lesions. There was no hemorrhagic metastasis identified.
Survival analysis and outcomes
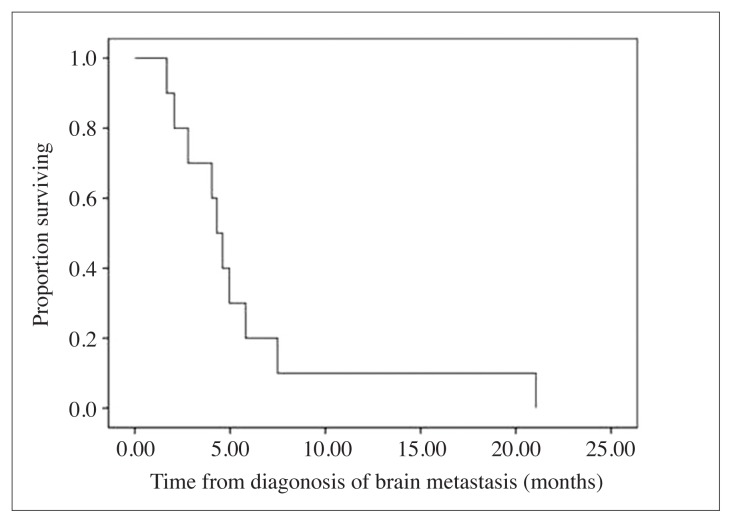
The median follow-up time was 27 (range; 3–108) months. All patients died due to the disease when the survival analysis was performed. The median OS was 19 (range; 1–45) months. The median time to diagnosis of brain metastasis was 24 (range; 0–24) months and the median time of survival from brain metastasis survival was 4.5 (range; 2–21; Figure 1) months. The median time of palliation of symptoms was 3.5 (range; 1–19) months.
Figure 1.
Survival rates of the patients with brain metastasis
At the time of diagnosis of brain metastasis, all patients had concurrent distant metastasis. Patients had concurrent bone metastasis (n=9; 90%), concurrent distant lymph node metastasis (n=5; 50%), concurrent lung metastasis (n=3; 30%), concurrent liver metastasis (n=3; 30%), concurrent adrenal gland metastasis (n=1; 10%). Otherwise, 8 extracranial disease continued to worsen in 8 patients, led a stable course in 2 patients at the time of diagnosis of brain metastasis.
Discussion
Metastases from prostate cancer to the brain are very unusual and the incidence of brain metastasis from prostate cancer is poorly documented.[6,7,14] In our study, 339 males received radiotherapy between January 2010 and November 2016 and in 2.9% of them brain metastases from prostate cancer were detected. Before our research, only one study investigated the proportion of brain metastasis from prostate cancer among the other brain metastasis in men.[15] In a study from Sweden, including 15.517 patients with brain metastasis diagnosed from 1987 to 2006, in 8.6% of these patients prostate cancer was detected. Prostate cancer was the fourth most common primary malignancy among men admitted with brain metastasis in their study.[15] Their rates of brain metastasis were higher than ours but the authors revealed in their study that they couldn’t avoid some degree of misclassification of skull or skull base metastases as brain metastases because of the similarity of symptoms’.
According to our results, patients had pure intraparenchymal metastasis (n=6; 60%) pure extensive dural metastasis (n=2; 20%) or both (n=2; 20%). But Lynes et al.[11] reported that the most common intracranial location of prostate cancer metastasis was the leptomeninges (65%), cerebrum (25%) and cerebellum (8%). A possible explanation for this difference is that Lynes et al.’s[11] study was conducted in 1986, thus this study included many patients diagnosed only with autopsy and/or CT imaging rather than MRI and it is possible that before introduction of MRI into clinical practice many intraparenchymal lesions in the brain might go undetected.
In addition, 75% of our patients with intraparenchymal metastases had multiple metastatic lesions. Similarly, in the study of Memorial-Sloan-Kettering Cancer Center, 71% of their patients had multiple intraparenchymal metastatic lesions.[6] But, in the study of Tremont-Lukats et al.[7], only 14% of the patients had multiple intraparenchymal metastatic lesions. The lower rate can be explained by the time interval of study which included in the patients treated between 1944 and 1998, so some parenchymal lesions could not be diagnosed because MRI was not used routinely at that time.
Our study patients had supratentorial metastasis (n=4; 40%), infratentorial lesions (n=1; 10%) and both (n=5; 50%). Similarly, in the study of Memorial-Sloan-Kettering Cancer Center, 48% of their patients had pure supratentorial metastases, 48% of them had both supratentorial and infratentorial metastases and none of the patients had pure infratentorial lesion.[6] But, in the study of Tremont-Lukats et al.[7], 67% of their patients had pure supratentorial lesions, 21% of them had pure infratentorial lesions and 3% of the cases had both of them. We know that, Tremont-Lukats et al.’s[7] study is the largest study that included in over than 16.000 prostate cancer patients and 131 of these patients had craniospinal metastases. So, the rarity of infratentorial metastasis can be explained by the scarce number of patients in our and Memorial- Sloan-Kettering Cancer Center’ studies which included 21 patients with brain metastasis from prostate cancer.
Any specific MRI characteristics have not been defined for brain metastasis from prostate cancer. In our study, the lesions of brain metastasis from prostate cancer had a large variety imaging presentations and it is very difficult to distinguish them from the other brain metastasis from other types of cancer. In the study of Memorial- Sloan-Kettering Cancer Center, the authors reported that 7 patients (33%) had hemorrhagic metastasis and the conntrast-enhancement pattern varied from purely solid, to mixed cystic and solid, to ring-like images.[6] Our results confirm the uncertainty of MRI characteristics of brain metastasis from prostate cancer.
The median survival time for patients with brain metastasis was 4.5 (range; 2–21) months in our study. In published studies, the median survival time for patients with brain metastasis from prostate cancer have ranged between 1 month to 7.7 months and our results confirm that the prognosis of brain metastasis from prostate cancer is poor like the other brain metastases from the other types of cancer.[6,7,10–14] Otherwise, at the time of diagnosis of brain metastasis, all patients had concurrent distant metastasis and extracranial disease of 80% of the patients continued to worsen. Similarly, in the study of Tremont-Lukats et al.[7], except one patient, all the other patients had concurrent distant metastases. The median baseline PSA level was 49.40 (range; 3–100) ng/mL in all patients and the median baseline PSA level was 61.75 ng/mL in non metastatic patients at the time of the diagnosis of prostate cancer. Besides, the Gleason score was ≥7 in all patients and ≥8 in 8 patients. Consequently, all of the patients were in the high risk group at the diagnosis of prostate cancer. Similarly, Hatzoglou et al.[6] reported in their study that patients with Gleason score <6 had lower risk than those having Gleason scores of ≥6. But the large case series did not show the PSA level of their patients so we could not compare our results with them. But these tumor characteristics can be meaningful for understanding the risk factors of brain metastasis originating from prostate cancer.
The major limitation of this study was that in none of the patients brain lesions were confirmed by pathology. But all of the patients had concurrent distant metastases at the time of detection of brain metastases, which made histopathological examination an unnecessary, and uncomfortable procedure for the patients.
In conclusion, although prostate cancer rarely metastasize to the brain, it may occur in disseminated disease and in cases with high risk prostate cancer. Our findings suggest that, the proportion of brain metastasis of prostate cancer among the other brain metastasis in men was 2.9%. The metastatic lesions of brain originating from prostate cancer had a large variety of imaging presentations and it is very difficult to distinguish them from the other brain metastasis arising from other types of cancer. Presence of a disseminated disease, high PSA level and high Gleason score can be useful parameters for the prediction of brain metastasis from prostate cancer.
Footnotes
Ethics Committee Approval: Ethics committee approval was received for this study from the ethics committee of Necmettin Erbakan University Meram School of Medicine in 16.12.16 (Protocol number: 2016/748).
Informed Consent: Written informed consent can not be obtained from patients because all patients died before initiation of the study.
Peer-review: Externally peer-reviewed.
Author Contributions: Concept – G.K., M.K.; Design – G.K., M.K.; Supervision – M.K.; Resources – G.K., M.A., B.B.Y., M.K.; Materials – G.K., M.A., B.B.Y.; Data Collection and/or Processing – G.K., M.A., B.B.Y.; Analysis and/or Interpretation – G.K., M.A., B.B.Y., M.K.; Literature Search – G.K.; Writing Manuscript – G.K.; Critical Review – G.K., M.K.
Conflict of Interest: No conflict of interest was declared by the authors.
Financial Disclosure: The authors have declared that they did not receive any financial support for this study.
References
- 1.Gavrilovic IT, Posner JB. Brain metastases: epidemiology and pathophysiology. J Neurooncol. 2005;75:5–14. doi: 10.1007/s11060-004-8093-6. [DOI] [PubMed] [Google Scholar]
- 2.Eichler AF, Loeffler JS. Multidisciplinary management of brain metastases. Oncologist. 2007;12:884–98. doi: 10.1634/theoncologist.12-7-884. [DOI] [PubMed] [Google Scholar]
- 3.Sawaya R, Bindal RK, Lang FF, Abi-Said D. Metastatic brain tumors. In: Kaye AH, Laws ER, editors. Brain Tumors An Encyclopedic Approach. 2nd ed. London: Churchill Livingstone; 2001. pp. 999–1026. [Google Scholar]
- 4.Turkish Institude of Public Health. Cancer statistics in Turkey. Ankara, Turkey: 2014. Available from: http://kanser.gov.tr/Dosya/kayitcilik/2009kanseraporu.pdf. [Google Scholar]
- 5.Gandaglia G, Abdollah F, Schiffmann J, Trudeau V, Shariat SF, Kim SP, et al. Distribution of Metastatic Sites in Patients with Prostate Cancer: A Population Based Analysis. Prostate. 2014;74:210–6. doi: 10.1002/pros.22742. [DOI] [PubMed] [Google Scholar]
- 6.Hatzoglou V, Patel GV, Morris MJ, Curtis K, Zhang Z, Shi W, et al. Brain metastasis from prostate cancer: An 11-Year Analysis in the MRI Era with Emphasis on Imaging Characteristics, Incidence, and Prognosis. J Neuroimaging. 2014;24:161–6. doi: 10.1111/j.1552-6569.2012.00767.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tremont-Lukats IW, Bobustuc G, Lagos GK, Lolas K, Kyritsis AP, Puduvalli VK. Brain metastasis from prostate carcinoma: the M.D. Anderson Cancer Center experience. Cancer. 2003;98:363–8. doi: 10.1002/cncr.11522. [DOI] [PubMed] [Google Scholar]
- 8.Catane R, Kaufman J, West C, Merrin C, Tsukada Y, Murphy GP. Brain metastasis from prostatic carcinoma. Cancer. 1976;38:2583–7. doi: 10.1002/1097-0142(197612)38:6<2583::aid-cncr2820380652>3.0.co;2-d. [DOI] [PubMed] [Google Scholar]
- 9.Saitoh H, Hida M, Shimbo T, Nakamura K, Yamagata J, Satoh T. Metastatic patterns of prostatic cancer. Correlation between sites and number of organs involved. Cancer. 1984;54:3078–84. doi: 10.1002/1097-0142(19841215)54:12<3078::aid-cncr2820541245>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- 10.Taylor HG, Lefkowitz M, Skoog SJ, Miles BJ, McLeod DG, Coggin JT. Intracranial metastases in prostate cancer. Cancer. 1984;53:2728–30. doi: 10.1002/1097-0142(19840615)53:12<2728::aid-cncr2820531231>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]
- 11.Lynes WL, Bostwick DG, Freiha FS, Stamey TA. Parenchymal brain metastases from adenocarcinoma of prostate. Urology. 1986;28:280–7. doi: 10.1016/0090-4295(86)90005-1. [DOI] [PubMed] [Google Scholar]
- 12.Demierre B, Berney J. Intracranial metastases of cancer of the prostate. Neurochirurgie. 1983;29:143–9. [PubMed] [Google Scholar]
- 13.Caffo O, Veccia A, Fellin G, Mussari S, Russo L, Tomio L, et al. Frequency of brain metastases from prostate cancer: an 18-year single-institution experience. J Neurooncol. 2013;111:163–7. doi: 10.1007/s11060-012-0994-1. [DOI] [PubMed] [Google Scholar]
- 14.Caffo O, Veccia A, Russo L, Galligioni E. Brain metastases form prostate cancer: an emerging clinical problem with implications for the future therapeutic scenario. Future Oncol. 2012;8:1585–95. doi: 10.2217/fon.12.156. [DOI] [PubMed] [Google Scholar]
- 15.Smedby KE, Brandt L, Bäcklund ML, Blomqvist P. Brain metastases admissions in Sweden between 1987 and 2006. Br J Cacer. 2009;101:1919–24. doi: 10.1038/sj.bjc.6605373. [DOI] [PMC free article] [PubMed] [Google Scholar]